The influence of pre- and perioperative administration of gabapentin on pain 3–4 years after total knee arthroplasty
-
Kristian Kjær Petersen
, Troels Haxholdt Lunn
Abstract
Background and aims:
Approximately 20% of patients having total knee arthroplasty (TKA) will experience chronic postoperative pain. Recently, preoperative pain facilitation has been associated with chronic pain after TKA, and gabapentin has been shown to decrease pain facilitation. The current study is a secondary follow-up of a primary RCT investigating the effect of gabapentin on acute postoperative pain after TKA and exploring the effect of pre- and perioperative administration of gabapentin on chronic postoperative pain and psychological state 3–4 years after TKA.
Methods:
Patients scheduled for TKA were randomized to either gabapentin 1,300 mg/day, gabapentin 900 mg/day, or placebo daily from 2-h before and 6 days after operation. Pre- and 3–4 years postoperatively pain scores related to pain while walking, at rest, when flexing the hip or the knee were collected. At the same time, the pain catastrophizing scale (PCS) and hospital anxiety and depression scale subscales for anxiety (HADS-A) and depression (HADS-D) were collected.
Results:
Lower postoperative pain while walking, flexing the hip, and at rest were found compared with preoperative scores (p<0.03), but these were not associated with gabapentin treatment (p>0.19). Significantly lower postoperative PCS and HADS-A scores were seen compared with preoperative scores (p<0.001), but these were not associated with gabapentin treatment (p>0.55).
Conclusions:
The current study found that pre- and perioperative administrations of gabapentin do not influence the pain or psychological state 3–4 years after TKA.
Implications:
The current study does not support that short-term pre- and perioperative use of gabapentin can reduce the development of chronic postoperative pain after TKA.
1 Introduction
Total knee arthroplasty (TKA) surgery is considered an effective intervention to improve function and reduce pain in the end-stage of knee osteoarthritis (KOA). In the US alone, the number of TKAs have increased from 31.2 per 100,000 persons in the period 1971–1976 to 220.9 in the period 2005–2008 [1]. By 2030, the incidence of TKA is expected to be increased by approximately 700% in the US [2], and as the incidence of chronic postoperative pain following TKA remains at approximately 20% [3], one of the main challenges is to reduce postoperative pain.
Several pre- and perioperative risk factors for chronic postoperative TKA have been suggested [4], [5] where the major risk factors are preoperative, heightened, psychological factors related to pain (e.g. pain catastrophizing, anxiety, or depression) [6], high pre- and perioperative pain intensities [7], [8], [9] and more recently preoperative pain sensitivity [8], [10], [11], [12]. Gabapentinoids have an anxiolytic effect [13], [14], [15] and reduce neuronal hyperexcitability [16], and it has been suggested that preoperative and perioperative administration of gabapentinoids may reduce the incidence of chronic postoperative pain. In this context, Buvanendran et al. [17] administered pregabalin on the day of surgery and 14 days following TKA and found a reduction in neuropathic pain 3 and 6 months after TKA compared with placebo.
Several older reviews and meta-analyses [18], [19], [20], [21], [22], concludes that gabapentinoids reduce pain in the first postoperative days. However, recent meta-analyses have shed critical light on most of the perioperative gabapentinoid literature and concluded that the quality of evidence is low, firm evidence for the use in postoperative pain management is lacking, the clinically relevant beneficial effect seems absent, and there may be a risk of harm [23], [24].
The mechanism-of-action for gabapentinoids is not completely understood, but gabapentinoids bind to the α2-δ subunit of voltage-sensitive calcium channels in the presynaptic dorsal horn neuron [25], [26] and inhibit the release of neurotransmitters, decreasing the pain facilitation of the dorsal horn neurons [27], and thereby reducing the pain facilitation [28]. In addition, studies support the effects on peripheral [29], primary afferent neurons [30], and a recent study found that gabapentin acts on both spinal and supraspinal mechanisms [31]. A measure of pain facilitation is temporal summation of pain (TSP), and gabapentin has been shown to decrease TSP in healthy subjects [16]. Preoperatively, facilitated TSP has been linked to the development of chronic postoperative pain following total hip arthroplasty surgery [10], TKA [8], [11], [12], and acute postoperative pain following thoracotomy [32], indicating that preoperative administration of gabapentinoids could potentially reduce the risk of developing chronic postoperative pain. Since, preoperative administration of pregabalin has been found to diminish neuropathic chronic postoperative pain following TKA [17], the current study hypothesized that different doses of gabapentin would reduce the prevalence of chronic postoperative pain following TKA and aimed to explore the effect of pre- and perioperatively administered gabapentin on the development of chronic postoperative pain in patients undergoing TKA. The current study is a secondary follow-up of a previously published primary RCT investigating the effect of gabapentin on acute postoperative pain after TKA [33].
2 Methods
2.1 Design and patients
This study is a secondary follow-up of a previously published primary RCT investigating the effect of gabapentin on acute postoperative pain after TKA [33] and patients who were reachable were included in the analysis. The primary study was approved by the Danish Medicines Agency, the regional Ethics Committee, and the Danish Data Protection Agency and was registered at EudraCT (2011-003105-22) and www.clinicaltrials.gov (NCT01507363, January 6, 2012). The primary RCT was conducted in accordance with good clinical practice principles and was monitored by the Danish good clinical practice monitoring units. Oral and written informed consents were obtained from all patients [33].
Patients scheduled to undergo elective, unilateral primary TKA were assessed for eligibility (by surgeons/project nurses) and recruited from participating centers at a prescheduled (study independent) hospital visit for clinical examination preceding admission for surgery at the Departments of Orthopedic Surgery, Copenhagen University Hospital, Hvidovre, Denmark; Aalborg University Hospital, Farsø, Denmark; and South of Denmark University Hospital, Grindsted, Denmark. Exclusion criteria were age above 85 and below 50 years, non-ethnic Danes, preoperative use of gabapentinoids, antiepileptics, anxiolytics, antidepressants, systemic glucocorticoids, or opioids of any kind (within 4 weeks), history of bipolar affective disorder, alcohol or drug abuse, history of malignant disease, body mass index (BMI) >40 kg/m2, diseases affecting central or peripheral nerve function, history of dementia, or other cognitive dysfunction, history of renal insufficiency, allergies to gabapentin, and fertile women (menstruation within 2 years).
2.2 Randomization, blinding, and study drug intervention
Detailed information regarding randomization, blinding, and study drug intervention has previously been described [33], but in brief, the randomization and blinding procedures together with the study drug preparations were handled by a state-registered and certificated pharmacy, The Capital Regional Pharmacy, not otherwise involved in the trial. A computer produced 1:1:1 random allocation sequence (A, B and C) was generated in blocks of 12 (25 blocks) without the use of stratification variables, using the free internet service “www. Randomization.com”. The study drugs, oral gabapentin 300 mg and 400 mg, and placebo, were prepared by the pharmacy as small capsules, identical in appearance. The capsules were administered twice a day for 7 days, starting 2 h preoperatively, again at 10 PM on the day of surgery, and thereafter at 8 AM and 10 PM on postoperative days 1–6 [33].
Group A: Gabapentin “high dose” group received gabapentin 1,300 mg/day: 900 mg preoperatively and 400 mg at 10:00 PM on the day of surgery, thereafter 400 mg at 8:00 AM and 900 mg at 10:00 PM.
Group B: Gabapentin “low dose” group received gabapentin 900 mg/day: 600 mg preoperatively and 300 mg at 10:00 PM, thereafter 300 mg at 8:00 AM and 600 mg at 10:00 PM.
Group C: Placebo group received placebo throughout the study period.
The dosage was determined based on the pharmacodynamics and kinetic characteristics of gabapentin [25] and on the few existing (smaller sized) dose-finding studies showing that higher perioperative dose regimens seem more effective than lower dose regimens, 1,200 or 900 mg reported in one study [34] and ≥600 mg reported in another [35].
2.3 Follow-up assessments
The primary study with data on pre- and six first postoperative days has previously been published [33]. The current secondary, exploratory work focuses on follow-up 3–4 years after TKA surgery. Prior to surgery, patients filled out questionnaires regarding pain intensities and psychological distress. At the same time, patients were contacted 3–4 years after surgery by phone, and the same questionnaires were completed during a telephone interview. Patients who did not respond to several attempts of telephone calls were contacted by letter. Patients who did not respond to the written invitation were classified as non-responders. Furthermore, patients who did not wish to participate in the telephone interview or were otherwise uncontactable were classified as non-responders.
2.4 Pain assessments
The pain intensity scores during walking, at rest (supine), upon 45 ° hip flexion with straight leg, and upon passive 60 ° knee flexion were collected. A 100-mm verbal numeric analog scale (NRS) was used (0=no pain and 100=worst pain imaginable).
2.5 Psychological assessments
Anxiety and depression symptoms were assessed using the hospital anxiety and depression scale (HADS) [36], which applies a subscale for anxiety (HADS-A) and a subscale for depression (HADS-D). The HADS ranges from 0 to 21; 0 to 7 indicate no symptoms of anxiety/depression, 8 to 10 indicate possible symptoms of anxiety/depression, and 11 to 21 indicate severe symptoms of anxiety/depression [36]. In addition, pain catastrophizing was assessed using the pain catastrophizing scale (PCS) [37]. A PCS score below 29 is considered a low pain catastrophizer whereas a score above 30 is considered a high pain catastrophizer [37].
2.6 Statistics
The data are presented as means and standard deviations (SD) unless otherwise stated. The revision rate since TKA surgery was examined between the three groups using a χ2-test. Repeated measures analysis of variance (rm-ANOVA) was used to assess the differences in pre- and postoperative pain, and psychological scores and adjustment for treatment groups were applied to assess if gabapentin influenced the outcome. The Bonferroni post hoc test was used in case of significant factors. Chi-squared (χ2)-tests investigated the differences in the number of patients reporting more than 30 mm at the NRS in the three groups.
Three prediction models assessing associations between pre- and post-operative factors were constructed. Initially, Pearson correlation analysis between preoperative PCS, preoperative HADS (A and D), pre-operative pain scores and postoperative pain scores was conducted. Secondly, the correlation analysis was adjusted for the treatment group to investigate if gabapentin influenced the predictive values. Finally, linear regressions were used to categorize independent parameters. p<0.05 was considered significant.
3 Results
Three-hundred patients were enrolled and randomized to administration of either high (group A) or low (group B) dose gabapentin or placebo (group C). Furthermore, 91 patients were included for primary analysis in group A, 92 patients in group B, and 91 patients in group C as previously described in the primary, original study by Lunn et al. [33]. For present follow-up study, the patients were re-contacted, and 68 patients in group A, 77 patients in group B, and 70 patients in group C responded to the invitation. A non-responder analysis found that responders and non-responders had similar preoperative NRS scores (t-test: p>0.21), BMI (t-test: p>0.74), gender distribution (χ2: p>0.62), age (t-test: p>0.26), PCS levels (t-test: p>0.69), HADS-A (t-test: p>0.38), and HADS-D (t-test: p>0.28).
Preoperative demographics for patients included in the follow-up are listed in Table 1. No significant differences were found between groups comparing age [ANOVA: F(2,212)<0.47, p>0.62], BMI [ANOVA: F(2,212)<0.44, p>0.63], time after surgery at follow-up [ANOVA: F(2,212)<0.59, p>0.55], or gender distribution (χ2: p>0.59).
Demographics for patients receiving high dose (1,300 mg/day, group A) or low dose (900 mg/day, group B) gabapentin or placebo (group C).
| Demographics |
||||
|---|---|---|---|---|
| Group A (n=68) | Group B (n=77) | Group C (n=70) | Statistics | |
| Age, years (mean±SD) | 66.9±7.7 | 67.8±8.5 | 66.7±7.6 | ANOVA: F(2,212)<0.47, p>0.62 |
| BMI, kg/m2 (mean±SD) | 29.1±4.1 | 29.6±4.0 | 29.7±4.5 | ANOVA: F(2,212)<0.44, p>0.63 |
| Gender distribution, females/males (percentage females of group) | 31/36 (46.3%) | 37/40 | 28/42 | χ 2: p>0.59 |
| Time from surgery to follow-up, months (mean±SD) | 45.3±5.1 | 45.2±5.0 | 46.2±7.3 | ANOVA: F(2,212)<0.59, p>0.55 |
-
BMI=body mass index.
3.1 Follow-up assessments – pain scores
At follow-up, three patients in group A (4.4% of group), four patients in group B (5.2% of group), and four patients in group C (5.7% of group) had received revision TKA surgery, which was not significantly different between the groups (χ2: p>0.94). Significantly lower postoperative pain scores were found for pain while walking [rm-ANOVA: F(1,212)>48.30, p<0.0001], at rest [rm-ANOVA: F(1,212)>4.93, p<0.027], and while flexing the hip [rm-ANOVA: F(1,212)>15.13, p<0.0001] compared with preoperative scores, Fig. 1. These results were independent of gabapentin treatment [rm-ANOVAs: F(2,212)<1.68, p>0.19], indicating that preoperative administration of gabapentin did not further improve the pain scores.
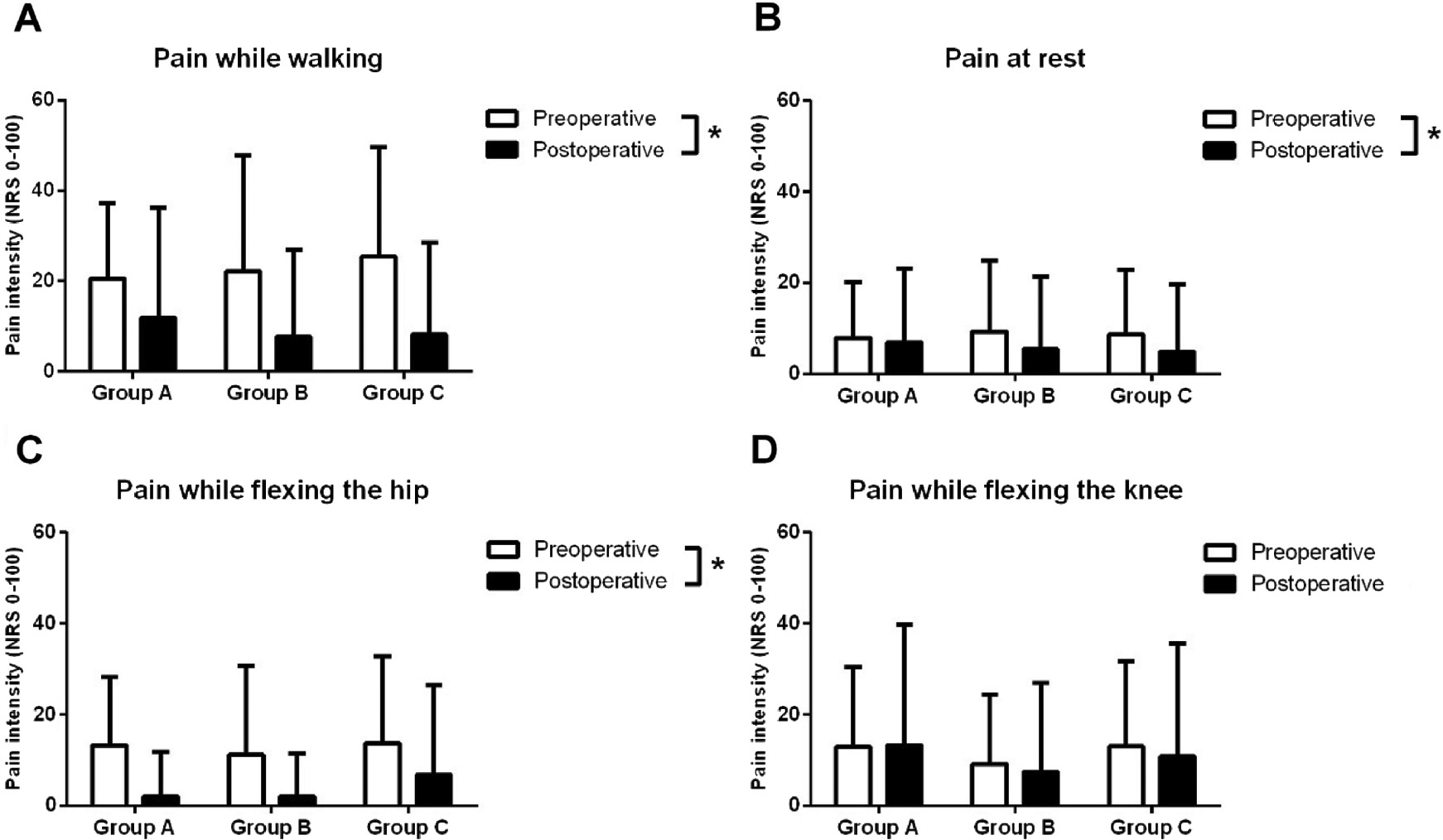
Comparison between preoperative (white bars) and postoperative (black bars) pain scores for pain while walking (A), pain at rest (B), pain while flexing the hip (C), and pain while flexing the knee (D). Knee osteoarthritis patients scheduled for total knee arthroplasty received pre- and perioperative high (group A) or low dose (group B) gabapentin or placebo (group C), and no differences in pain scores were seen between groups. *Indicates a significant difference (p<0.05) comparing pre- and postoperative scores.
Similarly, the changes in the pain scores from preoperative to follow-up assessments showed no significant differences for any pain scores when comparing the three treatment groups [ANOVA: F(2,212)<2.50, p>0.08].
No differences were found in number of patients reporting more than 30 mm at the NRS for pain while walking (χ2: p>0.19), at rest (χ2: p>0.41), while flexing the hip (χ2: p>0.27), and while flexing the knee (χ2: p>0.20).
3.2 Follow-up assessment – psychological scores
Significantly lower PCS [rm-ANOVA: F(1,198)>133.65, p<0.001] and HADS-A [rm-ANOVA: F(1,199)>33.20, p<0.001] scores were found when comparing postoperative to preoperative scores, Fig. 2. These findings were independent of treatment [rm-ANOVA: F(2,198)<0.60, p>0.55], indicating that gabapentin did not influence the postoperative psychological follow-up.
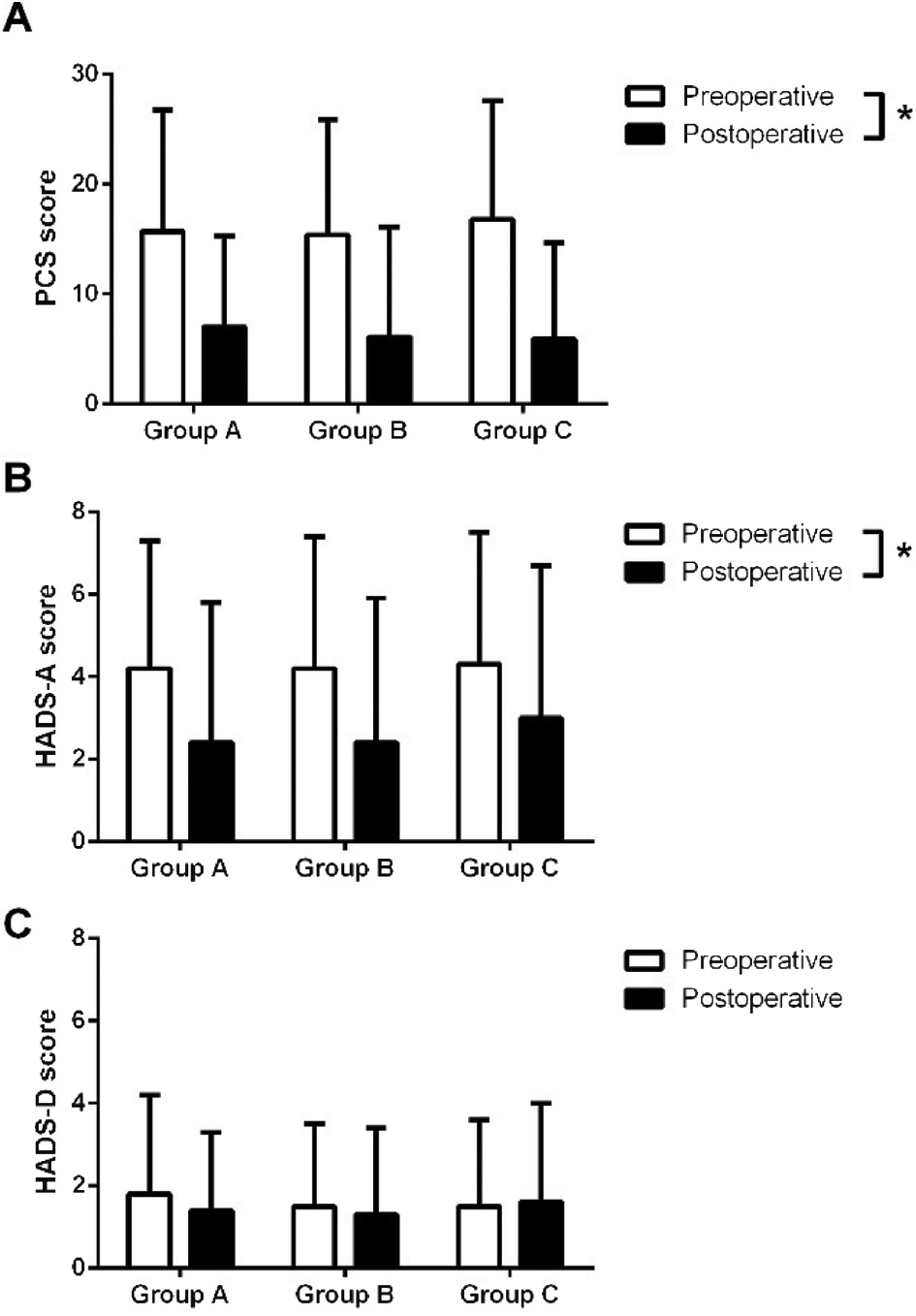
Comparison between preoperative (white bars) and postoperative (black bars) pain scores for (A) pain catastrophizing scale (PCS), (B) hospital anxiety and depression scale anxiety subscale (HADS-A), and (C) HADS depression subscale (HADS-D). Knee osteoarthritis patients scheduled for total knee arthroplasty received pre- and perioperative high (group A) or low dose (group B) gabapentin or placebo (group C), and no differences were seen comparing the groups for any of the psychological scores. *Indicates a significant difference (p<0.05) comparing pre- and postoperative scores.
Similarly, the changes from the preoperative to the follow-up assessments showed no significant differences for PCS [ANOVA: F(2,207)<0.38, p>0.67], HADS-A [ANOVA: F(2,204)<0.37, p>0.69], and HADS-D [ANOVA: F(2,204)<0.37, p>0.69] comparing the three treatment groups.
3.3 Associations between preoperative parameters and postoperative pain
The correlation analysis revealed weak, but significant correlations between preoperative HADS-D (r=0.146, p=0.034) and a trend towards significantly less pain during walking (r=0.133, p=0.051) and postoperative pain during walking. Further, preoperative pain at rest and postoperative pain at rest (r=0.147, p=0.032) were found to be associated. Finally, preoperative PCS (r=0.159, p=0.023) and a trend towards significant for preoperative HADS-D (r=0.133, p=0.055) were associated with postoperative pain during flexion of the hip (Table 2).
Pearson correlation coefficients (r) between pre- and post-operative factors.
| Preoperative factors associated with postoperative outcomes (unadjusted) | |||||
|---|---|---|---|---|---|
| Postoperative pain scores |
|||||
| Pain during walking, NRS-100 mm | Pain at rest, NRS-100 mm | Pain flex of the hip, NRS-100 mm | Pain flex of the knee, NRS-100 mm | ||
| Preoperative measures | Pain during walking, NRS-100 mm | r=0.133 p=0.051 |
|||
| Pain at rest, NRS-100 mm | r=0.147 p=0.032 |
||||
| Pain flex of the hip, NRS-100 mm | – | ||||
| Pain flex of the knee, NRS-100 mm | – | ||||
| PCS | – | – | r=0.159 p=0.023 |
– | |
| HADS-A | – | – | – | – | |
| HADS-D | r=0.146 p=0.034 |
– | r=0.133 p=0.055 |
– | |
-
PCS=pain catastrophizing scale; HADS-A=hospital anxiety and depression scale – anxiety subscale; HADS-D=hospital anxiety and depression scale – depression subscale.
Adjusting these correlations for the treatment group yielded similar results indicating that treatment with gabapentin did not affect these associations and further indicating that treatment with gabapentin had no effect on the associations between preoperative and postoperative factors (Table 3).
Correlations coefficients (r) between pre- and post-operative factors adjusted for gabapentin treatment.
| Preoperative factors associated with postoperative outcomes (adjusted for preoperative gabapentin treatment) |
|||||
|---|---|---|---|---|---|
| Postoperative pain scores |
|||||
| Pain during walking, NRS-100 mm | Pain at rest, NRS-100 mm | Pain flex of the hip, NRS-100 mm | Pain flex of the knee, NRS-100 mm | ||
| Preoperative measures | Pain during walking, NRS-100 mm | r=0.162 p=0.022 |
|||
| Pain at rest, NRS-100 mm | r=0.153 p=0.031 |
||||
| Pain flex of the hip, NRS-100 mm | – | ||||
| Pain flex of the knee, NRS-100 mm | – | ||||
| PCS | – | – | r=0.135 p=0.058 |
– | |
| HADS-A | – | – | – | – | |
| HADS-D | r=0.164 p=0.020 |
– | r=0.156 p=0.028 |
– | |
-
PCS=pain catastrophizing scale; HADS-A=hospital anxiety and depression scale – anxiety subscale; HADS-D=hospital anxiety and depression scale – depression subscale.
Linear regression models found preoperative HADS-D as an independent predictor for postoperative pain while walking (p=0.013) and pain at rest (p=0.013). In addition, preoperative HADS-A independently predicted postoperative pain at rest (p=0.015) and a trend for preoperative pain at rest as an independent preoperative predictor of postoperative pain at rest (p=0.051). The treatment with gabapentin was not found to influence these models (p>0.17), indicating that the gabapentin treatment did not improve the predictive value.
4 Discussion
Despite being a secondary follow-up of a primary RCT, this study is the first large trial to investigate the effect of pre- and perioperative administration of gabapentin on long-term pain after TKA. Gabapentin did not influence the chronic pain outcome or revision rate at 3–4 years follow-up. Further, preoperative HADS-D independently predicted postoperative pain while walking and at rest and preoperative HADS-A independently predicted pain at rest, but gabapentin was not associated with these predictions.
4.1 Effects of gabapentinoids on chronic postoperative pain
Yang et al. [38] studied the effect of continuous infusion of pregabalin on peripheral nerve injuries in rats and while pregabalin did affect the responses to an experimental pain test, there was no preventive effect of pregabalin on postoperative neuropathic pain compared with placebo. In the context of TKA, Buvanendran et al. [17] administered pregabalin 300 mg on the day of TKA surgery and 300 mg/daily in the following 10 postoperative days and found this to reduce the risk of postoperative neuropathic pain at 3- and 6-months follow-up compared with placebo. Several old reviews and meta-analyses have highlighted a pain-relieving effect of preoperative administration of gabapentin on pain in the first postoperative days [18], [19], [20], [21], [22]. In contrast, more recent reviews have highlighted that gabapentinoids might have minimal opioid sparring effect in the acute postoperative phase, but the severe adverse events are increased [24], and the evidence is based on low quality studies [23]. A recent review on the effect of preoperative administration of gabapentin and pregabalin prior to breast cancer surgery found no associations between the administration of either drug on the development of chronic postoperative pain [39]. Conclusively, a recent review and meta-analysis [40] summarized the administration of perioperative pregabalin across several surgical interventions and found no preventive effect on the development of pain 3, 6 and 12-months after surgery. The current study supports no additional effect by the administration of short time preoperative gabapentin on chronic postoperative pain and psychological factors such as pain catastrophizing, anxiety, or depression. The current study did not support the use of gabapentin for patients scheduled for TKA for the prevention of chronic postoperative pain.
4.2 Independent preoperative factors predicting chronic postoperative pain
Preoperative pain intensity has been associated with chronic postoperative pain after, e.g. amputation [41], [42], breast cancer surgery [43], and total joint replacement [4]. Recent studies indicate that other preoperative factors could contribute to the preoperative pain intensity and that these factors are more predictive for the chronic postoperative pain than the pain intensity itself [8], [11]. Contrary to this, Wylde et al. [44] found that both preoperative pain at rest and while moving were not associated with pain 12-months after TKA surgery. Similarly, too previously, the current study found that preoperative pain while walking and at rest was associated with chronic postoperative pain but this study added that none of these was independent predictive factors.
Several studies have found that postoperative pain catastrophizing scores are lower following e.g. spine surgery for lumbar spinal stenosis, abdominal surgery, and TKA surgery [45], [46]. In addition, preoperative psychological factors have been found to be associated with postoperative pain following various surgical procedures [4], [46], [47], [48]. In the context of TKA, Lewis et al. [49] in a meta-analysis of 32 studies and found significant effects for catastrophizing, preoperative pain, mental health, and comorbidities for pain of at least 3 months after surgery. In contrast, Wylde et al. [50] found preoperative HADS-A to influence postoperative pain at 1 year, but not at 5 year follow-up after TKA surgery, and preoperative PCS, HADS-D and pain self-efficacy were not found to be associated with pain at 1 year or at 5 year follow-up. Similarly, the current study found no association between preoperative PCS and postoperative pain scores. The current study did find HADS-D to be independently associated with postoperative pain while walking and at rest and preoperative HADS-A to be independently associated with postoperative pain at rest, but notably all of the associates were weak.
Osteoarthritic pain develops over many years [51], and pain duration is associated with increased pain intensity and pain sensitivity [52]. The mean maximum plasma concentration of gabapentin is reached 3 h after oral administration, and the half-life time is 5–9 h indicating that three daily administrations are needed to maintain an optimal plasma level [53]. Future studies could focus on long-term preoperative treatment to identify whether long-term gabapentin influences pain and pain sensitivity and whether this is associated with lowered risk of chronic postoperative pain.
5 Conclusion
The current study showed that pre- and perioperative administrated gabapentin does not influence the development of chronic postoperative pain or the revision rate at 3–4 year follow-up after TKA. Thus, it does not support that short-term pre- and perioperative use of gabapentin can reduce the development of chronic postoperative pain after TKA.
-
Authors’ statements
-
Research funding: The study was supported with a research grant from The Lundbeck Foundation, Hellerup, Denmark, which is independent of the pharmaceutical company, Lundbeck Pharma, Denmark.
-
Conflict of interest: None declared.
-
Informed Consent: Oral and written informed consents were obtained from all patients.
-
Ethical Approval: The primary study was approved by the Danish Medicines Agency, the regional ethics committee, and the Danish Data Protection Agency and was registered at EudraCT (2011-003105-22) and www.clinicaltrials.gov (NCT01507363, January 6, 2012).
References
[1] Singh JA, Vessely MB, Harmsen WS, Schleck CD, Melton LJ, Kurland RL, Berry DJ. A population-based study of trends in the use of total hip and total knee arthroplasty, 1969–2008. Mayo Clin Proc 2010;85:898–904.10.4065/mcp.2010.0115Suche in Google Scholar PubMed PubMed Central
[2] Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J bone Jt Surg 2007;89:780–5.10.2106/JBJS.F.00222Suche in Google Scholar PubMed
[3] Beswick AD, Wylde V, Gooberman-Hill R, Blom A, Dieppe P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open 2012;2:e000435.10.1136/bmjopen-2011-000435Suche in Google Scholar PubMed PubMed Central
[4] Petersen KK, Arendt-Nielsen L. Chronic postoperative pain after joint replacement. Pain Clin Updat 2016;24. Available at: https://s3.amazonaws.com/rdcms-iasp/files/production/public/AM/Images/PCU/PCU%2024-3_FINALFOR%20WEB.pdf.Suche in Google Scholar
[5] Lavand’homme P, Thienpont E. Pain after total knee arthroplasty. Bone Joint J 2015;97:45–8.10.1302/0301-620X.97B10.36524Suche in Google Scholar PubMed
[6] Baert IAC, Lluch E, Mulder T, Nijs J, Noten S, Meeus M. Does pre-surgical central modulation of pain influence outcome after total knee replacement? A systematic review. Osteoarthr Cartil 2016;24:213–23.10.1016/j.joca.2015.09.002Suche in Google Scholar PubMed
[7] Arendt-Nielsen L, Skou ST, Nielsen TA, Petersen KK. Altered central sensitization and pain modulation in the CNS in chronic joint pain. Curr Osteoporos Rep 2015;13:225–34.10.1007/s11914-015-0276-xSuche in Google Scholar PubMed
[8] Petersen KK, Arendt-Nielsen L, Simonsen O, Wilder-Smith O, Laursen MB. Presurgical assessment of temporal summation of pain predicts the development of chronic postoperative pain 12 months after total knee replacement. Pain 2015;156:55–61.10.1016/j.pain.0000000000000022Suche in Google Scholar PubMed
[9] Wylde V, Hewlett S, Learmonth ID, Dieppe P. Persistent pain after joint replacement: prevalence, sensory qualities, and postoperative determinants. Pain 2011;152:566–72.10.1016/j.pain.2010.11.023Suche in Google Scholar PubMed
[10] Izumi M, Petersen KK, Laursen MB, Arendt-Nielsen L, Graven-Nielsen T. Facilitated temporal summation of pain correlates with clinical pain intensity after hip arthroplasty. Pain 2017;158:323–32.10.1097/j.pain.0000000000000764Suche in Google Scholar PubMed
[11] Petersen KK, Graven-Nielsen T, Simonsen O, Laursen MB, Arendt-Nielsen L. Preoperative pain mechanisms assessed by cuff algometry are associated with chronic postoperative pain relief after total knee replacement. Pain 2016;157: 1400–6.10.1097/j.pain.0000000000000531Suche in Google Scholar PubMed
[12] Petersen KK, Simonsen O, Laursen MB, Arendt-Nielsen L. The role of preoperative radiological severity, sensory testing, and temporal summation on chronic postoperative pain following total knee arthroplasty. Clin J Pain 2018;34:193–7.10.1097/AJP.0000000000000528Suche in Google Scholar PubMed
[13] Ben-Menachem E. Pregabalin pharmacology and its relevance to clinical practice. Epilepsia 2004;45(s6):13–8.10.1111/j.0013-9580.2004.455003.xSuche in Google Scholar PubMed
[14] Nutt D, Mandel F, Baldinetti F. Early onset anxiolytic efficacy after a single dose of pregabalin: double-blind, placebo- and active-comparator controlled evaluation using a dental anxiety model. J Psychopharmacol 2009;23:867–73.10.1177/0269881108094722Suche in Google Scholar PubMed
[15] Gajraj NM. Pregabalin: its pharmacology and use in pain management. Anesth Analg 2007;105:1805–15.10.1213/01.ane.0000287643.13410.5eSuche in Google Scholar PubMed
[16] Arendt-Nielsen L, Frøkjær JB, Staahl C, Graven-Nielsen T, Huggins JP, Smart TS, Drewes AM. Effects of gabapentin on experimental somatic pain and temporal summation. Reg Anesth Pain Med 2007;32:382–8.10.1097/00115550-200709000-00004Suche in Google Scholar
[17] Buvanendran A, Kroin JS, Della Valle CJ, Kari M, Moric M, Tuman KJ. Perioperative oral pregabalin reduces chronic pain after total knee arthroplasty: a prospective, randomized, controlled trial. Anesth Analg 2010;110:199–207.10.1213/ANE.0b013e3181c4273aSuche in Google Scholar PubMed
[18] Ho K-Y, Gan TJ, Habib AS. Gabapentin and postoperative pain – a systematic review of randomized controlled trials. Pain 2006;126:91–101.10.1016/j.pain.2006.06.018Suche in Google Scholar PubMed
[19] Hurley RW, Cohen SP, Williams KA, Rowlingson AJ, Wu CL. The analgesic effects of perioperative gabapentin on postoperative pain: a meta-analysis. Reg Anesth Pain Med 2006;31:237–47.10.1016/j.rapm.2006.01.005Suche in Google Scholar PubMed
[20] Mathiesen O, Møiniche S, Dahl JB. Gabapentin and postoperative pain: a qualitative and quantitative systematic review, with focus on procedure. BMC Anesthesiol 2007;7:6–21.10.1186/1471-2253-7-6Suche in Google Scholar PubMed PubMed Central
[21] Straube S, Derry S, Moore RA, Wiffen PJ, McQuay HJ. Single dose oral gabapentin for established acute postoperative pain in adults. Cochrane database Syst Rev 2013;5:20464764.Suche in Google Scholar
[22] Tiippana EM, Hamunen K, Kontinen VK, Kalso E. Do surgical patients benefit from perioperative gabapentin/pregabalin? A systematic review of efficacy and safety. Anesth Analg 2007;104:1545–56.10.1213/01.ane.0000261517.27532.80Suche in Google Scholar PubMed
[23] Fabritius ML, Geisler A, Petersen PL, Nikolajsen L, Hansen MS, Kontinen V, Hamunen K, Dahl JB, Wetterslev J, Mathiesen O. Gabapentin for post-operative pain management – a systematic review with meta-analyses and trial sequential analyses. Acta Anaesthesiol Scand 2016;60:1188–208.10.1111/aas.12766Suche in Google Scholar PubMed
[24] Fabritius ML, Strøm C, Koyuncu S, Jæger P, Petersen PL, Geisler A, Wetterslev J, Dahl JB, Mathiesen O. Benefit and harm of pregabalin in acute pain treatment: a systematic review with meta-analyses and trial sequential analyses. BJA Br J Anaesth. 2017;0(September):1–17.10.1093/bja/aex227Suche in Google Scholar PubMed
[25] Bockbrader HN, Wesche D, Miller R, Chapel S, Janiczek N, Burger P. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin Pharmacokinet 2010;49:661–9.10.2165/11536200-000000000-00000Suche in Google Scholar PubMed
[26] Weinbroum AA. Non-opioid IV adjuvants in the perioperative period: pharmacological and clinical aspects of ketamine and gabapentinoids. Pharmacol Res 2012;65:411–29.10.1016/j.phrs.2012.01.002Suche in Google Scholar PubMed
[27] Schmidt PC, Ruchelli G, Mackey SC, Carroll IR. Perioperative gabapentinoids: choice of agent, dose, timing, and effects on chronic postsurgical pain. Anesthesiology 2013;119:1215–21.10.1097/ALN.0b013e3182a9a896Suche in Google Scholar PubMed
[28] Patel R, Dickenson AH. Mechanisms of the gabapentinoids and α 2 δ-1 calcium channel subunit in neuropathic pain. Pharmacol Res Perspect 2016;4:e00205.10.1002/prp2.205Suche in Google Scholar PubMed PubMed Central
[29] Carlton SM, Zhou S. Attenuation of formalin-induced nociceptive behaviors following local peripheral injection of gabapentin. Pain 1998;76:201–7.10.1016/S0304-3959(98)00043-8Suche in Google Scholar
[30] Sutton KG, Martin DJ, Pinnock RD, Lee K, Scott RH. Gabapentin inhibits high-threshold calcium channel currents in cultured rat dorsal root ganglion neurones. Br J Pharmacol 2002;135:257–65.10.1038/sj.bjp.0704439Suche in Google Scholar PubMed PubMed Central
[31] Bannister K, Qu C, Navratilova E, Oyarzo J, Xie JY, King T, Dickenson AH, Porreca F. Multiple sites and actions of gabapentin-induced relief of ongoing experimental neuropathic pain. Pain 2017;158:2386–95.10.1097/j.pain.0000000000001040Suche in Google Scholar PubMed PubMed Central
[32] Weissman-Fogel I, Granovsky Y, Crispel Y, Ben-Nun A, Best LA, Yarnitsky D, Granot M. Enhanced presurgical pain temporal summation response predicts post-thoracotomy pain intensity during the acute postoperative phase. J Pain 2009;10:628–36.10.1016/j.jpain.2008.12.009Suche in Google Scholar PubMed
[33] Lunn TH, Husted H, Laursen MB, Hansen LT, Kehlet H. Analgesic and sedative effects of perioperative gabapentin in total knee arthroplasty. Pain 2015;156:2438–48.10.1097/j.pain.0000000000000309Suche in Google Scholar PubMed
[34] Khan ZH, Rahimi M, Makarem J, Khan RH. Optimal dose of pre-incision/post-incision gabapentin for pain relief following lumbar laminectomy: a randomized study. Acta Anaesthesiol Scand 2011;55:306–12.10.1111/j.1399-6576.2010.02377.xSuche in Google Scholar PubMed
[35] Pandey CK, Navkar DV, Giri PJ, Raza M, Behari S, Singh RB, Singh U, Singh PK. Evaluation of the optimal preemptive dose of gabapentin for postoperative pain relief after lumbar diskectomy. J Neurosurg Anesthesiol 2005;17:65–8.10.1097/01.ana.0000151407.62650.51Suche in Google Scholar PubMed
[36] Zigmond A, Snaith R. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70.10.1111/j.1600-0447.1983.tb09716.xSuche in Google Scholar PubMed
[37] Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess 1995;7:524–32.10.1037//1040-3590.7.4.524Suche in Google Scholar
[38] Yang F, Whang J, Derry WT, Vardeh D, Scholz J. Analgesic treatment with pregabalin does not prevent persistent pain after peripheral nerve injury in the rat. Pain 2014;155:356–66.10.1016/j.pain.2013.10.024Suche in Google Scholar PubMed
[39] Rai AS, Khan JS, Dhaliwal J, Busse JW, Choi S, Devereaux P, Clarke H. Preoperative pregabalin or gabapentin for postoperative acute and chronic pain among patients undergoing breast cancer surgery: a systematic review and meta- analysis of randomized controlled trials. J Plast Reconstr Aesthetic Surg 2017;70:1317–28.10.1016/j.bjps.2017.05.054Suche in Google Scholar PubMed
[40] Martinez V, Pichard X, Fletcher D. Perioperative pregabalin administration does not prevent chronic postoperative pain: systematic review with a meta-analysis of randomized trials. Pain 2017;158:775–83.10.1097/j.pain.0000000000000838Suche in Google Scholar PubMed
[41] Nikolajsen L, Ilkjær S, Krøner K, Christensen JH, Jensen TS. The influence of preamputation pain on postamputation stump and phantom pain. Pain 1997;72:393–405.10.1016/S0304-3959(97)00061-4Suche in Google Scholar PubMed
[42] Jensen TS, Krebs B, Nielsen J, Rasmussen P. Immediate and long-term phantom limb pain in amputees: incidence, clinical characteristics and relationship to pre-amputation pain. Pain 1985;21:267–78.10.1016/0304-3959(85)90090-9Suche in Google Scholar PubMed
[43] Wang L, Guyatt GH, Kennedy SA, Mha BR, Romerosa B, Kwon HY, Kaushal A, Chang Y, Craigie S, de Almeida CPB, Couban RJ, Parascandalo SR, Izhar Z, Reid S, Khan JS, McGillion M, Busse JW. Predictors of persistent pain after breast cancer surgery: a systematic review and meta-analysis of observational studies. Can Med Assoc J 2016;188:1–10.10.1503/cmaj.151276Suche in Google Scholar PubMed PubMed Central
[44] Wylde V, Sayers A, Lenguerrand E, Gooberman-Hill R, Pyke M, Beswick AD, Dieppe P, Blom AW. Preoperative widespread pain sensitization and chronic pain after hip and knee replacement. Pain 2015;156:47–54.10.1016/j.pain.0000000000000002Suche in Google Scholar PubMed PubMed Central
[45] Kim H-J, Kwon OH, Chang B-S, Lee C-K, Chun H-J, Yeom JS. Change in pain catastrophizing in patients with lumbar spinal surgery. Spine J 2018;18:115–21.10.1016/j.spinee.2017.06.028Suche in Google Scholar PubMed
[46] Jackson T, Tian P, Wang Y, Iezzi T, Xie W. Toward identifying moderators of associations between presurgery emotional distress and postoperative pain outcomes: a meta-analysis of longitudinal studies. J Pain 2016;17:874–88.10.1016/j.jpain.2016.04.003Suche in Google Scholar PubMed
[47] Burns LC, Ritvo SE, Ferguson MK, Clarke H, Seltzer Z, Katz J. Pain catastrophizing as a risk factor for chronic pain after total knee arthroplasty: a systematic review. J Pain Res 2015;8:21–32.10.2147/JPR.S64730Suche in Google Scholar PubMed PubMed Central
[48] Rabbitts JA, Fisher E, Rosenbloom BN, Palermo TM. Prevalence and predictors of chronic postsurgical pain in children: a systematic review and meta-analysis. J Pain 2017;18:605–14.10.1016/j.jpain.2017.03.007Suche in Google Scholar PubMed PubMed Central
[49] Lewis GN, Rice DA, McNair PJ, Kluger M. Predictors of persistent pain after total knee arthroplasty: a systematic review and meta-analysis. Br J Anaesth 2015;114:551–61.10.1093/bja/aeu441Suche in Google Scholar PubMed
[50] Wylde V, Trela-Larsen L, Whitehouse MR, Blom AW. Preoperative psychosocial risk factors for poor outcomes at 1 and 5 years after total knee replacement. Acta Orthop 2017;3674(July):1–7.10.1080/17453674.2017.1334180Suche in Google Scholar PubMed PubMed Central
[51] Felson DT. The sources of pain in knee osteoarthritis. Curr Opin Rheumatol 2005;17:624–8.10.1097/01.bor.0000172800.49120.97Suche in Google Scholar PubMed
[52] Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, Graven-Nielsen T. Sensitization in patients with painful knee osteoarthritis. Pain 2010;149:573–81.10.1016/j.pain.2010.04.003Suche in Google Scholar PubMed
[53] McLean MJ. Clinical pharmacokinetics of gabapentin. Neurology 1994;44(6 Suppl 5):S17–22.Suche in Google Scholar
©2018 Scandinavian Association for the Study of Pain. Published by Walter de Gruyter GmbH, Berlin/Boston. All rights reserved.
Artikel in diesem Heft
- Frontmatter
- Topical review
- Reducing risk of spinal haematoma from spinal and epidural pain procedures
- Clinical pain research
- A multiple-dose double-blind randomized study to evaluate the safety, pharmacokinetics, pharmacodynamics and analgesic efficacy of the TRPV1 antagonist JNJ-39439335 (mavatrep)
- Reliability of three linguistically and culturally validated pain assessment tools for sedated ICU patients by ICU nurses in Finland
- Superior outcomes following cervical fusion vs. multimodal rehabilitation in a subgroup of randomized Whiplash-Associated-Disorders (WAD) patients indicating somatic pain origin-Comparison of outcome assessments made by four examiners from different disciplines
- Morning cortisol and fasting glucose are elevated in women with chronic widespread pain independent of comorbid restless legs syndrome
- Chronic pain experience and pain management in persons with spinal cord injury in Nepal
- The Standardised Mensendieck Test as a tool for evaluation of movement quality in patients with nonspecific chronic low back pain
- Exploring effect of pain education on chronic pain patients’ expectation of recovery and pain intensity
- Pain, psychological distress and motor pattern in women with provoked vestibulodynia (PVD) – symptom characteristics and therapy suggestions
- Relative and absolute test-retest reliabilities of pressure pain threshold in patients with knee osteoarthritis
- The influence of pre- and perioperative administration of gabapentin on pain 3–4 years after total knee arthroplasty
- Observational study
- CT guided neurolytic blockade of the coeliac plexus in patients with advanced and intractably painful pancreatic cancer
- Prescription of opioids to post-operative orthopaedic patients at time of discharge from hospital: a prospective observational study
- The psychological features of patellofemoral pain: a cross-sectional study
- Prevalence of self-reported musculoskeletal pain symptoms among school-age adolescents: age and sex differences
- The association between back muscle characteristics and pressure pain sensitivity in low back pain patients
- Postural control in subclinical neck pain: a comparative study on the effect of pain and measurement procedures
- Original experimental
- Exercise-induced hypoalgesia in women with varying levels of menstrual pain
- Exercise does not produce hypoalgesia when performed immediately after a painful stimulus
- Effectiveness of neck stabilisation and dynamic exercises on pain intensity, depression and anxiety among patients with non-specific neck pain: a randomised controlled trial
Artikel in diesem Heft
- Frontmatter
- Topical review
- Reducing risk of spinal haematoma from spinal and epidural pain procedures
- Clinical pain research
- A multiple-dose double-blind randomized study to evaluate the safety, pharmacokinetics, pharmacodynamics and analgesic efficacy of the TRPV1 antagonist JNJ-39439335 (mavatrep)
- Reliability of three linguistically and culturally validated pain assessment tools for sedated ICU patients by ICU nurses in Finland
- Superior outcomes following cervical fusion vs. multimodal rehabilitation in a subgroup of randomized Whiplash-Associated-Disorders (WAD) patients indicating somatic pain origin-Comparison of outcome assessments made by four examiners from different disciplines
- Morning cortisol and fasting glucose are elevated in women with chronic widespread pain independent of comorbid restless legs syndrome
- Chronic pain experience and pain management in persons with spinal cord injury in Nepal
- The Standardised Mensendieck Test as a tool for evaluation of movement quality in patients with nonspecific chronic low back pain
- Exploring effect of pain education on chronic pain patients’ expectation of recovery and pain intensity
- Pain, psychological distress and motor pattern in women with provoked vestibulodynia (PVD) – symptom characteristics and therapy suggestions
- Relative and absolute test-retest reliabilities of pressure pain threshold in patients with knee osteoarthritis
- The influence of pre- and perioperative administration of gabapentin on pain 3–4 years after total knee arthroplasty
- Observational study
- CT guided neurolytic blockade of the coeliac plexus in patients with advanced and intractably painful pancreatic cancer
- Prescription of opioids to post-operative orthopaedic patients at time of discharge from hospital: a prospective observational study
- The psychological features of patellofemoral pain: a cross-sectional study
- Prevalence of self-reported musculoskeletal pain symptoms among school-age adolescents: age and sex differences
- The association between back muscle characteristics and pressure pain sensitivity in low back pain patients
- Postural control in subclinical neck pain: a comparative study on the effect of pain and measurement procedures
- Original experimental
- Exercise-induced hypoalgesia in women with varying levels of menstrual pain
- Exercise does not produce hypoalgesia when performed immediately after a painful stimulus
- Effectiveness of neck stabilisation and dynamic exercises on pain intensity, depression and anxiety among patients with non-specific neck pain: a randomised controlled trial

