Abstract
Hydrothermal synthesis, crystal structure and properties of a new heterometallic coordination polymer [(VO(terpy))4(MoO4)2(Mo8O26)]·2H2O (1) (terpy = 2,2′;6′,2″-terpyridine) are reported. Compound 1 contains two crystallographically unique vanadium(IV) atoms, bonded to a terminal oxido ligand and further coordinated to a terpy ligand. The three N atoms of terpy occupy the meridional sites of a distorted {VN3O3} octahedron. A γ-octamolybdate (Mo8O26)4– located on an inversion centre and a tetraoxidomolybdate (MoO4)2– function as bridging ligands. The μ3-bridging tridentate binding of (MoO4)2– leads to the formation of a {V4Mo2O12}4+ cationic unit consisting of an eight-membered heterometallic {Mo2V2O4} ring with protruding oxidovanadium handles. A pair of {V4Mo2O12}4+ units are bridged by the centrosymmetric (Mo8O26)4– ligand, resulting in the formation of an infinite chain of alternating {V4Mo2O12}4+ cations and (Mo8O26)4– anions.
1 Introduction
Polyoxometalates (POMs) are discrete anionic transition metal clusters having unique topologies, nuclearities and electronic versatilities. The isopoly- and heteropolyanion clusters usually contain symmetrical core assemblies of metal-oxido motifs (M = V, Mo, W) and often adopt quasi-spherical structures of considerable topological interest [1, 2]. POMs are formed in condensation processes of simple anions in solution under different temperature and pH (mainly acidic) conditions [3, 4]. Due to their promising biological applications [5, 6] POMs are actively used as metallodrugs. In recent years, they are gaining importance for their antitumor, antiviral, antibacterial, anticancer activities [6]. In addition, these compounds exhibit remarkable redox properties and thus find potential applications in several areas viz. catalysis, medicine, electrochemistry, magnetic materials etc. [7], [8], [9], [10], [11], [12]. The concept of hybrid polyoxometalates has emerged in recent years. These post-functionalizable hybrid-POMs serve as building blocks due to structural diversity in such a way that they allow coupling of properties of POMs with different organic and inorganic metal cation functionalities making them useful in (photo) catalysis, bioinorganic chemistry and material science [13, 14]. The structural, electronic and magnetic properties of well-known polyoxovanadates (POVs), such as the robust [VIV 18O42]12− polyoxoanion can be altered by replacement of V by elements like Si-, Ge-, As- or Sb to form decorated heteroPOVs. The four general families of POVs are: fully-oxidised (VV), mixed-valent (VV/VIV or VIV/VIII), “fully-reduced” (VIV) and “highly-reduced” (VIII) species [15], [16], [17].
The chemistry of POVs is dominated by the decavanadate anion [V10O28]6– containing the fully oxidised VV ion [18], [19], [20] and several examples of structurally characterized decavanadates charge balanced by a variety of counter cations are listed in the Cambridge Structural Database [21]. As in the case of other early transition metals like Mo and W [22, 23], decavanadates can be readily obtained by treatment of V2O5 with a limited amount of base [24] or by acidification of tetraoxidovanadate/metavanadate solution in the presence of appropriate counter cations [25], [26], [27], [28]. Unlike decavanadates, the fully reduced POVs containing VIV are air-sensitive as evidenced by the report on the Na+ or K+ salt of [VIV 18O42]12− [29]. In this spherical polyoxoanion each of the eighteen V4+ cations is coordinated by five oxygen atoms, one of which is unshared pointing outwards of the cluster shell, which is a typical structural feature in the chemistry of the vanadyl [VO]2+ moiety. The stringent air-free conditions required to access V4+ species in aqueous chemistry can be overcome in hydrothermal reactions by use of a N-donor ligand as structure directing agent as evidenced by the synthesis of the heterometallic compounds [VO(η1-MoO4)(tren)]·H2O(tren = tris-2-aminoethylamine), [VO(μ3-MoO4)(bpy)] (bpy = 2,2′-bipyridine) etc. [30], [31], [32]. During the course of our investigations aimed at the synthesis of mixed metal hybrid organic compounds, we obtained a new heterometallic coordination polymer [(VO(terpy))4(MoO4)2(Mo8O26)]·2H2O (1), (terpy = 2,2′;6′,2″-terpyridine) containing bridging γ-octamolybdate (Mo8O26)4– and tetraoxidomolybdate (MoO4)2– ligands. Details of the hydrothermal synthesis, spectral characterization and structure of 1 are described in this report.
2 Results and discussion
2.1 Synthetic aspects, spectral and thermal studies
The oxidovanadium(IV) containing compound 1 was prepared by a hydrothermal reaction of molybdic acid, sodium metavanadate and 2,2′:6′,2′′-terpyridine as dark green crystalline blocks. A comparison of the experimental powder pattern of a bulk sample of 1 with that of the calculated pattern (Figure S1) reveals the formation of a phase pure product. In this reaction the N-donor serves as a structure directing ligand as well as a reducing agent. The formation of a [VO]2+ containing compounds starting from V5+ reactants in the presence of N-donor ligands under hydrothermal conditions is a well-known synthetic methodology in the literature [30, 32]. The presence of V4+ is confirmed by the characteristic ESR signal centred at g = 1.987 of a polycrystalline sample of 1 (Figure S2). The infrared (IR) and Raman spectra (Figures S3 and S4) of 1 exhibit several characteristic bands. The broad IR band centred at around 3410 cm−1 and the signal at 1609 cm−1 can be attributed to the stretching and bending vibrations respectively of the –OH moiety of lattice water. The IR spectrum also consists of peaks arising due to V–O (terminal) and various Mo–O stretching vibrations at 946, 893, 796, 725, 672, 543, 452 cm−1. The intense Raman signal at 968 cm−1 can be assigned for a vibration of the γ-[Mo8O26]4– unit [33]. The TG-DTA curves of 1 exhibit an initial mass loss of 1.2% (Calcd: 1.3%) accompanied by a weak endothermic signal centered at 92 °C assignable for the loss of coordinated water (Figure S5). This is followed by an exothermic peak at 440 °C corresponding to a large mass loss of 34.5%. This value is in close agreement with the calculated value of 34.0% for loss of the organic ligand. The residual mass of 64.3% (Calcd. 65.8%) can be assigned for the formation of a phase of composition V2Mo5O20.
2.2 Description of the crystal structure of 1
The title coordination polymer 1 [(VO(terpy))4(MoO4)2(Mo8O26)]·2H2O crystallizes in the centrosymmetric triclinic space group
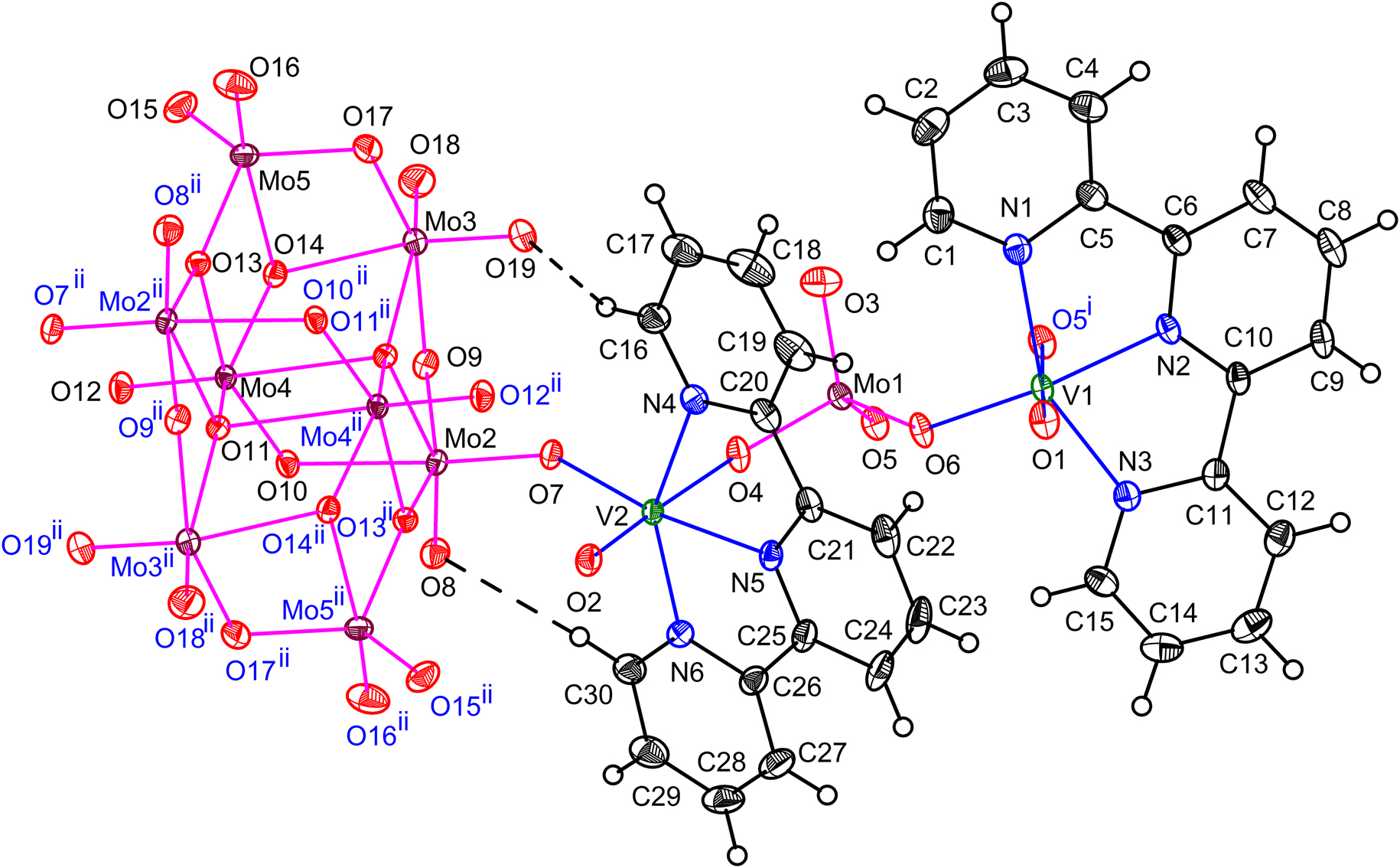
The asymmetric unit of 1 showing sixfold coordination around V1 and V2. Displacement ellipsoids are drawn at 30% probability level for all the non-hydrogen atoms. Intramolecular H-bonding is shown as broken lines. For clarity, symmetry generated atoms are labelled in blue.
Symmetry code: (i) –x, –y + 1, –z + 1 (ii) –x + 1, –y + 2, –z + 2.
The first unique V4+ (V1) is further bonded to two oxygen atoms (O5 and O6) of (MoO4)2– while the second independent V (V2) is bonded to O4 of (MoO4)2– and O7 of (Mo8O26)4– completing the {VN3O3} octahedron. The terminal oxido ligands O1 and O2 make the shortest V–O bonds (Table 1). Unlike the V–O distances which range from 1.5940(17) to 2.1833(16) Å for V1 (1.5998(16) to 2.1198(16) for V2), the V–N bond distances scatter in a narrow range between 2.0643(18) and 2.149(2) Å for V1 (2.0452(18) and 2.131(2) for V2). Bond valence sum of the V–N and V–O bonds for V1 and V2 were determined using the VaList program [34]. The values of 3.77 and 3.81 for V1 and V2 reveal that the unique vanadium atoms can be formulated as V(IV). The cis- as well as the trans- O–V–N or N–V–N angles deviate from ideal values and cover a wide range (76.36(8) to 101.97(7) and 152.35(8)° to 178.54(8)° for V1; 76.83(8)° to 104.11(7)° and 151.79(8)° to 177.15(9)° for V2) indicating a distortion of the {VN3O3} octahedron. The Mo–O bond lengths of the tetrahedral (MoO4)2– anion range from the shortest Mo–O3 bond of 1.7159(19) to 1.8042(16) Å for Mo–O6. The binding of O6 with V1 can explain the elongation of the Mo–O6 bond. The O–Mo–O bond angles range from 107.79(7)° to 112.53(8)° indicating a slight distortion of the {MoO4} tetrahedron.
Bond lengths and angles (Å, °) of {VN3O3}, (MoO4)2– and (Mo8O26)4– moieties in 1.
| V1–O1 | 1.5940(17) | Mo1–O4 | 1.7637(16) | Mo3–O18 | 1.6892(19) |
| V1–O5i | 2.1833(16) | Mo1–O5 | 1.7750(16) | Mo3–O19 | 1.7029(18) |
| V1–O6 | 1.9674(15) | Mo1–O6 | 1.8042(16) | Mo4–O10 | 1.7648(15) |
| V1–N1 | 2.142(2) | Mo2–O7 | 1.7605(15) | Mo4–O11 | 1.8973(14) |
| V1–N2 | 2.0643(18) | Mo2–O8 | 1.6884(16) | Mo4–O11ii | 2.5049(14) |
| V1–N3 | 2.1490(2) | Mo2–O9 | 1.8539(15) | Mo4–O12 | 1.6886(15) |
| V2–O2 | 1.5998(16) | Mo2–O10 | 2.2146(15) | Mo4–O14 | 1.8995(15) |
| V2–O4 | 2.1198(16) | Mo2–O11ii | 2.2450(14) | Mo4–O13 | 2.2101(15) |
| V2–O7 | 2.0096(15) | Mo2–O13ii | 2.0192(14) | Mo5–O13 | 1.9076(15) |
| V2–N4 | 2.1231(19) | Mo3–O9 | 1.9724(16) | Mo5–O14 | 2.3318(15) |
| V2–N5 | 2.0452(18) | Mo3–O11ii | 2.4960(15) | Mo5–O15 | 1.7032(18) |
| V2–N6 | 2.131(2) | Mo3–O14 | 2.1297(15) | Mo5–O16 | 1.6949(19) |
| Mo1–O3 | 1.7159(19) | Mo3–O17 | 1.9819(18) | Mo5–O17 | 1.8579(18) |
| O1–V1–O6 | 100.44(8) | O8–Mo2–O9 | 104.32(8) | O11–Mo4–O13 | 73.39(6) |
| O1–V1–N2 | 100.17(8) | O7–Mo2–O9 | 101.46(7) | O14–Mo4–O13 | 75.28(6) |
| O6–V1–N2 | 159.39(7) | O8–Mo2–O13ii | 101.14(7) | O12–Mo4–O11ii | 175.01(7) |
| O1–V1–N1 | 94.12(9) | O7–Mo2–O13ii | 90.94(6) | O10–Mo4–O11ii | 75.15(6) |
| O6–V1–N1 | 101.90(8) | O9–Mo2–O13ii | 148.10(6) | O11–Mo4–O11ii | 79.50(6) |
| N2–V1–N1 | 76.37(8) | O8–Mo2–O10 | 85.89(7) | O14–Mo4–O11ii | 71.37(5) |
| O1–V1–N3 | 95.08(9) | O7–Mo2–O10 | 168.50(6) | O13–Mo4–O11ii | 83.38(5) |
| O6–V1–N3 | 101.97(7) | O9–Mo2–O10 | 82.54(6) | O16–Mo5–O15 | 105.15(10) |
| N2–V1–N3 | 76.36(8) | O13ii–Mo2–O10 | 80.42(6) | O16–Mo5–O17 | 101.46(9) |
| N1–V1–N3 | 152.35(8) | O8–Mo2–O11ii | 158.66(7) | O15–Mo5–O17 | 112.62(9) |
| O1–V1–O5i | 178.54(8) | O7–Mo2–O11ii | 96.52(6) | O16–Mo5–O13 | 99.59(8) |
| O6–V1–O5i | 80.88(6) | O9–Mo2–O11ii | 78.91(6) | O15–Mo5–O13 | 116.32(8) |
| N2–V1–O5i | 78.51(7) | O13ii–Mo2–O11ii | 70.48(6) | O17–Mo5–O13 | 118.38(7) |
| N1–V1–O5i | 84.99(7) | O10–Mo2–O11ii | 73.51(5) | O16–Mo5–O14 | 166.35(9) |
| N3–V1–O5i | 85.21(7) | O18–Mo3–O19 | 105.13(10) | O15–Mo5–O14 | 88.39(8) |
| O2–V2–O7 | 95.20(7) | O18–Mo3–O9 | 98.92(9) | O17–Mo5–O14 | 74.17(6) |
| O2–V2–N5 | 95.15(8) | O19–Mo3–O9 | 96.60(8) | O13–Mo5–O14 | 72.22(6) |
| O7–V2–N5 | 169.48(7) | O18–Mo3–O17 | 99.81(9) | Mo1–O4–V2 | 146.34(9) |
| O2–V2–O4 | 177.15(9) | O19–Mo3–O17 | 93.97(9) | Mo1–O5–V1i | 144.17(10) |
| O7–V2–O4 | 85.34(6) | O9–Mo3–O17 | 155.26(7) | Mo1–O6–V1 | 134.78(9) |
| N5–V2–O4 | 84.42(7) | O18–Mo3–O14 | 100.75(8) | Mo2–O7–V2 | 145.82(9) |
| O2–V2–N4 | 98.67(9) | O19–Mo3–O14 | 153.66(8) | Mo2–O9–Mo3 | 119.09(8) |
| O7–V2–N4 | 99.70(7) | O9–Mo3–O14 | 84.02(6) | Mo4–O10–Mo2 | 118.35(7) |
| N5–V2–N4 | 76.83(8) | O17–Mo3–O14 | 76.72(6) | Mo4–O11–Mo2ii | 108.74(6) |
| O4–V2–N4 | 83.98(7) | O18–Mo3–O11ii | 165.24(8) | Mo4–O11–Mo3ii | 158.74(7) |
| O2–V2–N6 | 94.07(9) | O19–Mo3–O11ii | 86.91(8) | Mo2ii–O11–Mo3ii | 88.02(5) |
| O7–V2–N6 | 104.11(7) | O9–Mo3–O11ii | 70.76(5) | Mo4–O11–Mo4ii | 100.50(6) |
| N5–V2–N6 | 77.03(8) | O17–Mo3–O11ii | 87.60(6) | Mo2ii–O11–Mo4ii | 92.12(5) |
| O4–V2–N6 | 83.09(7) | O14–Mo3–O11ii | 68.34(5) | Mo3ii–O11–Mo4ii | 91.60(5) |
| N4–V2–N6 | 151.79(8) | O12–Mo4–O10 | 103.48(7) | Mo5–O13–Mo2ii | 142.53(8) |
| O3–Mo1–O4 | 109.17(10) | O12–Mo4–O11 | 105.48(7) | Mo5–O13–Mo4 | 108.46(6) |
| O3–Mo1–O5 | 109.80(9) | O10–Mo4–O11 | 99.25(7) | Mo2ii–O13–Mo4 | 105.69(6) |
| O4–Mo1–O5 | 107.93(8) | O12–Mo4–O14 | 104.37(7) | Mo4–O14–Mo3 | 125.60(7) |
| O3–Mo1–O6 | 109.54(9) | O10–Mo4–O14 | 100.23(7) | Mo4–O14–Mo5 | 104.03(6) |
| O4–Mo1–O6 | 107.79(7) | O11–Mo4–O14 | 139.13(6) | Mo3–O14–Mo5 | 93.44(6) |
| O5–Mo1–O6 | 112.53(8) | O12–Mo4–O13 | 98.19(7) | Mo5–O17–Mo3 | 115.67(8) |
| O8–Mo2–O7 | 103.33(8) | O10–Mo4–O13 | 158.30(6) |
-
Symmetry transformations used to generate equivalent atoms: Symmetry code: i) –x, –y + 1, –z + 1; ii) –x + 1, –y + 2, –z + 2.
The tetraanionic octamolybdate (Mo8O26)4– is well-known to exhibit polymorphism and several polymorphic modifications are well documented in the literature [35], [36], [37], [38], [39], [40], [41], [42], [43], [44]. Although the α- and β-modifications are more often encountered, other polymorphic forms like γ, δ, ε etc. are also known (Table S2). The centrosymmetric (Mo8O26)4– unit in 1 which consists of three unique octahedrally surrounded Mo atoms (Mo2, Mo3 and Mo4) and the penta-coordinated Mo5 atom (Figure S6) is a γ-modification of (Mo8O26)4– and the 13 unique O atoms can be classified under four types viz. i) the terminal oxido (Ot) ligands O7, O8, O12, O15, O16, O18 and O19, ii) μ2-bridging oxido ligands O9, O10, O17 iii) μ3-bridging O13 and O14 iv) μ4-bridging O11. The terminal Mo–Ot bond distances range from 1.6884(16) to 1.7605(15) Å. O7 which makes the longest Mo2–O7 distance of 1.7605(15) Å has also a bond to V2, which can explain its elongation. Unlike the terminal oxygen atoms, the bridging oxido ligands exhibit longer Mo–O distances which scatter in a wider range viz. 1.7648(15)–2.2146(15) Å for μ2-bridging ligands, and 1.8995(15)–2.3318(15) Å and 1.8973(14)–2.5049(14) Å for μ3- and μ4-bridging ligands respectively.
Oxidovandium(IV) compounds containing monodentate and bridging (MoO4)2– ligand are well documented in the literature [30, 32]. Compound 1 is unique due to the presence of two different Mo-based ligands viz. (MoO4)2– and (Mo8O26)4–. To the best of our knowledge no other V4+ compound is known containing coordinated (MoO4)2– and (Mo8O26)4– ligands. Zubieta and coworkers reported an example of a three-dimensional Cu(II) compound [{Cu2(L4)}2{γ-Mo8O26}{MoO4}2]·2H2O (entry 4 in Table S2) containing two differing molybdate ligands [37].
The μ3-bridging (MoO4)2– dianion binds to two symmetry related V1 atoms via O5 and O6 and a V2 through O4 while the centrosymmetric (Mo8O26)4– binds to V2 via O7 (Figure S7). The μ3-bridging tridentate binding of (MoO4)2– results in the formation of a {V4Mo2O12}4+ cationic unit which is composed of an eight membered heterometallic {Mo2V2O4} ring containing one of the unique V atoms (V1 in the ring) with the other unique V atom (V2) protruding as handles (Figure 2).
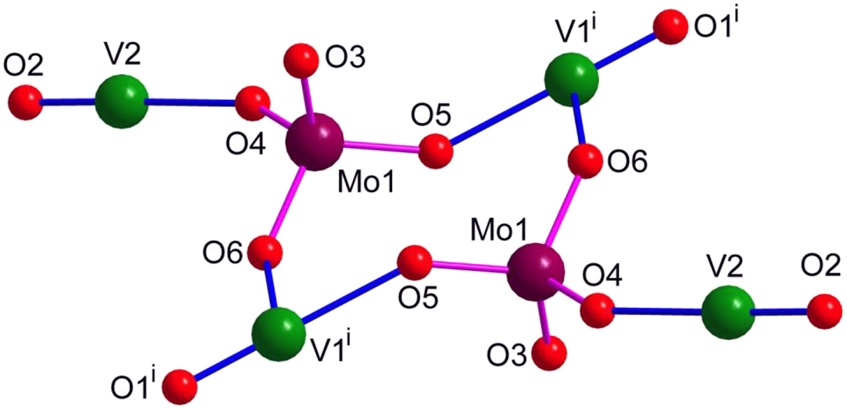
The {V4Mo2O12}4+ cationic unit made up of an eight-membered heterometallic {Mo2V2O4} ring with protruding oxidovanadium handles. The terpy ligands around V1 and V2 are not shown. Symmetry code i) –x, –y + 1, –z + 1.
A pair of {V4Mo2O12}4+ units are bridged by a (Mo8O26)4– ligand resulting in the formation of a polymeric chain which consists of alternating {V4Mo2O12}4+ cations and (Mo8O26)4– anions (Figure 3). The H atoms attached to the carbon atoms C4, C7, C9, C14, C16, C19, C24, C27, C29 and C30 of the unique terpy ligands function as hydrogen donors while the O atoms O1 and O2 attached to V1 and V2 and the O atoms of the γ-(Mo8O26)4– unit, viz. O8, O9, O10, O14, O15, O17 and O19 function as hydrogen acceptors resulting in a total of eleven C–H⋯O bonds (Table S3) of which two are intramolecular. The intermolecular C–H⋯O bonds serve to link parallel polymeric chains. The difficulty to locate the H atoms attached to the lattice water precludes a description of the O–H⋯O interactions.
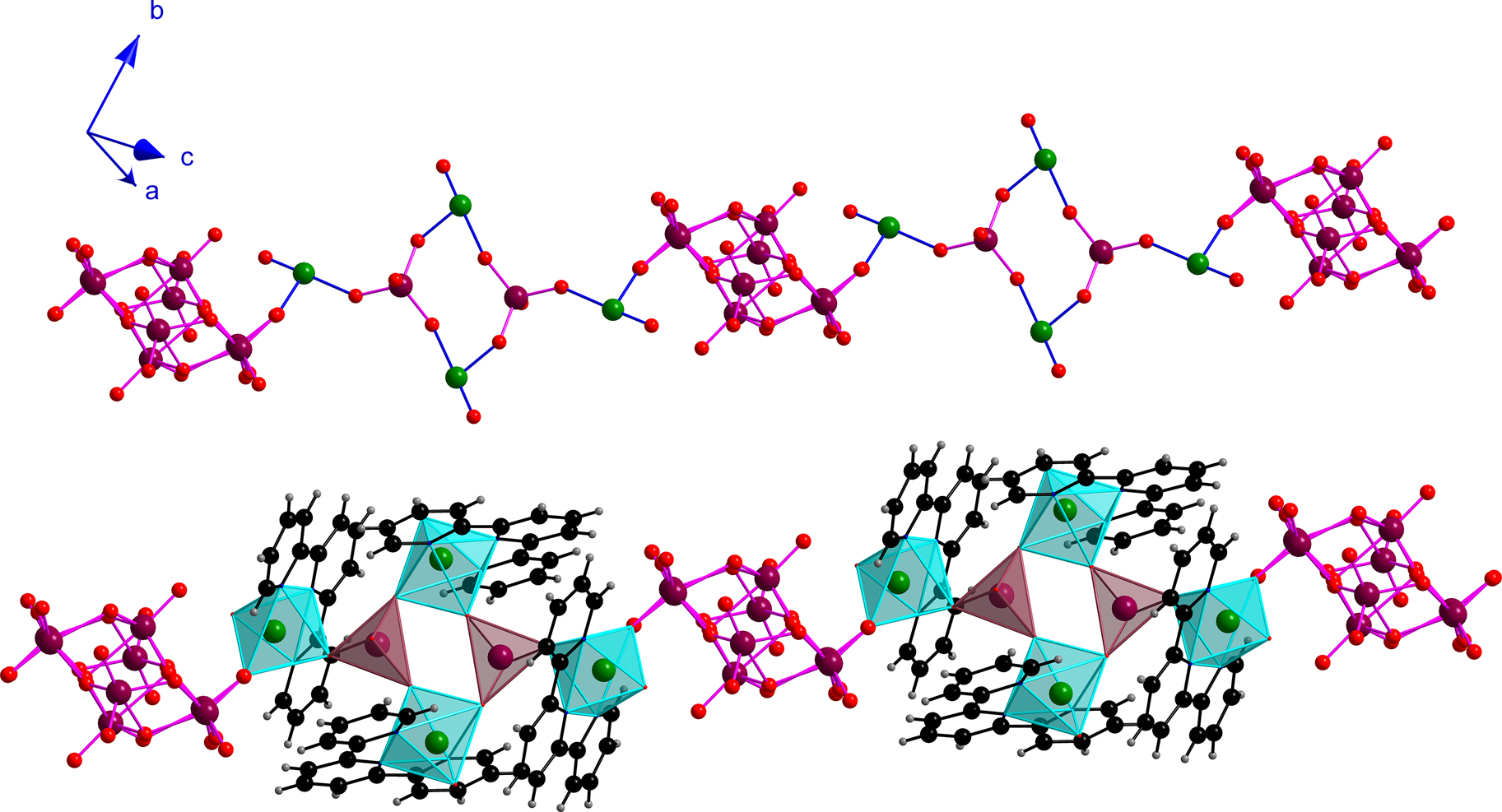
A portion of an infinite chain showing alternating (Mo8O26)4– anions and {V4Mo2O12}4+ cations. The tridentate terpy ligands on the unique V atoms are not shown (top). Same arrangement after inclusion of the terpy ligands. The {VO3N3} octahedra and (MoO4)2− tetrahedra are shown in polyhedral format (bottom).
3 Experimental
3.1 Materials and methods
All chemicals were used as received from commercial sources without any further purification. Infrared (IR) spectra of the solid samples diluted with KBr were recorded on a Shimadzu (IR Prestige-21) FT-IR spectrometer in the range 4000–400 cm−1 at a resolution of 4 cm−1. The Raman spectrum was recorded using a Labram HR Evolution Raman Spectrometer HORIBA Scientific at 785 nm laser radiation for excitation and laser power set to 5%. An ESR spectrum was recorded in a Jeol (JES, FA200) spectrometer using a microwave frequency of 9.5 GHz and modulation frequency of 100 kHz at Sophisticated Analytical Instruments Facility (SAIF), IIT-Madras. The elemental analysis (C, H and N) was performed on an Elementar Variomicro Cube CHNS Analyser. TG-DTA experiment was performed in air at the heating rate of 5 K/min in the temperature range of 30–700 °C on STA-409 PC simultaneous thermal analyser from Netzsch in an alumina crucible. Powder X-ray diffraction (PXRD) pattern was recorded on a Rigaku Smartlab powder diffractometer using Cu-Kα radiation. For single crystal structure determination, X-ray intensity data was collected on a Bruker D8 Quest Eco X-ray diffractometer, using graphite-monochromated Mo-Kα radiation. The structure was solved with Direct Methods using Shelxs-97 [45] and refinement was carried out against F 2 using Shelxl-2016 [45]. All non-hydrogen atoms were refined anisotropically. The H atoms bonded to the aromatic carbons were located in difference Fourier maps but were positioned with idealized geometry and refined isotropically with U iso(H) = 1.2 U eq(C) using a riding model. The hydrogen atoms attached to the lattice water O1W could not be located. Technical details of data acquisition and selected refinement results for 1 are given in Table 2.
Selected refinement results for [(VO(terpy))4(MoO4)2(Mo8O26)]·2H2O 1.
| Refinement result | Compound 1 |
|---|---|
| Empirical formula | C30 H24 Mo5 N6 O20 V2 |
| Formula weight (g mol−1) | 1370.14 |
| Temperature (K) | 296(2) |
| Wavelength (Å) | 0.71073 |
| Crystal system | Triclinic |
| Space group |
|
| Unit cell dimensions | |
| a (Å) | 11.4507(16) |
| b (Å) | 11.8541(17) |
| c (Å) | 14.675(2) |
| α (°) | 92.023(4) |
| β (°) | 98.321(4) |
| γ (°) | 91.804(4) |
| Volume (Å3) | 1968.4(5) |
| Z | 2 |
| D calc (g cm−3) | 2.31 |
| Absorption coefficient (mm−1) | 2.1 |
| F(000) | 1320 |
| Crystal size (mm3) | 0.24 × 0.16 × 0.14 |
| θ range for data collection (°) | 2.53–28.31 |
| Limiting indices | −15 ≤ h ≤ 15 −15 ≤ k ≤ 15 −19 ≤ l ≤ 19 |
| Reflections collected/unique | 66,874/9787 [R(int) = 0.0290] |
| Completeness θ = 25.242° | 99.9 |
| Refinement method | Full-matrix least-squares on F 2 |
| Data/restraints/parameters | 9787/0/568 |
| Goodness of fit on F 2 | 1.104 |
| Final R indices [I > 2σ(I)] |
R1 = 0.0198 wR2 = 0.0480 |
| R indices (all data) |
R1 = 0.0238 wR2 = 0.0507 |
| Largest diff. peak and hole (e Å 3) | 0.67 and −0.73 |
| Bond valence sum | V1 = 3.77; V2 = 3.81 |
| CCDC Deposition No | 2131584 |
4 Synthesis of [(VO(terpy))4(MoO4)2(Mo8O26)] 1
Compound 1 was synthesized under hydrothermal conditions in a steel autoclave with Teflon container (volume 25 mL). Sodium metavanadate (0.046 g, 0.38 mmol), 2,2′:6′,2′′-terpyridine (0.116 g, 0.497 mmol) and molybdic acid (0.094 g, 0.58 mmol) were taken in water (∼8 mL) and acetic acid (99.0%) was added to make the pH ∼ 3 and the reaction mixture was heated at 200 °C for 68 h. The green reaction mixture was filtered and washed with water to obtain dark green crystalline blocks of 1 (Yield = 0.092 g, 35%).
Analytical data (%) Calcd.: C, 26.29; N, 6.13; H, 1.77. Found: C, 26.97; N, 6.31; H, 1.83.
IR data (KBr, cm−1): 3410(br), 3084–2928(m), 1609(s), 1446(s), 1400(m), 1322(m), 1244(m), 1166(m), 1108(m), 1050(m), 946(s), 893(vs), 796(s), 725(s), 672(s), 543(m), 452(m).
Raman data (cm−1): 1047, 968(vs), 881, 825.
5 Supporting information
Deposition number CCDC 2131584 (1), contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures. Supplementary Data (Figs. S1–S7) and Tables S1–S3 associated with this article are available in electronic form.
Dedicated to Professor Christian Näther on the occasion of his 60th birthday.
Funding source: Council of Scientific and Industrial Research, India
Award Identifier / Grant number: 01(2923)/18/EMR-II
Acknowledgments
The authors thank the Department of Science and Technology (DST), New Delhi, for the sanction of a Bruker Eco D8 Quest instrument under the FIST program. Miss Nikita N. Harmalkar acknowledges Research Studentship from Goa University.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: SND thanks Council of Scientific and Industrial Research (CSIR), New Delhi, India (No. 01(2923)/18/EMR-II) for funding.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Pope, M. T., Müller, A. Polyoxometalate Chemistry from Topology via Self-Assembly to Applications; Kluver Academic Publishers: Amsterdam, 2002.10.1007/0-306-47625-8Search in Google Scholar
2. Pope, M. T., Müller, A. Polyoxometalate chemistry: an old field with new dimensions in several disciplines. Angew. Chem., Int. Ed. Engl. 1991, 30, 34–48; https://doi.org/10.1002/anie.199100341.Search in Google Scholar
3. Dolbecq, A., Dumas, E., Mayer, C. R., Mialane, P. Hybrid organic-inorganic polyoxometalate compounds: from structural diversity to applications. Chem. Rev. 2010, 110, 6009–6048; https://doi.org/10.1021/cr1000578.Search in Google Scholar PubMed
4. Xiao, L.-N., Zhao, C.-X., Shi, X.-M., Zhang, H., Wu, W., Cui, X.-B. Three new compounds based on similar molybdenum-vanadium clusters and several types of copper complexes. CrystEngComm 2018, 20, 969–977; https://doi.org/10.1039/C7CE01908D.Search in Google Scholar
5. Aureliano, M., Crans, D. C. Decavanadate (V10O28)6– and oxovanadates: oxometalates with many biological activities. J. Inorg. Biochem. 2009, 103, 536–546; https://doi.org/10.1016/j.jinorgbio.2008.11.010.Search in Google Scholar PubMed
6. Bijelic, A., Aureliano, M., Rompel, A. The antibacterial activity of polyoxometalates: structures, antibiotic effects and future perspectives. Chem. Commun. 2018, 54, 1153–1169; https://doi.org/10.1039/C7CC07549A.Search in Google Scholar PubMed PubMed Central
7. Cao, Y., Chen, Q., Shen, C., He, L. Polyoxometalate-based catalysts for CO2 conversion. Molecules 2019, 24, 2069–2095; https://doi.org/10.3390/molecules24112069.Search in Google Scholar PubMed PubMed Central
8. Zhou, Y., Chen, G., Long, Z., Wang, J. Recent advances in polyoxometalate-based heterogeneous catalytic materials for liquid-Phase organic transformations. RSC Adv. 2014, 4, 42092–42113; https://doi.org/10.1039/C4RA05175K.Search in Google Scholar
9. Lang, X., Chen, X., Zhao, J. Heterogeneous visible light photocatalysis for selective organic transformations. Chem. Soc. Rev. 2014, 43, 473–486; https://doi.org/10.1039/C3CS60188A.Search in Google Scholar
10. Wang, D., Liu, L., Jiang, J., Chen, L., Zhao, J. Polyoxometalate-based composite materials in electrochemistry: state-of-the-art progress and future outlook. Nanoscale 2020, 12, 5705–5718; https://doi.org/10.1039/C9NR10573E.Search in Google Scholar PubMed
11. Kӧgerler, P., Tsukerblat, B., Müller, A. Structure-related frustrated magnetism of nanosized polyoxometalates: aesthetics and properties in harmony. Dalton Trans. 2020, 39, 21–36; https://doi.org/10.1039/B910716A.Search in Google Scholar
12. Liang, Y., Li, Y., Wang, H., Dai, H. Strongly coupled inorganic/nanocarbon hybrid materials for advanced electrocatalysis. J. Am. Chem. Soc. 2013, 135, 2013–2036; https://doi.org/10.1021/ja3089923.Search in Google Scholar PubMed
13. Wang, S.-S., Yang, G.-Y. Recent advances in polyoxometalate-catalyzed reactions. Chem. Rev. 2015, 115, 4893–4962; https://doi.org/10.1021/cr500390v.Search in Google Scholar PubMed
14. Anyushin, A. V., Kondinski, A., Parac-Vogt, T. N. Hybrid polyoxometalates as post-functionalization platforms: from fundamentals to emerging applications. Chem. Soc. Rev. 2020, 49, 382–432; https://doi.org/10.1039/C8CS00854J.Search in Google Scholar
15. Monakhov, K. Y., Bensch, W., Kögerler, P. Semimetal-functionalised polyoxovanadates. Chem. Soc. Rev. 2015, 44, 8443–8483; https://doi.org/10.1039/C5CS00531K.Search in Google Scholar
16. Mahnke, L. K., Kondinski, A., Warzok, U., Näther, C., van Leusen, J., Schalley, C. A., Monakhov, K. Y., Kögerler, P., Bensch, W. Configurational isomerism in polyoxovanadates. Angew. Chem. Int. Ed. 2018, 57, 2972–2975; https://doi.org/10.1002/anie.201712417.Search in Google Scholar PubMed
17. Mahnke, L. K., Stehlíková, G., Synnatschke, K., Backes, C., Näther, C., Bensch, W. An exotic layered compound consisting of interconnected arsenato-polyoxovanadate clusters: thermal and magnetic properties and liquid phase exfoliation. ChemNanoMat 2021, 7, 78–84; https://doi.org/10.1002/cnma.202000563.Search in Google Scholar
18. Aureliano, M. Decavanadate: a journey in a search of a role. Dalton Trans. 2009, 9093–9100; https://doi.org/10.1039/B907581J.Search in Google Scholar
19. Day, V. W., Klemperer, W. G., Maltbie, D. J. Where are the protons in H3V10O283– ? J. Am. Chem. Soc. 1987, 109, 2991–3002; https://doi.org/10.1021/ja00244a022.Search in Google Scholar
20. Evans, H. T.Jr. The molecular structure of the isopoly complex ion, decavanadate (V10O28)6–. Inorg. Chem. 1966, 5, 967–977; https://doi.org/10.1021/ic50040a004.Search in Google Scholar
21. Groom, C. R., Bruno, I. J., Lightfoot, M. P., Ward, S. C. The Cambridge structural database. Acta Crystallogr. 2016, B72, 171–179; https://doi.org/10.1107/S2052520616003954.Search in Google Scholar PubMed PubMed Central
22. Wutkowski, A., Srinivasan, B. R., Naik, A. R., Schütt, C., Näther, C., Bensch, W. Synthesis, structure, and photochemistry of an organic heptamolybdate-monomolybdate. Eur. J. Inorg. Chem. 2011, 2254–2263; https://doi.org/10.1002/ejic.201001154.Search in Google Scholar
23. Srinivasan, B. R., Kundaikar, S. A., Morajkar, S. M., Näther, C., Bensch, W. Synthesis, crystal structure and properties of hepta(ammonium) penta(1H-imidazol-3-ium) paratungstate B tetrahydrate. J. Coord. Chem. 2021, 74, 2239–2252; https://doi.org/10.1080/00958972.2021.1965996.Search in Google Scholar
24. Pope, M. T., Dale, B. W. Isopoly-vanadates, -niobates, and -tantalates. Q. Rev. Chem. Soc. 1968, 22, 527–548; https://doi.org/10.1039/QR9682200527.Search in Google Scholar
25. Higami, T., Hashimoto, M., Okeya, S. [Ni(H2O)6]2[Na(H2O)3]2[V10O28]·4H2O, bis(nickel hexahydrate) bis(sodium trihydrate) decavanadate tetrahydrate. Acta Crystallogr. 2002, C58, i144–i146; https://doi.org/10.1107/S0108270102015603.Search in Google Scholar PubMed
26. Iida, A., Ozeki, T. Cu3V10O28·24H2O and CuNa4V10O28·23H2O. Acta Crystallogr. 2003, C59, i41–i44; https://doi.org/10.1107/S0108270103008552.Search in Google Scholar
27. Iida, A., Ozeki, T. Mg2Na2V10O28·20H2O and Mg3V10O28·28H2O. Acta Crystallogr. 2004, C60, i43–i46; https://doi.org/10.1107/S0108270104004676.Search in Google Scholar PubMed
28. Putrevu, N. R., Doedens, R. J., Khan, M. I. Decavanadate with a novel coordination complex: synthesis and characterization of (NH4)2[Ni(H2O)5(NH3)]2(V10O28)·4H2O. Inorg. Chem. Commun. 2013, 38, 5–7; https://doi.org/10.1016/j.inoche.2013.10.001.Search in Google Scholar
29. Johnson, G. K., Schlemper, E. O. Existence and structure of the molecular ion 18-vanadate(IV). J. Am. Chem. Soc. 1978, 100, 3645–3646; https://doi.org/10.1021/ja00479a083.Search in Google Scholar
30. Rasmussen, M., Näther, C., Bismayer, U., Bensch, W. Small non-polar complexes exhibiting significant piezoelectric properties: solvothermal synthesis and crystal structures of MO5V(tren)·H2O (M = Mo and W; tren = tris(2-aminoethyl)amine). J. Solid State Chem. 2012, 195, 108–114; https://doi.org/10.1016/j.jssc.2012.02.040.Search in Google Scholar
31. Finn, R. C., Zubieta, J. Solid state coordination chemistry: the hydrothermal synthesis and structure of a bimetallic oxide incorporating 2,2′:6′,2′′-terpyridine (terpy), [{VO(terpy)}MoO4]. Inorg. Chim. Acta 2002, 332, 186–190; https://doi.org/10.1016/S0020-1693(02)00703-X.Search in Google Scholar
32. Khan, M. I., Giri, S., Ayesh, S., Doedens, R. J. Synthesis and characterization of a new hybrid chain derived from oxovanadyl and molybdate moieties [VIVO(µ3-MoO4)(2,2′-bpy)]: topological equivalence of {MoO4} and {SO4} motifs. Inorg. Chem. Commun. 2004, 7, 721–724; https://doi.org/10.1016/j.inoche.2004.04.005.Search in Google Scholar
33. Himeno, S., Niiya, H., Ueda, T. Raman studies on the identification of isopolymolybdates in aqueous solution. Bull. Chem. Soc. Jpn. 1997, 70, 631–637; https://doi.org/10.1246/bcsj.70.631.Search in Google Scholar
34. Wills, A. S. VaList program. http://ccp14.cryst.bbk.ac.uk/solution/bond_valence/index.html.Search in Google Scholar
35. Yang, W., Lu, C., Zhuang, H. Hydrothermal synthesis and structures of three new copper complexes: [{Cu(2,2′-bipy}2(β-Mo8O26)], [{Cu(Py)3}2{Cu(Py)2}2(α-Mo8O26)] and [{Cu(Py)2}4]-[(SO4)(Mo12O36)]. J. Chem. Soc., Dalton Trans. 2002, 2879–2884; https://doi.org/10.1039/B111480H.Search in Google Scholar
36. Ying, J., Sun, C., Jin, L., Tian, A., Wang, X. Five compounds based on [TeMo6O24]6− and [β-Mo8O26]4− anions synthesized by using different symmetrical and asymmetric N-donor ligands. CrystEngComm 2021, 23, 5385–5396; https://doi.org/10.1039/D1CE00775K.Search in Google Scholar
37. Bartholomä, M., Jones, S., Zubieta, J. Construction of bimetallic oxide materials from molybdate building blocks and copper-ligand tethers with flexible spaces: structures of the two-dimensional [{Cu2(L4)(H2O)2}Mo8O26(H2O)2] and of the three-dimensional [{Cu2(L4)}2(Mo8O26)(MoO4)2] (L4 = N1, N1, N4, N4-tetrakis(pyridine-2-ylmethyl)butane-1,4-diamine). Inorg. Chem. Commun 2011, 14, 107–110; https://doi.org/10.1016/j.inoche.2010.09.043.Search in Google Scholar
38. Meng, J.-X., Lu, Y., Li, Y.-G., Fu, H., Wang, E.-B. Base-directed self-assembly of octamolybdate-based frameworks decorated by flexible N-containing ligands. Cryst. Growth Des. 2009, 9, 4116–4126; https://doi.org/10.1021/cg900352e.Search in Google Scholar
39. Hagrman, D., Zubeita, C., Rose, D. J., Zubieta, J., Haushalter, R. C. Composite solids constructed from one‐dimensional coordination polymer matrices and molybdenum oxide subunits: polyoxomolybdate clusters within [{Cu(4,4′‐bpy)}4Mo8O26] and [{Ni(H2O)2(4,4′‐bpy)2}2Mo8O26] and one‐dimensional oxide chains in [{Cu(4,4′‐bpy)}4Mo15O47]·8H2O. Angew. Chem. Int. Ed. Engl. 1997, 36, 873–876; https://doi.org/10.1002/anie.199708731.Search in Google Scholar
40. Allis, D. G., Rarig, R. S., Burkholder, E., Zubieta, J. A three-dimensional bimetallic oxide constructed from octamolybdate clusters and copper-ligand cation polymer subunits. A comment on the stability of the octamolybdate isomers. J. Mol. Struct. 2004, 688, 11–31; https://doi.org/10.1016/j.molstruc.2003.08.027.Search in Google Scholar
41. Rarig, R. S., Bewley, L., Burkholder, E., Zubieta, J. Organic-inorganic hybrid materials of the copper molybdate family. Syntheses and structures of [Cu(tpa)Mo2O7], [Cu(Me2bpy)Mo2O7] and [Cu(t-Bu2bpy)Mo4O13] (tpa = tri-2-pyridylamine, Me2bpy = 5,5′-dimethyl-2,2′-bipyridine, t-Bu2bpy = 4,4′-di-tert-butyl-2,2′-bipyridine). Indian J. Chem. 2003, 42A, 2235–2243.10.1016/S1293-2558(01)01214-6Search in Google Scholar
42. Lan, Y.-Q., Li, S.-L., Wang, X.-L., Shao, K.-Z., Su, Z.-M., Wang, E.-B. Supramolecular isomerism with polythreaded topology based on [Mo8O26]4− isomers. Inorg. Chem. 2008, 47, 529–534; https://doi.org/10.1021/ic701463h.Search in Google Scholar PubMed
43. Yue, Z.-C., Du, H.-J., Niu, Y.-Y., Jin, G.-X. An unprecedented ι-type octamolybdate: [TbI1]2[(β-Mo8O26)0.5(ι-Mo8O26)] directed by a new tricationic template. CrystEngComm 2013, 15, 9844–9848; https://doi.org/10.1039/C3CE41418C.Search in Google Scholar
44. Sarr, B., Diop, C. A. K., Melin, F., Sidibe, M., Hellwig, P., Michaud, F., Maury, F., Senocq, F., Mbaye, A., Rousselin, Y. Non-Hydrothermal synthesis and structure determination of two new β-octamolybdate (VI) stabilized with dialkylammonium counterions. J. Mol. Struct. 2018, 1170, 44–50; https://doi.org/10.1016/j.molstruc.2018.05.055.Search in Google Scholar
45. Sheldrick, G. M. Crystal structure refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/S2053229614024218.Search in Google Scholar PubMed PubMed Central
© 2022 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- In this issue
- Laudatio/Preface
- Christian Näther zum 60. Geburtstag gewidmet
- Research Articles
- Bismuth-rich bimetallic clusters (CuBi8)3+ and [MBi10]4+ (M = Pd, Pt) from ionothermal synthesis
- Crystal structure of phenanthrenide salts stabilized by 15-crown-5 and 18-crown-6
- Structure and properties of two new heteroleptic bismuth(III) dithiocabamates of the general composition Bi(S2CNH2)2X (X = Cl, SCN)
- Synthesis and structural characterization of three new mixed ligand alkaline-earth metal picrates
- Dimorphism of MnHAsO4(H2O): natural monoclinic krautite and its synthetic triclinic modification
- Synthesis, crystal structure, and topology of a polycatenated bismuth coordination polymer
- The unexpected crystal structure of thallium(I) tricyanomethanide Tl[C(CN)3]
- Synthesis, structure characterization and properties of a new oxidovanadium(IV) coordination polymer incorporating bridging (MoO4)2– and (Mo8O26)4– ligands
- Crystal structure of Dy11Ge4.33In5.67 and Tm11Ge4In6 from X-ray single-crystal and powder data
- Crystallisation of phosphates revisited: a multi-step formation process for SrHPO4
- Oxygen evolving reactions catalyzed by different manganese oxides: the role of oxidation state and specific surface area
- Synthesis and structural characterization of a new heterometallicmolybdate coordination polymer based on a µ3-bridging amino alcohol
- Chemically and Light-Driven Coordination-Induced Spin State Switching (CISSS) of a nonheme-iron complex
- Extracting information from X-ray diffraction patterns containing Laue oscillations
- Gadolinium trisilicide − a paramagnetic representative of the YbSi3 type series
Articles in the same Issue
- Frontmatter
- In this issue
- Laudatio/Preface
- Christian Näther zum 60. Geburtstag gewidmet
- Research Articles
- Bismuth-rich bimetallic clusters (CuBi8)3+ and [MBi10]4+ (M = Pd, Pt) from ionothermal synthesis
- Crystal structure of phenanthrenide salts stabilized by 15-crown-5 and 18-crown-6
- Structure and properties of two new heteroleptic bismuth(III) dithiocabamates of the general composition Bi(S2CNH2)2X (X = Cl, SCN)
- Synthesis and structural characterization of three new mixed ligand alkaline-earth metal picrates
- Dimorphism of MnHAsO4(H2O): natural monoclinic krautite and its synthetic triclinic modification
- Synthesis, crystal structure, and topology of a polycatenated bismuth coordination polymer
- The unexpected crystal structure of thallium(I) tricyanomethanide Tl[C(CN)3]
- Synthesis, structure characterization and properties of a new oxidovanadium(IV) coordination polymer incorporating bridging (MoO4)2– and (Mo8O26)4– ligands
- Crystal structure of Dy11Ge4.33In5.67 and Tm11Ge4In6 from X-ray single-crystal and powder data
- Crystallisation of phosphates revisited: a multi-step formation process for SrHPO4
- Oxygen evolving reactions catalyzed by different manganese oxides: the role of oxidation state and specific surface area
- Synthesis and structural characterization of a new heterometallicmolybdate coordination polymer based on a µ3-bridging amino alcohol
- Chemically and Light-Driven Coordination-Induced Spin State Switching (CISSS) of a nonheme-iron complex
- Extracting information from X-ray diffraction patterns containing Laue oscillations
- Gadolinium trisilicide − a paramagnetic representative of the YbSi3 type series

