Abstract
Objective
To prove that calcium alginate beads can be used as scaffolds during in vitro culture.
Methods
Mouse preosteoblastic cells (MC3T3-E1) were encapsulated in calcium alginate hydrogel beads. The Cell Counting Kit-8 (CCK-8) assay was used to assess cell viability at 2, 5, 8, 11, 14, and 21 days. Calcein-AM and propidium iodide (PI) were employed for live/dead staining.
Results
MC3T3-E1 cells were alive on day 21 and had the highest viability on day 14.
Conclusion
MC3T3-E1 cells could be encapsulated in calcium alginate hydrogel beads and cultured. Calcium alginate hydrogel beads can be used as scaffolds for three-dimensional in vitro culture.
1 Introduction
Three-dimensional (3D) culture technologies have been heralded as alternative and more accurate models than two-dimensional (2D) monolayer cell cultures. 3D cultures often provide more complete cell-to-cell and cell-to-matrix interactions than 2D cultures, mimicking the natural environment in which stem cells reside. Furthermore, many desired cellular characteristics are maintained or even promoted in 3D cultures, further supporting their use in basic and translational research. Different scaffolds have been used to study the 3D cultures of stem cells from different sources [1,2,3]. An Al2O3 scaffold with a pearl layer structure was prepared using a freezing–dry method [4]. An Al3Ti/ADC12 composite was synthesized by an ultrasonic-assisted direct melt reaction [5]. Furthermore, Guo et al. developed a novel superabsorbent copolymer by grafting acrylic acid onto pretreated soybean straw using gamma-ray irradiation [6]. Such scaffolds have been applied to the food, pharmaceutical, and medical industries.
Cell encapsulation into 3D scaffolds aims to entrap viable cells within immobilization devices surrounded by semipermeable spheroids. The scaffolds are expected to be permeable to molecules essential for cell survival, such as nutrients, oxygen, and growth factors but impermeable to the inward diffusion of molecules larger than a desired critical size. Hydrogel beads, in which biopolymers are crosslinked with divalent cations by injection [7], emulsion templating [8], electrostatic complexation [9], and antisolvent precipitation [10], have been designed to address this need. Alginate is unique among polysaccharides in terms of its chemical stability, pH sensitivity, capacity to form strong gel barriers to water and gases, and its biological functionality for appetite regulation [11].
Hydrogel bead composition was optimized to achieve the highest swelling capacity, greatest erythrocyte adhesion, and minimal in vitro cytotoxicity [12]. Fathi et al. suggested that a polymer hydrogel-aluminosilicate composite material may serve as a suitable platform for an effective hemostatic agent that incorporates multiple mechanisms of action [12]. Yadav et al. and Zhang et al. [13,14] fabricated sodium alginate beads to adsorb copper ions in water spontaneously. The beads were promising adsorbents for large-scale water remediation. Additionally, Jing et al. [15] described the facile synthesis of pH-responsive sodium alginate/carboxymethyl chitosan hydrogel beads. Those beads exhibited excellent pH sensitivity, pH reversibility, and lactoferrin loading capacity, revealing that the hydrogel beads could be used as potential protein carriers for oral delivery.
Calcium alginate gel beads are one of the sodium alginate microcapsule systems. Sodium alginate molecules contain free carboxyl groups and hydroxyl groups and can react with divalent metal ions such as calcium to form an “egg-box.” The structurally insoluble metal complex is also a gel.
The preparation of calcium alginate gel beads is uncomplicated, the process is mild, and the cell harvesting method is simple. Thus, calcium alginate gel beads have received attention as 3D scaffolds for cell culture in vitro. Currently, the cells that have been successfully cultured [16] using this system include bone marrow mesenchymal stem cells, adult cells chondrocytes, and Lactobacillus. Calcium alginate gel beads have the following characteristics: they are biodegradable; they require mild preparation conditions; they have porous permeable structures, and a microenvironment with internal hydrogel properties. The preparation of calcium alginate gel beads includes (1) drop methods, (2) backdrop methods, (3) double emulsification methods, and (4) complex coacervation methods. Drop methods mainly include the air spray encapsulation method and high-voltage electrostatic encapsulation method. The preparation of calcium alginate microgel beads by the high-voltage electrostatic method has the advantages of requiring mild preparation conditions, retaining high levels of biological activity, exhibiting high efficiency and rapid, and is often used in the pharmaceutical and biotechnology research fields. The application of calcium alginate gels include (1) cell culture, (2) controlled drug release, and (3) soft tissue filling materials. The most common use of calcium alginate gel beads to encapsulate cells in cell culture is for use with immobilized cultures, which are also known as 3D cultures. Weir and Xu [17] wrapped hBMSC in calcium alginate gel beads and found that the cells remained active, were osteogenic and differentiated, and were capable of synthesizing minerals.
In this study, calcium alginate gel beads were used to encapsulate mouse preosteoblastic cells (MC3T3-E1), which were cultured for 21 days and maintained viability in vitro. The Cell Counting Kit-8 (CCK-8) assay was used to detect cell activity. Calcein-AM and propidium iodide (PI) were used for the double staining of living and dead cells and to observe the cell activity. This study aimed to demonstrate that calcium alginate hydrogel beads can be used as scaffolds for 3D culture in vitro.
2 Materials and methods
2.1 MC3T3-E1 cell culture
MC3T3-E1 cells were purchased from the Basic Medical Cell Center, Chinese Union Medical University (Beijing, China). MC3T3-E1 cells were grown in a culture medium containing 10% fetal bovine serum and cultured in a 37°C, 5% CO2 incubator. The cell morphology and growth status were observed under an inverted phase contrast microscope. The medium was changed every two days, and the cells were subcultured every three to four days.
2.2 Preparation of calcium alginate gel beads loaded with MC3T3-E1 cells
Referring to the method reported by Zhang et al. [18], MC3T3-E1 cells in the logarithmic growth phase were collected and resuspended to 2.5 × 106 cells/mL in 1.5% (w/v) sodium alginate solution (produced by Dalian Aquaculture Plant, filtered and purified by Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, China). The bead preparation instrument consisted of a YD-05 high-voltage electrostatic microcapsule preparation instrument (Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, China), a WO109-1B injection pump execution unit, and a TJ-3A Syringe Pump Controller (Baoding Lange Constant Flow Pump Co., Ltd., Baoding, China). The cell suspension was added to 1.11% (w/v) CaCl2 solution (analytically pure anhydrous CaCl2, Shantou Xilong Chemical Plant, Shantou, China) using the bead-preparation instrument to perform the gelation reaction. Calcification was performed for 15 min to obtain calcium alginate gel beads (A-cell) containing cells.
2.3 Preparation of cell-free calcium alginate gel beads
We obtained cell-free calcium alginate gel beads (A-no cell) using the same method as above, without MC3T3-E1 cells.
2.4 CCK-8 assay to detect cell activity
Cells were divided into the experimental group (A-cell group) and the control group (A-no cell group), with eight replicates per group. A total of 100 µL of A-cell + 1,500 µL of culture medium was added to each well of the experimental group, while a total of 100 µL A-no cell + 1,500 µL of culture medium was added to each well of the control group. Cells were incubated at 37°C in a 5% CO2 incubator. Media was changed every 1–2 days. The corresponding 24-well plates were removed at 2, 5, 8, 11, 14, and 21 days, washed three times with Tyrode’s HEPES buffer, and 500 µL Tyrode’s HEPES buffer + 50 µL CCK-8 reagent (DOJINDO, Japan) was added to each well. The plates were next incubated in a CO2 incubator for 12 h at 37°C, after which a total of 300 µL of the liquid from each well was transferred to a 96-well plate. The optical density (OD) was then measured at 450 nm using a TECAN Sunrise microplate reader (Tecan, Switzerland).
2.5 Calcein-AM and PI double staining to detect live/dead cells
A-cell sets two replicates per group, except that there is no control group, the above operation before adding CCK-8 is the same as this experiment, plus 200 μL Tyrode’s HEPES buffer per well + 200 μL of staining solution containing Calcein-AM and PI (Calcein-AM with a final concentration of 2 μmol/L and PI of 2 μmol/L, DOJINDO, Japan), placed in a CO2 incubatorfor 12 h at 37°C for 15 min, observed by a Nikon TE 2000U inverted fluorescence microscope (Nikon Optiphot, Japan). Live cells were stained green, while dead cells were stained red. Images from each group were obtained using the same parameters. Blue light was used to excite live cells, and green light was used to excite dead cells within the same field of view. Image pro plus 6.0 software (Media Cybernetics, USA) was used for cell count, fluorescence amount, and area calculation, and Photoshop CS3 software (Adobe, USA) for image overlap.
2.6 Statistics
SPSS software 13.0 for Windows (IBM SPSS Statistics, USA) was used to perform t-tests or one-way analysis of variance on the experimental data. When the variance was homogeneity, the LSD test was used for between-group comparison, while the Games–Howell test was used if the variance was heterogeneity; α = 0.05.
3 Results
3.1 MC3T3-E1 cells
One day after the passage of MC3T3-E1 cells, long fusiform or polygonal cells were observed with good extension and clear cytoplasm (Figure 1).
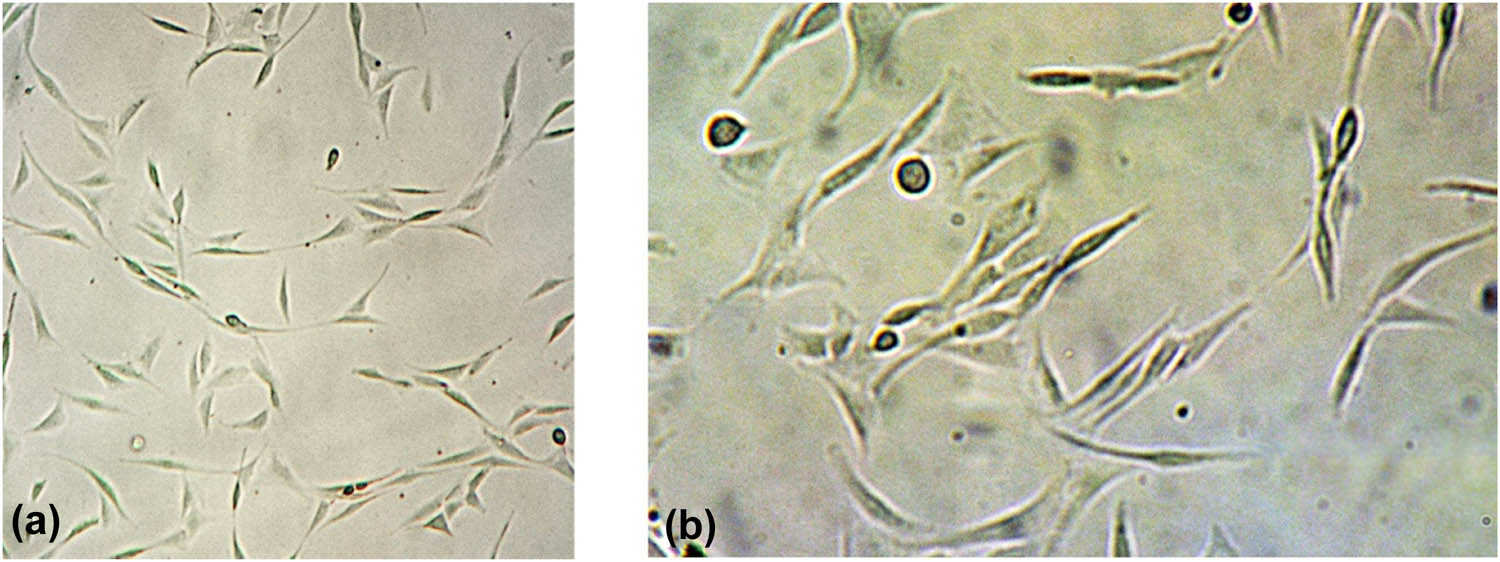
MC3T3-E1 cells 1 day after subculture. (a) (×40 magnification) and (b) (×100 magnification).

Principle and schematic of the preparation of calcium alginate hydrogel beads using the high-voltage electrostatic encapsulation method.
3.2 Calcium alginate gel beads
Sodium alginate droplets and calcium chloride form calcium alginate gel microspheres via a sol–gel phase transfer process (Figure 2), with the following reaction formula: 2nNaAlginate + nCa2+ → nCa(Alginate) + 2nNa+.
The particle size of the calcium alginate gel microspheres was related to the liquid flow rate and the viscosity. The particle size is also related to the electrostatic voltage, electrode distance, inner diameter of the needle, flow rate of the syringe, sodium alginate concentration and surface tension, and gelation volume and shrinkage coefficient.
3.3 Encapsulation of MC3T3-E1 cells by calcium alginate gel beads
Under an inverted phase contrast microscope, the calcium alginate gel microspheres appeared as semi-transparent circles with uniform size and smooth and continuous spherical contours. The cells in the microspheres were transparent and evenly dispersed (Figure 3).
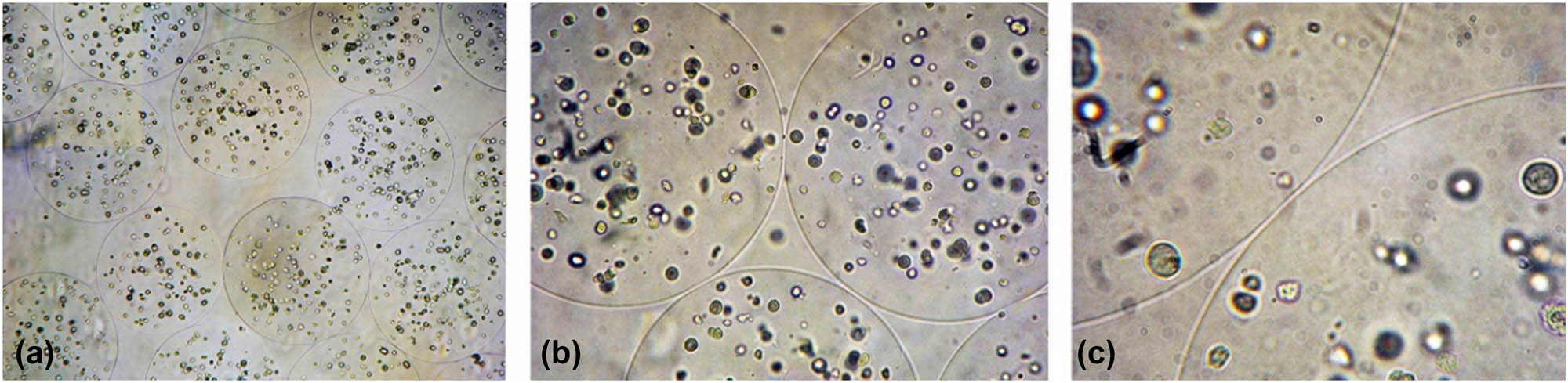
The alginate hydrogel beads with encapsulate MC3T3-E1 cells. (a) ×40 magnification; (b) ×100 magnification; (c) ×250 magnification.
3.4 CCK-8 method for the detection of cell activity
After different durations of in vitro culture, the cell OD values were obtained and are shown in Figure 4 (n = 8, X ± SD). The OD values of the A-cell group and the A-no cell group were significantly different at each time point (P < 0.001). The A-cell group had the highest OD value at 14 days. Compared to the OD values measured at other time points, the differences were significant. No significant differences were observed between the 2 day and 8 days OD values, while the 5 day OD value was significantly lower than the 2 and 8 day values. The OD value at 11 days was the lowest, while the OD value at 14 days increased until it was the highest, and then decreased. The OD value at 21 days was not significantly different than that at 5 days.

The alginate hydrogel beads with (A-cell) or without (A-no cell) MC3T3-E1 cells were cultured in vitro and the OD values are shown. Groups with different letters, a, b, c, and d, were significantly different (P < 0.001). Groups with the same letter were not significantly different (P > 0.05).
3.5 Calcein-AM and PI double staining method to detect cell activity
The results of double staining with Calcein-AM and PI at different times in A-cell cultures are shown in Figures 5 and 6. The green fluorescence of live cells was very bright at 2 days, the cells were dense, and the fluorescence intensity of live cells was the highest of all groups. Very few dead cells were observed. The green fluorescence intensity of live cells at 5 days was weaker than that at 2 days, while the red fluorescence intensity of dead cells was enhanced. The green fluorescence of viable cells at 8 days was stronger than that at 5 days but was weaker than that at 2 days. The number of dead cells did not change significantly from day 5. The green fluorescence of live cells at 11 days was weaker than that at 8 days, while dead cells were similar to that at 8 days. The number of live and dead cells was significantly increased at 14 days, while the intensity of green fluorescence was similar to that at 11 days. The number of viable cells was significantly decreased at 21 days compared with 14 days, while the fluorescence intensity was also decreased. The number of dead cells increased further, which was the most in all time points, and the red fluorescence intensity was not significantly different from that at 14 days.

The representative images of A-cells staining by Calcein-AM (green, live cell) and PI (red, dead cell) are shown. The A-cell were cultured in vitro for 2, 5, 8, 11, 14, and 21 days. A, B and C were ×40 magnified and displayed the same field; A and B were synthesized C. D, E and F were ×100 magnification and displayed the same field; D and E were synthesized F. The green fluorescence of live cells was very bright at 2 days, decreasing gradually to 8 days, then rise to the highest at 14 days, 21 days reduced to close to 8 days.

(a) Comparisons of the number of live (NL) A-cells cultured in vitro at different times (n = 8, X ± SD). (b) Comparisons of the fluorescence intensity of live (FL) A-cells cultured in vitro at different times. The difference between groups with different letters (a, b, c) was statistically significant (P < 0.05). Groups with the same letter were not significantly different (P > 0.05).
4 Discussion
4.1 Calcium alginate gel beads encapsulate MC3T3-E1 cells
The preparation of calcium alginate gel beads was simple, the preparation process was mild, and the method of cell harvesting was simple. It has received certain attention in cell culture. Currently, it has successfully cultured, chondrocytes [19], and many more [20,21,22].
Sodium alginate molecules contain free carboxyl groups and hydroxyl groups [23] and can react with divalent metal ions such as calcium to form a gel of insoluble metal complexes with an “egg-box” structure. During the coagulation of sodium alginate and CaCl2 into beads, the long-chain polysaccharide molecules collide, leaving many voids between the molecules. The formation of such voids provides a path for the penetration of solvent molecules. At the same time, because the matrix sodium alginate used is a biological polysaccharide, it is rich in hydrophilic groups such as hydroxyl, amino, and carboxyl groups. Thus, the hydrophilic gel microspheres formed from such biopolysaccharides have strong water absorption capacity and can quickly reach the swelling balance. The gel beads provide good hydrophilic swelling performance and porous characteristics to the spheres. During in vitro culture, oxygen, carbon dioxide, and macromolecular nutrients in the culture medium can enter the microspheres to supply the cells. Similarly, the metabolic waste of cells can also be discharged from the microspheres [23].
The supply of O2, CO2, and nutrients to the microspheres primarily depends on the permeability of the microspheres themselves, which depends on their pore size, their surface area (penetration area), and the distance between the surface of the microspheres and the cells within the microspheres (penetration distance). Permeability is proportional to the pore size and penetration area and inversely proportional to the penetration distance.
Abbah et al. [24] used alginate microspheres to encapsulate rat adipose tissue stem cells and induce their proliferation and differentiation to synthesize bone matrix. Weir and Xu [17] encapsulated hBMSCs in calcium alginate gel beads and found that the cells remained active, exhibited osteogenic differentiation, and synthesized minerals. Similarly, Wang et al. [25] induced the differentiation of BMSCs into osteoblasts using sodium alginate gel and detected the proliferation, growth morphology, and calcification of cells after the induction of Nodal and osteogenesis-related genes using MTT, toluidine blue staining, von Kossa staining, and RT-PCR assays, respectively. Thus, those data revealed that sodium alginate gel can promote the differentiation of BMSCs into osteoblasts.
4.2 Discussion of experimental results
In this study, MC3T3-E1 cells were encapsulated in calcium alginate gel microspheres, and cell viability was detected using CCK-8 assays at 2, 5, 8, 11, 14, and 21 days in vitro. MC3T3 was not encapsulated at the same time. Compared to the calcium alginate gel microspheres of E1 cells, the OD values were significantly different; Calcein-AM and PI was used to stain the live and dead MC3T3-E1 cells in the A-cell group, with a large number of live cells being observed at each time point. The results revealed that the cells in the calcium alginate gel microspheres were active after 21 days of culture, and that MC3T3-E1 cells could maintain activity in calcium alginate gel microspheres for a longer period of time.
The OD value was the highest on 14 days. Compared with the OD values measured at other time points, the difference was significant, thus, suggesting that calcium alginate gel microspheres encapsulated MC3T3-E1 cells during in vitro culture, and the activity was higher than at 14 days. Zhang et al. [18] prepared APA microcapsules encapsulating recombinant CHO cells (Chinese hamster ovary cells) using high-voltage electrostatic encapsulation and found that the recombinant CHO cells in the logarithmic growth phase could be used for encapsulation. It enters the logarithmic growth phase 2 days after encapsulation, following which, the growth rate gradually decreased, and the viable cell density was 4.17 × 107/mL of microcapsules at 14 days. In this study, calcium alginate gel microspheres were used to encapsulate MC3T3-E1 cells in the logarithmic growth phase, with the activity of the cells being highest at 14 days. Our result was consistent with Zhang Ying.
No significant difference was observed between the 2 and 8 days OD values in this study, while the 5 days OD value was lower than that at 2 and 8 days. This difference was significant, indicating that the encapsulation process may cause cell damage. From 2 to 5 days, the poorly viable cells slowly, at the same time, the cell proliferation rate was slower than the cell death rate, and the OD value decreased at 5 days. From 5 to 8 days, it is possible that as the poorly activity cells died, the cells in good condition gradually proliferated, and the number and activity of cells increased. Cell viability increased from 11 days and reached the highest point at 14 days, suggesting that cell growth reached a stable period at 14 days. From 14 to 21 days, the cell activity was reduced and no significant difference was observed between the OD value at 21 and 5 days. However, the OD value at 21 days remained significantly higher than that at 11 days. After the cells reached a stable period at 14 days, they gradually died, leading to an increase in the number of dead cells and a decrease in the number of live cells. The number of cells continued to increase, the nutrient content of the culture fluid decreased, and the supply of nutrients and oxygen to the calcium alginate gel microspheres could not keep pace with the rate of cell proliferation and the rate of metabolite production; thus, the cells were affected by nutrient depletion, causing cell division to stop.
Weir and Xu [17] encapsulated hBMSCs in calcium alginate gel beads with a diameter of 2.2 mm. Cells were then cultured in vitro, and their activity was assessed using the Wst-1 assay. The results at 1, 7, 14, and 21 days, and the OD values were all approximately 1.0. At 21 days, the cells were active, osteogenic and differentiated, and they synthesized minerals. However, our experiment was different from Weir. In this study, the calcium alginate gel microspheres had a diameter of 200–300 µm and were cultured for 21 days in vitro. Our alginate beads were smaller and only one-tenth compared with Weir. Additionally, the cell activity was measured by CCK-8 assays; the OD values were 1.157 ± 0.053 at 14 days; and the calcium alginate gel microspheres had different sizes, different cell types, different cell numbers, and different reagents for measuring cell activity. However, the difference in OD values did not imply that the MC3T3-E1 cells in this study had low activity.
5 Conclusions
This study reached the following conclusions: MC3T3-E1 cells were encapsulated by calcium alginate microspheres, and the cells were active until 21 days in vitro. The highest activity was observed at 14 days, suggesting that calcium alginate gel beads can be used as scaffolds for 3D cultivation in vitro.
Acknowledgments
We are grateful to the Peking University School and Hospital of Stomatology.
-
Funding information: This work supported by the National Natural Science Fund (30772447) and The Talent Introduction Project of Peking University Health Science Center (bmu2009139).
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: All data included in this study are available upon request from the corresponding author.
References
[1] Cutarelli A, Ghio S, Zasso J, Speccher A, Scarduelli G, Roccuzzo M, et al. Vertically-aligned functionalized silicon micropillars for 3D culture of human pluripotent stem cell-derived cortical progenitors. Cells. 2019;9:88.10.3390/cells9010088Suche in Google Scholar PubMed PubMed Central
[2] Ciardulli MC, Marino L, Lovecchio J, Giordano E, Forsyth NR, Selleri C, et al. Tendon and cytokine marker expression by human bone marrow mesenchymal stem cells in a hyaluronate/poly-lactic-co-glycolic acid (PLGA)/fibrin three-dimensional (3D) scaffold. Cells. 2020;9:1268.10.3390/cells9051268Suche in Google Scholar PubMed PubMed Central
[3] McKee C, Brown C, Chaudhry GR. Self-assembling scaffolds supported long-term growth of human primed embryonic stem cells and upregulated core and naïve pluripotent markers. Cells. 2019;8:1650.10.3390/cells8121650Suche in Google Scholar PubMed PubMed Central
[4] Zhang Q, Dong S, Ma S, Hou X, Yang W, Zhang Y, Wu G. Microstructure and compressive behavior of lamellar Al2O3p/Al composite prepared by freeze-drying and mechanical-pressure infiltration method. Sci Eng Compos Mater. 2020;27(1):1–9.10.1515/secm-2020-0001Suche in Google Scholar
[5] Sun YH, Yan H, Xiong JJ. Al3Ti/ADC12 composite synthesized by ultrasonic chemistry in situ reaction. Sci Eng Compos Mater. 2020;27(1):10–8.10.1515/secm-2020-0004Suche in Google Scholar
[6] Guo J, Huang JS, Liu TG, Chen JB, Janaswamy S. A novel superabsorbent material based on soybean straw: Synthesis and characterization. Sci Eng Compos Mater. 2022;29(1):65–73.10.1515/secm-2022-0006Suche in Google Scholar
[7] Zeeb B, Saberi AH, Weiss J, David JM. Formation and characterization of filled hydrogel beads based on calcium alginate: factors influencing nanoemulsion retention and release. Food Hydrocoll. 2015;50:27–36.10.1016/j.foodhyd.2015.02.041Suche in Google Scholar
[8] Sung MR, Xiao H, Decker EA, David JM. Fabrication, characterization and properties of filled hydrogel particles formed by the emulsion-template method. J Food Eng. 2015;155:16–21.10.1016/j.jfoodeng.2015.01.007Suche in Google Scholar
[9] Hosseini SMH, Djomeh ZE, Sabatino P, Meeren PVD. Nanocomplexes arising from protein-polysaccharide electrostatic interaction as a promising carrier for nutraceutical compounds. Food Hydrocoll. 2015;50:16–26.10.1016/j.foodhyd.2015.04.006Suche in Google Scholar
[10] Zou L, Zheng B, Zhang R, Zhang Z, Liu W, Liu C, et al. Food-grade nanoparticles for encapsulation, protection and delivery of curcumin: comparison of lipid, protein, and phospholipid nanoparticles under simulated gastrointestinal conditions. RSC Adv. 2016;6:3126–36.10.1039/C5RA22834DSuche in Google Scholar
[11] Ching SH, Bansal N, Bhandari B. Alginate gel particles–a review of production techniques and physical properties. Crit Rev Food Sci Nutr. 2017;57:1133–52.10.1080/10408398.2014.965773Suche in Google Scholar PubMed
[12] Fathi P, Sikorski M, Christodoulides K, Langan K, Choi YS, Titcomb M, et al. Zeolite-loaded alginate-chitosan hydrogel beads as a topical hemostat. J Biomed Mater Res B Appl Biomater. 2018;106(5):1662–71.10.1002/jbm.b.33969Suche in Google Scholar PubMed PubMed Central
[13] Yadav S, Asthana A, Singh AK, Chakraborty R, Vidya SS, Singh A, et al. Methionine-functionalized graphene oxide/sodium alginate bio-polymer nanocomposite hydrogel beads: synthesis, isotherm and kinetic studies for an adsorptive removal of fluoroquinolone antibiotics. Nanomaterials (Basel). 2021;11(3):568.10.3390/nano11030568Suche in Google Scholar PubMed PubMed Central
[14] Zhang H, Omer AM, Hu Z, Yang LY, Ji C, Ouyang XK. Fabrication of magnetic bentonite/carboxymethyl chitosan/sodium alginate hydrogel beads for Cu(ii) adsorption. Int J Biol Macromol. 2019;135:490–500.10.1016/j.ijbiomac.2019.05.185Suche in Google Scholar PubMed
[15] Jing H, Huang X, Du X, Mo L, Ma C, Wang H. Facile synthesis of pH-responsive sodium alginate/carboxymethyl chitosan hydrogel beads promoted by hydrogen bond. Carbohydr Polym. 2022;278:118993.10.1016/j.carbpol.2021.118993Suche in Google Scholar PubMed
[16] Andersen T, Auk-Emblem P, Dornish M. 3D cell culture in alginate hydrogels. Microarrays (Basel). 2015;4(2):133–61.10.3390/microarrays4020133Suche in Google Scholar PubMed PubMed Central
[17] Weir MD, Xu HHK. Human bone marrow stem cell-encapsulating calcium phosphate scaffolds for bone repair. Acta Biomaterialia. 2010;6:4118–26.10.1016/j.actbio.2010.04.029Suche in Google Scholar PubMed PubMed Central
[18] Zhang Y, Wang W, Xie Y, Yu W, Lv G, Guo X, et al. Optimization of the preparation and culture conditions of microencapsulated recombinant CHO cells. J Bioeng. 2007;23(3):502–7.10.1016/S1872-2075(07)60036-3Suche in Google Scholar
[19] Weber M, Steinert A, Jork A. Formation of cartilage matrix proteins by BMP-transfected murine mesenchymal stem cells encapsulated in a novel class of a1ginate. Biomaterials. 2002;23:2003–13.10.1016/S0142-9612(01)00329-5Suche in Google Scholar
[20] Allione M, Limongi T, Marini M, Torre B, Zhang P, Moretti M, et al. Micro/nanopatterned superhydrophobic surfaces fabrication for biomolecules and biomaterials manipulation and analysis. Micromachines (Basel). 2021;12(12):1501.10.3390/mi12121501Suche in Google Scholar PubMed PubMed Central
[21] Khanmohammadi CK, Sohrabi B, Rahmanzadeh A. Superhydrophobicity: Advanced biological and biomedical applications. Biomater Sci. 2019;7:3110–37.10.1039/C9BM00558GSuche in Google Scholar PubMed
[22] Kefallinou D, Ellinas K, Speliotis T, Stamatakis K, Gogolides E, Tserepi A. Optimization of antibacterial properties of “hybrid” metal-sputtered superhydrophobic surfaces. Coatings. 2020;10:25.10.3390/coatings10010025Suche in Google Scholar
[23] Wang R, Chai J, Luo B, Liu X, Zhang J, Wu M, et al. A review on slip boundary conditions at the nanoscale: recent development and applications. Beilstein J Nanotechnol. 2021;12:1237–51.10.3762/bjnano.12.91Suche in Google Scholar PubMed PubMed Central
[24] Abbah SA, Lu WW, Chan D, Cheung KM, Liu WG, Zhao F, et al. In vitro evaluation of alginate encapsulated adipose-tissue stromal cells for use as injectable bone graft substitute. Biochem Biophys Res Commun. 2006;347(1):185–91.10.1016/j.bbrc.2006.06.072Suche in Google Scholar PubMed
[25] Chen B, Pei GX, Wang K, Tang GH. Induce bone marrow mesenchymal stem cells to differentiate into osteoblasts on sodium alginate gel. Chin J Biol Eng. 2006;26(9):38–42.Suche in Google Scholar
© 2022 Fang-Fang Li et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Regular Articles
- Experimental investigations of a novel pressure microfoam preparation device for dust removal
- Influence of hydrothermal aging on the mechanical performance of foam core sandwich panels subjected to low-velocity impact
- Experimental study on surface wrapping strengthening of EPS particles and its concrete performance
- Modification of mechanical properties of Shanghai clayey soil with expanded polystyrene
- A new EPS beads strengthening technology and its influences on axial compressive properties of concrete
- A novel superabsorbent material based on soybean straw: Synthesis and characterization
- Use of line laser scanning thermography for the defect detection and evaluation of composite material
- Research on back analysis of meso-parameters of hydraulic cemented sand and gravel based on Box-Behnken design response surface
- Hot deformation behavior and microstructure of a 0.5 wt% graphene nanoplatelet reinforced aluminum composite
- Analysis of electromagnetic characteristics of the proposed composite four-rail electromagnetic launcher
- Preparation and characterization of a graphene hybridizing polyurethane damping composite
- Effects of layup parameters and interference value on the performance of CFRP–metal interference fit joints
- Vibration and noise reduction of pipelines using shape memory alloy
- Finite element analysis of behavior and ultimate strength of composite column
- Dynamic response of functionally graded plate under harmonic load with variable gradient parameters
- Deformation behavior of rubber composite based on FEA and experimental verification
- Effects of Z-pin on moisture absorption property and damage mode under flexural load for carbon fiber composite
- Design and testing of a smart rubber stave for marine water-lubricated bearings
- Study of carbon nano-modifier of fly ash in cement concrete mixtures of civil engineering
- Analysis of multiple impact tests’ damage to three-dimensional four-directional braided composites
- Theoretical analysis of aluminum honeycomb sandwich panel supported by reinforced concrete wall under low-speed impact load
- Effects of local fiber discontinuity on the fatigue strength parameter at the fiber inclusion corner in fiber-reinforced composites
- Experimental investigation on compressive properties of three-dimensional five-directional braided composites in hygrothermal environment
- Failure process of steel–polypropylene hybrid fiber-reinforced concrete based on numerical simulations
- A simple method for measuring the monofilament diameter of continuous filament yarn with high bending stiffness via synthetic laser imaging
- Span length effect on flexural properties of composite laminate reinforced with a plain weave carbon fiber fabric in a polymer matrix
- Mechanical properties improving and microstructure characterization of inorganic artificial stone binder
- Effect of thermal treatment process on the structure of C/SiO2 composite aerogels
- Mechanical and corrosion resistance analysis of laser cladding layer
- Wear and corrosion mechanisms of Ni–WC coatings modified with different Y2O3 by laser cladding on AISI 4145H steel
- Damage and failure analysis of composite stiffened panels under low-velocity impact and compression after impact with damp-heat aging
- In-situ CT characterization of 2D woven SiCf/SiC composite loading under compression
- Effect of the manufacturing process on the equivalency qualification of glass fiber reinforced polymer
- Study of concrete properties based on crushed stone sand mixture and fiber of fly ash of thermal power plants
- Establishment of wear mechanism distribution diagram of ZTAp-reinforced iron matrix composites
- Calculation method of elastic modulus for carbon fiber-reinforced plastics considering inhomogeneous interphase
- An experimental study on the failure and enhancement mechanism of bolt-strengthening GFRP T-joint subjected to tensile loading
- The viability of cell that encapsulated in calcium alginate hydrogel beads
- Discussion of ceramic bar reinforced TWIP steel composite structure
- A theoretical framework underlying an accelerated testing method and its application to composites under constant strain rates and fatigue loading
- Theoretical analysis of interfacial design and thermal conductivity in graphite flakes/Al composites with various interfacial coatings
- Multiscale heat conduction and fractal oxidation behaviors of needle-punched carbon/carbon composites
- Numerical simulation of composite grid sandwich structure under low-velocity impact
- Wear properties of Al/TiO2 composites fabricated via combined compo-casting and APB process
- Review Articles
- Application of melanin as biological functional material in composite film field
- Review on research progress of cemented sand and gravel dam
- Communication
- Fabrications and microstructure analysis of cobalt-based coatings by an easy-coating and sintering process
- Letter to the Editor
- Investigation on mechanical and conductive behaviors of nano-graphite-based concrete
Artikel in diesem Heft
- Regular Articles
- Experimental investigations of a novel pressure microfoam preparation device for dust removal
- Influence of hydrothermal aging on the mechanical performance of foam core sandwich panels subjected to low-velocity impact
- Experimental study on surface wrapping strengthening of EPS particles and its concrete performance
- Modification of mechanical properties of Shanghai clayey soil with expanded polystyrene
- A new EPS beads strengthening technology and its influences on axial compressive properties of concrete
- A novel superabsorbent material based on soybean straw: Synthesis and characterization
- Use of line laser scanning thermography for the defect detection and evaluation of composite material
- Research on back analysis of meso-parameters of hydraulic cemented sand and gravel based on Box-Behnken design response surface
- Hot deformation behavior and microstructure of a 0.5 wt% graphene nanoplatelet reinforced aluminum composite
- Analysis of electromagnetic characteristics of the proposed composite four-rail electromagnetic launcher
- Preparation and characterization of a graphene hybridizing polyurethane damping composite
- Effects of layup parameters and interference value on the performance of CFRP–metal interference fit joints
- Vibration and noise reduction of pipelines using shape memory alloy
- Finite element analysis of behavior and ultimate strength of composite column
- Dynamic response of functionally graded plate under harmonic load with variable gradient parameters
- Deformation behavior of rubber composite based on FEA and experimental verification
- Effects of Z-pin on moisture absorption property and damage mode under flexural load for carbon fiber composite
- Design and testing of a smart rubber stave for marine water-lubricated bearings
- Study of carbon nano-modifier of fly ash in cement concrete mixtures of civil engineering
- Analysis of multiple impact tests’ damage to three-dimensional four-directional braided composites
- Theoretical analysis of aluminum honeycomb sandwich panel supported by reinforced concrete wall under low-speed impact load
- Effects of local fiber discontinuity on the fatigue strength parameter at the fiber inclusion corner in fiber-reinforced composites
- Experimental investigation on compressive properties of three-dimensional five-directional braided composites in hygrothermal environment
- Failure process of steel–polypropylene hybrid fiber-reinforced concrete based on numerical simulations
- A simple method for measuring the monofilament diameter of continuous filament yarn with high bending stiffness via synthetic laser imaging
- Span length effect on flexural properties of composite laminate reinforced with a plain weave carbon fiber fabric in a polymer matrix
- Mechanical properties improving and microstructure characterization of inorganic artificial stone binder
- Effect of thermal treatment process on the structure of C/SiO2 composite aerogels
- Mechanical and corrosion resistance analysis of laser cladding layer
- Wear and corrosion mechanisms of Ni–WC coatings modified with different Y2O3 by laser cladding on AISI 4145H steel
- Damage and failure analysis of composite stiffened panels under low-velocity impact and compression after impact with damp-heat aging
- In-situ CT characterization of 2D woven SiCf/SiC composite loading under compression
- Effect of the manufacturing process on the equivalency qualification of glass fiber reinforced polymer
- Study of concrete properties based on crushed stone sand mixture and fiber of fly ash of thermal power plants
- Establishment of wear mechanism distribution diagram of ZTAp-reinforced iron matrix composites
- Calculation method of elastic modulus for carbon fiber-reinforced plastics considering inhomogeneous interphase
- An experimental study on the failure and enhancement mechanism of bolt-strengthening GFRP T-joint subjected to tensile loading
- The viability of cell that encapsulated in calcium alginate hydrogel beads
- Discussion of ceramic bar reinforced TWIP steel composite structure
- A theoretical framework underlying an accelerated testing method and its application to composites under constant strain rates and fatigue loading
- Theoretical analysis of interfacial design and thermal conductivity in graphite flakes/Al composites with various interfacial coatings
- Multiscale heat conduction and fractal oxidation behaviors of needle-punched carbon/carbon composites
- Numerical simulation of composite grid sandwich structure under low-velocity impact
- Wear properties of Al/TiO2 composites fabricated via combined compo-casting and APB process
- Review Articles
- Application of melanin as biological functional material in composite film field
- Review on research progress of cemented sand and gravel dam
- Communication
- Fabrications and microstructure analysis of cobalt-based coatings by an easy-coating and sintering process
- Letter to the Editor
- Investigation on mechanical and conductive behaviors of nano-graphite-based concrete

