Abstract
There are numerous sources of chemical pollutants which can impact the mangrove ecosystem through adjacent waters, industrial and sewage discharges and air depositions. Polycyclic aromatic hydrocarbons (PAHs) are semi volatile ubiquitous anthropogenic pollutants detected in all environmental compartments. In the monitoring framework for the mangrove ecosystem along the Red Sea coast of Saudi Arabia, nine mangrove stands were examined for the accumulation of PAHs. Polycyclic aromatic hydrocarbons were measured using Gas Chromatography-Mass Spectrometry (GC-MS). The mean values detected for total PAHs in sediments, roots and leaf were 2.98, 8.57 and 23.43 ng/g respectively. The trend of the total PAHs concentration in all sites showed the descending order: leaf > roots > sediments. Beside the sandy nature of the sediments, the presences of all stands in remote areas fare from the direct anthropogenic effects lead to these relative low values. PAH bio-concentration factors for leaf are two to three magnitudes higher than that in roots, suggesting atmosphere deposition /leaf uptake mechanism in addition to the sediment/root mechanism. The diagnostic ratios revealed that the sources of PAHs are mainly pyrogenic.
1 Introduction
Mangroves are intertidal wetland plants usually found in tropical and subtropical coastal areas. They are considered as one of the most important ecological habitats due to their unique features such as high productivity, global extent, also because they represent the connection between the terrestrial and marine environment [1,2]. They play an important role in the export of carbon and nutrients to the coastal zone and oceans [3]. Mangroves provide foods for marine animals as the primary producers do, serve as nursery habitats for marine biota, and enhancing the diversity of the terrestrial and aquatic organisms [4]. In the last few decades, mangrove ecosystems were exposed to high risk of extinction through anthropogenic activities near to mangrove swamps [5]. Organic carbon richness, availability of detritus, and high productivity are the most important characteristics which make the mangrove ecosystem liable to the accumulation of a wide range of organic pollutants like polycyclic aromatic hydrocarbons (PAHs) [6,7].
Polycyclic aromatic hydrocarbons are ubiquitous anthropogenic semi-volatile pollutants consisting of two or more fused benzene rings. Numerous inputs of PAHs to various environmental compartments represented in industrial emission, oil spills, ship traffic, urban runoff, wastewater and atmospheric deposition [8]. Sources of PAHs in the environment can naturally occur (biogenic) or anthropogenic due to human activities. Anthropogenic sources could be either petrogenic that directly associated with petroleum hydrocarbons and/or pyrogenic as a result of incomplete combustion of recent and fossil organic matter. Polycyclic aromatic hydrocarbons are categorized as one of the most dangerous and harmful substances to the environment and human health due to their toxicity, mutagenicity, and carcinogenicity [1]. That is why, 16 PAH congeners were classified as priority pollutants by United States Environmental Protection Agency (USEPA) [9]. In mangrove swamps, PAHs are transfered from sediments through roots and translocated into the upper part of the plants and cause a serious lesions like irregularity in growth rate [10], damage the cell membrane of the plant shoots [7], depletion in the exchange with the atmospheric gas [10], and increase the lethality and mutation percentage. The degree of contamination of PAHs in mangrove swamps varies significantly and hot spots could be found even in relatively clean swamps. Based on the proximity of a pollution source, very high levels of PAHs (11098 ng/g) were detected in mangrove sediments in Hong Kong [11], and mangrove plant (68518 ng/g) in India [12], while low levels of PAHs were detected in mangrove plants in China (24 ng/g) [13].
Saudi Arabia is one of the biggest countries in production, oil exploration and exploitation all over the world. Production and transportation of oil usually leads to considerable amounts of PAHs as well as other organic pollutants being introduced into the environment [14]. Despite the acuteness of the environmental activities in the Red Sea Saudi coast, only heavy metals levels were studied in the mangrove ecosystems [15,16]. To our knowledge, there has been no specific survey for PAHs levels in the mangrove swamps which are widespread along the Red Sea coast. The objectives of the present study are to (1) provide baseline data for PAHs levels in nine mangrove swamps (surface sediments, roots and leaf) along the eastern side of Red Sea (2) compare total and individual bio-concentrations and translocation of PAHs for different studied swamps with other swamps all over the world and (3) evaluate the probable sources of PAHs for the studied swamps.
2 Study Area
The coastal area of Saudi Arabia covers around 79% of the eastern part of the Red Sea coast, extending over a distance of 1840 km between Yemen in the South and Jordan in the North. Two mangrove species (Avicenna marina and Rhizophora mucronata) are spread over this area. Avicenna marina is the most dominant species at the coastal line area [17]. The present study covers mangrove swamps in the coastal area extended from 16 °43’50.49”N to 20°45’5.48”N and from 38°55’13.38”E to 42°42’22.91”E. All stations were located in remote areas far from direct anthropogenic effects, except station 1 (Jazan) which is located near to a fish farm and station 8 (Al-Kharrar Rabigh) which is located in the way of torrent stream. The mangrove samples had a mean height of 2.5-3.0 m and its age was estimated to be 25-30 years old, except station 8 (Al-Kharrar Rabigh) where the mangrove plants were characterized by longer and intensive leaf. The growth conditions of all swamps were comparable, therefore significant differences in PAHs contamination due to this factor is excluded. Figure 1 shows the map locations for the mangrove samples along the eastern Red Sea coast.
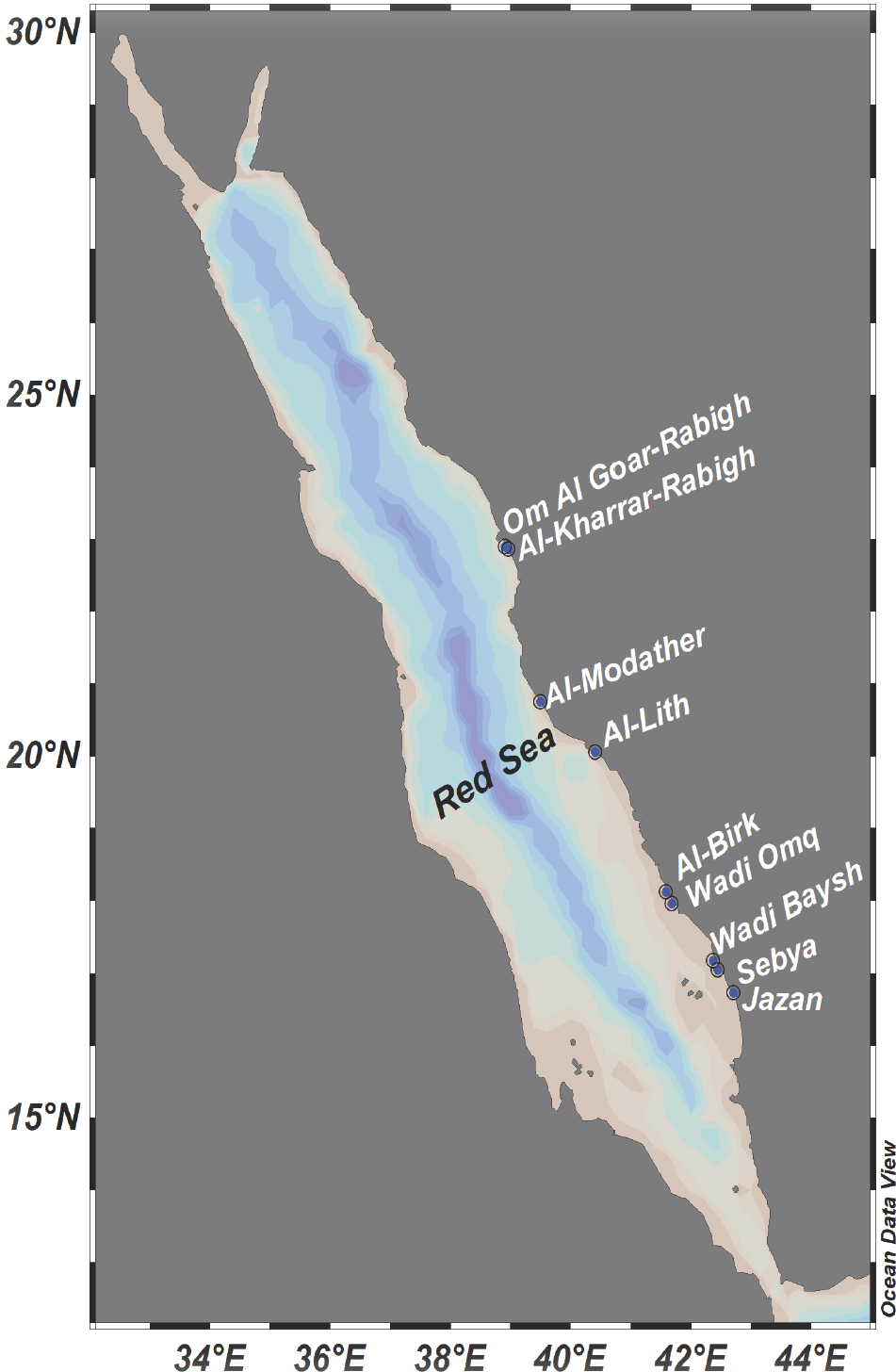
The map of the nine sampling locations of mangrove swamps along the Red Sea.
3 Material and Methods
3.1 Samples collection and pre-treatment
Approximately the top 0-5 cm of sediments were collected from each studied swamp and placed in aluminum bags, refrigerated, and transported to the laboratory within 8 hours of collection and kept frozen at -20°C. Plant samples were washed by tap water followed by deionized water before being freeze-dried. The dried plant samples were ground into powder using a blender. The sediment samples were freeze-dried for 24 hours, ground into a fine powder using a pestle and mortar, and filtered through an 80-mesh screen-sieve. All samples (sediments and plants) were transferred to glass bottles and kept at 4°C in a refrigerator for further analysis.
3.2 Organic carbon and grain size analysis
Total organic carbon in the sediments was analyzed by a wet dichromate-sulfuric acid oxidation method [18]. A standard dry sieving technique was used for sediment grain size fractions and were classified as fine (mud; < 0.063 mm), sand (0.063 – 2 mm) and gravel (> 2 mm) [19].
3.3 Extraction and purification of PAHs
Ten grams of each sediment sample (5 g leaf, 5 g root) was subjected to a soxhlet extraction using 300 ml dichloromethane (DCM) for 24 hours. All samples were spiked with 250μL deuterated surrogate standard mixture (naphthalene-d8, phenanthrene-d10 and chrysene-d12) before extraction [20]. The crude extract was concentrated on a rotavapor at 35°C. Particulate impurities for plant samples were filtered. The extracts were concentrated to approximately 1 ml and transferred to a silica column for cleanup and fractionation. The column was packed from top and bottom with pre-combusted anhydrous Na2SO4 at 450°C for 6 hours. Silica gel (230-400 mesh) was activated at 230°C for 12 hours then partially deactivated with 5% deionized water. The elution was done using n-hexane for the aliphatic fraction followed by n-hexane/DCM (70:30 v/v) for the PAH fraction [21]. PAH fractions were concentrated using a gentle stream of pure N2 to nearly 1 ml DCM. Deuterated internal standard mixture (acenaphthene-d10, flourene-d10 and perylene-d12) (100 μl, 5 ppm) was added just before injection to the GC-MS.
3.4 Identification and Quantification of PAHs
A GC-MS (Schimatzu 2010) with DB-5MS column (30 m* 0.25 μm, RTX) was used for analysis of the samples. The programmed temperature starts at 100°C with 1-minute hold, and then ramped at 6°C/minute to 300°C then hold for 3 minutes. The electron energy of the mass spectrometer was 70 eV. Individual PAHs were identified on both retention time and the mass spectrum of selected ions with the external calibrated standards for the priority 16 PAH parent targets in addition to 1 and 2- methylated naphthalene.
3.5 Calculation of Bio-concentration and Translocation Factors
The bio-concentration factor (BCF) for roots and leaf was calculated by the formula: BCFroot = Croot/CS and BCF leaf = C leaf/CS, where Croot and Cleaf are the concentration of total PAHs in mangrove roots and leaf respectively, and CS = concentration of PAHs in sediment [22]. The translocation factor (TF) was calculated from the formula TF = Cleaf/C to estimate the transfer of PAHs from root to leaf in mangrove plant [23].
3.6 Quality control and quality assurance
Duplicate samples (10% of the analyzed samples) and procedural blanks (1 blank for each 5 samples) were performed at the same time as the analysis. At the beginning of each working day, calibration standards were run before each sample batch to establish the calibration curves for PAHs. Before extraction, all samples were spiked with surrogate deuterated mixture for recovery calculations. Before GC-MS injection, deuterated internal standard mixture was added for all samples. The recoveries of sediment samples ranged between 66-104% with RSD% < 18%. The concentrations in the procedural blanks were no more than three times the method detection limit. Detection limits (DL) were calculated through a five-point calibration curve and extrapolated for determining the y axis intercept [24]. All results were expressed as dry weight basis, and those samples with concentrations less than the DL were reported as not detected (ND).
Ethical approval: The conducted research is not related to either human or animals use.
4 Results and Discussions
4.1 Levels of PAHs in Mangrove Swamps
From the 18 inspected PAH congeners (priority 16 PAHs in addition to 1 and 2- methylnaphthalenes), only 7 were detected either in sediments or in mangrove organs which are: naphthalene, 1-methylnaphthalene, phenanthrene, fluorine, anthracene, benzo[a]anthracene and pyrene, while the rest of congeners remained below the detection limit. Mangrove sediments present in variable compositions of silt, clay and sand contents [25]. Although several biogeochemical processes are available in mangrove swamps to host organic pollutants [26], sediment particle size is an important factor affecting the concentration of PAHs [27]. Table 1 represents proportional ratios for sand, mud, CaCO3, in addition to total organic carbon in the mangrove sediments for the nine studied stations. The particle size nature of sediments in the present study were found mainly sandy (range: 91.60-99.8%) with large particles and smaller surface area that diminishes adsorption of the PAHs [28]. The low values of total organic carbon detected in the mangrove sediments in the study area (range: 1.09-4.46%) was consistent with the sandy nature.
Sediment characteristics for the studied mangrove swamps along the Red Sea.
| Locations | Sand% | Mud% | TOC% | CaCO3% | |
|---|---|---|---|---|---|
| 1 | Jazan | 96.50 | 3.50 | 4.46 | 4.5 |
| 2 | Sebya | 96.20 | 3.80 | 3.67 | 15.26 |
| 3 | Wadi Baysh | 96.90 | 3.03 | 2.95 | 26.8 |
| 4 | Wadi Omq | 95.09 | 4.91 | 3.31 | 3.1 |
| 5 | Al-Birk | 98.38 | 1.62 | 2.24 | 2.2 |
| 6 | Al-Lith | 98.50 | 1.50 | 3.49 | 1.3 |
| 7 | Al-Modather | 99.80 | 0.20 | 1.27 | 41.5 |
| 8 | Al-Kharrar-Rabigh | 99.24 | 0.76 | 1.09 | 1.8 |
| 9 | Om Al-Goar-Rabigh | 91.60 | 8.40 | 1.88 | 2.7 |
Figure 2 represents the total PAHs concentrations (ΣPAHs) in the nine studied mangrove stands (sediments, roots and leaf) along the eastern Red Sea coast of Saudi Arabia. The total PAHs concentration in sediment samples ranged from 1.06 to 6.97 ng/g with an average value of 2.98 ng/g. These values seems to be very low relative to the extremely high PAHs levels detected in polluted mangrove sediments of different swamps in Hong Kong (11098 ng/g; 3785 ng/g) [11, 29]; and in Shenzhen, China (4480 ng/g) [6]. The detected levels from this study are also lower than the values detected in moderate polluted areas such as in Shantou mangrove wetlands in China (57 to 238 ng/g) [30], and in the Sunderban wetlands (Bay of Bengal, India, 20 to 839 ng/g) [31]. The low levels of PAHs in the sediments in the present study are attributed in principle to the fact that almost all studied swamps were in remote areas and far from direct anthropogenic effects, moreover, geochemical factors such as particle size and total organic carbon are consistent with the interpretation of these low levels. Aerosol deposition of PAHs could be the unique anthropogenic source in the studied mangrove swamps, especially for the low molecular weight PAH congeners [32,33]. Three member rings phenanthrene and anthracene were found as the most dominant congeners and represent 79.4%, 84.8% and 90.07% from total PAHs in mangrove sediments, roots and leaf respectively. Meanwhile, anthracene showed the highest detected concentrations in the sediment (2.39 ng/g in Al-Lith), roots (26.47 ng/g in Wadi Omq) and leaf (83.15 ng/g in Jazan). According to sediment quality guidelines (SQG), individual and total PAHs were found below the effect range low (ERL) [34].
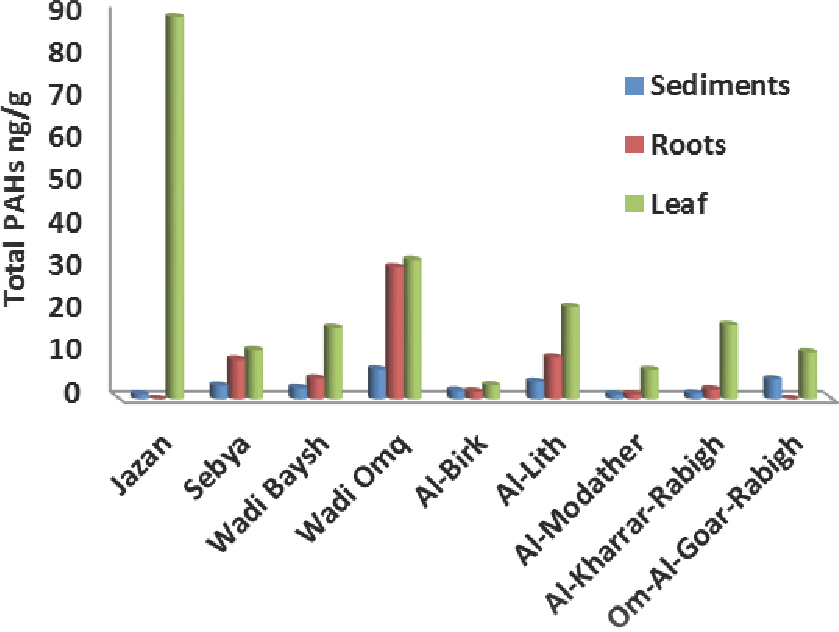
Total PAH concentrations (ng/g) for sediment, roots and leaf in the nine sites of the studied mangrove swamps.
Mangrove plant samples showed significant increase in the total PAHs levels, where roots values ranged from 1.11 to 30.96 ng/g with an average of 8.57 ng/g, and further increase in leaf (3.24 to 89.75 ng/g with an average of 23.43 ng/g). The trend of total PAHs concentrations in all sites showed the descending order: leaf > roots > sediments. The observed accumulation trend of PAHs in this study is compatible with the mangrove swamps in Shenzhen (Chania) [6]. On the other hand, the highest levels of PAHs were detected in the mangrove roots followed by leaf in Mumbai sediments (India) [12] explained by the fine particle size nature in this area.
4.2 Bio-concentration and translocation of PAHs in mangrove plant
The accumulation of PAHs in a mangrove plant takes place through two pathways; the substantial mechanism is the sediment/root uptake [7,35,36] and the other one is leaf/atmospheric uptake [33]. In the first mechanism, the PAH uptake prevails in the fine particle sediment around roots [33], and the low water solubility and lipophilic nature of PAHs smoothing the plant uptake that is easily accumulated in the lipid storing cells [37], then translocate in the upper shoots (leaf) [38]. In the leaf/atmospheric mechanism the uptake of the PAHs, either in gas phase and /or particulate phase, is through the stomata of the large surface areas of mangrove leaf that usually covered with thick waxy layers, which can accumulate the lipophilic PAHs from the atmosphere. The opportunity for PAHs uptake depends also on the volatility of PAH congeners [33]. It should be noted that both mechanisms are possible and that may lead to unsystematic correlations between PAHs in sediments and mangrove leaf and roots occasionally [39], as in stations 1 (Jazan) and 9 (Om Al-Goar Rabigh) in the present study.
Translocations (TF) and bio-concentration factors for roots (BCFroot) and leaf (BCFleaf) were calculated only for phenanthrene and anthracene congeners that were detected in most mangrove sediments, roots and leaf. A bio-concentration factor (BCF)>1 means that an organism absorbs PAH congener at a rate faster than that at which the substance is lost by catabolism and excretion. The results of the regression analysis for roots/sediments and leaf/roots indicated p-values ≤ 0.05 that confirm a significant correlation for both congeners, harmonize with sediment/root mechanism. BCFroot for both congeners was >1 in all stations except the Al-Birk station for anthracene (<1), and the highest value (4.13) was recorded at Wadi Baysh for phenanthrene; the mean value for BCFroot for anthracene (1.72) was lower than BCFroot for phenanthrene (2.68). On the other hand, the bio-concentration factors for leaf (BCFleaf) elucidate relatively high accumulation, where BCFleaf recorded 5.41 and 5.57 for phenanthrene and anthracene, respectively. BCFleaf was < 1 for phenanthrene at the Al-Kharrar-Rabigh and Om Al-Goar-Rabigh stations. The highest value for BCFleaf (18.37) was recorded at Al- Kharrar-Rabigh for anthracene. The high BCFleaf values may support the presence of both mechanisms for PAH accumulation, either from sediment/root way or leaf atmospheric deposition way [36,40]. TF consist with the bio-concentration factors that recorded 6.45 as the highest value at Al-Kharrar-Rabigh for anthracene (Figure 3).
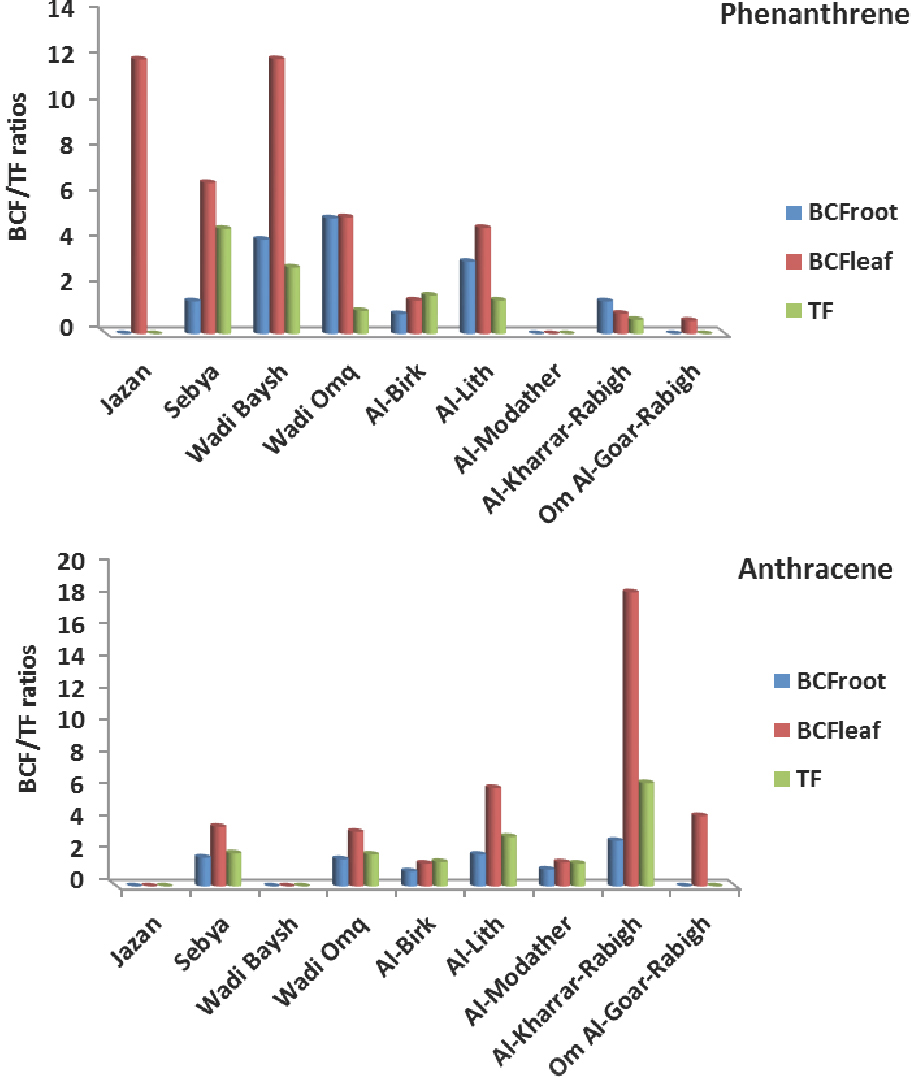
Bio-concentration factors (roots and leafs) and translocation factors for phenanthrene and anthracene in mangrove plants in the study area.
It is mentioning that the presence of flourene and phenanthrene in mangrove leaf at Al-Lith and Al-Modather stations, respectively, which were not detected in the roots and sediments preclude the sediment/root mechanism and outweigh the leaf/atmospheric deposition uptake mechanism especially for such light congener [41], that could represent the main source for three ring congeners to mangrove in these specific sites [42].
4.3 The probable origin of PAH in the study area
The common sources of PAHs in the marine environment originate from anthropogenic activities related to either pyrogenic and/or petrogenic processes [43]. It is very difficult to discriminate between different origins of PAH mixtures due to the complexity governing the distribution of PAHs in marine sediments and mangrove. However, isomeric ratios Phenanthrene/Anthathene and Anthrathene/(Phenanthrene+Anthrathene) were selected to differentiate between pyrogenic and petrogenic origin of PAHs due to the dominance of these congeners in most studied sites. Values higher than 10 for the Phenanthrene/Anthathene ratio indicate petrogenic origin, while the ratio of Anthrathene/(Phenanthrene + Anthrathene) indicate pyrogenic origin if higher than 0.1 [34]. In the present study, only roots at station 4 (Wadi Omq) and leaf at station 3 (Wadi Baysh) showed petrogenic origin, while the rest of PAHs in roots and leaf in all other sites including sediments showed pyrogenic origin (Figure 4). It is clear that the probable origin of PAHs is site specific depending on the anthropogenic sources; where mangrove swamps in Shenzhen (China) showed mainly pyrogenic origin as in the present case [6], while it showed mainly petrogenic origin in Mumbai (India) [12].
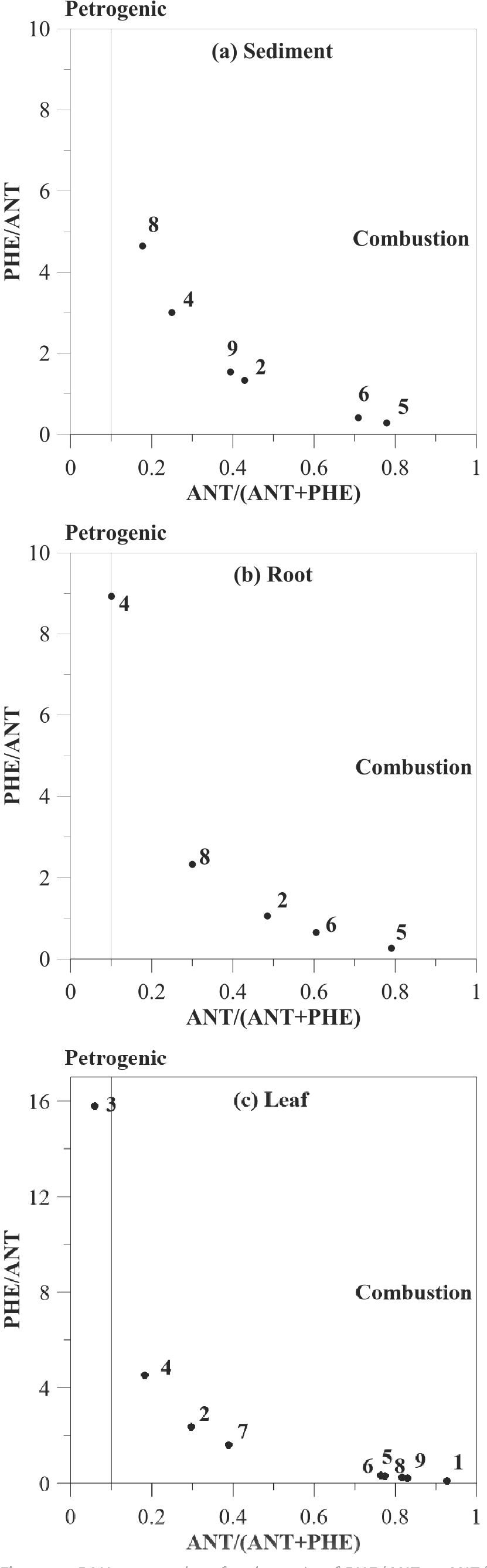
PAHs cross plots for the ratio of PHE/ANT vs ANT/(ANT+PHE) for sediment, root, and leaf samples.
5 Conclusion
The ranges of total PAHs in the studied mangrove swamps along the eastern coast of the Red Sea showed a gradual increase from sediment to root and the highest values were recorded in leaf. This trend supports PAH uptake through the sediment/root mechanism. The atmospheric deposition/leaf uptake mechanism found in a few stations where phenanthrene and anthracene were detected in mangrove leaf and not the sediments and roots. The relatively low levels of PAHs either in sediments or mangrove organs attribute to sandy nature and low organic carbon content of sediments in the studied swamps, moreover the presence of mangrove swamps apart from direct anthropogenic effects decrease the contamination probabilities. Despite the low recorded PAH values, bioconcentration factors in most stations showed values higher than one and reaching 18.37 for anthracene in the mangrove leaf as the maximum value for accumulation. PAH origin of most studied mangrove swamps was found mainly pyrogenic.
Acknowledgment
Mr. El-Amin Bashir and Mr. Rasiq K.T are grateful to deanship of graduate studies, King Abdulaziz University for providing the PhD fellowship.
Conflict of interest: Authors state no conflict of interest.
References
[1] Cuong D.T., Bayen S., Wurl O., Subramanian K., Wong K.K.S., Sivasothi N., et al., Heavy metal contamination in mangrove habitats of Singapore, Marine Pollution Bulletin, 2005, 50, 1732-1738.10.1016/j.marpolbul.2005.09.008Search in Google Scholar
[2] Abdulla P.A., Bjesse P., Eén N., Symbolic reachability analysis based on SAT-solvers, in International Conference on Tools and Algorithms for the Construction and Analysis of Systems, 2000, 411-425.10.1007/3-540-46419-0_28Search in Google Scholar
[3] Adame M.F.,. Lovelock C.E, Carbon and nutrient exchange of mangrove forests with the coastal ocean, Hydrobiologia, 2011, 663, 23-50.10.1007/s10750-010-0554-7Search in Google Scholar
[4] Dittmar T., Lara R.J., Do mangroves rather than rivers provide nutrients to coastal environments south of the Amazon River? Evidence from long-term flux measurements, Marine Ecology Progress Series, 2001, 213, 67-77.10.3354/meps213067Search in Google Scholar
[5] Polidoro B.A., Carpenter K.E., Collins L., Duke N.C., Ellison A.M., Ellison J.C., et al., The loss of species: mangrove extinction risk and geographic areas of global concern, PloS one, 2010, 5, e10095.10.1371/journal.pone.0010095Search in Google Scholar
[6] Li F., Zeng X., Yang J., Zhou K., Zan Q., Lei A., et al., Contamination of polycyclic aromatic hydrocarbons (PAHs) in surface sediments and plants of mangrove swamps in Shenzhen, China, Marine pollution bulletin, 2014, 85, 590-596.10.1016/j.marpolbul.2014.02.025Search in Google Scholar
[7] Kang F., Chen D., Gao Y., Zhang Y., Distribution of polycyclic aromatic hydrocarbons in subcellular root tissues of ryegrass (Lolium multiflorum Lam.), BMC plant biology, 2010, 10, p.210.10.1186/1471-2229-10-210Search in Google Scholar
[8] Boonyatumanond R., Wattayakorn G., Togo A., Takada H., Distribution and origins of polycyclic aromatic hydrocarbons (PAHs) in riverine, estuarine, and marine sediments in Thailand, Marine pollution bulletin, 2006, 52, 942-956.10.1016/j.marpolbul.2005.12.015Search in Google Scholar
[9] EPA U., Provisional guidance for quantitative risk assessment of polycyclic aromatic hydrocarbons, Development, 1993.Search in Google Scholar
[10] Naidoo G., Naidoo Y., Achar P., Responses of the mangroves Avicennia marina and Bruguiera gymnorrhiza to oil contamination, Flora-Morphology, Distribution, Functional Ecology of Plants, 2010, 205, 357-362.10.1016/j.flora.2009.12.033Search in Google Scholar
[11] Tam N., Ke L., Wang X., Wong Y., Contamination of polycyclic aromatic hydrocarbons in surface sediments of mangrove swamps, Environmental Pollution, 2001, 114, 255-263.10.1016/S0269-7491(00)00212-8Search in Google Scholar
[12] Shete A., Pandit G., Gunale V., Polycyclic Aromatic Hydrocarbons in the Mangrove Species: Avicennia Marina from Mumbai, India, J. Appl. Environ. Biol. Sci, 2016, 6, p.6.Search in Google Scholar
[13] Vane C., Harrison I., Kim A., Moss-Hayes V., Vickers B., Hong K., Organic and metal contamination in surface mangrove sediments of South China, Marine Pollution Bulletin, 2009, 58, 134-144.10.1016/j.marpolbul.2008.09.024Search in Google Scholar PubMed
[14] Kim K.-H., Jahan S.A., Kabir E., Brown R.J., A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects, Environment international, 2013, 60, 71-80.10.1016/j.envint.2013.07.019Search in Google Scholar PubMed
[15] Badr N.B., El-Fiky A.A., Mostafa A.R., Al-Mur B.A., Metal pollution records in core sediments of some Red Sea coastal areas, Kingdom of Saudi Arabia, Environmental monitoring and assessment, 2009, 155, 509-526.10.1007/s10661-008-0452-xSearch in Google Scholar PubMed
[16] Usman A.R., Alkredaa R.S., Al-Wabel M., Heavy metal contamination in sediments and mangroves from the coast of Red Sea: Avicennia marina as potential metal bioaccumulator, Ecotoxicology and environmental safety, 2013, 97, 263-270.10.1016/j.ecoenv.2013.08.009Search in Google Scholar PubMed
[17] Price A.R., Jobbins G., Shepherd A.R.D., Ormond R.F., An integrated environmental assessment of the Red Sea coast of Saudi Arabia, Environmental Conservation, 1998, 25, 65-76.10.1017/S0376892998000101Search in Google Scholar
[18] Aminot A., Chaussepied M., Manuel des analyses chimiques en milieu marin, 1983.Search in Google Scholar
[19] Syvitski J.P., Principles, methods and application of particle size analysis: Cambridge University Press, 2007.Search in Google Scholar
[20] Raza M., Zakaria M.P., Hashim N.R., Yim U.H., Kannan N., Ha S.Y., Composition and source identification of polycyclic aromatic hydrocarbons in mangrove sediments of Peninsular Malaysia: indication of anthropogenic input, Environmental earth sciences, 2013, 70, 2425-2436.10.1007/s12665-013-2279-1Search in Google Scholar
[21] Wu S., Tao S., Xu F., Dawson R., Lan T., Li B., et al., Polycyclic aromatic hydrocarbons in dustfall in Tianjin, China, Science of the Total Environment, 2005, 345, 115-126.10.1016/j.scitotenv.2004.11.003Search in Google Scholar PubMed
[22] Watts A.W., Ballestero T.P., Gardner K.H., Uptake of polycyclic aromatic hydrocarbons (PAHs) in salt marsh plants Spartina alterniflora grown in contaminated sediments, Chemosphere, 2006, 62, 1253-1260.10.1016/j.chemosphere.2005.07.006Search in Google Scholar PubMed
[23] Marchiol L., Assolari S., Sacco P., Zerbi G., Phytoextraction of heavy metals by canola (Brassica napus) and radish (Raphanus sativus) grown on multicontaminated soil, Environmental Pollution, 2004, 132, 21-27.10.1016/j.envpol.2004.04.001Search in Google Scholar PubMed
[24] Victoria U., Determination of Polychlorinated Biphenyls (PCBs) in Waste Oils by Gas Chromatography with Electron Capture Detector, EPA Victoria method, 2003.Search in Google Scholar
[25] Ranjan R.K., Routh J., Ramanathan A., Bulk organic matter characteristics in the Pichavaram mangrove–estuarine complex, south-eastern India, Applied Geochemistry, 2010, 25, 1176-1186.10.1016/j.apgeochem.2010.05.003Search in Google Scholar
[26] Weissenfels W.D., Klewer H.-J., Langhoff J., Adsorption of polycyclic aromatic hydrocarbons (PAHs) by soil particles: influence on biodegradability and biotoxicity, Applied Microbiology and Biotechnology, 1992, 36, 689-696.10.1007/BF00183251Search in Google Scholar PubMed
[27] Zhao H., Li X., Wang X., Tian D., Grain size distribution of road-deposited sediment and its contribution to heavy metal pollution in urban runoff in Beijing, China, Journal of Hazardous Materials, 2010, 183, 203-210.10.1016/j.jhazmat.2010.07.012Search in Google Scholar PubMed
[28] Bei L.B., Ping W., Li C., Yong Z., Effects of aging and flooding on sorption of PAHs in mangrove sediment, Fresenius Environmental Bulletin,2011, 20, 623-630.Search in Google Scholar
[29] Tam N.F., Wong T.W., Wong Y., A case study on fuel oil contamination in a mangrove swamp in Hong Kong, Marine pollution bulletin, 2005, 51, 1092-1100.10.1016/j.marpolbul.2005.06.005Search in Google Scholar PubMed
[30] Cao Q., Chen G., Wang H., Qin J., Huang X., Distribution and sources of PAHs in surface sediments of Shantou mangrove wetlands, China, Fresenius Environmental Bulletin, 2009, 18, 1788-1797.Search in Google Scholar
[31] Binelli A., Sarkar S.K., Chatterjee M., Riva C., Parolini M., deb Bhattacharya B., et al., A comparison of sediment quality guidelines for toxicity assessment in the Sunderban wetlands (Bay of Bengal, India), Chemosphere, 2008, 73, 1129-1137.10.1016/j.chemosphere.2008.07.019Search in Google Scholar PubMed
[32] Wang P., Wu T.-H., Zhang Y., Direct observation of the photodegradation of anthracene and pyrene adsorbed onto mangrove leaf, PloS one, 2014, 9, e104903.10.1371/journal.pone.0104903Search in Google Scholar PubMed PubMed Central
[33] Wang P., Du K.-Z., Zhu Y.-X., Zhang Y., A novel analytical approach for investigation of anthracene adsorption onto mangrove leaf, Talanta, 2008, 76, 1177-1182.10.1016/j.talanta.2008.05.021Search in Google Scholar PubMed
[34] Long E.R., Macdonald D.D., Smith S.L., Calder F.D., Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments, Environmental management, 1995, 190, 81-97.10.1007/BF02472006Search in Google Scholar
[35] Wang P.,. Zhang Y, Wu T.-H., Novel method for in situ visualization of polycyclic aromatic hydrocarbons in mangrove plants, Toxicological and Environ Chemistry, 2010, 92, 1825-1829.10.1080/02772248.2010.482833Search in Google Scholar
[36] Lohmann R., Dapsis M., Morgan E.J., Dekany V., Luey P.J., Determining air– water exchange, spatial and temporal trends of freely dissolved PAHs in an urban estuary using passive polyethylene samplers, Environmental science & technology, 2011, 45, 2655-2662.10.1021/es1025883Search in Google Scholar PubMed
[37] Chiou C.T., Sheng G., Manes M., A partition-limited model for the plant uptake of organic contaminants from soil and water, Environmental science & technology, 2001, 35, 1437-1444.10.1021/es0017561Search in Google Scholar PubMed
[38] Tam N., Wong Y.S., Effectiveness of bacterial inoculum and mangrove plants on remediation of sediment contaminated with polycyclic aromatic hydrocarbons, Marine Pollution Bulletin, 2008, 57, 716-726.10.1016/j.marpolbul.2008.02.029Search in Google Scholar PubMed
[39] Ahmed A., Ohlson M., Hoque S., Moula M.G., Chemical composition of leaf of a mangrove tree (Sonneratia apetala Buch.-Ham.) and their correlation with some soil variables, Bangladesh Journal of Botany, 2010, 39, 61-69.10.3329/bjb.v39i1.5528Search in Google Scholar
[40] Wang Y., Tao S., Jiao X., Coveney R., Wu S., Xing B., Polycyclic aromatic hydrocarbons in leaf cuticles and inner tissues of six species of trees in urban Beijing, Environmental pollution, 2008, 151, 158-164.10.1016/j.envpol.2007.02.005Search in Google Scholar PubMed
[41] Orecchio S., PAHs associated with the leaf of Quercus ilex L.: Extraction, GC–MS analysis, distribution and sources: Assessment of air quality in the Palermo (Italy) area, Atmospheric Environment, 2007, 41, 8669-8680.10.1016/j.atmosenv.2007.07.027Search in Google Scholar
[42] Kacálková L., Tlustoš P., The uptake of persistent organic pollutants by plants, Open Life Sciences, 2011, 6, 223-235.10.2478/s11535-010-0116-zSearch in Google Scholar
[43] Zakaria M.P., Takada H., Tsutsumi S., Ohno K., Yamada J., Kouno E., et al., Distribution of polycyclic aromatic hydrocarbons (PAHs) in rivers and estuaries in Malaysia: a widespread input of petrogenic PAHs, Environmental science & technology, 2001, 36, 1907-1918.10.1021/es011278+Search in Google Scholar PubMed
© 2017 M. El-Amin Bashir et al.
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Articles in the same Issue
- Regular Articles
- Rare Coumarins Induce Apoptosis, G1 Cell Block and Reduce RNA Content in HL60 Cells
- Regular Articles
- Evaluation of the photocatalytic ability of a sol-gel-derived MgO-ZrO2 oxide material
- Regular Articles
- Extraction Methods for the Isolation of Isoflavonoids from Plant Material
- Regular Articles
- Micro and nanocomposites of polybutadienebased polyurethane liners with mineral fillers and nanoclay: thermal and mechanical properties
- Regular Articles
- Effect of pH on Structural, Magnetic and FMR Properties of Hydrothermally Prepared Nano Ni Ferrite
- Regular Articles
- Statistical approach to study of lithium magnesium metaborate glasses
- Regular Articles
- The effectiveness of biodrying waste treatment in full scale reactor
- Regular Articles
- Chemical comparison of the underground parts of Valeriana officinalis and Valeriana turkestanica from Poland and Kazakhstan
- Regular Articles
- Phytochemical Characterization and Biological Evaluation of the Aqueous and Supercritical Fluid Extracts from Salvia sclareoides Brot
- Regular Articles
- Recent Microextraction Techniques for Determination and Chemical Speciation of Selenium
- Regular Articles
- Compost leachate treatment using polyaluminium chloride and nanofiltration
- Regular Articles
- Facile and Effective Synthesis of Praseodymium Tungstate Nanoparticles through an Optimized Procedure and Investigation of Photocatalytic Activity
- Regular Articles
- Computational Study on Non-linear Optical and Absorption Properties of Benzothiazole based Dyes: Tunable Electron-Withdrawing Strength and Reverse Polarity
- Regular Articles
- Comparative sorption studies of chromate by nano-and-micro sized Fe2O3 particles
- Regular Articles
- Recycling Monoethylene Glycol (MEG) from the Recirculating Waste of an Ethylene Oxide Unit
- Regular Articles
- Antimicrobial activity and thiosulfinates profile of a formulation based on Allium cepa L. extract
- Regular Articles
- The effect of catalyst precursors and conditions of preparing Pt and Pd-Pt catalysts on their activity in the oxidation of hexane
- Regular Articles
- Platinum and vanadate Bioactive Complexes of Glycoside Naringin and Phenolates
- Regular Articles
- Antimicrobial sesquiterpenoids from Laurencia obtusa Lamouroux
- Regular Articles
- Comprehensive spectroscopic (FT-IR, FT-Raman, 1H and 13C NMR) identification and computational studies on 1-acetyl-1H-indole-2,3-dione
- Regular Articles
- A combined experimental and theoretical study on vibrational and electronic properties of (5-methoxy-1H-indol-1-yl)(5-methoxy-1H-indol-2-yl)methanone
- Regular Articles
- Erratum to: Analysis of oligonucleotides by liquid chromatography with alkylamide stationary phase
- Regular Articles
- Non-isothermal Crystallization, Thermal Stability, and Mechanical Performance of Poly(L-lactic acid)/Barium Phenylphosphonate Systems
- Regular Articles
- Vortex assisted-supramolecular solvent based microextraction coupled with spectrophotometric determination of triclosan in environmental water samples
- Regular Articles
- Investigation on Two Compounds of O,O’-dithiophosphate Derivatives as Corrosion Inhibitors for Q235 Steel in Hydrochloric Acid Solution
- Regular Articles
- Evaluation of temporary seasonal variation of heavy metals and their potential ecological risk in Nzhelele River, South Africa
- Regular Articles
- Synthesis, characterization, second and third order non-linear optical properties and luminescence properties of 1,10-phenanthroline-2,9-di(carboxaldehyde phenylhydrazone) and its transition metal complexes
- Regular Articles
- Spectrodensitometric simultaneous determination of esomeprazole and domperidone in human plasma
- Regular Articles
- Computer-aided drug design of capuramycin analogues as anti-tuberculosis antibiotics by 3D-QSAR and molecular docking
- Regular Articles
- Synthesis, characterization, thermal degradation and urease inhibitory studies of the new hydrazide based Schiff base ligand 2-(2-hydroxyphenyl)-3-{[(E)-(2-hydroxyphenyl)methylidene]amino}-2,3-dihydroquinazolin-4(1H)-one
- Regular Articles
- Quaternary salts derived from 3-substituted quinuclidine as potential antioxidative and antimicrobial agents
- Regular Articles
- Bio-concentration of Polycyclic Aromatic Hydrocarbons in the grey Mangrove (Avicennia marina) along eastern coast of the Red Sea
- Regular Articles
- Quantitative Investigation of Roasting-magnetic Separation for Hematite Oolitic-ores: Mechanisms and Industrial Application
- Regular Articles
- Photobleaching characteristics of α-(8-quinolinoxy) zinc phthalocyanine, a new type of amphipathic complex
- Regular Articles
- Methane dry reforming over Ni catalysts supported on Ce–Zr oxides prepared by a route involving supercritical fluids
- Regular Articles
- Thermodynamic Compatibility, Crystallizability, Thermal, Mechanical Properties and Oil Resistance Characteristics of Nanostructure Poly (ethylene-co-methyl acrylate)/Poly(acrylonitrile-co-butadiene) Blends
- Regular Articles
- The crystal structure of compositionally homogeneous mixed ceria-zirconia oxides by high resolution X-ray and neutron diffraction methods
- Topical Issue on Agriculture
- Properties of the filtrate from treatment of pig manure by filtration method
- Topical Issue on Agriculture
- Monitoring content of cadmium, calcium, copper, iron, lead, magnesium and manganese in tea leaves by electrothermal and flame atomizer atomic absorption spectrometry
- Topical Issue on Catalysis
- Application of screen-printed carbon electrode modified with lead in stripping analysis of Cd(II)
- Topical Issue on Research for Natural Bioactive Products
- Burdock (Arctium lappa) Leaf Extracts Increase the In Vitro Antimicrobial Efficacy of Common Antibiotics on Gram-positive and Gram-negative Bacteria
- Topical Issue on Research for Natural Bioactive Products
- A survey of bacterial, fungal and plant metabolites against Aedes aegypti (Diptera: Culicidae), the vector of yellow and dengue fevers and Zika virus
- Topical Issue on Research for Natural Bioactive Products
- ‘Capiture’ plants with interesting biological activities: a case to go
- Topical Issue on Research for Natural Bioactive Products
- Volatile terpenoids as potential drug leads in Alzheimer’s disease
- Topical Issue on Research for Natural Bioactive Products
- Essential Oils as Immunomodulators: Some Examples
- Topical Issue on Research for Natural Bioactive Products
- Phenolic profiling and therapeutic potential of local flora of Azad Kashmir; In vitro enzyme inhibition and antioxidant
- Topical Issue on Research for Natural Bioactive Products
- Chemical profile, antioxidant activity and cytotoxic effect of extract from leaves of Erythrochiton brasiliensis Nees & Mart. from different regions of Europe
Articles in the same Issue
- Regular Articles
- Rare Coumarins Induce Apoptosis, G1 Cell Block and Reduce RNA Content in HL60 Cells
- Regular Articles
- Evaluation of the photocatalytic ability of a sol-gel-derived MgO-ZrO2 oxide material
- Regular Articles
- Extraction Methods for the Isolation of Isoflavonoids from Plant Material
- Regular Articles
- Micro and nanocomposites of polybutadienebased polyurethane liners with mineral fillers and nanoclay: thermal and mechanical properties
- Regular Articles
- Effect of pH on Structural, Magnetic and FMR Properties of Hydrothermally Prepared Nano Ni Ferrite
- Regular Articles
- Statistical approach to study of lithium magnesium metaborate glasses
- Regular Articles
- The effectiveness of biodrying waste treatment in full scale reactor
- Regular Articles
- Chemical comparison of the underground parts of Valeriana officinalis and Valeriana turkestanica from Poland and Kazakhstan
- Regular Articles
- Phytochemical Characterization and Biological Evaluation of the Aqueous and Supercritical Fluid Extracts from Salvia sclareoides Brot
- Regular Articles
- Recent Microextraction Techniques for Determination and Chemical Speciation of Selenium
- Regular Articles
- Compost leachate treatment using polyaluminium chloride and nanofiltration
- Regular Articles
- Facile and Effective Synthesis of Praseodymium Tungstate Nanoparticles through an Optimized Procedure and Investigation of Photocatalytic Activity
- Regular Articles
- Computational Study on Non-linear Optical and Absorption Properties of Benzothiazole based Dyes: Tunable Electron-Withdrawing Strength and Reverse Polarity
- Regular Articles
- Comparative sorption studies of chromate by nano-and-micro sized Fe2O3 particles
- Regular Articles
- Recycling Monoethylene Glycol (MEG) from the Recirculating Waste of an Ethylene Oxide Unit
- Regular Articles
- Antimicrobial activity and thiosulfinates profile of a formulation based on Allium cepa L. extract
- Regular Articles
- The effect of catalyst precursors and conditions of preparing Pt and Pd-Pt catalysts on their activity in the oxidation of hexane
- Regular Articles
- Platinum and vanadate Bioactive Complexes of Glycoside Naringin and Phenolates
- Regular Articles
- Antimicrobial sesquiterpenoids from Laurencia obtusa Lamouroux
- Regular Articles
- Comprehensive spectroscopic (FT-IR, FT-Raman, 1H and 13C NMR) identification and computational studies on 1-acetyl-1H-indole-2,3-dione
- Regular Articles
- A combined experimental and theoretical study on vibrational and electronic properties of (5-methoxy-1H-indol-1-yl)(5-methoxy-1H-indol-2-yl)methanone
- Regular Articles
- Erratum to: Analysis of oligonucleotides by liquid chromatography with alkylamide stationary phase
- Regular Articles
- Non-isothermal Crystallization, Thermal Stability, and Mechanical Performance of Poly(L-lactic acid)/Barium Phenylphosphonate Systems
- Regular Articles
- Vortex assisted-supramolecular solvent based microextraction coupled with spectrophotometric determination of triclosan in environmental water samples
- Regular Articles
- Investigation on Two Compounds of O,O’-dithiophosphate Derivatives as Corrosion Inhibitors for Q235 Steel in Hydrochloric Acid Solution
- Regular Articles
- Evaluation of temporary seasonal variation of heavy metals and their potential ecological risk in Nzhelele River, South Africa
- Regular Articles
- Synthesis, characterization, second and third order non-linear optical properties and luminescence properties of 1,10-phenanthroline-2,9-di(carboxaldehyde phenylhydrazone) and its transition metal complexes
- Regular Articles
- Spectrodensitometric simultaneous determination of esomeprazole and domperidone in human plasma
- Regular Articles
- Computer-aided drug design of capuramycin analogues as anti-tuberculosis antibiotics by 3D-QSAR and molecular docking
- Regular Articles
- Synthesis, characterization, thermal degradation and urease inhibitory studies of the new hydrazide based Schiff base ligand 2-(2-hydroxyphenyl)-3-{[(E)-(2-hydroxyphenyl)methylidene]amino}-2,3-dihydroquinazolin-4(1H)-one
- Regular Articles
- Quaternary salts derived from 3-substituted quinuclidine as potential antioxidative and antimicrobial agents
- Regular Articles
- Bio-concentration of Polycyclic Aromatic Hydrocarbons in the grey Mangrove (Avicennia marina) along eastern coast of the Red Sea
- Regular Articles
- Quantitative Investigation of Roasting-magnetic Separation for Hematite Oolitic-ores: Mechanisms and Industrial Application
- Regular Articles
- Photobleaching characteristics of α-(8-quinolinoxy) zinc phthalocyanine, a new type of amphipathic complex
- Regular Articles
- Methane dry reforming over Ni catalysts supported on Ce–Zr oxides prepared by a route involving supercritical fluids
- Regular Articles
- Thermodynamic Compatibility, Crystallizability, Thermal, Mechanical Properties and Oil Resistance Characteristics of Nanostructure Poly (ethylene-co-methyl acrylate)/Poly(acrylonitrile-co-butadiene) Blends
- Regular Articles
- The crystal structure of compositionally homogeneous mixed ceria-zirconia oxides by high resolution X-ray and neutron diffraction methods
- Topical Issue on Agriculture
- Properties of the filtrate from treatment of pig manure by filtration method
- Topical Issue on Agriculture
- Monitoring content of cadmium, calcium, copper, iron, lead, magnesium and manganese in tea leaves by electrothermal and flame atomizer atomic absorption spectrometry
- Topical Issue on Catalysis
- Application of screen-printed carbon electrode modified with lead in stripping analysis of Cd(II)
- Topical Issue on Research for Natural Bioactive Products
- Burdock (Arctium lappa) Leaf Extracts Increase the In Vitro Antimicrobial Efficacy of Common Antibiotics on Gram-positive and Gram-negative Bacteria
- Topical Issue on Research for Natural Bioactive Products
- A survey of bacterial, fungal and plant metabolites against Aedes aegypti (Diptera: Culicidae), the vector of yellow and dengue fevers and Zika virus
- Topical Issue on Research for Natural Bioactive Products
- ‘Capiture’ plants with interesting biological activities: a case to go
- Topical Issue on Research for Natural Bioactive Products
- Volatile terpenoids as potential drug leads in Alzheimer’s disease
- Topical Issue on Research for Natural Bioactive Products
- Essential Oils as Immunomodulators: Some Examples
- Topical Issue on Research for Natural Bioactive Products
- Phenolic profiling and therapeutic potential of local flora of Azad Kashmir; In vitro enzyme inhibition and antioxidant
- Topical Issue on Research for Natural Bioactive Products
- Chemical profile, antioxidant activity and cytotoxic effect of extract from leaves of Erythrochiton brasiliensis Nees & Mart. from different regions of Europe

