Introduction
Rili-BAEK 2008, in its current 2014 revised version, integrates the existing concept of control of results into an overall quality management system and supplements the previous editions of the Rili-BAEK guideline with pre- and post-analytics and the precise description of the analytical processes (1). In addition, all the areas of laboratory medicine are included in the concept through Sections B2-B5. The selection of so-called obligatory Rili-BAEK parameters was made according to the principle of marker parameters. Each obligatory Rili-BAEK parameter represents a particular group of analyses and basically stands for the random test with which the analytical quality of the represented group is validated. This concept assumes that by validating the quality of the marker parameter, the quality of the entire group represented is validated as well.
In this context, the external quality control plays an essential role towards ensuring that the internal quality control remains objective. As a purely independent control of accuracy, laboratories receive feedback as to the correctness of their measurements, independent of the calibration and control systems of the methods employed. This question, however, raises the issue of the true value. Figure 1 illustrates a schematic diagram for the measuring value theory and demonstrates that the true value in fact cannot be determined at all. Through metrologically advanced methods, one can very closely approximate this value for some analytes. This allows us to derive the method hierarchy depicted in Figure 2.
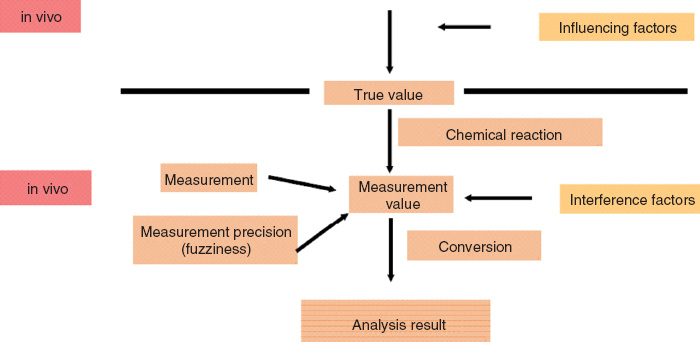
Measuring value theory.

Methods hierarchy.
Reference points
As for the question regarding the true or correct value, the Rili-BAEK guideline applies the reference method concept for certain parameters. In doing so, using a metrologically advanced method, an attempt is made to eliminate all possible interference factors and matrix effects, in order to determine a value that can be valid as a target value for all methods.
Given what at times are diametrically opposed guidelines of the Rili-BAEK for Germany on the one hand, and the in-vitro diagnostics directive of the EU on the other hand (2), the reference method concept may not necessarily be applicable in external quality controls. According to the IVD, the manufacturers are responsible for the traceability of the calibration and control material. In doing so, they are not required to refer to the reference methods. This means that, under certain circumstances, the reference method values cannot be used as reference points for the evaluation of external quality control. In these cases, only the consensus value of a method group can be applied as reference points. This consensus value is also used for the parameters for which no reference method has been developed. In certain exceptional cases (e.g. pharmaceuticals or drugs), the sample weights of these substances can be used as a reference point.
Problem areas
The above-mentioned problem concerning different concepts in the sets of rules leads to the fact that, for testosterone, for instance, the external quality controls cannot be evaluated on the basis of the reference method value (Figure 3). For an evaluation based on the reference method values, the pass rate would be very low at 56% and probably would not reflect the capability of the test systems.
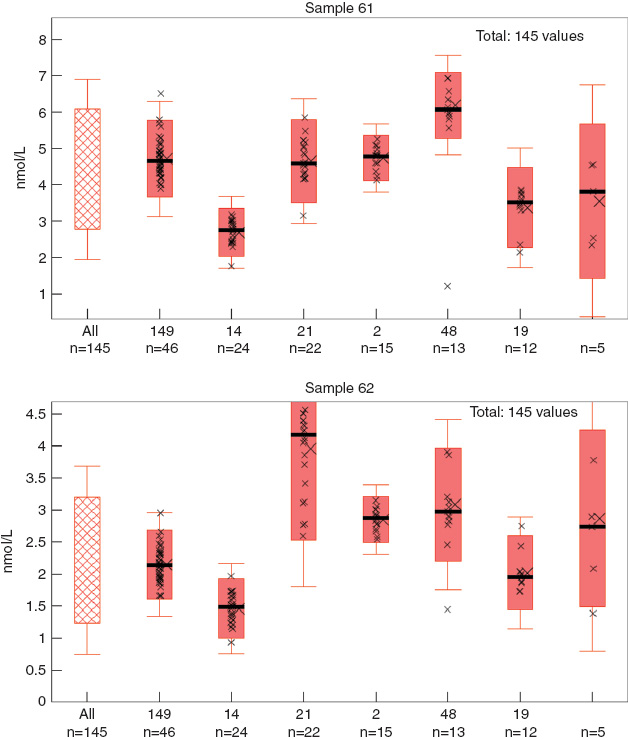
Example of testosterone.
The example of an HbA1c assay demonstrates an additional problem for external quality controls. The following effect was observed in the freeze-dried samples used over the years. The POCT systems required a proprietary control material and also could not be evaluated based on the reference method value. Yet even in the group of wet-chemical techniques, not all methods could be evaluated using the reference method concept. A remedy was found for this problem by using native materials in these external quality assurance tests. This observation points to an additional problem area in external quality control. In many cases, the samples used are processed samples. This processing is often necessary in order to stabilize the material and thus make it sustainable for the duration of the external quality assurance test. As a result, whilst a sample material on the basis of human specimens does exist, it is not identical to a native human sample. The samples processed in this manner can sometimes exhibit matrix effects, as seen in HbA1c, which may have different ramifications in each test system.
For most parameters evaluated by an external quality control, there is no reference point via a reference method. In cases such as these, a consensus value is calculated by means of the algorithm A as a stable mean value of a collective, which is then inputted as a target value for the corresponding collective. This concept is only possible, however, if the particular collective contains more than 6–8 participants.
Possible approaches and outlook
Possible approaches to a solution include increased use of native samples as demonstrated by HbA1c. In addition, the reference method concept ought to be further developed, and what’s more, the method-orientated research in the field of laboratory medicine should be intensified. The increased standards for quality assurance require clear reference points in external quality control for the evaluation of accuracy.
References
1. Guideline of the German Medical Association 2008 Version from [19.09.2014].Suche in Google Scholar
2. In-vitro Diagnostics Directive Guideline 98/79/EC.Suche in Google Scholar
©2015 by De Gruyter
Artikel in diesem Heft
- Frontmatter
- Editorial
- Editorial zur Rili-BÄK
- Neue Richtlinien der Bundesärztekammer zur Qualitätssicherung laboratoriumsmedizinischer Untersuchungen
- New Guidelines of the German Medical Association for Quality Assurance of Medical Laboratory Tests
- Qualitätssicherung im medizinischen Labor: eine Erfolgsgeschichte im Gesundheitssystem
- Quality assurance in the medical laboratory: a success story in the health-care system
- Die neue RiliBÄK – Anmerkungen des Referenzinstitutes für Bioanalytik (RfB)
- The new RiliBAEK- Remarks by the “Referenzinstitut für Bioanalytik” (RfB)
- Die RiliBÄK aus Sicht eines Ringversuchsanbieters
- The Rili-BAEK guideline from the perspective of an external quality assurance test provider
- Zur Veröffentlichung der englischen Fassung der Rili-BÄK – Sicht der Diagnostika Industrie auf die Rili-BÄK und Ihre Auswirkungen auf die Labormedizin in Deutschland
- Publication of the English version of the Rili-BAEK guideline – the diagnostics industry’s view on the Rili-BAEK guideline and its ramifications on laboratory medicine in Germany
- Qualitätssicherung für alle laboratoriumsmedizinischen Untersuchungen
- Quality assurance for all medical laboratory analyses
- Revision of the “Guideline of the German Medical Association on Quality Assurance in Medical Laboratory Examinations – Rili-BAEK” (unauthorized translation)
Artikel in diesem Heft
- Frontmatter
- Editorial
- Editorial zur Rili-BÄK
- Neue Richtlinien der Bundesärztekammer zur Qualitätssicherung laboratoriumsmedizinischer Untersuchungen
- New Guidelines of the German Medical Association for Quality Assurance of Medical Laboratory Tests
- Qualitätssicherung im medizinischen Labor: eine Erfolgsgeschichte im Gesundheitssystem
- Quality assurance in the medical laboratory: a success story in the health-care system
- Die neue RiliBÄK – Anmerkungen des Referenzinstitutes für Bioanalytik (RfB)
- The new RiliBAEK- Remarks by the “Referenzinstitut für Bioanalytik” (RfB)
- Die RiliBÄK aus Sicht eines Ringversuchsanbieters
- The Rili-BAEK guideline from the perspective of an external quality assurance test provider
- Zur Veröffentlichung der englischen Fassung der Rili-BÄK – Sicht der Diagnostika Industrie auf die Rili-BÄK und Ihre Auswirkungen auf die Labormedizin in Deutschland
- Publication of the English version of the Rili-BAEK guideline – the diagnostics industry’s view on the Rili-BAEK guideline and its ramifications on laboratory medicine in Germany
- Qualitätssicherung für alle laboratoriumsmedizinischen Untersuchungen
- Quality assurance for all medical laboratory analyses
- Revision of the “Guideline of the German Medical Association on Quality Assurance in Medical Laboratory Examinations – Rili-BAEK” (unauthorized translation)

