Abstract
In this paper, cationic organic montmorillonites (OMts) were prepared by dry, semi-dry, and wet methods. The structures and properties of these OMts according to the modification methods were studied. By the characterization of XRD, TG-DTA, lipophilicity, and thixotropy, the results showed that cationic OMt prepared by wet method with sufficient surfactants had better properties, including contact angle, swelling capacity, thixotropic loop area, and thermal stability compared with cationic OMt prepared by dry and semi-dry methods. Attention should be paid that with low ratio amount of surfactants used, cationic OMt prepared by the semi-dry method had favorable properties compared with cationic OMt prepared by wet method, and it had the potential to be applied in industrial field due to its environment-friendly and modification efficiency characteristics.
1 Introduction
Organic montmorillonites (OMts) were obtained from montmorillonite (Mt) modified by surfactants. OMts with good thixotropy, suspension, stability, and lubricity have attracted great attention since they have been found. And they have wide applications such as adsorbents of organic pollutants [1], polymer/layered silicate nano-composites [2], [3], [4], [5], thixotropic agent in coating, thickener used in ink [6], additives in oil-based drilling fluids [7], template in the preparation of graphite film [8], industrial waste water treatment agent, treatment agent to organic contaminants [9], [10], additives to enhance the mechanical properties of rubber [11], and so on.
Various factors may affect the structures and properties of OMts. Several literatures [12], [13], [14], [15], [16], [17], [18] have revealed the relationship between cation exchange capacity (CEC) of Mt, type of surfactants used in the modification process, and the structures and properties of OMts, while rare researches have studied the effect of different modification methods on the properties of OMts.
According to the characteristics of different modification methods, basic synthetic methods of OMts are three: dry, semi-dry, and wet methods [19]. The wet method, also known as the solution intercalation method, is reported as the most preferred method for OMts production in the industry [20]. As new methods for Mt modification, dry and semi-dry methods have aroused attention from the public due to their advantages in modification efficiency and water conservation compared with wet method. However, researches about performance of OMts prepared by dry, semi-dry, and wet methods and comparisons between three kinds of OMts are rarely reported. In this work, cationic OMts were prepared by three different methods, and the relationship between performances and modification methods of three different cationic OMts was studied comparatively.
2 Materials and methods
2.1 Mt
Mt was purchased from Linan, Zhejiang province, China. The Mt was ground in an agate mortar and passed through a 200-mesh sieve, without purification. Being analyzed by XRD, basal spacing of the sample was 1.50 nm (Figure 1). The CEC of the Mt, measured by the methylene blue method, was 106.4 mmol/100 g. The cationic surfactant was cetyltrimethyl ammonium bromide (CTAB), purchased from Shantou Xilong Co., Ltd., China.

XRD patterns of montmorillonite.
2.2 Preparation of the cationic OMts samples
The cationic OMts were prepared through the dry method by adding 10 g of Mt and CTAB into the ball mill and milling for 1 h. The amounts of surfactants were equivalent to 1.0 and 1.5 times the CEC of Mt. The products were ground in an agate mortar and passed through a 200-mesh sieve.
The cationic OMts were prepared through the semi-dry method by adding 10 g of Mt, CTAB, and 1 g water into the ball mill and milling for 1 h. The amount of surfactants and the procedure were the same to OMts prepared by dry method.
The cationic OMts prepared through the wet method were according to a previous study [21]. The amounts of surfactants used were the same as those in the dry method. The products were centrifuged and washed two times with deionized water, dried at 60°C, ground in an agate mortar, and passed through a 200-mesh sieve.
Modified by different methods (dry, semi-dry, and wet methods) under different amount of surfactants (1.0 and 1.5 CEC), samples were named as OMt (1.0)-D and OMt (1.5)-D, OMt (1.0)-S and OMt (1.5)-S, and OMt (1.0)-W and OMt (1.5)-W.
2.3 Characterization of the cationic OMts samples
XRD analysis was performed on a D/max-rA 12 kW diffraction (Rikagu, Japan) at 40 kV and 100 mA, using a Cu tube (Cu-Ka radiation, l=0.154 nm). Scans were recorded between 2° and 25° (2θ) with a step size of 0.02°, at a scanning rate of 4°/min.
Static contact angle measurements of OMts were made by ALB-XY-50-3JL (Zhongchen Co., Ltd., Shanghai, China). The sessile drop method was used for the wettability measurement of the OMts to determine the samples’ hydrophilicity or hydrophobicity. A video camera equipped with a homemade image analysis device allowed the determination of contact angle between deionized water and the surface of OMts.
Dispersibility measurement of OMts was determined according to GB27798-2011. First, 3-g samples were added into 80-g mixture of petroleum ether and xylene (petroleum ether:xylene=9:1), the colloid was stirred at 1500 r/min for 5 min, and then 67-g mixture of petroleum ether and xylene was added into the colloid and stirred for another 5 min. The colloid was transferred to a 100-ml graduated cylinderto mark 100 ml, stewing for 6 h.
Thixotropy of OMts was tested by RotoVisco1, by ThermoHAAKE RV1 rotary viscosimeter from Thermo Electron (Karlsruhe) GmbH, Germany, according to GB27798-2011. The difference of two thixotropic curve areas was the ring size, which measures thixotropy of OMts.
The TG-DTA analysis was conducted on Shimadzu (Japan) DTA-TG simultaneously measuring device DTG-60 under air atmosphere, starting at 30°C. A heating rate of 5°C/min was applied until the temperature reached 900°C.
3 Results and discussion
3.1 XRD analysis
Figure 2A shows XRD patterns of OMt (1.0)-D, OMt (1.0)-S, and OMt (1.0)-W. The typical XRD pattern of Ca-Mt (d001=1.50 nm, Figure 1) disappears, and a new group of reflections occurs in Figure 2A. d001 Values of samples are d001A=4.09 nm, d001B=4.12 nm, and d001C=3.91 nm, respectively. The d001 diffraction intensity of A and B curves are relatively higher than that of d002, while the intensity of d002C value is higher than that of d001C (Figure 2A). Under low surfactants addition, OMt (1.0)-W has smaller basal spacing than that of OMt (1.0)-D and OMt (1.0)-S. The results show that the degrees of the modification are influenced by different methods. In the wet modification method, a large part of surfactants dissolve in the water and dilute so part of them cannot be used to modify Mt, which led to insufficient modification, while for surfactants used in dry and semi-dry methods, under the mechanical force modification, none of surfactants are diluted, so surfactants equivalent to 1.0 CEC are sufficient to modify the Mt.
![Figure 2: XRD patterns of cationic OMts.(A) XRD patterns of low surfactants ratio organically modified montmorillonite [a: OMt (1.0)-D, b: OMt (1.0)-S, c: OMt (1.0)-W]. (B) XRD patterns of high surfactants ratio organically modified montmorillonite [a: OMt (1.5)-D, b: OMt (1.5)-S, c: OMt (1.5)-W].](/document/doi/10.1515/secm-2015-0027/asset/graphic/j_secm-2015-0027_fig_002.jpg)
XRD patterns of cationic OMts.
(A) XRD patterns of low surfactants ratio organically modified montmorillonite [a: OMt (1.0)-D, b: OMt (1.0)-S, c: OMt (1.0)-W]. (B) XRD patterns of high surfactants ratio organically modified montmorillonite [a: OMt (1.5)-D, b: OMt (1.5)-S, c: OMt (1.5)-W].
Figure 2B shows XRD patterns of OMt (1.5)-D, OMt (1.5)-S, and OMt (1.5)-W. When comparing Figure 2A and B, the effect of intercalation of OMt (1.0)-S and OMt (1.0)-D has changed slightly under different amount of surfactants; their basal spacings remain at d001A=4.11 nm and d001B=4.09 nm. Comparing basal reflection of OMt (1.0)-W with that of OMt (1.5)-W in Figure 2A and B, more regular diffraction curves are presented in Figure 2B. The reflection of d001C in Figure 2B has a relatively high intensity, accompanying with regular d002 and d003 diffraction peaks, and indicates that the OMts are modified efficiently by wet methods. In dry and semi-dry methods, surfactants beyond 1.0 CEC can hardly change the basal spacing because surfactants equivalent to 1.0 CEC have already modified Mt sufficiently, extra surfactants can only be attached to the surface of OMts. With surfactants equivalent to 1.5 CEC in the wet method, sufficient surfactants can be used to intercalate the Mt, so the basal spacing of OMt (1.5)-W is larger than that of OMt (1.0)-W. The results also verify the conclusion before that under low surfactants ratio, a large part of surfactants dissolve in the water and cannot be used to modify Mt.
As experiments described above, modification methods have an impact on the modification effect of cationic OMts. With surfactants equivalent to 1.0 CEC, Mt is modified sufficiently through dry and semi-dry methods but not with wet method. With a higher amount of surfactants used, Mt can be modified sufficiently by all three methods. For OMt (1.5)-W, basal spacing reaches 3.98 nm and the intensity of d001 reflection is higher than that of OMt (1.5)-D and OMt (1.5)-S, indicating that its modification effect is improved, while basal spacings of OMt (1.5)-S and OMt (1.5)-D have not changed significantly. It could be concluded that with more amount of surfactants, OMts prepared by wet method have similar interlayer space structures as OMts obtained by dry and semi-dry methods, while the same d001 value of OMts can be prepared with less dose of surfactants by dry and semi-dry methods.
3.2 TG-DTA analysis
The TG and DTA analysis results of OMts prepared by dry, semi-dry, and wet methods, respectively, are illustrated in Figure 3A and B.

TG and DTA diagrams of OMts.
(A) TG diagram of OMI (B) DT diagram of OMI. OMt (1.0)-W: montmorillonite modified by wet method with 1.0 CEC of cationic surfactants. OMt (1.0)-S: montmorillonite modified by semi-dry method with 1.0 CEC of cationic surfactants. OMt (1.0)-D: montmorillonite modified by dry method with 1.0 CEC of cationic surfactants. OMt (1.5)-W: montmorillonite modified by wet method with 1.5 CEC of cationic surfactants. OMt (1.5)-S: montmorillonite modified by semi-dry method with 1.5 CEC of cationic surfactants. OMt (1.5)-D: montmorillonite modified by dry method with 1.5 CEC of cationic surfactants.
Mass loss of the samples takes place in two steps, as shown in Figure 3A. In the first part of decomposition of OMts, mass loss below 205°C is related to the small organic molecule matter and water molecules adsorbed on the OMts particles [22]. The different mass loss percentage of OMts before 205°C reveals the different decomposition of residing small organic matter and water molecules on the surface of OMts. OMts prepared through wet method has the least mass loss percentage than OMts prepared by other methods regardless of the amount of organic modifier used during the modification process, while OMts prepared by dry method has the most mass loss. In TG curves of three OMts samples, the mass losses are insignificant between 100°C and 170°C, while in Figure 3B, there is no evident endothermic peaks appearing between 100°C and 170°C, indicating that there are hardly water molecules in the interlayer of OMts due to organic modification, which verifies the efficient organic modification [23], [24], [25].
The second-stage mass loss of three OMts samples in TG curves starts at approximately 170°C and ends at roughly 700°C. Starting temperatures of mass losses for OMt (1.0)-D, OMt (1.0)-S, and OMt (1.0)-W are 180°C, 187°C, and 205°C; mass losses end at 682°C, 684°C, and 687°C, respectively. Starting temperatures of mass losses for OMt (1.5)-D, OMt (1.5)-S, and OMt (1.5)-W are 171°C, 174°C, and 194°C; mass losses end at 680°C, 683°C, and 692°C, respectively. The more amount of surfactants remaining on the surface of OMts, the earlier the decomposition temperature of organic molecules in the OMts. Obviously, due to less organic molecules on the surface of OMts prepared by wet method, surfactants in the interlayer of OMt-W decompose at higher temperature. Furthermore, DTA results of different OMts in Figure 3B verify that the thermal stability of OMt-W is better than that of OMt-D and OMt-S; thermal stability of OMt-S is better than that of OMt-D. The results show that order of surfactants in the interlayer of OMt-W and structure regularity of OMt-W have a positive effect on the thermal stability of surfactants and the OMts. The results show that OMts-W has the best thermal stability among OMts prepared by dry, semi-dry, and wet methods.
3.3 Contact angle analysis
Figure 4 demonstrates contact angles of OMts modified through different methods under different amount of surfactants. Contact angles of OMt (1.0)-D, OMt (1.0)-S, and OMt (1.0)-W are 43.5±0.5°, 47.5±0.5°, and 49.0±0.5°, respectively. Among the results, OMt (1.0)-S and OMt (1.0)-W have similar contact angles. From the data above, during the process of modification, the more water used, the better coverage of surfactants on the surface of OMts, the better hydrophobicity of OMts achieved.

Contact angles of cationic OMts.
(A) Contact angles of OMt (1.0)-D. (B) Contact angles of OMt (1.0)-S. (C) Contact angles of OMt (1.0)-W. (D) Contact angles of OMt (1.5)-D. (E) Contact angles of OMt (1.5)-S. (F) Contact angles of OMt (1.5)-W.
Dispersity of surfactants during the modification process has a positive relationship with the coverage on the surface of OMts. As there is no dispersion medium in the dry method, the dispersion of surfactants on the surface of OMts obtained by dry method is worse than that of the other two methods, which leads to poor coverage and effect of modifications. For OMts prepared through semi-dry method, the small amount of water added as medium can help surfactants disperse more evenly on the surface of OMts, which leads to better hydrophobicity. For OMts prepared by wet method, over 90% water molecules in the modification process enhance the dispersity of surfactants on the surface of OMts. With the most even dispersity of surfactants on the surface of OMts, OMt (1.0)-W has the best hydrophobicity of all the three samples.
Contact angle of OMt (1.5)-D, OMt (1.5)-S, and OMt (1.5)-W are 43.5±0.5°, 46.0±0.5°, and 52.0±0.5°, respectively, which reveals that the hydrophobicity of OMts is OMt (1.5)-W>OMt (1.5)-S>OMt (1.5)-D. The comparison between Tables 1 and 2 shows that the contact angle of OMt (1.5)-W has changed significantly compared with OMt (1.0)-W, while the contact angle of OMt (1.5)-D and OMt (1.5)-S has little change compared with OMt (1.0)-D and OMt (1.0)-S. Obviously, increased lipophilicity indicates that in wet method modification, sufficient surfactants in liquid phase cover evenly on the surface of Mt and lead to good hydrophobility in the wet method, while for the preparation of OMts by dry and semi-dry methods, over-used surfactants can hardly change the surfactants coverage of the surface of OMts.
Low surfactants ratio OMts d001 value and dispersibility.
| Samples | d001 Value (Å) | Expanding volume (ml) |
|---|---|---|
| OMt (1.0)-D | 40.9 | 22.0 |
| OMt (1.0)-S | 41.2 | 39.5 |
| OMt (1.0)-W | 39.1 | 39.0 |
High surfactants ratio OMts d001 value and dispersibility.
| Samples | d001 Value (Å) | Expanding volume (ml) |
|---|---|---|
| OMt (1.5)-D | 41.1 | 30.0 |
| OMt (1.5)-S | 40.9 | 39.5 |
| OMt (1.5)-W | 39.8 | 67.0 |
3.4 Dispersibility analysis
Table 1 illustrates d001 values and dispersibility of OMt (1.0)-D, OMt (1.0)-S, and OMt (1.0)-W.
The expanding volume of samples in a graduated cylinder is 22.0 ml, 39.5 ml, and 39.0 ml, respectively. Dispersibility of OMt (1.0)-S and OMt (1.0)-W has no significant differences, which distributes around 40 ml and corresponds to the results in Table 1. The lipophilicity of OMt (1.0)-S and OMt (1.0)-W is better than that of OMt (1.0)-D, which also verifies the previous conclusion that the surfactants are unevenly distributed on the surface of OMts modified by the dry method.
Table 2 shows d001 values and dispersibility of OMt (1.5)-D, OMt (1.5)-S, and OMt (1.5)-W.
The expanding volumes of samples in a graduated cylinder are 30.0 ml, 39.5 ml, and 67.0 ml, respectively. The calibration of OMt (1.5)-W is about 67 ml, significantly higher than that of OMts obtained by the other two methods. The results are consistent with results in Table 2 that surfactants uniformly distributed on the surface of Mt in the wet method lead to good hydrophobicity.
In this part of the experiment, the dispersibility of OMts in petroleum ether and xylene solution is OMts-W>OMts-S>OMts-D. Thus, to a very real extent, modified methods affect the dispersion properties of the OMts.
3.5 Thixotropy analysis
Figure 5 shows thixotropic loop areas of OMts modified through different methods under different amounts of surfactants. Thixotropy as a crucial property illustrates the rheological property of the solution [26], [27]. From results in Figure 5, there are differences in the thixotropic loop between samples. Thixotropic loop areas of OMt (1.0)-D, OMt (1.0)-S, and OMt (1.0)-W in (A), (B), and (C) are 8.0 Pa/s, 19.3 Pa/s, and 21.6 Pa/s, respectively. Obviously, OMt (1.0)-D has a smaller thixotropic loop and worse thixotropy than that of OMt (1.0)-S and OMt (1.0)-W. OMt (1.0)-S and OMt (1.0)-W have little difference in the thixotropic loop area at roughly 20 Pa/s. Results show that OMt (1.0)-S and OMt (1.0)-W have better thixotropy than that of OMt (1.0)-D. And thixotropy of OMt (1.0)-W is slightly better than that of OMt (1.0)-S. Thixotropic loop areas of the OMt (1.5)-D, OMt (1.5)-S, and OMt (1.5)-W are 8.1 Pa/s, 22.6 Pa/s, and 28.7 Pa/s, respectively. Both OMt (1.5)-S and OMt (1.5)-W have an increase in thixotropy, while OMt (1.5)-W has increased more than the other OMts. The increase of surfactants concentration will lead to the increased interchain interaction and more ordered structures [28], [29]. OMts with well-ordered structure surfactants can disperse homogeneously in organic solvent and quickly form “house-of-cards” aggregate structure in the solvent [17], which shows better thixotropic property.
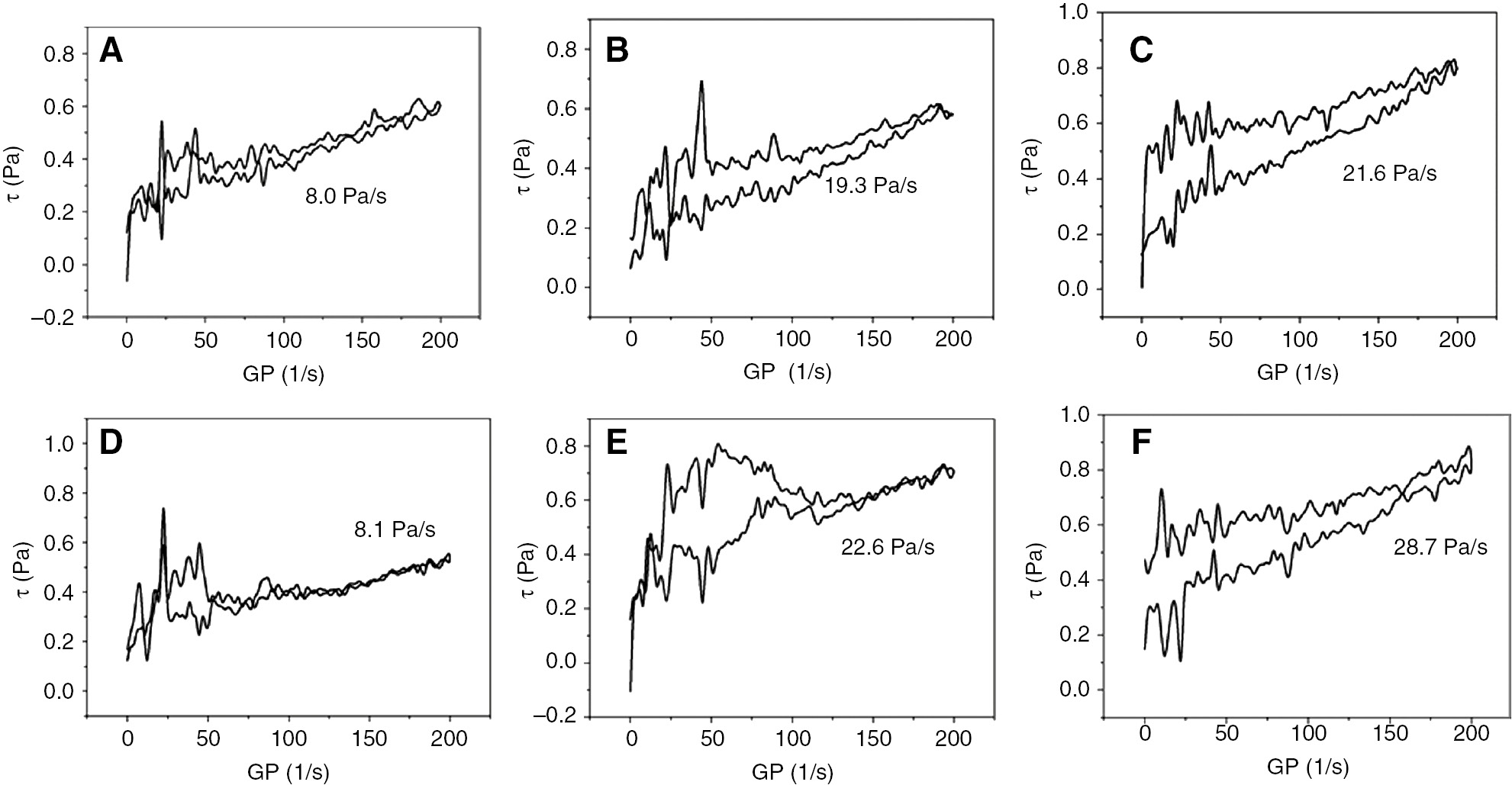
Thixotropic loop diagrams of cationic OMts.
(A) OMt (1.0)-D thixotropic loop diagrams. (B) OMt (1.0)-S thixotropic loop diagrams. (C) OMt (1.0)-W thixotropic loop diagrams. (D) OMt (1.5)-D thixotropic loop diagrams. (E) OMt (1.5)-S thixotropic loop diagrams. (F) OMt (1.5)-W thixotropic loop diagrams.
Thixotropy results show that the organic modification methods of Mt have an obvious impact on its thixotropy; order of thixotropy is OMts-W>OMts-S>OMts-D, while the differences between OMts-S and OMts-W are relatively small with less amount of surfactants used.
4 Conclusions
Cationic OMts were prepared through dry, semi-dry, and wet methods. Their structure and properties were studied by XRD, TG-DTA, contact angle, and viscometer. The results show that with surfactants equivalent to 1.0 CEC, OMts-S and OMts-W have similar properties, OMts-D has the worst properties, while OMts-W can achieve a better modification effect and properties by using sufficient surfactants (1.5 CEC). The order of properties including thermal stability, hydrophobicity, dispersibility, and thixotropy of OMts is in the sequence of OMt-W>OMts-S>OMts-D. Attention should be paid that with less amount of surfactants (1.0 CEC), OMts-S shows similar properties as OMts-W, while semi-dry modification method has advantages in energy conservation and environment protection, so it is also a method worthy of further research and it provides guidance on the proposed ideas on further scientific research and industrial use.
Acknowledgment
This work is financially supported by the Fundamental Research Funds for the Central Universities (no. 53200959784).
References
[1] Liu R, Frost RL, Martens WN. Mater. Chem. Phys. 2009, 113, 707–713.10.1016/j.matchemphys.2008.08.019Suche in Google Scholar
[2] Soundararajah QY, Karunaratne BSB, Rajapakse RMG. Mater. Chem. Phys. 2009, 113, 850–855.10.1016/j.matchemphys.2008.08.055Suche in Google Scholar
[3] Sinha RS, Okamoto M. Prog. Polym. Sci. 2003, 28, 1539–1641.10.1016/j.progpolymsci.2003.08.002Suche in Google Scholar
[4] Pradhan DK, Choudhary RNP, Samantaray BK. Mater. Chem. Phys. 2009, 115, 557–561.10.1016/j.matchemphys.2009.01.008Suche in Google Scholar
[5] Tsai TY, Li CH, Chang CH, Cheng WH, Hwang CL, Wu RJ. Adv. Mater. 2005, 17, 1769–1773.10.1002/adma.200401260Suche in Google Scholar
[6] Beall GW. Appl. Clay Sci. 2003, 24, 11–20.10.1016/j.clay.2003.07.006Suche in Google Scholar
[7] Silva IA, Sousa FKA, Menezes RR, Neves GA, Santana LNL, Ferreira HC. Appl. Clay Sci. 2014, 95, 71–377.10.1016/j.clay.2014.04.021Suche in Google Scholar
[8] Isayama M, Nomiyama K, Kunitake T. Adv. Mater. 1996, 8, 641–644.10.1002/adma.19960080807Suche in Google Scholar
[9] Chen Y, Ma YL. Mater. Rev. 2009, 23, 432–435.10.1007/s00540-009-0772-1Suche in Google Scholar
[10] He HP, Zhou Q, Martens WN, Kloprogge TJ, Yuan P, Xi YF, Zhu JX, Frost RL. Clays Clay Miner. 2006, 54, 689–696.10.1346/CCMN.2006.0540604Suche in Google Scholar
[11] Xu CY, Yu X, Fang X. Mult. Util. Miner Res. 1997, 4, 20–24.Suche in Google Scholar
[12] He HP, Ma Y, Zhu JX, Yuan P, Qing Y. Appl. Clay Sci. 2010, 48, 67–72.10.1016/j.clay.2009.11.024Suche in Google Scholar
[13] Lagaly G. Clay Miner. 1981, 16, 1–21.10.1180/claymin.1981.016.1.01Suche in Google Scholar
[14] Lee SY, Kim SJ. Colloid Surf. A 2002, 211, 19–26.10.1016/S0927-7757(02)00215-7Suche in Google Scholar
[15] Xi YF, Frost RL, He HP, Kloprogge T, Bostrom T. Langmuir 2005, 21, 8675–8680.10.1021/la051454iSuche in Google Scholar
[16] He HP, Frost RL, Bostrom T, Yuan P, Duong L, Yang D, Xi YF, Kloprogge TJ. Appl. Clay Sci. 2006, 31, 262–271.10.1016/j.clay.2005.10.011Suche in Google Scholar
[17] Zidelkheir B, Abdelgoad M. J. Therm. Anal. Calorim. 2008, 94, 181–187.10.1007/s10973-008-9053-8Suche in Google Scholar
[18] Ma YJ. Acta Minera. Sin. 1985, 5, 251–256.Suche in Google Scholar
[19] Wen P. Chem. Abstr. 2007, 6, 34–36.10.5089/9781451938203.023.A001Suche in Google Scholar
[20] Xiao W, Zhan MS, Li Z. Mater. Design 2003, 24, 455–462.10.1016/S0261-3069(03)00064-5Suche in Google Scholar
[21] Zhang Z, Zhang J, Liao L, Xia Z. Mater. Res. Bull. 2013, 48, 1811–1816.10.1016/j.materresbull.2013.01.029Suche in Google Scholar
[22] Yariv S. Appl. Clay Sci. 2004, 24, 225–236.10.1016/j.clay.2003.04.002Suche in Google Scholar
[23] Xie W, Xie RC, Pan WP, Hunter D, Koene B, Tan LS, Vaia R. Chem. Mater. 2012, 14, 4837–4845.10.1021/cm020705vSuche in Google Scholar
[24] He HP, Ding Z, Zhu JX, Yuan P, Xi YF, Yang D, Frost RL. Clays Clay Miner. 2005, 53, 286–292.10.1346/CCMN.2005.0530308Suche in Google Scholar
[25] Hedley CB, Yuan G, Theng BKG. Appl. Clay Sci. 2005, 35, 180–188.10.1016/j.clay.2006.09.005Suche in Google Scholar
[26] Van Olphen H. Discuss Faraday Soc. 1951, 11, 82–84.10.1039/df9511100082Suche in Google Scholar
[27] Juang RS, Lin SH, Tsao KH. Colloid Interface Sci. 2002, 254, 234–241.10.1006/jcis.2002.8629Suche in Google Scholar
[28] Tasi G, Mizukami F. J. Math. Chem. 1999, 25, 55–64.10.1023/A:1019163812482Suche in Google Scholar
[29] Okamura E, Umemura J, Takenaka T. Biochim. Biophys. Acta. 1985, 812, 139–146.10.1016/0005-2736(85)90531-0Suche in Google Scholar
©2018 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Frontmatter
- Review
- A model on the curved shapes of unsymmetric laminates including tool-part interaction
- Original articles
- Enhanced catalytic performance of β-FeOOH by coupling with single-walled carbon nanotubes in a visible-light-Fenton-like process
- Investigation of the microstructure and properties of W75-Cu/W55-Cu brazed joint with Cu-Mn-Co filler metal
- In situ polymerization approach to poly(arylene ether nitrile)-functionalized multiwalled carbon nanotube composite films: thermal, mechanical, dielectric, and electrical properties
- Fabrication and characterization properties of polypropylene/polycarbonate/clay nanocomposites prepared with twin-screw extruder
- Torsional vibration of functionally graded carbon nanotube reinforced conical shells
- A comparative study performance of cationic organic montmorillonite prepared by different methods
- Design and analysis with different substrate materials of a new metamaterial for satellite applications
- An investigation on dry sliding wear behaviour of pressure infiltrated AA1050-XMg/B4C composites
- Investigation of mechanical performances of composite bolted joints with local reinforcements
- Effect of alkali treatment on the flexural properties of a Luffa cylindrica-reinforced epoxy composite
- Thermal expansion, electrical conductivity and hardness of Mn3Zn0.5Sn0.5N/Al composites
- Effect of modified layered double hydroxide on the flammability and mechanical properties of polypropylene
- A unified formulation for free vibration of functionally graded plates
- Friction-stir welding of aluminum alloy with an iron-based metal as reinforcing material
- Hybridization effect of coir fiber on physico-mechanical properties of polyethylene-banana/coir fiber hybrid composites
- Micromechanical properties of unidirectional composites filled with single and clustered shaped fibers
- Structure and microwave absorbing properties of carbon-filled ultra-high molecular weight polyethylene
- Investigation and optimization of the electro-discharge machining parameters of 2024 aluminum alloy and Al/7.5% Al2O3 particulate-reinforced metal matrix composite
- Structural behavior of load-bearing sandwich wall panels with GFRP skin and a foam-web core
- Synthesis, thermal and magnetic behavior of iron oxide-polymer nanocomposites
- High-temperature damping capacity of fly ash cenosphere/AZ91D Mg alloy composites
- Investigation of penetration into woven fabric specimens impregnated with shear thickening fluid
Artikel in diesem Heft
- Frontmatter
- Review
- A model on the curved shapes of unsymmetric laminates including tool-part interaction
- Original articles
- Enhanced catalytic performance of β-FeOOH by coupling with single-walled carbon nanotubes in a visible-light-Fenton-like process
- Investigation of the microstructure and properties of W75-Cu/W55-Cu brazed joint with Cu-Mn-Co filler metal
- In situ polymerization approach to poly(arylene ether nitrile)-functionalized multiwalled carbon nanotube composite films: thermal, mechanical, dielectric, and electrical properties
- Fabrication and characterization properties of polypropylene/polycarbonate/clay nanocomposites prepared with twin-screw extruder
- Torsional vibration of functionally graded carbon nanotube reinforced conical shells
- A comparative study performance of cationic organic montmorillonite prepared by different methods
- Design and analysis with different substrate materials of a new metamaterial for satellite applications
- An investigation on dry sliding wear behaviour of pressure infiltrated AA1050-XMg/B4C composites
- Investigation of mechanical performances of composite bolted joints with local reinforcements
- Effect of alkali treatment on the flexural properties of a Luffa cylindrica-reinforced epoxy composite
- Thermal expansion, electrical conductivity and hardness of Mn3Zn0.5Sn0.5N/Al composites
- Effect of modified layered double hydroxide on the flammability and mechanical properties of polypropylene
- A unified formulation for free vibration of functionally graded plates
- Friction-stir welding of aluminum alloy with an iron-based metal as reinforcing material
- Hybridization effect of coir fiber on physico-mechanical properties of polyethylene-banana/coir fiber hybrid composites
- Micromechanical properties of unidirectional composites filled with single and clustered shaped fibers
- Structure and microwave absorbing properties of carbon-filled ultra-high molecular weight polyethylene
- Investigation and optimization of the electro-discharge machining parameters of 2024 aluminum alloy and Al/7.5% Al2O3 particulate-reinforced metal matrix composite
- Structural behavior of load-bearing sandwich wall panels with GFRP skin and a foam-web core
- Synthesis, thermal and magnetic behavior of iron oxide-polymer nanocomposites
- High-temperature damping capacity of fly ash cenosphere/AZ91D Mg alloy composites
- Investigation of penetration into woven fabric specimens impregnated with shear thickening fluid

