Abstract
In Florida, strawberries are prone to infestation by Tetranychus urticae Koch (twospotted spider mite; Trombidiformes: Tetranychidae) and Scirtothrips dorsalis Hood (chilli thrips; Thysanoptera: Thripidae). Management of these pests using conventional insecticides is becoming difficult, thereby forcing many growers to adopt the use of commercially available biological control agents including Neoseiulus cucumeris Oudemans and Amblyseius swirskii Athias-Henriot (both Mesostigmata: Phytoseiidae). These predators are mass reared on prey different from T. urticae, and their prey switching capacity is unknown. Therefore, the objective of this study was to compare the predation capacity of A. swirskii and N. cucumeris feeding on familiar and unfamiliar prey. Thus, using T. urticae as a non-familiar prey model organism, a no choice test was conducted where A. swirskii and N. cucumeris were each provided with Acarus spp. (commonly used as prey for commercial rearing of predatory mites) as familiar prey and eggs, deutonymphs, and adult females of T. urticae as unfamiliar prey. Prey consumption was recorded at 12, 24, 36 and 48 h. Our results show that both A. swirskii and N. cucumeris exhibited higher prey consumption when prey was familiar (Acarus spp.) compared to unfamiliar prey. Additionally, both predators showed higher consumption of eggs compared to T. urticae nymphs and adults. In 12 h, both predators had consumed 50 % of eggs, however, 36–48 h were required for 50 % of the T. urticae adults and nymphs to be consumed. Therefore, strawberry growers utilizing these predatory mites ought to refrain from the use of insecticides known to be detrimental to predatory mites for a period of at least 48 h to allow the predators to adjust to the new prey and environment.
Resumen
En Florida, las fresas son propensas a la infestación por Tetranychus urticae Koch (arañita roja de dos manchas; Trombidiformes: Tetranychidae) y Scirtothrips dorsalis Hood (trips del chile; Thysanoptera: Thripidae). El manejo de estas plagas utilizando insecticidas convencionales se está volviendo difícil, lo que obliga a muchos productores a adoptar el uso de agentes de control biológico disponibles comercialmente, incluidos Neoseiulus cucumeris Oudemans y Amblyseius swirskii Athias-Henriot (ambos Mesostigmata: Phytoseiidae). Estos depredadores se crían en masa usando presas diferentes a T. urticae y por lo tanto se desconoce su capacidad de cambiar de presa. El objetivo de este estudio fue comparar la capacidad de depredación de A. swirskii y N. cucumeris alimentándose de presas familiares o desconocidas. Por lo tanto, utilizando T. urticae como organismo modelo presa no familiar, se realizó una prueba sin elección en la que A. swirskii y N. cucumeris recibieron Acarus spp. (comúnmente utilizada como presa para la cría comercial de ácaros depredadores) como presa familiar; y huevos, deutoninfas y hembras adultas de T. urticae como presa desconocida. El consumo de presas se registró a las 12, 24, 36 y 48 horas. Nuestros resultados muestran que tanto A. swirskii como N. cucumeris exhibieron un mayor consumo de presas cuando estas eran familiares (Acarus spp.) en comparación con presas desconocidas. Además, ambos depredadores mostraron un mayor consumo de huevos en comparación con las ninfas y los adultos de T. urticae. En 12 horas, ambos depredadores habían consumido el 50 % de los huevos, sin embargo, se necesitaron de 36 a 48 horas para que se consumiera el 50 % de los adultos y ninfas de T. urticae. Por lo tanto, los productores de fresas que utilizan estos ácaros depredadores deben abstenerse del uso de insecticidas, que se sabe que son perjudiciales para los ácaros depredadores, durante un período de al menos 48 horas para permitir que los depredadores se adapten a la nueva presa y al entorno.
1 Introduction
In 2021, strawberry (Fragaria x ananassa Rosaceae) production in Florida was valued at approximately US $399 million dollars (Hudson 2022), making the state the second largest producer of strawberries in the USA and the highest producer of winter strawberries (Guan et al. 2016). Similar to other crops grown in the state, strawberries face various arthropod pests such as phytophagous thrips (Thysanoptera: Thripidae), particularly Frankliniella occidentalis Pergande and Scirtothrips dorsalis Hood (Lahiri and Panthi 2020; Lahiri et al. 2022; Panthi and Renkema 2020; Renkema et al. 2020). In addition, spider mites (Trombidiformes: Tetranychidae), especially Tetranychus urticae Koch commonly known as twospotted spider mites, are frequently found damaging strawberries (Lahiri et al. 2022; Nyoike and Liburd 2013; Zhou et al. 2023). During the strawberry season, it is common to find numerous fields experiencing simultaneous infestations of S. dorsalis and T. urticae (Lahiri 2023).
Management of these pests has centered around the use of insecticides and miticides, however there is growing evidence that these pests have already developed resistance to these compounds (Kaur and Lahiri 2022; Kaur et al. 2023; Lahiri and Panthi 2020; Lahiri et al. 2024). Twospotted spider mites have been reported to already exhibit resistance to a variety of miticides used in their management (Van Leeuwen et al. 2010). In addition to development of resistance, chemical management of these pests can have unintended effects on beneficial insects found in strawberry such as Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) and Chrysoperla sinica (Tjeder) (Neuroptera: Chrysopidae) used in aphid management (Bommarco et al. 2011; Liu et al. 2016) and predatory mites released for S. dorsalis and T. urticae management (Busuulwa et al. 2024). Furthermore, the environmental fate of these insecticides has continuously raised concern as their increased persistence in soils could have implications for human health (Adeyinka et al. 2023; Hasnaki et al. 2023).
Over the years, considerable attention has been given to the use of biological control agents in managing various strawberry pests, both in the greenhouse and in the field. Currently, augmentative biological control is gaining popularity as an alternative to chemical control in strawberry pest management (Lahiri et al. 2022). Special attention has been given to predatory mites in the Phytoseiidae family due to their capacity to prey on many phytophagous mites and different thrips species (Cock et al. 2010).
For example, the specialist predatory mite Phytoseiulus persimilis Athias-Henriot (Mesostigmata: Phytoseiidae) has been used successfully in managing T. urticae populations both in greenhouses and fields (Cloyd et al. 2006; Raworth 2001; Rhodes and Liburd 2006). Similarly, commercially available generalist predators such as Neoseiulus cucumeris Oudemans, Amblyseius swirskii (Athias-Henriot), and Neoseiulus californicus McGregor (all Mesostigmata: Phytoseiidae) have been used to control a variety of soft bodied arthropod pests such as western flower thrips (F. occidentalis), common blossom thrips Frankliniella schultzei Trybom (Thysanoptera: Thripidae), broad mites Polyphagotarsonemus latus (Banks) (Trombidiformes: Tarsonemidae), and twospotted spider mites on various crops (Arthurs et al. 2009; Rhodes and Liburd 2006).
The unique ability of these generalist predatory mites to feed not only on insects and mites, but also on pollen has contributed to their wide adaptation for use in many biological control programs (McMurtry et al. 2013). Given that strawberries in Florida experience simultaneous infestation from both S. dorsalis and T. urticae (Lahiri et al. 2022), using a specialist predatory mite such as P. persimilis would not be effective in managing both pests. Therefore, reliance on the generalist predatory mites, A. swirskii and N. cucumeris for management of both S. dorsalis and T. urticae has become prevalent (Lahiri and Yambisa 2021). This is because both A. swirskii and N. cucumeris have been found to be effective in feeding on both pests (Buitenhuis et al. 2015; Dalir et al. 2021; Easterbrook et al. 2001). Although N. californicus is a generalist, it has been shown to have a higher affinity for T. urticae, compared to thrips (Blackwood et al. 2001; Cloyd et al. 2006; McMurtry et al. 2013), therefore limiting its use in strawberries for management of S. dorsalis and T. urticae simultaneous infestations.
Given that A. swirskii and N. cucumeris control a wide variety of soft bodied arthropod pests (McMurtry et al. 2013), improving their predation efficacies has become an important aspect of biocontrol. The most common approach for achieving this has been through predator behavioral modifications resulting from early life exposure of the predatory mites to pests (Schausberger et al. 2021). Schausberger et al. (2010) showed that N. californicus and A. swirskii posed the ability to not only learn to prey on a new food source but also imprint on prey that they experienced early in life. The exposure of generalist predatory mites to a variety of potential prey, early on in life, permits them to learn to recognize and feed on the prey more efficiently in their adulthood (Rahmani et al. 2009; Reichert et al. 2017; Schausberger et al. 2021, 2010; Seiter and Schausberger 2015). This comes as a result of positive modulation of trophic top-down cascades where learned predators become more efficient, thus facilitating high optimization of large-scale augmentative biocontrol (Schausberger et al. 2021; Seiter and Schausberger 2016).
Nonetheless, commercially available generalist predatory mites especially N. californicus, N. cucumeris, and A. swirskii are mass reared using factitious prey, such as the flour mite Acarus siro L. (Acari: Acaridae), the dried fruit mite Carpoglyphus lactis L. (Acari: Carpoglyphidae) (Nguyen et al. 2013), and Thyreophagus entomophagus Laboulbène (Acari: Acaridae) (Fidgett and Stinson 2008). Furthermore, commercial producers do not include protocols that allow predatory mites to readjust to their new target prey and environment. Therefore, this creates the need to understand the predation capability of commercially available generalist predatory mites before their release and how long it would take them to learn new prey in order to provide a baseline of expectations which may have vital implications for integrated pest management practices.
It is important to mention that previous research on this topic has centered around the exposure of prey-naïve and prey-experienced predators to the same prey species (Reichert et al. 2017; Schausberger et al. 2021, 2018; Schausberger and Peneder 2017; Seiter and Schausberger 2016, 2015). Therefore, the objective of this study was to compare the predation capability of commercially available N. cucumeris and A. swirskii feeding on known prey (Acarus spp.) and various stages of T. urticae (unfamiliar prey) in the context of strawberry production. We hypothesize that commercially available N. cucumeris and A. swirskii will possess low predation capacity on T. urticae due to prey unfamiliarity.
2 Materials and methods
2.1 Pre-experimental procedures
2.1.1 Bean plant propagation
Lima bean seeds (Phaseolus vulgaris L; Fabales: Fabaceae) (Henderson Bush Kellogg Seed Co., California, USA) were sown in plastic pots measuring 14.6 × 15.24 cm (Kord Regal Standard plastic pots, The HC Companies, Twinsburg, Ohio, USA) filled with general purpose potting soil (ProMix BX, Sun Gro Horticulture, Agawam, Massachusetts, USA). The plastic pots were then placed on trays measuring 30.48 × 40.64 cm (Choice Teal Plastic Fast Food products, Michigan, USA), with each tray containing four plastic pots. Each tray was then placed in a rearing and observation cage measuring 61 × 61 × 61 cm (Bioquip, Compton, California, USA). The whole setup was placed in a growth room maintained at 25 ± 1 °C, relative humidity (RH) of 65 ± 5 %, and 14:10 h L:D photoperiod. Watering of the plants was done twice a week by filling the tray with approximately 1.5 L of water mixed with 16 ml of fertilizer (J R Peters Classic 20-20-20 All Purpose Fertilizer, Allentown, Pennsylvania, USA). The bean plants were allowed to grow for 3 weeks.
2.1.2 Tetranychus urticae rearing
T. urticae was selected as the model prey due to its suitability as a food source for both predators. Colonies of T. urticae used in the experiments were obtained from a natural population that had established on non-treated strawberry plants in the greenhouse. The identification of T. urticae was confirmed using the key published by Alatawi and Kamran (2018) by examining the male aedeagus. The knob of the aedeagus was found to have a small posterior projection, with a length shorter than the width of the aedeagal neck.
The populations were examined for healthy individuals that were transferred onto bean plants by taking a heavily infested strawberry leaf and placing it in a rearing and observation cage containing four pots of the 3-week-old bean plants. T. urticae was reared on bean plants because of their rapid establishment on beans in comparison to strawberries (personal observation by A. Busuulwa). The newly established colony was maintained in a growth room at 27.1 ± 1 °C, 51.45 ± 4.77 % RH, and a photoperiod of 14:10 (L:D). The temperature and humidity data were recorded using a HOBO U23 Pro V2 Temperature/Relative Humidity Data Logger (Onset, Bourne, Massachusetts, USA). T. urticae colonies were allowed a period of 3 weeks in order to fully establish. Senescing bean plants were replaced with fresh bean plants in the rearing cages to allow T. urticae colonization of the new plant with minimum handling.
2.1.3 N. cucumeris and A. swirskii colonies
Commercially available predatory mites (N. cucumeris and A. swirskii) used in the experiments were purchased from Arbico Organics (Arizona Biological Control Inc., Tucson, Arizona, USA). On arrival to the laboratory, the predatory mites were kept in their original containers in a 45 L ice cooler (Island Breeze Family, Igloo, Katy, Texas, USA) packed with eight foam refrigerants (Polar Tech Industries Inc., Genoa, Illinois, USA) that had been kept in a freezer overnight. The foam refrigerants were replaced every two days until the beginning of experiments. Both predatory mite packages came with live food (Acarus spp.), intermixed with bran and vermiculite.
2.2 Experimental procedure
Sixty active gravid females of N. cucumeris and A. swirskii were randomly selected from the coolers. Each N. cucumeris and A. swirskii was placed separately on an individual strawberry leaf disc arena using a 3/0 fine paint brush (Artist Brush Keep Smiling, Shenzhen Eseng International Co. Ltd., Shenzhen, China), and starved for 48 h in a growth chamber at a temperature of 25 ± 1 °C, 65 ± 5 % RH and a photoperiod of 14:10 (L:D). The leaf disc arenas were made following original designs by Montemayor et al. (2023). The arenas consisted of a 1.9 cm circular leaf disc of a commonly grown strawberry cultivar ‘Florida Brilliance’ (Whitaker et al. 2019), placed with the abaxial surface facing downwards on top of 1 % agar (Fisher BioReagents Fair Lawn, New Jersey, USA). The agar was placed on a 2.2 cm (diameter) piece of floral foam (FloraCraft Corporation, Ludington, Michigan, USA) that had been glued to a 59.2 ml plastic condiment cup (Comfy packages Brooklyn, New York, USA) half filled with deionized water to create a moat that prevented mites from escaping. In order to facilitate proper ventilation, a circular opening approximately 0.5 cm was cut into the lid using an electric plastic cutter (Winons, WHK-0005, China) and covered with clear vinyl. An individual T. urticae-naïve N. cucumeris or A. swirskii from the starved colony was selected and placed on a new leaf disc arena and provisioned randomly with one of the four diet options in a no-choice test as shown in Table 1. Each prey type was replicated fifteen times. The predators were considered naïve due to lack of previous experience in handling and feeding on any of the life stages of T. urticae.
Prey provided to Neoseiulus cucumeris or Amblyseius swirskii in leaf-disc arenas.
| Treatment | Amount provided |
|---|---|
| Tetranychus urticae eggs | 50 |
| Tetranychus urticae adult gravid females | 5 |
| Tetranychus urticae deutonymphs | 10 |
| Acarus spp. adult gravid females (control) | 5 |
Prey familiar to the predatory mites used in the experiment as the control (Acarus spp.) were those that were shipped with N. cucumeris and A. swirskii colonies. Deutonymphs of T. urticae used in the experiment were obtained by randomly transferring ten T. urticae gravid females onto strawberry leaf disc arenas, allowing them to lay eggs for 24 h and subsequently removing the females. These eggs were then placed in a growth chamber and maintained at a temperature of 25 ± 1 °C, 85 ± 1 % RH and a photoperiod of 14:10 h L:D, for seven days to allow development of similarly aged deutonymphs. T. urticae eggs also were obtained as described above. Upon commencement of the experiment, the number of T. urticae adults, eggs, deutonymphs, or Acarus spp. (control) consumed by N. cucumeris and A. swirskii were recorded at 12, 24, 36, and 48 h and the proportion of each prey item consumed every 12 h was calculated. Consumed prey was not replaced throughout the duration of the experiment. Two experimental trials were carried out. The recording of prey observations extended up to 48 h, as it became evident that after this time frame, a notable shift occurred with prey, primarily eggs and nymphs undergoing transformative changes into different life phases.
2.3 Data analysis
All analyses were conducted in R version 4.2.2 (R Core Team 2022) to test our hypothesis that commercially available N. cucumeris and A. swirskii have low predation capacity for T. urticae due to prey unfamiliarity, a generalized linear mixed effects model (GLMM) was constructed. First, the proportion of prey consumed was calculated as prey consumed divided by the total number of prey provided at the beginning of the experiment. As a result, a value of 1 indicated 100 % consumption and a value of 0 indicated 0 % consumption. Proportion of prey consumed was considered as the response variable assuming a beta error distribution. Given that the beta distribution does not accept proportions of exactly one or zero, a small correction was applied to the data to remove absolute zeros and ones. The correction was done as follows, y″ =
Model fitting was done using the glmmTMB package (Brooks et al. 2017). Analysis of deviance was performed using car:Anova (Fox et al. 2013). The model was inspected for autocorrelation using performance: Check_autocorrelation (Lüdecke et al. 2021), residuals were found to be independent and not autocorrelated (p = 0.286). Model fitness was assessed by visual inspection of residuals using the DHARMa package (Hartig 2022). The assessment showed that both assumptions of normality and homoscedasticity were reasonably met. Linear contrasts were done using the Tukey adjustment for multiple comparisons using emmeans:emmeans (Lenth 2020). To determine pooled prey consumption across both species of predatory mites, a post hoc means contrast was performed, comparing consumption of different prey. To estimate consumption of different prey types by different species of predatory mites, a post hoc means contrast was done comparing consumption of different prey by each species of predatory mites. To further understand the effect of time on prey consumption, a post hoc means contrast was performed, comparing consumption of different prey between the two species of predatory mites at individual time points. All the above contrasts were constructed by pooling across all other factors in the model. Means and confidence intervals (CI) were back transformed before being reported in text and figures.
3 Results
Our analysis showed that there was an effect of prey type (χ2 = 432.42, df = 3, P < 0.001) and duration (χ2 = 703.36, df = 3, P < 0.001) on the proportion of prey consumed by both predators. Nonetheless, there was no effect of predator species on prey consumption (χ2 = 2.401, df = 1, P = 0.12). The effect of trial was also not significant (χ2 = 0.58, df = 1, P = 0.45), and, as a result, trial was subsequently removed from the model. On the other hand, the interaction of prey type with species of predatory mite was significant (χ2 = 117.51, df = 3, P < 0.001). Similarly, the interaction of prey type (treatment) with duration (χ2 = 75.99, df = 9, P < 0.001) and predatory mite species with time (χ2 = 12.75, df = 3, P = 0.0052) were significant. In addition, the three-way interaction of prey type (treatment) with species of predatory mites and time was significant (χ2 = 24.54, df = 9, P = 0.0035).
On average, the proportion of pooled prey consumption across both predatory mite species and time was higher when the prey was Acarus spp. (familiar prey control) compared to the other three prey items (Table 2). No statistical differences were observed between pooled average consumption of nymphs (38.8 % CI: 35.41–42.34 %) nor adults (36.9 % CI: 33.60–40.43 %). At the species level, consumption of prey varied depending on the type of prey (Figure 1). In both predatory mite species, the proportion of Acarus spp. (familiar prey “control”) consumed was higher than any other prey (A. swirskii (69.7 % CI: 65.5–73.6 %) and N. cucumeris (88.2 % CI: 85.8–90.3 %), followed by T. urticae eggs (A. swirskii (52.0 % CI: 47.4–56.6 %) and N. cucumeris (57.6 % CI: 52.7–62.4 %), nymphs (A. swirskii (38.1 % CI: 33.8–42.6 %) and N. cucumeris (35.9 % CI: 31.4–40.6 %), and adults (A. swirskii (48.9 % CI: 44.3–53.5 %) and N. cucumeris (29.7 % CI: 25.7–34.1 %)). However, in N. cucumeris there was no difference in consumption between nymphs (35.8 % CI: 31.40–40.60 %) and adults (29.7 % CI: 25.7–34.1 %). On the contrary, in A. swirskii significantly higher consumption of adults (48.9 % CI: 44.2–53.4 %) was observed compared to nymphs (38.1 % CI: 33.80–42.60 %).
Average proportion of unfamiliar prey (Tetranychus urticae) consumed pooled across both species of predatory mites (Neoseiulus cucumeris or Amblyseius swirskii) and time.
| Prey type | Proportion of prey consumed | LCL | UCL |
|---|---|---|---|
| Control (Acarus spp.) | 0.806 a | 0.781 | 0.83 |
| Eggs | 0.548 b | 0.512 | 0.58 |
| Adults | 0.388 c | 0.35 | 0.42 |
| Nymphs | 0.369 c | 0.34 | 0.41 |
-
The column “Prey type” contains the two species of prey (Acarus spp. adults and T. urticae eggs, nymphs and adults) provided to the predatory mites. The columns “LCL and UCL” correspond to the lower and upper confidence intervals, respectively. Proportions of prey consumed that differ based on linear contrasts (P < 0.05) are differentiated by having different letters.
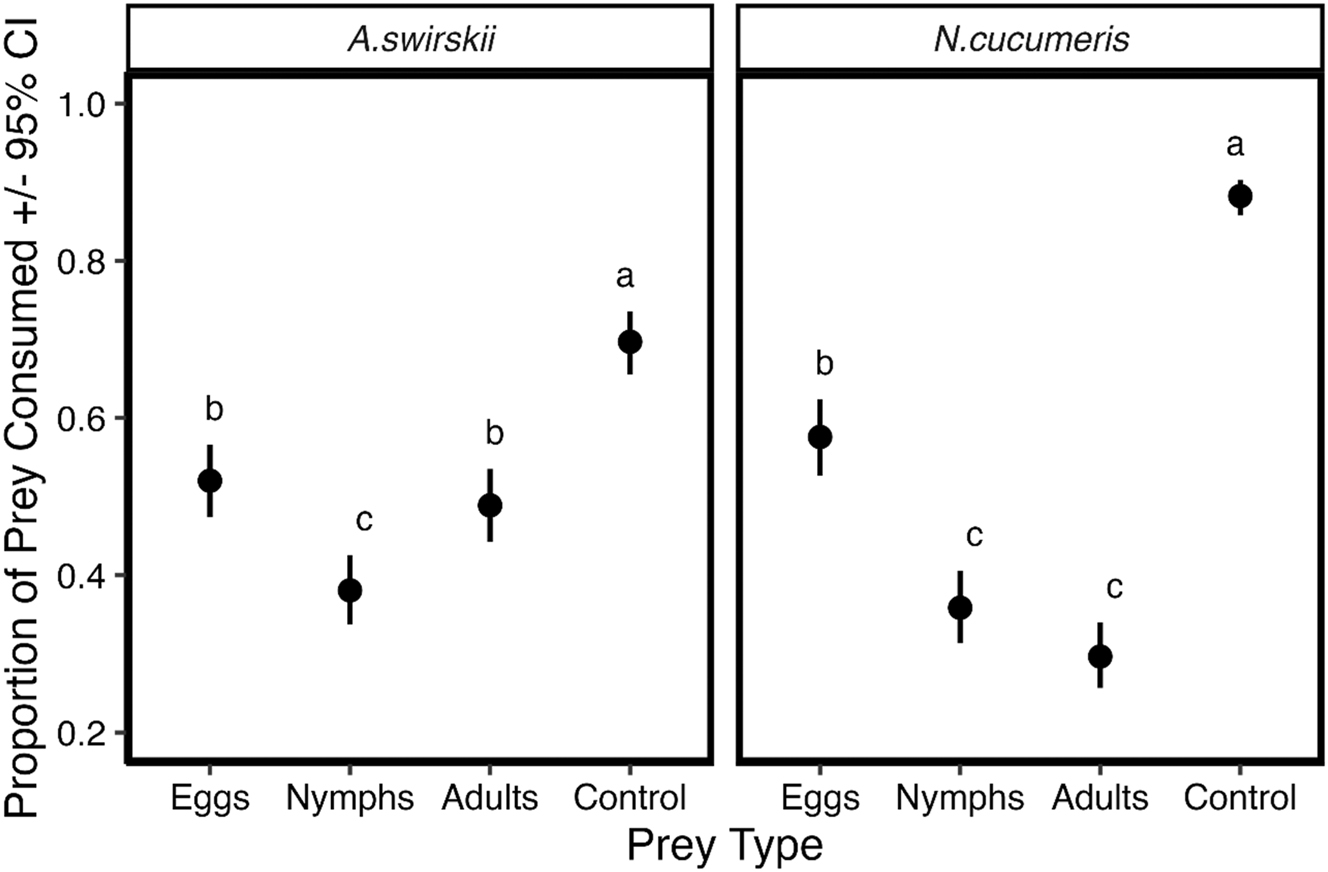
Proportion of egg, nymph and adult Tetranychus urticae and adult Acarus spp. (control) consumed by each species of predatory mite (Neoseiulus cucumeris and Amblyseius swirskii) averaged across time. Proportions of prey consumed that differ based on linear contrasts (Tukey P < 0.05) are differentiated by having different letters.
Overall, when we compared prey consumption for adult T. urticae (unfamiliar prey) and adult Acarus spp. (familiar prey control), we observed that the proportion of prey consumed by both A. swirskii and N. cucumeris increased over time (Figure 2). However, the rate of increase in prey consumption was higher for familiar prey compared to unfamiliar prey. Additionally, when provided with adult T. urticae as prey, we observed a difference in prey consumption between the two predators. The proportion of prey consumed at all time intervals was higher for A. swirskii compared to N. cucumeris. Conversely, when Acarus spp. was provided as a food source, the opposite trend was observed in that prey consumption was higher for N. cucumeris compared to A. swirskii, with the exception that there were no differences in the proportion of Acarus spp. consumed by both predatory mite species at 48 h of observation (Figure 2).
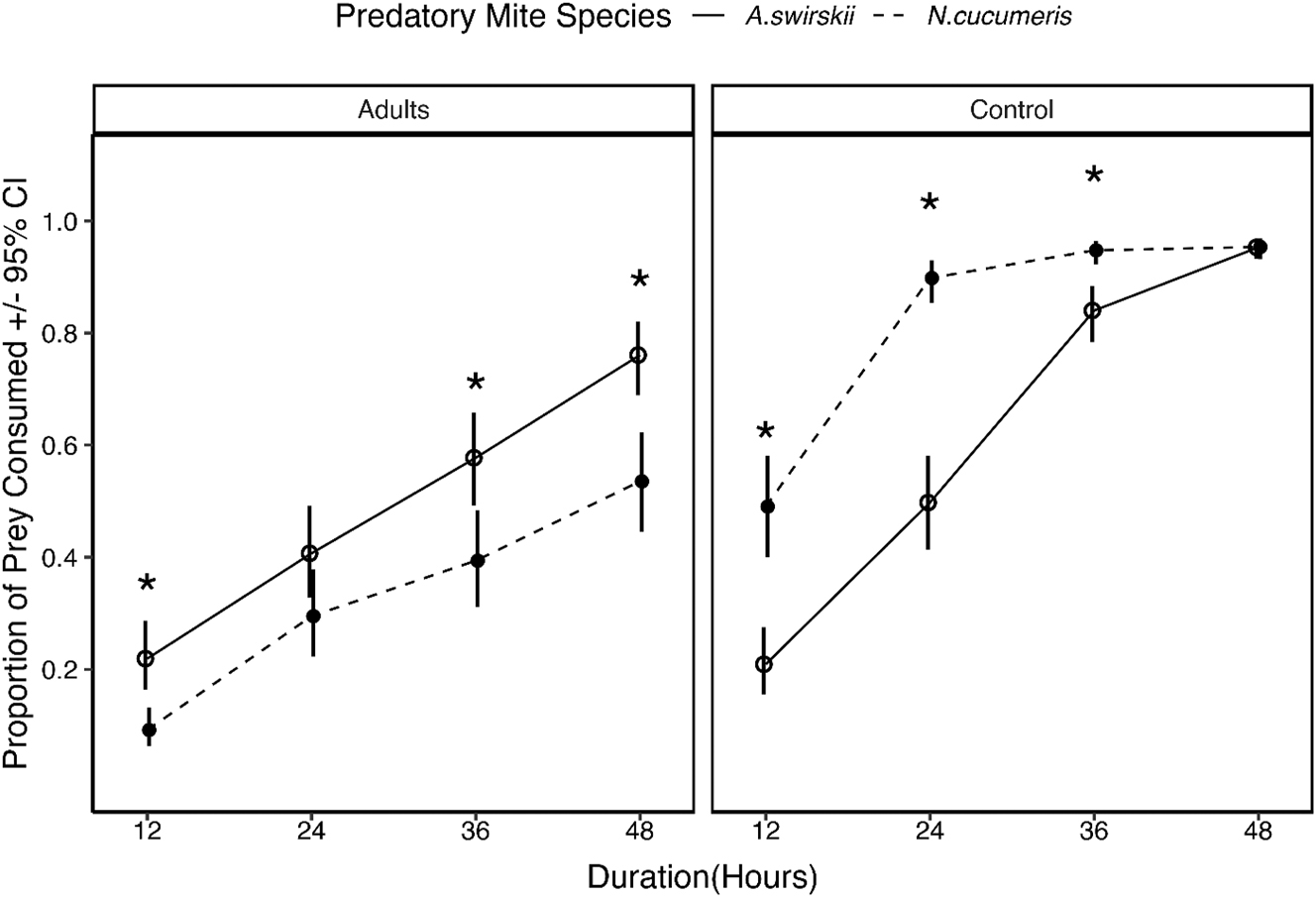
Consumption of adult Tetranychus urticae and adult Acarus spp. (control) by Neoseiulus cucumeris and Amblyseius swirskii at different time points. *Comparisons that are significantly different (Tukey P < 0.05).
4 Discussion
Our results indicate that prey familiarity is important in determining predation efficacy of A. swirskii and N. cucumeris feeding on T. urticae. In both predatory mites, prey consumption was higher when prey was familiar (control) compared to unfamiliar prey. Both A. swirskii and N. cucumeris showed higher consumption of T. urticae eggs compared to nymphs and adults. Nonetheless, in both predatory mite species, we observed an increase in the proportion of prey consumed with time (Figure 2). Higher consumption of familiar prey (Acarus spp.) by both species of predatory mites can be attributed to food imprinting, a phenomenon common in many groups of insects such as predatory mites and parasitoids (Ishii and Shimada 2012; Schausberger et al. 2010; Schausberger et al. 2018). This phenomenon is vital for the survival of many predatory insect species given that it allows predators to effectively adjust to not only qualitative, but also quantitative variations in food availability, which directly improves their survival (Immelmann 1975).
Given that commercially available predatory mites are in most cases mass reared on food sources different from the target prey, our study shows that this is disadvantageous given that it results in low predator efficacies. To address this, we suggest that commercial producers of predatory mites modify rearing techniques to allow for exposure of their predators to various cues of target pests to allow them to learn these prey species early on in life (Rahmani et al. 2009; Reichert et al. 2017; Seiter and Schausberger 2016). This could be done by rearing predatory mites on a variety of potential prey species to be targeted upon augmentative releases or dissemination of artificial herbivore-induced plant volatiles corresponding to a particular pest into rearing arenas. Use of synthetic herbivore-induced plant volatiles in priming biological control agents has been widely studied (Vet and Dicke 1992) and successfully used to improve efficacy of some biological control agents such as parasitoids (Hare and Morgan 1997). This would be advantageous in that it would lead to increased predation and numerical response of predatory mites, therefore improving their efficacy (Rahmani et al. 2009; Schausberger et al. 2021). For instance, it was shown that early life exposure of the predatory mite N. californicus to F. occidentalis resulted in higher predation rates (Schausberger et al. 2021). Similarly, early exposure of A. swirskii to kairomones emanating from F. occidentalis lead to an increase in predation of F. occidentalis by A. swirskii (Schausberger et al. 2020).
On the other hand, we observed high consumption of T. urticae eggs by both predatory mites compared to other T. urticae life stages (Figure 1). Egg preference by predators has been reported in many oligophagous spider mite specialists including P. persimilis, Neoseiulus fallacis (Garman), Phytoseiulus macropilis (Banks), and Neoseiulus longispinosus (Evans) (Blackwood et al. 2001). Similarly, the phytoseiid mite, Amblyseius largoensis Muma, preferred to feed on eggs of the red palm mite Raoiella indica Hirst (Trombidiformes: Tenuipalpidae) in comparison to its other life stages (Carrillo and Peña 2012). This can be attributed to the fact that eggs are of more nutritional value to predators than nymphs and adults of T. urticae (McMurtry and Rodriquez 1987; Xiao et al. 2013). In addition, other factors such as reduced time of prey attack and nutrient digestion may explain the observed trend (Soleymani et al. 2016). It has been demonstrated that some phytoseiid mites such as A. swirskii, prefer feeding on prey of smaller size (Nomikou et al. 2003). Our results contradict this previous finding, as we observed a higher proportion of adults consumed by A. swirskii compared to nymphs. This discrepancy could potentially be attributed to the lower number of adults (5) provided in our study compared to the number of nymphs (10) presented. Prey size affecting predator prey choice was further demonstrated in citrus, where various phytoseiid mites were observed consuming T. urticae eggs more than other life stages (Grafton-Cardwell et al. 1997).
Overall, the proportion of unfamiliar prey consumed by both predatory mites increased over time (Figure 2). We attributed this increase in predation to the fact that the predators had learned to recognize the provided prey as a potential food source (Schausberger et al. 2021, 2010; Schausberger and Peneder 2017). However, it should be noted that the efficacy of using A. swirskii and N. cucumeris in the field to manage T. urticae can be affected by the webbing produced by T. urticae, given that these two predators cannot easily escape getting entangled in these webs (Shimoda et al. 2010). A. swirskii consumed higher proportions of T. urticae adults compared to N. cucumeris. This is because, A. swirskii females are usually more aggressive while feeding compared to other generalist phytoseiid mites (Calvo et al. 2015; Wimmer et al. 2008). Our results, in addition, show that with familiar prey (control), both predatory mite species were able to consume 50 % of prey provided in 12 h. Besides T. urticae eggs, where 50 % consumption occurred at approximately 24 h, 36–48 h were required for 50 % of the T. urticae adults and nymphs to be consumed. This finding has great implications at the field level as it suggests that strawberry growers utilizing these predatory mites ought to refrain from the using any management practice that would be detrimental to predatory mites for a period of at least 48 h. This approach would potentially allow predatory mites to rapidly adjust to their new environment with less stress, allowing for rapid establishment of predator populations. This, in turn, could improve the efficacy of these biocontrol agents leading to season-long suppression of S. dorsalis in strawberry fields, and potentially eliminating the need for multiple releases of predatory mites.
Funding source: USDA National Institute of Food and Agriculture
Award Identifier / Grant number: Hatch Project No. FLA-GCR-005888
Acknowledgments
The authors would like to thank Dr. Vance Whitaker for providing strawberry transplants.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: This study was supported by funding from USDA National Institute of Food and Agriculture Hatch Project No. FLA-GCR-005888.
-
Data availability: The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Adeyinka, G.C., Afolabi, F., and Bakare, B.F. (2023). Evaluating the fate and potential health risks of organochlorine pesticides and triclosan in soil, sediment, and water from Asa Dam River, Ilorin Kwara State, Nigeria. Environ. Monit. Assess. 195: 189, https://doi.org/10.1007/S10661-022-10783-5.Suche in Google Scholar PubMed
Alatawi, F.J. and Kamran, M. (2018). Spider mites (Acari: Tetranychidae) of Saudi Arabia: two new species, new records and a key to all known species. J. Nat. Hist. 52: 429–455, https://doi.org/10.1080/00222933.2018.1434251.Suche in Google Scholar
Arthurs, S., McKenzie, C.L., Chen, J., Dogramaci, M., Brennan, M., Houben, K., and Osborne, L. (2009). Evaluation of Neoseiulus cucumeris and Amblyseius swirskii (Acari: Phytoseiidae) as biological control agents of chilli thrips, Scirtothrips dorsalis (Thysanoptera: Thripidae) on pepper. Biol. Control. 49: 91–96, https://doi.org/10.1016/j.biocontrol.2009.01.002.Suche in Google Scholar
Blackwood, J.S., Schausberger, P., and Croft, A. (2001). Prey-stage preference in generalist and specialist phytoseiid mites (Acari: Phytoseiidae) when offered Tetranychus urticae (Acari: Tetranychidae) eggs and larvae. Environ. Entomol. 30: 1103–1111, https://doi.org/10.1603/0046-225x-30.6.1103.Suche in Google Scholar
Bommarco, R., Miranda, F., Bylund, H., and Björkman, C. (2011). Insecticides suppress natural enemies and increase pest damage in cabbage. J. Econ. Entomol. 104: 782–791, https://doi.org/10.1603/EC10444.Suche in Google Scholar PubMed
Brooks, M.E., Kristensen, K., Van Benthem, K.J., Magnusson, A., Berg, C.W., Nielsen, A., Skaug, H.J., Machler, M., Bolker, B.M., Brooks, M.E., et al.. (2017). glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R. J. 9: 378–400, https://doi.org/10.3929/ETHZ-B-000240890.Suche in Google Scholar
Buitenhuis, R., Murphy, G., Shipp, L., and Scott-Dupree, C. (2015). Amblyseius swirskii in greenhouse production systems: a floricultural perspective. Exp. Appl. Acarol. 65: 451–464, https://doi.org/10.1007/s10493-014-9869-9.Suche in Google Scholar PubMed
Busuulwa, A., Revynthi, A., Liburd, O., and Lahiri, S. (2024). Residual effect of commonly used fungicides in strawberries on Amblyseius swirskii, Neoseiulus cucumeris, and Neoseiulus californicus (Mesostigmata: Phytoseiidae). Exp. Appl. Acarol. 93: 253–272, https://doi.org/10.1007/s10493-024-00928-1.Suche in Google Scholar PubMed PubMed Central
Calvo, F.J., Knapp, M., van Houten, Y.M., Hoogerbrugge, Á.H., and Belda, E.J. (2015). Amblyseius swirskii: what made this predatory mite such a successful biocontrol agent? Exp. Appl. Acarol. 65: 419–433, https://doi.org/10.1007/s10493-014-9873-0.Suche in Google Scholar PubMed
Carrillo, D. and Peña, J.E. (2012). Prey-stage preferences and functional and numerical responses of Amblyseius largoensis (Acari: Phytoseiidae) to Raoiella indica (Acari: Tenuipalpidae). Exp. Appl. Acarol. 57: 361–372, https://doi.org/10.1007/s10493-011-9488-7.Suche in Google Scholar PubMed
Cloyd, R.A., Galle, C.L., and Keith, S.R. (2006). Compatibility of three miticides with the predatory mites Neoseiulus californicus McGregor and Phytoseiulus persimilis Athias-Henriot (Acari: Phytoseiidae). HortScience 41: 707–710, https://doi.org/10.21273/hortsci.41.3.707.Suche in Google Scholar
Cock, M.J.W., Van Lenteren, J.C., Brodeur, J., Barratt, B.I.P., Bigler, F., Bolckmans, K., Cônsoli, F.L., Haas, F., Mason, P.G., and Parra, J.R.P. (2010). Do new access and benefit sharing procedures under the convention on biological diversity threaten the future of biological control? BioControl 55: 199–218, https://doi.org/10.1007/s10526-009-9234-9.Suche in Google Scholar
Dalir, S., Hajiqanbar, H., Fathipour, Y., and Khanamani, M. (2021). A comprehensive picture of foraging strategies of Neoseiulus cucumeris and Amblyseius swirskii on western flower thrips. Pest Manag. Sci. 77: 5418–5429, https://doi.org/10.1002/ps.6581.Suche in Google Scholar PubMed
Easterbrook, M.A., Fitzgerald, J.D., and Solomon, M.G. (2001). Biological control of strawberry tarsonemid mite Phytonemus pallidus and two spotted spider mites Tetranychus urticae on strawberry in the UK using species of Neoseiulus (Amblyseius) (Acari: Phytoseiidae). Exp. Appl. Acarol. 25: 25–36, https://doi.org/10.1023/a:1010685903130.10.1023/A:1010685903130Suche in Google Scholar PubMed
Fidgett, M. and Stinson, C. (2008). Method for rearing predatory mites. WO Patent WO/2008/015393.Suche in Google Scholar
Fox, J., Friendly, M., and Weisberg, S. (2013). Hypothesis tests for multivariate linear models using the car package. R J. 5: 39–52, https://doi.org/10.32614/rj-2013-004.Suche in Google Scholar
Grafton-Cardwell, E.E., Ouyang, Y., and Striggow, R.A. (1997). Predaceous mites (Acari: Phytoseiidae) for control of spider mites (Acari: Tetranychidae) in nursery citrus. Environ. Entomol. 26: 121–130, https://doi.org/10.1093/ee/26.1.121.Suche in Google Scholar
Guan, Z., Feng, W., and Whidden, A.J. (2016). Top challenges facing the Florida strawberry industry. Insights from a comprehensive industry survey. UF/IFAS EDIS FE972: 2–3, https://doi.org/10.32473/edis-fe972-2015.Suche in Google Scholar
Hare, J.D. and Morgan, D.J.W. (1997). Mass priming Aphytis: behavioral improvement of insectary reared biological control agents. Biol. Control. 10: 207–214, https://doi.org/10.1006/bcon.1997.0565.Suche in Google Scholar
Hartig, F. (2022). DHARMa: Residual diagnostics for hierarchical (Multi-Level/Mixed) regression models. R package version 0.4.6, https://cran.r-project.org/package=dharma.Suche in Google Scholar
Hasnaki, R., Ziaee, M., and Mahdavi, V. (2023). Pesticide residues in corn and soil of corn fields of Khuzestan, Iran, and potential health risk assessment. J. Food Compos. Anal. 115: 104972, https://doi.org/10.1016/j.jfca.2022.104972.Suche in Google Scholar
Hudson, M. (2022). USDA’s national agricultural statistics service Florida field office 2022 Annual Statistical Bulletin, https://www.nass.usda.gov/Statistics_by_State/Florida/Publications/Annual_Statistical_Bulletin/2022/_Content-2022.pdf (Accessed 29 April 2023).Suche in Google Scholar
Immelmann, K. (1975). Ecological significance of imprinting and early learning. Annu. Rev. Ecol. Evol. Syst. 6: 15–37, https://doi.org/10.1146/annurev.es.06.110175.000311.Suche in Google Scholar
Ishii, Y. and Shimada, M. (2012). Learning predator promotes coexistence of prey species in host-parasitoid systems. Proc. Natl. Acad. Sci. U.S.A. 109: 5116–5120, https://doi.org/10.1073/pnas.1115133109.Suche in Google Scholar PubMed PubMed Central
Kaur, G. and Lahiri, S. (2022). Chilli thrips, Scirtothrips dorsalis Hood (Thysanoptera: Thripidae) management practices for Florida strawberry crops. UF/IFAS EDIS. ENY2076, https://doi.org/10.32473/edis-in1346-2022.Suche in Google Scholar
Kaur, G., Stelinski, L.L., Martini, X., Boyd, N., and Lahiri, S. (2023). Reduced insecticide susceptibility among populations of Scirtothrips dorsalis Hood (Thysanoptera: Thripidae) in strawberry production. J. Appl. Entomol. 147: 271–278, https://doi.org/10.1111/jen.13108.Suche in Google Scholar
Lahiri, S. (2023). Arthropod pest management practices of strawberry growers in Florida: a survey of the 2019-2020 field season. UF/IFAS EDIS. ENY2097, https://doi.org/10.32473/edis-in1391-2023.Suche in Google Scholar
Lahiri, S. and Panthi, B. (2020). Insecticide efficacy for chilli thrips management in strawberry, 2019. Arthropod Manag. Tests. 45: tsaa046, https://doi.org/10.1093/amt/tsaa046.Suche in Google Scholar
Lahiri, S. and Yambisa, A. (2021). Efficacy of a biopesticide and predatory mite to manage chilli thrips, Scirtothrips dorsalis Hood (Thysanoptera: Thripidae) in Strawberry. Fla. Entomol. 104: 322–324, https://doi.org/10.1653/024.104.0410.Suche in Google Scholar
Lahiri, S., Smith, H.A., Gireesh, M., Kaur, G., and Montemayor, J.D. (2022). Arthropod pest management in strawberry. Insects 13: 475, https://doi.org/10.3390/insects13050475.Suche in Google Scholar PubMed PubMed Central
Lahiri, S., Kaur, G., and Busuulwa, A. (2024). Field efficacy of a biopesticide and a predatory mite for suppression of Scirtothrips dorsalis (Thysanoptera: Thripidae) in strawberry. J. Econ. Entomol. 117: 1623–1627, https://doi.org/10.1093/jee/toae144.Suche in Google Scholar PubMed PubMed Central
Lenth, R. (2020). emmeans: estimated marginal means, aka Least-Squares Means. R package version 1.5.2-1, https://cran.r-project.org/package=emmeans.Suche in Google Scholar
Liu, Y., Li, X., Zhou, C., Liu, F., and Mu, W. (2016). Toxicity of nine insecticides on four natural enemies of Spodoptera exigua. Sci. Rep. 6: 39060, https://doi.org/10.1038/srep39060.Suche in Google Scholar PubMed PubMed Central
Lüdecke, D., Ben-Shachar, M.S., Patil, I., Waggoner, P., and Makowski, D. (2021). performance: an R package for assessment, comparison and testing of statistical models. J. Open Source Softw. 6: 3139, https://doi.org/10.21105/joss.03139.Suche in Google Scholar
McMurtry, J.A., De Moraes, G.J., and Sourassou, N.F. (2013). Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol. 18: 297–320, https://doi.org/10.11158/saa.18.4.1.Suche in Google Scholar
McMurtry, J.A and Rodriquez, J. (1987). Nutritional ecology of phytoseiid mites. In: Nutritional ecology of insects, mites and spiders and related invertebrates, 1st ed. John Wiley & Sons, Inc., New York, pp. 609–644.Suche in Google Scholar
Montemayor, J.D., Smith, H.A., Peres, N.A., Rossitto De Marchi, B., and Lahiri, S. (2023). Is UV-C light compatible with biological control of twospotted spider mite? Biol. Control 183: 105269, https://doi.org/10.1016/j.biocontrol.2023.105269.Suche in Google Scholar
Nguyen, D.T., Vangansbeke, D., Lü, X., and de Clercq, P. (2013). Development and reproduction of the predatory mite Amblyseius swirskii on artificial diets. BioControl 58: 369–377, https://doi.org/10.1007/S10526-012-9502-y.Suche in Google Scholar
Nomikou, M., Janssen, A., and Sabelis, M.W. (2003). Phytoseiid predators of whiteflies feed and reproduce on non-prey food sources. Exp. Appl. Acarol. 31: 15–26, https://doi.org/10.1023/b:appa.0000005142.31959.e8.10.1023/B:APPA.0000005142.31959.e8Suche in Google Scholar
Nyoike, T.W. and Liburd, O.E. (2013). Effect of Tetranychus urticae (Acari: Tetranychidae), on marketable yields of field-grown strawberries in north-central Florida. J. Econ. Entomol. 106: 1757–1766, https://doi.org/10.1603/ec12033.Suche in Google Scholar PubMed
Panthi, B. and Renkema, J. (2020). Managing Scirtothrips dorsalis Hood (Thysanoptera: Thripidae) in Florida strawberry with flupyradifurone. Int. J. Fruit Sci. 20: 967–977, https://doi.org/10.1080/15538362.2020.1755768.Suche in Google Scholar
R Core Team (2022). R: a language and environment for statistical computing, Available at: https://www.r-project.org/ (Accessed 10 June 2022).Suche in Google Scholar
Rahmani, H., Hoffmann, D., Walzer, A., and Schausberger, P. (2009). Adaptive learning in the foraging behavior of the predatory mite Phytoseiulus persimilis. Behav. Ecol. 20: 946–950, https://doi.org/10.1093/beheco/arp081.Suche in Google Scholar
Raworth, D.A. (2001). Control of twospotted spider mite by Phytoseiulus persimilis. J. Asia. Pac. Entomol. 4: 157–163, https://doi.org/10.1016/s1226-8615(08)60117-x.Suche in Google Scholar
Reichert, M.B., Christiansen, I.C., Seiter, M., and Schausberger, P. (2017). Transgenerational loss and recovery of early learning ability in foraging predatory mites. Exp. Appl. Acarol. 71: 243–258, https://doi.org/10.1007/S10493-017-0122-1.Suche in Google Scholar
Renkema, J.M., Krey, K., Devkota, S., Liburd, O.E., and Funderburk, J. (2020). Efficacy of insecticides for season-long control of thrips (Thysanoptera: Thripidae) in winter strawberries in Florida. Crop Prot. 127: 104945, https://doi.org/10.1016/j.cropro.2019.104945.Suche in Google Scholar
Rhodes, E.M. and Liburd, O.E. (2006). Evaluation of predatory mites and acramite for control of twospotted spider mites in strawberries in North Central Florida. J. Econ. Entomol. 99: 1291–1298, https://doi.org/10.1093/jee/99.4.1291.Suche in Google Scholar
Schausberger, P. and Peneder, S. (2017). Non-associative versus associative learning by foraging predatory mites. BMC Ecology 17: 2, https://doi.org/10.1186/s12898-016-0112-x.Suche in Google Scholar PubMed PubMed Central
Schausberger, P., Walzer, A., Hoffmann, D., and Rahmani, H. (2010). Food imprinting revisited: early learning in foraging predatory mites. Behaviour 147: 883–897, https://doi.org/10.1163/000579510x495799.Suche in Google Scholar
Schausberger, P., Davaasambuu, U., Saussure, S., and Christiansen, I.C. (2018). Categorizing experience-based foraging plasticity in mites: age dependency, primacy effects and memory persistence. R. Soc. Open Sci. 5: 172110, https://doi.org/10.1098/rsos.172110.Suche in Google Scholar PubMed PubMed Central
Schausberger, P., Seiter, M., and Raspotnig, G. (2020). Innate and learned responses of foraging predatory mites to polar and non-polar fractions of thrips’ chemical cues. Biol. Control. 151: 104371, https://doi.org/10.1016/j.biocontrol.2020.104371.Suche in Google Scholar
Schausberger, P., Çekin, D., and Litin, A. (2021). Learned predators enhance biological control via organizational upward and trophic top-down cascades. J. Appl. Ecol. 58: 158–166, https://doi.org/10.1111/1365-2664.13791.Suche in Google Scholar PubMed PubMed Central
Seiter, M. and Schausberger, P. (2015). Maternal intraguild predation risk affects offspring anti-predator behavior and learning in mites. Sci. Rep. 5: 15046, https://doi.org/10.1038/srep15046.Suche in Google Scholar PubMed PubMed Central
Seiter, M. and Schausberger, P. (2016). Constitutive and operational variation of learning in foraging predatory mites. PLoS One 12: e0171450, https://doi.org/10.1371/journal.pone.0166334.Suche in Google Scholar PubMed PubMed Central
Shimoda, T., Kishimoto, H., Takabayashi, J., Amano, H., and Dicke, M. (2010). Relationship between the ability to penetrate complex webs of Tetranychus spider mites and the ability of thread-cutting behavior in phytoseiid predatory mites. Biol. Control 53: 273–279, https://doi.org/10.1016/j.biocontrol.2010.02.007.Suche in Google Scholar
Smithson, M. and Verkuilen, J. (2006). A better lemon squeezer? Maximum-likelihood regression with beta-distributed dependent variables. Psychol. Methods 11: 54–71, https://doi.org/10.1037/1082-989x.11.1.54.Suche in Google Scholar
Soleymani, S., Hakimitabar, M., and Seiedy, M. (2016). Prey preference of predatory mite Amblyseius swirskii (Acari: Phytoseiidae) on Tetranychus urticae (Acari: Tetranychidae) and Bemisia tabaci (Hemiptera: aleyrodidae). Biocontrol Sci. Technol. 26: 562–569, https://doi.org/10.1080/09583157.2015.1133808.Suche in Google Scholar
Van Leeuwen, T., Vontas, J., Tsagkarakou, A., Dermauw, W., and Tirry, L. (2010). Acaricide resistance mechanisms in the two-spotted spider mite Tetranychus urticae and other important Acari: a review. Insect Biochem. Mol. Biol. 40: 563–572, https://doi.org/10.1016/j.ibmb.2010.05.008.Suche in Google Scholar PubMed
Vet, L.E.M. and Dicke, M. (1992). Ecology of infochemical use by natural enemies in a tritrophic context. Annu. Rev. Entomol. 37: 141–172, https://doi.org/10.1146/annurev.en.37.010192.001041.Suche in Google Scholar
Whitaker, V.M., Peres, N.A., Osorio, L.F., Fan, Z., Do Nascimento Nunes, M.C., Plotto, A., and Sims, C.A. (2019). Florida Brilliance’ strawberry. HortScience 54: 2073–2077, https://doi.org/10.21273/hortsci14327-19.Suche in Google Scholar
Wimmer, D., Hoffmann, D., and Schausberger, P. (2008). Prey suitability of western flower thrips, Frankliniella occidentalis, and onion thrips, Thrips tabaci, for the predatory mite Amblyseius swirskii. Biocontrol Sci. Technol. 18: 533–542, https://doi.org/10.1080/09583150802029784.Suche in Google Scholar
Xiao, Y., Osborne, L.S., Chen, J., and McKenzie, C.L. (2013). Functional responses and prey-stage preferences of a predatory gall midge and two predacious mites with twospotted spider mites, Tetranychus urticae, as host. J. Insect Sci. 13: 9–12, https://doi.org/10.1673/031.013.0801.Suche in Google Scholar PubMed PubMed Central
Zhou, C., Lee, W.S., Liburd, O.E., Aygun, I., Zhou, X., Pourreza, A., Schueller, J.K., and Ampatzidis, Y. (2023). Detecting two-spotted spider mites and predatory mites in strawberry using deep learning. Smart Agr. Technol. 4: 100229, https://doi.org/10.1016/j.atech.2023.100229.Suche in Google Scholar
© 2024 the author(s), published by De Gruyter on behalf of the Florida Entomological Society
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Research Articles
- Distribution and dispersal of adult spotted wing drosophila, Drosophila suzukii (Diptera: Drosophilidae), in organically grown strawberries in Florida
- A comparison of the capture of non-target arthropods between control methods and monitoring traps of Anastrepha ludens in citrus agroecosystems
- Development of microsatellite markers for colony delineation of the invasive Asian subterranean termite (Blattodea: Rhinotermitidae) in South Florida and Taiwan
- Biology and life table of Oligonychus punicae Hirst (Trombidiformes: Tetranychidae) on three host plants
- Relative captures and detection of male Ceratitis capitata using a natural oil lure or trimedlure plugs
- Evaluation of HOOK SWD attract-and-kill on captures, emergence, and survival of Drosophila suzukii in Florida
- Rearing Neoseiulus cucumeris and Amblyseius swirskii (Mesostigmata: Phytoseiidae) on non-target species reduces their predation efficacy on target species
- Response of male Bactrocera zonata (Diptera: Tephritidae) to methyl eugenol: can they be desensitized?
- Monitoring of coccinellid (Coleoptera) presence and syrphid (Diptera) species diversity and abundance in southern California citrus orchards: implications for conservation biological control of Asian citrus psyllid and other citrus pests
- Topical treatment of adult house flies, Musca domestica L. (Diptera: Muscidae), with Beauveria bassiana in combination with three entomopathogenic bacteria
- Laboratory evaluation of 15 entomopathogenic fungal spore formulations on the mortality of Drosophila suzukii (Diptera: Drosophilidae), related drosophilids, and honeybees
- Effect of diatomaceous earth on diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae), larval feeding and survival on cabbage
- Bioactivity of seed extracts from different genotypes of Jatropha curcas (Euphorbiaceae) against Spodoptera frugiperda (Lepidoptera: Noctuidae)
- Assessment of sugarberry as a host tree of Halyomorpha halys (Hemiptera: Pentatomidae) in southeastern USA agroecosystems
- The importance of multigeneration host specificity testing: rejection of a potential biocontrol agent of Nymphaea mexicana (Nymphaeaceae) in South Africa
- Endophytic potential of entomopathogenic fungi associated with Urochloa ruziziensis (Poaceae) for spittlebug (Hemiptera: Cercopidae) control
- The first complete mitogenome sequence of a biological control agent, Pseudophilothrips ichini (Hood) (Thysanoptera: Phlaeothripidae)
- Exploring the potential of Delphastus davidsoni (Coleoptera: Coccinellidae) in the biological control of Bemisia tabaci MEAM 1 (Hemiptera: Aleyrodidae)
- Behavioral responses of Ixodiphagus hookeri (Hymenoptera; Encyrtidae) to Rhipicephalus sanguineus nymphs (Ixodida: Ixodidae) and dog hair volatiles
- Illustrating the current geographic distribution of Diaphorina citri (Hemiptera: Psyllidae) in Campeche, Mexico: a maximum entropy modeling approach
- New records of Clusiidae (Diptera: Schizophora), including three species new to North America
- Photuris mcavoyi (Coleoptera: Lampyridae): a new firefly from Delaware interdunal wetlands
- Bees (Hymenoptera: Apoidea) diversity and synanthropy in a protected natural area and its influence zone in western Mexico
- Temperature-dependent development and life tables of Palpita unionalis (Lepidoptera: Pyralidae)
- Orchid bee collects herbicide that mimics the fragrance of its orchid mutualists
- Importance of wildflowers in Orius insidiosus (Heteroptera: Anthocoridae) diet
- Bee diversity and abundance in perennial irrigated crops and adjacent habitats in central Washington state
- Comparison of home-made and commercial baits for trapping Drosophila suzukii (Diptera: Drosophilidae) in blueberry crops
- Miscellaneous
- Dr. Charles W. O’Brien: True Pioneer in Weevil Taxonomy and Publisher
- Scientific Notes
- Nests and resin sources (including propolis) of the naturalized orchid bee Euglossa dilemma (Hymenoptera: Apidae) in Florida
- Impact of laurel wilt on the avocado germplasm collection at the United States Department of Agriculture, Agricultural Research Service, Subtropical Horticulture Research Station
- Monitoring adult Delia platura (Diptera: Anthomyiidae) in New York State corn fields using blue and yellow sticky cards
- New distribution records and host plants of two species of Hypothenemus (Coleoptera: Curculionidae: Scolytinae) in mangrove ecosystems of Tamaulipas, Mexico
- First record of Trichogramma pretiosum parasitizing Iridopsis panopla eggs in eucalyptus in Brazil
- Spodoptera cosmioides (Lepidoptera: Noctuidae) as an alternative host for mass rearing the parasitoid Palmistichus elaeisis (Hymenoptera: Eulophidae)
- Effects of biochar on ambrosia beetle attacks on redbud and pecan container trees
- First report of Diatraea impersonatella (Lepidoptera: Crambidae) on sugarcane (Saccharum officinarum L.) in Honduras
- Book Reviews
- Kratzer, C. A.: The Cicadas of North America
Artikel in diesem Heft
- Frontmatter
- Research Articles
- Distribution and dispersal of adult spotted wing drosophila, Drosophila suzukii (Diptera: Drosophilidae), in organically grown strawberries in Florida
- A comparison of the capture of non-target arthropods between control methods and monitoring traps of Anastrepha ludens in citrus agroecosystems
- Development of microsatellite markers for colony delineation of the invasive Asian subterranean termite (Blattodea: Rhinotermitidae) in South Florida and Taiwan
- Biology and life table of Oligonychus punicae Hirst (Trombidiformes: Tetranychidae) on three host plants
- Relative captures and detection of male Ceratitis capitata using a natural oil lure or trimedlure plugs
- Evaluation of HOOK SWD attract-and-kill on captures, emergence, and survival of Drosophila suzukii in Florida
- Rearing Neoseiulus cucumeris and Amblyseius swirskii (Mesostigmata: Phytoseiidae) on non-target species reduces their predation efficacy on target species
- Response of male Bactrocera zonata (Diptera: Tephritidae) to methyl eugenol: can they be desensitized?
- Monitoring of coccinellid (Coleoptera) presence and syrphid (Diptera) species diversity and abundance in southern California citrus orchards: implications for conservation biological control of Asian citrus psyllid and other citrus pests
- Topical treatment of adult house flies, Musca domestica L. (Diptera: Muscidae), with Beauveria bassiana in combination with three entomopathogenic bacteria
- Laboratory evaluation of 15 entomopathogenic fungal spore formulations on the mortality of Drosophila suzukii (Diptera: Drosophilidae), related drosophilids, and honeybees
- Effect of diatomaceous earth on diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae), larval feeding and survival on cabbage
- Bioactivity of seed extracts from different genotypes of Jatropha curcas (Euphorbiaceae) against Spodoptera frugiperda (Lepidoptera: Noctuidae)
- Assessment of sugarberry as a host tree of Halyomorpha halys (Hemiptera: Pentatomidae) in southeastern USA agroecosystems
- The importance of multigeneration host specificity testing: rejection of a potential biocontrol agent of Nymphaea mexicana (Nymphaeaceae) in South Africa
- Endophytic potential of entomopathogenic fungi associated with Urochloa ruziziensis (Poaceae) for spittlebug (Hemiptera: Cercopidae) control
- The first complete mitogenome sequence of a biological control agent, Pseudophilothrips ichini (Hood) (Thysanoptera: Phlaeothripidae)
- Exploring the potential of Delphastus davidsoni (Coleoptera: Coccinellidae) in the biological control of Bemisia tabaci MEAM 1 (Hemiptera: Aleyrodidae)
- Behavioral responses of Ixodiphagus hookeri (Hymenoptera; Encyrtidae) to Rhipicephalus sanguineus nymphs (Ixodida: Ixodidae) and dog hair volatiles
- Illustrating the current geographic distribution of Diaphorina citri (Hemiptera: Psyllidae) in Campeche, Mexico: a maximum entropy modeling approach
- New records of Clusiidae (Diptera: Schizophora), including three species new to North America
- Photuris mcavoyi (Coleoptera: Lampyridae): a new firefly from Delaware interdunal wetlands
- Bees (Hymenoptera: Apoidea) diversity and synanthropy in a protected natural area and its influence zone in western Mexico
- Temperature-dependent development and life tables of Palpita unionalis (Lepidoptera: Pyralidae)
- Orchid bee collects herbicide that mimics the fragrance of its orchid mutualists
- Importance of wildflowers in Orius insidiosus (Heteroptera: Anthocoridae) diet
- Bee diversity and abundance in perennial irrigated crops and adjacent habitats in central Washington state
- Comparison of home-made and commercial baits for trapping Drosophila suzukii (Diptera: Drosophilidae) in blueberry crops
- Miscellaneous
- Dr. Charles W. O’Brien: True Pioneer in Weevil Taxonomy and Publisher
- Scientific Notes
- Nests and resin sources (including propolis) of the naturalized orchid bee Euglossa dilemma (Hymenoptera: Apidae) in Florida
- Impact of laurel wilt on the avocado germplasm collection at the United States Department of Agriculture, Agricultural Research Service, Subtropical Horticulture Research Station
- Monitoring adult Delia platura (Diptera: Anthomyiidae) in New York State corn fields using blue and yellow sticky cards
- New distribution records and host plants of two species of Hypothenemus (Coleoptera: Curculionidae: Scolytinae) in mangrove ecosystems of Tamaulipas, Mexico
- First record of Trichogramma pretiosum parasitizing Iridopsis panopla eggs in eucalyptus in Brazil
- Spodoptera cosmioides (Lepidoptera: Noctuidae) as an alternative host for mass rearing the parasitoid Palmistichus elaeisis (Hymenoptera: Eulophidae)
- Effects of biochar on ambrosia beetle attacks on redbud and pecan container trees
- First report of Diatraea impersonatella (Lepidoptera: Crambidae) on sugarcane (Saccharum officinarum L.) in Honduras
- Book Reviews
- Kratzer, C. A.: The Cicadas of North America

