Abstract
Purification of the organic extract of Laurencia obtusa Lamouroux by column chromatography and preparative thin layer chromatography provided four new compounds: a eudesmane-type sesquiterpenoid [eudesma-4(15),11-diene-5,7-diol (1)], a cuparane-type sesquiterpenoid [10-hydroxycuparaldehyde (2)], and two nor-cuparanes [3-hydroxy-15-nor-cuparan-10β-ol (3) and 2-bromo-3-hydroxy-15-nor-cuparan-10β-ol (4)]. Structural identification was made possible by comparison of spectral data with those reported in the literature. Compounds 3 and 4 are significant as nor-cuparanes are rarely isolated from marine environment. 1 showed moderate anticandidal activity, whereas 2 exhibited reasonable antibacterial activity against multidrug-resistant bacteria (especially Gram-positive). All the compounds are nontoxic to Artemia salina.
1 Introduction
Laurencia is a common genus of marine red algae, taxonomically classified as Rhodophyta, Rhodophyceae, Ceramiales, Rhodomelaceae. Laurencia obtusa is one of the most widely investigated marine species by natural product chemists. It provides a unique source of halogenated secondary metabolites such as C15-acetogenises , diterpenes, and sesquiterpenoid skeletons [1, 2, 3, 4, 5, 6, 7, 8, 9]. Many of these compounds are biologically active, showing antioxidant, antimalarial, antimicrobial, and cytotoxic activity [3,6,10].
To find new bioactive compounds in marine macroorganisms, Laurencia obtusa Lamouroux was collected from the Saudi Red Sea and its organic fraction extracted to provide four new sesquiterpenoids (Figure 1). The antimicrobial and anticandidal activities of the new compounds were also investigated.

Structure of compounds 1–4.
2 Experimental
2.1 General
Column chromatography was performed with aluminum oxide Fluka, neutral type 507C. Fractions were analyzed by? TLC on silica gel 60 F254. Preparative TLC was performed on glass plates (20 cm × 20 cm) coated with silica gel (250μm thickness). Spots were visualized using UV light (254 nm), then p-anisaldehyde-sulfuric acid spray reagent. High resolution electron impact mass spectra (HREIMS) were recorded on a Krators EIMS-25 instrument at an ionizing voltage of 70 eV. 1D and 2D NMR spectra were recorded on a Bruker 850 MHz spectrometer. Chemical shifts are reported in parts per million (ppm) using the solvent residual signal as the internal standard (CDCl3: δ 7.26 for 1H and δ 77.0 for 13C).
2.2 Extraction and Isolation
Laurencia obtusa Lamouroux was collected in May 2016 from Salman Gulf, north of Jeddah, Saudi Arabia (21°51′39.8″ N; 38°58′42.7″ E). Reference standard (JAD 03060) was stored at the Faculty of Marine Sciences, King Abdulaziz University (Jeddah, Saudi Arabia). L. obtusa was dried, then extracted with equal volumes of dichloromethane and methanol. The residue (6 g) was purified first by column chromatography on Sephadex LH-20 (MeOH/CHCl3 = 9.5:0.5), then by column chromatography on neutral aluminum oxide using gradient elution (n-hexane/diethyl ether to n-hexane/EtOAc). Fractions containing the product were combined and dried under reduced pressure. Final purification by PTLC afforded the pure product.
2.3 Chemical characterization
Eudesma-4(15),11-diene-5,7-diol (1)
purified by column chromatography (eluent: 30% diethyl ether) followed by PTLC (eluent: 30% diethyl ether). The violet band (p-anisaldehyde-sulfuric acid reagent) was collected to provide 1 as a colorless liquid (1.4 mg, 0.0007% yield). Rf 0.30; [α]D +54.0 (c 0.014, CH2Cl2); IR ν (cm−1) 3424, 2924, 2854, 1712, 1640, 1455, 1377, 1261, 1172, 1074, 904; EI-MS m/z 236; HREIMS m/z 236.1764 (Calcd. 236.1776, C15H24O2); 1H and 13C NMR (Tables 1 and 2, respectively).
1H NMR data of compounds 1–4 (CDCl3, 850 MHz).
| No. | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| δH ppm (mult., J/Hz) | δH ppm (mult., J/Hz) | δH ppm (mult., J/Hz) | δH ppm (mult., J/Hz) | |
| 1 | Ha 2.05 (ddd, 17.9, 13.6, 4.3) | 7.39 (d, 8.5) | 7.07 (d, 8.5) | 7.14 (d, 2.6) |
| Hb1.25-1.27 (m) | ||||
| 2 | Ha 1.99 (ddd, 17.9, 13.6, 4.3) | 7.79 (d, 8.5) | 6.74 (d, 8.5) | - |
| Hb 1.55-1.57 (m) | ||||
| 3 | Ha2.32-2.35 (m) | - | - | - |
| Hb 2.17-2.22 (m) | ||||
| 4 | - | 7.79 (d, 8.5) | 6.74 (d, 8.5) | 6.92 (d, 8.5) |
| 5 | - | 7.39 (d, 8.5) | 7.07 (d, 8.5) | 7.02 (dd, 8.5, 2.6) |
| 6 | 2.25 (d,14.5) | - | - | - |
| 1.50 (dd,14.5, 2.6) | ||||
| 7 | - | - | - | - |
| 8 | 1.59-1.62 (m) | Ha 2.34 (ddd, 14.5, 9.4, 5.1) | Ha 2.22-2.27 (m) | Ha 2.22-2.27 (m) |
| Hb 2.02 (ddd, 13.6, 12.8, 5.1) | Hb 1.94-1.98 (m) | Hb 1.94-1.98 (m) | ||
| 9 | Ha 1.88 (ddd, 17.9, 12.8, 5.1) | Ha 2.57 (ddd, 14.5, 9.4, 6.0) | Ha 2.48-2.52 (m) | Ha 2.48-2.52 (m) |
| Hb 1.21-1.24 (m) | Hb 2.28-2.31 (m) | Hb 2.19-2.25 (m) | Hb 2.19-2.25 (m) | |
| 10 | - | 4.01 (dd, 9.4, 9.4) | 4.04 (dd, 10.2, 9.4) | 4.02 (dd, 10.2, 9.4) |
| 11 | - | - | - | - |
| 12 | Ha 5.04 (s) | 1.13 (s) | 1.08 (s) | 1.08 (s) |
| Hb 4.83 (s) | ||||
| 13 | 1.82 (s) | 0.62 (s) | 0.61 (s) | 0.63 (s) |
| 14 | 0.93 (s) | 1.48 (s) | 1.41 (s) | 1.40 (s) |
| 15 | Ha 5.06 (s) | 9.99 (s) | - | - |
| Hb 4.84 (s) |
13C NMR data of compounds 1–4 (CDCl3, 212 MHz).
| No. | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1 | 31.6, CH2 | 128.2, CH | 128.7, CH | 127.9, CH |
| 2 | 31.2, CH2 | 128.9, CH | 114.2, CH | 119.1, C |
| 3 | 33.3, CH2 | 134.4, C | 153.6, C | 149.4, C |
| 4 | 151.6, C | 128.9, CH | 114.2, CH | 115.1, CH |
| 5 | 78.3, C | 128.2, CH | 128.7, CH | 127.6, CH |
| 6 | 40.6, CH2 | 154.5, C | 139.3, C | 140.6, C |
| 7 | 75.0, C | 48.8, C | 47.6, C | 47.6, C |
| 8 | 22.3, CH2 | 36.5, CH2 | 36.6, CH2 | 36.5, CH2 |
| 9 | 34.2, CH2 | 33.1, CH2 | 33.1, CH2 | 33.0, CH2 |
| 10 | 38.5, C | 61.1, CH | 62.0, CH | 61.5, CH |
| 11 | 150.9, C | 49.6, C | 48.3, C | 48.5, C |
| 12 | 109.6, CH2 | 21.1, CH3 | 20.9, CH3 | 21.0, CH3 |
| 13 | 18.9, CH3 | 22.6, CH3 | 22.5, CH3 | 22.7, CH3 |
| 14 | 22.2, CH3 | 25.1, CH3 | 25.2, CH3 | 25.2, CH3 |
| 15 | 108.2, CH2 | 191.8, CH | - | - |
10-Hydroxycuparaldehyde (2)
purified by column chromatography (eluent: 15% diethyl ether) followed by PTLC (eluent: 10% diethyl ether). The brown band (p-anisaldehyde-sulfuric acid reagent) was collected to provide 2 as a pale yellow liquid (4.5 mg, 0.0023% yield). Rf 0.53; [α]D +79.0 (c 0.023, CH2,Cl2); UV (MeOH) λmax 252, 259 and 290 nm; IR ν (cm−1) 3274, 2972, 2927, 1701, 1606, 1461, 1392, 1369, 1223, 1178, 1075, 854; EI-MS m/z 232; HREIMS m/z 232.1451 (Calcd. 232.1463, C15H20O2); 1H and 13C NMR (Tables 1 and 2, respectively).
3-Hydroxy-15-nor-cuparan-10β-ol (3)
purified by column chromatography (eluent: 40% diethyl ether) followed by PTLC (eluent: 30% diethyl ether). The light blue band (p-anisaldehyde-sulfuric acid reagent) was collected to provide 3 as a colorless liquid (0.7 mg, 0.0004% yield). Rf 0.4; [α]D +61.8 (c 0.007, CH2Cl2); UV (MeOH) λmax 230 and 280 nm; IR ν (cm−1) 3383, 2921, 2851, 1736, 1605, 1463, 1377, 1286, 1239, 1183, 1075, 832; EI-MS m/z 220; HREIMS m/z 220.1451 (Calcd. 220.1463, C14H20O2); 1H and 13C NMR (Tables 1 and 2, respectively).
2-Bromo-3-hydroxy-15-nor-cuparan-10β-ol (4);
purified by column chromatography (eluent: 40% diethyl ether) followed by PTLC (eluent: 30% diethyl ether). The light blue band (p-anisaldehyde-sulfuric acid reagent) was collected to provide 4 as a colorless liquid (0.7 mg, 0.0004% yield). Rf 0.4; [α]D +50.4 (c 0.007, CH2,Cl2); UV (MeOH) λmax 230 and 280 nm; IR ν (cm−1) 3383, 2921, 2851, 1736, 1605, 1463, 1377, 1286, 1239, 1183, 1075, 832, 557; HREIMS m/z 298.0556 and 300.0536 (1:1) (Calcd. 298.0568 and 300.0548 for C14H1979BrO2 and C14H1981BrO2, respectively); 1H and 13C NMR (Tables 1 and 2, respectively).
2.4 Biological evaluation
2.4.1 Antibacterial activity
Gram-negative and Gram-positive bacteria (Table 3) were obtained from urine samples of patients at the King Fahad General Hospital (Saudi Arabia) and identified according to standard guidelines [11].
Antimicrobial activity and minimal inhibitory concentrations (MICs) of 2 and amoxicillin on multidrug-resistant bacteria.
| Bacterium[a] | Antimicrobial activity (mm)[b] | MIC (mM) | ||
|---|---|---|---|---|
| 2 | amoxicillin[c] | 2 | amoxicillin | |
| E. coli | 12.3±2.33 | 17.0±2.13 | 0.15 | 0.01 |
| K. pneumoniae | 10.0±2.00 | 22.0±4.22 | 0.11 | 0.002 |
| P. mirabilis | 14.0±3.70 | 24.0±1.34 | 0.09 | 0.01 |
| P. aeruginosa | 13.0±3.12 | 17.0±2.21 | 0.12 | 0.02 |
| E. faecalis | 15.5±2.57 | 19.0±1.49 | 0.08 | 0.04 |
| S. aureus | 15.0±2.56 | 17.0±1.09 | 0.09 | 0.08 |
The antibacterial activity was tested on Muller-Hinton agar using agar diffusion well test [12]. The minimum inhibitory concentration (MIC) was determined by ELISA using 96-well plates and fluorescein diacetate (FDA, 5 μl of a 0.25% w/w in acetone) as an indicator. The green color due to FDA hydrolysis was estimated at λmax 490 nm using the ELISA tray reader [13,14].
Compound toxicity was assessed using Artemisia salina as test creature and dimethylsulfoxide (DMSO) as a negative control [15].
2.4.2 Anticandidal activity
MIC was determined using the method described by Chand et al. [13] and modified by Aly and Gumgumji [14].
3 Results and discussion
Four new sesquiterpenoids (1–4) were isolated from Laurencia obtusa Lamouroux after extraction and purification of its organic fraction. Spectroscopic analysis of the four compounds afforded the following results.
Compound 1 was isolated as a liquid with specific rotation [α]D +54 (c 0.014, CH2Cl2). HREIMS analysis of the molecular ion peak at m/z 236.1764 provided the molecular formula C15H24O2, which requires four degrees of unsaturation. The lack of absorption in the UV spectrum revealed the absence of conjugation. Hydroxyl group (ν 3424 cm−1) and exocyclic C=C bond (ν 1640 cm−1) were assigned from the IR spectrum. The 13C NMR spectrum displayed 15 signals, which were categorized by DEPT into 5 quaternary, 8 methylene and 2 methyl carbons. HSQC experiment indicated the presence of two tertiary methyls (δH/δC = 1.82/18.9 and 0.93/22.2), two oxygenated quaternary carbons (δC 78.3 and 75.0), two olefinic quaternary carbons (δC 151.6 and 150.9), and two exocyclic methylene groups (δH/δC 5.06 and 4.84/108.2; δH/δC 5.04 and 4.83/109.6; Tables 1 and 2). The 1H─1H correlation experiment highlighted three sequences: three correlated methylene groups, two correlated methylene groups, and an isolated methylene group (δH 1.50 and 2.25). Overall, these results suggest that compound 1 is an eudesmane-type sesquiterpenoid with two C=C bonds fulfilling four degrees of unsaturation. HMBC experiment revealed additional correlations: the methyl protons at δH 0.93 (Me-14) correlate to carbons at δC 78.3, 34.2 and 31.6, confirming that Me-14 is an angular methyl; methyl protons at δH 1.82 (Me-13) correlate to carbons at δC 150.9, 109.6 and 75.0, confirming that Me-13 is part of the isopropylidene group (Figure 2). The relative configuration of 1 was assigned by NOESY experiment as well as by analogy with known compounds. The absence of correlation between Me-13 and Me-14 in NOESY spectrum suggests the two groups are not co-facially oriented. As in natural eudesmanes Me-14 is β-oriented,7 Me-14 and OH group at C-5 must be trans correlated. Based on structure, 1 was named eudesma-4(15),11-diene-5,7-diol.
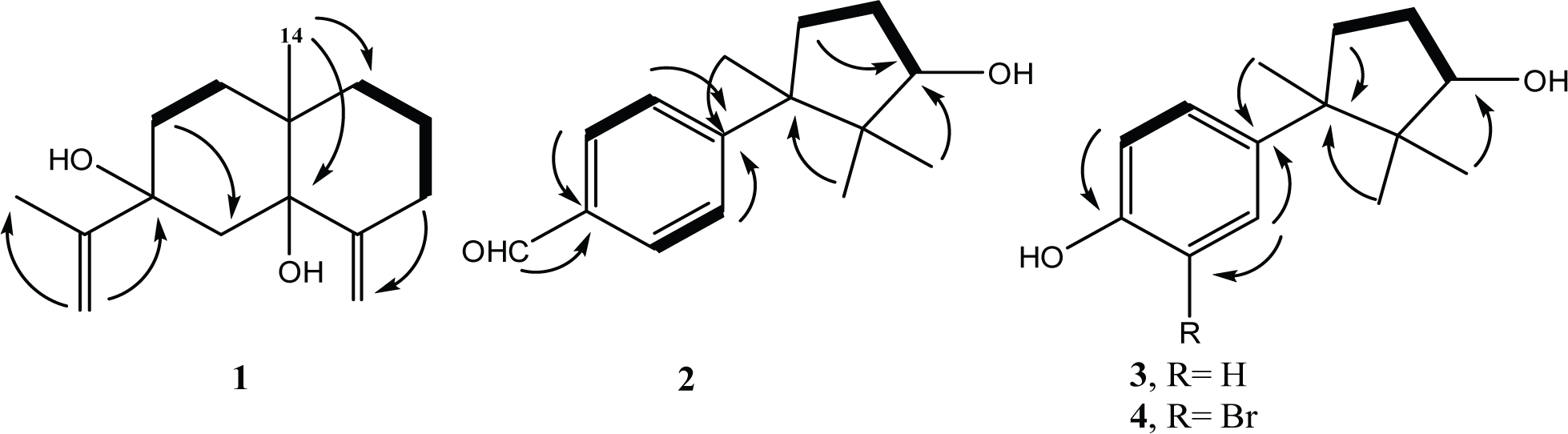
Selected H-H COSY ( ) and HMBC correlations of compounds 1-4 (
) and HMBC correlations of compounds 1-4 ( )
)
Compound 2 was isolated as a liquid with specific rotation [α]D +79.2 (c 0.025, CH2Cl2). HREIMS analysis of the molecular ion peak (m/z 232) provided the molecular formula C15H20O2, which requires six degrees of unsaturation. The UV absorptions at 252, 259 and 290 nm indicated a substituted benzene ring. The IR spectrum showed the characteristic bands of OH, C=O and gem dimethyl groups (ν 3274, 1701 and 1369 cm−1, respectively). The presence of the OH group was confirmed by a peak at m/z 214 [M–H2O] in the EI-MS spectrum. The 13C NMR spectrum of 2 showed 15 signals, categorized by DEPT into 4 quaternary, 6 methine, 2 methylene and 3 methyl carbons. The 1H─13C HSQC experiment revealed the presence of the following groups (Tables 1 and 2): three aliphatic methyl groups (singlets at δH/δC 1.48/25.1, 1.13/21.1 and 0.62/22.6), a formyl group (singlet at δH/δC 9.99/191.8), and a p-disubstituted benzene ring (two doublets in the aromatic region at δH/δC 7.79 [J = 8.5 Hz, 2H]/128.9 and δH 7.39 [J = 8.5 Hz, 2H]/128.2). In addition, the proton-proton correlation spectrum revealed two proton sequences: the aromatic system and a CH2─CH2─CH fragment. The aforementioned analysis points to a cuparane-type sesquiterpenoid structure containing a cyclopentane ring connected to a benzaldehyde skeleton. The location of the aldehyde group in the p-position of the benzene ring was confirmed by HMBC correlation of the aldehyde proton (H-15, δH 9.99) to C-3 (δC 134.4), C-2 (δC 128.9), and C-4 (δC 128.9). Correlations of H-14 (δH 1.48) to C-7 (δC 48.8), C-11 (δC 49.6), C-8 (δC 36.5), and C-6 (δC 154.5) are also in agreement with the structure of 2.
Comparison of the spectral data of 2 with those reported for cuparane [16] showed similarity, except for the presence of an aldehyde group in 2 in the place of a methyl group in cuparane. The increase in chemical shift value of Me-14 in compound 2 (δH 1.48) with respect to cuparane (δH 1.23) is likely explained in terms of anisotropy created by the aromatic ring as well as the electron withdrawing effect of the aldehyde group. The relative configuration of 2 was determined by NOESY experiment, which showed the correlation of Me-14 (δH 1.48) to Me-12 (δH 1.13), and of Me-13 (δH 0.62) to H-10 (δH 4.01). These correlations indicate that Me-14, Me-12 and the hydroxyl group on C-10 have the same orientation. Consequently, the relative configurations of C-7 and C-10 were assigned as 7R* and 10R*, respectively. Compound 2 was given the trivial name 10-hydroxycuparaldehyde.
Metabolite 3 was isolated as a liquid with specific rotation [α]D +61.8 (c 0.007, CH2Cl2). Its molecular formula was determined as C14H20O2 (with five degrees of unsaturation) by HREIMS. The UV absorptions at 230 and 280 nm indicated a substituted benzene ring. The IR spectrum revealed the presence of OH and gem dimethyl groups (ν 3383 and 1377 cm−1, respectively). The 13C NMR spectrum of 3 showed 14 signals, categorized by DEPT into 4 quaternary, 5 methine, 2 methylene and 3 methyl carbons. The 1H─13C HSQC spectrum allowed to identify three tertiary methyls (singlets at δH/δC 1.41/25.2, 1.08/20.9 and 0.61/22.5) and a p-disubstituted benzene ring (two doublets in the aromatic region at δH/δC 6.74 [2H, J = 8.5 Hz]/114.2 and at δH/δC 7.07 [2H, J = 8.5 Hz]/128.7; Tables 1 and 2). Two OH groups are located on C-3 (δC 153.6) and C-10 δH/δC 4.04/62.0). In addition, the proton-proton correlation spectrum revealed two proton sequences: the aromatic system and the CH2─CH2─CH fragment. With five degrees of unsaturation, the carbon skeleton of compound 3 must contain a (p-OH)C6H4 group attached to a 1,2,2-trimethylcyclopentan-3-ol moiety. The position of the hydroxyl groups was confirmed by HMBC correlation of the phenolic carbon C-3 (δC 153.6) to the aromatic protons H-2 and H-4 (δH 6.74), and that of H-10 (δH 4.04) to C-11 (δC 48.3) and C-9 (δC 33.1).
The abovementioned analysis indicates that compound 3 is a nor-cuparane-type sesquiterpenoid. Compound 3 was named 3-hydroxy-15-nor-cuparan-10β-ol.
Compound 4 (isolated as a liquid with specific rotation [α]D +50.4 [c 0.007, CH2Cl2]) was identified by comparison with the spectroscopic data of 3 (Tables 1 and 2). Their IR, UV and NMR spectra were similar, with a difference in the aromatic region of the 1H NMR spectrum. The 1H─13C HSQC spectrum of 4 exhibited three methine carbons in the aromatic region δC/δC 7.14 [d, J = 2.6 Hz]/127.9, 6.92 [d, J = 8.5 Hz]/115.1 and 7.02 [dd, J = 8.5 Hz, J = 2.6 Hz]/127.6) and three quaternary carbons (δC 140.6, 119.1, and 149.4) indicating a 1,2,4-trisubstituted benzene ring. The molecular formula of 4 was assigned as C14H19BrO2 by HREIMS. The parent peaks at m/z 298 and 300 in the EI-MS spectrum, with relative intensities in the 1:1 ratio, alongside the absorption band at ν 557 cm−1 in the IR spectrum, clearly indicate the presence of a bromine atom.
The analysis indicates that compound 4 is a nor-cuparane bearing two hydroxyl groups and a bromine atom. The position of bromine was assigned to C-2 (δC 119.1). 4 was named 2-bromo-3-hydroxy-15-nor-cuparan-10β-ol.
The antibacterial activity of 1 and 2 was tested against several strains of multidrug-resistant bacteria (Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, Enterococcus faecalis and Staphylococcus aureus). 1 displayed weak activity against all tested bacteria (results not shown), whereas 2 showed an inhibition zone of 10.0-15.5 mm diameter on Muller-Hinton agar (Table 3).
The MICs of 2 were in the range of 0.08–0.15 mM for all tested bacteria. Noteworthy, 2 was more active against Gram-positive bacteria (Table 3). All compounds showed no toxicity (LD50 >0.5 mM) against Artemia salina as test creature.
Compound 1 displayed anticandidal activity against Candida albicans and Candida tropicalis (MICs = 8.27 and 10.13 μM, respectively. This activity is relatively high if compared to amphotericin B (positive control; MICs = 4.63 and 5.27 μM, respectively).
4 Conclusions
Four new compounds were isolated from the organic extract of the red alga L. obtusa Lamouroux. These compounds belong to two major classes of sesquiterpenoids: eudesmanes [eudesma-4(15),11-diene-5,7-diol (1)] and cuparanes [10-hydroxycuparaldehyde (2), 3-hydroxy-15-nor-cuparan-10β-ol (3), and 2-bromo-3-hydroxy-15-nor-cuparan-10β-ol (4)]. Compounds 1 and 2 displayed relatively high antimicrobial activities, whereas 3 and 4 belong to a specific group of compounds (nor-cuparane sesquiterpenoids) that are rarely isolated from the marine environment.
Acknowledgements
The authors are indebted to Prof. Mohsen El-Sherbiny, Faculty of Marine Sciences (King Abdulaziz University) for the collection and identification of the alga sample. They also thank King Fahd Center for Medical Research for giving them the opportunity to work in its central laboratory.
Conflict of interest: Authors declare no conflict of interest.
References
[1] Kladi, M.; Vagias, C.; Papazafiri, P.; Furnari, G.; Serio, D.; Roussis, V. New sesquiterpenes from the red alga Laurencia microcladia, Tetrahedron 2007, 63, 7606–7611.10.1016/j.tet.2007.05.051Suche in Google Scholar
[2] Alarif, W. M.; Abou-Elnaga, Z. Sh.; Ayyad, S.-E. S.; Al-lihaibi, S. S. New larvicidal acetogenin from the red alga Laurencia papillosa, CLEAN – Soil, Air, Water 2011, 38, 548–557.10.1002/clen.201000033Suche in Google Scholar
[3] Alarif, W. M.; Al-lihaibi, S. S.; Ayyad, S.-E. S.; Abdel-Rhman, M. H.; Badria, F. A. Laurene-type sesquiterpenes from the Red Sea red alga Laurencia obtusa as potential antitumor-antimicrobial agents, Eur. J. Med. Chem. 2012, 55, 462–466.10.1016/j.ejmech.2012.06.060Suche in Google Scholar PubMed
[4] Ayyad, S.-E. S.; Al-Footy, K. O.; Alarif, W. M.; Sobahi, T. R.; Basaif, S. A.; Makki, M. et. al. Bioactive C15 Acetogenins from Red Alga Laurencia obtusa, Chem. Pharm. Bull. 2011, 59, 1294–1298.10.1248/cpb.59.1294Suche in Google Scholar PubMed
[5] König, G. M.; Wright, A.D. Laurencia rigida: Chemical investigations of its antifouling dichloromethane extract, J. Nat. Prod. 1997, 60, 967–970.10.1021/np970181rSuche in Google Scholar PubMed
[6] Angawi, R. F.; Alarif, W. M.; Hamza, R. I.; Badria, F. A.; Ayyad, S.-E. N. New cytotoxic laurene, cuparene and laurokamurene type-sesquiterpenes from the red alga Laurencia obtusa, Helv. Chem. Acta. 2014, 97, 1388–1395.10.1002/chin.201516284Suche in Google Scholar
[7] Alarif, W. M.; Al-Footy, K. O.; Zubair, M. S.; PH M. H.; Ghandourah, M. A. ; Basaif, S. A.; Al-Lihaibi, S. S.; Ayyad, S.-E. N.; Badria, F. A. The role of new eudesmane-type sesquiterpenoid and known eudesmane derivatives from the red alga Laurencia obtusa as potential antifungal–antitumour agents, Nat. Prod. Res. 2015, 30, 1150–1155.10.1080/14786419.2015.1046378Suche in Google Scholar PubMed
[8] El Sayed K. A.; Dunbar, D. C., Perry, T. L., Wilkins, S. P., Hamann, M. T.; Greenplate, J. T. Marine natural products as prototype insecticidal agents J . Agri. Food Chem. 1997, 45, 2735–2739.10.1021/jf960746+Suche in Google Scholar
[9] Vairappan, C. S.; Kawamoto, T.; Miwa, H.; Suzuki, M. Potent antibacterial activity of halogenated compounds against antibiotic-resistant bacteria, Planta Med. 2004, 70, 1087–1090.10.1055/s-2004-832653Suche in Google Scholar PubMed
[10] Esselin, H.; Sutour, S.; Liberal, J.; Cruz, M. T.; Salgueiro, L.; Siegler, B. et al. Chemical Composition of Laurencia obtusa Extract and Isolation of a New C15-Acetogenin, Molecules 2017, 22, 779–790.10.3390/molecules22050779Suche in Google Scholar PubMed PubMed Central
[11] Christopher K., Bruno E. (2003) Identification of bacterial species. In O’Donnell MA (Editor) Tested studies for laboratory teaching. Proceedings of the 24th Workshop/Conference of the Association for Biology Laboratory Education (ABLE); pp 103–130.Suche in Google Scholar
[12] Holder, I.; Boyce, S. Agar well diffusion assay testing of bacterial susceptibility to various antimicrobials in concentrations non-toxic for human cells in culture Burns 1994, 20, 426–429.10.1016/0305-4179(94)90035-3Suche in Google Scholar
[13] Chand, S.; Lusunzi, I.; Veal, D. A. L.; Williams, R.; Karuso, P. Rapid screening of the antimicrobial activity of extracts and natural products, J. Antibiotics 1994, 47, 1295–1304.10.7164/antibiotics.47.1295Suche in Google Scholar PubMed
[14] Aly, M.; Gumgumjee, N. M. Antimicrobial efficacy of Rheum palmatum, Curcuma longa and Alpinia officinarum extracts against some pathogenic microorganisms African J. Biotechnol. 2011, 10, 12058–12063.Suche in Google Scholar
[15] Meyer, B.; Ferrigni, N.; Putnam, J.; Jacobsen, L.; Nichols, D. J.; McLaughlin, J. L. Brine shrimp: A convenient general bioassay for active plant constituents, Planta Med. 1982, 45, 31–34.10.1055/s-2007-971236Suche in Google Scholar PubMed
[16] Ichiba, T.; Higa, T. New cuparene-derived sesquiterpenes with unprecedented oxygenation pattern from the sea hare Aplysia dactylomela, J. Org. Chem. 1986, 51, 3364–3366.10.1021/jo00367a021Suche in Google Scholar
© 2017 Nahed O. Bawakid et al.
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Artikel in diesem Heft
- Regular Articles
- Rare Coumarins Induce Apoptosis, G1 Cell Block and Reduce RNA Content in HL60 Cells
- Regular Articles
- Evaluation of the photocatalytic ability of a sol-gel-derived MgO-ZrO2 oxide material
- Regular Articles
- Extraction Methods for the Isolation of Isoflavonoids from Plant Material
- Regular Articles
- Micro and nanocomposites of polybutadienebased polyurethane liners with mineral fillers and nanoclay: thermal and mechanical properties
- Regular Articles
- Effect of pH on Structural, Magnetic and FMR Properties of Hydrothermally Prepared Nano Ni Ferrite
- Regular Articles
- Statistical approach to study of lithium magnesium metaborate glasses
- Regular Articles
- The effectiveness of biodrying waste treatment in full scale reactor
- Regular Articles
- Chemical comparison of the underground parts of Valeriana officinalis and Valeriana turkestanica from Poland and Kazakhstan
- Regular Articles
- Phytochemical Characterization and Biological Evaluation of the Aqueous and Supercritical Fluid Extracts from Salvia sclareoides Brot
- Regular Articles
- Recent Microextraction Techniques for Determination and Chemical Speciation of Selenium
- Regular Articles
- Compost leachate treatment using polyaluminium chloride and nanofiltration
- Regular Articles
- Facile and Effective Synthesis of Praseodymium Tungstate Nanoparticles through an Optimized Procedure and Investigation of Photocatalytic Activity
- Regular Articles
- Computational Study on Non-linear Optical and Absorption Properties of Benzothiazole based Dyes: Tunable Electron-Withdrawing Strength and Reverse Polarity
- Regular Articles
- Comparative sorption studies of chromate by nano-and-micro sized Fe2O3 particles
- Regular Articles
- Recycling Monoethylene Glycol (MEG) from the Recirculating Waste of an Ethylene Oxide Unit
- Regular Articles
- Antimicrobial activity and thiosulfinates profile of a formulation based on Allium cepa L. extract
- Regular Articles
- The effect of catalyst precursors and conditions of preparing Pt and Pd-Pt catalysts on their activity in the oxidation of hexane
- Regular Articles
- Platinum and vanadate Bioactive Complexes of Glycoside Naringin and Phenolates
- Regular Articles
- Antimicrobial sesquiterpenoids from Laurencia obtusa Lamouroux
- Regular Articles
- Comprehensive spectroscopic (FT-IR, FT-Raman, 1H and 13C NMR) identification and computational studies on 1-acetyl-1H-indole-2,3-dione
- Regular Articles
- A combined experimental and theoretical study on vibrational and electronic properties of (5-methoxy-1H-indol-1-yl)(5-methoxy-1H-indol-2-yl)methanone
- Regular Articles
- Erratum to: Analysis of oligonucleotides by liquid chromatography with alkylamide stationary phase
- Regular Articles
- Non-isothermal Crystallization, Thermal Stability, and Mechanical Performance of Poly(L-lactic acid)/Barium Phenylphosphonate Systems
- Regular Articles
- Vortex assisted-supramolecular solvent based microextraction coupled with spectrophotometric determination of triclosan in environmental water samples
- Regular Articles
- Investigation on Two Compounds of O,O’-dithiophosphate Derivatives as Corrosion Inhibitors for Q235 Steel in Hydrochloric Acid Solution
- Regular Articles
- Evaluation of temporary seasonal variation of heavy metals and their potential ecological risk in Nzhelele River, South Africa
- Regular Articles
- Synthesis, characterization, second and third order non-linear optical properties and luminescence properties of 1,10-phenanthroline-2,9-di(carboxaldehyde phenylhydrazone) and its transition metal complexes
- Regular Articles
- Spectrodensitometric simultaneous determination of esomeprazole and domperidone in human plasma
- Regular Articles
- Computer-aided drug design of capuramycin analogues as anti-tuberculosis antibiotics by 3D-QSAR and molecular docking
- Regular Articles
- Synthesis, characterization, thermal degradation and urease inhibitory studies of the new hydrazide based Schiff base ligand 2-(2-hydroxyphenyl)-3-{[(E)-(2-hydroxyphenyl)methylidene]amino}-2,3-dihydroquinazolin-4(1H)-one
- Regular Articles
- Quaternary salts derived from 3-substituted quinuclidine as potential antioxidative and antimicrobial agents
- Regular Articles
- Bio-concentration of Polycyclic Aromatic Hydrocarbons in the grey Mangrove (Avicennia marina) along eastern coast of the Red Sea
- Regular Articles
- Quantitative Investigation of Roasting-magnetic Separation for Hematite Oolitic-ores: Mechanisms and Industrial Application
- Regular Articles
- Photobleaching characteristics of α-(8-quinolinoxy) zinc phthalocyanine, a new type of amphipathic complex
- Regular Articles
- Methane dry reforming over Ni catalysts supported on Ce–Zr oxides prepared by a route involving supercritical fluids
- Regular Articles
- Thermodynamic Compatibility, Crystallizability, Thermal, Mechanical Properties and Oil Resistance Characteristics of Nanostructure Poly (ethylene-co-methyl acrylate)/Poly(acrylonitrile-co-butadiene) Blends
- Regular Articles
- The crystal structure of compositionally homogeneous mixed ceria-zirconia oxides by high resolution X-ray and neutron diffraction methods
- Topical Issue on Agriculture
- Properties of the filtrate from treatment of pig manure by filtration method
- Topical Issue on Agriculture
- Monitoring content of cadmium, calcium, copper, iron, lead, magnesium and manganese in tea leaves by electrothermal and flame atomizer atomic absorption spectrometry
- Topical Issue on Catalysis
- Application of screen-printed carbon electrode modified with lead in stripping analysis of Cd(II)
- Topical Issue on Research for Natural Bioactive Products
- Burdock (Arctium lappa) Leaf Extracts Increase the In Vitro Antimicrobial Efficacy of Common Antibiotics on Gram-positive and Gram-negative Bacteria
- Topical Issue on Research for Natural Bioactive Products
- A survey of bacterial, fungal and plant metabolites against Aedes aegypti (Diptera: Culicidae), the vector of yellow and dengue fevers and Zika virus
- Topical Issue on Research for Natural Bioactive Products
- ‘Capiture’ plants with interesting biological activities: a case to go
- Topical Issue on Research for Natural Bioactive Products
- Volatile terpenoids as potential drug leads in Alzheimer’s disease
- Topical Issue on Research for Natural Bioactive Products
- Essential Oils as Immunomodulators: Some Examples
- Topical Issue on Research for Natural Bioactive Products
- Phenolic profiling and therapeutic potential of local flora of Azad Kashmir; In vitro enzyme inhibition and antioxidant
- Topical Issue on Research for Natural Bioactive Products
- Chemical profile, antioxidant activity and cytotoxic effect of extract from leaves of Erythrochiton brasiliensis Nees & Mart. from different regions of Europe
Artikel in diesem Heft
- Regular Articles
- Rare Coumarins Induce Apoptosis, G1 Cell Block and Reduce RNA Content in HL60 Cells
- Regular Articles
- Evaluation of the photocatalytic ability of a sol-gel-derived MgO-ZrO2 oxide material
- Regular Articles
- Extraction Methods for the Isolation of Isoflavonoids from Plant Material
- Regular Articles
- Micro and nanocomposites of polybutadienebased polyurethane liners with mineral fillers and nanoclay: thermal and mechanical properties
- Regular Articles
- Effect of pH on Structural, Magnetic and FMR Properties of Hydrothermally Prepared Nano Ni Ferrite
- Regular Articles
- Statistical approach to study of lithium magnesium metaborate glasses
- Regular Articles
- The effectiveness of biodrying waste treatment in full scale reactor
- Regular Articles
- Chemical comparison of the underground parts of Valeriana officinalis and Valeriana turkestanica from Poland and Kazakhstan
- Regular Articles
- Phytochemical Characterization and Biological Evaluation of the Aqueous and Supercritical Fluid Extracts from Salvia sclareoides Brot
- Regular Articles
- Recent Microextraction Techniques for Determination and Chemical Speciation of Selenium
- Regular Articles
- Compost leachate treatment using polyaluminium chloride and nanofiltration
- Regular Articles
- Facile and Effective Synthesis of Praseodymium Tungstate Nanoparticles through an Optimized Procedure and Investigation of Photocatalytic Activity
- Regular Articles
- Computational Study on Non-linear Optical and Absorption Properties of Benzothiazole based Dyes: Tunable Electron-Withdrawing Strength and Reverse Polarity
- Regular Articles
- Comparative sorption studies of chromate by nano-and-micro sized Fe2O3 particles
- Regular Articles
- Recycling Monoethylene Glycol (MEG) from the Recirculating Waste of an Ethylene Oxide Unit
- Regular Articles
- Antimicrobial activity and thiosulfinates profile of a formulation based on Allium cepa L. extract
- Regular Articles
- The effect of catalyst precursors and conditions of preparing Pt and Pd-Pt catalysts on their activity in the oxidation of hexane
- Regular Articles
- Platinum and vanadate Bioactive Complexes of Glycoside Naringin and Phenolates
- Regular Articles
- Antimicrobial sesquiterpenoids from Laurencia obtusa Lamouroux
- Regular Articles
- Comprehensive spectroscopic (FT-IR, FT-Raman, 1H and 13C NMR) identification and computational studies on 1-acetyl-1H-indole-2,3-dione
- Regular Articles
- A combined experimental and theoretical study on vibrational and electronic properties of (5-methoxy-1H-indol-1-yl)(5-methoxy-1H-indol-2-yl)methanone
- Regular Articles
- Erratum to: Analysis of oligonucleotides by liquid chromatography with alkylamide stationary phase
- Regular Articles
- Non-isothermal Crystallization, Thermal Stability, and Mechanical Performance of Poly(L-lactic acid)/Barium Phenylphosphonate Systems
- Regular Articles
- Vortex assisted-supramolecular solvent based microextraction coupled with spectrophotometric determination of triclosan in environmental water samples
- Regular Articles
- Investigation on Two Compounds of O,O’-dithiophosphate Derivatives as Corrosion Inhibitors for Q235 Steel in Hydrochloric Acid Solution
- Regular Articles
- Evaluation of temporary seasonal variation of heavy metals and their potential ecological risk in Nzhelele River, South Africa
- Regular Articles
- Synthesis, characterization, second and third order non-linear optical properties and luminescence properties of 1,10-phenanthroline-2,9-di(carboxaldehyde phenylhydrazone) and its transition metal complexes
- Regular Articles
- Spectrodensitometric simultaneous determination of esomeprazole and domperidone in human plasma
- Regular Articles
- Computer-aided drug design of capuramycin analogues as anti-tuberculosis antibiotics by 3D-QSAR and molecular docking
- Regular Articles
- Synthesis, characterization, thermal degradation and urease inhibitory studies of the new hydrazide based Schiff base ligand 2-(2-hydroxyphenyl)-3-{[(E)-(2-hydroxyphenyl)methylidene]amino}-2,3-dihydroquinazolin-4(1H)-one
- Regular Articles
- Quaternary salts derived from 3-substituted quinuclidine as potential antioxidative and antimicrobial agents
- Regular Articles
- Bio-concentration of Polycyclic Aromatic Hydrocarbons in the grey Mangrove (Avicennia marina) along eastern coast of the Red Sea
- Regular Articles
- Quantitative Investigation of Roasting-magnetic Separation for Hematite Oolitic-ores: Mechanisms and Industrial Application
- Regular Articles
- Photobleaching characteristics of α-(8-quinolinoxy) zinc phthalocyanine, a new type of amphipathic complex
- Regular Articles
- Methane dry reforming over Ni catalysts supported on Ce–Zr oxides prepared by a route involving supercritical fluids
- Regular Articles
- Thermodynamic Compatibility, Crystallizability, Thermal, Mechanical Properties and Oil Resistance Characteristics of Nanostructure Poly (ethylene-co-methyl acrylate)/Poly(acrylonitrile-co-butadiene) Blends
- Regular Articles
- The crystal structure of compositionally homogeneous mixed ceria-zirconia oxides by high resolution X-ray and neutron diffraction methods
- Topical Issue on Agriculture
- Properties of the filtrate from treatment of pig manure by filtration method
- Topical Issue on Agriculture
- Monitoring content of cadmium, calcium, copper, iron, lead, magnesium and manganese in tea leaves by electrothermal and flame atomizer atomic absorption spectrometry
- Topical Issue on Catalysis
- Application of screen-printed carbon electrode modified with lead in stripping analysis of Cd(II)
- Topical Issue on Research for Natural Bioactive Products
- Burdock (Arctium lappa) Leaf Extracts Increase the In Vitro Antimicrobial Efficacy of Common Antibiotics on Gram-positive and Gram-negative Bacteria
- Topical Issue on Research for Natural Bioactive Products
- A survey of bacterial, fungal and plant metabolites against Aedes aegypti (Diptera: Culicidae), the vector of yellow and dengue fevers and Zika virus
- Topical Issue on Research for Natural Bioactive Products
- ‘Capiture’ plants with interesting biological activities: a case to go
- Topical Issue on Research for Natural Bioactive Products
- Volatile terpenoids as potential drug leads in Alzheimer’s disease
- Topical Issue on Research for Natural Bioactive Products
- Essential Oils as Immunomodulators: Some Examples
- Topical Issue on Research for Natural Bioactive Products
- Phenolic profiling and therapeutic potential of local flora of Azad Kashmir; In vitro enzyme inhibition and antioxidant
- Topical Issue on Research for Natural Bioactive Products
- Chemical profile, antioxidant activity and cytotoxic effect of extract from leaves of Erythrochiton brasiliensis Nees & Mart. from different regions of Europe


