Abstract
C5H12NNaO3S2, monoclinic, P21/c (no. 14), a = 28.8222(3) Å, b = 5.6782(3) Å, c = 12.3810(9) Å, β = 102.074(2)°, V = 1987.43(17) Å3, Z = 8, Rgt(F) = 0.0269, wRref(F2) = 0.0591, T = 100(2) K.
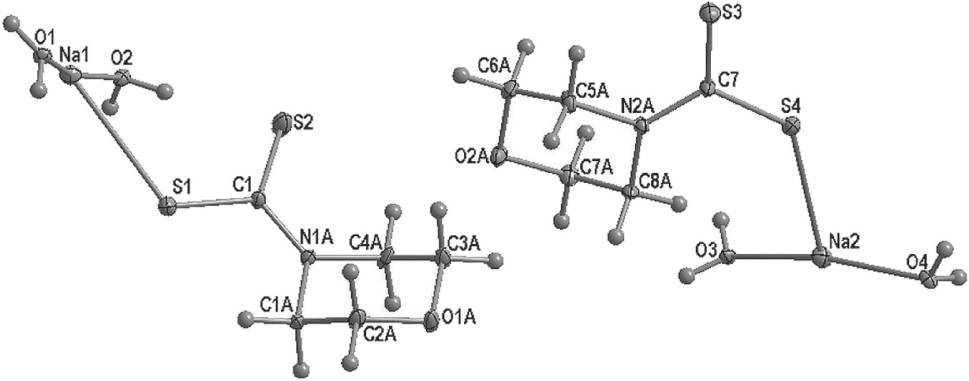
The asymmetric unit of the title crystal structure is shown in the figure (The disorder in the morpholine moieties is amitted for clarity.). Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Source of material
The title compound was synthesized by dispersing (4.33 mL, 50 mmol) of morpholine into 20 mL of diethyl ether and stirred for 10 minutes. Followed by the addition of 20 mL of an ice cold methanolic solution of sodium hydroxide (2.00 g, 50 mmol) and stirred for 15 minutes at an ice-cold temperature. To this mixture, cold carbon disulfide (3 mL, 50 mmol) was added dropwise. The mixture was stirred for 4 h while maintaining an ice-cold temperature resulting in a formation of white precipitate which was collected by filtration. The product obtained was rinsed with diethyl ether and dried in a desiccator. The crystals of the title compound were formed after four days of extracting the filtrate with diethyl ether. White powder. Yield = 61%. m.p. 308.4−310.3 °C. 1H NMR D2O: δ = 3.77 (4H, t, N—CH2), 4.36 (4H, t, O—CH2). 13C NMR D2O: δ = 51.40 (N—CH2), 66.13 (O—CH2), 209.36 (C-SSNa). IR v(cm−1): 972 (C—S), 1108 (C=S), 1414 (C—N). MS (m/z): 162.0045 [M+].
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.36 × 0.23 × 0.14 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.55 mm−1 |
| Diffractometer, scan mode: | Bruker SMART, φ and ω-scans |
| θmax, completeness: | 27.4°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 31639, 4506, 0.024 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 4308 |
| N(param)refined: | 150 |
| Programs: | Bruker programs [1], SHELX [2], WinGX and ORTEP [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| S1 | 0.55623(2) | 0.03105(6) | 0.78588(3) | 0.01240(8) |
| O1 | 0.45297(4) | −0.09325(19) | 0.91778(9) | 0.0155(2) |
| S2 | 0.63255(2) | 0.23405(7) | 0.96360(3) | 0.01908(9) |
| S4 | 0.94367(2) | −0.03262(6) | 1.23051(3) | 0.01196(8) |
| S3 | 0.86773(2) | −0.24153(6) | 1.33344(3) | 0.01535(8) |
| Na1 | 0.49327(2) | 0.25887(10) | 0.91592(4) | 0.01334(12) |
| Na2 | 0.99301(2) | 0.24134(10) | 1.07727(4) | 0.01347(12) |
| O2 | 0.54519(4) | 0.5878(2) | 0.94798(9) | 0.0150(2) |
| O3 | 0.95269(4) | 0.59302(19) | 1.03569(9) | 0.0151(2) |
| O4 | 1.04453(4) | −0.0892(2) | 1.09677(9) | 0.0155(2) |
| O1Aa | 0.71454(6) | −0.3683(3) | 0.76431(14) | 0.0275(4) |
| C2Aa | 0.66508(7) | −0.3968(4) | 0.72608(17) | 0.0224(4) |
| H2AAa | 0.651540 | −0.473359 | 0.784375 | 0.027* |
| H2ABa | 0.659231 | −0.501320 | 0.660638 | 0.027* |
| C1Aa | 0.64031(8) | −0.1632(5) | 0.69550(19) | 0.0179(5) |
| H1AAa | 0.652399 | −0.088940 | 0.634480 | 0.022* |
| H1ABa | 0.605753 | −0.188696 | 0.670403 | 0.022* |
| N1Aa | 0.64941(6) | −0.0095(3) | 0.79245(16) | 0.0150(4) |
| C1 | 0.61594(5) | 0.0703(3) | 0.84542(12) | 0.0172(3) |
| C3Ab | 0.72386(8) | −0.2202(5) | 0.85912(19) | 0.0268(5) |
| H3AAb | 0.758564 | −0.200031 | 0.884208 | 0.032* |
| H3ABb | 0.711654 | −0.296028 | 0.919601 | 0.032* |
| C4Ab | 0.70071(7) | 0.0202(4) | 0.83457(18) | 0.0221(4) |
| H4AAb | 0.706321 | 0.116435 | 0.902748 | 0.027* |
| H4ABb | 0.714882 | 0.103137 | 0.778969 | 0.027* |
| C7b | 0.88425(5) | −0.0648(3) | 1.23605(13) | 0.0200(3) |
| N1Bb | 0.6461(3) | −0.1046(17) | 0.8302(6) | 0.0229(17) |
| C3Bb | 0.6741(3) | −0.1908(19) | 0.6646(7) | 0.033(2) |
| H3Ab | 0.668880 | −0.283507 | 0.595410 | 0.039* |
| H3Bb | 0.670723 | −0.021693 | 0.645134 | 0.039* |
| O1Bb | 0.7211(2) | −0.2362(17) | 0.7295(6) | 0.0385(19) |
| C4Bb | 0.7287(3) | −0.083(2) | 0.8248(7) | 0.035(2) |
| H12Ab | 0.761660 | −0.100481 | 0.867208 | 0.042* |
| H12Bb | 0.724018 | 0.083056 | 0.800456 | 0.042* |
| C5Bb | 0.6959(3) | −0.1413(18) | 0.8945(7) | 0.032(2) |
| H13Ab | 0.701842 | −0.039784 | 0.960984 | 0.038* |
| H13Bb | 0.700271 | −0.307453 | 0.918704 | 0.038* |
| C2Bb | 0.6398(3) | −0.2571(19) | 0.7294(8) | 0.023(2) |
| H15Ab | 0.644200 | −0.424523 | 0.751503 | 0.027* |
| H15Bb | 0.607240 | −0.237908 | 0.684652 | 0.027* |
| O2Ac | 0.7808(2) | 0.3381(8) | 1.0415(4) | 0.0241(8) |
| N2Ac | 0.85009(7) | 0.0104(4) | 1.15107(17) | 0.0150(4) |
| C5Ac | 0.79895(8) | −0.0287(5) | 1.1431(2) | 0.0213(5) |
| H5AAc | 0.793880 | −0.112700 | 1.209790 | 0.026* |
| H5ABc | 0.786137 | −0.127670 | 1.077873 | 0.026* |
| C8Ac | 0.85855(8) | 0.1566(5) | 1.0594(2) | 0.0183(5) |
| H8AAc | 0.848921 | 0.069446 | 0.988992 | 0.022* |
| H8ABc | 0.892800 | 0.193185 | 1.070167 | 0.022* |
| C7Ac | 0.83047(8) | 0.3831(5) | 1.0539(2) | 0.0223(5) |
| H7ACc | 0.841840 | 0.474885 | 1.122310 | 0.027* |
| H7ADc | 0.835826 | 0.478690 | 0.990767 | 0.027* |
| C6Ac | 0.77320(12) | 0.2054(7) | 1.1329(3) | 0.0238(7) |
| H6ACc | 0.738765 | 0.176893 | 1.124981 | 0.029* |
| H6ADc | 0.784221 | 0.297518 | 1.201369 | 0.029* |
| N2Bd | 0.85459(12) | 0.1221(7) | 1.1920(3) | 0.0129(7) |
| C6Bd | 0.86342(15) | 0.2834(9) | 1.1047(4) | 0.0178(9) |
| H2Cd | 0.895502 | 0.255411 | 1.090399 | 0.021* |
| H2Dd | 0.861639 | 0.448896 | 1.128646 | 0.021* |
| C7Bd | 0.82628(16) | 0.2389(9) | 1.0006(4) | 0.0217(9) |
| H9AAd | 0.831825 | 0.346724 | 0.941786 | 0.026* |
| H9ABd | 0.829497 | 0.075408 | 0.975394 | 0.026* |
| C9Bd | 0.80589(14) | 0.1546(8) | 1.2098(3) | 0.0172(8) |
| H6AAd | 0.802222 | 0.316016 | 1.237083 | 0.021* |
| H6ABd | 0.799714 | 0.041440 | 1.265959 | 0.021* |
| C8Bd | 0.7710(2) | 0.1153(12) | 1.1023(5) | 0.0211(12) |
| H7AAd | 0.773330 | −0.049684 | 1.078147 | 0.025* |
| H7ABd | 0.738366 | 0.141104 | 1.113547 | 0.025* |
| O2Bd | 0.7800(4) | 0.2726(15) | 1.0174(8) | 0.0224(15) |
| H1A | 0.4492(7) | −0.179(4) | 0.8673(17) | 0.026(5)* |
| H3C | 0.9494(7) | 0.679(4) | 1.0831(17) | 0.026(5)* |
| H2A | 0.5467(7) | 0.691(4) | 0.9045(17) | 0.028(5)* |
| H4A | 1.0458(7) | −0.198(4) | 1.1437(18) | 0.033(6)* |
| H2B | 0.5699(8) | 0.514(4) | 0.9506(16) | 0.030(6)* |
| H4B | 1.0680(8) | −0.015(4) | 1.1179(17) | 0.034(6)* |
| H3D | 0.9294(8) | 0.608(4) | 0.9877(18) | 0.032(6)* |
| H1B | 0.4288(8) | −0.110(4) | 0.9439(18) | 0.039(6)* |
Occupancies: a = 0.8, b = 0.2, c = 0.65, d = 0.35.
Experimental details
Crystal evaluation and data collection were done on a Bruker Smart APEX2 diffractometer [1]. The structure was solved by the direct method using the SHELXS [2] program and refined using SHELXL [2]. The visual crystal structure information was obtained using ORTEP-3 [3] system software. All hydrogen atoms were placed in idealized positions and refined in riding models with Uiso assigning values of 1.2 times those of their parent atoms and the distances of C—Hs were constrained to 0.99 Å for all the methylene H atoms and 0.84 Å for water hydrogens.
Both morpholine moieties are disordered over two positions (Table 2).
Discussion
Dithiocarbamate ligands are known to be flexible ligands that are able to form diverse types of complexes and be able to stabilize transition metal in various oxidation states [4], [5]. They may possess electrochemical and optical properties because of their redox behaviour and strong coordination ability [6]. They are known to be planar and sterically non-demanding ligands that can be automatically changed by having choices of substituents [7]. Their functionalization of substituents on the nitrogen atom of the dithiocarbamate moeity, can result in various complex structures through secondary interactions hence desirable physical properties [8]. The ease of formation of metal complexes is due to the electron delocalization around the N(CSS)- moiety which is also transferred to the metal centre [9]. This ability is recognized to the approval of dithiocarbamates and thioureide tautomers [10]. In dithiocarbamates, the sulfur atoms act as soft donor atoms containing a lone pair of electron localized on nitrogen (sp3) resulting in pyramidal arrangement of substituents. In thioureide systems there are donor ligands which are planar with lone pair of electron localized in the backbone of carbon-nitrogen bond and onto the sulfur atoms [10]. Dithiocarbamate ligands have been widely used in coordination chemistry due to diverse applications, for example used in dyes, agricultural and pharmaceutical industries and to chelate with heavy metals [11], [12], [13].
Dithiocarbamate ligands are mostly synthesized from nucleophilic addition reaction of primary or secondary amines with carbon disulfide in the presence of a strong base i.e. sodium/ potassium hydroxide acting as a proton acceptor [14]. The formation of these compounds is often accompanied by release of heat, hence the syntheses are carried out at low temperatures [15]. The title compound was as such prepared by the reaction morpholine 4-dithiocarbamate with sodium hydroxide in ethanol.
The asymmetric unit of the title compound contains two fragments of two chains of coordination polymers (see the figure). The fragments contain a morpholine 4-dithiocarbamate moiety and two water molecules coordinated to a sodium centre. The coordination of the two water molecules to the Na center results in four member metallacycles connected in alternating orthogonal fashion in the b crystallographic direction. Two S atoms from two morpholine moieties also in the same chains occupy the other coordination sites such that adjacent chains have the O containing sides of the chair conformed morpholine facing each other. The interaction between the sodium cation and water molecules does not disturb the geometric conformation of the ligand [16]. The S—Na bond distances are 3.0291(7) Å, while the S—C ones are 1.7151(15) Å and 1.7390(15) Å indicating a tendency towards a double bond character of electron density in the S—C—S group [17]. The C—S—Na bond angle is 132.42(5)°, while the S—C—S bond angle is 120.40(8)° and is similar to what is observed for similar complexes.
Acknowledgements
We acknowledge the University of KwaZulu-Natal and National Research Foundation of South Africa for financial support for Ekemini Akpan and Sizwe J. Zamisa.
References
1. Bruker. APEX3, SAINT-Plus, XPREP. Bruker AXS Inc., Madison, WI, USA (2016).Search in Google Scholar
2. Sheldrick, G. M.: SHELXT – Integrated space-group and crystal-structure determination. Acta Crystallogr. A71 (2015) 3–8.10.1107/S2053273314026370Search in Google Scholar PubMed PubMed Central
3. Farrugia, L. J.: WinGX and ORTEP for Windows. Appl. Crystallogr. 45 (2012) 849–854.10.1107/S0021889812029111Search in Google Scholar
4. Al-Hasani, T. J.; Al-Taie, M. K.: Synthesis, structural and antibacterial study of some metal ion dithiocarbamate-azo complexes. J. Al-Nahrain University-Scie. 18 (2015) 1–12.10.22401/JNUS.18.4.01Search in Google Scholar
5. Beyramabadi, S.; Morsali, A.; Vahidi, S.: Dft characterization of 1-acetylpiperazinyl-dithiocarbamate ligand and its transition metal complexes. J. Struct. Chem. 53 (2012) 665–675.10.1134/S0022476612040087Search in Google Scholar
6. Tiwari, S.; Reddy, K.; Bajpai, A.; Khare, K.; Nagaraju, V.: Synthesis and characterization of bisdithiocarbamates from weak nitrogen bases and its metal complexes. Int. Res. J. Pure. Appl. Chem. 7(2) (2015) 78–91.10.9734/IRJPAC/2015/6588Search in Google Scholar
7. Venugopal, K.; Rameshbabu, K.; Sreeramulu, J.: Synthesis and characterization of (novel heterocyclic) 3-amino-9-ethyl carbazole dithiocarbamate [aeczdtc] ligand and its metal complexes. Der. Pharma. Chemica. 7 (2015) 252–60.Search in Google Scholar
8. Manar, K. K.; Yadav, M. K.; Drew, M. G.; Singh, N.: Influence of functionalities over polymer, trimer, dimer formation and optical properties of cadmium dithiocarbamates. Polyhedron 117 (2016) 592–599.10.1016/j.poly.2016.06.047Search in Google Scholar
9. Vojta, D.; Višnjevac, A.; Leka, Z.; Kosović, M.; Vazdar, M.: Temperature-induced release of crystal water in the Co, Mo and Pt complexes of N,N-diacetatedithiocarbamate. FTIR spectroscopy and quantum chemical study. J. Mol. Struct. 1103 (2016) 245–253.10.1016/j.molstruc.2015.09.038Search in Google Scholar
10. Hogarth, G.: Metal-dithiocarbamate complexes: chemistry and biological activity. Mini Rev. Med. Chem. 12 (2012) 1202–1215.10.2174/138955712802762095Search in Google Scholar PubMed
11. Rani, P. J.; Thirumaran, S.; Ciattini, S.: Synthesis and characterization of Ni(II) and Zn(II) complexes of (furan-2-yl)methyl (2-(thiophen-2-yl)ethyl) dithiocarbamate (ftpedtc): X-ray structures of [Zn(ftpedtc)2(py)] and [Zn(ftpedtc)Cl(1,10-phen)]. Spectrochim. Acta A 137 (2015) 1164–1173.10.1016/j.saa.2014.09.019Search in Google Scholar PubMed
12. Abu-El-Halawa, R.; Zabin, S. A.: Removal efficiency of Pb, Cd, Cu and Zn from polluted water using dithiocarbamate ligands. J. Taibah. Univ. Sci. 11 (2017) 57–65.10.1016/j.jtusci.2015.07.002Search in Google Scholar
13. Hou, X.; Li, X.; Hemit, H.; Aisa, H. A.: Synthesis, characterization, and antitumor activities of new palladium (ii) complexes with 1-(alkyldithiocarbonyl)-imidazoles. J. Coord. Chem. 67 (2014) 461–469.10.1080/00958972.2014.890717Search in Google Scholar
14. Andrew, F. P.; Ajibade, P. A.: Metal complexes of alkyl-aryl dithiocarbamates: Structural studies, anticancer potentials and applications as precursors for semiconductor nanocrystals. J. Mol. Struct. 1155 (2018) 843–855.10.1016/j.molstruc.2017.10.106Search in Google Scholar
15. Nabipour, H.: Synthesis of a new dithiocarbamate cobalt complex and its nanoparticles with the study of their biological properties. Int. J. Nano Dimension 1 (2011) 225–232.10.1049/mnl.2010.0224Search in Google Scholar
16. Amim, R. S.; Oliveira, M. R.; Perpétuo, G. J.; Janczak, J.; Miranda, L. D.; Rubinger, M. M.: Syntheses, crystal structure and spectroscopic characterization of new platinum (II) dithiocarbimato complexes. Polyhedron 27 (2008) 1891–1897.10.1016/j.poly.2008.02.030Search in Google Scholar
17. Andrew, F. P.; Ajibade, P. A.: Synthesis, characterization and anticancer studies of bis (1-phenylpiperazine dithiocarbamato) Cu(II), Zn(II) and Pt(II) complexes: Crystal structures of 1-phenylpiperazine dithiocarbamato-S,S′ Zn(II) and Pt(II). J. Mol. Struct. 1170 (2018) 24–29.10.1016/j.molstruc.2018.05.068Search in Google Scholar
© 2019 Nolwazi Solomane et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Frontmatter
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-5-fluorophenol, C14H13FN2O
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-4,6-dichlorophenol, C14H12Cl2N2O
- The crystal structure of (E)-1-(4-methoxyphenyl)-3-(2-nitrophenyl)triaz-1-ene C8H8N2O4
- Crystal structure of (E)-2-(((6-bromopyridin-2-yl)methylene)amino)-3′,6′-bis(ethylamino)-2′,7′-dimethylspiro[isoindoline-1,9′-xanthen]-3-one—methanol (1:1), C32H30N5O2Br ⋅ CH4O
- Crystal structure of 2,4-pentanedione bis(2,4-dinitrophenylhydrazone), C17H16N8O8
- Crystal structure of sodium morpholine-4-carbodithioate, (C5H12NNaO3S2)
- Crystal structure of 1,1′-(hexane-1,6-diyl)bis(3-ethyl-1H-imidazol-3-ium) bis(hexafluorido phosphate), C16H28F12N4P2
- Crystal structure of 5-(4-chlorophenyl)-3-(4-fluorophenyl)-1-phenyl-4,5-dihydro-1H-pyrazole, C21H16ClFN2
- Crystal structure of catena-poly[diaqua-bis(3-carboxy-5-methoxybenzoato-κO)-(1,2-bis(imidazol-1-yl)ethane-κ2N:N′)cobalt(II)], C26H28CoN4O12, [Co(C9H6O5)2(H2O)2(C8H10N4)]
- The crystal structure of 3-cyclohexyl-1,5-dioxaspiro[5.5]undecane-2,4-dione, C15H22O4
- Crystal structure of (2,4-dimethoxybenzyl)triphenylphosphonium trifluoroacetate — trifluoroacetic acid (1/1), C31H27F6O6P
- Crystal structure of 4-tert-butyl-1-(2,6-dimethylphenyl)-1H-1,2,3-triazole, C14H19N3
- Crystal structure of 1,1′-methylenebis(4-tert-butylpyridinium) tetrachloridocobaltate(II) – dichloromethane (1:1), C20H30Cl6CoN2
- Crystal structure of (4,4′-(ethane-1,2-diylbis((nitrilo)(2-furylmethylylidene)))bis(3-methyl-1-phenyl-1H-pyrazol-5-olato-κ4N,N′,O,O′))-nickel(II)), C32H26N6NiO4
- Synthesis and crystal structure of bis{((E)-((4-((E)-1-(benzyloxyimino)ethyl)phenyl)imino)methyl)-2-phenolato-κ2N,O}copper(II), C44H38CuN4O4
- Crystal structure of catena-poly[diaqua-bis(3,5-dichloropyridine-4-carboxylato-κ1O)-bis(μ2-4,4′-bipyridine-κ2N:N′)cobalt(II)], C22H16Cl4CoN4O6
- The crystal structure of 2-(4-chloro-2,6-dinitrophenyl)-1-(4-chloro-3,5-dinitrophenyl)diazene 1-oxide, C12H4Cl2N6O9
- The crystal structure of 3-(1H-benzo[d]imidazol-2-yl)-7-chloro-1-cyclopropyl-6-fluoro-1,4-dihydroquinolin — dimethylsulfoxide (1/1), C21H19ClFN3O2S
- The crystal structure of dichlorido-bis(1-butyl-1H-imidazole-κN)zinc(II), C14H24Cl2ZnN4
- (Z)-N-tert-butyl-1-(2-(3,5-dichlorobenzamido)phenyl) methanimine oxide, C18H18Cl2N2O2
- Crystal structure of diaqua-bis(3-carboxy-5-bromoisophthalato-κO)-bis(1-(3-(1H-benzo[d]imidazol-1-yl)propyl)-1H-benzo[d]imidazol-3-ium-κN)nickel(II) bis(3-carboxy-5-bromoisophthalate), C66H54Br4N8NiO18
- Crystal structure of poly[aqua(μ2-5-methoxyisophthalato-κ2O,O′:O′′)-(1,2-bis(imidazol-1′-yl)ethane-κ2N:N′)cobalt(II), C34H36Co2N8O12
- Crystal structure of poly[diaqua-bis(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N′)manganese(II)] terephthalate tetrahydrate, MnC32H38N10O10
- Crystal structure of the fluorescent fipronil derivative 5,5′-(methylenebis(azanediyl))bis(1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carbonitrile), C25H6N8O2Cl4F12S2
- Crystal structure of the phosphorescent complex diethyldithiophosphonato-κ2S,S′-bis(2-phenylpyridinato-κ2C,N)iridium(III), C26H26N2O2PS2Ir
- The crystal structure of 4,10-diethoxy-6H,12H-6,12-epoxydibenzo[b,f][1,5]dioxocine, C18H18O5
- Crystal structure of dichlorido-bis(N-benzyl-2-(quinolin-8-yloxy)acetamide-κ2N,O)copper(II) — ethyl acetate (1/1), C38H36N4O6Cl2Cu
- Synthesis and crystal structure of bis{4-methyl-2-((E)-((4-((E)-1-(benzyloxyimino)ethyl)phenyl)imino)methyl)phenolato-κ3N,O,O}copper(II), C92H84Cu2N8O8
- The crystal structure of 1,3,5-trinitro-4,6-diazidobenzene, C6HN9O6
- Crystal structure 1-cinnamyl-2-((Z)-styryl)-1H-benzo[d]imidazole — methanol (1/1), C24H20N2 ⋅ CH4O
- The crystal structure of poly[m2-aqua-tetraaqua-bis(m9-4-formylbenzene-1,3-disulfonato)tetrasodium(I) hydrate, C14H18O19S4Na4
- Crystal structure of 2-((2,8-bis(trifluoromethyl)quinolin-4-yl)(hydroxy)methyl)piperidin-1-ium trifluoroacetate, [C17H17F6N2O][C2F3O2]
- The crystal structure of bis(ferrocenecarboxylato-κ2O,O′)bis[4-(dimethylamino)pyridine-κN]copper(II) — acetonitrile(1/2), C40H44CuO4Fe2N6
- Crystal structure of poly[di-μ2-aqua)-diaqua-bis(μ6-4,4′,4′′-(benzene-1,3,5-triyltris(oxy))tribenzoato-κ6O1:O2:O3:O3:O5:O6)tricadmium(II)] dihydrate, C54H42Cd3O24
- The crystal structure of dichlorido(1,3-bis(2,6-diisopropyl-phenyl)-1H-3λ4-imidazol-2-yl)(3-phenyl-pyridine-κN)palladium(IV), C38H45N3Cl2Pd
- The crystal structure of 2-chloro-4-(prop-2-yn-1-yloxy)-6-phenyl-1,3,5-triazine, C12H8ClN3O
- The crystal structure of 2,6-di-tert-butyl-4-(phenyl(phenylsulfonyl)methyl)phenol, C27H32O3S
- Crystal structure of bis{μ2-bis{(((((1-methoxyimino)ethyl)phenyl)imino)methyl)-2-phenolato-κ3N,O:O}copper(II)}, C68H68Cu2N8O8
- Crystal structure of catena-poly[tetraaqua-bis(μ2-2-(4-carboxylatophenoxy)benzoato-κ2O:O′)-pentakis(pyridine-κ1N)dinickel(II)], C53H47N5Ni2O13
- Synthesis and crystal structure of 1-(2,6-dichloro-4-trifluoromethyl-phenyl)-5-(3-methoxy-benzylamino)-4-trifluoromethanesulfinyl-1H-pyrazole-3-carbonitrile, C20H12N4Cl2F6O2S
- Redetermination of the crystal structure of bis(μ2-di-ethyldithiocarbamato-κ3S,S′:S;κ3S:S: S′)-hexacarbonyl-di-rhenium(I), C16H20N2O6Re2S4
- The crystal structure of (E)-N′-((2-hydroxynaphthalen-1-yl)methylene)-2-phenylacetohydrazide, C19H16O2N2
- Crystal structure of 6-hydroxy-4,8,11b-trimethyltetradecayhdro-8,11-epoxy-6a,9-methanocyclohepta[a]naphthalene-4-carboxylic acid – methanol (1/1), C20H30O4
- The crystal structure of aqua-bis(3-acetyl-2-oxo-2H-chromen-4-olato-κ2O,O′)zinc(II) monohydrate, C22H18O10Zn
- Crystal structure of poly[bis(μ2-4-bromoisophthalate-κ2O:O′)-tris(μ2-1-(3-((1H-1,2,4-triazol-1-yl)methyl)benzyl)-1H-1,2,4-triazole-κ2N:N′)dicobalt(II)] monohydrate, C26H23CoN9O5Br
- A cyclic I102− anion in the layered crystal structure of theophyllinium pentaiodide, C7H9I5N4O2
- Crystal structure of catena-poly[diaqua-bis(μ2-4-((4-(pyridin-2-ylmethoxy)phenyl)diazenyl)benzoato-κ3O,O′:N)cadmium(III)], Cd(C19H14O3N3)2(H2O)
- Crystal structure of catena-poly[(μ2-4,4′-bipyridyl-κN,N′)-bis(O,O′-dimethyldithiophosphato-κS)-zinc(II)], {C14H20N2O4P2S4Zn}n
- Crystal structure of 3-amino-2-hydroxy-6-methoxybenzamide hydrate, C16H22N4O7
- Crystal structure of hemikis(cyclohexane-1,4-diammonium) (pyridine-2-carboxylate), [C6H16N2]0.5[C6H4NO2]
- Crystal structure of 2-chloro-4-(prop-2-yn-1-yloxy)-6-(thiophen-2-yl)-1,3,5-triazine, C10H6ClN3OS
- The crystal structure of 3-butyl-1-methyl-1H-imidazol-3-ium catena-poly[tris(μ2-bromido-κ2Br:Br)lead(II)], C8H15Br3N2Pb
- Crystal structure of 3-(5-amino-1H-1,2,4-triazol-3-yl)-1-(piperidin-1-yl)propan-1-one, C10H17N5O
- Crystal structure of aqua-2,2′,2′′-(((nitrilo-κN-tris(ethane-2,1-diyl))tris(azanylylidene-κ3N′,N′′,N′′′))tris(methanylylidene))tris(4-chlorophenolato-κ3O,O′,O′′)neodymium(III), C27H26Cl3N4NdO4
- Crystal structure of dichlorido-(μ2-2,2′-(diazene-1,2-diyl)bis(benzen-1-ido)-κ2C:C′)dimercury(II), C12H8Cl2Hg2N2
- Crystal structure of (3E,5E)-3,5-bis(4-cyanobenzylidene)-1-((4-fluorophenyl)sulfonyl)piperidin-4-one, C27H18FN3O3S
- Crystal structure of dichlorido(pyridine-κN)(2,4,6-tri-2-pyridyl-1,3,5-triazine-κ3N2,N1,N6)nickel(II), C23H17Cl2N7Ni
- Redetermination of the crystal structure of tetrakis(4-chlorobenzyl)tin(IV), C28H24Cl4Sn
- The crystal structure of 2,6-bis(pyridin-1-ium-3-ylmethyl)hexahydro-4,8-ethenopyrrolo-[3,4-f] isoindole-1,3,5,7-tetrone tetrachloridocuprate(II) monohydrate, C24H24Cl4CuN4O5
- Crystal structure of cyclo-[octaaqua-tetrakis(μ2-5,5′-(1H-imidazole-4,5-diyl)bis(tetrazol-2-ido)-κ4N,N′,N′′,N′′′)tetramagnesium(II)], C20H24N40O8Mg4
- The crystal structure of a matrine derivative, 13-(4-Cl-pyrrole)-matrine, C18H26ClN4O
- Crystal structure of (dibenzyl sulphoxide-κO)bis(2-chlorobenzyl-κC1)dichloridotin(IV), C28H26Cl4OSSn
- Crystal structure of catena-poly[(μ2-azido-κ2N:N)(μ2-4-cyanobenzoato-κ2O:O′)-(μ2-methanol-κ2O:O)copper(II)], C9H8CuN4O3
- Crystal structure of 1,1′-dibenzyl-3,3′-dicyano-1,1′,4,4′-tetrahydro-4,4′-bipyridine, C26H22N4
- Crystal structure of (2-bromobenzyl)((1-bromonaphthalen-2-yl)methyl)sulfane, C18H14Br2S
- Crystal structure of 2-(4-ammoniocyclohexyl)-3-(pyridin-2-yl)imidazo[1,5-a]pyridin-2-ium 2-[(2-carboxylatophenyl)disulfanyl]benzoate dihydrate, [C18H22N4][C14H8O4S2] ⋅ 2H2O
- Crystal structure of (E)-N-((3R,5S,10S, 13S,14S,17S)-17-((S)-1-(dimethylamino)ethyl)-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-3-yl)-2-methylbut-2-enamide – water – methanol (1/1/1), C29H54N2O3
- Crystal structure of methyl 2-(4-(3-(2,4-difluorophenyl)pyrazolo[1,5-a]pyrimidin-6-yl)phenyl)acetate, C21H15F2N3O2
- Crystal structure of poly[triaqua-(μ4-benzene-1,3,5-tricarboxylato-κ5O1,O2:O3:O4:O5)-(μ2-5-(3-pyridyl)tetrazolato-κ2N1:N3)dizinc(II)], C15H13N5O9Zn2
- Crystal structure of N-(3-methylphenyl)(propan-2-yloxy)carbothioamide, C11H15NOS
- Crystal structure of poly[(μ2-1,3-bis(imidazol-1-ylmethyl)benzene-κ2N:N′)(nitrato-κ1O)cadmium(II)] — water (2/1), C28H32CdN10O7
- Crystal structure of 4-phenyl-2,4-dihydro-3H-1,2,4-triazole-3-thione, C8H7N3S
- Crystal structure of benzyltrichloridobis(1H-pyrazole-κ2N)tin(IV), C13H15Cl3N4Sn
- Crystal structure of chlorido-4-fluorobenzyl-bis(2-methylquinolin-8-olato-κ2N,O)tin(IV), C27H22ClFN2O2Sn
- Crystal structure of tetrakis(O,O′-diisopropyldithiophosphato-κ2S,S′)-(μ2-1,2-bis(4-pyridylmethylene)hydrazine-κ2N:N′)zinc(II), C36H66N4O8P4S8Zn2
- Crystal structure of tetrabutylammonium 4,4-oxydibenzoate – boric acid – water (1/2/6) C46H98B2N2O17
- Redetermination of the crystal structure of catena-poly[[tribenzyltin(IV)]-(μ2-pyridine-4-carboxylato-κ2N:O)], C27H25NO2Sn
- The synthysis and crystal structure of cyclohexyl 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carboxylate, C18H15N3Cl2F6O3S
- The crystal structure of 5,7-bis(2-hydroxyethoxy)-2-phenyl-4H-chromen-4-one, C19H18O6
- Synthesis and crystal structure of (±)-Ethyl 5′-(difluoromethyl)-2-oxo-4′,5′-dihydrospiro[indoline-3,3′-pyrazole]-4′-carboxylate, C14H13F2N3O3
Articles in the same Issue
- Frontmatter
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-5-fluorophenol, C14H13FN2O
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-4,6-dichlorophenol, C14H12Cl2N2O
- The crystal structure of (E)-1-(4-methoxyphenyl)-3-(2-nitrophenyl)triaz-1-ene C8H8N2O4
- Crystal structure of (E)-2-(((6-bromopyridin-2-yl)methylene)amino)-3′,6′-bis(ethylamino)-2′,7′-dimethylspiro[isoindoline-1,9′-xanthen]-3-one—methanol (1:1), C32H30N5O2Br ⋅ CH4O
- Crystal structure of 2,4-pentanedione bis(2,4-dinitrophenylhydrazone), C17H16N8O8
- Crystal structure of sodium morpholine-4-carbodithioate, (C5H12NNaO3S2)
- Crystal structure of 1,1′-(hexane-1,6-diyl)bis(3-ethyl-1H-imidazol-3-ium) bis(hexafluorido phosphate), C16H28F12N4P2
- Crystal structure of 5-(4-chlorophenyl)-3-(4-fluorophenyl)-1-phenyl-4,5-dihydro-1H-pyrazole, C21H16ClFN2
- Crystal structure of catena-poly[diaqua-bis(3-carboxy-5-methoxybenzoato-κO)-(1,2-bis(imidazol-1-yl)ethane-κ2N:N′)cobalt(II)], C26H28CoN4O12, [Co(C9H6O5)2(H2O)2(C8H10N4)]
- The crystal structure of 3-cyclohexyl-1,5-dioxaspiro[5.5]undecane-2,4-dione, C15H22O4
- Crystal structure of (2,4-dimethoxybenzyl)triphenylphosphonium trifluoroacetate — trifluoroacetic acid (1/1), C31H27F6O6P
- Crystal structure of 4-tert-butyl-1-(2,6-dimethylphenyl)-1H-1,2,3-triazole, C14H19N3
- Crystal structure of 1,1′-methylenebis(4-tert-butylpyridinium) tetrachloridocobaltate(II) – dichloromethane (1:1), C20H30Cl6CoN2
- Crystal structure of (4,4′-(ethane-1,2-diylbis((nitrilo)(2-furylmethylylidene)))bis(3-methyl-1-phenyl-1H-pyrazol-5-olato-κ4N,N′,O,O′))-nickel(II)), C32H26N6NiO4
- Synthesis and crystal structure of bis{((E)-((4-((E)-1-(benzyloxyimino)ethyl)phenyl)imino)methyl)-2-phenolato-κ2N,O}copper(II), C44H38CuN4O4
- Crystal structure of catena-poly[diaqua-bis(3,5-dichloropyridine-4-carboxylato-κ1O)-bis(μ2-4,4′-bipyridine-κ2N:N′)cobalt(II)], C22H16Cl4CoN4O6
- The crystal structure of 2-(4-chloro-2,6-dinitrophenyl)-1-(4-chloro-3,5-dinitrophenyl)diazene 1-oxide, C12H4Cl2N6O9
- The crystal structure of 3-(1H-benzo[d]imidazol-2-yl)-7-chloro-1-cyclopropyl-6-fluoro-1,4-dihydroquinolin — dimethylsulfoxide (1/1), C21H19ClFN3O2S
- The crystal structure of dichlorido-bis(1-butyl-1H-imidazole-κN)zinc(II), C14H24Cl2ZnN4
- (Z)-N-tert-butyl-1-(2-(3,5-dichlorobenzamido)phenyl) methanimine oxide, C18H18Cl2N2O2
- Crystal structure of diaqua-bis(3-carboxy-5-bromoisophthalato-κO)-bis(1-(3-(1H-benzo[d]imidazol-1-yl)propyl)-1H-benzo[d]imidazol-3-ium-κN)nickel(II) bis(3-carboxy-5-bromoisophthalate), C66H54Br4N8NiO18
- Crystal structure of poly[aqua(μ2-5-methoxyisophthalato-κ2O,O′:O′′)-(1,2-bis(imidazol-1′-yl)ethane-κ2N:N′)cobalt(II), C34H36Co2N8O12
- Crystal structure of poly[diaqua-bis(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N′)manganese(II)] terephthalate tetrahydrate, MnC32H38N10O10
- Crystal structure of the fluorescent fipronil derivative 5,5′-(methylenebis(azanediyl))bis(1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carbonitrile), C25H6N8O2Cl4F12S2
- Crystal structure of the phosphorescent complex diethyldithiophosphonato-κ2S,S′-bis(2-phenylpyridinato-κ2C,N)iridium(III), C26H26N2O2PS2Ir
- The crystal structure of 4,10-diethoxy-6H,12H-6,12-epoxydibenzo[b,f][1,5]dioxocine, C18H18O5
- Crystal structure of dichlorido-bis(N-benzyl-2-(quinolin-8-yloxy)acetamide-κ2N,O)copper(II) — ethyl acetate (1/1), C38H36N4O6Cl2Cu
- Synthesis and crystal structure of bis{4-methyl-2-((E)-((4-((E)-1-(benzyloxyimino)ethyl)phenyl)imino)methyl)phenolato-κ3N,O,O}copper(II), C92H84Cu2N8O8
- The crystal structure of 1,3,5-trinitro-4,6-diazidobenzene, C6HN9O6
- Crystal structure 1-cinnamyl-2-((Z)-styryl)-1H-benzo[d]imidazole — methanol (1/1), C24H20N2 ⋅ CH4O
- The crystal structure of poly[m2-aqua-tetraaqua-bis(m9-4-formylbenzene-1,3-disulfonato)tetrasodium(I) hydrate, C14H18O19S4Na4
- Crystal structure of 2-((2,8-bis(trifluoromethyl)quinolin-4-yl)(hydroxy)methyl)piperidin-1-ium trifluoroacetate, [C17H17F6N2O][C2F3O2]
- The crystal structure of bis(ferrocenecarboxylato-κ2O,O′)bis[4-(dimethylamino)pyridine-κN]copper(II) — acetonitrile(1/2), C40H44CuO4Fe2N6
- Crystal structure of poly[di-μ2-aqua)-diaqua-bis(μ6-4,4′,4′′-(benzene-1,3,5-triyltris(oxy))tribenzoato-κ6O1:O2:O3:O3:O5:O6)tricadmium(II)] dihydrate, C54H42Cd3O24
- The crystal structure of dichlorido(1,3-bis(2,6-diisopropyl-phenyl)-1H-3λ4-imidazol-2-yl)(3-phenyl-pyridine-κN)palladium(IV), C38H45N3Cl2Pd
- The crystal structure of 2-chloro-4-(prop-2-yn-1-yloxy)-6-phenyl-1,3,5-triazine, C12H8ClN3O
- The crystal structure of 2,6-di-tert-butyl-4-(phenyl(phenylsulfonyl)methyl)phenol, C27H32O3S
- Crystal structure of bis{μ2-bis{(((((1-methoxyimino)ethyl)phenyl)imino)methyl)-2-phenolato-κ3N,O:O}copper(II)}, C68H68Cu2N8O8
- Crystal structure of catena-poly[tetraaqua-bis(μ2-2-(4-carboxylatophenoxy)benzoato-κ2O:O′)-pentakis(pyridine-κ1N)dinickel(II)], C53H47N5Ni2O13
- Synthesis and crystal structure of 1-(2,6-dichloro-4-trifluoromethyl-phenyl)-5-(3-methoxy-benzylamino)-4-trifluoromethanesulfinyl-1H-pyrazole-3-carbonitrile, C20H12N4Cl2F6O2S
- Redetermination of the crystal structure of bis(μ2-di-ethyldithiocarbamato-κ3S,S′:S;κ3S:S: S′)-hexacarbonyl-di-rhenium(I), C16H20N2O6Re2S4
- The crystal structure of (E)-N′-((2-hydroxynaphthalen-1-yl)methylene)-2-phenylacetohydrazide, C19H16O2N2
- Crystal structure of 6-hydroxy-4,8,11b-trimethyltetradecayhdro-8,11-epoxy-6a,9-methanocyclohepta[a]naphthalene-4-carboxylic acid – methanol (1/1), C20H30O4
- The crystal structure of aqua-bis(3-acetyl-2-oxo-2H-chromen-4-olato-κ2O,O′)zinc(II) monohydrate, C22H18O10Zn
- Crystal structure of poly[bis(μ2-4-bromoisophthalate-κ2O:O′)-tris(μ2-1-(3-((1H-1,2,4-triazol-1-yl)methyl)benzyl)-1H-1,2,4-triazole-κ2N:N′)dicobalt(II)] monohydrate, C26H23CoN9O5Br
- A cyclic I102− anion in the layered crystal structure of theophyllinium pentaiodide, C7H9I5N4O2
- Crystal structure of catena-poly[diaqua-bis(μ2-4-((4-(pyridin-2-ylmethoxy)phenyl)diazenyl)benzoato-κ3O,O′:N)cadmium(III)], Cd(C19H14O3N3)2(H2O)
- Crystal structure of catena-poly[(μ2-4,4′-bipyridyl-κN,N′)-bis(O,O′-dimethyldithiophosphato-κS)-zinc(II)], {C14H20N2O4P2S4Zn}n
- Crystal structure of 3-amino-2-hydroxy-6-methoxybenzamide hydrate, C16H22N4O7
- Crystal structure of hemikis(cyclohexane-1,4-diammonium) (pyridine-2-carboxylate), [C6H16N2]0.5[C6H4NO2]
- Crystal structure of 2-chloro-4-(prop-2-yn-1-yloxy)-6-(thiophen-2-yl)-1,3,5-triazine, C10H6ClN3OS
- The crystal structure of 3-butyl-1-methyl-1H-imidazol-3-ium catena-poly[tris(μ2-bromido-κ2Br:Br)lead(II)], C8H15Br3N2Pb
- Crystal structure of 3-(5-amino-1H-1,2,4-triazol-3-yl)-1-(piperidin-1-yl)propan-1-one, C10H17N5O
- Crystal structure of aqua-2,2′,2′′-(((nitrilo-κN-tris(ethane-2,1-diyl))tris(azanylylidene-κ3N′,N′′,N′′′))tris(methanylylidene))tris(4-chlorophenolato-κ3O,O′,O′′)neodymium(III), C27H26Cl3N4NdO4
- Crystal structure of dichlorido-(μ2-2,2′-(diazene-1,2-diyl)bis(benzen-1-ido)-κ2C:C′)dimercury(II), C12H8Cl2Hg2N2
- Crystal structure of (3E,5E)-3,5-bis(4-cyanobenzylidene)-1-((4-fluorophenyl)sulfonyl)piperidin-4-one, C27H18FN3O3S
- Crystal structure of dichlorido(pyridine-κN)(2,4,6-tri-2-pyridyl-1,3,5-triazine-κ3N2,N1,N6)nickel(II), C23H17Cl2N7Ni
- Redetermination of the crystal structure of tetrakis(4-chlorobenzyl)tin(IV), C28H24Cl4Sn
- The crystal structure of 2,6-bis(pyridin-1-ium-3-ylmethyl)hexahydro-4,8-ethenopyrrolo-[3,4-f] isoindole-1,3,5,7-tetrone tetrachloridocuprate(II) monohydrate, C24H24Cl4CuN4O5
- Crystal structure of cyclo-[octaaqua-tetrakis(μ2-5,5′-(1H-imidazole-4,5-diyl)bis(tetrazol-2-ido)-κ4N,N′,N′′,N′′′)tetramagnesium(II)], C20H24N40O8Mg4
- The crystal structure of a matrine derivative, 13-(4-Cl-pyrrole)-matrine, C18H26ClN4O
- Crystal structure of (dibenzyl sulphoxide-κO)bis(2-chlorobenzyl-κC1)dichloridotin(IV), C28H26Cl4OSSn
- Crystal structure of catena-poly[(μ2-azido-κ2N:N)(μ2-4-cyanobenzoato-κ2O:O′)-(μ2-methanol-κ2O:O)copper(II)], C9H8CuN4O3
- Crystal structure of 1,1′-dibenzyl-3,3′-dicyano-1,1′,4,4′-tetrahydro-4,4′-bipyridine, C26H22N4
- Crystal structure of (2-bromobenzyl)((1-bromonaphthalen-2-yl)methyl)sulfane, C18H14Br2S
- Crystal structure of 2-(4-ammoniocyclohexyl)-3-(pyridin-2-yl)imidazo[1,5-a]pyridin-2-ium 2-[(2-carboxylatophenyl)disulfanyl]benzoate dihydrate, [C18H22N4][C14H8O4S2] ⋅ 2H2O
- Crystal structure of (E)-N-((3R,5S,10S, 13S,14S,17S)-17-((S)-1-(dimethylamino)ethyl)-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-3-yl)-2-methylbut-2-enamide – water – methanol (1/1/1), C29H54N2O3
- Crystal structure of methyl 2-(4-(3-(2,4-difluorophenyl)pyrazolo[1,5-a]pyrimidin-6-yl)phenyl)acetate, C21H15F2N3O2
- Crystal structure of poly[triaqua-(μ4-benzene-1,3,5-tricarboxylato-κ5O1,O2:O3:O4:O5)-(μ2-5-(3-pyridyl)tetrazolato-κ2N1:N3)dizinc(II)], C15H13N5O9Zn2
- Crystal structure of N-(3-methylphenyl)(propan-2-yloxy)carbothioamide, C11H15NOS
- Crystal structure of poly[(μ2-1,3-bis(imidazol-1-ylmethyl)benzene-κ2N:N′)(nitrato-κ1O)cadmium(II)] — water (2/1), C28H32CdN10O7
- Crystal structure of 4-phenyl-2,4-dihydro-3H-1,2,4-triazole-3-thione, C8H7N3S
- Crystal structure of benzyltrichloridobis(1H-pyrazole-κ2N)tin(IV), C13H15Cl3N4Sn
- Crystal structure of chlorido-4-fluorobenzyl-bis(2-methylquinolin-8-olato-κ2N,O)tin(IV), C27H22ClFN2O2Sn
- Crystal structure of tetrakis(O,O′-diisopropyldithiophosphato-κ2S,S′)-(μ2-1,2-bis(4-pyridylmethylene)hydrazine-κ2N:N′)zinc(II), C36H66N4O8P4S8Zn2
- Crystal structure of tetrabutylammonium 4,4-oxydibenzoate – boric acid – water (1/2/6) C46H98B2N2O17
- Redetermination of the crystal structure of catena-poly[[tribenzyltin(IV)]-(μ2-pyridine-4-carboxylato-κ2N:O)], C27H25NO2Sn
- The synthysis and crystal structure of cyclohexyl 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carboxylate, C18H15N3Cl2F6O3S
- The crystal structure of 5,7-bis(2-hydroxyethoxy)-2-phenyl-4H-chromen-4-one, C19H18O6
- Synthesis and crystal structure of (±)-Ethyl 5′-(difluoromethyl)-2-oxo-4′,5′-dihydrospiro[indoline-3,3′-pyrazole]-4′-carboxylate, C14H13F2N3O3

