Abstract
Background and aims:
Exercise-induced hypoalgesia (EIH) and conditioned pain modulation (CPM) are assumed to reflect descending pain inhibition. Potential interactions between EIH and CPM may be important in the therapy of chronic pain, as reduced CPM and increased pain after exercise are frequently observed. This study compared the EIH response after CPM was activated using a cold pressor task with the EIH response after a control condition.
Methods:
Thirty-one participants (age: 27.7±9.8; 15 female) completed two sessions: a cold pressor task (CPT) session, i.e. testing EIH with preceding CPM activation induced using a 2 min CPT at approximately 2°C, and a control session, i.e. testing EIH after a control condition (2 min of quiet rest). EIH was induced using a 15 min bicycling exercise at a target heart rate corresponding to 75% VO2 max. Repeated measures ANOVAs on pressure pain thresholds (PPTs) at the hand, back and leg were used to determine the effects of exercise after the cold pressor test and control condition. Furthermore, correlations between CPM and EIH, in the CPT session as well as control session, were calculated at each assessment site.
Results:
A significant time x condition interaction (F(1, 30)=43.61, p<0.001, partial η2=0.59), with Bonferroni-corrected post-hoc t-tests showed that PPTs increased after exercise in the control session (p<0.001), but not in the CPT session (p=0.125). Furthermore, there was a small positive correlation of EIH in the control session and CPM at the hand (r=0.37, p=0.043). There was a moderate negative correlation of EIH in the CPT session and CPM at the hand (r=−0.50, p=0.004), and smaller negative correlations at the back (r=−0.37, p=0.036) and at the leg (r=−0.35, p=0.054).
Conclusions:
Attenuated EIH after the CPM activation in comparison to a control condition suggests that EIH and CPM may share underlying pain inhibitory mechanisms on a systemic level. This assumption is further supported by the finding of small to moderate significant correlations between EIH and CPM at the hand. The attenuated EIH response furthermore suggests that these mechanisms are exhaustible, i.e. that its effects decline after a certain amount of inhibition.
Implications:
In patients with chronic pain, assessing the current capacity of the descending pain inhibitory system – as indicated by the CPM response – may aid to make better predictions about how patients will respond to exercise with respect to acute pain reduction.
1 Introduction
Physical exercise is an important component in treatment and rehabilitation of patients with chronic pain [1]. In pain-free individuals, acute exercise can transiently reduce pain sensitivity, known as exercise-induced hypoalgesia (EIH) [2]. EIH is often reported when assessed at non-exercising muscles, but greater increases in pressure pain thresholds (PPTs) are seen at the exercising muscles [3], [4], [5], [6], [7]. This suggests that EIH involves – in addition to peripheral or segmental pain modulatory processes – a systemic component of central descending inhibition, which is reflected in EIH effects at non-exercising muscles.
The existence of EIH in subjects with chronic pain is still controversial [1]. Clinically, it is well known that some patients report increasing pain after exercise. This is in agreement with studies observing that a subset of patients with chronic pain demonstrates impaired EIH responses [2], [8], [9], [10], or even hyperalgesia after exercise [11] compared with pain-free controls.
A reason for EIH being reduced when pain is present could be related to aberrant conditioned pain modulation (CPM). CPM delineates a decrease in pain sensitivity after a painful stimulus. As CPM is observable at stimulated and non-stimulated body parts, it has been suggested that it mainly operates on a systemic level and reflects descending pain inhibition [12]. More consistently than attenuated EIH, reduced CPM responses characterize patients with chronic pain [13], [14]. Taken together, these observations have led to the question whether EIH and CPM share underlying mechanisms. Accordingly, small positive correlations were reported between EIH and CPM in pain-free subjects [15], [16], [17] and subjects with musculoskeletal pain [18]. These findings imply that EIH and CPM may be related to each other on a systemic level [19].
Furthermore, it has been proposed before that CPM might be exhaustible, meaning that once a certain amount of descending inhibition is reached, no further inhibition is possible [20], [21], [22], [23]. In chronic pain, the CPM system might be constantly active due to clinical pain, which is reflected in reduced CPM responses. Likewise, EIH responses might be reduced in chronic pain because they vary depending on pre-existing activation of the CPM system. A recent study supports this hypothesis by demonstrating that patients with knee osteoarthritis, who showed no CPM response, also showed hyperalgesia after exercise at remote assessment sites [24].
By contrast, however, there are also studies suggesting that EIH and CPM affect pain sensitivity independently [25], showing no correlations between EIH and CPM responses [3], [20], [26]. Still, studies testing direct interactions between EIH and CPM in pain-free participants are rare. However, such studies might contribute to better understand EIH in chronic pain, as it is possibly related to aberrant CPM. Thus, the aim of this study was to investigate EIH responses at exercising and non-exercising body parts after CPM was activated in pain-free subjects. We hypothesized that EIH would be reduced when exercise was performed immediately after activation of CPM compared to EIH after a control condition. Furthermore, we expected that CPM activation would affect the subsequent EIH response at non-exercising muscles to a greater extent than at the exercising muscles.
2 Materials and methods
2.1 Subjects
Thirty-one healthy subjects (age: 27.7±9.8 years; 15 women) participated in this study. Subjects were recruited via personal contacts of KN as a part of her medical dissertation. All subjects were naive to experimental pain testing. None of the included subjects suffered from neurological, psychological or cardiovascular diseases or had experienced pain during the weeks prior to participation. Before the experiment, participants were asked to refrain from any pain medication and vigorous exercise for 24 h, and caffeine for 4 h. The study was conducted in accordance with the Declaration of Helsinki and approved by ethical review board of the psychological faculty at the Ruhr-University of Bochum (application #242). All subjects gave written informed consent before participating in the study.
2.2 Protocol
In this repeated-measures within-subject study design, all participants completed two experimental sessions separated by 1–2 weeks. However, due to scheduling conflicts, in one subject the sessions were separated by 6 weeks. In each of the two sessions, subjects performed an aerobic bicycling exercise with a duration of 15 min, preceded by either a cold pressor task (CPT) or a duration-matched control condition (Fig. 1). The order of the sessions was randomized and counterbalanced. Pressure pain thresholds (PPTs) were used to assess pain sensitivity before and after the control condition, the cold pressor task and the bicycling exercise.
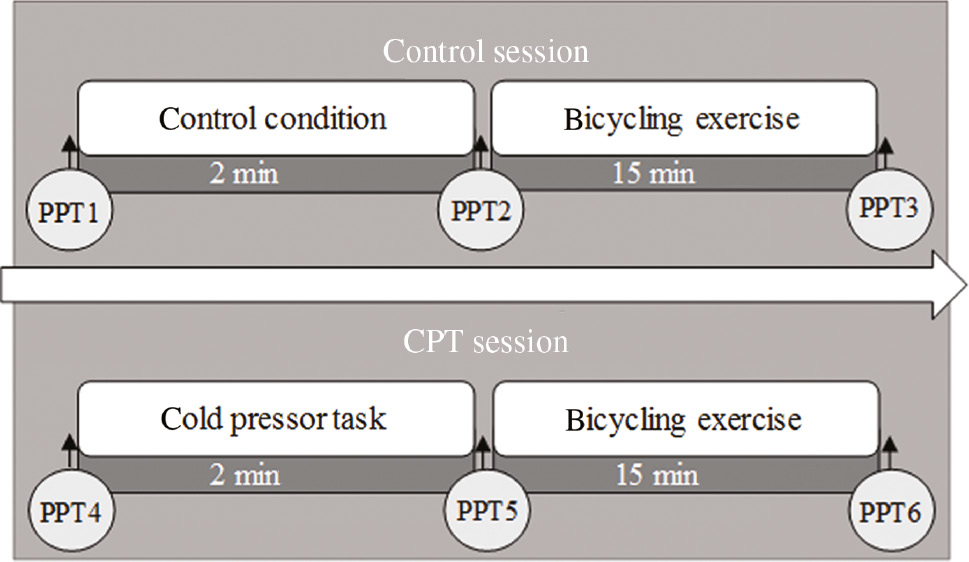
Schematic illustration of the experimental protocol performed during the two experimental sessions. Pressure pain thresholds (PPTs) were assessed on three assessment sites (hand, back and leg) before and immediately after cold pressor task, a control condition, and exercise.
First, all subjects were introduced to the protocol for assessment of PPTs, where they were told that the experimenter would slowly increase the pressure on the respective muscles. Subjects were instructed to say “stop” as soon as the perception of pressure changed into the first perception of pain. The experimenter emphasized that the PPT was not a measure of how much pain they could tolerate. After the instruction subjects completed a practice trial at the middle of the thenar eminence of the right hand to ensure that they understood the procedure. In each session, subjects were familiarized with the PPT procedure 5 min before the first assessment.
2.3 Assessment of pressure pain thresholds
PPTs were assessed at 1) the thenar eminence of the non-dominant hand, 2) the lower back at the non-dominant side approximately 2 cm adjacent to the spine at the level of the 3rd lumbar vertebra, and 3) the middle of the non-dominant biceps femoris muscle. PPTs were assessed with a handheld algometer (Somedic Sales AB, Horby, Sweden). The stimulation area was 1 cm2 and the rate of pressure increase was kept to approximately 50 kPa/s. PPTs were assessed with the subject lying in prone position on the examination table and the order of assessment was counterbalanced and randomized. Two PPT assessments were completed for each assessment site and the mean of these was used for analysis [27]. Twenty-second intervals between assessments were maintained. All assessments were performed by female assessors (KN and HG).
2.4 Bicycling exercise
The bicycling exercise lasted for 15 min. Prior to the exercise, the age-related target heart rate was determined for each subject. Based on a previously used aerobic exercise protocol demonstrating robust EIH [16], a target heart rate of approximately 86% of the maximal age-related heart rate was chosen. This target heart rate corresponds to 75% VO2max [28]. Subjects performed the exercise on a stationary ergometer with a build in heart rate monitor using a heart rate belt that was strapped around the chest (Corival cpet, Lode, Groningen, the Netherlands). The subjects were asked to maintain a pedal rate of 70 rounds per minute throughout the 15 min.
The first 2 min of the exercise were used as a warm-up. After the 2 min, resistance was increased over the next 3 min until the target heart rate was reached. The heart rate was monitored continuously and resistance was altered in order to maintain the target heart rate if needed. Thirty seconds before completion of the exercise, subjects were asked to rate their level of perceived exertion due to the exercise (Borg 6–20 RPE scale [29]).
2.5 Cold pressor task and the control condition
Subjects performed the 2 min cold pressor task (CPT) in a sitting position. Subjects were asked to immerse their dominant hand to 2 cm above the wrist into an in-house custom made water bath with circulating ice water. The temperature was kept constant at approximately 2°C. The instruction was to not move the hand or the joints of the fingers while performing the cold pressor task. Just before removing the hand, subjects were asked to rate the pain intensity caused by the ice water bath on a numerical rating scale ranging from 0 to 10, with 0 indicating no pain and 10 indicating the worst pain imaginable.
For the control condition, subjects were instructed to relax comfortably in sitting position for 2 min in an undisturbed room, which was the same in which the CPT was performed.
2.6 Statistical analysis
All statistical analyses were performed using SPSS (Version 25, IBM, Armonk, NY, USA). Results are presented as means and standard deviations, unless otherwise specified. To assess potential differences in PPT values at baseline (PPT1 vs. PPT4, see Fig. 1), a two-way repeated measures analysis of variance (rm-ANOVA) was conducted with the assessment site (hand, back and leg) and session (control session, CPT session) as within-subject factors.
In order to validate the CPM protocol in a first step, a three-way rm-ANOVA with the within-subject factors time (pre, post), session (CPT session, control session) and assessment site (hand, back, leg) was performed. This analysis was used to determine whether the activation of CPM was successful, i.e. whether the cold pressor task induced a significant increase in PPTs compared to the control condition at each assessment site.
In the main analysis, the effect of a preceding CPT vs. control condition on the subsequent EIH response was analyzed using a three-way rm-ANOVA with the within-subject factors time (pre, post exercise), session (CPT session, control session) and assessment site (hand, back, leg).
Greenhouse-Geisser corrections were used in case of sphericity violations and p-values less than 0.05 were considered significant. Partial η2 (ηp2) was used to estimate the effect size of main effects or interactions in the rm-ANOVAs. In case of significant factors or interactions in the rm-ANOVAs, post-hoc comparisons incorporating Bonferroni-corrections for the multiple comparisons were performed with paired t-tests. Cohen’s d was calculated in order to estimate the effect sizes of pairwise comparisons.
Furthermore, absolute change scores were calculated in order to quantify the EIH responses following the control and the CPT condition, as well as to quantify the CPT alone. The EIH response after the control condition was calculated as PPT3 minus PPT2; the EIH response after the CPT condition was calculated as PPT6 minus PPT5. The CPM response was calculated as PPT5 minus PPT4 (see Fig. 1).
Finally, bivariate correlation coefficients were used to determine the relationship between the EIH response in both conditions and the CPM response, for each assessment site. In case of normally distributed change scores, Pearson’s r was computed, and in case of non-normally distributed change scores, Spearman’s rho was computed.
3 Results
3.1 Baseline characteristics
All subjects completed both sessions. At baseline, a significant main effect of assessment site was found (F(2, 58)=24.91, p<0.001, ηp2=0.46), with lower PPTs at the hand (302±103 kPa) than at the back (391±178 kPa, p<0.001), and at the leg (414±177 kPa, p<0.001). There further was a trend towards a significant difference in baseline PPTs between sessions (F(1, 30)=3.31, p=0.086, ηp2=0.10) with higher PPTs in the CPT session. Table 1 shows the raw mean PPTs and SD across time, conditions and assessment sites.
Means (M) and standard deviations (SD) of pressure pain thresholds (PPTS), at baseline, after CPT or control condition and after exercise across conditions and assessment sites.
| Session | Assessment site | Baseline |
After control condition or CPT |
After exercise |
|---|---|---|---|---|
| M (SD) |
||||
| PPT1 | PPT2 | PPT3 | ||
| Control | Hand | 287.68 (107.47) | 272.13 (101.71) | 312.31 (99.76) |
| Back | 377.95 (179.11) | 380.73 (184.12) | 444.50 (210.59) | |
| Leg | 388.63 (169.67) | 387.18 (175.86) | 440.77 (187.50) | |
| PPT4 | PPT5 | PPT6 | ||
|
|
||||
| CPT | Hand | 315.77 (109.63) | 373.66 (116.48) | 332.73 (103.20) |
| Back | 404.87 (201.57) | 465.31 (184.44) | 457.56 (197.71) | |
| Leg | 439.37 (197.45) | 452.50 (171.25) | 465.44 (216.95) | |
-
The numerations (PPT1-PP6) correspond to the schematic experimental protocol in Fig. 1.
3.2 Conditioned pain modulation
The mean pain intensity reported during cold pressor task was 7.5±1.7. Table 1 shows the raw mean PPTs and SD across time, conditions and assessment sites. Results of the three-way rm-ANOVA on PPTs with the within-subject factors time (pre, post), session (CPT session, control session) and assessment site (hand, back, leg) showed a significant time×session×assessment site interaction (Fig. 2; F(2, 60)=6.78, p=0.002, ηp2=0.18).
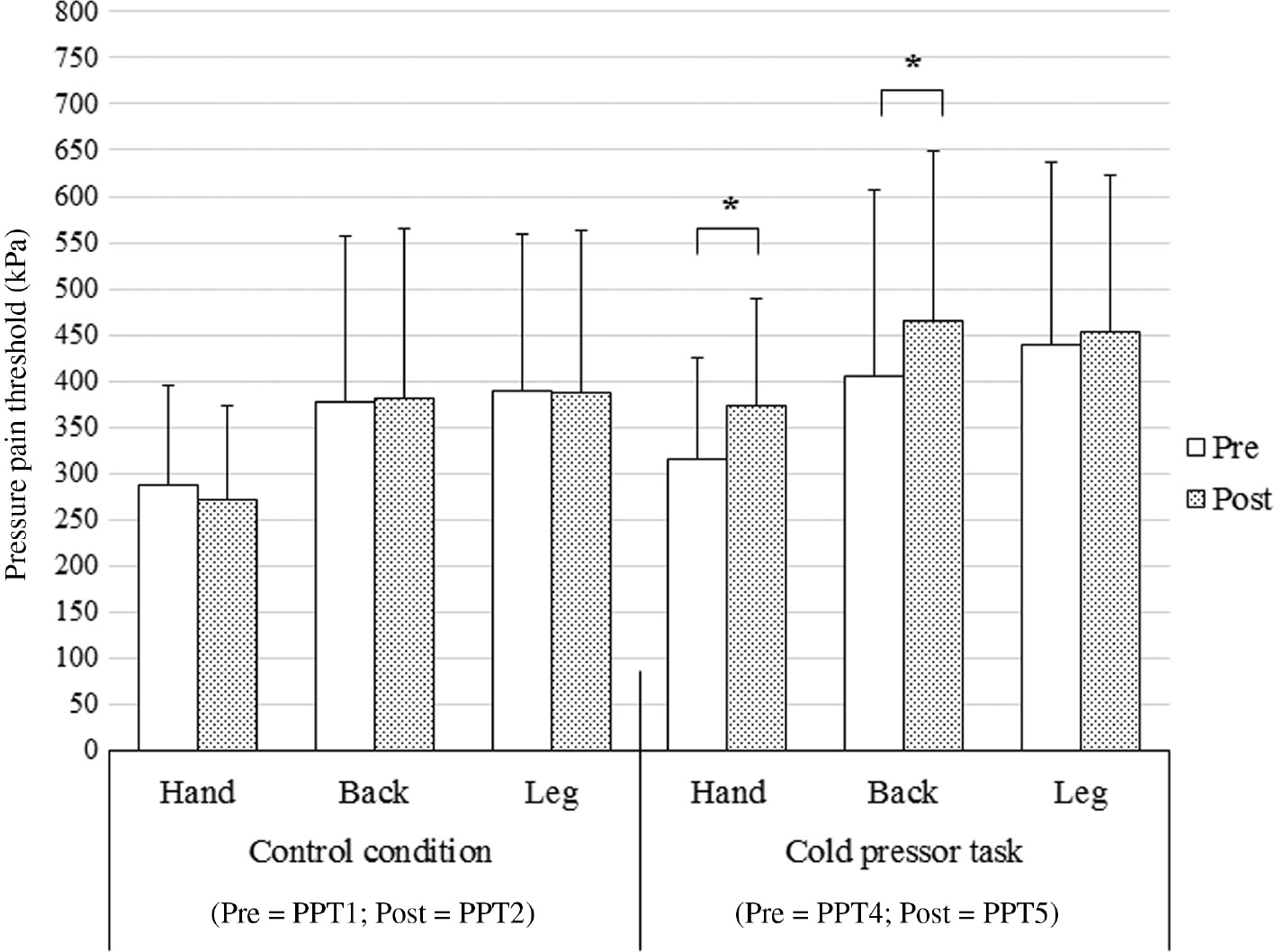
Mean (±SD) pressure pain threshold (PPT) recorded at three assessment sites (hand, back and leg) before and immediately after a 2 min control condition and a 2 min cold pressor task. Significantly different between pre and post (*p<0.001).
Post-hoc tests with a Bonferroni-adjusted α level of α=0.008 for six comparisons showed no significant change in PPTs at the hand (p=0.022, Cohen’s d=0.15), the back (p=0.700, Cohen’s d=0.02), nor at the leg (p=0.892, Cohen’s d=0.01) after the control condition compared with before the control condition. After the CPT, there was a significant increase compared to before the CPT in PPTs at the hand (p<0.001, Cohen’s d=0.28), and at the back (p<0.001, Cohen’s d=0.31). However, there was no significant increase in PPTs at the leg (p=0.344, Cohen’s d=0.07).
3.3 Exercise-induced hypoalgesia in control session and cold pressor task session
Subjects reported a mean perceived exertion after the bicycling exercise in both conditions of 15.87±1.23 on the Borg 6-20 RPE scale. The perceived exertion due to exercise in the CPT condition (15.81±1.42) was comparable to the perceived exertion during exercise in the control condition (15.94±1.36; p=0.587, Cohen’s d=0.09).
Table 1 shows the raw mean PPTs and standard deviations (SDs) across time, session and assessment site. The three-way rm-ANOVA showed an interaction between the factors time×condition (F(1, 30)=43.61, p<0.001, ηp2=0.59). Bonferroni-corrected post-hoc tests with an adjusted α level of α=0.025 for two comparisons indicated that in the control condition, there was a significant increase in PPTs after exercise (p<0.001, Cohen’s d=0.34), while there was no significant change in PPTs after exercise in the CPT condition (p=0.125, Cohen’s d=0.07).
Furthermore, there was a trend towards a significant time×condition×assessment site interaction (F(1.54, 46.06)=2.72, p=0.089, ηp2=0.083). Subsequent analyses, with a Bonferroni-corrected α level of α=0.008 for six comparisons, indicated that in the control condition, an increase in PPTs after exercise occurred at each assessment site (hand: p<0.001, Cohen’s d=0.40; back: p<0.001, Cohen’s d=0.29; leg: p<0.001, Cohen’s d=0.29; Fig. 3). By contrast, in the CPT condition, there was a significant decline in PPTs after exercise at the hand (p<0.001, Cohen’s d=0.36), while there was no significant change in PPTs at the back (p=0.448, Cohen’s d=0.04), nor at the leg (p=0.401, Cohen’s d=0.06, Fig. 3).
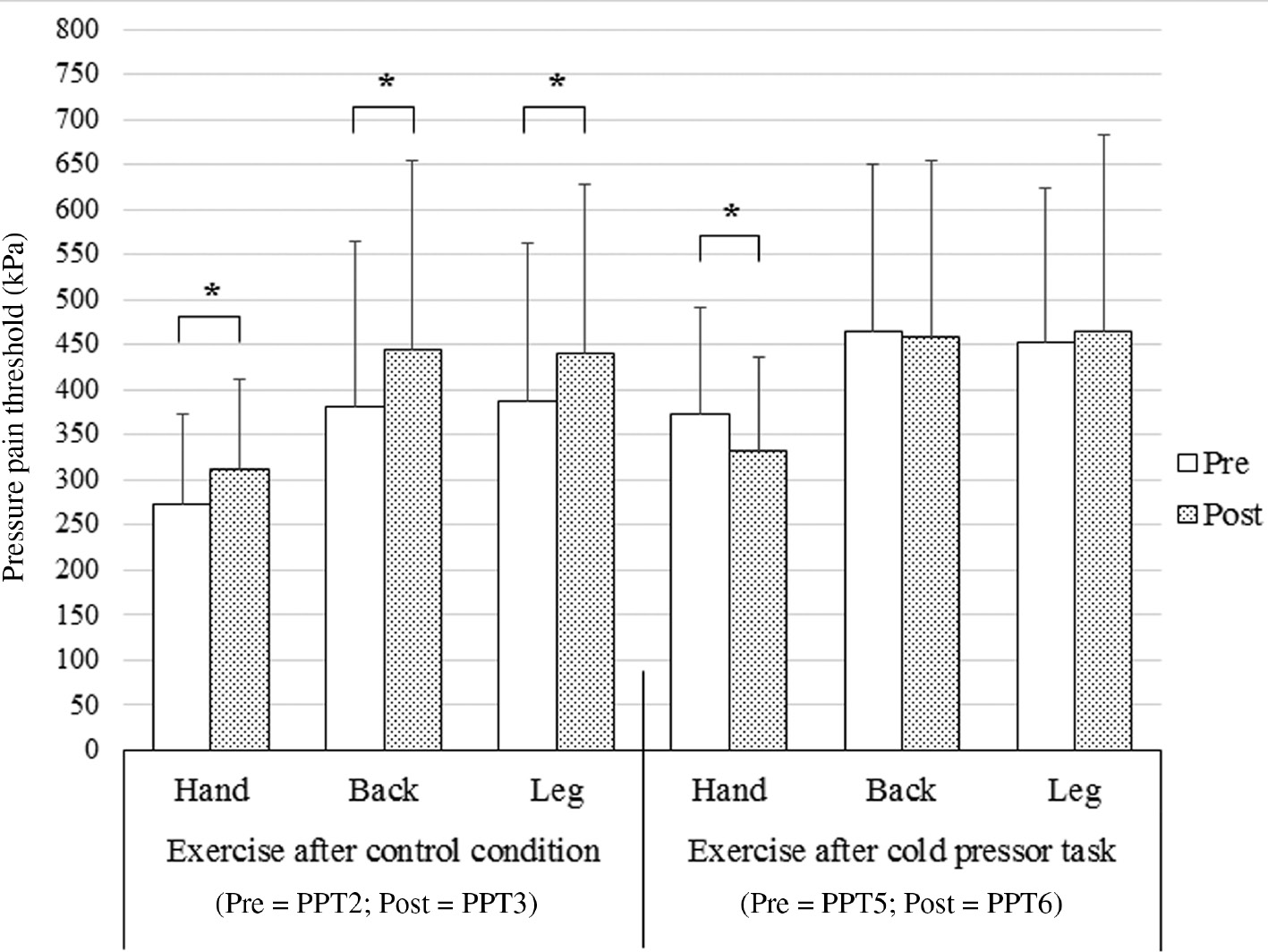
Mean (±SD) pressure pain threshold (PPT) recorded at three assessment sites (hand, back and leg) after bicycling in the CPT session and the control session. Significantly different between pre and post (*p<0.001).
3.4 EIH change scores and correlations between the CPM and EIH responses
Table 2 shows the EIH absolute change scores across conditions and assessment sites, as well as CPM absolute change scores at each site. Furthermore, the correlation coefficients between EIH responses in both conditions and the CPM responses at each assessment site are displayed.
Means (M) and standard deviations (SD) of absolute change scores, before and after CPT (CPM response) as well as before and after exercise in the control condition (EIH response in the control condition) and the CPT condition (EIH response in the CPT condition) at each assessment site.
| CPM response | EIH response in control condition |
EIH response in CPT condition |
|||||
|---|---|---|---|---|---|---|---|
| Hand | Back | Leg | Hand | Back | Leg | ||
| M (SD) | 40.18 (42.49) | 63.77 (70.15) | 53.60 (67.98) | −40.94 (45.77) | −7.74 (56.02) | 12.94 (84.56) | |
| Hand | 57.89 (54.81) | r=0.37b | – | – | r=−0.50a | – | – |
| Back | 60.44 (73.47) | – | rho=−0.09 | – | – | rho=−0.37b | – |
| Leg | 13.13 (76.03) | – | – | rho=0.25 | – | – | rho=−0.35c |
-
Significant correlation coefficients (ap<0.001; bp<0.05; cp<0.100). The correlation coefficients between the CPM responses and the EIH responses in the control condition or CPT condition, respectively, are displayed for each assessment site (hand, back, leg).
Regarding the correlation between the EIH response in the control condition and the CPM response, there was a small positive correlation at the hand (r=0.37, p=0.043), but no significant correlations were seen at the back (rho=−0.09, p=0.641), nor at the leg (rho=0.25, p=0.179).
In contrast, there was a moderate negative correlation between the CPM change score and the EIH change score following the CPT condition at the hand (r=−0.50, p=0.004). At the back, there was a small negative but significant correlation (r=−0.37, p=0.036). At the leg, there was a borderline significant negative correlation (r=−0.35, p=0.054).
4 Discussion
4.1 Attenuated EIH response after cold pressor task (CPT)
This study sought to compare exercise-induced hypoalgesia (EIH) with and without preceding activation of conditioned pain modulation (CPM). Our data suggest that the EIH and CPM protocols in the present study, for the most part, induced the expected hypoalgesic responses when performed independently. In line with our first hypothesis, an attenuated EIH response occurred after activation of CPM compared to the EIH response without preceding activation of CPM. Previous research reported small positive correlations between independently assessed EIH and CPM responses [15], [16], [19], suggesting shared mechanisms. A similar correlation also emerged in the present study. Moreover, the activation of the CPM system affected the subsequent EIH response, implying that the EIH and CPM protocols have shared mechanisms.
Furthermore, the finding that EIH was attenuated after activation of the CPM system could suggest that the pain inhibitory systems was exhausted. Previous research has given some evidence for this notion. For instance, Valencia and colleagues reported that when a CPM protocol was performed twice within one experimental session, the second CPM response was reduced [30]. Furthermore, Arendt-Nielsen and colleagues observed a reduced CPM response when two concomitant conditioning stimuli were applied compared to one conditioning stimulus alone [21]. Moreover, studies reported a reduction in the CPM response after exercise [20], [31], while in one study, this was only the case in individuals who showed systemic EIH [31]. In sum, these findings suggest that EIH and CPM protocols may target similar descending pain inhibitory mechanisms in an exhaustive manner.
Several observations regarding the mechanisms of EIH and CPM support this assumption. Both have been related to activation changes in similar cerebral structures associated with pain inhibition [32], [33], [34]; this coincides with the observation that both EIH and CPM show systemic effects [3], [5], [12]. The periaqueductal gray (PAG) is an opioid-sensitive midbrain structure which plays a pivotal role in descending pain inhibition [35], [36]. Cutaneous cold pain, as induced by CPT, activates thermal ascending pathways projecting to the PAG [21], [37], and CPM has been related to activity alterations in the PAG [34], [38], [39]. Similarly, nociceptive muscle afferents project to the PAG via the dorsal horn [37], and activity changes in the PAG have been related to EIH [32]. Therefore, opiodergic processes triggered in the PAG may be a candidate for descending pain inhibition that is common to both EIH and CPM.
Furthermore, the present study suggests that EIH and CPM may be exhaustible. Another study provides a further hint to opioidergic involvement in exhausted pain inhibition. Ram and colleagues [40] reported that a continuous intake of opioids resulted in reduced CPM responses in patients with chronic pain, proposing a mechanism for opioid-induced hyperalgesia. Taken together with this finding, the notion of exhaustibility emerging in the present study could aid to explain deficient descending pain inhibition in chronic pain. It has been proposed that a reduced capacity for pain inhibition is a precursor, not a result of chronic pain [13], [41]. However, a study by Kosek and Ordeberg [42] reported that patients with osteoarthritis showed reduced CPM in a painful state, but that CPM responses recovered in patients who experienced pain relief after surgery. Hence, in some individuals with chronic pain, descending pain inhibition may be in an exhausted state, possibly due to constant stimulation by nociceptive afferents that trigger the CPM system. A recent study by Fingleton and colleagues [24] reported that patients with knee ostheoarthritis, who had an impaired CPM response, showed a systemic hyperalgesic response after an isometric exercise. They concluded that in some patients with chronic pain, there might be a reduced capacity for descending pain inhibition, leading to both reduced CPM and EIH responses.
4.2 Site-specific attenuation of EIH after CPM
Unlike at the non-exercising body parts, PPTs did not decrease at the exercising body parts after exercise in the CPT condition, but remained stable. As the CPM response is rather short-living, not lasting longer than a few minutes after termination of the conditioning stimulus [3], [12], it seems like the exercise may have stabilized the CPM response which would otherwise have declined. Possibly, additional peripheral or segmental sources of pain inhibition, triggered by exercise, may have caused this effect. This is in accordance with previous research indicating that the EIH response is greater at exercising body parts, compared to non-exercising body parts [3]. This assumption is supported by another result of the Fingleton study [24]. They reported that CPM non-responders showed decreases in PPTs after exercise at non-exercising body parts, while there was no change in PPTs after exercise at exercising body parts. This result is somewhat comparable to the current findings, assuming that patients who are CPM non-responders have an exhausted capacity for systemic, descending pain inhibition.
Taken together, these finding suggest that in the exercising body parts, additional peripheral processes may undermine an otherwise stronger relationship between EIH and CPM. The finding that there were higher correlations between EIH and CPM at the remote assessment site than at the assessment sites at the exercising body parts, further corroborates this assumption. Stolzman and colleagues [19] have reported similar site-specific correlations of EIH and CPM, while another study did not observe site-specific differences in the correlations [16]. Therefore, future studies should investigate the relationship between EIH and CPM in a site-specific manner.
4.3 Conditioned pain modulation
After the CPT, there was an increase in PPTs at the hand and at the back, indicating that CPM was successfully activated. The amount of this increase was comparable at the hand and at the back, which is in accordance to the existing literature suggesting a systemic CPM effect [12]. However, no change in PPTs was measurable at the leg. This is an unexpected finding and could be related to the sequential methodology of the CPM protocol: unlike in many CPM protocols [43], the test stimulus, i.e. the PPT procedure, was applied immediately after, and not during the conditioning stimulus, i.e. the CPT. However, guidelines for the practice of CPM assessment suggest to measure the CPM response after termination of the conditioning stimulus [12], [44]. Furthermore, there was an observable effect of the CPT on EIH at the leg compared to the control condition, which suggests that activation of CPM may have been successful nonetheless.
4.4 Limitations
Some methodological limitations should be considered when interpreting the results. First, a quiet rest condition after termination of the CPT without further stimulation by exercise, would allow more definite interpretations about the effect of CPM on the subsequent EIH response. Without a quiet rest control condition before exercise, our interpretations, especially regarding site-specific effects of CPM on EIH, remain somewhat speculative.
Furthermore, ceiling effects in the PPTs may account for the observation of no change or a decrease in pain sensitivity after exercise in the CPT condition; the PPTs before exercise in the CPT condition were elevated as a result of the immediately preceding CPT compared to the control condition. This account implies that PPTs cannot further increase after reaching a certain maximum, which may explain the present findings without an involvement of shared, exhaustible mechanisms [31]. A ceiling may result from hand algometry methodology: as PPTs increase, they progressively depend on the experimenter’s manual strength to exert pressure on the respective muscles. However, this explanation seems improbable, as throughout the experiment, PPTs at the hand were significantly lower than at the back and at the leg. In contrast, a decrease in PPTs after exercise in the CPT condition only occurred at the hand, while at the back and at the leg, PPTs remained stable. Ceiling effects in PPTs due to limits in the experimenter’s strength to exert pressure would imply the opposite, namely that the most pronounced ceiling effects would occur at those sites where the highest PPTs are assessed.
A third point of concern is that there was a trend towards higher baseline PPTs in the CPT session than in the control session. A systematic influence of habituation to the PPT procedure seems improbable, as the order of the sessions was randomized. However, there may have been effects of expectations, as the CPT was already visible at the time of assessment of baseline PPTs. To adjust these differences in future studies, a sham CPT should be incorporated as a control condition for the CPT in favor of quiet rest.
4.5 Conclusions and clinical implications
The current study is the first to study the direct effect of a painful stimulus on the subsequent EIH response in pain-free individuals. The results implicate that the systemic hypoalgesic effect of exercise is attenuated if CPM is activated before. If CPM responses in patients with chronic pain are reduced because the processes underlying the CPM response are continuously active in chronic pain, this may imply that these patients may not benefit from exercise with regard to acute pain reduction. This further supports the recent claim that the capacity for descending pain, as indicated by the CPM response, should be assessed in patients with chronic pain [13], [45], [46]. Specifically, the individual magnitude of the CPM response may aid clinicians to make individual treatment decisions as to whether exercise, or which kind of exercise will help to acutely reduce pain sensitivity and if counter-effects are to be expected.
-
Authors’ statements
-
Research funding: None declared.
-
Conflict of interest: There are no actual or potential conflicts of interest for any of the authors.
-
Informed consent: All subjects gave written informed consent before participating in the study.
-
Ethical approval: The study was conducted in accordance with the Declaration of Helsinki and approved by the ethical review board of the psychological faculty at the Ruhr-University of Bochum (application #242).
References
[1] Daenen L, Varkey E, Kellmann M, Nijs J. Exercise, not to exercise, or how to exercise in patients with chronic pain? Applying science to practice. Clin J Pain 2015;31:108–14.10.1097/AJP.0000000000000099Suche in Google Scholar PubMed
[2] Naugle KM, Fillingim RB, Riley JL. A meta-analytic review of the hypoalgesic effects of exercise. J Pain 2012;13:1139–50.10.1016/j.jpain.2012.09.006Suche in Google Scholar PubMed PubMed Central
[3] Vaegter HB, Handberg G, Graven-Nielsen T. Similarities between exercise-induced hypoalgesia and conditioned pain modulation in humans. Pain 2014;155:158–67.10.1016/j.pain.2013.09.023Suche in Google Scholar PubMed
[4] Micalos PS, Arendt-Nielsen L. Differential pain response at local and remote muscle sites following aerobic cycling exercise at mild and moderate intensity. Springerplus 2016;5:91.10.1186/s40064-016-1721-8Suche in Google Scholar PubMed PubMed Central
[5] Kosek E, Lundberg L. Segmental and plurisegmental modulation of pressure pain thresholds during static muscle contractions in healthy individuals. Eur J Pain 2003;7:251–8.10.1016/S1090-3801(02)00124-6Suche in Google Scholar PubMed
[6] Jones MD, Taylor JL, Booth J, Barry BK. Exploring the mechanisms of exercise-induced hypoalgesia using somatosensory and laser evoked potentials. Front Physiol 2016;7:581.10.3389/fphys.2016.00581Suche in Google Scholar PubMed PubMed Central
[7] Koltyn KF, Umeda M. Contralateral attenuation of pain after short-duration submaximal isometric exercise. J Pain 2007;8:887–92.10.1016/j.jpain.2007.06.003Suche in Google Scholar PubMed
[8] Vierck CJ, Staud R, Price DD, Cannon RL, Mauderli AP, Martin A. The effect of maximal exercise on temporal summation of second pain (windup) in patients with fibromyalgia syndrome. J Pain 2001;2:334–44.10.1054/jpai.2001.25533Suche in Google Scholar PubMed
[9] Cook DB, Stegner AJ, Ellingson LD. Exercise Alters pain sensitivity in Gulf war veterans with chronic musculoskeletal pain. J Pain 2010;11:764–72.10.1016/j.jpain.2009.11.010Suche in Google Scholar PubMed
[10] Meeus M, Roussel NA, Truijen S. Reduced pressure pain thresholds in response to exercise in chronic fatigue syndrome but not in chronic low back pain: an experimental study. J Rehabil Med 2010;42:884–90.10.2340/16501977-0595Suche in Google Scholar PubMed
[11] Lannersten L, Kosek E. Dysfunction of endogenous pain inhibition during exercise with painful muscles in patients with shoulder myalgia and fibromyalgia. Pain 2010;151:77–86.10.1016/j.pain.2010.06.021Suche in Google Scholar PubMed
[12] Pud D, Sprecher E, Yarnitsky D. Homotopic and heterotopic effects of endogenous analgesia in healthy volunteers. Neurosci Lett 2005;380:209–13.10.1016/j.neulet.2005.01.037Suche in Google Scholar PubMed
[13] Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Curr Opin Anaesthesiol 2010;23:611–5.10.1097/ACO.0b013e32833c348bSuche in Google Scholar PubMed
[14] Parent AJ, Beaudet N, Daigle K, Sabbagh R, Sansoucy Y, Marchand S, Sarret P, Goffaux P. Relationship between blood- and cerebrospinal fluid-bound neurotransmitter concentrations and conditioned pain modulation in pain-free and chronic pain subjects. J Pain 2015;16:436–44.10.1016/j.jpain.2015.01.007Suche in Google Scholar PubMed
[15] Lemley KJ, Hunter SK, Bement MKH. Conditioned pain modulation predicts exercise-induced hypoalgesia in healthy adults. Med Sci Sports Exerc 2015;47:176–84.10.1249/MSS.0000000000000381Suche in Google Scholar PubMed
[16] Vaegter HB, Handberg G, Jorgensen MN, Kinly A, Graven-Nielsen T. Aerobic exercise and cold pressor test induce hypoalgesia in active and inactive men and women. Pain Med 2015;16:923–33.10.1111/pme.12641Suche in Google Scholar PubMed
[17] Stolzman S, Bement MH. Does exercise decrease pain via conditioned pain modulation in adolescents? Pediatr Phys Ther 2016;28:470–3.10.1097/PEP.0000000000000312Suche in Google Scholar PubMed PubMed Central
[18] Vaegter HB, Handberg G, Graven-Nielsen T. Hypoalgesia after exercise and the cold pressor test is reduced in chronic musculoskeletal pain patients with high pain sensitivity. Clin J Pain 2016;32:58–69.10.1097/AJP.0000000000000223Suche in Google Scholar PubMed
[19] Stolzman S, Danduran M, Hunter SK, Bement MH. Pain response after maximal aerobic exercise in adolescents across weight status. Med Sci Sports Exerc 2015;47: 2431–40.10.1249/MSS.0000000000000678Suche in Google Scholar PubMed PubMed Central
[20] Meeus M, Hermans L, Ickmans K, Struyf F, van Cauwenbergh D, Bronckaerts L, DeClerck LS, Moorken G, Hans G, Grosemans S, Njis J. Endogenous pain modulation in response to exercise in patients with rheumatoid arthritis, patients with chronic fatigue syndrome and comorbid fibromyalgia, and healthy controls: a double-blind randomized controlled trial. Pain Pract 2015;15:98–106.10.1111/papr.12181Suche in Google Scholar PubMed
[21] Arendt-Nielsen L, Sluka KA, Nie HL. Experimental muscle pain impairs descending inhibition. Pain 2008;140:465–71.10.1016/j.pain.2008.09.027Suche in Google Scholar PubMed PubMed Central
[22] Granot M, Weissman-Fogel I, Crispel Y, Pud D, Granovsky Y, Sprecher E, Yarnitsky D. Determinants of endogenous analgesia magnitude in a diffuse noxious inhibitory control (DNIC) paradigm: do conditioning stimulus painfulness, gender and personality variables matter? Pain 2008;136:142–9.10.1016/j.pain.2007.06.029Suche in Google Scholar PubMed
[23] Nir R-R, Yarnitsky D. Conditioned pain modulation. Curr Opin Support Palliat Care 2015;9:131–7.10.1097/SPC.0000000000000126Suche in Google Scholar PubMed
[24] Fingleton C, Smart KM, Doody CM. Exercise-induced hypoalgesia in people with knee osteoarthritis with normal and abnormal conditioned pain modulation. Clin J Pain 2017;33:395–404.10.1097/AJP.0000000000000418Suche in Google Scholar PubMed
[25] Ellingson LD, Koltyn KF, Kim J-S, Cook DB. Does exercise induce hypoalgesia through conditioned pain modulation? Psychophysiol 2014;51:267–76.10.1111/psyp.12168Suche in Google Scholar PubMed
[26] Smith A, Ritchie C, Pedler A, McCamley K, Roberts K, Sterling M. Exercise induced hypoalgesia is elicited by isometric, but not aerobic exercise in individuals with chronic whiplash associated disorders. Scand J Pain 2017;15:14–21.10.1016/j.sjpain.2016.11.007Suche in Google Scholar PubMed
[27] Ohrbach R, Gale EN. Pressure pain thresholds in normal muscles: reliability, measurement effects, and topographic differences. Pain 1989;37:257–63.10.1016/0304-3959(89)90189-9Suche in Google Scholar PubMed
[28] Swain DP, Abernathy KS, Smith CS, Lee SJ, Bunn SA. Target heart rates for the development of cardiorespiratory fitness. Med Sci Sports Exerc 1994;26:112–6.10.1249/00005768-199401000-00019Suche in Google Scholar
[29] Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;14:377–81.10.1249/00005768-198205000-00012Suche in Google Scholar
[30] Valencia C, Kindler LL, Fillingim RB, George SZ. Stability of conditioned pain modulation in two musculoskeletal pain models: investigating the influence of shoulder pain intensity and gender. BMC Musculoskelet Disord 2013;14:182.10.1186/1471-2474-14-182Suche in Google Scholar PubMed PubMed Central
[31] Alsouhibani A, Vaegter H, Hoeger-Bement M. (290) Attenuated conditioned pain modulation in young healthy adults that reported systemic pain relief following exercise. J Pain 2017;18:S48.10.1016/j.jpain.2017.02.183Suche in Google Scholar
[32] Scheef L, Jankowski J, Daamen M, Weyer G, Klingenberg M, Renner J, Mueckter S, Schürmann B, Musshof F, Wagner M, Schild HH, Zimmer A, Boecker H. An fMRI study on the acute effects of exercise on pain processing in trained athletes. Pain 2012;153:1702–14.10.1016/j.pain.2012.05.008Suche in Google Scholar PubMed
[33] Moont R, Crispel Y, Lev R, Pud D, Yarnitsky D. Temporal changes in cortical activation during conditioned pain modulation (CPM), a LORETA study. Pain 2011;152:1469–77.10.1016/j.pain.2011.01.036Suche in Google Scholar PubMed
[34] Piché M, Arsenault M, Rainville P. Cerebral and cerebrospinal processes underlying counterirritation analgesia. J Neurosci 2009;29:14236–46.10.1523/JNEUROSCI.2341-09.2009Suche in Google Scholar PubMed PubMed Central
[35] Ossipov MH, Morimura K, Porreca F. Descending pain modulation and chronification of pain. Curr Opin Support Palliat Care 2014;8:143–51.10.1097/SPC.0000000000000055Suche in Google Scholar PubMed PubMed Central
[36] Basbaum AI, Fields HL. Endogenous pain control mechanisms: review and hypothesis. Ann Neurol 1978;4:451–62.10.1002/ana.410040511Suche in Google Scholar PubMed
[37] Keay KA, Bandler R. Deep and superficial noxious stimulation increases Fos-like immunoreactivity in different regions of the midbrain periaqueductal grey of the rat. Neurosci Lett 1993;154:23–6.10.1016/0304-3940(93)90162-ESuche in Google Scholar PubMed
[38] Wilder-Smith CH, Schindler D, Lovblad K, Redmond SM, Nirkko A. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut 2004;53:1595–601.10.1136/gut.2003.028514Suche in Google Scholar PubMed PubMed Central
[39] Sprenger C, Bingel U, Büchel C. Treating pain with pain: supraspinal mechanisms of endogenous analgesia elicited by heterotopic noxious conditioning stimulation. Pain 2011;152:428–39.10.1016/j.pain.2010.11.018Suche in Google Scholar PubMed
[40] Ram KC, Eisenberg E, Haddad M, Pud D. Oral opioid use alters DNIC but not cold pain perception in patients with chronic pain – new perspective of opioid-induced hyperalgesia. Pain 2008;139:431–8.10.1016/j.pain.2008.05.015Suche in Google Scholar PubMed
[41] Vaegter HB, Handberg G, Emmeluth C, Graven-Nielsen T. Preoperative hypoalgesia after cold pressor test and aerobic exercise is associated with pain relief 6 months after total knee replacement. Clin J pain 2017;33:475–84.10.1097/AJP.0000000000000428Suche in Google Scholar PubMed
[42] Kosek E, Ordeberg G. Lack of pressure pain modulation by heterotopic noxious conditioning stimulation in patients with painful osteoarthritis before, but not following, surgical pain relief. Pain 2000;88:69–78.10.1016/S0304-3959(00)00310-9Suche in Google Scholar PubMed
[43] Yarnitsky D, Bouhassira D, Drewes AM, Fillingim RB, Granot M, Hansson P, Landau R, Treede RD, Wilder-Smith OHG. Recommendations on practice of conditioned pain modulation (CPM) testing. Eur J Pain 2015;19:805–6.10.1002/ejp.605Suche in Google Scholar PubMed
[44] Yarnitsky D, Arendt-Nielsen L, Bouhassira D, Edwards RR, Fillingim RB, Granot M, Hansson P, Lautenbacher S, Marchand S, Wilder-Smith OHG. Recommendations on terminology and practice of psychophysical DNIC testing. Eur J Pain 2010;14:339.10.1016/j.ejpain.2010.02.004Suche in Google Scholar PubMed
[45] Vaegter HB, Graven-Nielsen T. Pain modulatory phenotypes differentiate subgroups with different clinical and experimental pain sensitivity. Pain 2016;157:1480–8.10.1097/j.pain.0000000000000543Suche in Google Scholar PubMed
[46] Edwards RR, Dworkin RH, Turk DC, Angst MS, Dionne R, Freeman R, Hansson P, Haroutounian S, Arendt-Nielsen L, Attal N, Baron R, Brell J, Bujanover S, Burke L, Carr D, Chappell AS, Cowan P, Etropolski M, Fillingim RB, Gewandter JS, et al. Patient phenotyping in clinical trials of chronic pain treatments: IMMPACT recommendations. Pain 2016;157:1851–71.10.1097/j.pain.0000000000000602Suche in Google Scholar PubMed PubMed Central
©2018 Scandinavian Association for the Study of Pain. Published by Walter de Gruyter GmbH, Berlin/Boston. All rights reserved.
Artikel in diesem Heft
- Frontmatter
- Topical review
- Reducing risk of spinal haematoma from spinal and epidural pain procedures
- Clinical pain research
- A multiple-dose double-blind randomized study to evaluate the safety, pharmacokinetics, pharmacodynamics and analgesic efficacy of the TRPV1 antagonist JNJ-39439335 (mavatrep)
- Reliability of three linguistically and culturally validated pain assessment tools for sedated ICU patients by ICU nurses in Finland
- Superior outcomes following cervical fusion vs. multimodal rehabilitation in a subgroup of randomized Whiplash-Associated-Disorders (WAD) patients indicating somatic pain origin-Comparison of outcome assessments made by four examiners from different disciplines
- Morning cortisol and fasting glucose are elevated in women with chronic widespread pain independent of comorbid restless legs syndrome
- Chronic pain experience and pain management in persons with spinal cord injury in Nepal
- The Standardised Mensendieck Test as a tool for evaluation of movement quality in patients with nonspecific chronic low back pain
- Exploring effect of pain education on chronic pain patients’ expectation of recovery and pain intensity
- Pain, psychological distress and motor pattern in women with provoked vestibulodynia (PVD) – symptom characteristics and therapy suggestions
- Relative and absolute test-retest reliabilities of pressure pain threshold in patients with knee osteoarthritis
- The influence of pre- and perioperative administration of gabapentin on pain 3–4 years after total knee arthroplasty
- Observational study
- CT guided neurolytic blockade of the coeliac plexus in patients with advanced and intractably painful pancreatic cancer
- Prescription of opioids to post-operative orthopaedic patients at time of discharge from hospital: a prospective observational study
- The psychological features of patellofemoral pain: a cross-sectional study
- Prevalence of self-reported musculoskeletal pain symptoms among school-age adolescents: age and sex differences
- The association between back muscle characteristics and pressure pain sensitivity in low back pain patients
- Postural control in subclinical neck pain: a comparative study on the effect of pain and measurement procedures
- Original experimental
- Exercise-induced hypoalgesia in women with varying levels of menstrual pain
- Exercise does not produce hypoalgesia when performed immediately after a painful stimulus
- Effectiveness of neck stabilisation and dynamic exercises on pain intensity, depression and anxiety among patients with non-specific neck pain: a randomised controlled trial
Artikel in diesem Heft
- Frontmatter
- Topical review
- Reducing risk of spinal haematoma from spinal and epidural pain procedures
- Clinical pain research
- A multiple-dose double-blind randomized study to evaluate the safety, pharmacokinetics, pharmacodynamics and analgesic efficacy of the TRPV1 antagonist JNJ-39439335 (mavatrep)
- Reliability of three linguistically and culturally validated pain assessment tools for sedated ICU patients by ICU nurses in Finland
- Superior outcomes following cervical fusion vs. multimodal rehabilitation in a subgroup of randomized Whiplash-Associated-Disorders (WAD) patients indicating somatic pain origin-Comparison of outcome assessments made by four examiners from different disciplines
- Morning cortisol and fasting glucose are elevated in women with chronic widespread pain independent of comorbid restless legs syndrome
- Chronic pain experience and pain management in persons with spinal cord injury in Nepal
- The Standardised Mensendieck Test as a tool for evaluation of movement quality in patients with nonspecific chronic low back pain
- Exploring effect of pain education on chronic pain patients’ expectation of recovery and pain intensity
- Pain, psychological distress and motor pattern in women with provoked vestibulodynia (PVD) – symptom characteristics and therapy suggestions
- Relative and absolute test-retest reliabilities of pressure pain threshold in patients with knee osteoarthritis
- The influence of pre- and perioperative administration of gabapentin on pain 3–4 years after total knee arthroplasty
- Observational study
- CT guided neurolytic blockade of the coeliac plexus in patients with advanced and intractably painful pancreatic cancer
- Prescription of opioids to post-operative orthopaedic patients at time of discharge from hospital: a prospective observational study
- The psychological features of patellofemoral pain: a cross-sectional study
- Prevalence of self-reported musculoskeletal pain symptoms among school-age adolescents: age and sex differences
- The association between back muscle characteristics and pressure pain sensitivity in low back pain patients
- Postural control in subclinical neck pain: a comparative study on the effect of pain and measurement procedures
- Original experimental
- Exercise-induced hypoalgesia in women with varying levels of menstrual pain
- Exercise does not produce hypoalgesia when performed immediately after a painful stimulus
- Effectiveness of neck stabilisation and dynamic exercises on pain intensity, depression and anxiety among patients with non-specific neck pain: a randomised controlled trial

