Monitoring adult Delia platura (Diptera: Anthomyiidae) in New York State corn fields using blue and yellow sticky cards
-
Paola Olaya-Arenas
Abstract
The seedcorn maggot, Delia platura (Meigen; Diptera: Anthomyiidae), is a polyphagous pest that feeds on decaying organic matter, seeds, and seedlings of several crops, including corn (Zea mays L.; Poaceae). In corn and soybeans, D. platura is a sporadic pest. To make recommendations as to which sticky trap color is more effective at trapping D. platura, we sampled 52 corn fields in four regions across New York State with blue and yellow sticky cards. More adults were caught with blue (77.6 %) than yellow cards (22.4 %). The same pattern was observed when all individuals were combined and separated by females and males. The higher captures with blue sticky cards were consistent across space and time, indicating the effectiveness of trapping adults and monitoring them at a regional scale.
Resumen
La mosca, Delia platura (Meigen; Diptera: Anthomyiidae), es una plaga polífaga que se alimenta de materia orgánica en descomposición, semillas y plántulas de varios cultivos, incluyendo el maíz (Zea mays L.; Poaceae). En maíz y soja, D. platura es una plaga esporádica. Para poder hacer recomendaciones de cual color de trampa pegajosa es más efectiva an capturar D. platura, muestreamos 52 campos de maíz en cuatro regiones del estado de Nueva York con tarjetas pegajosas azules y amarillas. Se atraparon más adultos con tarjetas azules (77.6 %) que con tarjetas amarillas (22.4 %). El mismo patrón se observó cuando se combinaron todos los individuos y se separaron por hembras y machos. Las capturas con tarjetas pegajosas azules fueron consistentemente más altas independiente del lugar o tiempo de muestreo, lo que indica la efectividad de atrapar adultos y monitorearlos a escala regional con tarjetas azules.
Corn (Poaceae: Zea mays L.) is a vital crop in New York State, with a significant impact on both grain and silage production. In 2022, New York State producers harvested over 1 million acres of corn, valued at USD $544 million (USDA/NASS 2022). Among the early-season pests that pose a particular concern for corn, Delia platura (Meigen; Diptera: Anthomyiidae) stands out due to its unpredictable nature and the limited rescue options available once seed damage occurs (Bessin 2019; Hodgson 2016; Koch and Wold-Burkness 2015; Krupke et al. 2017; Schmidt et al. 2017). Producers typically become aware of the damage when affected plants fail to germinate, leading to visible stand losses (Schmidt et al. 2017). A 10 % stand loss represents a 1–5 % decrease in yield and an economic loss of $8 to $40 per acre (Shields 2021). In New York State, the first generation of adult D. platura emerges from the soil typically by early April, followed by mating and oviposition in May. This period coincides with the planting of corn and edible beans in most areas of the state, providing an opportunity for the developing maggots to feed on these recently planted crops (NYS IPM 2024). Traditionally, D. platura has been controlled using insecticides containing neonicotinoids such as thiamethoxam and clothianidin (Grout et al. 2020; Krupke et al. 2017; Wise et al. 2014). However, with the enactment of the ‘Birds and Bees Protection Act’ in New York State (Holyman-Sigal et al. 2023), to protect pollinators by limiting the use of neonicotinoid pesticides on certain crop seeds, ornamental plants, and turf, these insecticides will no longer be a possible control method for D. platura.
Sticky traps are an important tool in the integrated pest management (IPM) strategy toolkit for a wide range of insect pests. These traps supply an early warning signal of pest presence and population density, facilitating the development of pesticide-free control strategies (Bashir et al. 2014; Epsky et al. 2008), such as adjusting planting times to lower-risk periods (Nault et al. 2011; Silver et al. 2018). Sticky trap cost-effectiveness and low labor requirements make them ideal for early detection and monitoring of D. platura adults. Blue and yellow are colors that have been shown to effectively attract D. platura in the field (Finch 1992; Vernon and Borden 1983). For this reason, we used blue and yellow sticky cards to monitor adult D. platura in corn fields across the state to make recommendations as to which sticky trap color is more effective at trapping D. platura.
In 2022, a total of 596 (298 yellow, 298 blue) commercial dry touch, lure-free, rectangular, small-sized, sticky cards (10.2 cm W × 16.5 cm L cm; Solida, Saint- Ferréol-les-Neiges, Québec, Canada) were placed in 52 corn fields within 22 counties in New York State (Figure 1A). At each corn field, two wooden stakes were placed 30 m from each other. Each stake had one blue and one yellow sticky card secured using a 6-inch clamp, with the lowest card placed 25.4 cm off the ground (Figure 1B). To ensure the traps did not interfere with corn planting, we placed them at the edges of each field. Field crop specialists placed sticky cards in the field on 19 April 2022, and replaced them weekly until 4 June or 2 July 2022, for a total sampling time of 6–10 weeks. Each set of sticky cards was labeled with the date and the trap identification number, wrapped individually in parchment paper, and mailed to the laboratory in resealable plastic bags. All bags with sticky cards were stored at 4 °C until identification. D. platura individuals (both females and males) were counted on one yellow and one blue sticky card out of the two pairs of sticky cards placed in each field at each time point. The second pair of cards was a back-up in case the first pair was damaged or got lost. We identified D. platura using the taxonomic key for Delia species (Savage et al. 2016).

Spatial distribution of planted corn (yellow) and 52 sampled corn fields (black dots) in 22 counties in New York State in 2022 (A) and visualization of an individual corn field with blue and yellow sticky cards placed at the edge (B).
The effectiveness of blue and yellow sticky cards in trapping adult D. platura in the field was assessed using a negative binomial generalized linear mixed model (glmer.nb) to account for overdispersion (variance/mean > 1) in the data. The response variables were the total number of adults, females, and males per card, the predictor was the color of the card, and sites were treated as the random effect to take into account the non-independence of the repeated measurements within a site. Data analyses were conducted in R 4.1.2 (R Core Team 2021), with the glm.nb model from the MASS package (Venables and Ripley 2002).
During the sampling season, a total of 16,944 adult D. platura individuals were trapped, comprising 8,110 females and 8,834 males. The blue cards captured a mean (±1 SE) of 44.1 ± 3.93 D. platura adults compared to the yellow cards which captured a mean (±1 SE) of 12.7 ± 1.45 adults (see Figure 2A; z = −13.9, p < 0.001). Both females (Figure 2B; z = −11.5, p < 0.001) and males (Figure 2C; z = −13.9, p < 0.001) were captured more frequently on the blue sticky cards than on the yellow cards.
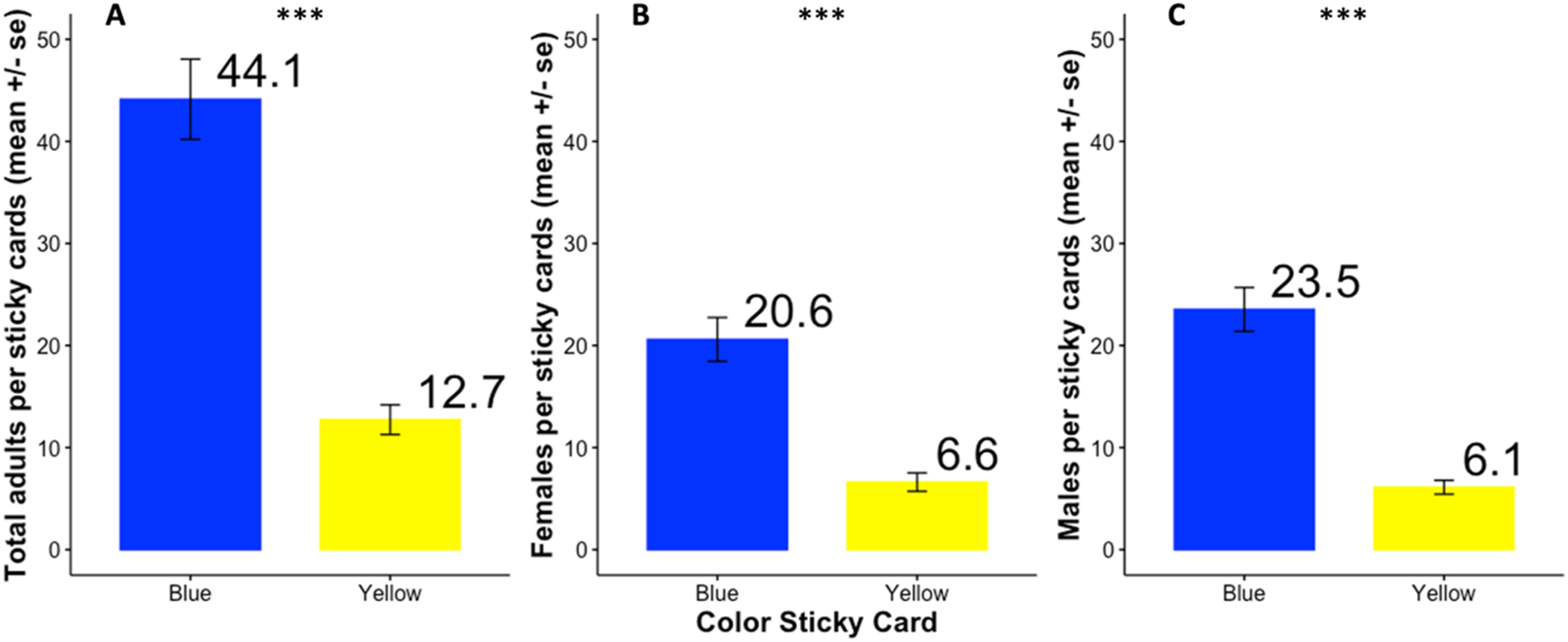
Adult Delia platura (mean ± 1 SE) trapped on blue and yellow sticky cards between 19 April and 2 July 2022 in 22 counties in New York State. Total D. platura (A), D. platura females (B), and D. platura males (C). The numbers on top of each bar are the mean number of flies trapped on each card color. Significance at ***p < 0.001. Number of observations = 596 (298 blue, 298 yellow), sites = 52.
Delia species feeding on various crops have been monitored using different trapping methods (water pans, sticky cards), traps of varying shapes, sizes, and colors, with differences in color intensity and reflectance, in combination with or without lures, and varied orientations and angles from the ground (Finch and Collier 1989; Harris and Miller 1983; Vernon 1986; Willett et al. 2020). In this study, we used blue and yellow sticky cards without a lure to monitor D. platura in 52 corn fields covering the main areas in New York State growing corn (Figure 1A). Blue sticky traps consistently outperformed yellow traps in capturing higher numbers of D. platura, and this result is unlikely to be due to random variation, based on the high negative z-scores. Our results align with a previous study in canola (Brassica rapa cv. Tobin; Brassicaceae) indicating D. platura preference for blue sticky cards (Vernon and Broatch 1996). Our study differed from Vernon and Broatch (1996) in several aspects. First, we used commercial sticky cards instead of white cardboard painted and coated with non-toxic insect-trapping adhesive. The use of commercial cards allowed us to make recommendations that others can easily follow as the cards can be purchased and do not need to be manufactured. Second, we conducted a broader study, sampling 52 corn fields across New York State over 10 weeks, in contrast to Vernon and Broatch (1996), that sampled one canola field for 14 days. However, we did not explore the orientation of sticky cards, nor did we consider the impact of background color (i.e., brown/gray of the soil, green of the plant, or yellow of the tassels) in combination with sticky card color on captured individuals, both factors considered by Vernon and Broatch (1996). A similar study to ours, testing the use of yellow and blue cards to catch Delia planipalpis (Meigen) found that yellow cards were more effective than blue cards in trapping this species (Lasa et al. 2024). This is contrary to what we found in our study and indicates that species within the same genus can respond differently to colors, making it clear that we cannot extrapolate results even in related species.
In conclusion, we found that rectangular, lure-free, small-sized blue sticky cards resulted in much higher adult captures than rectangular, lure-free, small-sized yellow sticky cards, but a combination of shape, size, and orientation has not been tested for D. platura. In future studies, it is recommended to test different attributes of the sticky cards in combination with color to evaluate which other attributes can further increase catches of D. platura in the field.
Funding source: National Institute of Food and Agriculture
Award Identifier / Grant number: Hatch Project Nr 7003547
Funding source: New York state Department of Agriculture and Markets
Acknowledgments
We thank Abby Seaman, Jodi Letham, and Tim Williams for help with logistics, data collection, and insect identification.
-
Research ethics: Not applicable.
-
Author contributions: K.P. conceptualized and administered the project. K.P. and K.W. acquired funding. K.P. H.S, A.D, K.W., J.D., E.S., J.M., A.G., J.L., K.O., M.S., M.Z. contributed to the experimental design and field logistics; S.C., J.D., A.D., A.G., T.L., J.M., K.O., K.P.; T.R.; H.S., E.S., M.S., K.W., M.Z. performed the field collections; S.C., L.E.V., L.M., H.M., A.P., A.D., T.L., O.R., P.O.A., H.S. processed the samples and identified the flies; P.O.A. performed the data analyses and wrote the first manuscript draft and edited versions; K.P., H.S., L.E.V., E.S., S.C., T.L. provided edits to the manuscript. All authors have read and agreed to the published version of the manuscript.
-
Competing interests: The authors state no conflict of interest.
-
Research funding: This research received funding from the New York State Department of Agriculture and Markets through the New York State Integrated Pest Management (NYSIPM) at Cornell University and by the intramural research program of the U.S. Department of Agriculture, National Institute of Food and Agriculture, Hatch Project Nr 7003547.
-
Data availability: The raw data can be obtained on request from the corresponding author.
References
Bashir, M.A., Alvi, A.M., and Naz, H. (2014). Effectiveness of sticky traps in monitoring insects. J. Environ. Agric. Sci. 1: 5.Search in Google Scholar
Bessin, R. (2019). Seedcorn maggots. University of Kentucky, College of Agriculture, Food, and Environment, Lexington, Kentucky, https://entomology.ca.uky.edu/files/ef309.pdf (Accessed 8 June 2024).Search in Google Scholar
Epsky, N.D., Morrill, W.L., and Mankin, R.W. (2008). Traps for capturing insects. USDA/ARS Subtropical Horticulture Research Station, Miami, Florida; Montana State University, Bozeman, Montana; USDA/ARS Center for Medical, Agricultural, and Veterinary Entomology, Gainesville, Florida.Search in Google Scholar
Finch, S. and Collier, R.H. (1989). Effects of the angle of inclination of traps on the numbers of large Diptera caught on sticky boards in certain vegetable crops. Entomologia Experimentalis et Applicata 52: 23–27, https://doi.org/10.1111/j.1570-7458.1989.tb01245.x.Search in Google Scholar
Finch, S. (1992). Improving the selectivity of water traps for monitoring populations of the cabbage root fly. Ann. Appl. Biol. 120: 1–7, https://doi.org/10.1111/j.1744-7348.1992.tb03397.x.Search in Google Scholar
Grout, T.A., Koenig, P.A., Kapuvari, J.K., and McArt, S.H. (2020). Neonicotinoid insecticides in New York State: economic benefits and risk to pollinators. Report No. 432, Cornell University, College of Agriculture and Life Sciences, Ithaca, New York.Search in Google Scholar
Harris, M.O. and Miller, J.R. (1983). Color stimuli and oviposition behavior of the onion fly Delia antiqua (Diptera: Anthomyiidae). Ann. Entomol. Soc. Am. 76: 766–771, https://doi.org/10.1093/aesa/76.4.766.Search in Google Scholar
Hodgson, E. (2016). Look for seedcorn maggot in corn and soybean. Iowa State University Extension and Outreach, Ames, Iowa, https://crops.extension.iastate.edu/cropnews/2016/04/look-seedcorn-maggot-corn-and-soybean (Accessed 8 June 2024).Search in Google Scholar
Holyman-Sigal, B., Addabbo, P.Jr., Bailey, J.T., Breslin, N.D., Brisport, J., Brouk, S.G., Cleare, C., Comrie, L., Fernandez, N., Gonzalez, K., et al.. (2023). NYS Senate Bill S1856A: enacts the birds and bees protection act, https://www.nysenate.gov/legislation/bills/2023/S1856/amendment/A (Accessed 8 June 2024).Search in Google Scholar
Koch, R. and Wold-Burkness, S. (2015). Seedcorn maggot. University of Minnesota Extension, St Paul, Minnesota, https://extension.umn.edu/soybean-pest-management/seedcorn-maggot (Accessed 8 June 2024).Search in Google Scholar
Krupke, C.H., Obermeyer, J.L., and Bledsoe, L.W. (2017). Corn insect control recommendation. Purdue University Extension, Entomology, West Lafayette, Indiana, https://extension.entm.purdue.edu/publications/E−219.pdf (Accessed 8 June 2024).Search in Google Scholar
Lasa, R., Córdova-García, G., Navarro-de-la-Fuente, L., and Williams, T. (2024). Sticky traps and water pan traps to monitor Delia planipalpis (Diptera: Anthomyiidae), an emerging pest of broccoli in Mexico. Crop Prot. 176: 106495, https://doi.org/10.1016/j.cropro.2023.106495.Search in Google Scholar
Nault, B.A., Werling, B.P., Straub, R.W., and Nyrop, J.P. (2011). Delaying onion planting to control onion maggot (Diptera: Anthomyiidae): efficacy and underlying mechanisms. J. Econ. Entomol. 104: 1622–1632, https://doi.org/10.1603/ec11175.Search in Google Scholar PubMed
NYS IPM (New York State Integrated Pest Management) (2024). Seedcorn maggot IPM. Cornell College of Agriculture and Life Sciences, Ithaca, New York, https://cals.cornell.edu/new-york-state-integrated-pest-management/outreach-education/fact-sheets/seedcorn-maggot-ipm (Accessed 17 July 2024).Search in Google Scholar
R Core Team (2021). R: a language and environment for statistical computing. R Foundation for Statistical Computing, http://www.R-project.org (Accessed 19 March 2024).Search in Google Scholar
Savage, J., Fortier, A., Fournier, F., and Bellavance, V. (2016). Identification of Delia pest species (Diptera: Anthomyiidae) in cultivated crucifers and other vegetable crops in Canada. Can. J. Arthropod Identif. 29: 1–40.Search in Google Scholar
Schmidt, E., Regan, K., and Barbercheck, M. (2017). Seedcorn maggot as a pest of field corn. PennState Extension. The Pennsylvania State University, University Park, Pennsylvania, https://extension.psu.edu/seedcorn-maggot-as-a-pest-of-field-corn (Accessed 8 June 2024).Search in Google Scholar
Shields, E.J. (2021). Seed corn maggot, stand losses and the need for insecticide seed treatments. Cornell University, Ithaca, New York, https://blogs.cornell.edu/whatscroppingup/2021/10/08/seed-corn-maggot-stand-losses-and-the-need-for-insecticide-seed-treatments/ (Accessed 8 June 2024).Search in Google Scholar
Silver, N., Hillier, K., and Blatt, S. (2018). Management of Delia (Diptera: Anthomyiidae) through selectively timed planting of Phaseolus vulgaris (Fabaceae) in Atlantic Canada. Can. Entomol. 150: 663–674, https://doi.org/10.4039/tce.2018.36.Search in Google Scholar
USDA/NASS (United States Department of Agriculture, National Agricultural Statistics Service) (2022). New York State overview. United States Department of Agriculture, National Agricultural Statistics Service, Washington D.C, https://www.nass.usda.gov/Quick_Stats/Ag_Overview/stateOverview.php?state=NEW%20YORK (Accessed 8 June 2024).Search in Google Scholar
Venables, W.N. and Ripley, B.D. (2002). Modern applied statistics with S, 4th ed. Springer, New York, NY.10.1007/978-0-387-21706-2Search in Google Scholar
Vernon, R.S. (1986). A spectral zone of color preference for the onion fly, Delia antiqua (Diptera: Anthomyiidae), with reference to the reflective intensity of traps. Can. Entomol. 118: 849–856, https://doi.org/10.4039/ent118849-9.Search in Google Scholar
Vernon, R. and Broatch, J. (1996). Responsiveness of Delia spp. (Diptera: Anthomyiidae) to colored sticky traps in flowering and rosette stage canola. Can. Entomol. 128: 1077–1085, https://doi.org/10.4039/Ent1281077-6.Search in Google Scholar
Vernon, R.S. and Borden, J.H. (1983). Spectral specific discrimination by Hylemya antiqua (Meigen) (Diptera: Anthomyiidae) and other vegetable-infesting species. Environ. Entomol. 12: 650–655, https://doi.org/10.1093/ee/12.3.650.Search in Google Scholar
Willett, D.S., Filgueiras, C.C., Nyrop, J.P., and Nault, B.A. (2020). Field monitoring of onion maggot (Delia antiqua) fly through improved trapping. J. Appl. Entomol. 144: 382–387, https://doi.org/10.1111/jen.12740.Search in Google Scholar
Wise, K., Waldron, K., and Woodsen, M. (2014). Early season insect pests of corn management guide. New York State IPM Program, Cornell University, College of Agriculture and Life Sciences, Ithaca, New York, https://ecommons.cornell.edu/handle/1813/42380 (Accessed 8 June 2024).Search in Google Scholar
© 2024 the author(s), published by De Gruyter on behalf of the Florida Entomological Society
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Research Articles
- Distribution and dispersal of adult spotted wing drosophila, Drosophila suzukii (Diptera: Drosophilidae), in organically grown strawberries in Florida
- A comparison of the capture of non-target arthropods between control methods and monitoring traps of Anastrepha ludens in citrus agroecosystems
- Development of microsatellite markers for colony delineation of the invasive Asian subterranean termite (Blattodea: Rhinotermitidae) in South Florida and Taiwan
- Biology and life table of Oligonychus punicae Hirst (Trombidiformes: Tetranychidae) on three host plants
- Relative captures and detection of male Ceratitis capitata using a natural oil lure or trimedlure plugs
- Evaluation of HOOK SWD attract-and-kill on captures, emergence, and survival of Drosophila suzukii in Florida
- Rearing Neoseiulus cucumeris and Amblyseius swirskii (Mesostigmata: Phytoseiidae) on non-target species reduces their predation efficacy on target species
- Response of male Bactrocera zonata (Diptera: Tephritidae) to methyl eugenol: can they be desensitized?
- Monitoring of coccinellid (Coleoptera) presence and syrphid (Diptera) species diversity and abundance in southern California citrus orchards: implications for conservation biological control of Asian citrus psyllid and other citrus pests
- Topical treatment of adult house flies, Musca domestica L. (Diptera: Muscidae), with Beauveria bassiana in combination with three entomopathogenic bacteria
- Laboratory evaluation of 15 entomopathogenic fungal spore formulations on the mortality of Drosophila suzukii (Diptera: Drosophilidae), related drosophilids, and honeybees
- Effect of diatomaceous earth on diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae), larval feeding and survival on cabbage
- Bioactivity of seed extracts from different genotypes of Jatropha curcas (Euphorbiaceae) against Spodoptera frugiperda (Lepidoptera: Noctuidae)
- Assessment of sugarberry as a host tree of Halyomorpha halys (Hemiptera: Pentatomidae) in southeastern USA agroecosystems
- The importance of multigeneration host specificity testing: rejection of a potential biocontrol agent of Nymphaea mexicana (Nymphaeaceae) in South Africa
- Endophytic potential of entomopathogenic fungi associated with Urochloa ruziziensis (Poaceae) for spittlebug (Hemiptera: Cercopidae) control
- The first complete mitogenome sequence of a biological control agent, Pseudophilothrips ichini (Hood) (Thysanoptera: Phlaeothripidae)
- Exploring the potential of Delphastus davidsoni (Coleoptera: Coccinellidae) in the biological control of Bemisia tabaci MEAM 1 (Hemiptera: Aleyrodidae)
- Behavioral responses of Ixodiphagus hookeri (Hymenoptera; Encyrtidae) to Rhipicephalus sanguineus nymphs (Ixodida: Ixodidae) and dog hair volatiles
- Illustrating the current geographic distribution of Diaphorina citri (Hemiptera: Psyllidae) in Campeche, Mexico: a maximum entropy modeling approach
- New records of Clusiidae (Diptera: Schizophora), including three species new to North America
- Photuris mcavoyi (Coleoptera: Lampyridae): a new firefly from Delaware interdunal wetlands
- Bees (Hymenoptera: Apoidea) diversity and synanthropy in a protected natural area and its influence zone in western Mexico
- Temperature-dependent development and life tables of Palpita unionalis (Lepidoptera: Pyralidae)
- Orchid bee collects herbicide that mimics the fragrance of its orchid mutualists
- Importance of wildflowers in Orius insidiosus (Heteroptera: Anthocoridae) diet
- Bee diversity and abundance in perennial irrigated crops and adjacent habitats in central Washington state
- Comparison of home-made and commercial baits for trapping Drosophila suzukii (Diptera: Drosophilidae) in blueberry crops
- Miscellaneous
- Dr. Charles W. O’Brien: True Pioneer in Weevil Taxonomy and Publisher
- Scientific Notes
- Nests and resin sources (including propolis) of the naturalized orchid bee Euglossa dilemma (Hymenoptera: Apidae) in Florida
- Impact of laurel wilt on the avocado germplasm collection at the United States Department of Agriculture, Agricultural Research Service, Subtropical Horticulture Research Station
- Monitoring adult Delia platura (Diptera: Anthomyiidae) in New York State corn fields using blue and yellow sticky cards
- New distribution records and host plants of two species of Hypothenemus (Coleoptera: Curculionidae: Scolytinae) in mangrove ecosystems of Tamaulipas, Mexico
- First record of Trichogramma pretiosum parasitizing Iridopsis panopla eggs in eucalyptus in Brazil
- Spodoptera cosmioides (Lepidoptera: Noctuidae) as an alternative host for mass rearing the parasitoid Palmistichus elaeisis (Hymenoptera: Eulophidae)
- Effects of biochar on ambrosia beetle attacks on redbud and pecan container trees
- First report of Diatraea impersonatella (Lepidoptera: Crambidae) on sugarcane (Saccharum officinarum L.) in Honduras
- Book Reviews
- Kratzer, C. A.: The Cicadas of North America
Articles in the same Issue
- Frontmatter
- Research Articles
- Distribution and dispersal of adult spotted wing drosophila, Drosophila suzukii (Diptera: Drosophilidae), in organically grown strawberries in Florida
- A comparison of the capture of non-target arthropods between control methods and monitoring traps of Anastrepha ludens in citrus agroecosystems
- Development of microsatellite markers for colony delineation of the invasive Asian subterranean termite (Blattodea: Rhinotermitidae) in South Florida and Taiwan
- Biology and life table of Oligonychus punicae Hirst (Trombidiformes: Tetranychidae) on three host plants
- Relative captures and detection of male Ceratitis capitata using a natural oil lure or trimedlure plugs
- Evaluation of HOOK SWD attract-and-kill on captures, emergence, and survival of Drosophila suzukii in Florida
- Rearing Neoseiulus cucumeris and Amblyseius swirskii (Mesostigmata: Phytoseiidae) on non-target species reduces their predation efficacy on target species
- Response of male Bactrocera zonata (Diptera: Tephritidae) to methyl eugenol: can they be desensitized?
- Monitoring of coccinellid (Coleoptera) presence and syrphid (Diptera) species diversity and abundance in southern California citrus orchards: implications for conservation biological control of Asian citrus psyllid and other citrus pests
- Topical treatment of adult house flies, Musca domestica L. (Diptera: Muscidae), with Beauveria bassiana in combination with three entomopathogenic bacteria
- Laboratory evaluation of 15 entomopathogenic fungal spore formulations on the mortality of Drosophila suzukii (Diptera: Drosophilidae), related drosophilids, and honeybees
- Effect of diatomaceous earth on diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae), larval feeding and survival on cabbage
- Bioactivity of seed extracts from different genotypes of Jatropha curcas (Euphorbiaceae) against Spodoptera frugiperda (Lepidoptera: Noctuidae)
- Assessment of sugarberry as a host tree of Halyomorpha halys (Hemiptera: Pentatomidae) in southeastern USA agroecosystems
- The importance of multigeneration host specificity testing: rejection of a potential biocontrol agent of Nymphaea mexicana (Nymphaeaceae) in South Africa
- Endophytic potential of entomopathogenic fungi associated with Urochloa ruziziensis (Poaceae) for spittlebug (Hemiptera: Cercopidae) control
- The first complete mitogenome sequence of a biological control agent, Pseudophilothrips ichini (Hood) (Thysanoptera: Phlaeothripidae)
- Exploring the potential of Delphastus davidsoni (Coleoptera: Coccinellidae) in the biological control of Bemisia tabaci MEAM 1 (Hemiptera: Aleyrodidae)
- Behavioral responses of Ixodiphagus hookeri (Hymenoptera; Encyrtidae) to Rhipicephalus sanguineus nymphs (Ixodida: Ixodidae) and dog hair volatiles
- Illustrating the current geographic distribution of Diaphorina citri (Hemiptera: Psyllidae) in Campeche, Mexico: a maximum entropy modeling approach
- New records of Clusiidae (Diptera: Schizophora), including three species new to North America
- Photuris mcavoyi (Coleoptera: Lampyridae): a new firefly from Delaware interdunal wetlands
- Bees (Hymenoptera: Apoidea) diversity and synanthropy in a protected natural area and its influence zone in western Mexico
- Temperature-dependent development and life tables of Palpita unionalis (Lepidoptera: Pyralidae)
- Orchid bee collects herbicide that mimics the fragrance of its orchid mutualists
- Importance of wildflowers in Orius insidiosus (Heteroptera: Anthocoridae) diet
- Bee diversity and abundance in perennial irrigated crops and adjacent habitats in central Washington state
- Comparison of home-made and commercial baits for trapping Drosophila suzukii (Diptera: Drosophilidae) in blueberry crops
- Miscellaneous
- Dr. Charles W. O’Brien: True Pioneer in Weevil Taxonomy and Publisher
- Scientific Notes
- Nests and resin sources (including propolis) of the naturalized orchid bee Euglossa dilemma (Hymenoptera: Apidae) in Florida
- Impact of laurel wilt on the avocado germplasm collection at the United States Department of Agriculture, Agricultural Research Service, Subtropical Horticulture Research Station
- Monitoring adult Delia platura (Diptera: Anthomyiidae) in New York State corn fields using blue and yellow sticky cards
- New distribution records and host plants of two species of Hypothenemus (Coleoptera: Curculionidae: Scolytinae) in mangrove ecosystems of Tamaulipas, Mexico
- First record of Trichogramma pretiosum parasitizing Iridopsis panopla eggs in eucalyptus in Brazil
- Spodoptera cosmioides (Lepidoptera: Noctuidae) as an alternative host for mass rearing the parasitoid Palmistichus elaeisis (Hymenoptera: Eulophidae)
- Effects of biochar on ambrosia beetle attacks on redbud and pecan container trees
- First report of Diatraea impersonatella (Lepidoptera: Crambidae) on sugarcane (Saccharum officinarum L.) in Honduras
- Book Reviews
- Kratzer, C. A.: The Cicadas of North America

