Abstract
Palpita unionalis Hubner (Lepidoptera: Pyralidae) is one of the primary pests in olive orchards, damaging buds and fruits of the olive tree. The present study investigated the biology of P. unionalis across a temperature gradient and elaborated on the corresponding life tables for a better understanding of its population dynamics in the environment. The biology of the pest was studied at six temperatures (17, 20, 22, 25, 27, and 30 °C). The duration and survival of immature stages, sex ratio, and survival of emerged adults were evaluated. The female fecundity and longevity (pre-oviposition, oviposition, and post-oviposition periods) were calculated. The life tables were elaborated from these parameters. The life cycle of P. unionalis was significantly longer at 17 °C and shorter at 30 °C. Larval survival was significantly reduced at 30 °C, however, adult survival was at a maximum, yet their sex ratio was not affected by temperature. The lower temperature, 20 °C, was optimal for fecundity (586.3 eggs), which was reflected in the highest net reproductive rate (105.7 offspring per individual). The thermal constant (K) and the lower thermal threshold (t 0) for egg to adult period were 582.9 degree days and 7.1 °C, respectively. Based on the current study, it is concluded from the parameters of biology and life tables that temperature has a significant role in the population growth of P. unionalis. This should be considered throughout the monitoring and management of P. unionalis at different agro-ecologies in the context of global warming.
Resumen
Palpita unionalis Hubner (Lepidoptera: Pyralidae) es una de las principales plagas del cultivo del olivo, que causa daño a las yemas y frutos de los arboles de olivo. El presente estudio investigó la biología de P. unionalis a través de un gradiente de temperatura y elaboró las tablas de vida correspondientes para una mejor comprensión de la dinámica de su población en el medio ambiente. Se estudió la biología de la plaga a 6 temperaturas (17, 20, 22, 25, 27 y 30 °C). Se evaluaron la duración y sobrevivencia de los estadios inmaduros, la proporción de sexos y la sobrevivencia de los adultos emergidos. Se calcularon la fecundidad y longevidad de las hembras (períodos de preoviposición, oviposición y posoviposición). A partir de estos parámetros se elaboraron las tablas de vida. El ciclo de vida de P. unionalis fue significativamente más largo a 17 °C y más corto a 30 °C. La sobrevivencia de las larvas se redujo significativamente a 30 °C, sin embargo, la sobrevivencia de los adultos fue máxima, aunque su proporción de sexos no se vio afectada por la temperatura. La temperatura baja de 20 °C, se consideró óptima para la fecundidad (586.3 huevos), lo que se reflejó en la tasa reproductiva neta más alta (105.7 crías por individuo). La constante térmica (K) y el umbral térmico inferior (t 0) para el período de huevo a adulto fueron 582.9 grados d y 7.1 °C, respectivamente. Basados en este estudio, a partir de los parámetros biológicos y las tablas de vida, se concluye que la temperatura tiene un papel importante en el crecimiento de la población de P. unionalis. Esto debe considerarse durante el monitoreo y manejo de P. unionalis en diferentes agroecologías en el contexto del calentamiento global.
1 Introduction
Olive (Olea europaea L.; Oleaceae) farming has expanded and intensified from its traditional distribution area in Mediterranean countries. Furthermore, olives are being planted in areas where climatic conditions differ markedly from that ideal for its growth and production to face the progressive global demand for both olive oil and olives. Over the last fifty years, the acreage dedicated to olive orchards has tripled, with cultivation practices adopted in 56 countries, some of which have climates quite different from the traditional olive-producing regions. Despite these challenges, olive farming has been making inroads in the southeastern United States. Particularly in Florida, a state better known for its citrus groves, the olive industry is emerging as a viable agricultural endeavor. This expansion into Florida, along with areas of South Carolina, Georgia, Alabama, and Mississippi, signifies a notable shift in olive cultivation, embracing regions that mirror the latitudes of successful groves in Texas (Sánchez-Martínez and Garrido-Almonacid 2019). Insect pests are one of the limiting factors for olive expansion and production, affecting both established and emerging growth areas.
The olive leaf moth, Palpita unionalis Hubner (Lepidoptera: Pyralidae), is one of the main pests of olive groves. There are reports of 156 Palpita species worldwide, but only a few are of economic importance and P. unionalis is the most prominent (Scheunemann et al. 2021). This pest used to be one of the secondary pests of olives; nevertheless, it is now considered a main pest, especially in nurseries and young plantations. Additionally, it has become more significant in mature olive trees. Synonyms of P. unionalis are Margaronia unionalis (Hubner) or Palpita vitrealis (Rossi) (Yilmaz and Genc 2012). P. unionalis also attacks other genera of the family Oleaceae including Jasminum, Ligustrum, Phillyrea, and Fraxinus (Athanassiou et al. 2004; Tzanakakis 2003), hence the pest has an alternative common name “Jasmine moth”. Larvae also attack leaves and flower buds of ornamentals (Hegazi et al. 2007). The pest usually has a moderate population density in olive orchards; however, sporadic outbreaks happen, inflicting severe damage in olive orchards and nurseries (Hegazi et al. 2012; Kovanci and Kumral 2004; Kovanci et al. 2006). Larvae attack tender leaves and buds, where neonate larvae feed upon the lower surface parenchyma, turning the leaves dry and brown. When P. unionalis feeds on apical buds in nurseries, stunted growth of seedlings occurs. Usually, infested flower buds abscise before fruit set. In the case of heavy infestations, the larvae attack olive fruits, particularly the table varieties, eating the fruit sometimes right down to the stone, which makes them unacceptable for the market (Athanassiou et al. 2004; Badawi et al. 1976; Kumral et al. 2007). The most significant damage takes place in nurseries, young olive trees, and shoots of old trees (Khaghaninia and Pourabad 2009). Feeding damage may reach 90 % of the leaf area in nurseries and young orchards, and in older fruiting orchards, the reduction of olive production may reach up to 30 %, especially in the fruit ripening time of late summer and early fall (Arambourg 1986; López-Villalta 1999).
Several research studies have focused on the biological aspects of P. unionalis with respect to general biology (Badawi et al. 1976; Khaghaninia and Pourabad 2009; Shehata et al. 2003; Yilmaz and Genc 2012), population fluctuations (Hegazi et al. 2011, 2012; Kovanci et al. 2006), and life tables on various hosts (Kumral et al. 2007). However, the effect of temperature on these biological aspects is not yet well known. Temperature plays a significant role in insect development, survival, reproduction, behavior, fitness, abundance, and distribution (Barbosa et al. 2019). Accordingly, the study of temperature effects on insect life-history variables is crucial to predict pest occurrence and to develop effective management strategies (Kang et al. 2009; Nava et al. 2007). Consequently, the objective of the present study was to gain a better understanding of P. unionalis thermal demands and to elucidate the effect of temperature on its growth, survival, and reproductive success for better prediction and management of this pest.
2 Materials and methods
This study was done at the Laboratory of Entomology of the Desert Research Center (Cairo, Egypt), under controlled conditions of temperature, relative humidity (RH, 70–80 %) and photoperiod 16:8 h (L:D).
2.1 Insect collection and rearing
Larvae and pupae of P. unionalis were collected from olive groves at Matrouh Governorate, Northwestern Coast of Egypt (31.450805 °N, 26.621500 °E). Larvae were kept in the laboratory in transparent plastic cages (20 cm diam × 30 cm high) and were provided with tender olive leaves daily. The pupae collected from the cages, along with additional pupae collected directly from the field, were transferred to new cages until the adults emerged. The adult moths were offered a piece of cotton saturated with 10 % sugar solution. Filter papers and tender olive branches; served as egg deposition sites in moth cages. The filter paper (24 cm diameter) was attached horizontally to the roof of the cage with rubber bands. Filter papers and olive branches with eggs were removed and replaced every morning, and these eggs were used in the experiments.
2.2 Biology of P. unionalis at different temperatures
Ten eggs per Petri dish were incubated at 17, 20, 22, 25, 27, and 30 °C. Thirty replications were made at each temperature regime (300 eggs total per temperature). Fresh tender olive leaves were provided after 2 days and replaced as needed throughout the experimental period. For each temperature regime, the number of live and dead individuals of each stage (eggs, larvae, and pupae) were recorded daily until the adults emerged. Once the adults emerged the survival and development time of each immature stage was recorded. In addition, the sex ratio of emerged adults was determined by calculating number of female/(female + male) of the emerged adults. To study the reproduction potential and moth survival, emerged males and females were separated, and individual couples were released in plastic jars. Adult feeding and egg collection were done as described above and eggs were counted daily. Survival, pre-oviposition, oviposition, and post-oviposition periods were recorded. These biological parameters of immatures and adult stages were used for life tables to estimate the net reproductive rate (R 0), finite rate of increase (λ), mean generation time (T), intrinsic rate of increase (r m), and doubling time (DT). Life table parameters were computed using standard methodology (Birch 1948).
2.3 Statistical analysis
The data were subjected to ANOVA (P ≤ 0.05), and when statistical significance occurred, the temperature effects were differentiated by Tukey’s HSD test (P ≤ 0.05). The sex ratio was analyzed with generalized linear model (GLM) assuming a binomial distribution. The longevity of adult males and females was analyzed by means of survival curves (Kaplan–Meier estimator), then compared by the log-rank test. The median survival time for each temperature reflected the time upon which the survival probability was 0.5. The linear regression model was used to calculate the lower thermal threshold (t 0) and thermal constant (K). The data analyses were done using the software R, version 4.1.2 (R Development Core Team, Vienna, Austria).
3 Results
3.1 Biology of P. unionalis at different temperatures
The duration of all P. unionalis developmental stages, and the survival of larval stages were found to be temperature dependent (Table 1). Out of all the evaluated temperatures, 17 °C significantly prolonged the developmental periods for eggs (from when the egg is laid until the larvae hatches; 7.8 days) (F = 42.37; df = 5; P < 0.01), larvae (from when the larvae hatches until it pupates; 43.0 days) (F = 53.96; df = 5; P < 0.01) and pupae (from when the pupae is formed until the adult emerges; 27.0 days) (F = 37; df = 5; P < 0.01). In contrast, at 30 °C the shortest developmental periods were recorded for egg (3.0 days) and pupal (6.0 days) stages. The shortest developmental period for the larval stages was at 27 °C (15.4 days), which did not significantly differ from the developmental period at 30 °C (18.9 days). Furthermore, larval survival (17.1 %) was negatively affected at 30 °C when compared to the other considered temperatures (F = 53.96; df = 5; P < 0.01). Nevertheless, no statistical differences related to temperature were detected regarding egg (F = 0.69; df = 5; P < 0.63) and pupal (F = 2.02; df = 5; P < 0.09) survival.
Biological parameters of Palpita unionalis at different temperatures (mean value ± SE).
| Biological parameters | Temperature (°C) | ||||||
|---|---|---|---|---|---|---|---|
| 17 | 20 | 22 | 25 | 27 | 30 | ||
| Duration (days) | Egg stage | 7.82 ± 0.13a | 5.28 ± 0.08b | 3.88 ± 0.13c | 3.67 ± 0.10c | 3.42 ± 0.21cd | 3.01 ± 0.01d |
| Larval stage | 43.00 ± 3.33a | 22.44 ± 0.55b | 22.08 ± 0.83b | 17.20 ± 0.35bc | 15.35 ± 0.69bc | 18.93 ± 0.65bc | |
| Pupal stage | 27.03 ± 0.34a | 11.85 ± 0.17b | 9.51 ± 0.09c | 7.75 ± 0.12d | 8.06 ± 0.09d | 6.02 ± 0.33e | |
| Survival (%) | Egg stage | 82.72 ± 5.50 | 86.79 ± 3.63 | 93.19 ± 4.13 | 85.51 ± 4.23 | 79.22 ± 7.86 | 83.20 ± 2.38 |
| Larval stage | 89.11 ± 6.58a | 63.64 ± 7.43ab | 40.22 ± 10.15b | 58.46 ± 5.01b | 47.14 ± 7.10b | 17.14 ± 2.53c | |
| Pupal stage | 68.00 ± 8.00 | 69.58 ± 8.87 | 81.86 ± 4.69 | 90.00 ± 3.27 | 78.33 ± 8.33 | 60.71 ± 9.67 | |
| Fecundity | 229.00 ± 9.87ab | 586.25 ± 148.25a | 144.29 ± 69.00b | 260.77 ± 58.37ab | 434.50 ± 69.55ab | 81.00 ± 62.28b | |
| Pre-oviposition period | 3.00 ± 0.58 | 2.00 ± 0.00 | 2.71 ± 0.57 | 2.15 ± 0.41 | 3.13 ± 0.35 | 3.29 ± 0.47 | |
| Oviposition period | 17.00 ± 1.73a | 14.75 ± 2.06a | 4.57 ± 0.81b | 6.85 ± 1.03b | 6.75 ± 0.65b | 2.14 ± 0.83b | |
| Post-oviposition period | 2.33 ± 0.67 | 2.50 ± 0.65 | 4.57 ± 1.90 | 2.00 ± 0.44 | 1.88 ± 0.23 | 2.43 ± 0.90 | |
| Sex ratio | 0.516 ± 0.09 | 0.528 ± 0.06 | 0.430 ± 0.05 | 0.500 ± 0.06 | 0.462 ± 0.05 | 0.537 ± 0.07 | |
-
Rows without letters had no significant differences by the F-test (P ≤ 0.05). Means (±SE) followed by the same letters in the row did not differ by Tukey’s test (P ≤ 0.05). The sex ratio values did not differ by temperature regime according to pairwise comparison test of proportions (P ≤ 0.05). Fecundity is number of eggs. Oviposition periods in days. Sex ratio was determined by calculating number of female/(female + male) of the emerged adults.
For adult moths, females kept at 20 °C had the highest fecundity, 586.3 eggs which significantly reduced to 81.0 eggs at 30 °C (F = 4.95; df = 5; P < 0.01). Females presented the longest oviposition period at 17 °C (17.0 days), which did not significantly differ from the oviposition period recorded at 20 °C (14.8 days). All other temperatures significantly reduced the oviposition period (F = 17.09; df = 5; P < 0.09). Meanwhile, the durations of both pre-oviposition (F = 1.24; df = 5; P < 0.31) and post-oviposition (F = 1.161; df = 5; P < 0.35) periods were not significantly affected by temperature. The sex ratio at all temperatures was close to 0.5 and with no significant differences (Z = 0.20; P < 0.01).
Regarding female longevity (χ 2 = 46; df = 5; P < 0.01; Figure 1A) and male longevity (χ 2 = 20.7; df = 5; P < 0.01; Figure 1B), survival was inversely proportional to temperature. Females lived longer than males at all the tested temperatures. Females survived until 27 days (mean survival time “MST” = 17 days) and males survived until 21 days (MST = 14 days) at the lowest temperature 17 °C. Longevity of both females and males was significantly shortened to 11 days (MST = 7 days) and 10 days (MST = 5 days) at the highest temperature 30 °C, respectively, compared to the other tested temperatures.
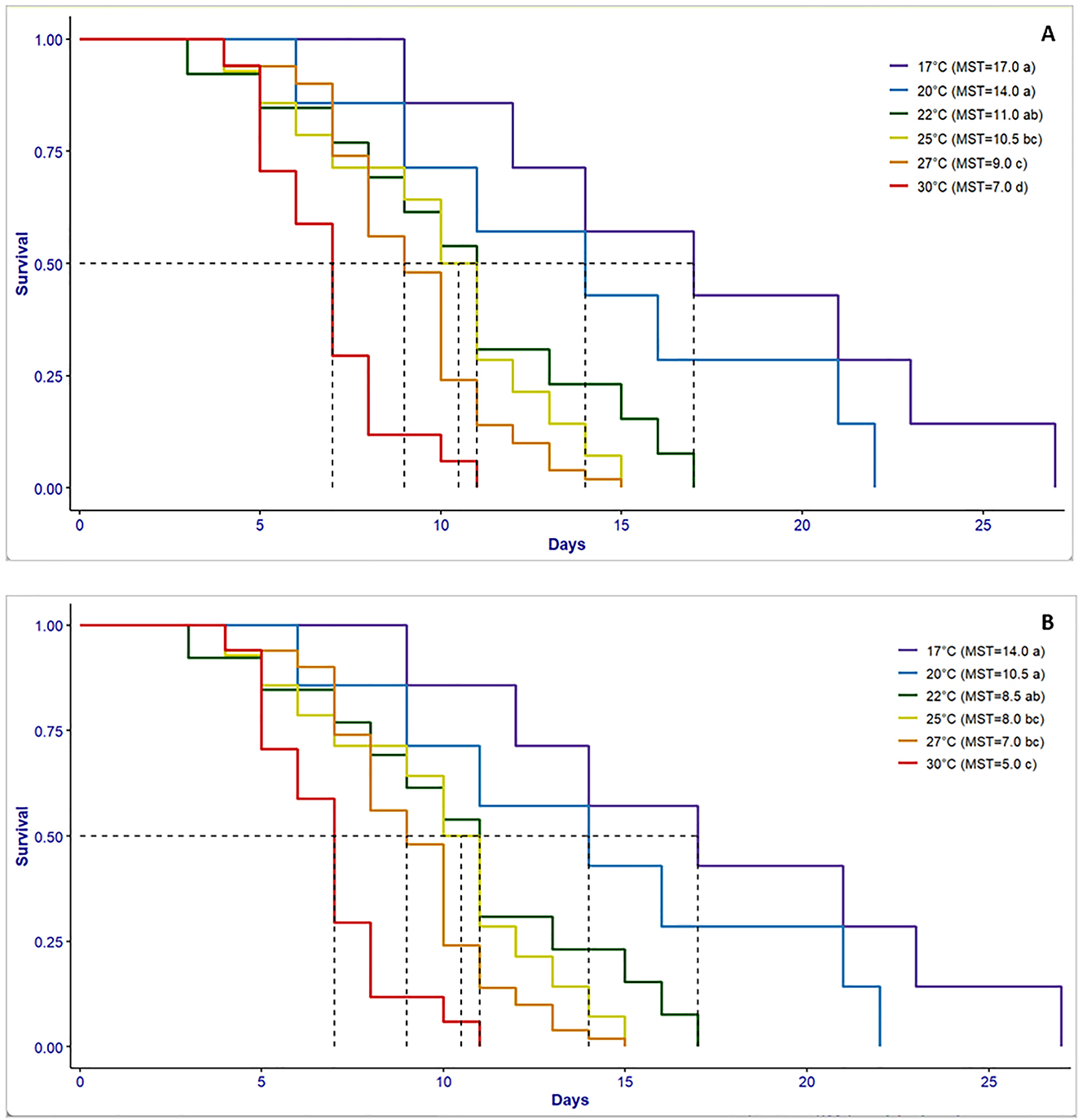
Survival curves for females (A) and males (B) of Palpita unionalis reared at different temperatures. Vertical dotted lines represent the mean survival time (MST) for each temperature. Curves followed by the same letters for each sex (see figure key) did not differ from one another by the log-rank test.
All the life table parameters (Table 2) significantly differed at the evaluated temperatures. For net reproductive rate (R 0; F = 13.63, df = 5; P < 0.01), intrinsic rate of increase (r m; F = 6.72, df = 5; P < 0.01), finite rate of increase (λ; F = 6.77, df = 5; P < 0.01), generation time (T; F = 25.51, df = 5; P < 0.01) and doubling time (DT; F = 5.73, df = 5; P < 0.01). It was possible to observe the variation of the R 0 from 105.7 to 35.5 and T from 37.0 days to 31.6 days at 20 and 30 °C, respectively. Despite the values of r m and λ (0.136; 1.145) being the highest at 25 °C, these values did not significantly differ from those calculated for the lower temperatures. Meanwhile, the highest DT value (6.2) was recorded for the P. unionalis kept at 30 °C.
Influence of six different temperatures on the life table parameters of Palpita unionalis.
| Parameter | Temperature (°C) | |||||
|---|---|---|---|---|---|---|
| 17 | 20 | 22 | 25 | 27 | 30 | |
| Net reproductive rate (R 0) | 77.83 ± 12.37ab | 105.72 ± 3.69a | 92.30 ± 4.74a | 75.79 ± 6.40ab | 59.29 ± 5.32bc | 35.53 ± 3.19c |
| Intrinsic rate of increase (r m) (day−1) | 0.120 ± 0.006ab | 0.126 ± 0.003ab | 0.135 ± 0.002a | 0.136 ± 0.002a | 0.122 ± 0.003ab | 0.113 ± 0.003b |
| Finite rate of increase (λ) (day−1) | 1.127 ± 0.007ab | 1.134 ± 0.004ab | 1.145 ± 0.002a | 1.145 ± 0.002a | 1.130 ± 0.003ab | 1.119 ± 0.003b |
| Generation time (T) (day) | 36.21 ± 0.42a | 36.98 ± 0.78a | 33.40 ± 0.15b | 31.84 ± 0.61b | 33.33 ± 0.01b | 31.56 ± 0.05b |
| Doubling time (DT) (day) | 5.83 ± 0.32ab | 5.50 ± 0.14ab | 5.12 ± 0.08b | 5.11 ± 0.08b | 5.67 ± 0.12ab | 6.15 ± 0.16a |
-
Means (±SE) followed by the same letters in the row do not differ by Tukey’s test (P ≤ 0.05).
3.2 Determination of thermal requirements
The lower thermal threshold (t 0) of eggs and its thermal constant (K) were 7.20 °C and 67.2 degree days (y = −10.83 + 1.51x; R 2 = 0.94; P < 0.03); of the larvae were 4.2 °C and 416.0 degree days (y = −1.047 + 0.25x; R 2 = 0.75; P < 0.02); of the pupae were 11.5 °C and 115.6 degree days (y = −0.22 + 0.89x; R 2 = 0.94; P < 0.03); and of the egg to adult period were 7.1 °C and 582.9 degree days (y = −1.26 + 0.18x; R 2 = 0.85; P < 0.03), respectively.
4 Discussion
Studying the thermal requirements of pests can help to improve their monitoring, define their initial existence, and predict their population outbreaks (Scheunemann et al. 2021). These data can also help in the development of model-based risk assessment tools, such as distribution maps (Azrag and Babin 2023). This knowledge could be helpful for the development of suitable management strategies that support the effectiveness of biocontrol programs and that rationalize the use of potentially harmful synthetic pesticides. In the present study, overall, the parameters measured for the biology of P. unionalis concur with the findings of many authors who studied the same pest with variations that may be attributed to factors like diet and environmental conditions. In laboratory studies, Fouda (1973) and Badawi et al. (1976) found that the incubation period lasted between 3 days at 30 °C to 12 days at 15 °C, and all larvae failed to hatch at 35 °C. Moreover, the duration of the last (6th) larval instar (5–7 days at 25 °C) was nearly twice that of the first instar (3 days at 25 °C). The intermediate larval instars were the lowest in duration and all larvae died at 35 °C after the first molt. The pupal stage lasted for 23.4, 15.7, 7.5, and 7.0 days for females and 31.2, 17.1, 8.5, and 8.0 days for males at 15, 20, 25 and 30 °C, respectively. Vassilaina-Alexopoulou and Santorini (1973) recorded 21–26 days of larval development at 26 °C. El Khawas (2000) observed that the larvae hatch within 1–3 days after deposition at 25 °C. The mean duration of larval development was 15.8 days, and the total developmental period (from egg to adult) was 23.4 days. The developmental time of immature stages was 30 days at 25 °C (Kumral et al. 2007). Khaghaninia and Pourabad (2009) recorded 34.9 days for the mean total developmental time at 27 °C. Yilmaz and Genc (2012) reported 4.16 days incubation period and 23.3 days for larval development at 24 °C.
The current results showed that the lower temperatures favored the survival of adults and larval stages of P. unionalis, yet eggs and pupal stages were not significantly affected. This was in support of Yilmaz and Genc (2012), who recorded 60 % larval survival at 24 °C compared to 59 % larval survival at 25 °C during the present study. There were also similarities in adult female survival as Yilmaz and Genc (2012) recorded 16 d of survival and females survived for 15 d in our study. Kumral et al. (2007) found the immature survival at 25 °C to be 73 % and the mean number of deposited eggs was 390 eggs. This was similar to the fecundity recorded by Yilmaz and Genc (2012), 352 eggs at 24 °C, but higher than the number of eggs that we recorded, 260 eggs at 25 °C. However, the fecundity (586 eggs) as well as the net reproductive rate, R 0 (105), were the highest at 20 °C during our study. This was similar to the results obtained by Scheunemann et al. (2021) for Palpita forficifera Munroe as its highest net reproductive rate, 121, was recorded at 25 °C as compared to its values, 21, 81, and 99 at 15, 20, and 30 °C, respectively. The net reproductive rate expresses the ability of the population to grow with each succeeding generation, and it is a crucial marker of population dynamics that sums up the insect physiological potential for reproduction (Kumral et al. 2007; Richards 1961). The comparison between these net reproductive rates along different temperatures allows evaluation of the potential for population increase under climate stress. The present results reveal the high biotic potential of the olive leaf moth at 22 °C, where this rate was significantly reduced at high temperatures. It is worth to note that adverse unstable climate and the presence of natural enemies out in the field are important limiting factors of this biotic potential (Scheunemann et al. 2019). All the other demographic parameters obtained from the current study indicate a negative impact of high temperature on P. unionalis.
In conclusion, higher temperature negatively affected the growth, survival, and reproduction of the olive leaf moth. This was observed from the evaluated biological parameters and the calculated life table parameters. However, despite developing at a slower rate, P. unionalis subjected to the lowest temperature, 17 °C did not differ significantly from those kept at slightly higher temperatures for other biological and life table parameters. Nevertheless, the biological parameters recorded for P. forficifera revealed that the ideal temperatures for development were 25 and 30 °C (Scheunemann et al. 2021). This variation in heat preferences between the two species may be due to the difference in their range of distribution. P. unionalis was reported to spread in the Mediterranean region, and P. forficifera presents in South America (Ricalde et al. 2015; Scheunemann et al. 2019). This was confirmed as the lower thermal threshold of P. unionalis in the present experiments for the egg to adult period was 7.10 °C, but it was 10.7 °C for P. forficifera (Scheunemann et al. 2021). According to the reported thermal requirements of P. unionalis, the number of annual generations should be calculated for each region separately. A lower number of generations are expected to be found in regions where the temperature is lower. In general, P. unionalis seems to be a multivoltine species that has many overlapping generations throughout the year (Hegazi et al. 2007). López-Villalta (1999) reported asynchronous development of larvae due to temperature, which resulted in concurrent presence of all stages throughout the season. Future studies are needed to conduct field observations of pest activity as temperature variations in the field differ from the constant temperatures used in the laboratory. The ecological relationship between the pest and its host plant (olive) also should be considered. The web formed by larvae, for example, during the feeding process may provide a suitable microclimate, allowing the larvae to survive critical periods, especially extreme cold and hot weather conditions (Scheunemann et al. 2021). Still, considering the expected scenario of global climate change that proposes temperature rising, the biology, thermal regime and number of generations are expected to change. This requires continuous monitoring of the bioecological behavior of the pest and the change of its thermal requirements for the future safety of global olive production.
Acknowledgments
The author would like to extend sincere gratitude to the Desert Research Center (DRC), Egypt, for their invaluable support in facilitating field visits essential for sample collection. Additionally, heartfelt thanks are owed to the Plant Protection Department for providing access to laboratory facilities and equipment. The generous support of my colleagues played a pivotal role in enabling comprehensive experimental work and analysis, which were crucial in bringing this research to completion. Special acknowledgment is also extended to the anonymous reviewers and the editor for their insightful comments and suggestions, which have significantly contributed to enhancing the quality and clarity of this manuscript. Their expertise and thoughtful critiques were invaluable in guiding the revisions of this work.
-
Research ethics: Not applicable.
-
Author contributions: The author has accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: The author declares no competing interests.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
Arambourg, Y. (1986) Pyralidae. Margaronia unionalis Hübn. In: Arambourg, Y. (Ed.). Traite d entomologie oleicole. International Olive Oil Council, Madrid, Spain, pp. 75–80.Suche in Google Scholar
Athanassiou, C.G., Kavallieratos, N.G., and Mazomenos, B.E. (2004). Effect of trap type, trap color, trapping location, and pheromone dispenser on captures of male Palpita unionalis (Lepidoptera: Pyralidae). J. Econ. Entomol. 97: 321–329, https://doi.org/10.1603/0022-0493-97.2.321.Suche in Google Scholar PubMed
Azrag, A.G.A. and Babin, R. (2023). Integrating temperature-dependent development and reproduction models for predicting population growth of the coffee berry borer, Hypothenemus hampei Ferrari. Bull. Entomol. Res. 113: 79–85, https://doi.org/10.1017/S0007485322000293.Suche in Google Scholar PubMed
Badawi, A., Awadallah, A.M., and Foda, S.M. (1976). On the biology of the olive leaf moth Palpita unionalis Hb. (Lep., Pyralidae). Zeitschrift für Angew. Entomol. 80: 103–110, https://doi.org/10.1111/j.1439-0418.1976.tb03306.x.Suche in Google Scholar
Barbosa, L.R., Santos, F., Soliman, E.P., Rodrigues, A.P., Wilcken, C.F., Campos, J.M., Zanuncio, A.J.V., and Zanuncio, J.C. (2019). Biological parameters, life table and thermal requirements of Thaumastocoris peregrinus (Heteroptera: Thaumastocoridae) at different temperatures. Sci. Rep. 9: 10174, https://doi.org/10.1038/s41598-019-45663-5.Suche in Google Scholar PubMed PubMed Central
Birch, L.C. (1948). The intrinsic rate of natural increase of an insect population. J. Anim. Ecol. 17: 15–26, https://doi.org/10.2307/1605.Suche in Google Scholar
El Khawas, M.A.M. (2000). Integrated control of insect pests on olive trees in Egypt with emphasis on biological control, PhD thesis, Faculty of Science, Cairo University, Egypt.Suche in Google Scholar
Fouda, S.M.A. (1973). Studies on Margaronia (Glyphodes) unionalis and its control, MSc dissertation. Faculty of Agriculture, Ain Shams Universty, Egypt.Suche in Google Scholar
Hegazi, E.M., Konstantopoulou, M.A., Herz, A., Khafagi, W.E., Agamy, E., Showiel, S., Atwa, A., Abd El-Aziz, G.M., and Abdel-Rahman, S.M. (2011). Seasonality in the occurrence of two lepidopterous olive pests in Egypt. Insect Sci. 18: 565–574, https://doi.org/10.1111/j.1744-7917.2010.01398.x.Suche in Google Scholar
Hegazi, E.M., Konstantopoulou, M.A., Khafagi, W.E., Schlyter, F., Herz, A., Raptopoulos, D.G., Hassan, S., and Atwa, A. (2012). The population trend of Palpita unionalis in different olive varieties in Egypt. Phytoparasitica 40: 451–459, https://doi.org/10.1007/s12600-012-0246-0.Suche in Google Scholar
Hegazi, E.M., Konstantopoulou, M.A., Milonas, P., Herz, A., Mazomenos, B.E., Khafagi, W.E., Zaitun, A., Abdel-Rahman, S.M., Helal, I., and El-Kemny, S. (2007). Mating disruption of the jasmine moth Palpita unionalis (Lepidoptera: Pyralidae) using a two pheromone component blend: a case study over three consecutive olive growing seasons in Egypt. Crop Prot. 26: 837–844, https://doi.org/10.1016/j.cropro.2006.08.003.Suche in Google Scholar
Khaghaninia, S. and Pourabad, R. (2009). Investigation on biology of olive leaf worm Palpita unionalis Hb. (Lepidoptera: Pyralidae) in constant laboratory conditions. Mun. Entomol. Zool. 4: 320–326.Suche in Google Scholar
Kovanci, B. and Kumral, N.A. (2004). Insect pests in olive groves of Bursa (Turkey). Acta Hortic. 791: 569–576, https://doi.org/10.17660/ActaHortic.2008.791.88.Suche in Google Scholar
Kovanci, B., Kumral, N.A., and Akbudak, B. (2006). Investigations on the population fluctuation of olive pyralid, Palpita unionalis (Hubner) (Lepidoptera: Pyralidae) in olive groves in Bursa Province of Turkey. Turk. Entomol. Derg. 30: 23–32.Suche in Google Scholar
Kumral, N.A., Kovanci, B., and Akbudak, B. (2007). Life tables of the olive leaf moth, Palpita unionalis (Huebner) (Lepidoptera: Pyralidae), on different host plants. J. Biol. Environ. Sci. 1: 105–110.Suche in Google Scholar
Kang, L., Chen, B., Wei, J.-N., and Liu, T.-X. (2009). Roles of thermal adaptation and chemical ecology in Liriomyza distribution and control. Ann. Rev. Entomol. 54: 127–145, https://doi.org/10.1146/annurev.ento.54.110807.090507.Suche in Google Scholar PubMed
López-Villalta, M.C. (1999). Olive pest and disease management. International Olive Oil Council, Madrid, Spain.Suche in Google Scholar
Nava, D.E., Nascimento, A.M., Stein, C.P., Haddad, M.L., Bento, J.M.S., and Parra, J.R.P. (2007). Biology, thermal requirements, and estimation of the number of generations of Zaprionus indianus (Diptera: Drosophilidae) for the main fig producing regions of Brazil. Fla. Entomol. 90: 495–501. https://doi.org/10.1653/0015-4040(2007)90[495:Btraeo]2.0.Co;2.10.1653/0015-4040(2007)90[495:BTRAEO]2.0.CO;2Suche in Google Scholar
Ricalde, M.P., Nava, D.E., Loeck, A.E., Coutinho, E.F., Bisognin, A., and Garcia, F.R.M. (2015). Insects related to olive culture in Rio Grande do Sul state, Brazil. Cienc. Rural 45: 2125–2130, https://doi.org/10.1590/0103-8478cr20141477.Suche in Google Scholar
Richards, O.W. (1961). The theoretical and practical study of natural insect populations. Ann. Rev. Entomol. 6: 147–162, https://doi.org/10.1146/annurev.en.06.010161.001051.Suche in Google Scholar
Sánchez-Martínez, J. and Garrido-Almonacid, A. (2019). Olive cultivation in the era of globalization. VNUHCM J. Soc. Sci. Humanit. 2: 60–71, https://doi.org/10.32508/stdjssh.v2i1.478.Suche in Google Scholar
Scheunemann, T., Kruger, A.P., Perleberg, V.A., Ritzel, A.G., Bernardi, D., and Nava, D.E. (2021). Effect of different thermal conditions on biology and number of generations of Palpita forficifera (Lepidoptera: Crambidae). Fla. Entomol. 104: 282–288, https://doi.org/10.1653/024.104.0405.Suche in Google Scholar
Scheunemann, T., Manica-Berto, R., Nornberg, S.D., Goncalves, R.D., Grutzmacher, A.D., and Nava, D.E. (2019). Biology and fertility life tables for Palpita forficifera (Lepidoptera: Crambidae) reared on three olive cultivars and privet. J. Econ. Entomol. 112: 450–456, https://doi.org/10.1093/jee/toy327.Suche in Google Scholar
Shehata, W.A., Abou-Elkhair, S.S., Stefanos, S.S., Youssef, A.A., and Nasr, F.N. (2003). Biological studies on the olive leaf moth, Palpita unionalis Hubner (Lepid., Pyralidae), and the olive moth, Prays oleae Bernard (Lepid., Yponomeutidae). Anz. Schadl.-J. Pest Sci. 76: 155–158, https://doi.org/10.1007/s10340-003-0011-8.Suche in Google Scholar
Tzanakakis, M.E. (2003). Seasonal development and dormancy of insects and mites feeding on olive: a review. Neth. J. Zool. 52: 87–224, https://doi.org/10.1163/156854203764817670.Suche in Google Scholar
Vassilaina-Alexopoulou, P. and Santorini, A. (1973). Some data on the biology of Palpita unionalis Hubner (Lepidoptera: Pyralidae), under laboratory conditions. Ann. Inst. Phytopathol. Benaki 10: 320–326.Suche in Google Scholar
Yilmaz, C. and Genc, H. (2012). Determination of the life cycle of the olive fruit leaf moth, Palpita unionalis (Lepidoptera: Pyralidae) in the laboratory. Fla. Entomol. 95: 162–170, https://doi.org/10.1653/024.095.0125.Suche in Google Scholar
© 2024 the author(s), published by De Gruyter on behalf of the Florida Entomological Society
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Research Articles
- Distribution and dispersal of adult spotted wing drosophila, Drosophila suzukii (Diptera: Drosophilidae), in organically grown strawberries in Florida
- A comparison of the capture of non-target arthropods between control methods and monitoring traps of Anastrepha ludens in citrus agroecosystems
- Development of microsatellite markers for colony delineation of the invasive Asian subterranean termite (Blattodea: Rhinotermitidae) in South Florida and Taiwan
- Biology and life table of Oligonychus punicae Hirst (Trombidiformes: Tetranychidae) on three host plants
- Relative captures and detection of male Ceratitis capitata using a natural oil lure or trimedlure plugs
- Evaluation of HOOK SWD attract-and-kill on captures, emergence, and survival of Drosophila suzukii in Florida
- Rearing Neoseiulus cucumeris and Amblyseius swirskii (Mesostigmata: Phytoseiidae) on non-target species reduces their predation efficacy on target species
- Response of male Bactrocera zonata (Diptera: Tephritidae) to methyl eugenol: can they be desensitized?
- Monitoring of coccinellid (Coleoptera) presence and syrphid (Diptera) species diversity and abundance in southern California citrus orchards: implications for conservation biological control of Asian citrus psyllid and other citrus pests
- Topical treatment of adult house flies, Musca domestica L. (Diptera: Muscidae), with Beauveria bassiana in combination with three entomopathogenic bacteria
- Laboratory evaluation of 15 entomopathogenic fungal spore formulations on the mortality of Drosophila suzukii (Diptera: Drosophilidae), related drosophilids, and honeybees
- Effect of diatomaceous earth on diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae), larval feeding and survival on cabbage
- Bioactivity of seed extracts from different genotypes of Jatropha curcas (Euphorbiaceae) against Spodoptera frugiperda (Lepidoptera: Noctuidae)
- Assessment of sugarberry as a host tree of Halyomorpha halys (Hemiptera: Pentatomidae) in southeastern USA agroecosystems
- The importance of multigeneration host specificity testing: rejection of a potential biocontrol agent of Nymphaea mexicana (Nymphaeaceae) in South Africa
- Endophytic potential of entomopathogenic fungi associated with Urochloa ruziziensis (Poaceae) for spittlebug (Hemiptera: Cercopidae) control
- The first complete mitogenome sequence of a biological control agent, Pseudophilothrips ichini (Hood) (Thysanoptera: Phlaeothripidae)
- Exploring the potential of Delphastus davidsoni (Coleoptera: Coccinellidae) in the biological control of Bemisia tabaci MEAM 1 (Hemiptera: Aleyrodidae)
- Behavioral responses of Ixodiphagus hookeri (Hymenoptera; Encyrtidae) to Rhipicephalus sanguineus nymphs (Ixodida: Ixodidae) and dog hair volatiles
- Illustrating the current geographic distribution of Diaphorina citri (Hemiptera: Psyllidae) in Campeche, Mexico: a maximum entropy modeling approach
- New records of Clusiidae (Diptera: Schizophora), including three species new to North America
- Photuris mcavoyi (Coleoptera: Lampyridae): a new firefly from Delaware interdunal wetlands
- Bees (Hymenoptera: Apoidea) diversity and synanthropy in a protected natural area and its influence zone in western Mexico
- Temperature-dependent development and life tables of Palpita unionalis (Lepidoptera: Pyralidae)
- Orchid bee collects herbicide that mimics the fragrance of its orchid mutualists
- Importance of wildflowers in Orius insidiosus (Heteroptera: Anthocoridae) diet
- Bee diversity and abundance in perennial irrigated crops and adjacent habitats in central Washington state
- Comparison of home-made and commercial baits for trapping Drosophila suzukii (Diptera: Drosophilidae) in blueberry crops
- Miscellaneous
- Dr. Charles W. O’Brien: True Pioneer in Weevil Taxonomy and Publisher
- Scientific Notes
- Nests and resin sources (including propolis) of the naturalized orchid bee Euglossa dilemma (Hymenoptera: Apidae) in Florida
- Impact of laurel wilt on the avocado germplasm collection at the United States Department of Agriculture, Agricultural Research Service, Subtropical Horticulture Research Station
- Monitoring adult Delia platura (Diptera: Anthomyiidae) in New York State corn fields using blue and yellow sticky cards
- New distribution records and host plants of two species of Hypothenemus (Coleoptera: Curculionidae: Scolytinae) in mangrove ecosystems of Tamaulipas, Mexico
- First record of Trichogramma pretiosum parasitizing Iridopsis panopla eggs in eucalyptus in Brazil
- Spodoptera cosmioides (Lepidoptera: Noctuidae) as an alternative host for mass rearing the parasitoid Palmistichus elaeisis (Hymenoptera: Eulophidae)
- Effects of biochar on ambrosia beetle attacks on redbud and pecan container trees
- First report of Diatraea impersonatella (Lepidoptera: Crambidae) on sugarcane (Saccharum officinarum L.) in Honduras
- Book Reviews
- Kratzer, C. A.: The Cicadas of North America
Artikel in diesem Heft
- Frontmatter
- Research Articles
- Distribution and dispersal of adult spotted wing drosophila, Drosophila suzukii (Diptera: Drosophilidae), in organically grown strawberries in Florida
- A comparison of the capture of non-target arthropods between control methods and monitoring traps of Anastrepha ludens in citrus agroecosystems
- Development of microsatellite markers for colony delineation of the invasive Asian subterranean termite (Blattodea: Rhinotermitidae) in South Florida and Taiwan
- Biology and life table of Oligonychus punicae Hirst (Trombidiformes: Tetranychidae) on three host plants
- Relative captures and detection of male Ceratitis capitata using a natural oil lure or trimedlure plugs
- Evaluation of HOOK SWD attract-and-kill on captures, emergence, and survival of Drosophila suzukii in Florida
- Rearing Neoseiulus cucumeris and Amblyseius swirskii (Mesostigmata: Phytoseiidae) on non-target species reduces their predation efficacy on target species
- Response of male Bactrocera zonata (Diptera: Tephritidae) to methyl eugenol: can they be desensitized?
- Monitoring of coccinellid (Coleoptera) presence and syrphid (Diptera) species diversity and abundance in southern California citrus orchards: implications for conservation biological control of Asian citrus psyllid and other citrus pests
- Topical treatment of adult house flies, Musca domestica L. (Diptera: Muscidae), with Beauveria bassiana in combination with three entomopathogenic bacteria
- Laboratory evaluation of 15 entomopathogenic fungal spore formulations on the mortality of Drosophila suzukii (Diptera: Drosophilidae), related drosophilids, and honeybees
- Effect of diatomaceous earth on diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae), larval feeding and survival on cabbage
- Bioactivity of seed extracts from different genotypes of Jatropha curcas (Euphorbiaceae) against Spodoptera frugiperda (Lepidoptera: Noctuidae)
- Assessment of sugarberry as a host tree of Halyomorpha halys (Hemiptera: Pentatomidae) in southeastern USA agroecosystems
- The importance of multigeneration host specificity testing: rejection of a potential biocontrol agent of Nymphaea mexicana (Nymphaeaceae) in South Africa
- Endophytic potential of entomopathogenic fungi associated with Urochloa ruziziensis (Poaceae) for spittlebug (Hemiptera: Cercopidae) control
- The first complete mitogenome sequence of a biological control agent, Pseudophilothrips ichini (Hood) (Thysanoptera: Phlaeothripidae)
- Exploring the potential of Delphastus davidsoni (Coleoptera: Coccinellidae) in the biological control of Bemisia tabaci MEAM 1 (Hemiptera: Aleyrodidae)
- Behavioral responses of Ixodiphagus hookeri (Hymenoptera; Encyrtidae) to Rhipicephalus sanguineus nymphs (Ixodida: Ixodidae) and dog hair volatiles
- Illustrating the current geographic distribution of Diaphorina citri (Hemiptera: Psyllidae) in Campeche, Mexico: a maximum entropy modeling approach
- New records of Clusiidae (Diptera: Schizophora), including three species new to North America
- Photuris mcavoyi (Coleoptera: Lampyridae): a new firefly from Delaware interdunal wetlands
- Bees (Hymenoptera: Apoidea) diversity and synanthropy in a protected natural area and its influence zone in western Mexico
- Temperature-dependent development and life tables of Palpita unionalis (Lepidoptera: Pyralidae)
- Orchid bee collects herbicide that mimics the fragrance of its orchid mutualists
- Importance of wildflowers in Orius insidiosus (Heteroptera: Anthocoridae) diet
- Bee diversity and abundance in perennial irrigated crops and adjacent habitats in central Washington state
- Comparison of home-made and commercial baits for trapping Drosophila suzukii (Diptera: Drosophilidae) in blueberry crops
- Miscellaneous
- Dr. Charles W. O’Brien: True Pioneer in Weevil Taxonomy and Publisher
- Scientific Notes
- Nests and resin sources (including propolis) of the naturalized orchid bee Euglossa dilemma (Hymenoptera: Apidae) in Florida
- Impact of laurel wilt on the avocado germplasm collection at the United States Department of Agriculture, Agricultural Research Service, Subtropical Horticulture Research Station
- Monitoring adult Delia platura (Diptera: Anthomyiidae) in New York State corn fields using blue and yellow sticky cards
- New distribution records and host plants of two species of Hypothenemus (Coleoptera: Curculionidae: Scolytinae) in mangrove ecosystems of Tamaulipas, Mexico
- First record of Trichogramma pretiosum parasitizing Iridopsis panopla eggs in eucalyptus in Brazil
- Spodoptera cosmioides (Lepidoptera: Noctuidae) as an alternative host for mass rearing the parasitoid Palmistichus elaeisis (Hymenoptera: Eulophidae)
- Effects of biochar on ambrosia beetle attacks on redbud and pecan container trees
- First report of Diatraea impersonatella (Lepidoptera: Crambidae) on sugarcane (Saccharum officinarum L.) in Honduras
- Book Reviews
- Kratzer, C. A.: The Cicadas of North America

