Abstract
Copper-aluminum layered double hydroxides (Cu-LDHs) and nickel-aluminum layered double hydroxides (Ni-LDHs) were synthesized using co-precipitation method. LDHs were organically modified by long chain sodium stearate. Polypropylene (PP)/layered double hydroxides (LDHs) and polypropylene (PP)/organically-modified layered double hydroxides (m.Cu-LDHs or m.Ni-LDHs) were prepared through the melt bending of the PP with either nanosized LDHs or m.LDHs without any other additives. The effect of stearate on the dispersibility of LDHs was investigated by X-ray diffraction (XRD). The surface morphology of LDHs was also studied using scanning electron microscope (SEM), and the thermal stability properties of PP/LDHs composites were studied by thermogravimetric analysis (TGA). The mechanical properties of the PP/LDH composites, tensile strength, and modulus of elasticity were investigated. The flammability properties were investigated using the cone calorimeter test. The intercalation of modified LDHs was determined by XRD in the presence of stearate. Results showed that modified LDHs presented good disperasbility in the PP matrix. The thermal stability of PP has been improved by up to 6% using m.Ni-LDHS. Unmodified and modified nanosized LDHs decreased the fire growth rate of PP from 10.8 kW/m2.s to 4.1 kW/m2.s and 4.5 kW/m2.s, respectively.
1 Introduction
The incorporation of layered double hydroxide (LDH) into polymers to form polymer –LDH nanocomposites has gained increasing research interest, along with the improvement of their properties like thermal, mechanical, and fire retardant is increasing interest in the research of material chemistry. LDHs, also known as hydrotalcite-like materials, are anionic clays that are inexpensive and environment friendly [1], [2], [3], [4], [5]. The introduction of long-chain organic anions into LDH leads to the formation of alternating metal hydroxide layer-organic anion layer hybrids, in which the intercalation of many long-chain surfactant anions, including alkyl carboxylates, alkyl sulfate, and alkyl sulfonates, have been reported [6], [7].
The interlayer anions can be exchanged by other organic anions, a process that leads to the modification of their chemical and environmental properties. Furthermore, increasing the compatibility of LDH with the polymer is difficult, because the clay is polar, whereas the polymer is non-polar. Many nanocomposites were prepared using non-polar polymers with compatiblizers such as maleic anhydride were published [8], [9]. The compatibility of the polymer with inorganic filler is increased by the surface modification of inorganic fillers with small organic compounds, which have a polar group [10], [11], [12], [13].The harmonization of organically-modified Mg-Al-layered double hydroxides with polypropylene was carried out by melt blending the polymers with LDH [14], [15]. The creation of PP/LDH nanocomposites was carried out by melt intercalation using PP-g-MAH as a compatible agent. The interaction between the LDH and PP matrix was improved via organic modification, the mechanical properties of tensile strength and modulus increased as LDH content increased, and the thermal stability of the PP/LDH composites was improved [16], [17]. Different types of PP/LDH nanocomposites have been reported, including PP/Mg-Al-LDH [18], [19], and PP/Co-Al-LDH [20].
The effects of nanosized Ni-Al LDHs, Cu-Al LDHs, modified Ni-Al LD, and modified Cu-Al LDH by sodium stearate on the mechanical and flame retardancy of PP are reported in this study. The results of this study should stimulate future research on the further development of polymer/LDH composites for a wide range of practical applications.
2 Materials and methods
2.1 Materials
All materials were used as received without any further purification. Anhydrous aluminum chloride (AlCl3) sodium chloride (NaCl), and methanol (CH3OH) were purchased from Alfa Aesar Co. Sodium stearate (C18H35O2Na) and sodium hydroxide (NaOH) were received from Merck Aldrich (Germany), whereas nickel chloride (Co. NiCl2.6H2O) and copper chloride (CuCl2.2H2O) were supplied by Sdfine. Chem Limited, Co (UK).
2.2 Preparation of nanosized copper aluminum LDH and nickel-aluminum LDH
Nanosized LDHs were synthesized by using the co-precipitation method [21]. Anhydrous aluminum chloride (13.33 g) was dissolved in 200 ml distilled water in the presence of 19.99 g (0.342 m) of NaCl in a three-neck round-bottom flask under magnetic stirrer (800 rpm). Copper chloride dehydrate (34.52 g) was separately dissolved in 200 ml distilled water. Copper solution was mixed with aluminum solution for 30 min at 800 rpm and at room temperature. The pH of the resulting copper and aluminum salt mixture solution was around 3.95. Sodium hydroxide (50 wt%) solution was added drop-wise to the salt mixture solution with vigorous stirring to reach pH of 10.28. The resulting slurry was aged in the mother liquid for 24 h at 70°C. LDH precipitate was collected by filtration using a Buchner funnel. The collected LDH precipitate was washed several times by using distilled water and dried at 70°C in an oven for 24 h. Ni-Al LDH was typically synthesized and processed as Cu-Al LDH. The resulting LDHs were marked as Cu-LDH and Ni-LDH for Cu and Ni ion, respectively.
2.3 Preparation of nanosized modified copper-aluminum LDH and nickel- aluminum LDH by sodium stearate
Cu-LDH (15 g) or Ni-LDH (15 g) was dispersed in 400 ml of sodium stearate solution (0.008 m) and then vigorously stirred for 48 h at 100°C. The resulting organic modified LDHs were collected by filtration, washed several times using distilled water, and then dried at 70°C in an oven for 24 h. The resulting modified LDHs were labeled as m.Cu-LDH and m.Ni-LDH.
2.4 Processing of PP/LDH nanocomposites
Different percentages (0.5, 1, 1.5 and 2 wt%) of LDHs without further additives were mixed with polypropylene powder. The PP/LDHs mixture was fed into an extruder (at 220°C and 36 rpm). The PP/LDH composites were shredded using a crusher and injected using injection molding. The composition of the PP/LDH composites is listed in Table 1. Organic m.Ni-LDH with three different loadings (0.5, 1, and 1.5 wt%) are illustrated in Figure 1. The critical limit of Ni-LDHs and Cu-LDHs in the PP matrix was found to be 1.5%, at which LDHs aggregated at 2% and were no longer completely spattered by the PP matrix loading. Therefore, in this study, different percentages of LDHs were dispersed and processed in neat PP, namely, 0, 0.5, 1.0 and 1.5 (wt./wt)%.
Compositions of the PP/LDH nanocomposites.
| Sample code | PP (wt%) | Ni-LDH (wt%) | m.Ni-LDH (wt%) | Cu-LDH (wt%) | m.Cu-LDH (wt%) |
|---|---|---|---|---|---|
| H0 | 100 | – | – | – | – |
| H1 | 99.5 | 0.5 | – | – | – |
| H2 | 99 | 1 | – | – | – |
| H3 | 98.5 | 1.5 | – | – | – |
| H4 | 99.5 | – | 0.5 | – | – |
| H5 | 99 | – | 1 | – | – |
| H6 | 98.5 | – | 1.5 | – | – |
| H7 | 99.5 | – | – | 0.5 | – |
| H8 | 99 | – | – | 1 | – |
| H9 | 98.5 | – | – | 1.5 | – |
| H11 | 99.5 | – | – | – | 0.5 |
| H12 | 99 | – | – | – | 1 |
| H13 | 98.5 | – | – | – | 1.5 |

PP/m.Ni-LDHs (H1:0.5 wt%, H2:1 wt%, and H3:1.5 wt%).
2.5 Characterization
2.5.1 X-ray diffraction (XRD)
XRD patterns were recorded using Philips XPERT-PRO (USA) with nickel-filtered CuKa (k=1.5405) radiation.
2.5.2 Thermogravimetric analysis (TGA)
Thermal stability was studied by TGA (TA Instruments-Q50, USA). The thermal degradation process was carried out under N2 atmosphere from 30°C to 500°C at a heating rate of 20°C/min.
2.5.3 Mechanical test
The tensile strength of the PP/LDH composites was measured using universal mechanical testing machine (ZWIC B066550) at 25°C. Specimens were manufactured according to ISO 37. Tensile speed for the tests was 2 mm/min. Each datum of the tensile test was the average of the three values.
2.5.4 Scanning electron microscopy (SEM)
The morphological studies for Ni-LDH and Cu-LDH powder were carried out using scanning electron microscope (SEM), FE-SEM Zeiss Leo Supra 55 (American University in Cairo unit). Samples were sputtered by thin film made of gold to improve conductivity.
2.5.5 Flammability testing
Cone calorimeter represents a small-scale testing configuration, which provides important correlating parameters with real fire scenarios. The values provided by a cone calorimeter mainly consist of time to ignition (TTI), which corresponds to the period at which combustible materials can withstand the heat when exposed to a constant radiant heat flux before igniting and undergoing sustained flaming combustion. Peak of heat release rate (PHRR) and heat release rate average (HRR average) are quantitative measures of thermal energy released by a material per unit area when exposed to a fire radiating at constant heat flux (or temperature).
Flammability cone calorimeter testing was conducted based on ISO 5660-I standard using a cone calorimeter (Fire Testing Technology, Ltd., UK). Square samples with a dimension of 100×100 (mm) were placed in a horizontal direction. Samples were irritated with the heat flux of 50 kw/m2. Measurable parameters were detected as follows: (i) TTI, (ii) HRR and PHRR, (iii) mass loss rate (MLR), (iv) specific extension area (SEA), (v) effective heat of combustion (EHC), (vi) total heat release (THR), and (vii) carbon monoxide and carbon dioxide yield. Measurements were conducted before and after the addition of the flame retardants.
3 Results and discussion
3.1 X-ray diffraction (XRD)
The XRD patterns of nanosized Ni-LDH, m.Ni-LDH, Cu-LDH, and m.Cu-LDH, are shown in Figure 2(A) and (B). The peaks of Ni-LDH are recorded at 2θ=32°, 46°, and 56°. The sharp peaks of Cu-LDH are recorded at 2θ=15°, 32°, 40°, and 46°. The peaks of m.Ni-LDH and m.Cu-LDH are broad, and the peaks at 46° and 56° could not be detected for the m.Ni-LDH sample. Meanwhile, the peak at 2θ=46° is not found in m.Cu-LDH. These broad and non-detectable peaks in the m-LDH sample can be attributed to the intercalation of modified hydroxide layers in the presence of stearate. The extension between the layers of double hydroxide was achieved as a result of the intercalation of stearate chains onto the layered double hydroxide [22]. The shifting and broadening of peaks due to an increase of basal spacing) and the increase of inter layer space depended on the intercalation nature of the functional group, the size of the surfactant, and the alkyl chain length of sodium stearate [23].
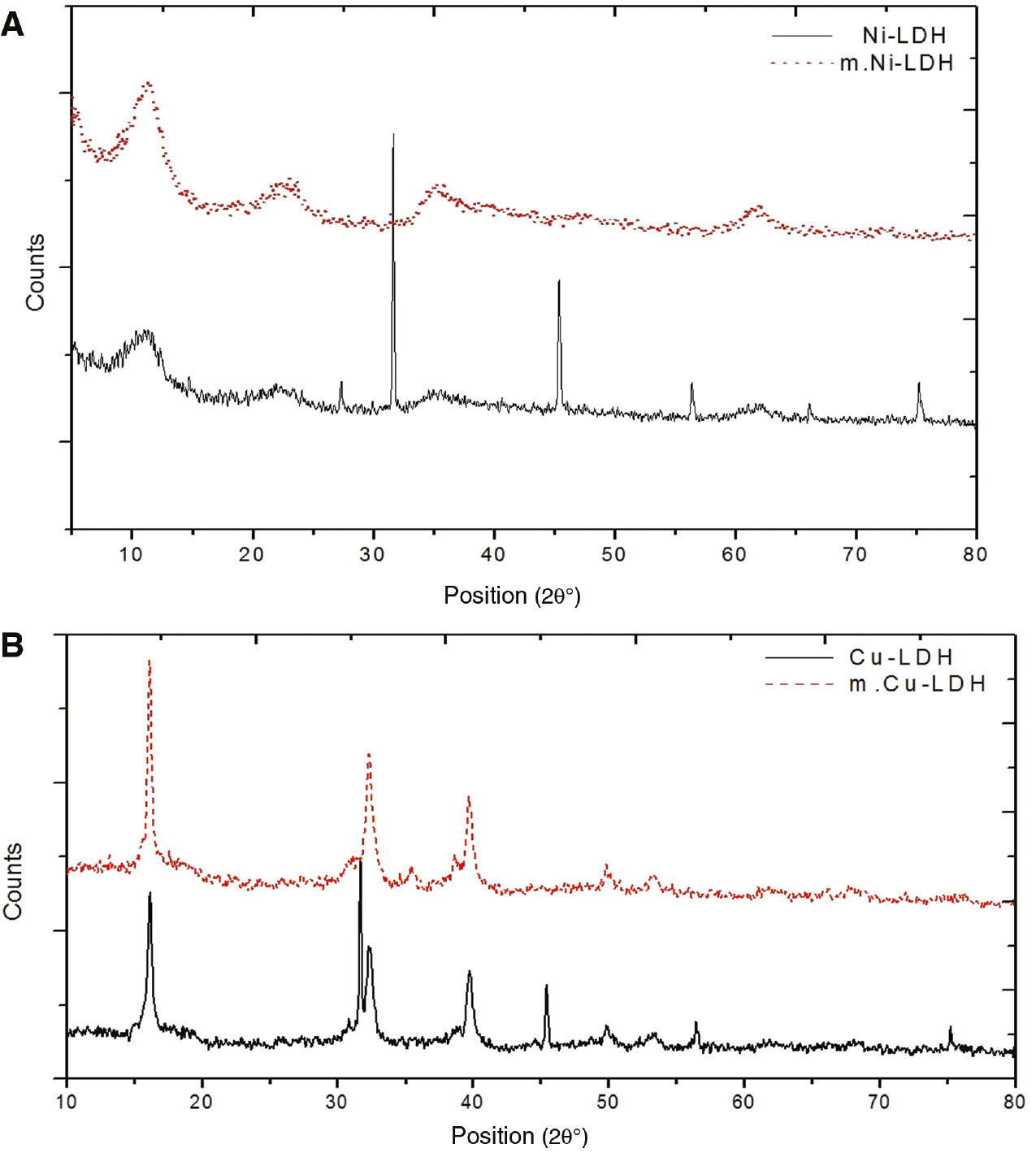
XRD patterns of (A) nanosized Ni-LDH and m.Ni-LDH and (B) Cu-LDH and m.Cu-LDH.
3.2 Thermogravimetric analysis (TGA)
Figure 3(A) shows that the thermal stability of PP can be significantly improved in the presence of m.Ni-LDH compared with PP, PP/Ni-LDH, and PP/Cu-LDH. This can be ascribed to the enhanced interfacial force between PP and Ni-LDH in the presence of stearate. PP and PP/LDH composites exhibit a one-step degradation process. The thermal degradation of neat PP starts dramatically at 425°C. The degradation stage of neat polypropylene shifts from 421.85°C to 426.6°C, 450°C and 421.85°C for H3, H9, and H6, respectively. The highest thermal stability of the PP/m.Ni-LDH composite is observed when the weight decreased significantly at 437.9°C. The values of onset decomposition temperature values are presented in Table 2. The temperature values at weight loss (10%) and (50%) are presented as (T10) and (T50), respectively.

(A) Thermogravimetric analysis (TGA) and (B) derivative TGA of the PP/LDH composites containing different LDH contents.
TGA data of neat PP and the PP/Ni-LDH, PP/m.Ni-LDH, and PP/Cu-LDH composites.
| Sample | T10 (°C) | T50 (°C) |
|---|---|---|
| H0 | 421.85 | 451.18 |
| H3 | 426.68 | 452.99 |
| H6 | 450.17 | 458.18 |
| H9 | 421.85 | 454.68 |
3.3 Scanning electron microscopy (SEM)
Figure 4(A) shows the SEM images of Ni-LDH. As can be seen, the unit cell of the LDH is in the nanoscale range of <50 nm. Figure 4(B) shows that the LDH size is <100 nm in the Cu-LDH. These results are similar with those reported by Choi et al. [24].

(A) SEM of nanosized Ni-LDH, (B) SEM of nanosized Cu-LDH.
3.4 Mechanical properties
The results of tensile modulus, as presented in Figure 5, suggest that LDHs are able to impart stiffness to the composites. The addition of stearate to PP does not seem to contribute to the significant improvement of the tensile strength of the composites. The mechanical properties of PP and the PP/LDH nanocomposites were tabulated in Table 3. Bassyouni [23] suggests that stiffness does not primarily depend on the particle-matrix interface, but more likely on the entire particle’s contents in the tensile loading direction, because the modulus of elasticity is defined as a tangent modulus at low strain values (off set 0.05%–0.25%). According to Abalov et al. and Zoromba et al. [25], [26], [27], the incorporation of inorganic fillers into a thermoplastic matrix can increase or decrease the tensile strength of the resulting nanocomposites. A slight improvement in tensile strength has been detected in presence of stearate (PP/m.Cu-LDH and PP/m.Ni-LDH). For the H3 composite, the tensile strength improved to 33.7 [N/mm2] and elongation at break decreased to 31%. For the H6 composite, the elongation at break was enhanced to 62%; its modulus of elasticity also improved by 18% with 489 [N/mm2] compared with neat PP.

Stress–strain curve for the PP and PP/LDH nanocomposites.
Mechanical properties of PP and the PP/LDH nanocomposites.
| Modulus of elasticity | Tensile strength | Elongation at break | |
|---|---|---|---|
| E | σM | εB | |
| [N/mm2] | [N/mm2] | [%] | |
| H0 | 410 | 33.3 | 419 |
| H3 | 484 | 33.7 | 31 |
| H6 | 489 | 34.0 | 62 |
| H9 | 488 | 33.4 | 16 |
| H13 | 488 | 34.3 | 49 |
3.5 Flammability properties of PP/Ni-LDH and PP/m.Ni-LDH nanocomposites
The flammability properties of the PP/Ni-LDH and PP/m.Ni-LDH nanocomposites were evaluated using a cone calorimeter. The cone calorimeter data are listed in Table 4. The neat PP (H0) burns very fast after ignition, and a sharp PHHR appears at 1831.96 kW/m2. For the PP/Ni-LDH samples (H1, H2, and H3 for 0.5%, 1.0%, and 1.5% Ni, respectively), all composites showed a reduction in PHHR compared with neat PP. The reduction rates are 10.72%, 21.90%, and 30.80% for H1, H2, and H3, respectively, indicating that, with the increase in Ni-LDH loading to PP, the PHHR decreases, as shown in Figure 6. The same behavior reduction of PHHR is detected (39.06%, 43.95%, and 31.59%) for the modified composites PP/m.Ni-LDH (H4, H5, and H6, respectively, as shown in Figure 7. The PHHR values increase in relation to PP/Ni-LDH and PP/m/Ni-LDH nanocomposites by increasing the percentage of Ni-LDH or m.Ni-LDH. The increasing PHHR values in case of PP/m/Ni-LDH is lower than in the case of PP/Ni-LDH. This can be attributed to the existence of stearate in the modified LDH. The H4 and H5 composites show THR of 70.2 MJ/m2 (36.64% reduction) and 81.24 MJ/m2 (26.67% reduction), respectively, in comparison to neat PP, which has a THR of 110.8 MJ/m2. Meanwhile, the H4 and H5 composites showed reduced EHC and SEA values compared with H0, as shown in Table 4.
Cone calorimetric data for the PP/Ni-LDH and PP/m.NiLDH nanocomposites.
| Parameter | H0 | H1 | H2 | H3 | H4 | H5 | H6 |
|---|---|---|---|---|---|---|---|
| Tign (s) | 45 | 53 | 92 | 41 | 59 | 45 | 49 |
| PHHR (kW/m2) | 1831.96 | 1635.53 | 1430.59 | 1266.66 | 1116.37 | 1026.86 | 1254.95 |
| AHRR (kW/m2) | 615.47 | 368.37 | 361.16 | 368.78 | 255.25 | 358.45 | 358.45 |
| THR (MJ/m2) | 110.8 | 106.8 | 117.8 | 129.1 | 70.2 | 81.24 | 111.1 |
| EHC (MJ/kg) | 62.13 | 69.02 | 76.89 | 68.48 | 61.21 | 60.9 | 74.12 |
| SEA (m2/kg) | 2308.50 | 1464.23 | 3507.77 | 2845.69 | 1114.55 | 1124.55 | 3857.66 |
| Time to PHHR (s) | 170 | 225 | 235 | 225 | 175 | 160 | 200 |
| Time to end (s) | 225 | 228 | 304 | 273 | 192 | 335 | 242 |
| FIGRA (kW/m2s) | 10.776 | 7.269 | 6.087 | 5.629 | 6.379 | 6.417 | 6.274 |
| FPI (s) | 0.02456 | 0.06430 | 0.03236 | 0.05244 | 0.5284 | 0.04382 | 0.03904 |
| Residue yield (%) | 0.05 | 0.25 | 0.21 | 0.27 | 0.27 | 0.26 | 0.21 |

Heat release rate curves of neat PP (H0) and the PP/Ni-LDH nanocomposites.
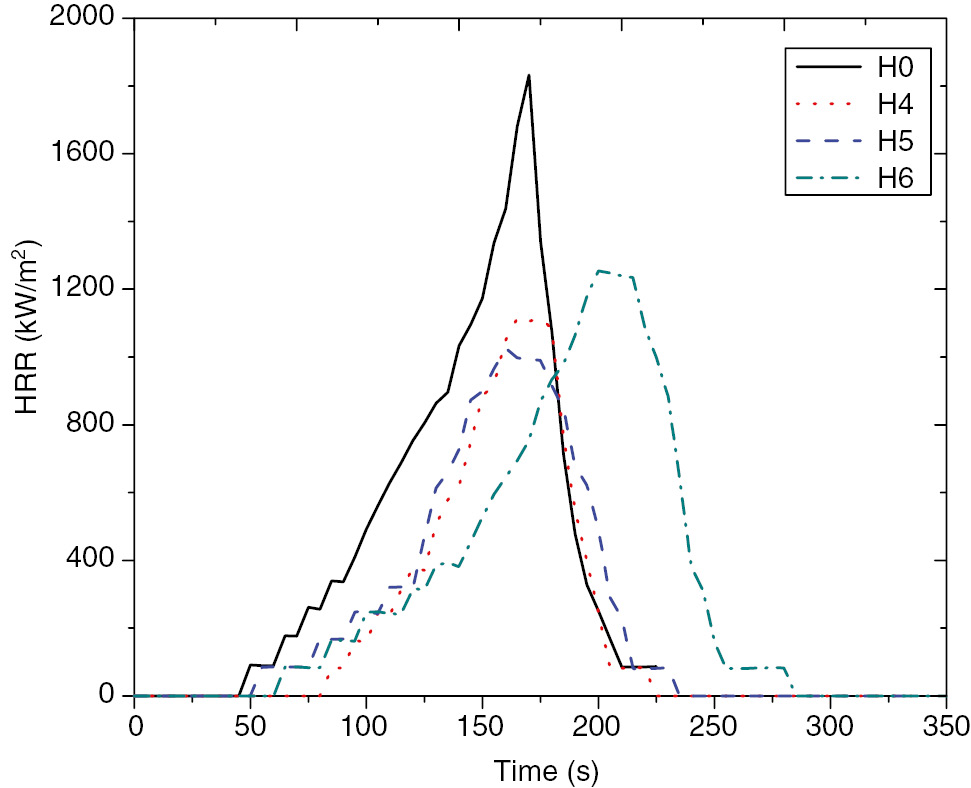
Heat release rate curves of neat PP (H0) and the PP/m.Ni-LDH nanocomposites.
The fire growth rate (FIGRA) was calculated as PHRR/time to PHRR. This can serve as the basis of predicting fire spread rate and the size of a fire [28]. The FIGRA values for the PP/Ni-LDH and PP/m.Ni-LDH composites show a positive sign in terms of the contribution to the fire growth of materials, as listed in Table 4. The decrease in FIGRA value presents better cone calorimeter performance. Table 4 shows that all PP/Ni-LDH and PP/m.Ni-LDH composites have decreased FIGRA values when the content of Ni-LDH loading increased in the following order H1>H2>H3 for PP/Ni-LDH and H4>H6>H7 for PP/m.Ni-LDH compared with neat PP (H0). Furthermore, the fire performance index (FPI) was calculated as the ratio between TTI to PHRR, as given in Equation (1) below.
This FPI value gives interesting information about all PP/Ni-LDH and PP/m.Ni-LDH compositions. These results are used in this study as a reference for the degree of fire hazard involved [29], [30], [31]. The properties of the final masses of the samples are presented in Table 4. The highest final masses corresponding to the PP show the highest FPI values. Hence, all PP/Ni-LDH and PP/m.Ni-LDH composites (H1, H3, H4, and H5) are completely effective.
3.6 Flammability properties of PP/Cu-LDH and PP/m.Cu-LDH nanocomposites
PP/Cu-LDH composites (H7, H8, and H9), show a reduction in PHHR compared with neat PP (H0). The reductions are detected by 43.94% for H7, 30.32% for H8, and 20.85% for H9, as shown in Figure 8. These values refer to the increase in PHHR with increasing Cu-LDH % loadings, as shown in Figure 9. The modified composites (H11, H12, and H13) show a reduction in PHHR values by 46.18%, 35.81%, and 26.57%, respectively, as shown in Figure 9. With increasing percentage of m.Cu-LDH, the PHHR also increases with respect to PP/Cu-LDHs. The H7 sample shows THR of 81.2 MJ/m2 (36.64% reduction), whereas all samples have a THR value greater than that of neat PP. All PP/Cu-LDH and PP/m.Cu-LDH composites showed slightly changed EHC and SEA values compared with neat PP.

Heat release rate curves of neat PP and the PP/Cu-LDH nanocomposites.

Heat release rate curves of neat PP and the PP/m.Cu-LDH nanocomposites.
The fire growth rates (FIGRA) of all PP/Cu-LDH and PP/m.Cu-LDH composites are shown in Table 5. As can be seen, FIGRA decreases with the increase in the unmodified Cu-LDH loadings in the following order: H7>H9>H8 for PP/Cu-LDH. The FPI values of all PP/Cu-LDH and PP/m.Cu-LDH are presented in Table 5.
Cone calorimetric data for the PP/Cu-LDH and PP/m.Cu-LDH nanocomposites.
| Parameter | H0 | H7 | H8 | H9 | H11 | H12 | H13 |
|---|---|---|---|---|---|---|---|
| Tign (s) | 45 | 45 | 57 | 50 | 69 | 54 | 54 |
| PHHR (kW/m2) | 1831.96 | 1026.86 | 1276.46 | 1449.98 | 985.91 | 1175.99 | 1345.14 |
| AHRR (kW/m2) | 615.47 | 280.15 | 487.59 | 487.03 | 358.72 | 328.55 | 289.29 |
| THR (MJ/m2) | 110.8 | 81.2 | 123.0 | 121.8 | 120 | 121.6 | 114.3 |
| EHC (MJ/kg) | 62.13 | 74.47 | 67.86 | 66.80 | 68.17 | 76.36 | 67.70 |
| SEA (m2/kg) | 2308.50 | 4514.18 | 3401.63 | 2209.30 | 3524.45 | 2742.48 | 2742.48 |
| Time to PHHR (s) | 170 | 160 | 230 | 225 | 240 | 260 | 250 |
| Time to end (s) | 225 | 215 | 243 | 240 | 405 | 305 | 305 |
| FIGRA (kW/m2s) | 10.776 | 6.417 | 5.549 | 6.444 | 4.107 | 4.523 | 5.380 |
| FPI (s) | 0.02456 | 0.04382 | 0.02663 | 0.03448 | 0.06998 | 0.04591 | 0.04014 |
| Residue yield (%) | 0.05 | 0.29 | 0.21 | 0.23 | 0.25 | 0.24 | 0.21 |
4 Conclusions
Nanosized copper-aluminum layered double hydroxides (Cu-LDH) and nickel-aluminum layered double hydroxides (Ni-LDH) were synthesized by using the co-precipitation method. LDHs were organically modified by chain sodium stearate. The intercalation of the stearate was carried out into the layered double hydroxides (LDHs). Polypropylene nanocomposites were prepared with 0.5%, 1%, and 1.5% of unmodified LDHs and stearate-modified LDHs. Thermal stability can be significantly improved using modified LDHs by up to 6% in the presence of m.Ni-LDH. Modulus of elasticity is improved as LDH particle loadings increased. Modified and un-modified LDHs have no effect on the tensile strength of PP. The intercalation of sodium stearate among LDHs is successfully demonstrated and proven by XRD analysis. The flame retardancy of PP can be improved significantly in the presence of nanosized Cu-LDHs and Ni-LDHs. However, modified LDHs have less effect on flame retardnacy because of the presence stearate chains.
References
[1] Zhang R, Huang H, Yang W, Xiao X, Yang J, Hu H. High Perform. Polym. 2012, 25, 104–112.10.1177/0954008312457581Search in Google Scholar
[2] Zimmermann A, Silvia J, Sonia F. Z, Wypych F. J Polym. Res. 2013, 20, 224.10.1007/s10965-013-0224-3Search in Google Scholar
[3] Yang Y, Duan H, Wang X, Liu Y, Yang J. High Perform. Polym. 2015, 27, 782–789.10.1177/0954008314566435Search in Google Scholar
[4] Niranjana Prabhu T, Demappa T, Harish V, Prashantha K. High Perform. Polym. 2013, 25, 559–565.10.1177/0954008313475830Search in Google Scholar
[5] Cimini Jr. CA. Sci. Eng. Compos. Mater. 2011, 18, 247–257.10.1515/SECM.2011.048Search in Google Scholar
[6] Meyn M, Beneke K, Lagaly G. Inorg. Chem. 1990, 29, 5201–5207.10.1021/ic00351a013Search in Google Scholar
[7] Zhang J, Wilkie CA. Polym. Degrad. Stab. 2003, 80, 163–169.10.1016/S0141-3910(02)00398-1Search in Google Scholar
[8] Wang D, Wilkie CA. Polym. Degrad. Stab. 2003, 80, 171–182.10.1016/S0141-3910(02)00399-3Search in Google Scholar
[9] Javid U, Khan ZM, Khan MB, Bassyouni M, Abdel-Hamid SMS, Abdel-Aziz MH, Ul Hasan SW. Compos. Part B Eng. 2016, 91, 257–265.10.1016/j.compositesb.2015.12.034Search in Google Scholar
[10] Zoromba MSh, Belal AAM, Ali AEM, Helaly FM, Abd El-Hakim AA, Badran AS. Polym.-Plast. Technol. Eng. 2007, 46, 529–535.10.1080/03602550701298622Search in Google Scholar
[11] Zoromba MSh, Bassyouni M, Abdel-Hamid SMS. Rubber Chem. Technol. 2015, 88, 449–462.10.5254/rct.15.84910Search in Google Scholar
[12] Al-Qabandi O, De Silva A, Al-Enezi S, Bassyouni M. J. Reinf. Plast. Compos. 2014, 33, 2287–2299.10.1177/0731684414561538Search in Google Scholar
[13] Bassyouni M, Sherif Sayed A, Sadek MA, Ashour FH. Compos. Part B Eng. 2012, 43, 1439–44.10.1016/j.compositesb.2011.08.014Search in Google Scholar
[14] Nyambo C, Wang D, Wilkie CA. Polym. Adv. Technol. 2009, 20, 332–340.10.1002/pat.1272Search in Google Scholar
[15] Khan AI, Hare D. J. Mater. Chem. 2002, 12, 3191–3198.10.1039/B204076JSearch in Google Scholar
[16] Leroux X, Gardette J, Singh RP. Polym. Eng. Sci. 2012, 9, 2006–2014.10.1002/pen.23147Search in Google Scholar
[17] Carneiro JR, Almeida PJ, de Lurdes Lopes M. Sci. Eng. Compos. Mater. 2011, 18, 241–245.10.1515/SECM.2011.047Search in Google Scholar
[18] Wang Q, Zhang X, Zhu J, Guo Z, O’Hare D. Chem. Commun. 2012, 48, 7450–7452.10.1039/c2cc32708bSearch in Google Scholar PubMed
[19] Wang Q, Zhang X, Wang CJ, Zhu J, O’Hare D, Guo Z. J. Mater. Chem. 2012, 22, 19113–19121.10.1039/c2jm33493cSearch in Google Scholar
[20] Wang G, Wang C, Chen C. Polymer 2005, 46, 5065–5074.10.1016/j.polymer.2005.04.054Search in Google Scholar
[21] Agroui K, Maallemi A, Boumaour M, Collins G, Salama M. Sol. Energ. Mat. Sol. Cells 2006, 90, 2509–2514.10.1016/j.solmat.2006.03.023Search in Google Scholar
[22] Su S, Jiang DD, Wilkie CA. Polymer. Degrad. Stab. 2004, 83, 321–33.10.1016/S0141-3910(03)00277-5Search in Google Scholar
[23] Anbarasan R, Lee WD, Im SS. Bull. Mater. Sci. 2005, 28, 154–149.10.1007/BF02704234Search in Google Scholar
[24] Choi SJ, Jeong H, Yu J. Int J Nanomedicine 2015, 10, 3217–3229.10.2147/IJN.S82061Search in Google Scholar PubMed PubMed Central
[25] Bassyouni M, Taha I, Shereen MS, Abdel-hamid X, Steuernagel L. J. Reinf. Plast. Compos. 2012, 31, 303–312.10.1177/0731684411436024Search in Google Scholar
[26] Gofman JV, Abalov IV. Acta Geodyn. Geomater. 2009, 6, 187–192.Search in Google Scholar
[27] Iqbal N, Sagar S, Bassyouni M, Khan ZM. J. Appl. Mol. Sci. 2013, 130, 4392–4400.10.1002/app.39363Search in Google Scholar
[28] Hirschler M. J. Fire Sci. 1991, 9, 183–222.10.1177/073490419100900302Search in Google Scholar
[29] Nour MA. Polym. J. 2003, 6, 439–442.Search in Google Scholar
[30] Nour MA, Gaafer MS, Eid A, El-Ebissy AA. 14th European Conference on Composite Materials (ECCM-14), 7–10 June 2010, Budapest, Hungary.Search in Google Scholar
[31] Hasan EH, Helal MA, Nour MA, Shokry KM. Key Eng. Mat. 2014, 600, 547–557.10.4028/www.scientific.net/KEM.600.547Search in Google Scholar
©2018 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Review
- A model on the curved shapes of unsymmetric laminates including tool-part interaction
- Original articles
- Enhanced catalytic performance of β-FeOOH by coupling with single-walled carbon nanotubes in a visible-light-Fenton-like process
- Investigation of the microstructure and properties of W75-Cu/W55-Cu brazed joint with Cu-Mn-Co filler metal
- In situ polymerization approach to poly(arylene ether nitrile)-functionalized multiwalled carbon nanotube composite films: thermal, mechanical, dielectric, and electrical properties
- Fabrication and characterization properties of polypropylene/polycarbonate/clay nanocomposites prepared with twin-screw extruder
- Torsional vibration of functionally graded carbon nanotube reinforced conical shells
- A comparative study performance of cationic organic montmorillonite prepared by different methods
- Design and analysis with different substrate materials of a new metamaterial for satellite applications
- An investigation on dry sliding wear behaviour of pressure infiltrated AA1050-XMg/B4C composites
- Investigation of mechanical performances of composite bolted joints with local reinforcements
- Effect of alkali treatment on the flexural properties of a Luffa cylindrica-reinforced epoxy composite
- Thermal expansion, electrical conductivity and hardness of Mn3Zn0.5Sn0.5N/Al composites
- Effect of modified layered double hydroxide on the flammability and mechanical properties of polypropylene
- A unified formulation for free vibration of functionally graded plates
- Friction-stir welding of aluminum alloy with an iron-based metal as reinforcing material
- Hybridization effect of coir fiber on physico-mechanical properties of polyethylene-banana/coir fiber hybrid composites
- Micromechanical properties of unidirectional composites filled with single and clustered shaped fibers
- Structure and microwave absorbing properties of carbon-filled ultra-high molecular weight polyethylene
- Investigation and optimization of the electro-discharge machining parameters of 2024 aluminum alloy and Al/7.5% Al2O3 particulate-reinforced metal matrix composite
- Structural behavior of load-bearing sandwich wall panels with GFRP skin and a foam-web core
- Synthesis, thermal and magnetic behavior of iron oxide-polymer nanocomposites
- High-temperature damping capacity of fly ash cenosphere/AZ91D Mg alloy composites
- Investigation of penetration into woven fabric specimens impregnated with shear thickening fluid
Articles in the same Issue
- Frontmatter
- Review
- A model on the curved shapes of unsymmetric laminates including tool-part interaction
- Original articles
- Enhanced catalytic performance of β-FeOOH by coupling with single-walled carbon nanotubes in a visible-light-Fenton-like process
- Investigation of the microstructure and properties of W75-Cu/W55-Cu brazed joint with Cu-Mn-Co filler metal
- In situ polymerization approach to poly(arylene ether nitrile)-functionalized multiwalled carbon nanotube composite films: thermal, mechanical, dielectric, and electrical properties
- Fabrication and characterization properties of polypropylene/polycarbonate/clay nanocomposites prepared with twin-screw extruder
- Torsional vibration of functionally graded carbon nanotube reinforced conical shells
- A comparative study performance of cationic organic montmorillonite prepared by different methods
- Design and analysis with different substrate materials of a new metamaterial for satellite applications
- An investigation on dry sliding wear behaviour of pressure infiltrated AA1050-XMg/B4C composites
- Investigation of mechanical performances of composite bolted joints with local reinforcements
- Effect of alkali treatment on the flexural properties of a Luffa cylindrica-reinforced epoxy composite
- Thermal expansion, electrical conductivity and hardness of Mn3Zn0.5Sn0.5N/Al composites
- Effect of modified layered double hydroxide on the flammability and mechanical properties of polypropylene
- A unified formulation for free vibration of functionally graded plates
- Friction-stir welding of aluminum alloy with an iron-based metal as reinforcing material
- Hybridization effect of coir fiber on physico-mechanical properties of polyethylene-banana/coir fiber hybrid composites
- Micromechanical properties of unidirectional composites filled with single and clustered shaped fibers
- Structure and microwave absorbing properties of carbon-filled ultra-high molecular weight polyethylene
- Investigation and optimization of the electro-discharge machining parameters of 2024 aluminum alloy and Al/7.5% Al2O3 particulate-reinforced metal matrix composite
- Structural behavior of load-bearing sandwich wall panels with GFRP skin and a foam-web core
- Synthesis, thermal and magnetic behavior of iron oxide-polymer nanocomposites
- High-temperature damping capacity of fly ash cenosphere/AZ91D Mg alloy composites
- Investigation of penetration into woven fabric specimens impregnated with shear thickening fluid

