Abstract
The dissolution of alkaline-earth metal carbonate in aqueous picric acid followed by reaction with nicotinamide results in the formation of [M(H2O) n (nic)2(pic)2] (nic = nicotinamide; pic = picrate; n = 1 and M = Ba 1; n = 2 and M = Ca (or Sr) 2 (or 3)). In [Ba(H2O)(nic)2(pic)2] 1, the barium and the oxygen atoms of a terminal aqua ligand are located on a two-fold axis. Compound 1 exhibits a {BaO7N2} coordination sphere, where the barium atom is bonded to a unique bidentate picrate and the crystallographically independent nicotinamide bridges to two symmetry related barium atoms with a Ba···Ba separation of 9.799 Å via the pyridine nitrogen and the amide oxygen atoms leading to the formation of a two-dimensional coordination polymer. The compounds 2 and 3 are isostructural with discrete molecules. The central Ca atom in 2 (or Sr in 3) located on a two-fold axis is bonded to a crystallographically unique terminal aqua ligand, an independent monodentate nicotinamide and a unique bidentate picrate anion resulting in a distorted {MO8} polyhedron. The mixed ligand alkaline-earth metal picrates 1–3 exhibit three varieties of hydrogen bonding and π–π stacking interactions. Several alkaline-earth metal picrates are compared in this study.
1 Introduction
The past two decades have witnessed a rapid growth of publications in the area of alkaline-earth metal chemistry. Synthetic methodologies, crystal structures, important properties and related applications of alkaline-earth metal compounds are described in a recent review article titled, “Chemistry of alkaline earth metals: It is not all ionic and definitely not boring!” by Fromm [1]. The relevance of the present work on the mixed ligand alkaline-earth metal picrates can be evidenced by the following statement from Fromm’s review which is quoted verbatim “While magnesium and calcium are the best studied among the alkaline earth metal compounds, the heavier alkaline earth metals have not yet been studied in detail, and recent results show the potential progress to be done” [1].
Several research groups are currently investigating alkaline-earth based materials with a view to unravel the rich chemistry of the structurally flexible alkaline-earths [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]. The lighter element Be (which is known to be toxic) adopts mainly a tetrahedral coordination, while the biologically important Mg is known to prefer hexa-coordination in a majority of its compounds. In contrast, the heavier congeners namely Ca, Sr and Ba are structurally flexible and exhibit variable coordination numbers as evidenced by several structurally characterized alkaline-earth materials listed in the Cambridge Structural Database (CSD) [16].
A variety of synthetic methods including hydrothermal/solvothermal, mechanochemical, sonochemical processes etc. have been employed for compound preparation [17], [18], [19], [20], [21], [22], [23], [24], [25]. In our alkaline-earth metal chemistry research program, we have employed an aqueous reaction protocol, which involves the dissolution of alkaline-earth metal carbonates by reaction with an acidic ligand. For this purpose, we have chosen the readily available nitroaromatics as acidic ligands and have developed an extensive chemistry of alkaline-earth metal 4-nitrobenzoates [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36]. We have demonstrated that coligands like dimethylformamide, N-methylformamide etc. can be incorporated in the coordination sphere of the alkaline-earth metals under ambient conditions resulting in the formation of coordination polymers which exhibit very short M–O(amide) (M = alkaline-earth metal) bonds [37], [38], [39]. We have extended these studies using picric acid (picH, for 2,4,6-trinitrophenol) for dissolution of metal carbonate and reported on the structure characterization of a barium coordination polymer containing a µ2-tetradentate bridging picrate [40]. The neutral molecule nicotinamide (nic) also known as niacinamide is a biologically important compound [41] and has been chosen as a coligand in the present study of the MCO3/picH/H2O reaction system. The details of the syntheses, vibrational spectra and crystal structures of three new mixed ligand alkaline-earth metal picrates [M(H2O) n (nic)2(pic)2] (n = 1 and M = Ba 1; n = 2 and M = Ca (or Sr) 2 or (3)) are described in this report.
2 Experimental
2.1 Materials and methods
All chemicals were used as received from commercial sources without any further purification. Infrared (IR) spectra of the solid samples taken up in KBr pellets were recorded on a Shimadzu (IR Prestige-21) FT-IR spectrometer in the range 4000–400 cm−1 at a resolution of 4 cm−1. Raman spectra of the solids and their aqueous solutions were measured by using an Agiltron PeakSeeker Pro Raman instrument with 785 nm laser irradiation and a laser power of 100 mW. X-ray powder patterns were recorded, on a Rigaku Miniflex II powder diffractometer using Cu-Kα radiation with a Ni filter. For single crystal structure determination, X-ray intensity data was collected on a Bruker D8 Quest Eco X-ray diffractometer, using graphite-monochromated Mo-Kα radiation. The structures were solved with Direct Methods using Shelxs-97 [42] and refinement was carried out against F2 using Shelxl-2016 [42]. All non-hydrogen atoms were refined anisotropically. The H atoms bonded to the aromatic carbon atoms and the H atoms of the amide nitrogen atoms were located in difference maps but were positioned with idealized geometry and refined isotropically with Uiso(H) = 1.2 Ueq(C,N) using a riding model. The O–H hydrogen atoms were located in difference maps, and were refined using a riding model. Technical details of data acquisition and selected refinement results for 1–3 are given in Table 1.
Crystal data and structure refinement for 1–3.
| Refinement result | Compound 1 | Compound 2 | Compound 3 |
|---|---|---|---|
| Empirical formula | C24H18BaN10O17 | C24H20CaN10O18 | C24H20N10O18Sr |
| Formula weight (g mol−1) | 855.82 | 776.58 | 824.12 |
| Temperature (K) | 293(2) | 293(2) | 293(2) |
| Wavelength (Å) | 0.71073 | 0.71073 | 0.71073 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic |
| Space group | C2/c | C2/c | C2/c |
| Unit cell dimensions | |||
| a (Å) | 16.3113(5) | 27.2850(17) | 26.985(3) |
| b (Å) | 10.8621(4) | 9.8636(6) | 10.0221(12) |
| c (Å) | 18.1809(6) | 12.5517(8) | 12.6504(15) |
| β (°) | 105.0960(10) | 113.627(2) | 111.810(4) |
| Volume (Å3) | 3110.04(18) | 3094.9(3) | 3176.4(7) |
| Z | 4 | 4 | 4 |
| Dcalc (g cm−3) | 1.83 | 1.67 | 1.72 |
| Absorption coefficient (mm−1) | 1.4 | 0.3 | 1.8 |
| F(000) (e) | 1696 | 1592 | 1664 |
| Crystal size (mm3) | 0.40 × 0.21 × 0.12 | 0.17 × 0.13 × 0.08 | 0.21 × 0.19 × 0.14 |
| θ range for data collection (°) | 2.587–27.131 | 3.037–28.311 | 2.975–28.324 |
| Limiting indices | −20 ≤ h ≤ 20 | −36 ≤ h ≤ 35 | −36 ≤ h ≤ 24 |
| −13 ≤ k ≤ 13 | −13 ≤ k ≤ 13 | −13 ≤ k ≤ 13 | |
| −23 ≤ l ≤ 23 | −14 ≤ l ≤ 16 | −16 ≤ l ≤ 13 | |
| Reflections collected/unique | 27075/3435 [R(int) = 0.0215] | 13381/3843 [R(int) = 0.0427] | 6637/3639 [R(int) = 0.0349] |
| Completeness θ = 25.242° | 99.70 | 99.90 | 93.10 |
| Refinement method | Full-matrix least-squares on F2 | ||
| Data/parameters | 3435/236 | 3843/246 | 3639/241 |
| Goodness of fit on F2 | 1.081 | 1.04 | 1.047 |
| Final R indices [I > 2σ(I)] | R1 = 0.0169 wR2 = 0.0432 | R1 = 0.0483, wR2 = 0.1007 | R1 = 0.0529, wR2 = 0.1212 |
| R indices (all data) | R1 = 0.0178 wR2 = 0.0440 | R1 = 0.0834, wR2 = 0.1158 | R1 = 0.0767, wR2 = 0.1317 |
| Largest diff. peak and hole (e Å−3) | 0.30 and −0.33 | 0.40 and −0.32 | 0.97 and −1.21 |
| CCDC deposition no | 2131550 | 2131551 | 2131552 |
2.2 Synthesis of [M(H2O) n (nic)2(pic)2] (n = 1 and M = Ba 1; n = 2 and M = Ca (or Sr) 2 or (3))
Picric acid (picH) is known to be an explosive chemical. In view of this, it is quite essential to follow appropriate safety regulations in the handling, storage and disposal of picric acid and compounds derived from it. In this study, all experiments were performed on a gram scale of picric acid. A mixture of BaCO3 (0.395 g, 2 mmol) and picH (0.916 g, 4 mmol) was taken up in water (∼30 mL) to obtain a slurry. The slurry was stirred well and brisk effervescence could be observed accompanied by the formation of a yellow solution. Slow warming of the reaction mixture on a water bath maintained at ∼60 °C resulted in the formation of a clear, bright yellow solution after ∼20 min. The pH was close to neutral and the reaction mixture was filtered into a clean beaker containing an aqueous solution of nicotinamide (0.244 g, 2 mmol; ∼5 mL) and left undisturbed for crystallization. The yellow crystalline product was isolated by filtration, washed with ether and air-dried (yield: 1.22 g, 71%). The use of CaCO3 (0.200 g, 2 mmol) or SrCO3 (0.295 g, 2 mmol) instead of BaCO3 afforded compounds 2 and 3 in good yields (1.08 g (78%) for 2 and 1.05 g for 3 (72%)).
Compound 1 IR data (KBr cm−1): 3603(s), 3444(s), 3344(m), 3089(m), 1666(s), 1614(s), 1556(s), 1514(s), 1477(s), 1427(s), 1334(s), 1259(s), 1163(s), 1080(s), 1031(s), 968(w), 912(s), 831(m), 788(s), 744(m), 702(s), 642(w), 584(m), 518(w); Raman (cm−1): 1554(w), 1333(s), 1309(s), 1159(w), 941(w), 823(m), 719(w).
Compound 2 IR data (KBr cm−1): 3626(s), 3469(s), 3323–2500(br), 1693(s), 1612(s), 1565(s), 1514(w), 1487(w), 1429(s), 1334(s), 1267(s), 1163(s), 1080(m), 1029(m), 939(m), 914(s), 835(w), 790(s), 744(s), 704(s), 642(m), 582(w), 522(m); Raman (cm−1): 1554(w), 1314(s), 1164(w), 941(w), 823(m), 702(w).
Compound 3 IR data (KBr cm−1): 3624(s), 3473(s), 3361–2500(br), 1693(s), 1614(s), 1568(s), 1514(w), 1487(w), 1427(s), 1363(m), 1334(s), 1261(s), 1161(s), 1080(m), 1029(m), 939(w), 912(s), 835(w), 790(s), 744(s), 704(s), 6940(m), 520(m); Raman (cm−1): 1559(w), 1309(s), 1159(w), 941(w), 823(m), 702(w).
3 Results and discussion
3.1 Description of the crystal structures of compounds 1–3
The three compounds (1–3) described in this study crystallize in the centrosymmetric monoclinic space group C2/c. The crystal structures consist of an alkaline-earth metal located on a two-fold axis, a terminal aqua ligand, a crystallographically independent picrate anion and a unique nicotinamide moiety. The geometric parameters of the organic ligands (Table S1) in 1–3 are in the normal range [16, 43]. In the monoaqua compound [Ba(H2O)(nic)2(pic)2] 1, the barium atom and the oxygen atom O1 of the water molecule are situated on a two-fold axis.
The central Ba atom is bonded to a terminal aqua ligand (O1), two symmetry related picrate ligands via the phenolate (O3) and a nitro oxygen atom (O4), four symmetry related nicotinamide ligands via their amide oxygen atom (O2) and the pyridine nitrogen atom (N2) resulting in a {BaO7N2} coordination polyhedron (Figure 1). The Ba–O/N bond lengths are scattered in a wide range between 2.6793(11) and 2.9329(14) Å (Table 2). The amide oxygen atom makes the shortest Ba1–O2 bond which is shorter than the Ba1–O3(phenolate) and Ba1–O1(aqua) bond lengths at 2.7107(12) and 2.834(2) Å, respectively. The nitro oxygen atom is involved in the longest Ba1–O4 distance of 2.9282(14) Å. The Ba–N distances are slightly longer (Ba1–N2ii = 2.9329(14) Å) than the longest Ba–O distance. The distinct Ba–O/N bond lengths and O–Ba–O/N bond angles ranging from 56.38(4) to 151.66(6)° reveal that the tricapped trigonal prismatic {BaO7N2} coordination polyhedron is quite distorted (Figure 2).
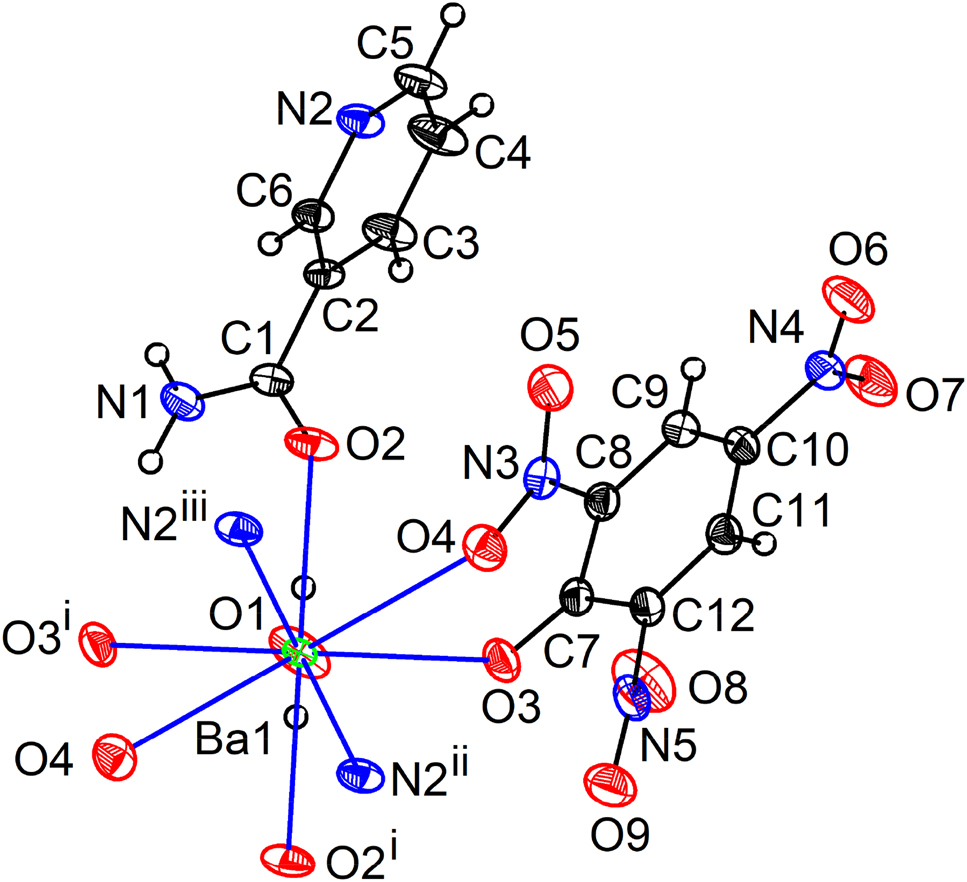
The asymmetric unit of 1 showing nine-fold coordination around the Ba atom. Displacement ellipsoids are drawn at the 30% probability level for all non-hydrogen atoms.
Symmetry code: (i) −x+1, y, −z+3/2 (ii) x+1/2, y+1/2, z (iii) −x+1/2, y+1/2, −z+3/2.
Selected geometric parameters for 1–3.
| Bond lengths and bond angles (Å, °) of {BaO7N2} and {MO8} polyhedra | |||
|---|---|---|---|
| [Ba(H2O)(nic)2(pic)2] 1 | |||
| Ba1–O2 | 2.6793(11) | Ba1–O4i | 2.9281(14) |
| Ba1–O2i | 2.6794(11) | Ba1–O4 | 2.9282(14) |
| Ba1–O3 | 2.7107(12) | Ba1–N2ii | 2.9329(14) |
| Ba1–O3i | 2.7107(12) | Ba1–N2iii | 2.9329(14) |
| Ba1–O1 | 2.834(2) | Ba···Baiv | 9.799 |
| O2–Ba1–O2i | 151.66(6) | O3i–Ba1–O4 | 150.79(4) |
| O2–Ba1–O3 | 83.84(5) | O1–Ba1–O4 | 115.31(3) |
| O2i–Ba1–O3 | 85.67(4) | O4i–Ba1–O4 | 129.37(5) |
| O2–Ba1–O3i | 85.67(4) | O2–Ba1–N2ii | 133.69(4) |
| O2i–Ba1–O3i | 83.84(5) | O2i–Ba1–N2ii | 72.95(4) |
| O3–Ba1–O3i | 136.17(7) | O3–Ba1–N2ii | 92.53(5) |
| O2–Ba1–O1 | 75.83(3) | O3i–Ba1–N2ii | 124.20(4) |
| O2i–Ba1–O1 | 75.83(3) | O1–Ba1–N2ii | 144.30(3) |
| O3–Ba1–O1 | 68.09(3) | O4i–Ba1–N2ii | 67.89(4) |
| O3i–Ba1–O1 | 68.08(3) | O4–Ba1–N2ii | 71.45(4) |
| O2–Ba1–O4i | 125.37(4) | O2–Ba1–N2iii | 72.95(4) |
| O2i–Ba1–O4i | 68.32(4) | O2i–Ba1–N2iii | 133.69(4) |
| O3–Ba1–O4i | 150.79(4) | O3–Ba1–N2iii | 124.20(4) |
| O3i–Ba1–O4i | 56.38(4) | O3i–Ba1–N2iii | 92.53(5) |
| O1–Ba1–O4i | 115.31(3) | O1–Ba1–N2iii | 144.30(3) |
| O2–Ba1–O4 | 68.31(4) | O4i–Ba1–N2iii | 71.45(4) |
| O2i–Ba1–O4 | 125.37(4) | O4–Ba1–N2iii | 67.89(4) |
| O3–Ba1–O4 | 56.38(4) | N2ii–Ba1–N2iii | 71.40(6) |
| [Ca(H2O)2(nic)2(pic)2] 2 | |||
| Ca1–O2 | 2.3578(16) | Ca1–O1 | 2.4141(17) |
| Ca1–O2i | 2.3578(16) | Ca1–O1i | 2.4141(17) |
| Ca1–O3 | 2.3916(14) | Ca1–O4i | 2.5391(17) |
| Ca1–O3i | 2.3916(14) | Ca1–O4 | 2.5391(17) |
| O2–Ca1–O2i | 78.94(8) | O1–Ca1–O1i | 138.07(9) |
| O2–Ca1–O3 | 80.10(6) | O2–Ca1–O4i | 122.67(6) |
| O2i–Ca1–O3 | 84.07(6) | O2i–Ca1–O4i | 135.00(5) |
| O2–Ca1–O3i | 84.07(6) | O3–Ca1–O4i | 134.56(6) |
| O2i–Ca1–O3i | 80.10(6) | O3i–Ca1–O4i | 65.57(5) |
| O3–Ca1–O3i | 159.46(8) | O1–Ca1–O4i | 76.80(6) |
| O2–Ca1–O1 | 149.98(6) | O1i–Ca1–O4i | 69.54(6) |
| O2i–Ca1–O1 | 71.73(6) | O2–Ca1–O4 | 135.00(5) |
| O3–Ca1–O1 | 102.66(6) | O2i–Ca1–O4 | 122.67(6) |
| O3i–Ca1–O1 | 84.75(6) | O3–Ca1–O4 | 65.57(5) |
| O2–Ca1–O1i | 71.73(6) | O3i–Ca1–O4 | 134.56(6) |
| O2i–Ca1–O1i | 149.98(6) | O1–Ca1–O4 | 69.54(6) |
| O3–Ca1–O1i | 84.75(6) | O1i–Ca1–O4 | 76.80(6) |
| O3i–Ca1–O1i | 102.66(6) | O4i–Ca1–O4 | 72.27(8) |
| [Sr(H2O)2(nic)2(pic)2] 3 | |||
| Sr1–O2 | 2.481(3) | Sr1–O1i | 2.553(3) |
| Sr1–O2i | 2.481(3) | Sr1–O1 | 2.553(3) |
| Sr1–O3i | 2.514(2) | Sr1–O4 | 2.648(3) |
| Sr1–O3 | 2.514(2) | Sr1–O4i | 2.648(3) |
| O2–Sr1–O2i | 78.75(14) | O1i–Sr1–O1 | 138.23(12) |
| O2–Sr1–O3i | 82.01(9) | O2–Sr1–O4 | 137.23(8) |
| O2i–Sr1–O3i | 84.04(9) | O2i–Sr1–O4 | 118.73(10) |
| O2–Sr1–O3 | 84.04(9) | O3i–Sr1–O4 | 134.98(8) |
| O2i–Sr1–O3 | 82.01(9) | O3–Sr1–O4 | 62.61(8) |
| O3i–Sr1–O3 | 161.93(11) | O1i–Sr1–O4 | 77.05(9) |
| O2–Sr1–O1i | 72.46(9) | O1–Sr1–O4 | 70.37(8) |
| O2i–Sr1–O1i | 148.47(9) | O2–Sr1–O4i | 118.73(10) |
| O3i–Sr1–O1i | 104.04(9) | O2i–Sr1–O4i | 137.23(8) |
| O3–Sr1–O1i | 82.49(9) | O3i–Sr1–O4i | 62.61(8) |
| O2–Sr1–O1 | 148.47(9) | O3–Sr1–O4i | 134.98(8) |
| O2i–Sr1–O1 | 72.46(9) | O1i–Sr1–O4i | 70.37(8) |
| O3i–Sr1–O1 | 82.49(9) | O1–Sr1–O4i | 77.04(9) |
| O3–Sr1–O1 | 104.05(9) | O4–Sr1–O4i | 76.42(12) |
-
Symmetry transformations used to generate equivalent atoms: Symmetry code for 1: (i) −x+1, y, −z+3/2 (ii) x+1/2, y+1/2, z (iii) −x+1/2, y+1/2, −z+3/2 (iv) x−1/2, y−1/2, z for 2: (i) −x+1, y, −z+3/2 for 3: (i) −x+1, y, −z+1/2.
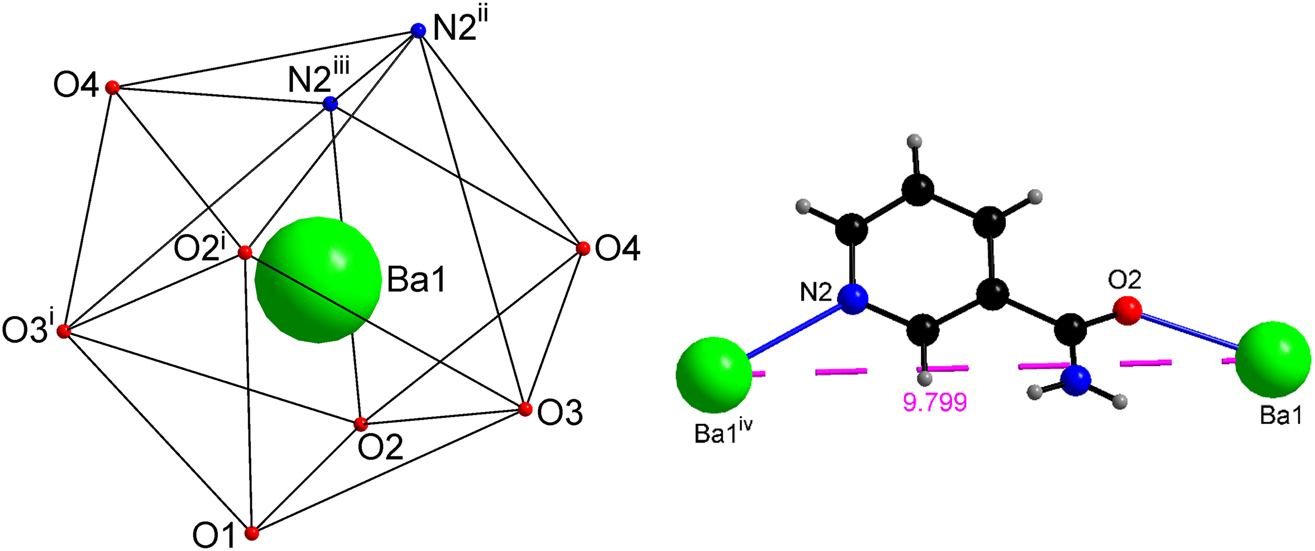
The distorted tricapped trigonal prismatic {BaO7N2} coordination polyhedron (left). The (N, O) donor nic ligand bridges two symmetry related Ba cations (right). For symmetry code see Table 2.
The O-donor aqua and picrate ligands are bound to the Ba atom in monodentate and bidentate fashion, respectively. In contrast, the unique nicotinamide functions as a (N, O) donor and bridges two symmetry related Ba cations separated by 9.799 Å (Figure 2). This bridging mode of nic via the amide oxygen and pyridine N atom organizes the Ba cations into a layer (Figure 3) in the crystallographic ab plane, resulting in the formation of a two-dimensional coordination polymer (Figure 3). In view of its special position, each Ba cation is bridged via a nic ligand to four symmetry related Ba cations and all Ba···Ba separations in the layer are identical.
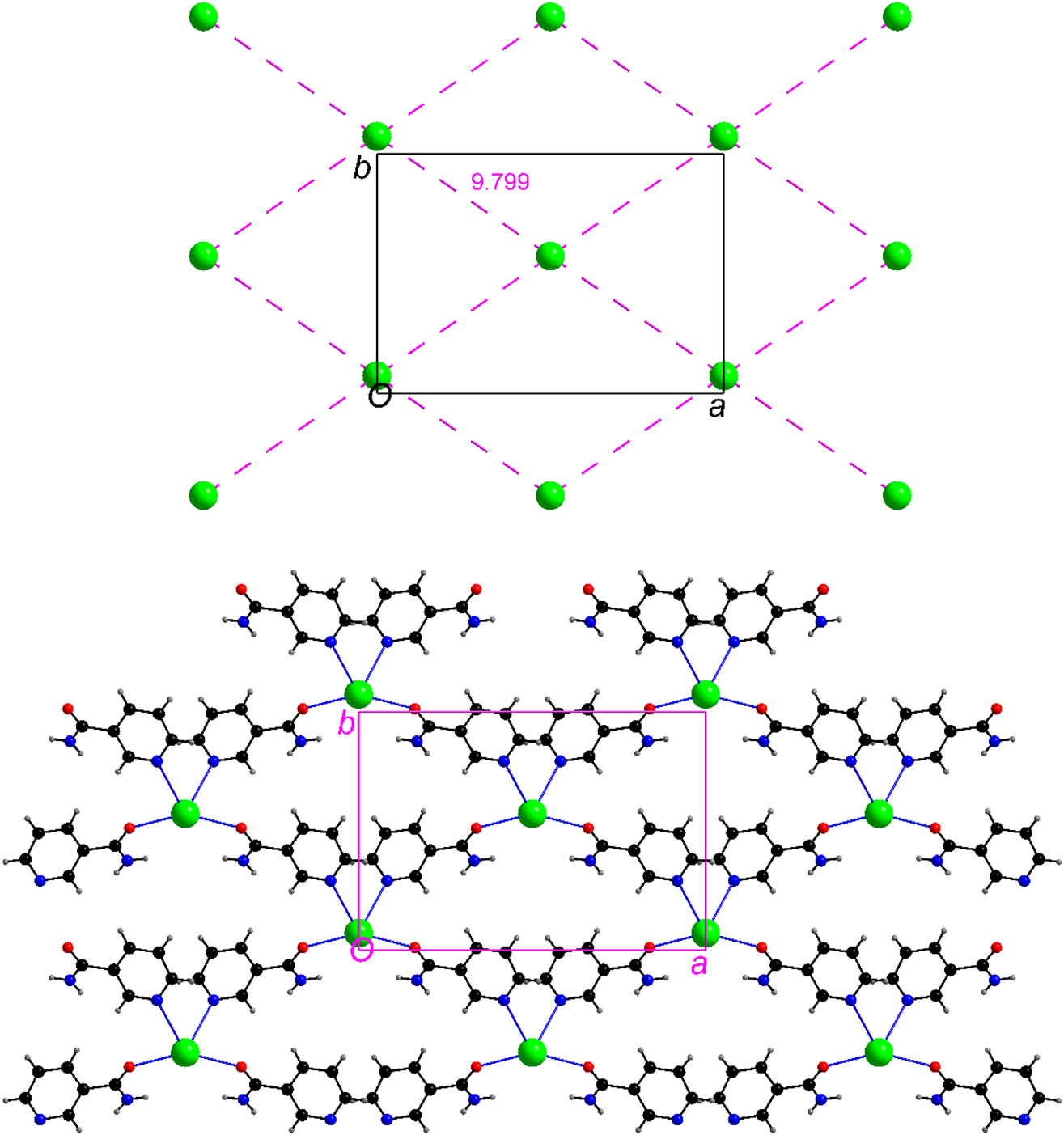
A portion of the layer of Ba cations in 1 (top). The same arrangement showing only bridging nic ligands (bottom). For clarity, terminal ligands around the Ba atoms (Figure S1) are not shown.
Each Ba cation in the layer is further bonded to a terminal aqua and two symmetry related picrate ligands (Figure S1) accounting for the remaining five coordination sites. In view of the μ2-bridging coordination, the {BaO7N2} polyhedra are discrete (Figure 4).
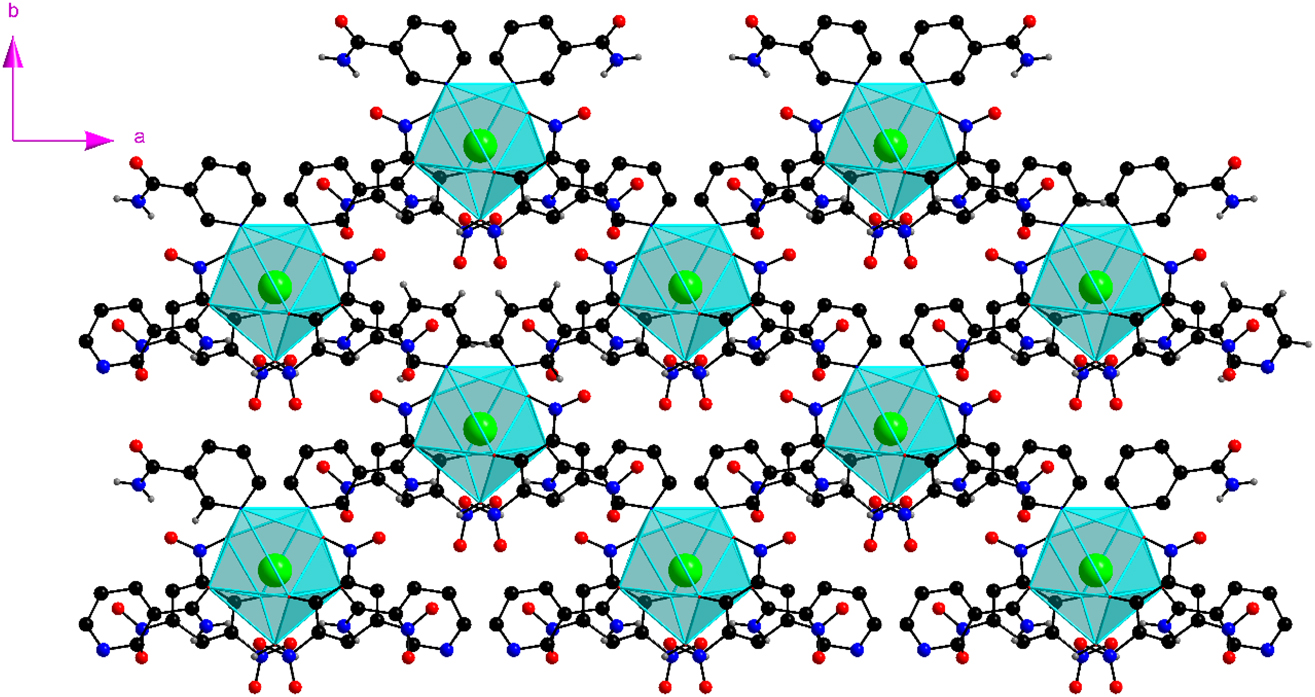
The discrete {BaO7N2} polyhedra in 1. For clarity, the H atoms of the aromatic ring are not shown.
Unlike the monoaqua compound 1, which is a two-dimensional coordination polymer, the diaqua compounds [M(H2O)2(nic)2(pic)2] (M = Ca (or Sr) 2 (or 3)) are monomers and are isostructural. No pyridine N coordination is observed in 2 (or 3). As in 1, the water molecule and the picrate anion function as monodentate and bidentate O-donor ligands, respectively. However, the nicotinamide in 2 (or 3) functions as a monodentate O-donor ligand and is bonded to Ca (or Sr) atoms via the amide oxygen atom (O2) of the unique nicotinamide ligand resulting in a {MO8} coordination polyhedron (Figure 5, Figure S2). The absence of any bridging ligands in 2 (or 3) explains their discrete nature.
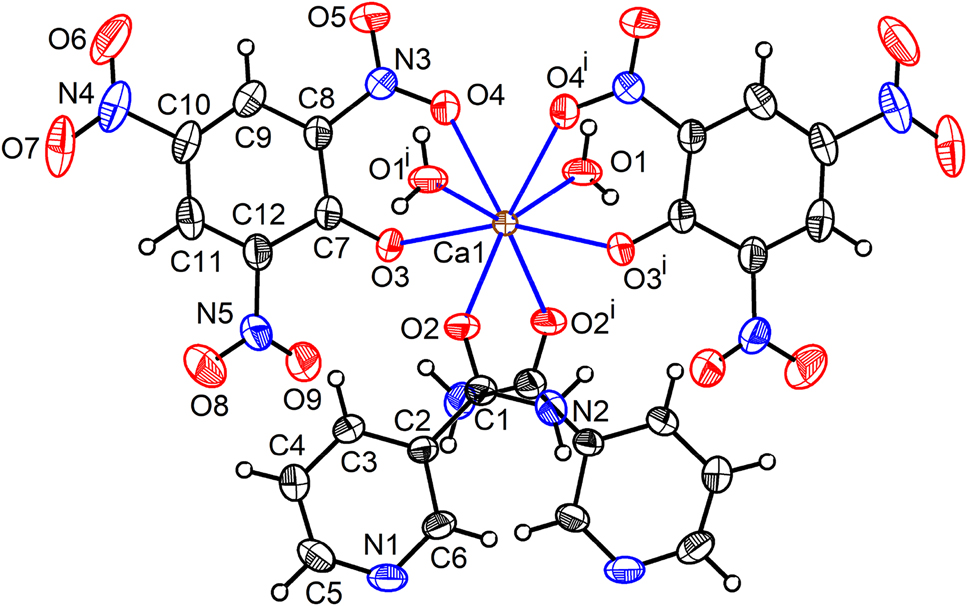
The crystal structure of 2 showing eight-fold coordination around the Ca atom. Displacement ellipsoids are drawn at 50% probability level for all the non-hydrogen atoms.
Symmetry code: (i) −x+1, y, −z+3/2. For the crystal structure of the isostructural 3 see Figure S2.
The amide oxygen atom makes the shortest M–O2 bond (Ca1–O2 = 2.3578(16); Sr1–O2 = 2.481(3) Å) while the nitro oxygen atom exhibits the longest M–O4 bond length (Ca1–O4 = 2.5391(17); Sr1–O4 = 2.648(3) Å). The M–O3(phenolate) and M–O1(aqua) distances in the isostructural molecuels 2 and 3 are intermediate between the shortest and longest M–O bonds as in 1, with the M–O4 bond slightly longer than M–O3 (Table 2). The distinct M–O bond lengths and a wide range of bond angles (65.57(5)–159.46(8)° in 2; 62.61(8)–161.93(11)° in 3) indicate that the square-antiprismatic {MO8} coordination polyhedron is distorted (Figure S3). The amide oxygen atom is involved in the shortest bond in the three compounds 1–3 described in this study. A similar observation has been made by us in our studies of alkaline-earth 4-nitrobenzoates [38], [39], [40].
In all three compounds 1–3, the H atoms of the aqua ligands, the hydrogen atoms bonded to the nitrogen atom of the amine group of nic and the H atoms attached to the carbon atoms of the pyridine ring (C4, C5 and C6 in 1; C6 in 2 (or 3)) of the nic ligand function as H-donors, while the phenolate O (O3) and the oxygen atoms of the nitro groups of the picrate anion function as hydrogen bond acceptors resulting in three varieties of H-bonding interactions (Table S2). An analysis of the short ring interactions [44] in 1–3 was performed using the program Platon [45], to determine the ring centroid to ring centroid distances (Cg···Cg) between adjacent aromatic rings. The values of these are found to be in the range 4.5669(2)–5.9138(2) Å in 1, (3.5476(2)–5.2823(3) Å in 2 and 3.5435(4)–5.9676(7) Å in 3) (Table S3; Figures S4–S6). As it has been reported that stacking interactions between aromatic rings can exist at a very long Cg···Cg distances [46], the observed data reveal the presence of π···π stacking in crystals of 1–3.
Several alkaline-earth metal picrates [47], [48], [49], [50], [51], [52], [53], [54] have already been structurally characterized (Table 3). An analysis of these structures and their atomic coordinates available in the CSD reveals a rich structural chemistry of this group of compounds. The free ligand picric acid which crystallizes in the polar space group Pca21 [55] forms several picrates, a majority of which crystallize in centrosymmetric space groups [16]. This trend can also be observed in alkaline-earth metal picrates. In addition to functioning as a charge balancing anion, the picrate anion is bonded to the alkaline-earth metal cations except for the case of [Mg(H2O)6](pic)2·3H2O. In this compound containing an octahedral [Mg(H2O)6]2+ moiety, the picrate is outside the coordination sphere of the cation. In [Ca(H2O)(teg)(pic)](pic)·3H2O a picrate anion is not bonded to Ca cation while the second one is coordinated in a bidentate manner. The bidentate (η2) binding mode of picrate, which involves the coordination of the phenolic oxygen atom and an oxygen atom of an ortho nitro group is observed in 12 of the 14 compounds in Table 3, especially in all the calcium picrates. Two barium picrates (entry no. 10, 11) containing two crystallographically unique picrates exhibit both monodentate (η1) and bidentate binding modes (η2).
Binding mode of picrate units in alkaline-earth metal compounds.
| No. | Compound | Space group | Coordination sphere | Binding mode of picrate | D a | Ref |
|---|---|---|---|---|---|---|
| 1 | [Mg(H2O)6](pic)2·3H2O | P21/c | {MgO6} | uncoordinated | Mb | [47] |
| 2 | [Ca(H2O)4(pic)2]·H2O | Pmab | {CaO8} | bidentate (η2-O,O′) | Mb | [48] |
| 3 | [Ca(H2O)4(pic)2](L) | C2/c | {CaO8} | bidentate (η2-O,O′) | Mb | [49] |
| 4 | [Ca(byp)2(pic)2] | Pbca | {CaO4N4} | bidentate (η2-O,O′) | Mb | [50] |
| 5 | [Ca(H2O)(teg)(pic)]picc |
|
{CaO8} | uncoordinated bidentate (η2-O,O′) | [51] | |
| 6 | [Ca(H2O)2(nic)2(pic)2] 2 | C2/c | {CaO8} | bidentate (η2-O,O′) | Mb | This work |
| 7 | [Sr(H2O)4(pic)2]·H2O | C2/c | {SrO8} | bidentate (η2-O,O′) | 1D | [48] |
| 8 | [Sr(H2O)2(nic)2(pic)2] 3 | C2/c | {SrO8} | bidentate (η2-O,O′) | Mb | This work |
| 9 | [Ba(H2O)5(pic)2]·H2Oc |
|
{BaO10} | bidentate (η2-O,O′) tridentate (μ2-O,O′,O′′) | 1D | [48] |
| 10 | [Ba(ace)(phen)2(pic)2]c | Pbca | {BaO4N4} | monodentate (η1)bidentate (η2-O,O′) | Mb | [52] |
| 11 | [Ba(H2O)2(dbc)(pic)2]c |
|
{BaO10} | monodentate (η1)bidentate (η2-O,O′) | Mb | [53] |
| 12 | [Ba(L1)(pic)2] |
|
{BaO9N2} | bidentate (η2-O,O′) | Mb | [54] |
| 13 | [Ba(DMSO)(pic)2] | P21/m | {BaO10} | tetradentate (μ2-O,O,O′,O′′) | 1D | [40] |
| 14 | [Ba(H2O)(nic)2(pic)2] 1 | C2/c | {BaO7N2} | bidentate (η2-O,O′) | 2D | This work |
-
aD = Dimensionality; bM = discrete monomer pic = picrate; L = 18-crown-6; bpy = 2,2′-Bipyridyl; teg = tetraethylene glycol; ace = acetone; phen = 1,10-phenanthroline; dbc = dibenzo-24-crown-8; L1 = diaza 21-crown-7 ether; nic = nicotinamide; ctwo crystallographically unique picrates.
A survey of the metal coordination polyhedra in these compounds reveals many interesting features. Ten of the compounds are bonded to only O-donor ligands while in four cases including 1, coordination of N is observed. The Mg compound exhibits six-fold coordination while all Ca and Sr compounds exhibit eight-fold coordination. In the case of Ba the coordination number ranges from 8 (entry no. 10) to 11 in the Ba-picrate containing the diaza21-crown-7 ether (entry no. 12). A majority (10) of the compounds are discrete monomers which includes the single Mg-picrate and the picrates of Ca, while one Sr-picrate (entry no. 7) and three Ba picrates (entry no. 9, 13 and 14) are coordination polymers. The picrate ligand exhibits a µ2-tridentate and µ2-tetradentate bridging mode, respectively, in the water-rich [Ba(H2O)5(pic)2]·H2O (entry no. 9) and the anhydrous [Ba(DMSO)(pic)2] (entry no. 13) coordination polymers. The µ2-tridentate binding of picrate results in discrete {BaO10} polyhedra as in 1, while the µ2-tetradentate binding of the unique picrate and the bridging DMSO ligand in [Ba(DMSO)(pic)2] results in the formation of a chain of face-sharing {BaO10} polyhedra flanked by the picrate ligands. In [Sr(H2O)4(pic)2]·H2O a bridging aqua ligand is responsible for the polymeric structure while in 1, the neutral coligand nic is responsible for the two-dimensional structure. The monoaqua Ba compound 1 is the only example of a two-dimensional coordination polymer in this series of compounds.
3.2 Synthetic aspects and spectral characteristics of 1–3
The reaction of MCO3 (M = Ca, Sr or Ba) with picric acid in water leads to a dissolution of the insoluble MCO3 to afford water-rich metal picrates (entry nos. 2, 7 and 9 in Table 3) as documented in the literature [47, 48]. In recent work we have shown that solubilization of BaCO3 in aqueous picric acid followed by reaction with DMSO results in the formation of the anhydrous compound [Ba(DMSO)(pic)2]. In this study, the synthesis of compounds 1–3 was performed as shown below.
First, the insoluble metal carbonate was dissolved in aqueous picric acid by slow warming to obtain a yellow solution which was filtered. This was further reacted with nicotinamide to obtain 1–3 as yellow crystalline materials in good yield. The products thus obtained by slow evaporation of the reaction mixture were suitable for single crystal work. In the case of M = Mg, the above reaction resulted in the formation of a fine powder. A comparison of the experimental powder pattern of the bulk samples thus obtained with the theoretical pattern (Figures S7–S9) revealed that the materials are phase pure. The IR and Raman spectra of 1–3 (Figures S10 and S11) exhibit several bands which indicate the presence of the organic moieties. The IR bands at around 3600 and 3444 cm−1 in 1 can be assigned to the stretching vibrations of the water molecule(s) OH2 and of the –NH2 group of the amide, respectively. The intense band at 1666 cm−1 in 1 can be assigned for the amide carbonyl vibration of nic which occurs at a lower energy compared to that of the free nic ligand which exhibits a strong signal at 1690 cm−1. Pure picric acid exhibits a strong signal at 1190 cm−1 for the vibration of the C–O group and this occurs at 1160 cm−1 in 1–3. The Raman spectra of all compounds exhibit an intense signal at ∼1309 cm−1, which can be assigned for the symmetric stretching vibration of the nitro functionality.
4 Conclusions
The use of the neutral nicotinamide in the MCO3/picH/H2O reaction system has resulted in the formation of three new mixed ligand alkaline-earth metal picrates 1–3. The monoaqua Ba compound [Ba(H2O)(nic)2(pic)2] 1 is a two-dimensional coordination polymer, while the isostructural diaqua compounds [M(H2O)2(nic)2(pic)2] (M = Ca 2; M = Sr 3) are discrete monomers. A comparative study of several alkaline-earth metal picrates reveals intrinsic characteristics of the structural chemistry of this group of compounds.
5 Supporting information
Deposition numbers CCDC 2131550 (1), CCDC 2131551 (2) and CCDC 2131552 contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures. Supplementary Data (Figures S1–S11) and Tables (Tables S1–S3) associated with this article are available in electronic form.
Dedicated to Professor Christian Näther on the occasion of his 60th birthday.
Funding source: University Grants Commission http://dx.doi.org/10.13039/501100001501
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Financial assistance was provided to the School of Chemical Sciences (formerly Department of Chemistry), Goa University at the level of DSA-I under the Special Assistance Programme (SAP) by the University Grants Commission, New Delhi.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Fromm, K. M. Chemistry of alkaline earth metals: it is not all ionic and definitely not boring. Coord. Chem. Rev. 2020, 408, 213193–213214; https://doi.org/10.1016/j.ccr.2020.213193.Suche in Google Scholar
2. Fromm, K. M., Gueneau, E. D. Structures of alkali and alkaline earth metal clusters with oxygen donor ligands. Polyhedron 2004, 23, 1479–1504; https://doi.org/10.1016/j.poly.2004.04.014.Suche in Google Scholar
3. Fromm, K. M. Coordination polymer networks with s-block metal ions. Coord. Chem. Rev. 2008, 252, 856–885; https://doi.org/10.1016/j.ccr.2007.10.032.Suche in Google Scholar
4. Datta, S., Gamer, M. T., Roesky, P. W. Aminotroponiminate calcium and strontium complexes. Dalton Trans. 2008, 2839–2843; https://doi.org/10.1039/b719552d.Suche in Google Scholar PubMed
5. Banerjee, D., Parise, J. B. Recent advances in s-block metal carboxylate networks. Cryst. Growth Des. 2011, 11, 4704–4720; https://doi.org/10.1021/cg2008304.Suche in Google Scholar
6. Arlin, J. B., Florence, A. J., Johnston, A., Kennedy, A. R., Miller, G. J., Patterson, K. Systematic data set for structure-property investigations: solubility and solid-state structure of alkaline earth metal salts of benzoates. Cryst. Growth Des. 2011, 11, 1318–1327; https://doi.org/10.1021/cg101547r.Suche in Google Scholar
7. Banerjee, D., Zhang, Z., Plonka, A. M., Li, J., Parise, J. B. A calcium coordination framework having permanent porosity and high CO2/N2 selectivity. Cryst. Growth Des. 2012, 12, 2162–2165; https://doi.org/10.1021/cg300274n.Suche in Google Scholar
8. Foo, M. L., Horike, S., Inubushi, Y., Kitagawa, S. An alkaline earth I3O0 porous coordination polymer: [Ba2TMA(NO3)(DMF)]. Angew. Chem. Int. Ed. 2012, 51, 6107–6111; https://doi.org/10.1002/anie.201202285.Suche in Google Scholar PubMed
9. Foo, M. L., Horike, S., Duan, J., Chen, W., Kitagawa, S. Tuning the dimensionality of inorganic connectivity in barium coordination polymers via biphenyl carboxylic acid ligands. Cryst. Growth Des. 2013, 13, 2965–2972; https://doi.org/10.1021/cg4003803.Suche in Google Scholar
10. Chanthapally, A., Quah, H. S., Vittal, J. J. Solid-state reactivity of supramolecular isomers: a study of the s-block coordination polymers. Cryst. Growth Des. 2014, 14, 2605–2613; https://doi.org/10.1021/cg500307q.Suche in Google Scholar
11. Bauer, H., Thum, K., Alonso, M., Fischer, C., Harder, S. Alkene transfer hydrogenation with alkaline-earth metal catalysts. Angew. Chem. Int. Ed. 2016, 58, 4248–4253; https://doi.org/10.1002/anie.201813910.Suche in Google Scholar PubMed
12. Balendra, Banday, A., Murugavel, S., Kanaujia, P. K., Prakash, G. V., Ramanan, A. Calcium and strontium coordination polymers based on rigid and flexible aromatic dicarboxylates: synthesis, structure, photoluminescence and dielectric properties. ChemistrySelect 2017, 2, 8567–8576; https://doi.org/10.1002/slct.201701232.Suche in Google Scholar
13. Georg, P., Das, R. K., Chowdhury, P. Facile microwave synthesis of Ca-BDC metal organic framework for adsorption and controlled release of Curcumin. Microporous Mesoporous Mater. 2019, 281, 161–171; https://doi.org/10.1016/j.micromeso.2019.02.028.Suche in Google Scholar
14. Balendra, Banday, M., Tewari, S., Singh, B., Murugavel, S., Ramanan, A. Alkaline-earth metal based coordination polymers assembled from two different V-shaped ligands: synthesis, structure, and dielectric properties. Inorg. Chim. Acta. 2019, 495, 118940; https://doi.org/10.1016/j.ica.2019.05.039.Suche in Google Scholar
15. Xian, S., Lin, Y., Wang, H., Li, J. Calcium-based metal-organic frameworks and their potential applications. Small 2021, 17, 2005165; https://doi.org/10.1002/smll.202005165.Suche in Google Scholar PubMed
16. Groom, C. R., Bruno, I. J., Lightfoot, M. P., Ward, S. C. The Cambridge structural database. Acta Crystallogr. 2016, B72, 171–179; https://doi.org/10.1107/s2052520616003954.Suche in Google Scholar
17. Dey, C., Kundu, T., Biswal, B. P., Mallick, A., Banerjee, R. Crystalline metal-organic frameworks (MOFs): synthesis, structure and function. Acta Crystallogr. 2014, B70, 3–10; https://doi.org/10.1107/s2052520613029557.Suche in Google Scholar
18. Scholz, G., Abdulkader, A., Kemnitz, E. Synthesis and crystal structure of two CuII-benzene-1,2,4,5-tetracarboxylates with three-dimensional open frameworks. Z. Anorg. Allg. Chem. 2014, 640, 310–316; https://doi.org/10.1002/zaac.201300537.Suche in Google Scholar
19. Scholz, G., Abdulkader, A., Kemnitz, E. Mechanochemical synthesis and characterization of alkaline earth metal terephthalates: M(C8H4O4)·nH2O (M = Ca, Sr, Ba). Z. Anorg. Allg. Chem. 2014, 640, 317–324; https://doi.org/10.1002/zaac.201300537.Suche in Google Scholar
20. Al-Terkawi, A. A., Scholz, G., Emmerling, F., Kemnitz, E. Mechanochemical synthesis, characterization, and structure determination of new alkaline earth metal-tetrafluoroterephthalate frameworks: Ca(pBDC-F4)·4H2O, Sr(pBDC-F4)·4H2O, and Ba(pBDC-F4). Cryst. Growth Des. 2016, 16, 1923–1933; https://doi.org/10.1021/acs.cgd.5b01457.Suche in Google Scholar
21. Li, T.-T., Cai, S.-L., Zeng, R.-H., Zheng, S.-R. Structures and luminescent properties of two new main group coordination polymers based on 2-(hydroxymethyl)-1H-imidazole-4,5-dicarboxylic acid. Inorg. Chem. Commun. 2014, 48, 40–43; https://doi.org/10.1016/j.inoche.2014.07.031.Suche in Google Scholar
22. Halake, S., Ok, K. M. Crystal growth, differential gas adsorption, high thermal stability, and reversible coordination of two new barium-organic frameworks, Ba(SBA)(DMF)4 and Ba2(BTEC)(H2O). J. Solid State Chem. 2015, 231, 132–137; https://doi.org/10.1016/j.jssc.2015.08.021.Suche in Google Scholar
23. Kelly, N. R., Goetz, S., Batten, S. R., Kruger, P. E. Coordination behaviour and network formation with 4,4′,6,6′-tetracarboxy-2,2′-bipyridine and 4,4′-dicarboxy-2,2′-bipyridine ligands with rare and alkaline earth metals. CrystEngComm 2008, 10, 68–78; https://doi.org/10.1039/b711469a.Suche in Google Scholar
24. Moghzi, F., Soleimannejad, J. Sonochemical synthesis of a new nano-sized barium coordination polymer and its application as a heterogeneous catalyst towards sono-synthesis of biodiesel. Ultrason. Sonochem. 2018, 42, 193–200; https://doi.org/10.1016/j.ultsonch.2017.11.023.Suche in Google Scholar
25. Drzewiecka-Antonika, A., Koziol, A. E., Rejmak, P., Lawniczak-Jablonska, K., Nittlera, L., Lisc, T. Novel Ba(II) and Pb(II) coordination polymers based on citric acid: synthesis, crystal structure and DFT studies. Polyhedron 2017, 132, 1–11; https://doi.org/10.1016/j.poly.2017.04.024.Suche in Google Scholar
26. Srinivasan, B. R., Sawant, S. C. Thermal and spectroscopic characterization of Mg(II) complexes of nitro-substituted benzoic acids. Thermochim. Acta 2003, 402, 45–55; https://doi.org/10.1016/s0040-6031(02)00533-6.Suche in Google Scholar
27. Srinivasan, B. R., Sawant, J. V., Raghavaiah, P. Synthesis, spectroscopy, thermal and X-ray structure studies of a seven coordinated hydrated Ca(II)-para-nitrobenzoate complex showing mono and bidentate carboxylate ligation. Indian J. Chem. 2006, 45A, 2392–2399.Suche in Google Scholar
28. Srinivasan, B. R., Sawant, J. V., Nather, C., Bensch, W. Synthesis, spectroscopy and supramolecular structures of two magnesium 4-nitrobenzoate complexes. J. Chem. Sci. 2007, 119, 243–252; https://doi.org/10.1007/s12039-007-0032-6.Suche in Google Scholar
29. Srinivasan, B. R., Raghavaiah, P., Sawant, J. V. Heptaaqua(4-nitrobenzoato-κ2O,O’)-strontium(II) 4-nitrobenzoate dehydrate. Acta Crystallogr. 2007, E63, m2251–m2252; https://doi.org/10.1107/s1600536807036872.Suche in Google Scholar
30. Srinivasan, B. R., Sawant, J. V., Raghavaiah, P. Synthesis, spectroscopy, thermal studies and supramolecular structures of two new alkali-earth 4-nitrobenzoate complexes containing coordinated imidazole. J. Chem. Sci. 2007, 119, 11–20; https://doi.org/10.1007/s12039-007-0003-y.Suche in Google Scholar
31. Srinivasan, B. R., Sawant, J. V., Sawant, S. C., Raghavaiah, P. Catena-Poly[[(pentaaqua)(4-nitrobenzoato-O,O′)barium(II)](μ-4-nitrobenzoato-O,O′)]: a barium(II) coordination polymer showing O–H⋅⋅⋅O and C–H⋅⋅⋅O interactions. J. Chem. Sci. 2007, 119, 593–601; https://doi.org/10.1007/s12039-007-0074-9.Suche in Google Scholar
32. Srinivasan, B. R., Shetgaonkar, S. Y., Sawant, J. V., Synthesis, Raghavaiah. P. X-ray structure and properties of a calcium(II) coordination polymer showing μ2-η1:η1 and μ3-η2:η1 coordination modes of 4-nitrobenzoate. Polyhedron 2008, 27, 3299–3305; https://doi.org/10.1016/j.poly.2008.07.020.Suche in Google Scholar
33. Srinivasan, B. R., Shetgaonkar, S. Y., Näther, C., Bensch, W. Solid state synthesis and characterization of a triple chain calcium(II) coordination polymer showing two different bridging 4-nitrobenzoate coordination modes. Polyhedron 2009, 28, 534–540; https://doi.org/10.1016/j.poly.2008.11.022.Suche in Google Scholar
34. Srinivasan, B. R., Shetgaonkar, S. Y., Näther, C. Preparation, crystal structures, and thermal studies of alkaline earth nitrocarboxylates. Z. Anorg. Allg. Chem. 2011, 637, 130–136; https://doi.org/10.1002/zaac.201000214.Suche in Google Scholar
35. Srinivasan, B. R., Shetgaonkar, S. Y., Saxena, M., Näther, C. A new calcium(II) coordination polymer based on a μ2-bridging tridentate 4-nitrobenzoate. Indian J. Chem. 2012, 51A, 435–443.Suche in Google Scholar
36. Srinivasan, B. R., Raghavaiah, P., Dhavskar, K. T. Structural characterization of catena-[bis(μ-4-nitrobenzoato)-diaqua-calcium 4,4’-bipyridine] and catena-[bis(μ-4-nitrobenzoato)-diaqua-calcium 1H-1,2,4-triazole]. Indian J. Chem. 2021, 60A, 785–796.Suche in Google Scholar
37. Srinivasan, B. R., Dhavskar, K. T. On the syntheses and structures of two calcium coordination polymers containing terminal amide ligands. Indian J. Chem. 2017, 56A, 387–393.Suche in Google Scholar
38. Bhargao, P. H., Srinivasan, B. R. Synthesis and structural characterization of a barium coordination polymer based on a μ2-monoatomic bridging 4-nitrobenzoate. J. Coord. Chem. 2019, 72, 2599–2615; https://doi.org/10.1080/00958972.2019.1666980.Suche in Google Scholar
39. Parsekar, N. U., Bhargao, P. H., Näther, C., Bensch, W., Srinivasan, B. R. Synthesis and structural characterization of three new strontium(II) coordination polymers based on 4-nitrobenzoate. J. Inorg. Organomet. Polym. Mater. 2021, 32, 200–215; https://doi.org/10.1007/s10904-021-02097-9.Suche in Google Scholar
40. Srinivasan, B. R., Parsekar, N. U., Narvekar, K. U. Catena-poly(μ2-dimethylsulfoxide)bis(μ2-2,4,6-trinitrophenolato)barium(II). IUCrData 2020, 5, x201498; https://doi.org/10.1107/s2414314620014984.Suche in Google Scholar
41. MacKay, D., Hathcock, J., Guarneri, E. Niacin: chemical forms, bioavailability, and health effects. Nutr. Rev. 2012, 70, 357–366; https://doi.org/10.1111/j.1753-4887.2012.00479.x.Suche in Google Scholar PubMed
42. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar PubMed PubMed Central
43. Srinivasan, B. R., Parsekar, N. U., Apreyan, R. A., Petrosyan, A. M. On the second harmonic generation activity in centrosymmetric crystals. Mol. Cryst. Liq. Cryst. 2019, 680, 75–84; https://doi.org/10.1080/15421406.2019.1624027.Suche in Google Scholar
44. Hunter, C. A., Sanders, J. K. M. The nature of π-π interactions. J. Am. Chem. Soc. 1990, 112, 5525–5534; https://doi.org/10.1021/ja00170a016.Suche in Google Scholar
45. Spek, A. L. Structure validation in chemical crystallography. Acta Crystallogr. 2009, D65, 148–155; https://doi.org/10.1107/s090744490804362x.Suche in Google Scholar
46. Ninković, D. B., Janjić, G. V., Veljković, D. Z., Sredojević, D. N., Zarić, S. D. What are the preferred horizontal displacements in parallel aromatic–aromatic interactions? Significant interactions at large displacements. ChemPhysChem 2011, 12, 3511–3514; https://doi.org/10.1002/cphc.201100777.Suche in Google Scholar PubMed
47. Harrowfield, J. M., Skelton, B. W., White, A. H. Structural studies of alkaline earth metal picrates. Aust. J. Chem. 1995, 48, 1333–1347; https://doi.org/10.1071/ch9951333.Suche in Google Scholar
48. Diakiw, V., Hambley, T., Kepert, D., Raston, C., White, A. Crystal structure of calcium picrate pentahydrate: a new eight-coordinate stereochemistry for [M(bidentate)2(unidentate)4]. Aust. J. Chem. 1979, 32, 301–309; https://doi.org/10.1071/ch9790301.Suche in Google Scholar
49. Chekhlov, A. N. Koordinatsionna Khim. 2001, 27, 809; https://doi.org/10.1023/a:1012558820726.10.1023/A:1012558820726Suche in Google Scholar
50. Poonia, N. S., Chandra, R., Padmanabhan, V. M., Yadav, V. S. Coordination chemistry of alkali and alkaline earth cations: X-ray structural analysis of calcium(picrate)2(2,2′-bipyridyl)2. J. Coord. Chem. 1989, 21, 167–174.10.1080/00958979009409185Suche in Google Scholar
51. Singh, T. P., Reinhardt, R., Poonia, N. S. Crystal & molecular structure of calcium (picrate)2-tetraethylene glycol monohydrate. Indian J. Chem. 1984, 23A, 976–982.Suche in Google Scholar
52. Postma, R., Kanters, J. A., Duisenberg, A. J. M., Venkatasubramanian, K., Poonia, N. S. Structure of bis(1,10-phenanthroline)bis(2,4,6-trinitrophenolato)barium(II) acetone (1:1), [Ba(C12H8N2)2(C6H2N3O7)2].C3H6O. Acta Crystallogr. 1983, C39, 1221–1225; https://doi.org/10.1107/s0108270183008008.Suche in Google Scholar
53. Hughes, D. L., Wingfield, J. N. Crystal structure of a complex showing simultaneous water-barium ion co-ordination to 6,7,9,10,12,13,20,21,23,24,26,27-dodecahydrodibenzo[b,n][1,4,7,10,13,16,19,22] octaoxacyclotetracosin (dibenzo-24-crown-8). J. Chem. Soc. Chem. Commun. 1977, 804–805; https://doi.org/10.1039/c39770000804.Suche in Google Scholar
54. Chandler, C. J., Gable, R. W., Gulbis, J. M., Mackay, M. F. Preparation and structural characterization of a diaza 21-crown-7 ether complex with barium picrate - (3,6,9,12,15-pentaoxa-26,29-diazatetracyclo[15.8.4.020,28.023,27]-nonaco sa-17,19,21,23,25(1),26,28-heptaene-2,16-dione)barium(II) picrate. Aust. J. Chem. 1988, 41, 799–806; https://doi.org/10.1071/ch9880799.Suche in Google Scholar
55. Srikrishnan, T., Soriano-Garcia, M., Parthasarathy, R. Orientation and intramolecular hydrogen bonding of nitro groups in the crystal structure of picric acid C6H3N3O7. Z. Kristallogr. 1980, 151, 317–323.10.1524/zkri.1980.151.3-4.317Suche in Google Scholar
Supplementary Material
The online version of this article offers supplementary material (https://doi.org/10.1515/znb-2022-0003).
© 2022 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- In this issue
- Laudatio/Preface
- Christian Näther zum 60. Geburtstag gewidmet
- Research Articles
- Bismuth-rich bimetallic clusters (CuBi8)3+ and [MBi10]4+ (M = Pd, Pt) from ionothermal synthesis
- Crystal structure of phenanthrenide salts stabilized by 15-crown-5 and 18-crown-6
- Structure and properties of two new heteroleptic bismuth(III) dithiocabamates of the general composition Bi(S2CNH2)2X (X = Cl, SCN)
- Synthesis and structural characterization of three new mixed ligand alkaline-earth metal picrates
- Dimorphism of MnHAsO4(H2O): natural monoclinic krautite and its synthetic triclinic modification
- Synthesis, crystal structure, and topology of a polycatenated bismuth coordination polymer
- The unexpected crystal structure of thallium(I) tricyanomethanide Tl[C(CN)3]
- Synthesis, structure characterization and properties of a new oxidovanadium(IV) coordination polymer incorporating bridging (MoO4)2– and (Mo8O26)4– ligands
- Crystal structure of Dy11Ge4.33In5.67 and Tm11Ge4In6 from X-ray single-crystal and powder data
- Crystallisation of phosphates revisited: a multi-step formation process for SrHPO4
- Oxygen evolving reactions catalyzed by different manganese oxides: the role of oxidation state and specific surface area
- Synthesis and structural characterization of a new heterometallicmolybdate coordination polymer based on a µ3-bridging amino alcohol
- Chemically and Light-Driven Coordination-Induced Spin State Switching (CISSS) of a nonheme-iron complex
- Extracting information from X-ray diffraction patterns containing Laue oscillations
- Gadolinium trisilicide − a paramagnetic representative of the YbSi3 type series
Artikel in diesem Heft
- Frontmatter
- In this issue
- Laudatio/Preface
- Christian Näther zum 60. Geburtstag gewidmet
- Research Articles
- Bismuth-rich bimetallic clusters (CuBi8)3+ and [MBi10]4+ (M = Pd, Pt) from ionothermal synthesis
- Crystal structure of phenanthrenide salts stabilized by 15-crown-5 and 18-crown-6
- Structure and properties of two new heteroleptic bismuth(III) dithiocabamates of the general composition Bi(S2CNH2)2X (X = Cl, SCN)
- Synthesis and structural characterization of three new mixed ligand alkaline-earth metal picrates
- Dimorphism of MnHAsO4(H2O): natural monoclinic krautite and its synthetic triclinic modification
- Synthesis, crystal structure, and topology of a polycatenated bismuth coordination polymer
- The unexpected crystal structure of thallium(I) tricyanomethanide Tl[C(CN)3]
- Synthesis, structure characterization and properties of a new oxidovanadium(IV) coordination polymer incorporating bridging (MoO4)2– and (Mo8O26)4– ligands
- Crystal structure of Dy11Ge4.33In5.67 and Tm11Ge4In6 from X-ray single-crystal and powder data
- Crystallisation of phosphates revisited: a multi-step formation process for SrHPO4
- Oxygen evolving reactions catalyzed by different manganese oxides: the role of oxidation state and specific surface area
- Synthesis and structural characterization of a new heterometallicmolybdate coordination polymer based on a µ3-bridging amino alcohol
- Chemically and Light-Driven Coordination-Induced Spin State Switching (CISSS) of a nonheme-iron complex
- Extracting information from X-ray diffraction patterns containing Laue oscillations
- Gadolinium trisilicide − a paramagnetic representative of the YbSi3 type series

