Endophytic potential of entomopathogenic fungi associated with Urochloa ruziziensis (Poaceae) for spittlebug (Hemiptera: Cercopidae) control
-
Pedro M. de Oliveira Netto
, Michelle O. Campagnani de Mendonça
Abstract
Spittlebugs are pests that affect forage plants in tropical America, causing damage to such plants and causing significant annual losses in milk and meat production. One of the alternatives for combating these insect pests with minimal environmental impacts is the use of entomopathogenic fungi. The objectives of this research were: (i) to evaluate the endophytic potential of entomopathogenic fungi applied to Urochloa ruziziensis (R.Germ. & C.M.Evrard) Crins (synonymous with Brachiaria ruziziensis; Poaceae) through seed treatment for the control of the spittlebug species, Mahanarva spectabilis (Distant) and Deois schach (F.) (Hemiptera: Cercopidae); (ii) to analyze the efficiency of banker plants as a means of spreading the fungi to the field; and (iii) to determine the frequency of infection after the storage of treated seeds. U. ruziziensis seeds were treated with a suspension containing 1 × 108 conidia of the fungi Fusarium sp. (Hypocreales: Nectriaceae), Metarhizium anisopliae (Metschn.) Sorokīn (Hypocreales: Clavicipitaceae) or a commercial strain of M. anisopliae for 30 min, after which they were planted in 2 L planters and kept in a greenhouse. The surplus of treated seeds was conditioned at 22 °C and sown monthly for 10 months. Insects were fed plants from treated seeds. The entomopathogenic fungi were found to be endophytic and to equally infect the two species of spittlebugs from pastures, M. spectabilis and D. schach, at different stages of development; however, they caused low nymphal mortality. The banker plant technique with plants from seeds treated with entomopathogenic fungi was efficient. Furthermore, it was observed that it is possible to store seeds treated with fungi for 12 months. These results open the perspective of using entomopathogenic fungi with endophytic action as an auxiliary tool in reducing the populations of pasture spittlebugs in tropical regions.
Resumo
As cigarrinhas-das-pastagens são pragas que atingem as forrageiras na América Tropical, causando danos promovendo elevadas perdas anuais na produção de leite e de carne. Uma das alternativas para o combate destes insetos-praga é o uso de fungos entomopatogênicos que não geram impactos ambientais. Sendo assim, os objetivos da pesquisa foram: i-avaliar o potencial endofítico de fungos entomopatogênicos aplicados em Urochloa ruziziensis (R.Germ. & C.M.Evrard) Crins (sinonímia de Brachiaria ruziziensis; Poaceae), por meio do tratamento de sementes, para o controle de Mahanarva spectabilis (Distant) e Deois schach (F.) (Hemiptera: Cercopidae); ii-analisar a eficiência de plantas banqueiras como meio de disseminação do fungo à campo e, iii-determinar a frequência de infecção após o armazenamento de sementes tratadas. As sementes de braquiária foram tratadas com a suspensão, contendo 1 × 108 conídeos por 30 minutos, dos fungos Fusarium sp. (Hypocreales: Nectriaceae) Metarhizium anisopliae (Metschn.) Sorokīn (Hypocreales: Clavicipitaceae) e Metarhizium anisopliae (cepa comercial), foram plantadas em vasos com capacidade de 2 L, e mantidas em casa-de-vegetação. O excedente de sementes tratadas foi devidamente acondicionado à 22 °C e semeadas, mensalmente, por 10 meses. Ninfas e adultos dos insetos-praga foram alimentadas com as plantas advindas de sementes tratadas. Constatou-se que os fungos entomopatogênicos foram endofíticos, infectaram igualmente as espécies de cigarrinhas das pastagens M. spectabilis e D. schach nos diferentes estágios de desenvolvimento; porém, ocasionaram baixa mortalidade ninfal. O uso da técnica de planta banqueira contendo plantas advindas de sementes tratadas com os fungos entomopatogênicos foi eficiente. Além disso, observou-se que é possível armazenar as sementes tratadas com os fungos por 12 meses. Esses resultados abrem a perspectiva do uso de fungos entomopatogênicos com ação endofítica como uma ferramenta auxiliar na redução das populações das cigarrinhas das pastagens nas regiões tropicais.
1 Introduction
Brazil, currently the main exporter of beef (EMBRAPA 2021), has the largest herd in the world and ranks third in milk production (MAPA 2022). According to the Instituto Brasileiro de Geografia e Estatística (Instituto Brasileiro de Geografia e Estatística 2020), the coverage of natural pastures in Brazil was reduced by 17.9 % from 57,633,189 ha in 2006 to 47,323,399 ha in 2017. On the other hand, the area of planted pastures increased by 9.5 %, which represents an increase of 9,765,275 ha, especially in the northern region, which experienced strong expansion of cattle raising. However, despite the efforts undertaken to expand forage grass cultivation, the mortality of Urochloa spp. (Hochst. ex A. Rich.) R. D. Webster = Brachiaria spp. (Hochst. ex A. Rich Stapf) (Poaceae), due to the presence of pests, has been described as a factor with a negative impact on the growth of national livestock (Dias Filho 2017).
Resende et al. (2013) highlighted spittlebugs as the main pests of forage grasses in tropical America. During the act of sucking, various species of spittlebugs (Hemiptera: Cercopidae), including Mahanarva spectabilis (Distant) and Deois schach (F.), inject toxins into the plants that promote cell death, yellowing, and consequently complete desiccation of the plants (Fazolin et al. 2016). Pastures consequently suffer a significant reduction in the volume of dry matter production; in addition, they also face a drop in the nutritional quality and palatability of the forage produced (Valério 2009). The problems caused in pastures by the annual attack of these insect pests are recurrent, which drastically reduce the production and quality of susceptible forage pastures, causing approximately US $2 billion yearly damage worldwide (Thompson 2004).
To mitigate the problem caused by spittlebugs in pastures, some strategies based on plant resistance mechanisms (Valverde 2006; Auad et al. 2007; Congio et al. 2012; Resende et al. 2013; Silva et al. 2017; Paladini et al. 2018; Alvarenga et al. 2019; Congio et al. 2020), the diversification of pastures with the inclusion of grasses resistant to spittlebugs (Alvarenga et al. 2019), the use of compounds of plant origin (Dias et al. 2019; Nascimento et al. 2021), and entomopathogenic fungi (Pereira et al. 2008; Campagnani et al. 2017; Pitta et al. 2019; Ribeiro and Cazarotto 2019), have been proposed. The last alternative, however, has a serious limitations due to its dependence on abiotic factors, which are directly related to the efficiency of spittlebug control. In this regard, it is important to consider that some fungal species live under endophytic conditions, which can alleviate this instability driven by abiotic factors. Based on the aforementioned context, this study was performed considering the hypothesis that fungi with endophytic potential for the control of spittlebugs can reduce problems related to climatic factors.
It is also noteworthy that, in addition to the natural form, biological control agents can be introduced through spraying, seed treatments, and banker plants. Banker plants are established and managed adjacent to the crops, which may attract natural enemies for pest control. The method has been widely investigated over many years and used to facilitate the establishment, development and dispersal of organisms that are beneficial in the biological control of pests. Banker plants act directly or indirectly, providing resources such as prey or hosts and food for natural enemies that are deliberately added to the cropping system to aid in the reproduction and maintenance of natural enemy populations that live close to the crop, providing specific pest control (Huang et al. 2011; Andorno and López 2014). Based on this system, it was hypothesized that the inclusion of plants from seeds treated with entomopathogenic fungi showing endophytic potential would reduce the adult population of spittlebugs in pastures.
Furthermore, maintaining the viability and presence of endophytic fungi in seeds is a strategy to be investigated in the seed storage process, considering that in a forage breeding program, seed storage involves selecting materials with desirable characteristics over time.
Therefore, the objectives of this research were: (i) to evaluate the endophytic potential of entomopathogenic fungi applied to Urochloa ruziziensis through seed treatment for the control of M. spectabilis and D. schach; (ii) to analyze the efficiency of banker plants as a means of spreading fungi to the field; and (iii) to determine the frequency of infection after the storage of treated seeds.
2 Materials and methods
The present study was carried out in a greenhouse at Embrapa Dairy Cattle and at the Experimental Field José Henrique Bruschi-CEJHB (21.5565833 °S, 43.2692222 °W; Coronel Pacheco, Minas Gerais). Steps related to obtaining and confirming the entomopathogenic fungi were conducted at the Federal University of São João Del Rei, São João Del Rei, Minas Gerais (UFSJ).
2.1 Origin of entomopathogenic fungi and seeds of U. r uziziensis
Fungi isolated in a silvopastoral system in the state of Maranhão, Brazil, Fusarium sp. (Hypocreales: Nectriaceae; UFMG 11443, GenBank = ON831395) and Metarhizium anisopliae (Metschn.) Sorokīn (Hypocreales: Clavicipitaceae; UFMG 11444, GenBank = ON831396) were provided by the entomopathogens collection of the Department of Biosystems Engineering (DEPEB), UFSJ, maintained since 2017. These fungi are deposited in the Microorganism Collection of the Federal University of Minas Gerais, Brazil (World Data Center for Microorganisms - WDCM 1029. CM-UFMG). The DNA sequences were analyzed and compared with the type culture sequences deposited in GenBank using the BLASTn program (Basic Local Alignment Search Tool version 2.215 of BLAST 2.0) available from the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/blast/). The other strain of M. anisopliae, strain E9 (1.39 × 108 conidia/g), was purchased commercially from KOPPERT do Brasil Holding S.A® (Piracicaba, São Paulo, Brazil). The U. ruziziensis cv. Kennedy seeds were purchased commercially from SOESP Company (Presidente Prudente, São Paulo, Brazil).
2.2 Fungal inoculation of seeds
For each fungus, 100 g of seeds was used. After weighing the seeds, superficial disinfestation was performed by washing the seeds in sodium hypochlorite (2 %) for 2 min and then in 70 % alcohol for 1 min. After these steps, the seeds were rinsed with distilled water according to the methodology adapted from Carvalho et al. (2012) and Ferreira et al. (2017). Batches of conidia were tested for their viability according to the methodology proposed by Lopes et al. (2013). For fungal inoculation, 400 mL of sterile distilled water suspension containing 1 × 108 conidia/mL was added, as specified for the commercially acquired M. anisopliae strain, and 0.05 % Tween 80 was included as a surfactant. A control suspension without the presence of fungi was prepared, for a total of four treatments. The seeds were in contact with the suspension for 30 min according to an adaptation of the methodology by Keyser et al. (2014).
2.3 Cultivation of U. ruziziensis containing entomopathogenic fungi
Ten U. ruziziensis seeds treated with entomopathogenic fungi were deposited on a soil, sand, and manure mixture at a ratio of 3:1:1 (Resende et al. 2012) and were covered with a layer of vermiculite (Micron® Jacareí, São Paulo, Brazil) in a 2L planter. Every 45 days the soil was fertilized with urea (0.09 g/planter), simple superphosphate (0.04 g/planter), and potassium chloride (0.04 g/planter). The plants were irrigated by MA-50 micro-sprinklers with anti-drops (Photogenesis, Belo Horizonte, Minas Gerais, Brazil) with a capacity of 50 L/h/m2, automatically, three times a day for 15 min throughout the experiment. After 45 days, the first pruning was performed, leaving the plants 15 cm tall.
2.4 Pest insect collection
In October 2022, adults of M. spectabilis and D. schach were collected from the experimental field of Embrapa Dairy Cattle in Coronel Pacheco, Minas Gerais State, Brazil with an entomological net. The captured adults were placed in entomological cages at the Entomology Laboratory at Embrapa Dairy Cattle. After five days, eggs of the two pest species were collected following the methodology of Auad et al. (2007). Then, 50 eggs of each species were placed on the U. ruziziensis, in the vegetative phase (40 cm high), without the presence of fungi, in the greenhouse and covered with voile fabric to prevent the nymphs from escaping after hatching. Forty planters were infested, totaling 2,000 eggs/species. After 35 days, the nymphs obtained from the planters were used in the experiments.
2.5 Molecular analysis of fungi
After macro- and micromorphological analysis, the fungal isolates were grouped and from each group a random sample was taken with characteristics similar to the fungi applied for molecular analysis, totaling: 12 samples for experiment 1; eight samples for experiment 2, 3, and 4. Filamentous fungi were inoculated on dextrose (4 %), casein (1 %) and agar (1.5 %; Sabouraund dextrose agar, KASVI®, Spain) for seven days. The DNA of the isolates was extracted from the microorganism grown in culture medium according to the method described by Doyle and Doyle (1987). The extracted genomic DNA sample was submitted to the polymerase chain reaction (PCR) to amplify the internal transcribed spacer (ITS) region of the rDNA using the SR6R (5′ – AAGWAAAAGTCGTAACAAGG – 3′) and LR1 (5′ – GGTTGGTTTCTTTTCCT – 3′) primer oligonucleotides (Vilgalys and Hester 1990). The PCR mixture consisted of 1 µL of DNA, 1 µL of each primer at 10 µM, 10 µL of 5X PCR buffer, 1 µL of dNTPs at 10 mM, 0.2 µL of GoTaq DNA polymerase 5U/µL (Promega), and 35.8 µL autoclaved MilliQ H2O, for a final volume of 50 µL. The amplification program consisted of initial denaturation at 94 °C for 2 min followed by 40 cycles of denaturation at 94 °C for 10 s, annealing at 54 °C for 30 s, extension at 72 °C for 45 s, and final extension at 72 °C for 4 min. The amplified products were verified by electrophoresis on a 0.8 % agarose gel stained with ethidium bromide. The amplified products were purified by precipitation with polyethylene glycol (Schmitz and Riesner 2006), submitted to the sequencing reaction by the chain termination method using the Big Dye 3.1 reagent (Applied Biosystems) and analyzed in a 3,500 xL automatic capillary sequencer (Applied Biosystems).
2.6 Experiment 1: evaluation of the frequency of infection and persistence of fungi in plant tissues
U. ruziziensis seeds were treated with the three fungal strains and the control solution without the presence of fungi. The seeds were deposited on the soil, sand and manure mixture at a ratio of 3:1:1 (Resende et al. 2012), and were covered with a layer of vermiculite (Micron®, Jacareí, São Paulo, Brazil), in a 2 L planter, and kept in a greenhouse.
Plant tissue samples (top leaf) were taken at random points from plants in 15 planters, totaling 60 leaf samples (one sample × four treatments × 15 pots/replication) at 45, 120, and 150 days after sowing. These leaves were put into sterile plastic tubes, placed in a thermal box and transported to DEPEB (UFSJ) for testing. Each leaf was surface disinfected by immersion in 70 % ethanol (1 min) and 2 % sodium hypochlorite (1 min), followed by washing with sterile distilled water (2 min) (Carvalho et al. 2012; Ferreira et al. 2017). After disinfection, a fragment of each leaf was placed in a Petri dish containing Sabouraud dextrose agar culture medium (4 % dextrose, 1 % casein, and 1.5 % agar; Kasvi®). The plates were incubated at 25 °C ± 2 °C for approximately 7 days in an EletroLab® BOD-type climatized chamber. To verify the presence of the inoculated fungi, the macromorphology and micromorphology of the fungi present in the fragments were compared to the three inoculated fungal strains according to methodology adapted from Fróhlich et al. (2000). The fungal species were grouped using identification keys according to Alves (1998) for subsequent molecular analysis (see section above).
The experimental design used was completely randomized with plants from seeds treated with different fungi or left untreated (control). The treatments were represented by the leaves collected randomly from each pot for the analysis of the frequency of infection by the fungi applied. Endophytic confirmation was adopted when more than 60 % of the samples presented the characteristics of the fungi applied.
2.7 Experiment 2: efficacy of entomopathogenic fungi applied via seed and confirmation of infection, for the control of M. spectabilis and D. schach in a greenhouse
The experimental design was completely randomized in a factorial scheme (4 × 2) consisting of seeds treated with the three fungal strains or the control solution and two species of spittlebugs, M. spectabilis or D. schach, with 10 replications per treatment, totaling 80 planters.
Ninety days after sowing, 10 nymphs (from the third to fifth instar) of each species were placed in each planter. Daily, for a period of 10 days, the nymphs that died and the adults that emerged and died were removed, packed in 1.5 mL microcentrifuge tubes and labeled according to treatment. After this period, on the 10th day, dead and alive nymphs and adults were collected for analysis of the presence of the fungi. Ten adults coming from nymphs that did not die under the action of the fungi were placed on the aerial part of the same plant from each treatment. Ten days after placing for a second time, the insects that were dead were removed (after 20 days of exposure for that individual), packed in 1.5 mL microcentrifuge tubes and labeled according to treatment. The mortality data (%) in the different treatments were used to calculate the efficiency of the fungi in the greenhouse bioassay.
Samples of nymphs and/or adults of spittlebugs were collected into sterile 1.5 mL microcentrifuge tubes to analyze the cause of mortality and placed in thermal boxes. The samples were taken to the DEPEB at UFSJ for incubation in the middle of culture medium selective for fungi (Sabouraud dextrose agar, 4 % dextrose, 1 % casein, and 1.5 % agar; Kasvi®) to evaluate the presence of fungi in the nymphs and adults of spittlebugs, which were applied in the treatment of U. ruziziensis seeds. Superficial disinfestation of insects, fungal isolation and identification were performed as described for Experiment 1.
2.8 Experiment 3: efficacy of entomopathogenic fungi applied via seeds and used on banker plants to control M. spectabilis under field conditions
Ten U. ruziziensis seeds treated with the three fungal strains and seeds from the control group, without any fungus, were planted in the greenhouse, as described for Experiment 1. When the plants had been sown for 100 days, 10 pots from each treatment plus the control group were taken to the experimental field and placed in clumps of elephant grass (Pennisetum purpureum Schumach.; Poaceae) with proven attack by M. spectabilis (to prove attack, screening and a visual search were carried out on the foam containing nymphs at the base of the plants). The pots were placed in a straight line with a distance of 5 m between them.
Every 7 days for a period of 3 weeks, the numbers of dead spittlebug adults from nymphs fed on elephant grass and that, upon emerging as adults, migrated to plants that were in planters, were determined. This count was performed in a radius of 2 m around each planter. Five samples were collected from each replicate per treatment, which were packaged and sent for analysis of the presence of the fungi in the insect tissues, as described in Experiment 1.
2.9 Experiment 4: analysis of the persistence of fungi in tissues of U. ruziziensis and infection of M. spectabilis on plants of different ages
After treatment with the three fungi strains or no fungal treatment, the U. ruziziensis seeds were stored for 60 days in a climatized chamber (22 °C). From this moment on, seed samples were collected to germinate every 30 days for 10 consecutive months. The plants obtained on each of the sowing dates were kept in 2 L planters in a greenhouse with controlled fertilization, irrigation and pruning to maintain an approximate height of 25 cm. The aerial part of the plants of batch from one to nine were pruned at 45 days, leaving the plants 15 cm tall. Plants in the last batch of seeds (the youngest plants) were 45 days old and so were not pruned. So, when infested with M. spectabilis nymphs the aerial part of all plants was 45 days-old.
A randomized block design was used with plants from seeds treated with different fungi strains or untreated (control) with plants of different ages (1–10 months), with five replications. When the plants of the last batch of seeds were 45 days old, the plants of all 10 batches (plant age from 45 days to 10 months, sowing times) were infested with four nymphs of the third and fourth instars of M. spectabilis per pot to evaluate the presence of the fungus and its efficiency in controlling the insect and to define the persistence of the fungus in stored seeds and in vivo plants managed with elimination of the aerial part (cuts) to simulate natural conditions in a pasture.
For analysis of the frequency of fungal infection in plant tissue (leaves), a random sample was taken from each plant, totaling five samples per treatment, placed in a sterile 1.5 mL microcentrifuge tube, placed in a thermal box and taken to the Microbiology Laboratory of the Federal University of São João Del Rei for testing.
For analysis of the frequency of fungal infection in the pest insects fed on the plants from the different sowing lots, four nymphs of M. spectabilis were allocated per planter, and to prevent the nymphs of the insect pest from escaping, the planters were covered with voile. After 10 days of insect feeding in different treatments, dead and live nymphs were removed and placed in 1.5 mL microcentrifuge tubes. They were then frozen at −22 °C and sent to DEPEB, UFSJ, Minas Gerais, Brazil, to verify the presence of fungi in insect tissues. Superficial disinfestation of insects and leaves of U. ruziziensis and fungal isolation and identification were performed as described for Experiment 1.
2.10 Statistical analyses
All data analyses were performed with R version 4.2.2 (R Core Team 2022). The data obtained did not meet normality assumptions related to the residuals and homogeneity of variances (Shapiro–Wilk test and Bartlett test, p < 0.01). A generalized linear model (GLM) followed by analysis of variance (ANOVA) was used to verify whether there were significant differences in the proportions of plants and spittlebugs expressing the presence of endophytic fungi and the mortality of spittlebugs that fed on plants inoculated with the endophytic fungi. For these comparisons, a GLM was used in a logistic regression model with a binomial distribution, and the “hnp” package (Moral et al. 2017) was previously used to choose the best model. In this model, the effects of treatments (fungal species, spittlebug species, developmental stage, and storage time) were tested individually, as were their interactions. In instances where the impact of interactions did not attain statistical significance, the effects of treatments were assessed independently. The treatments were separated using logistic regression, which allows the prediction of values taken by a categorical variable, normally binary, from a series of continuous and/or binary explanatory variables. This is a regression model for binomially distributed dependent variables. Thus, it is a generalized linear model that uses the logit function as the link function. This model does not assume normality of residuals or homogeneity of variances. The presence or absence of the endophytic fungi in the plants or spittlebugs was included as a variable in the model. The packages visreg (Breheny and Burchett 2019), MASS (Ripley 2019), ggplot2 (Wickham 2016), and GGally (Schloerke et al. 2021) were used for the logistic regression.
3 Results
3.1 Frequency of infection and persistence of fungi in plant tissues
The interactions (fungal species and period of permanence of fungi in plants) did not attain statistical significance; hence, the effects of treatments were assessed independently. The entomopathogenic fungi Fusarium sp. (UFMG 11443), M. anisopliae (UFMG 11444), and M. anisopliae (commercial strain) were isolated from the tissues of U. ruziziensis 45 days after seed treatment, which confirmed their endophytic capacity. The presence of the fungi used in the treatments was confirmed by molecular analysis (Table 1). The percentage of plants with the fungi in their tissues was above 60 %, which was significantly higher (χ2 = 399.72; df = 3; P < 0.0001) than the percentage of plants with fungi in their tissues obtained from untreated seeds (Figure 1A). This percentage did not differ significantly among the plants obtained from the seeds that were treated with different fungi. In addition, it was found that the fungi in plants was reduced significantly 150 days after sowing compared with 45 days after sowing (χ2 = 386.12; df = 2; P = 0.0011) (Figure 1B).
Confirmation of fungi applied via Urochloa ruziziensis seeds in Mahanarva spectabilis nymphs fed on these plants in greenhouse and field, and plant tissue after infested. GenBank accessions of sequences.
| Fungi applied | Experiment | Isolate habitat/host | Molecular analysis | GenBank |
|---|---|---|---|---|
| Metarhizium anisopliae | Exp#01 | Plant tissues | Metarhizium anisopliae or M. robertsii a | OR755961 |
| Metarhizium anisopliae | Plant tissues | Metarhizium anisopliae or M. robertsii | OR755962 | |
| Metarhizium anisopliae | Plant tissues | Metarhizium anisopliae or M. robertsii | OR755963 | |
| Fusarium sp. | Plant tissues | Fusarium sp. | OR755966 | |
| Fusarium sp. | Plant tissues | Fusarium sp. | OR755967 | |
| Fusarium sp. | Plant tissues | Fusarium sp. | OR755968 | |
| Metarhizium anisopliae | Plant tissues | Metarhizium anisopliae or M. robertsii | OR755969 | |
|
|
||||
| Metarhizium anisopliae | Exp#02 | Nymphs | Metarhizium anisopliae or M. robertsii | OR755940 |
| Metarhizium anisopliae | Nymphs | Metarhizium anisopliae or M. robertsii | OR755941 | |
| Fusarium sp. | Nymphs | Fusarium sp. | OR755943 | |
| Fusarium sp. | Nymphs | Fusarium sp. | OR755944 | |
| Metarhizium anisopliae | Nymphs | Metarhizium anisopliae or M. robertsii | OR755945 | |
| Metarhizium anisopliae | Nymphs | Metarhizium anisopliae or M. robertsii | OR755946 | |
|
|
||||
| Metarhizium anisopliae | Exp#03 | Nymphs | Metarhizium anisopliae or M. robertsii | OR755933 |
| Metarhizium anisopliae | Nymphs | Metarhizium anisopliae or M. robertsii | OR755947 | |
| Fusarium sp. | Nymphs | Fusarium sp. | OR755936 | |
| Fusarium sp. | Nymphs | Fusarium sp. | OR755937 | |
| Metarhizium anisopliae | Nymphs | Metarhizium anisopliae or M. robertsii | OR755938 | |
| Metarhizium anisopliae | Nymphs | Metarhizium anisopliae or M. robertsii | OR755939 | |
|
|
||||
| Metarhizium anisopliae | Exp#04 | Plant tissues | Metarhizium anisopliae or M. robertsii | OR755948 |
| Metarhizium anisopliae | Plant tissues | Metarhizium anisopliae or M. robertsii | OR755949 | |
| Fusarium sp. | Plant tissues | Fusarium sp. | OR755952 | |
| Fusarium sp. | Plant tissues | Fusarium sp. | OR755953 | |
| Metarhizium anisopliae | Plant tissues | Metarhizium anisopliae or M. robertsii | OR755954 | |
| Metarhizium anisopliae | Plant tissues | Metarhizium anisopliae or M. robertsii | OR755955 | |
|
|
||||
| Metarhizium anisopliae | Exp#04 | Adults | Metarhizium anisopliae or M. robertsii | OR755956 |
| Fusarium sp. | Adults | Fusarium sp. | OR755957 | |
| Metarhizium anisopliae | Adults | Metarhizium anisopliae or M. robertsii | OR755958 | |
| Fusarium sp. | Adults | Fusarium sp. | OR755959 | |
| Metarhizium anisopliae | Adults | Metarhizium anisopliae or M. robertsii | OR755960 | |
-
Exp#2 Nymphs from the greenhouse; Exp#3 Nymphs from the field; a ITS region does not allow these species to be distinguished.
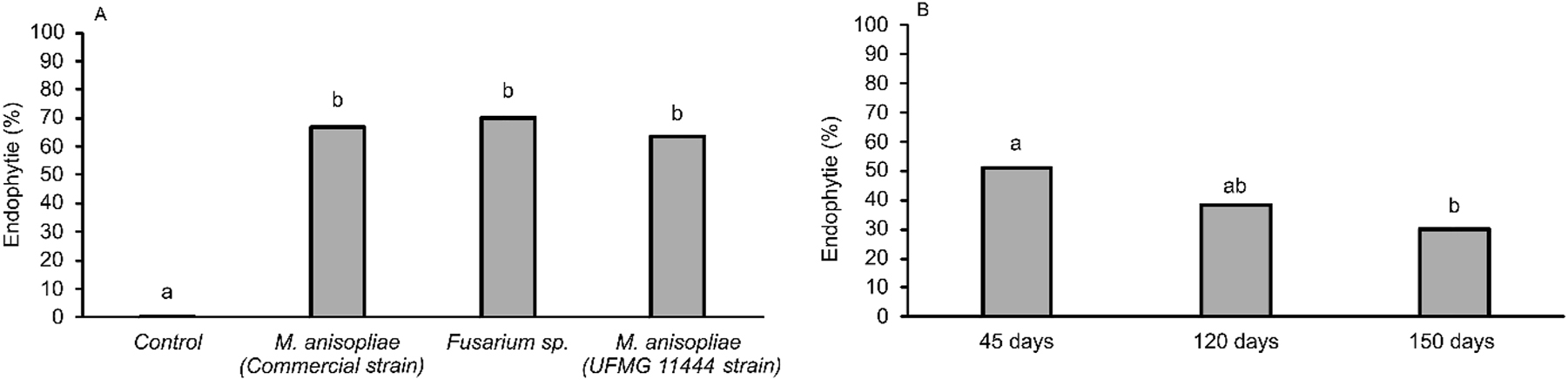
Entomopathogenic fungi Fusarium sp. (UFMG 11443, GenBank = ON831395), Metarhizium anisopliae (UFMG 11444, GenBank = ON831396), and Metarhizium anisopliae (commercial strain) present on the leaves of Urochloa ruziziensis at 45 days of age (A). Presence of entomopathogenic fungi in plants of different ages (B). Significant differences are indicated by different letters above bars.
3.2 Isolation of fungi from spittlebugs and insect pest mortality in a greenhouse
The interactions (spittlebug species and insect developmental stage) did not attain statistical significance; hence, the effects of treatments were assessed independently. The presence of the entomopathogenic fungi isolated from nymphs was confirmed by molecular analysis (Table 1). The entomopathogenic fungi Fusarium sp. (UFMG 11443), M. anisopliae (UFMG 11444), and M. anisopliae (commercial strain) were isolated for up to and including 10 days from live nymphs and adults of M. spectabilis and D. schach fed on plants derived from seeds treated with the respective fungi. The presence of fungi did not differ significantly (χ2 = 162.16; df = 1; P = 0.1839) between M. spectabilis and D. schach (Figure 2A) or between the developmental stages (χ2 = 162.05; df = 1; P = 0.7419) of these insects (Figure 2B), demonstrating that the samples of the entomopathogenic fungi used affected both nymphs and adults of the spittlebug species. However, despite the confirmation of the presence of the fungi on the spittlebugs, the nymphal mortalities were below 20 % and did not differ significantly (χ2 = 17.24; df = 3; P = 0.9853) from those of spittlebugs fed on plants from seeds not treated with the fungi (control) (Figure 2C).

Infection and mortality of spittlebugs fed on plants grown from seeds treated with the entomopathogenic fungi Fusarium sp. (UFMG 11443, GenBank = ON831395), Metarhizium anisopliae (UFMG 11444, GenBank = ON831396), and Metarhizium anisopliae (commercial strain) in the greenhouse. Infection frequency of Mahanarva spectabilis and Deois schach (A). Adults and nymphs with the presence of entomopathogenic fungi (B). Mortality of spittlebug nymphs and adults in 10 days due to entomopathogenic fungi (C). Adult mortality (%) coming from nymphs that did not die under the action of the fungi in each treatment within 10 days, 10 days after placing the insects back on the aerial part of the plant (20 days of total exposure for that individual; D). Significant differences are indicated by different letters above bars.
Adults that died within 10 days, coming from nymphs that did not die under the action of the fungi within 10 days (20 days of exposure for that individual) were confirmed as infected by the endophytic fungi, with no significant differences in infection among the fungi applied. Only the control differed statistically from the other treatments, where the insects were not infected by the fungi used in the experiments (χ2 = 39.14; df = 3; P = 0.0010) (Figure 2D).
3.3 Fungal isolation from spittlebugs and pest insect mortality under field conditions
The introduction of planters of banker plants from seeds treated with the fungi Fusarium sp. (UFMG 11443), M. anisopliae (UFMG 11444), and M. anisopliae (commercial strain) caused infection in M. spectabilis adults reared in elephant grass clumps in the field. In the M. spectabilis adults that fed on these plants and were collected dead nearby or on the plants, mortality was above 75 % for the three fungi, with infection confirmed by molecular analysis (Table 1), showing a significant difference (χ2 = 48.27; df = 3; P < 0.0001) from the control treatment (Figure 3).
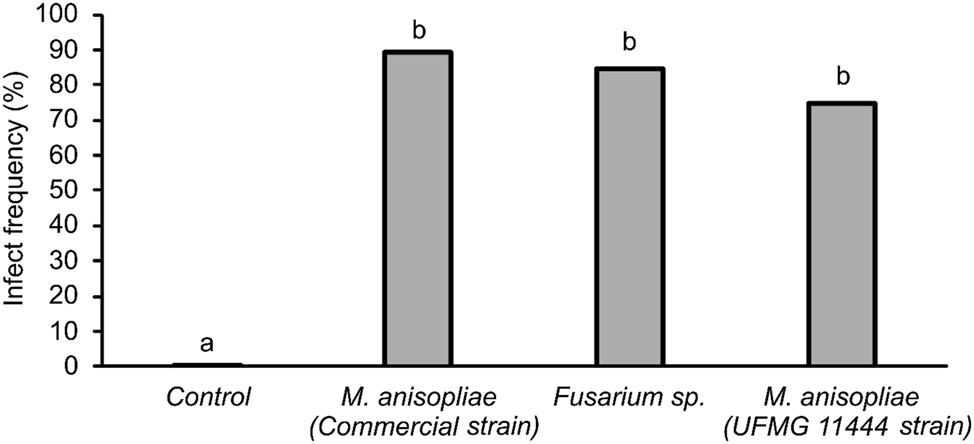
Dead adults of Mahanarva spectabilis collected in the field around banker plants grown from seeds treated with entomopathogenic fungi Fusarium sp. (UFMG 11443, GenBank = ON831395), Metarhizium anisopliae (UFMG 11444, GenBank = ON831396), and Metarhizium anisopliae (commercial strain). Insects confirmed to be infected with entomopathogenic fungi. Significant differences are indicated by different letters above bars.
3.4 Analysis of the persistence of fungi in the tissues of U. ruziziensis and infection of M. spectabilis on plants of different ages
There was no significant interaction (χ2 = 255.11; df = 27; P = 0.9235) between the species of entomopathogenic fungi and the storage period of treated seeds/plant ages. Therefore, these factors were analyzed separately. The frequency of infection by entomopathogenic and endophytic fungi ranged from 5 % to 40 % in plants derived from seeds stored for 90 days after inoculation of the fungi (plants for 330 days) or stored for 360 days after inoculation (plants for 60 days); however, it was not significantly different (χ2 = 295.88; df = 9; P = 0.1692) between plants measured on different days after inoculation. This evidence indicates the persistence of these fungi in plants and stored seeds, which was confirmed by molecular analysis (Table 1) of the fungi isolated from the plants over time (Table 2).
Frequency of endophytic fungal infection in the tissues of Urochloa ruziziensis plants or in Mahanarva spectabilis nymphs when fed on plants derived from seeds stored for 12 months after treatment with endophytic fungi.
| Storage period of the treated seeds (days) | Age of the plant used in the experiment (days) | Infection frequency (%) | |
|---|---|---|---|
| In-plants | In-insects | ||
| 90 | 330 | 5.0 | 21.8 |
| 120 | 300 | 15.0 | 45.9 |
| 150 | 270 | 15.0 | 33.3 |
| 180 | 240 | 15.0 | 57.1 |
| 210 | 210 | 25.0 | 28.5 |
| 240 | 180 | 20.0 | 36.3 |
| 270 | 150 | 30.0 | 54.1 |
| 300 | 120 | 20.0 | 36.3 |
| 330 | 90 | 30.0 | 58.3 |
| 360 | 60 | 40.0 | 36.6 |
| Significance | P = 0.46 | P = 0.1692 | |
| Control | 0 a | 0 a | |
| Metarhizium anisopliae | Commerical | 30 b | 54.5 b |
| Fusarium sp. | UFMG 11443 | 26 b | 55.3 b |
| Metarhizium anisopliae | UFMG11444 | 30 b | 51.7 b |
| Significance | P < 0.0001 | P < 0.0001 | |
-
Frequency values (%) of endophytic fungal infection in plant tissues or insects followed by the same letters are not significantly different as per the analysis of variance.
Insects fed on plants derived from seeds stored for 90 days (plants for 330 days) or stored for 360 days (plants for 60 days) showed a frequency of infection by entomopathogenic fungi from 21.8 % and 58.3 %, respectively; however, they did not present significant differences (χ2 = 202.04; df = 9; P = 0.4636) for the time since fungal inoculation, which verified the persistence of fungi in seeds stored for up to 300 days and in plants 300 days after sowing (Table 2). The frequencies of fungal infection in plant tissues and M. spectabilis nymphs were significantly higher than that in the control treatment and did not differ from each other, with a mean of 28.6 % in plant tissues (χ2 = 179.74; df = 3; P < 0.0001) and 53.8 % in insect pests (χ2 = 48.27; df = 3; P < 0.0001) (Table 2).
4 Discussion
The use of entomopathogenic fungi for biological control of spittlebugs can benefit the milk and meat production chain, as it would reduce the use of phytosanitary products, such as pesticides, that are harmful to nonhuman animals, humans, and the environment, in addition to being, most of the time, uneconomical and anti-ecological.
The spittlebug control efficiencies of classical application of M. anisopliae to plants before infestation by the insects and conidial spraying are between 10 % and 60 % (Alves 1998). The quality of the fungus applied per unit area, the application method, the isolate used and the climatic conditions during the applications are factors that promote this large variation in efficiency (Dinardo-Miranda et al. 2004). Despite this, the strategy is efficient when used at an appropriate time and with products from reputable sources (Auad and da Silva 2019).
Pereira et al. (2008) reported the influence of dry conditions and the time of application on the efficiency of the fungus M. anisopliae (isolate IBCB 425) in controlling Deois flavopicta Stål (Hemiptera: Cercopidae) in Urochloa pasture. These authors found that sunlight is harmful to entomopathogenic fungi. Greenfield et al. (2016) confirmed that the effectiveness of entomopathogenic fungi is limited by abiotic factors (e.g., UV radiation, temperature, and low humidity) that reduce the viability of fungal conidia. These authors also pointed out an alternative, the inoculation of plants with entomopathogenic fungi that act as endophytes, providing protection to the fungus against these abiotic factors. Corroborating these findings, it was demonstrated in the present study that the fungi Fusarium sp. (UFMG 11443), M. anisopliae (UFMG 11444), and M. anisopliae (commercial strain) were able to colonize the tested plants and cause epizootics in spittlebugs that fed on the plants.
Over the course of evolution, fungi have evolved several mechanisms to interact with a variety of living organisms, including plants. Different genera of entomopathogenic fungi have been identified as endophytic. In this regard, the multifaceted lifestyle of entomopathogenic fungi, such as saprophytes, endophytes, and biocontrol agents, can offer several benefits to the host plant, such as promoting its growth and protection against pathogens and insect pests (González-Pérez et al. 2022).
The use of fungi in biological pest control is under development, and there may be an future increase in the production of mycoinsecticides to avoid the use of chemical elements harmful to nature in agricultural processes (Barão et al. 2022). Parra (2014) reports that, in Brazil, Mahanarva fimbriolata Stål (Hemiptera: Cercopidae) is controlled with the fungus M. anisopliae, covering an area of two million ha. With the seed treatment methodology presented in this work, the endophytic fungi can be applied more efficiently than with traditional topical applications. However, further studies are needed to determine the length of time that these entomopathogenic fungi remain viable endophytes in plant tissue.
Mantzoukas and Lagogiannis (2019) pointed out that entomopathogenic species such as Beauveria bassiana (Bals.-Criv.) Vuill. (Hypocreales: Cordicipitaceae), Metarhizium spp., and Isaria fumosorosea Wize (Hypocreales: Cordicipitaceae) have high pathogenic potential against insects when inoculated into plants. These species were able to enter the plant tissues after being sprayed as conidia, with significantly reduced infection after 14 days. In contrast to these results, the fungi Fusarium sp. (UFMG 11443), M. anisopliae (UFMG 11444), and M. anisopliae (commercial strain) used in the present research, had reduced infection of plants at 120 and 150 days after inoculation of conidia via seed. In addition, it is noteworthy that the percentage of infection was above 60 %, which proved that fungi used in this research were endophytic and maintained their virulence. According to Campagnani et al. (2017), the success of a mycoinsecticide depends on several criteria, the main one being its virulence. The virulence of endophytic fungi present in the plant tissue is important in determining whether insects feed on the plants and ingest and become infected with the fungi.
Pitta et al. (2019) used M. anisopliae to combat M. spectabilis infestation and obtained satisfactory results for the infection of both nymphs and adults. This result was corroborated in the present study, in which the infection caused by the fungal strains tested was the same for the nymphal and adult stage of M. spectabilis and D. schach in a greenhouse. These fungi were collected for the first time in a silvopastoral system in Maranhão, naturally occurring in spittlebug adults, and were recorded as efficient and endophytic (Campagnani et al. 2024). Reiterating the statements by Branine et al. (2019) for Metarhizium robertsii J.F. Bisch., S.A. Rehner & Humber (Hypocreales: Clavicipitaceae), the fungi tested in this study colonized the plants and infected the insects, forming a three-way interaction between fungus, plant, and insect.
In the present study, the frequency of insect pest infection was above 50 % in the greenhouse and above 60 % in the field. However, despite the confirmation of the presence of fungi in spittlebugs, these fungi promoted mortalities below 20 % in the greenhouse, which did not differ significantly from those consuming plants derived from the seeds not treated by the fungi, perhaps more time was required for the epizootic to occur. This was confirmed with the remaining adults, which, despite coming from nymphs that did not die within 10 days after being placed on the aerial part of the plant, were infected and were confirmed to have been killed by the fungi applied in the subsequent 10 days, by carrying out isolation and subsequent molecular identification of the fungi. These results differ from those obtained by Campagnani et al. (2024), which suggested that the same fungal isolates had potential for use as biopesticides, as they promoted nymphal mortality of M. spectabilis above 88 %. This difference can be attributed to the fact that in the present work, the nymphs used in the experiments were obtained in the same study environment, while those used by Campagnani et al. (2024) came from an experimental field, which possibly provided a population of nymphs experiencing a certain degree of stress. This stress may have facilitated infection by the entomopathogenic fungi. Another factor that may explain such a large difference between the results is the virulence of the fungi used, as those used in the present research were stored in a freezer (−2 °C) and came from the third subculture, and those used by Campagnani et al. (2024) came from the first subculture after infection of the host. Therefore, it is essential to establish new research directions to understand and define optimal conditions for storing and subculturing fungi. This will maintain their virulence, enabling their use across the three population peaks of spittlebugs that occur during the rainy season. The duration of the experiment may have also influenced this result. Specifically, in this work, the insects were evaluated until the 10th day, and in the other study, the evaluation was performed only at 15 days, perhaps we did not provide enough time for the fungi to kill sufficient insects or to sporulate.
It is believed that another way to lessen the dependence of entomopathogenic fungi on abiotic factors in the control of spittlebugs in pastures is to introduce the fungi into the crop with the use of banker plants, among other options. Banker plants act directly or indirectly, providing resources such as prey or hosts and food for the natural enemies that are deliberately added to a cropping system, assisting in the reproduction and maintenance of the communities of these natural enemies close to the crop; hence, they provide pest control (Zheng et al. 2017). This same strategy was corroborated in the present study, where we inserted planters of banker plants from seeds treated with entomopathogenic fungi in the middle of a field infested with spittlebugs, to combat the infestation by M. spectabilis through the infection of those that fed on the introduced plants. We obtained positive results, with infection of 75 % of the dead adults collected around or inside the planters inserted in the field.
In a forage breeding program, seed storage consists of maintaining genetic, morphological, and physiological characteristics to support the selection of materials with desirable characteristics over time. Furthermore, the maintenance of viability and the presence of endophytic fungi in the seeds are factors to be investigated in the seed storage process. Based on this, Cheplick (2017) found that endophytic fungi (Epichloe festucae Leuchtm., Schardl & M.R. Siegel var. lolii; Hypocreales: Clavicipitaceae) persist at frequencies from 58 % to 73 % in Lolium (Poaceae) seeds stored for 22 years. In the present study, the entomopathogenic fungi used in the seed treatments had a frequency of infection between 5 % and 40 % in the tissues of U. ruziziensis and 21.8 %–58.3 % in the M. spectabilis nymphs, which fed on these plants, 12 months after fungal inoculation of the seeds. This proves that despite changes in the frequency of infection over time, fungi are still able to persist and maintain a certain level of virulence.
It is noteworthy that this persistence is linked to the cultivars used and/or abiotic factors occurring during storage. Latch and Christensen (1982) found that in perennial ryegrass plants, viable endophytes were found in 40 % of the seeds of cultivar Nui stored at −5 °C for 7 years and in 80 % of the plants from seeds of cv. Ellett stored for 6 years. Clement et al. (2008) also found variation in infection by Neotyphodium (Hypocreales: Clavicipitaceae) after storage among 20 accessions of Lolium arundinaceum (Schreb.) Darbysh. (Poaceae). In addition, the viability of endophytic fungi can be reduced due to certain abiotic factors. This was confirmed in a study by Welty (1987), who used Festuca arundinacea (Poaceae) seeds stored for 18 months and recorded the interaction of temperature, moisture content, and storage time in influencing the viability of an endophytic fungus (Acremonium coenophialum Morgan-Jones & W. Gams; Hypocreales: Clavicipitaceae); they observed a significant reduction in viability at high temperatures.
The aforementioned biotic and abiotic factors should be included in new studies involving the storage of seeds of U. ruziziensis inoculated with entomopathogenic fungi. The results of the present research, in which the treated seeds were stored at 22 °C for 12 months, provided an optimistic scenario for the storage of U. ruziziensis seeds infected with entomopathogenic fungi, which can act in more than one cycle of the well-defined period of the insect pest from October to March of each year. This reinforces that treated seeds can be stored for a year, and plants from the previous year can form pastures that negatively impact the insect pest, considering that plants with the shoots eliminated (cut) to simulate natural pasture conditions still presented entomopathogenic fungi after 10 months.
Thus, it became evident that the entomopathogenic fungi tested in this study were endophytic, equally infecting the spittlebugs M. spectabilis and D. schach from pastures at different stages of development; however, they caused low nymphal mortality within the time constraints of our study. The use of banker plants from seeds treated with entomopathogenic fungi was efficient in controlling spittlebugs. Furthermore, it was possible to store seeds treated with the fungi for 12 months. During this period, it was also possible to detect infection by endophytic fungi in pastures formed in the previous year. These results open up the prospect of using entomopathogenic fungi with endophytic action and are promising candidates for bioproducts as an auxiliary tool in reducing these pests.
Funding source: Conselho Nacional de Desenvolvimento CientÃ-fico e Tecnológico
Funding source: Fundação de Amparo à Pesquisa do Estado de Minas Gerais
Acknowledgments
The authors express their gratitude to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil (Finance Code 307956/2023-7) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG, Brazil (Finance Code CAG APQ-00732-18 and APQ 03630-23).
-
Research ethics: Not applicable.
-
Author contributions: The authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: The authors state no conflict of interest.
-
Research funding: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil (Finance Code 307956/2023-7) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Brazil) (Finance Code CAG APQ-00732-18 and APQ 03630-23).
-
Data availability: The raw data can be obtained on request from the corresponding author.
References
Alvarenga, R., Auad, A.M., Moraes, J.C., and Silva, S.E. (2019). Do silicon and nitric oxide induce resistance to Mahanarva spectabilis (Hemiptera: Cercopidae) in forage grasses? Pest Manage. Sci. 75: 3282–3292, https://doi.org/10.1002/ps.5450.Search in Google Scholar PubMed
Alves, S.B. (1998). Fungos entomopatogênicos. In: Controle microbiano de insetos. Fundação de Estudos Agrários “Luiz de Queiroz, Piracicaba, Brazil, pp. 289–381.Search in Google Scholar
Andorno, A.V. and López, S.N. (2014). Biological control of Myzus persicae (Hemiptera: Aphididae) through banker plant system in protected crops. Biol. Control 78: 9–14, https://doi.org/10.1016/j.biocontrol.2014.07.003.Search in Google Scholar
Auad, A.M. and da Silva, S.E.B. (2019). Pasture. In: Natural enemies of insect pests in neotropical agroecosystems. Springer International Publishing, Cham, pp. 369–382.10.1007/978-3-030-24733-1_30Search in Google Scholar
Auad, A.M., Simões, A.D., Pereira, A.V., Braga, A.L.F., Souza Sobrinho, F., Lédo, F.J.da S., Paula-Moraes, S.V., Oliveira, S.A., and Ferreira, R.B. (2007). Seleção de genótipos de capim-elefante quanto à resistência à cigarrinha-das-pastagens. Pesqui. Agropecu. Bras. 42: 1077–1081, https://doi.org/10.1590/s0100-204x2007000800003.Search in Google Scholar
Barão, N.C., Carla, R., and Everlon, C. (2022). Fungos endofíticos: uma ferramenta para promoção do crescimento de plantas e agricultura sustentável. Micologia 13: 39–55.Search in Google Scholar
Branine, M., Bazzicalupo, A., and Branco, S. (2019). Biology and applications of endophytic insect-pathogenic fungi. PLoS Pathog. 15: e1007831, https://doi.org/10.1371/journal.ppat.1007831.Search in Google Scholar PubMed PubMed Central
Breheny, P. and Burchett, W. (2019). Package “visreg”, Available at: https://cran.r-project.org/web/packages/visreg/visreg.pdf (Accessed 8 January 2023).Search in Google Scholar
Campagnani, M.O., Auad, A.M., Maurício, R.M., Madureira, A.P., Cangussú, M.A., Rosa, L.H., Pereira, M.F.A., Muniz, M., Souza, S.R.O., Silva, N.B.M., et al.. (2024). Endophytic capacity of entomopathogenic fungi in a pasture grass and their potential to control the spittlebug Mahanarva spectabilis (Hemiptera: Cercopidae). Agronomy 14: 943, https://doi.org/10.3390/agronomy14050943.Search in Google Scholar
Campagnani, M.O., Campos, W.G., Amorim, S.S., Rosa, L.H., Auad, A.M., Cangussú, M.A., and Maurício, R.M. (2017). Prospection and fungal virulence associated with Mahanarva spectabilis (Hemiptera: Cercopidae) in an Amazon silvopastoral system. Fla. Entomol. 100: 426–432, https://doi.org/10.1653/024.100.0204.Search in Google Scholar
Carvalho, C.R., Gonçalves, V.N., Pereira, C.B., Johann, S., Galliza, I.V., Alves, T.M.A., Rabello, A., Sobral, M.E.G., Zani, C.L., Rosa, C.A., et al.. (2012). The diversity, antimicrobial and anticancer activity of endophytic fungi associated with the medicinal plant Stryphnodendron adstringens (Mart.) Coville (Fabaceae) from the Brazilian savannah. Symbiosis 57: 95–107, https://doi.org/10.1007/s13199-012-0182-2.Search in Google Scholar
Cheplick, G.P. (2017). Persistence of endophytic fungi in cultivars of Lolium perenne grown from seeds stored for 22 years. Am. J. Bot. 104: 627–631, https://doi.org/10.3732/ajb.1700030.Search in Google Scholar PubMed
Clement, S.L., Martin, R.C., Dombrowski, J.E., Elberson, L.R., Kynaston, M., and Azevedo, M.D. (2008). Neotyphodium endophytes in tall fescue seed: viability after seed production and prolonged cold storage. Seed Sci. Technol. 36: 710–720, https://doi.org/10.15258/sst.2008.36.3.20.Search in Google Scholar
Congio, G.F.S., Almeida, P.C., Barreto, T.R., Tinazo, V.A., Silva, T.A.C.C., Costa, D.F.A., and Corsi, M. (2012). Regrowth of Marandu palisadegrass submitted to spittlebugs attack. Arq. Inst. Biol. 79: 389–396, https://doi.org/10.1590/s1808-16572012000300009.Search in Google Scholar
Congio, G.F.S., de Almeida, P.C., Barreto, T.R., Tinazo, V.A., da Silva, T.A.C.C., Costa, D.F.A., and Corsi, M. (2020). Spittlebug damage on tropical grass and its impact in pasture-based beef production systems. Sci. Rep. 10: 10758, https://doi.org/10.1038/s41598-020-67490-9.Search in Google Scholar PubMed PubMed Central
Dias Filho, M.B. (2017). Soluções para problemas recorrentes em pastagens no Pará. In: EMBRAPA Amazônia Oriental. Brasília, Brazil.Search in Google Scholar
Dias, M.L., Auad, A.M., Magno, M.C., Resende, T.T., Fonseca, M.G., and Silva, S.E.B. (2019). Insecticidal activity of compounds of plant origin on Mahanarva spectabilis (Hemiptera: Cercopidae). Insects 10: 360, https://doi.org/10.3390/insects10100360.Search in Google Scholar PubMed PubMed Central
Dinardo-Miranda, L.L., Vasconcelos, A.C.M., Ferreira, J.M.G., Garcia, C.A.Jr., Coelho, Á.L., and Gil, M.A. (2004). Eficiência de Metarhizium anisopliae (Metsch.) no controle de Mahanarva fimbriolata (Stål) (Hemiptera: Cercopidae) em cana-de-açúcar. Neotrop. Entomol. 33: 743–749, https://doi.org/10.1590/s1519-566x2004000600012.Search in Google Scholar
Doyle, J.J. and Doyle, J.L. (1987). A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 19: 11–15.Search in Google Scholar
EMBRAPA (Empresa Brasileira de Pesquisa Agropecuária) (2021). Brasil é o quarto maior produtor de grãos e o maior exportador de carne bovina do mundo, diz estudo. Brasília, https://www.embrapa.br/busca-de-noticias/-/noticia/62619259/brasil-e-o-quarto-maior-produtor-de-graos-e-o-maior-exportador-de-carne-bovina-do-mundo-diz-estudo (Accessed 20 June 2022).Search in Google Scholar
Fazolin, M., Santos, R.S., Andrade, C.M.S., Assis, G.M.L., and Valentim, J.F. (2016). Cigarrinhas-das-pastagens: como identificar e controlar a principal praga das Pastagens. Embrapa Acre, Rio Branco, AC, Brazil.Search in Google Scholar
Ferreira, M.C., Cantrell, C.L., Wedge, D.E., Gonçalves, V.N., Jacob, M.R., Khan, S., Rosa, C.A., and Rosa, L.H. (2017). Diversity of the endophytic fungi associated with the ancient and narrowly endemic neotropical plant Vellozia gigantea from the endangered Brazilian rupestrian grasslands. Biochem. Syst. Ecol. 71: 163–169, https://doi.org/10.1016/j.bse.2017.02.006.Search in Google Scholar
Fróhlich, J., Hyde, K.D., and Petrini, O. (2000). Endophytic fungi associated with palms. Mycol. Res. 104: 1202–1212, https://doi.org/10.1017/s095375620000263x.Search in Google Scholar
González-Pérez, E., Ortega-Amaro, M.A., Bautista, E., Delgado-Sánchez, P., and Jiménez-and Bremont, J.F. (2022). The entomopathogenic fungus Metarhizium anisopliae enhances Arabidopsis, tomato, and maize plant growth. Plant Physiol. Biochem. 176: 34–43, https://doi.org/10.1016/j.plaphy.2022.02.008.Search in Google Scholar PubMed
Greenfield, M., Gómez-Jiménez, M.I., Ortiz, V., Vega, F.E., Kramer, M., and Parsa, S. (2016). Beauveria bassiana and Metarhizium anisopliae endophytically colonize cassava roots following soil drench inoculation. Biol. Control 95: 40–48, https://doi.org/10.1016/j.biocontrol.2016.01.002.Search in Google Scholar PubMed PubMed Central
Huang, N., Enkegaard, A., Osborne, L.S., Ramakers, P.M.J., Messelink, G.J., Pijnakker, J., and Murphy, G. (2011). The banker plant method in biological control. Crit. Rev. Plant Sci. 30: 259–278, https://doi.org/10.1080/07352689.2011.572055.Search in Google Scholar
Instituto Brasileiro de Geografia e Estatística (2020). Atlas do espaço rural Brasileiro/IBGE 2020, IBGE, Rio de Janeiro, Available at: https://biblioteca.ibge.gov.br/index.php/biblioteca-catalogo?view=detalhes&id=2101773 (Accessed 23 July 2021).Search in Google Scholar
Keyser, C.A., Thorup-Kristensen, K., and Meyling, N.V. (2014). Metarhizium seed treatment mediates fungal dispersal via roots and induces infections in insects. Fungal Ecol. 11: 122–131, https://doi.org/10.1016/j.funeco.2014.05.005.Search in Google Scholar
Latch, G.C.M. and Christensen, M.J. (1982). Ryegrass endophyte, incidence, and control. New Zeal. J. Agr. Res. 25: 443–448, https://doi.org/10.1080/00288233.1982.10417910.Search in Google Scholar
Lopes, R.B., Martins, I., Souza, D.A., and Faria, M. (2013). Influence of some parameters on the germination assessment of mycopesticides. J. Invertebr. Pathol. 112: 236–242, https://doi.org/10.1016/j.jip.2012.12.010.Search in Google Scholar PubMed
Mantzoukas, S. and Lagogiannis, I. (2019). Endophytic colonization of pepper (Capsicum annum) controls aphids (Myzus persicae Sulzer). Appl. Sci. 9: 2239, https://doi.org/10.3390/app9112239.Search in Google Scholar
MAPA (Ministério da Agricultura, Pecuária e Abastecimento, Brasil) (2022). Mapa do leite: políticas públicas e privadas para o leite. Brasília, Brasil, https://www.gov.br/agricultura/pt-br/assuntos/producao-animal/mapa-do-leite (Accessed 30 October 2022).Search in Google Scholar
Moral, R.A., Hinde, J., and Demétrio, C.G.B. (2017). Half-normal plots and overdispersed models in R : the hnp Package. J. Stat. Softw. 81, https://doi.org/10.18637/jss.v081.i10.Search in Google Scholar
Nascimento, V.F., Auad, A.M., and de Resende, T.T. (2021). Olfactory response of Mahanarva spectabilis (Distant, 1909) (Hemiptera: Cercopidae) to volatile aqueous extracts of plant origin applied to elephant grass plants (Pennisetum purpureum Schum). Agronomy 11: 856, https://doi.org/10.3390/agronomy11050856.Search in Google Scholar
Paladini, A., Takiya, D.M., Urban, J.M., and Cryan, J.R. (2018). New World spittlebugs (Hemiptera: Cercopidae: Ischnorhininae): dated molecular phylogeny, classification, and evolution of aposematic coloration. Mol. Phylogenet. Evol. 120: 321–334, https://doi.org/10.1016/j.ympev.2017.12.020.Search in Google Scholar PubMed
Parra, J.R.P. (2014). Biological control in Brazil: an overview. Sci. Agric. (Piracicaba, Braz.) 71: 420–429, https://doi.org/10.1590/0103-9016-2014-0167.Search in Google Scholar
Pereira, M.F.A., Benedetti, R.A.L., and Almeida, J.E.M. (2008). Eficiência de Metarhizium anisopliae (Metsch.) Sorokin no controle de Deois flavopicta (Stal., 1854), em pastagem de Capim-Braquiária (Brachiária decumbens). Arq. Inst. Biol. 75: 465–469, https://doi.org/10.1590/1808-1657v75p4652008.Search in Google Scholar
Pitta, R.M., Matiero, S.C., Corassa, J.N., and Rampelotti-Ferreira, F.T. (2019). Influence of pastoral systems on Mahanarva spectabilis (distant) (Hemiptera: Cercopidae) and the entomopathogen Metarhizium anisopliae (Metsch.) Sorokin. Sci. Electron. Arch. 12: 13–20, https://doi.org/10.36560/1252019956.Search in Google Scholar
R Core Team (2022). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, URL https://www.R-project.org/Research104:1202–1212.Search in Google Scholar
Resende, T.T., Auad, A.M., Fonseca, M.das G., dos Santos, T.H., and Vieira, T.M. (2012). Impact of the spittlebug Mahanarva spectabilis on Signal grass. Sci. World J. 2012: 926715–926716, https://doi.org/10.1100/2012/926715.Search in Google Scholar PubMed PubMed Central
Resende, T.T., Auad, A.M., Fonseca, M.das G., Souza Sobrinho, F., Ribeiro dos Santos, D., and da Silva, S.E.B. (2013). The damage capacity of Mahanarva spectabilis (Distant, 1909) (Hemiptera: Cercopidae) adults on Brachiaria ruziziensis pasture. Sci. World J. 2013: 281295, https://doi.org/10.1155/2013/281295.Search in Google Scholar PubMed PubMed Central
Ribeiro, L.do P. and Cazarotto, A.R. (2019). Cigarrinhas-das-pastagens em Santa Catarina: avaliação do complexo de espécies e da incidência natural de fungos entomopatogênicos. Agropecuária Catarinense 32: 2, https://doi.org/10.22491/rac.2019.v32n2.11.Search in Google Scholar
Ripley, B. (2019). Package “MASS.” title: support functions and datasets for venables and Ripley’s MASS. In: R package version: 7.3-51.4, https://cran.rproject.org/web/packages/MASS/MASS.pdf.Search in Google Scholar
Schloerke, B., Cook, D., Larmarange, J., Briatte, F., Marbach, M., Thoen, E., Elberg, A., and Crowley, J. (2021). GGally: extension to ’ggplot2. In: R package version 2.1.2, https://CRAN.R-project.org/package=GGally.Search in Google Scholar
Schmitz, A. and Riesner, D. (2006). Purification of nucleic acids by selective precipitation with polyethylene glycol 6000. Anal. Biochem. 354: 311–313, https://doi.org/10.1016/j.ab.2006.03.014.Search in Google Scholar PubMed
Silva, I., Noboa, C.S., Vale, J.P.I., Matta, F., Vigna, B.B.Z., Favero, A.P., and Gusmao, M.R. (2017). Antibiose em genótipos de Paspalum spp. à cigarrinha Mahanarva spectabilis (Hemiptera: Cercopidae). In: Anais da nona Jornada Científica – EMBRAPA, 2017. EMBRAPA Pecuária Sudeste, São Carlos, SP.Search in Google Scholar
Thompson, V. (2004). Associative nitrogen fixation, C 4 photosynthesis, and the evolution of spittlebugs (Hemiptera: Cercopidae) as major pests of neotropical sugarcane and forage grasses. Bull. Entomol. Res. 94: 189–200, https://doi.org/10.1079/ber2004293.Search in Google Scholar PubMed
Valério, J.R. (2009). Cigarrinhas- das- pastagens, Vol. 1. Embrapa Gado de corte, Campo Grande, MS, Brazil.Search in Google Scholar
Valverde, A.H.P. (2006). Resistência em genótipos de Brachiaria a ninfas de três espécies de cigarrinha-das-pastagens (Hemiptera: Cercopidae). Dissertação. Universidade Federal de Viçosa, Viçosa, MG, Brazil.Search in Google Scholar
Vilgalys, R. and Hester, M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 172: 4238–4246, https://doi.org/10.1128/jb.172.8.4238-4246.1990.Search in Google Scholar PubMed PubMed Central
Welty, R.E. (1987). Influence of moisture content, temperature, and length of storage on seed germination and survival of endophytic fungi in seeds of tall Fescue and perennial Ryegrass. Phytopathology 77: 893, https://doi.org/10.1094/phyto-77-893.Search in Google Scholar
Wickham, H. (2016). ggplot2: elegant graphics for data analysis. Springer-Verlag, New York, USA.10.1007/978-3-319-24277-4_9Search in Google Scholar
Zheng, X., Lu, Y., Zhu, P., Zhang, F., Tian, J., Xu, H., Chen, G., Nansen, C., and Lu, Z. (2017). Use of banker plant system for sustainable management of the most important insect pest in rice fields in China. Sci. Rep. 7: 45581, https://doi.org/10.1038/srep45581.Search in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter on behalf of the Florida Entomological Society
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Research Articles
- Distribution and dispersal of adult spotted wing drosophila, Drosophila suzukii (Diptera: Drosophilidae), in organically grown strawberries in Florida
- A comparison of the capture of non-target arthropods between control methods and monitoring traps of Anastrepha ludens in citrus agroecosystems
- Development of microsatellite markers for colony delineation of the invasive Asian subterranean termite (Blattodea: Rhinotermitidae) in South Florida and Taiwan
- Biology and life table of Oligonychus punicae Hirst (Trombidiformes: Tetranychidae) on three host plants
- Relative captures and detection of male Ceratitis capitata using a natural oil lure or trimedlure plugs
- Evaluation of HOOK SWD attract-and-kill on captures, emergence, and survival of Drosophila suzukii in Florida
- Rearing Neoseiulus cucumeris and Amblyseius swirskii (Mesostigmata: Phytoseiidae) on non-target species reduces their predation efficacy on target species
- Response of male Bactrocera zonata (Diptera: Tephritidae) to methyl eugenol: can they be desensitized?
- Monitoring of coccinellid (Coleoptera) presence and syrphid (Diptera) species diversity and abundance in southern California citrus orchards: implications for conservation biological control of Asian citrus psyllid and other citrus pests
- Topical treatment of adult house flies, Musca domestica L. (Diptera: Muscidae), with Beauveria bassiana in combination with three entomopathogenic bacteria
- Laboratory evaluation of 15 entomopathogenic fungal spore formulations on the mortality of Drosophila suzukii (Diptera: Drosophilidae), related drosophilids, and honeybees
- Effect of diatomaceous earth on diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae), larval feeding and survival on cabbage
- Bioactivity of seed extracts from different genotypes of Jatropha curcas (Euphorbiaceae) against Spodoptera frugiperda (Lepidoptera: Noctuidae)
- Assessment of sugarberry as a host tree of Halyomorpha halys (Hemiptera: Pentatomidae) in southeastern USA agroecosystems
- The importance of multigeneration host specificity testing: rejection of a potential biocontrol agent of Nymphaea mexicana (Nymphaeaceae) in South Africa
- Endophytic potential of entomopathogenic fungi associated with Urochloa ruziziensis (Poaceae) for spittlebug (Hemiptera: Cercopidae) control
- The first complete mitogenome sequence of a biological control agent, Pseudophilothrips ichini (Hood) (Thysanoptera: Phlaeothripidae)
- Exploring the potential of Delphastus davidsoni (Coleoptera: Coccinellidae) in the biological control of Bemisia tabaci MEAM 1 (Hemiptera: Aleyrodidae)
- Behavioral responses of Ixodiphagus hookeri (Hymenoptera; Encyrtidae) to Rhipicephalus sanguineus nymphs (Ixodida: Ixodidae) and dog hair volatiles
- Illustrating the current geographic distribution of Diaphorina citri (Hemiptera: Psyllidae) in Campeche, Mexico: a maximum entropy modeling approach
- New records of Clusiidae (Diptera: Schizophora), including three species new to North America
- Photuris mcavoyi (Coleoptera: Lampyridae): a new firefly from Delaware interdunal wetlands
- Bees (Hymenoptera: Apoidea) diversity and synanthropy in a protected natural area and its influence zone in western Mexico
- Temperature-dependent development and life tables of Palpita unionalis (Lepidoptera: Pyralidae)
- Orchid bee collects herbicide that mimics the fragrance of its orchid mutualists
- Importance of wildflowers in Orius insidiosus (Heteroptera: Anthocoridae) diet
- Bee diversity and abundance in perennial irrigated crops and adjacent habitats in central Washington state
- Comparison of home-made and commercial baits for trapping Drosophila suzukii (Diptera: Drosophilidae) in blueberry crops
- Miscellaneous
- Dr. Charles W. O’Brien: True Pioneer in Weevil Taxonomy and Publisher
- Scientific Notes
- Nests and resin sources (including propolis) of the naturalized orchid bee Euglossa dilemma (Hymenoptera: Apidae) in Florida
- Impact of laurel wilt on the avocado germplasm collection at the United States Department of Agriculture, Agricultural Research Service, Subtropical Horticulture Research Station
- Monitoring adult Delia platura (Diptera: Anthomyiidae) in New York State corn fields using blue and yellow sticky cards
- New distribution records and host plants of two species of Hypothenemus (Coleoptera: Curculionidae: Scolytinae) in mangrove ecosystems of Tamaulipas, Mexico
- First record of Trichogramma pretiosum parasitizing Iridopsis panopla eggs in eucalyptus in Brazil
- Spodoptera cosmioides (Lepidoptera: Noctuidae) as an alternative host for mass rearing the parasitoid Palmistichus elaeisis (Hymenoptera: Eulophidae)
- Effects of biochar on ambrosia beetle attacks on redbud and pecan container trees
- First report of Diatraea impersonatella (Lepidoptera: Crambidae) on sugarcane (Saccharum officinarum L.) in Honduras
- Book Reviews
- Kratzer, C. A.: The Cicadas of North America
Articles in the same Issue
- Frontmatter
- Research Articles
- Distribution and dispersal of adult spotted wing drosophila, Drosophila suzukii (Diptera: Drosophilidae), in organically grown strawberries in Florida
- A comparison of the capture of non-target arthropods between control methods and monitoring traps of Anastrepha ludens in citrus agroecosystems
- Development of microsatellite markers for colony delineation of the invasive Asian subterranean termite (Blattodea: Rhinotermitidae) in South Florida and Taiwan
- Biology and life table of Oligonychus punicae Hirst (Trombidiformes: Tetranychidae) on three host plants
- Relative captures and detection of male Ceratitis capitata using a natural oil lure or trimedlure plugs
- Evaluation of HOOK SWD attract-and-kill on captures, emergence, and survival of Drosophila suzukii in Florida
- Rearing Neoseiulus cucumeris and Amblyseius swirskii (Mesostigmata: Phytoseiidae) on non-target species reduces their predation efficacy on target species
- Response of male Bactrocera zonata (Diptera: Tephritidae) to methyl eugenol: can they be desensitized?
- Monitoring of coccinellid (Coleoptera) presence and syrphid (Diptera) species diversity and abundance in southern California citrus orchards: implications for conservation biological control of Asian citrus psyllid and other citrus pests
- Topical treatment of adult house flies, Musca domestica L. (Diptera: Muscidae), with Beauveria bassiana in combination with three entomopathogenic bacteria
- Laboratory evaluation of 15 entomopathogenic fungal spore formulations on the mortality of Drosophila suzukii (Diptera: Drosophilidae), related drosophilids, and honeybees
- Effect of diatomaceous earth on diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae), larval feeding and survival on cabbage
- Bioactivity of seed extracts from different genotypes of Jatropha curcas (Euphorbiaceae) against Spodoptera frugiperda (Lepidoptera: Noctuidae)
- Assessment of sugarberry as a host tree of Halyomorpha halys (Hemiptera: Pentatomidae) in southeastern USA agroecosystems
- The importance of multigeneration host specificity testing: rejection of a potential biocontrol agent of Nymphaea mexicana (Nymphaeaceae) in South Africa
- Endophytic potential of entomopathogenic fungi associated with Urochloa ruziziensis (Poaceae) for spittlebug (Hemiptera: Cercopidae) control
- The first complete mitogenome sequence of a biological control agent, Pseudophilothrips ichini (Hood) (Thysanoptera: Phlaeothripidae)
- Exploring the potential of Delphastus davidsoni (Coleoptera: Coccinellidae) in the biological control of Bemisia tabaci MEAM 1 (Hemiptera: Aleyrodidae)
- Behavioral responses of Ixodiphagus hookeri (Hymenoptera; Encyrtidae) to Rhipicephalus sanguineus nymphs (Ixodida: Ixodidae) and dog hair volatiles
- Illustrating the current geographic distribution of Diaphorina citri (Hemiptera: Psyllidae) in Campeche, Mexico: a maximum entropy modeling approach
- New records of Clusiidae (Diptera: Schizophora), including three species new to North America
- Photuris mcavoyi (Coleoptera: Lampyridae): a new firefly from Delaware interdunal wetlands
- Bees (Hymenoptera: Apoidea) diversity and synanthropy in a protected natural area and its influence zone in western Mexico
- Temperature-dependent development and life tables of Palpita unionalis (Lepidoptera: Pyralidae)
- Orchid bee collects herbicide that mimics the fragrance of its orchid mutualists
- Importance of wildflowers in Orius insidiosus (Heteroptera: Anthocoridae) diet
- Bee diversity and abundance in perennial irrigated crops and adjacent habitats in central Washington state
- Comparison of home-made and commercial baits for trapping Drosophila suzukii (Diptera: Drosophilidae) in blueberry crops
- Miscellaneous
- Dr. Charles W. O’Brien: True Pioneer in Weevil Taxonomy and Publisher
- Scientific Notes
- Nests and resin sources (including propolis) of the naturalized orchid bee Euglossa dilemma (Hymenoptera: Apidae) in Florida
- Impact of laurel wilt on the avocado germplasm collection at the United States Department of Agriculture, Agricultural Research Service, Subtropical Horticulture Research Station
- Monitoring adult Delia platura (Diptera: Anthomyiidae) in New York State corn fields using blue and yellow sticky cards
- New distribution records and host plants of two species of Hypothenemus (Coleoptera: Curculionidae: Scolytinae) in mangrove ecosystems of Tamaulipas, Mexico
- First record of Trichogramma pretiosum parasitizing Iridopsis panopla eggs in eucalyptus in Brazil
- Spodoptera cosmioides (Lepidoptera: Noctuidae) as an alternative host for mass rearing the parasitoid Palmistichus elaeisis (Hymenoptera: Eulophidae)
- Effects of biochar on ambrosia beetle attacks on redbud and pecan container trees
- First report of Diatraea impersonatella (Lepidoptera: Crambidae) on sugarcane (Saccharum officinarum L.) in Honduras
- Book Reviews
- Kratzer, C. A.: The Cicadas of North America

