Abstract
To enhance the high-temperature stability of hot-work die steel, a novel hot-work die steel was designed on the basis of 55NiCrMoV7 steel by increasing the content of Mo and V. The hardness variation trend of the two steels during high-temperature service was investigated through a thermal stability test. Test methods such as X-ray diffraction, scanning electron microscope, high-resolution transmission electron microscope, and energy spectrum analysis were employed to analyze the microstructure and carbide evolution. Combined with the Johnson–Mehl–Avrami equation, the influence of Mo and V on the high-temperature aging kinetic process was analyzed. The results indicated that the evolution of carbide and the matrix recovery process exhibit a coupling appearance. The thermal stability and tempering resistance of material experiment steel (MES) 2 steel at the test temperature were optimized, and its tempering activation energy is 34.9 kJ·mol−1 higher than that of MES 1 steel. Under the same tempering conditions, the matrix recovery of MES 2 steel is slow, and carbide coarsening is effectively inhibited. This is mainly because Mo and V are strong carbide-forming elements that preferentially combine with C to form MC over Cr, Mn, and other elements. As a result, more Cr and Mn dissolve in the matrix. This not only plays the role of solid solution strengthening but also reduces the diffusion rate of C in the matrix at high temperatures and effectively inhibits the precipitation and growth of carbides.
1 Introduction
Hot work die steel is employed to press solid or liquid metal under high temperature and high pressure conditions [1,2,3]. The mold cavity is in contact with high-temperature forgings and also subjected to substantial impact force. The working temperature can reach 600–700°C [4,5,6], which is much higher than the tempering temperature of ordinary materials. This will result in changes in the internal structure of the material and lead to the softening of die steel [7,8]. To prevent deformation caused by a rapid decrease in hardness during the use of molds, which can significantly reduce their service life, it is essential for mold steel to possess good high-temperature stability to enhance its anti-softening ability [9,10,11].
55NiCrMoV7 steel has long been widely used as a preferred material for hot forging molds in aerospace, military, and other forging fields due to its high hardness, strength, excellent wear resistance, and high usability [12,13]. In recent years, with the development of the mold industry toward large-scale, precision, complexity, high efficiency, and rapid pace, the service environment of mold steel has become increasingly demanding [14,15]. Many scholars have improved the service life of 55NiCrMoV7 material by means of optimizing process parameters, laser deposition and other methods, achieving good results and maximizing the application potential of 55NiCrMoV7 material in mold steel [16,17].
As the production pace increases and high-temperature alloy forgings are more widely applied, mold steel is required to have higher thermal stability and tempering resistance. Consequently, the 55NiCrMoV7 material can no longer meet the growing high-performance requirements. Considering the actual working conditions of hot work die steel, our team has independently developed an improved 55NiCrMoV7 die steel that is suitable for the high stability requirements of large forgings compared with advanced die steel. This steel is based on 55NiCrMoV7 and has an increased content of strong carbide-forming elements Mo and V. These elements are preferentially combined with C atoms rather than Cr, Mn, and other elements to form MC, which has a coherent/semi-coherent relationship with the matrix and can enhance thermal stability [18,19,20]. Moreover, more Cr and Mn are dissolved in the matrix to play the role of solid solution strengthening [21].
Literature suggests that the addition of Mo and V significantly improves the thermal stability of steel. The research team led by Wu Xiaochun developed SDH2 steel through careful compositional control [22,23]. Their findings showed that Mo and V in the alloy effectively delay the decomposition of tempered martensite and the transformation of residual austenite, thereby stabilizing (Mo/V) C-type carbides at high temperatures. This stabilization inhibits dislocation movement within the matrix, suppresses recrystallization and recovery processes, and ultimately enhances the thermal stability of mold steel during high-temperature applications. Liu et al. [24,25,26,27] investigated how different Mo contents affect the solubility of M6C and M23C6 carbides in mold steel. Their results indicated that increased Mo promotes the formation of various secondary phase carbides (such as MC, M2C, M6C). A higher Mo content increases the driving force for M2C carbide formation while delaying the transformations from MC and M2C to M23C6. The fine and dispersed precipitates of M2C carbides formed at high temperatures produce a significant secondary hardening effect, greatly improving both the recrystallization stability and thermal fatigue life of the material. Min et al. [28] successfully developed SDPH13 steel with exceptional high-temperature strength and toughness by increasing the Mo content. This modification enhances resistance to thermal cracking and tempering softening, thus prolonging the service life of mold steels under high-temperature conditions. Hu et al. [29,30] discovered in their study on H13 mold steel that V reacts to form face-centered cubic VC in the alloy during annealing at 500–700°C. When these precipitated VC particles are located near dislocation lines, their high hardness leads to strong interactions with dislocations, hindering dislocation slip and increasing the diffusion activation energy of the α phase. As a result, the thermal stability of the steel is enhanced. Japanese scholars Park and Ahn [30,31,32] developed SKD61 steel by increasing the Mo content in 4Cr5MoSiV1 steel to 2% and SH71 steel by raising the V content in 3Cr2W8V steel. Through heat treatment, finer dispersed carbides can be obtained, significantly enhancing the tempering stability and thermal fatigue resistance of the steel. By optimizing the alloying element composition and adding new strong carbide-forming elements while precisely controlling the types of second-phase precipitates in steels, it has become a common and highly effective technical approach both domestically and internationally for developing new high-performance die steels. Recent studies have shown that adding molybdenum (Mo) to H13 mold steel effectively inhibits precipitation at the interface between the matrix and precipitates, thereby reducing carbide coarsening; vanadium (V) has a similar effect. However, an excessive amount of V may lead to the formation of large unmelted carbides, which can increase the brittleness of the steel. Additionally, due to its high cost, an excessive amount of V can significantly increase production costs. Scholars have also noted that Mo and V elements have certain effects on improving the thermal stability of steels; however, there are limited reports on optimal design strategies and thermal stability mechanisms for 55NiCrMoV7.
Based on the foregoing analysis, the composition of material experiment steel (MES) 1 steel alloy was refined by increasing the contents of molybdenum (Mo) and vanadium (V) to 1.1 and 0.7%, respectively, thus obtaining MES 2 steel. The influence of Mo and V on the post-heat treatment resistance of 55NiCrMoV7 steel was systematically examined through a comparative analysis of thermal stability data, and the Johnson–Mehl–Avrami (JMA) equation was used for quantitative assessment. Moreover, a comprehensive investigation into microstructure, precipitates, and thermal stability was carried out.
2 Experimental materials and procedure
2.1 Materials and heat treatment
A 50 kg experimental steel ingot was produced using a vacuum induction furnace. After annealing, the water riser was removed. The blank was then heated to 1,240°C in a natural gas furnace and maintained at this temperature for 4 h. Subsequently, the blank underwent two upsetting and two pulling forging operations and was ultimately formed into a test specimen measuring 160 × 160 × 240 mm with a forging ratio of 16:1. Finally, the test specimen was heated to 800°C and held at that temperature for 8 h before being cooled in the furnace down to 250°C and then air-cooled. The chemical composition of the experimental material is presented in Table 1. Subsequently, it was processed into test specimens measuring 30 × 30 × 30 mm using cutting machine equipment.
Chemical compositions of the tested steel (wt%)
| Element | C | Si | Mn | S | P | Cr | Ni | Mo | V | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| MES 1 | 0.56 | 0.20 | 0.81 | 0.0010 | 0.0065 | 1.17 | 1.75 | 0.52 | 0.10 | Bal. |
| MES 2 | 0.56 | 0.20 | 0.83 | 0.0063 | 0.0046 | 1.20 | 1.73 | 1.09 | 0.71 | Bal. |
The experimental materials of both steels were quenched at the temperatures specified in Table 2 and underwent heat treatment twice in a box furnace at temperatures of 100, 200, 300, 400, 450, 500, 520, 540, 560, 580, 600, and 700°C for 3 h each time to study the relationship between hardness and temper temperature.
Heat treatment process of tempering resistance specimens
| Quenching temperature (°C) | Holding time (h) | Tempering temperature (°C) | Holding time (h) | |
|---|---|---|---|---|
| MES 1 | 850 | 1.5 | 100, 200, 300, 400, 450, 500, 520, 540, 560, 580, 600, 700 | 3 |
| MES 2 | 920 |
Note: All temperings were performed twice under the same process.
To accurately compare the thermal stability of the two steels, they were first maintained in the same tempering state (i.e., the same tempering process was performed) before the thermal stability test, as shown in Table 3. Then, the high-temperature thermal stability test was carried out in the resistance furnace. The samples were held at 550, 600, 650, and 700°C for 3, 6, 9, 12, 18, 24, 48, 72, 96, and 120 h, respectively, to study the variation law of tempering hardness with holding time.
Heat treatment process of thermal stability specimens
| Quenching temperature (°C) | Holding time (h) | Tempering temperature (°C) | Holding time (h) | Hardness (HRC) | |
|---|---|---|---|---|---|
| MES 1 | 850 | 1.5 | 540 | 3 | 44.6 ± 0.3 |
| MES 2 | 920 | 1.5 | 540 | 3 | 48.4 ± 0.3 |
Note: All temperings were performed twice under the same process.
2.2 Mechanical properties
After heat treatment, the two types of steels were cut into 15 × 15 × 15 mm samples by a wire cutting machine. The hardness of the samples in different states was detected by an HRC-150 Rockwell hardness tester, and hardness curves were drawn. Five points on each sample were tested, and the average hardness value was taken.
2.3 Characterization of microstructure
To understand the precipitation of carbides in the test steel during quenching-tempering heat treatment, we utilized the electrolytic diagram of carbides in steel as shown in Figure 1 to electrolyze the samples under various tempering process parameters, electrolyze the iron matrix, and collect the precipitated carbides from the steel. During electrolysis, an aqueous solution containing 50 g·L−1 potassium chloride and 10 g·L−1 citric acid was selected. The electrolytic current was set at 0.02 A·cm−2, and the temperature within the electrolytic cell was maintained in the range of 0–5°C. After electrolysis, microporous filter membranes were used to collect the carbides precipitated from the steel under different tempering process parameters. Phase analysis of the extracted carbides was carried out by a D-8 X-ray diffractometer from Germany, and the X-ray diffraction (XRD) spectra of the carbides precipitated from the test steel at different tempering temperatures were drawn.

Schematic diagrams for collecting of carbides in steel by electrolysis.
After testing the hardness of the samples, metallographic samples were prepared by mechanical grinding, polishing, and corrosion with 4% nitric acid alcohol. The SM-5610LV scanning electron microscope and JEM-2100 HRTEM transmission electron microscope (TEM) (Carl Zeiss AG, Oberkochen, GER) were used to observe the changes in martensitic shape and the morphology, size, and distribution of the precipitation phase of MES 1 and MES 2 steels in different states. Electron diffraction patterns were selected using an electronic imaging system, and the composition of the matrix and precipitates was analyzed using a matching energy spectrometer equipment.
3 Results and discussion
3.1 Tempering temperature and hardness curve
Figure 2 presents the hardness curves of two types of test steels at different tempering temperatures. As can be seen from the curve, the hardness changes of the two steels are similar. In the quenched state, they have the highest hardness, reaching 55.4 HRC and 57.0 HRC respectively. As the tempering temperature increases, the hardness gradually decreases. When the tempering temperature reaches a certain level, secondary hardening begins to occur. The secondary hardening temperature of MES 1 steel is around 500°C, and the peak hardness of secondary hardening is 45.3 HRC. The secondary hardening temperature of MES 2 steel is around 540°C, corresponding to a peak hardness of 48.4 HRC. When the tempering temperature exceeds the secondary hardening temperature, the hardness of both steels decreases with the increase in tempering temperature. When the temperature reaches 700°C, the hardness of the two steels is 24.6 HRC and 31.5 HRC, respectively.

Relationship curve between tempering temperature and hardness.
The notable differences include an increase in the secondary hardening peak hardness of MES 2 by 3.1 HRC compared to MES 1, as well as an increase in the secondary hardening peak temperature from 500 to 540°C, indicating an elevation of 40°C. Wu et al. [33] explained that the alloying combination of low chromium and high molybdenum in SDCM steel effectively stabilizes MC and M2C carbides, thereby delaying their transformation into M23C6 and consequently reducing hardness degradation. Additionally, Wang et al. [16] noted that during the quenching process of titanium microalloyed 4Cr5MoSiV1 steel, titanium, recognized as a potent carbide-forming element, can replace certain vanadium elements preferentially to form MC-type strong carbides. This phenomenon facilitates enhanced dissolution of alloying elements in the matrix, which is beneficial for increasing the secondary hardening characteristics of steel. In this study, we increased the contents of molybdenum and vanadium, both recognized as strong carbide-forming elements, which substitute specific chromium elements to produce additional MC-type carbides. This modification enhances the solubility of chromium in the α phase matrix and further influences the secondary hardening phenomenon by increasing its peak hardness. This observation corroborates previous research suggesting that incorporating titanium leads to its substitution for some vanadium, resulting in the formation of VC compounds. The enhancement in secondary hardening peak hardness positively contributes to both the thermal stability and recrystallization temperature of the steel, thus enabling it to achieve higher service temperatures and an extended service life. Therefore, increasing the content of molybdenum and vanadium in MES 2 significantly strengthens the heat resistance of heat-resistant mold steel against heat treatment.
3.2 Thermal stability curve
To ensure the two steels are in the same tempering state, based on the hardness curve in Figure 2, the two steels were initially tempered at 540°C, and then held at 550, 600, 650, and 700°C, respectively for 120 h. The thermal stability curves are shown in Figure 3. It can be observed that when both types of steel are held at four different temperatures, as the tempering time increases, the hardness values display a downward trend. When the tempering time reaches 48 h, the hardness reaches a relatively stable plateau. Holding below 600°C leads to a continuous and slow decrease in hardness values for both types of steel. Holding above 600°C results in a two-stage change in hardness. The hardness drops rapidly in the first 12 h and then the trend slows down, ultimately reaching a stable plateau. From the hardness change curve, it can be seen that the tempering softening behavior of the two steels is similar at the same tempering temperature. However, under the same tempering process, the hardness value of MES 2 steel is higher than that of MES 1 steel. At the same tempering temperature, with the extension of tempering time, the hardness decay curve of MES 2 steel is smoother than that of MES 1 steel. After the hardness value reaches a stable plateau, the hardness value of MES 2 steel basically remains unchanged with the extension of time, while MES 1 steel still shows a slight decrease over time. When held at 700°C for 48 h, the hardness values of the two steels decrease to 29.4 HRC and 20.5 HRC, respectively, with a decrease of 20.6 HRC and 26.1 HRC. The thermal stability of MES 2 steel is 20.1% higher than that of MES 1 steel. In summary, when held below 700°C, MES 2 steel demonstrates better thermal stability than MES 1 steel.

Thermal stability curve of two tested steels. (a) MES 1 and (b) MES 2.
In addition, under the same tempering time, the hardness value of the two steels decreases with the increase in tempering temperature, and the reduction of MES 2 steel is less than that of MES 1 steel, showing better tempering resistance and thermal stability than MES 1 steel.
3.3 High temperature aging kinetics
Avrami [34,35] simultaneously proposed the tempering dynamics law to describe the solid-state phase transition controlled by diffusion. According to their law, Watte et al. [36]. proposed the tempering dynamics law equation based on the JMA equation.
where τ is the tempering ratio; t is the tempering time; m is the Avrami exponent; and D is a parameter related to the tempering temperature, following the Arrhenius equation.
The following equation can be used to describe the relationship between tempering rate and hardness [37]:
where H is the hardness in the quenched state, H 0 is the hardness after tempering, and H 1 is the hardness in the annealed state.
Taking the logarithm of both sides of equation (1)
Taking the logarithm of both sides of equation (3):
Equation (4) reveals that at the same tempering temperature, the relationship between lnln(1/(1 – τ)) and lnt is linear. The hardness values of two tested steels under different tempering processes in Figure 3 are, respectively, input into equations (2) and (4), and Figure 4(a) and (b) are plotted. The slope of the linear fitting is m, and the m values of the two steels at temperatures of 550, 600, 650, and 700°C are presented in Table 4. The data indicate that under identical annealing conditions, the m value for MES 2 steel is consistently higher than that of MES 1 steel. This observation suggests that the diffusion of alloying elements in MES 2 steel requires a higher energy input during the annealing process. Lindsley and Marder [38] reported that when the n value is within the range of 0.2–0.3 during martensitic annealing, carbon atoms diffuse along dislocations and grain boundaries to regulate carbide coarsening. This finding also corroborates the relatively flat curve observed at 700°C. Moreover, numerous studies have demonstrated that molybdenum (Mo) and vanadium (V) have an inhibitory effect on recrystallization grain growth within the α phase during high-temperature annealing by preferentially precipitating near dislocations, grain boundaries, and undissolved carbides [39]. Consequently, the increased concentrations of Mo and V in MES 2 steel significantly inhibit carbide coarsening, thereby delaying this phenomenon and enhancing overall annealing stability.

Avrami index m of two tested steels at different temperatures. (a) MES 1; and (b) MES 2.
Avrami index m of two steels at different temperatures
| Steel | M | |||
|---|---|---|---|---|
| 550°C | 600°C | 650°C | 700°C | |
| MES 1 | 0.52 | 0.46 | 0.31 | 0.22 |
| MES 2 | 0.65 | 0.53 | 0.35 | 0.23 |
Previous studies [40,41] have shown that when interstitial atoms move from one phase plane to another, the Avrami index m = 0.4. According to the experimental data, it was found that the m values of MES 2 and MES 1 are very close to 0.4, indicating that during the thermal stability process, both experimental materials exhibit a phenomenon of matrix defect diffusion segregation, which belongs to a typical martensitic recovery. This can also be confirmed in the subsequent microscopic analysis.
Based on Watte’s study of tempering kinetics, Avrami [42,43] established a relationship between the second phase growth kinetics and growth activation energy.
where D 0 is the tempering treatment coefficient, ΔQ is the activation energy of the tempering transformation, R is the ideal gas constant (8.314 J·mol−1·K−1), and T is the tempering isothermal temperature in K.
Taking the logarithm of both sides of equation (5)
According to equation (4), the intercept of the lnln(1/(1 – τ)) – lnt line is lnD. The lnD values at different temperatures can be obtained through Figure 4(a) and (b). The temperature T and corresponding lnD values are entered into equation (6) and fitted into the relationship curve of ln D – 1/T as shown in Figure 5. The slope of the curve is −ΔQ/R. The slopes of the two curves are calculated to be 13.465 and 17.621, respectively. Therefore, the tempering activation energies ΔQ of MES 1 and MES 2 steel are 110.6 and 146.5 KJ·mol−1, respectively. The tempering activation energy of MES 2 is 34.9 KJ·mol−1 higher than that of MES 1, indicating that carbon elements in MES 2 steel need to cross higher energy barriers through diffusion and alloy element binding. This suppresses the formation and growth of carbides and exhibits higher tempering resistance. This is consistent with the conclusion drawn by Zhang et al. [44] that V alloying can effectively improve the thermal stability of steel.

Activation energy of two tested steels.
Equation (7) was obtained by the conversion of equations (1) and (2).
According to Figure 4, the lnD of MES 1 steel at 550, 600, 650, and 700°C were −9.52738, −7.27226, −4.77856, and −2.75953, respectively. The lnD of MES 2 steel at 550, 600, 650, and 700°C were −7.55774, −5.2952, −3.7769, and −2.2889, respectively. By substituting m, ln D, H 1, and H in equation (7), the tempering softening equations for MES 1 and MES 2 steels could be obtained.
3.4 Influence of tempering temperature on the microstructure of tested steels
3.4.1 Microstructure analysis
Figure 6 presents the as-hardened microstructures and those after 6 h of heat treatment at 550 and 650°C for two types of steel. As can be seen from the figure, the primary microstructures of the two steels after quenching and tempering are martensite and precipitated carbides. When the tempering temperature is 550°C, the plate-like morphology of martensite in MES 2 steel is pronounced, and the original austenite grain boundaries are clearly visible. Nano-sized particle-like carbides are uniformly distributed within the martensitic bundles, showing no significant change from the as-hardened microstructures, as shown in Figure 6(e). In contrast, in MES 1 steel, the original austenite grain boundaries are faintly visible. Its tempered martensitic bundles are sparse and contain larger precipitated carbides that have coarsened compared to the as-hardened microstructures, as shown in Figure 6(b). When the tempering temperature is increased to 650°C, most of the martensite in MES 2 steel has decomposed, and recovery and recrystallization begin to occur in local areas. However, the matrix morphology of martensite is still retained, and the phenomenon of carbide coarsening is not obvious, as shown in Figure 6(f). The recrystallization phenomenon of the matrix in MES 1 steel is more obvious, and the size of carbide increases significantly, as shown in Figure 6(c). According to the hardness curve analysis in Figure 4, as the temperature increases, the main reasons for the decrease in hardness of the test steel are: the gradual elimination of internal stress, the recovery of the matrix, the accumulation and growth of precipitated carbides, the weakening of precipitation strengthening and the strengthening of tempering softening, resulting in the continuous decline in the hardness of steel. Compared with MES 2 steel, the matrix of MES 1 steel has complete recovery and carbide coarsening is more serious, which also indicates that MES 2 steel has higher tempering resistance than MES 1 steel.

Microstructure of two steels at different tempering temperatures. (a) As-hardened microstructure of MES 1; (b) MES 1 (550°C/6 h); (c) MES 1 (650°C/6 h); (d) As-hardened microstructure of MES 2; (e) MES 2 (550°C/6 h); and (f) MES 2(650°C/6 h).
3.4.2 Precipitated phase analysis
Figure 7 shows the XRD spectra of the second-phase carbides in MES 2 steel at different tempering temperatures. The carbides are collected through the extraction method. The obtained data indicate that at a tempering temperature of 500°C, the predominant type of carbide precipitated in the steel is the MC carbide. When the tempering temperature reaches 540°C, the peaks corresponding to M2C carbide begin to appear. As the tempering temperature rises to 600°C, the M2C peak disappears, while the peaks of M7C3 and M23C6 carbides start to emerge. When the tempering temperature further increases to 700°C, the diffraction peak characteristics are similar to those at 600°C. TEM analysis of the sample tempered at 540°C (as shown in Figure 8 reveals numerous rod-shaped precipitates within the martensite laths, uniformly dispersed with widths ranging from 20 to 30 nm and lengths approximately between 60 and 95 nm. Figure 8b presents a dark field image. Calibration and EDS analysis confirm that these precipitates are Cr-containing M2C carbides (as shown in Figure 8(c) and (d)).
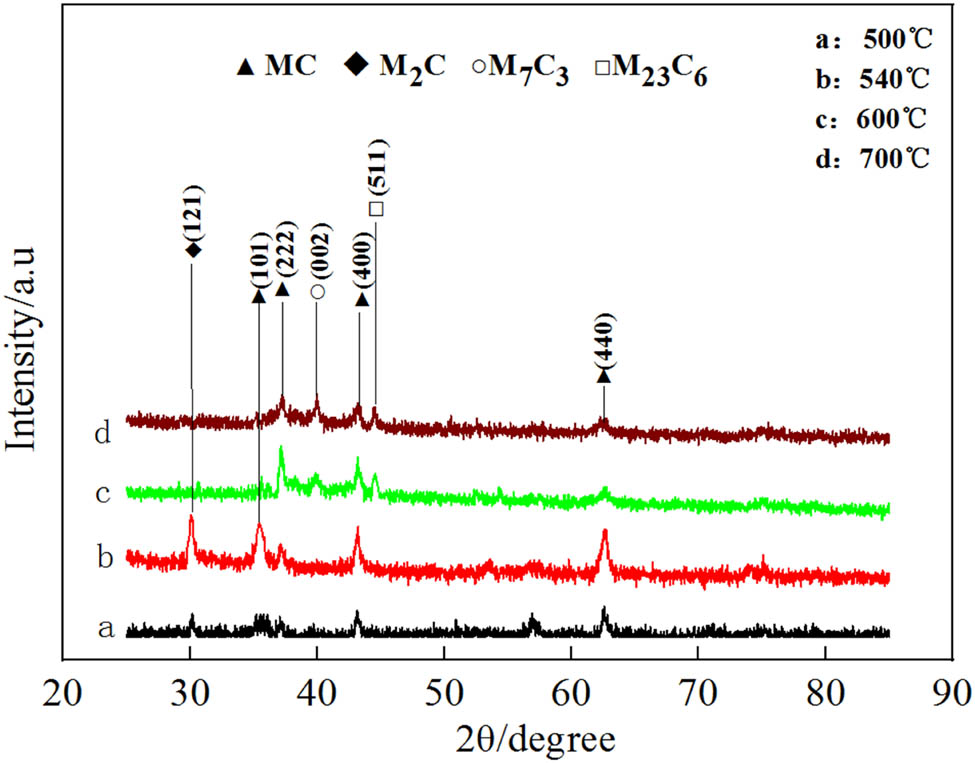
XRD pattern of the second phase precipitated in MES 2 steel.

TEM micrographs of precipitated phase and EDS elements image (540°C). (a) Bright field image; (b) dark field image; (c) SAED pattern; and (d) EDS.
The influence of alloying elements significantly affects both the type and morphology of the precipitated phases in the matrix. The carbide formation ability of the alloying elements in this test steel follows the sequence: V ≥ Mo ≥ Cr ≥ Fe. At a tempering temperature of 540°C, precipitation of M2C carbides occurs in the matrix, resulting in a significant secondary hardening phenomenon in this steel variant. With the increase in the tempering temperature, the diffusion kinetics of Mo and V is enhanced due to their strong tendency to form carbides. Consequently, an increased concentration of Mo and V accumulates in the M2C carbides by seizing carbon atoms, thereby facilitating the transformation of most of the M2C carbides into the more structurally stable MC carbides. A small portion of the remaining M2C carbides undergoes aggregation as the temperature continues to rise, evolving into larger structures such as M7C3 and M23C6 carbides. This phenomenon mainly accounts for the rapid decline in hardness once the tempering temperature exceeds 600°C.
3.5 Influence of tempering time on microstructure of test steel
The resistance to tempering softening of hot work die steel mainly depends on the recovery of high-density dislocation martensite and the coarsening degree of precipitated carbides. To further analyze the high-temperature thermal stability mechanism of the two materials, the microstructure and carbide evolution of the two materials at 600°C were observed by TEM, as shown in Figure 9. Both materials exhibited lath martensite wrapped with high-density dislocations, and precipitates were dispersed on the matrix. As can be seen from Figure 9(a), after being insulated at 600°C for 6 h, most of the matrix in MES 1 steel recovered, while the martensitic lath boundary and a small number of entangled dislocations were retained in local areas, and the carbide was distributed in granular dispersion. After insulation for 48 h, obvious recovery and recrystallization occurred, high-density dislocations almost disappeared, the martensitic lath boundary was difficult to distinguish, the ferrite structure appeared, and most of the carbides were distributed along the grain boundary and coarsened obviously. The EDS analysis in Figure 9(c) and (e) showed that the alloying elements in carbides are mainly Cr and Mn. According to the calibration of the diffraction pattern, the carbides were M23C6 containing Cr, as shown in Figure 9(g). Relatively, after MES 2 steel was insulated at 600°C for 6 h, most of the martensitic slats were still clearly visible, a large number of high-density dislocations were distributed on the matrix, and the precipitated carbides were uniformly distributed in granular form on the matrix. With the extension of insulation time, there was no obvious coarsening of granular carbide. According to the EDS spectra in Figure 9(f) and the diffraction pattern calibration in Figure 9(h), it could be seen that the precipitated phase was MC carbide containing mainly V and a small amount of Mo elements. Some studies [45,46,47] have shown that alloying elements V and Mo can reduce the formation energy of MC carbide. The interface energy between MC carbide and matrix is reduced, and high coherence is maintained, which makes MC have high coarsening resistance and thermal stability, which is consistent with the conclusion of this study.

TEM diagram of two kinds of steel at different tempering time (600°C). (a) MES 1 steel 600°C/6 h; (b) MES 2 steel 600°C/6 h; (c) MES 1 steel 600°C/48 h; (d) MES 2 steel 600°C/48 h; (e) energy spectrum at point A in (c); (f) energy spectrum at point B in (d); (g) electron diffraction pattern at point A in (c); and (h) electron diffraction pattern at point B in (d).
To facilitate a more intuitive analysis of carbide changes in steel, the average size of carbides was statistically analyzed after different tempering times at 600°C for two types of steel, as depicted in Figure 10. It is evident that under the same holding time, the carbide size of MES 1 steel is significantly larger than that of MES 2 steel. Moreover, with an increase in holding time, the growth rate of carbide size in MES 1 steel accelerates. Specifically, when the tempering time reaches 48 h, the average carbide sizes for MES 1 and MES 2 steels are approximately 152 and 96 nm, respectively. This indicates that the carbide coarsening of MES 1 steel was obvious with the extension of the insulating time, and the coarsened carbides had a weak pinning effect on the phase boundary and grain boundary. Furthermore, the recrystallization of the matrix was promoted, and the hardness of the steel decreased significantly during the thermal holding process, showing low thermal stability. In the process of heat preservation, the matrix of MES2 steel gradually underwent recovery-recrystallization, and the degree of it was obviously smaller than that of MES1 steel. At the same time, carbide coarsening was not obvious with the extension of insulating time. Most of the carbides were still dispersed in the matrix in the form of fine particles, and there were sparse dislocation lines on the matrix, which also showed the dragging and pinning effect of fine carbides on dislocations.
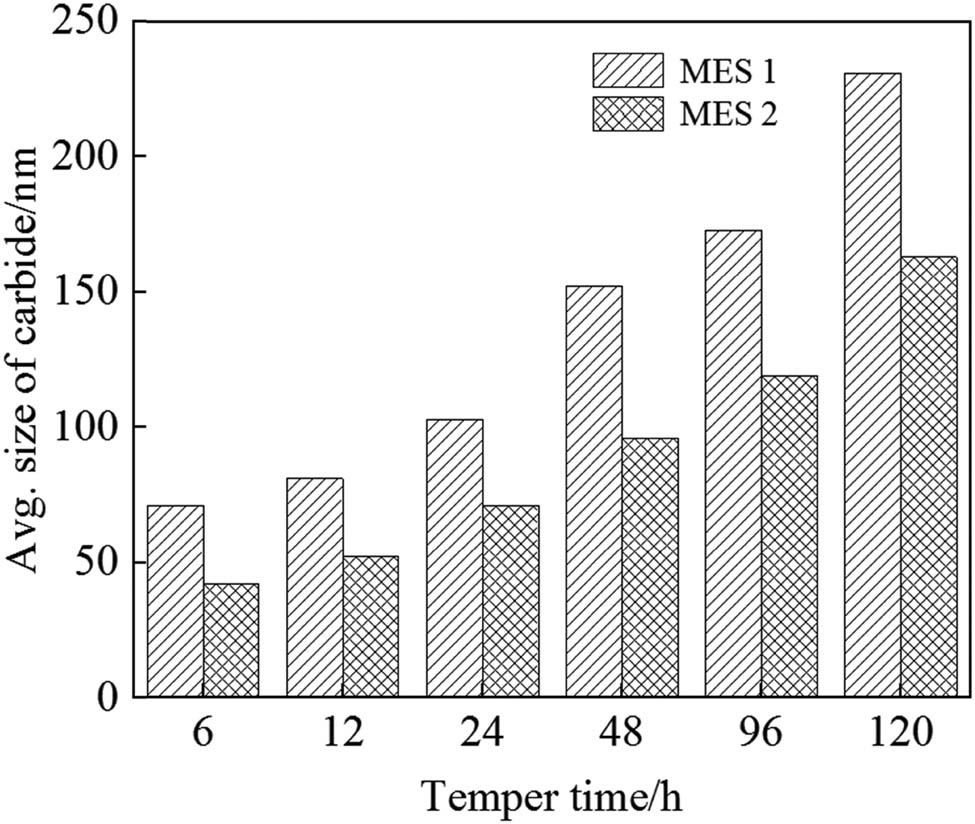
The average carbide size of two kinds of steel after different tempering time at 600°C.
As the tempering time extends, the precipitation of intermetallic compounds in the matrix increases. The size of the precipitated phase gradually grows, and the weakening effect on the matrix progressively becomes greater than the precipitation strengthening. Consequently, the matrix gradually softens, leading to a decrease in the hardness of the steel. When the tempering time is 48 h, the recrystallization phenomenon becomes evident, and a second phase of nanometer scale begins to precipitate in the new grain. The precipitation strengthening of the second phase mitigates the softening of the matrix due to the reduction in the solid solution of alloying elements. This is also the main reason for the gradual decline in hardness with the extension of tempering time.
Compared with MES 1, the contents of Mo and V in MES 2 are higher. Moreover, the quenching temperature of MES 2 steel is higher than that of MES 1 steel, which makes more alloying elements dissolve in the matrix, raises the decomposition temperature of the matrix, and effectively inhibits matrix recovery and carbide coarsening. With the increase in tempering temperature and the extension of tempering time, the C element and alloying element undergo diffusion phase transformation to form carbide precipitation and grow. This causes the alloying element content in the matrix to gradually decrease and weaken the solid solution strengthening effect of the matrix. In MES 2, while the C content remains unchanged compared with MES 1, the content of strong carbide-forming elements Mo and V increases. This leads to the formation of relatively small carbides during the tempering process. In this case, Mo and V preferentially occupy Mn and Cr in the C element. More Mn and Cr elements are mainly dissolved in the matrix, which hinders grain boundary migration, slows down matrix coarsening, and plays the role of solid solution strengthening. Ghost and Olson pointed out that the content of Mn and Cr elements will effectively increase the driving force of grain nucleation and block grain coarsening [48].
4 Conclusion
The aim of this study was to design a new type of MES 2 steel with excellent performance based on 55NiCrMoV7 (MES 1) steel through alloying. By analyzing its macro hardness, microstructure, and high-temperature aging kinetics, it is clarified that MES 2 steel has better tempering resistance and thermal stability. The main conclusions are as follows:
The increase in the concentrations of Mo and V in MES 2 steel shifts the secondary hardening peak to the right, raising the peak temperature by 40°C and increasing the peak hardness by 3.1 HRC, thus indicating better resistance to softening during heat treatment.
As the heat treatment temperature increases and the holding time is extended, both steels exhibit similar annealing softening behavior. However, the hardness of MES 2 steel is consistently higher than that of MES 1 steel. Additionally, the activation energy for annealing in MES 2 steel is 34.9 KJ·mol−1 higher than that in MES 1 steel, indicating greater thermal stability for MES 2.
Calculations using the JMA equation and microstructural analysis show that increasing the concentrations of Mo and V in MES 2 steel significantly hinders the recovery of the α phase matrix. The precipitated fine and dispersed carbides are (Mo/V)C, which have high thermal stability. Meanwhile, Cr and Mn mainly dissolve in the matrix, enhancing solute strengthening.
By calculating the tempering kinetics of both steels, their softening equations are obtained and validated. These equations can be used to effectively predict the hardness of the dies after a prolonged service period at high temperature.
Acknowledgements
The authors acknowledegments the Major science and technology innovation project of Luoyang (2301020A), and China Postdoctoral Science Foundation (2021T140779). Authors thank Luoyang CITIC HIC Casting and Forging Co., Ltd for supplying the materials used in this study.
-
Funding information: This work is funded by the Major science and technology innovation project of Luoyang (2301020A), and China Postdoctoral Science Foundation (2021T140779).
-
Author contributions: Y.Y.: conceptualization, experimental design, and writing. W.W.: supervision and methodology. M.Z.: investigation, and translation. R.S.: methodology, visualization, and investigation. Z.W.: methodology, investigation, and translation. Y.Z.: data curation and investigation. J.X.: project administration and methodology.
-
Conflict of interest: The authors state no conflict of interest.
-
Ethical approval: Not applicable.
-
Date availability statement: Data will be made available on request.
References
[1] Wu, X. and Y. Shi. Development status and trend of hot forging die materials. Die and Mould Industry, Vol. 41, 2015, pp. 1–10.Search in Google Scholar
[2] Wang, B. Development status of hot work die steel. Die & Mould Manufacture, Vol. 17, 2017, pp. 79–82.Search in Google Scholar
[3] Wang, H., H. Feng, H. Li, G. Zhou, H. Zhu, S. Zhang, et al. Nitrogen-substituting carbon significantly improves softening resistance of H13 hot-work die steel. Metallurgical and Materials Transactions, Vol. 55, 2024, pp. 1916–1931.10.1007/s11661-024-07367-ySearch in Google Scholar
[4] Wu, X. and P. Zuo. Development status and trend of hot working die steels at home and abroad. Die & Mould Industry, Vol. 39, 2013, pp. 1–9.Search in Google Scholar
[5] Peter, J. and H. Mária. Characterization of microstructure and fracture performance of boronized H11 grade hot-work tool steel. Materials Performance and Characterization, Vol. 9, 2020, pp. 126–135.10.1520/MPC20190086Search in Google Scholar
[6] Chen, R., Z. Wang, H. Wang, L. Qi, and F. Zhu. Effects of yttrium on the microstructures, internal fraction and martensitic transformation in H13 die steel. Journal of Materials Science, Vol. 56, 2021, pp. 1–12.10.1007/s10853-020-05731-ySearch in Google Scholar
[7] Takuro, M., T. Toshihiro, T. Setsuo, K. Tamotsu, and A. Kazuhiko. Difference between carbon and nitrogen in thermal stability of metastable 18%Cr-8%Ni austenite. Scripta Materialia, Vol. 154, 2018, pp. 8–11.10.1016/j.scriptamat.2018.05.019Search in Google Scholar
[8] Song, Y., X. Mao, W. Wang, Z. Ouyang, H. Liang, and L. Li. Effect of Re-Nb on the strength and toughness of 3Cr2MoNiWV cast hot working die steel. Journal of Materials Science, Vol. 41, 2006, pp. 4185–4190.10.1007/s10853-006-6974-0Search in Google Scholar
[9] Shi, N., X. Wu, and N. Min. Effect of carbide evolution on thermal-stability of 4Cr2Mo2W2V hot work steel. Transactions of Materials and Heat Treatment, Vol. 32, 2011, pp. 68–72.Search in Google Scholar
[10] Liang, H., G. Zhao, D. Qin, E. Shang, and Q. Jiang. Effect of mechanical vibration on the microstructure and thermal fatigue behavior of cast hot work die steel. Journal of Materials Science, Vol. 41, 2006, pp. 2529–2532.10.1007/s10853-006-5312-xSearch in Google Scholar
[11] Jiang, Q., J. Fang, and Q. Guan. Thermomechanical fatigue behavior of Cr–Ni–Mo cast hot work die steel. Scripta Materialia, Vol. 45, 2001, pp. 199–204.10.1016/S1359-6462(01)01015-6Search in Google Scholar
[12] Zhang, W., Y. Zhang, G. Cheng, G. Wang, T. Qian, and R. Shi. Formation mechanism of inspection defects in hundred-ton class 55NiCrMoV7 die steel forging. Ironmaking & Steelmaking, Vol. 51, 2024, pp. 316–327.10.1177/03019233241245213Search in Google Scholar
[13] Jebaraj, M., K. M. Pradeep, N. Yuvaraj, and R. Anburaj. Investigation of surface integrity in end milling of 55NiCrMoV7 die steel under the cryogenic environments. Machining Science and Technology, Vol. 24, 2020, pp. 465–488.10.1080/10910344.2019.1698612Search in Google Scholar
[14] Yu, X., C. Wu, R. Shi, and Y. Yuan. Microstructural evolution and mechanical properties of 55NiCrMoV7 hot-work die steel during quenching and tempering treatments. Advances in Manufacturing, Vol. 9, 2021, pp. 520–537.10.1007/s40436-021-00352-3Search in Google Scholar
[15] Yuan, Y., W. Wang, R. Shi, Y. Zhang, and J. Xie. Microstructure evolution and fracture mechanism of 55NiCrMoV7 hot-working die steel during high-temperature tensile. Metals, Vol. 13, 2023, id. 1056.10.3390/met13061056Search in Google Scholar
[16] Wang, Y., K. Song, Y. Zhang, and G. Wang. Microstructure evolution and fracture mechanism of H13 steel during high temperature tensile deformation. Materials Science & Engineering A, Vol. 746, 2019, pp. 127–133.10.1016/j.msea.2019.01.027Search in Google Scholar
[17] Han, R. and X. Wu. Research status and development trend of die steel for plastic material forming at domestic and foreign. Die and Mould Industry, Vol. 44, 2018, pp. 1–7.Search in Google Scholar
[18] Xia, X., M. Liu, T. Zhang, H. Wen, and Q. Dong. Improvement of thermal stability in hot-deformed Nd-Fe-B magnets by grain refinement. Scripta Materialia, Vol. 178, 2019, pp. 129–133.10.1016/j.scriptamat.2019.11.018Search in Google Scholar
[19] Wang, M., B. You, Y. Wu, B. Liang, X. Gao, W. Li, et al. Effect of Cr, Mo, and V elements on the microstructure and thermal fatigue properties of the chromium hot-work steels processed by selective laser melting. Metals, Vol. 12, 2022, pp. 735–735.10.3390/met12050735Search in Google Scholar
[20] Gong, W., J. Yue, J. Tian, J. Liao, Y. Yu, and Z. Jiang. The effect of nickel on the carbide precipitation behavior in Cr-Mo-V hot-working die steel. Journal of Materials Research and Technology, Vol. 27, 2023, pp. 4452–4460.10.1016/j.jmrt.2023.10.229Search in Google Scholar
[21] Chang, F., H. Zhang, Y. Gao, S. Shu, F. Qiu, and Q. Jiang. Microstructure evolution and mechanical property enhancement of high-Cr hot work die steel manipulated by trace amounts of nano-sized TiC. Materials Science & Engineering A, Vol. 824, 2021, id. 141788.10.1016/j.msea.2021.141788Search in Google Scholar
[22] Chen, J., X. Wu, Y. Min, and H. Wang. Comparison of thermal fatigue behavior of SDH2 and 8407 steel. Transactions of Materials and Heat Treatment, Vol. 28, 2007, pp. 71–75.Search in Google Scholar
[23] Chen, J., X. Wu, Y. Min, H. Wang, and G. Lv. Study on hot-working die steel SDH2 with high strength and high toughness. Heat Treatment, Vol. 21, 2007, pp. 17–21.Search in Google Scholar
[24] Liu, Z., Y. Wang, and S. Shang. Origin of negative thermal expansion phenomenon in solids. Scripta Mater, Vol. 65, 2011, pp. 664–667.10.1016/j.scriptamat.2011.07.001Search in Google Scholar
[25] Lan, H., W. Liu, and X. Liu. Ultrafine grained steels produced by tempering cold rolled martensite and their thermal stability. Transactions of Materials and Heat Treatment, Vol. 22, 2008, pp. 279–286.Search in Google Scholar
[26] Wang, Y., K. Song, and Y. Zhang. High-temperature softening mechanism and kinetic of 4Cr5MoSiV1 steel during tempering. Materials Research Express, Vol. 6, 2019, id. 096513.10.1088/2053-1591/ab2bb9Search in Google Scholar
[27] Chen, Y., B. Huang, and Y. Tsai. Microstructural evolutions of low carbon Nb/Mo-containing bainitic steels during high-temperature tempering. Materials Characterization, Vol. 131, 2017, pp. 298–305.10.1016/j.matchar.2017.07.022Search in Google Scholar
[28] Min, Y., X. Wu, and L. Xu. Oxidation and thermal fatigue behaviors of two type hot work steels during thermal cycling. Journal of Iron and Steel Research International, Vol. 20, 2013, pp. 8–13.10.1016/S1006-706X(13)60202-2Search in Google Scholar
[29] Hu, X., L. Li, and X. Wu. Coarsening kinetics of carbides in 4Cr5MoSiV1 hot work tool steel during thermal fatigue. Transactions of Materials and Heat Treatment, Vol. 26, 2005, pp. 57–62.Search in Google Scholar
[30] Telasang, G., G. Padmanabham, and I. Manna. Structure-property correlation in laser surface treated AISI H13 tool steel for improved mechanical properties. Materials Science & Engineering, A: Structural Materials: Properties, Microstructure and Processing, Vol. 599, 2014, pp. 255–267.10.1016/j.msea.2014.01.083Search in Google Scholar
[31] Park, N. and D. Ahn. Wear characteristics of Stellite6 and NOREM02 hardfaced SKD61 hot working tool steel at the elevated temperature. International Journal of Precision Engineering and Manufacturing, Vol. 15, 2014, pp. 2549–2558.10.1007/s12541-014-0626-0Search in Google Scholar
[32] Kang, C., S. Choi, and K. Lee. Effects of powder feeding rate on the crack formation in laser-surface alloying-hardened SKD61 hot die steel using SKH51 powder. Korean Journal of Metals and Materials, Vol. 54, 2016, pp. 194–203.10.3365/KJMM.2016.54.3.194Search in Google Scholar
[33] He, M., S. Li, and X. Wu. Thermal stability of SDCM hot work tool steel. Shanghai Metals, Vol. 37, No. 4, 2015, pp. 44–51.Search in Google Scholar
[34] Avrami, M. Granulation, phase change, and microstructure kinetics of phase change. III. The Journal of Chemical Physics, Vol. 9, No. 2, 1941, pp. 177–184.10.1063/1.1750872Search in Google Scholar
[35] Johnson, W. and R. Mehl. Reaction kinetics in processes of nucleation and growth. American Institute of Mining and Metallvrgical Engineers, Vol. 135, 1939, pp. 416–442.Search in Google Scholar
[36] Watte, P., P. Humbeeck, E. Aernoudt, and I. Lefever. Strain aging in heavily drawn eutectoid steel wires. Scripta Mater, Vol. 34, 1996, pp. 89–95.10.1016/1359-6462(95)00479-3Search in Google Scholar
[37] Li, H., K. Gai, L. He, C. Zhang, H. Cui, and M. Li. Non-isothermal phase-transformation kinetics model for evaluating the austenization of 55CrMo steel based on Johnson-Mehl-Avrami equation. Materials & Design, Vol. 92, 2016, pp. 731–741.10.1016/j.matdes.2015.12.110Search in Google Scholar
[38] Lindsley, B. A. and A. R. Marder. The morphology and coarsening kinetics of spheroidized Fe-C binary alloys. Acta Materialia, Vol. 46, No. 1, 1998, pp. 341–351.10.1016/S1359-6454(97)00165-1Search in Google Scholar
[39] Xie, Y., G. Cheng, L. Chen, Y. Zhang, and Q. Yan. Characteristics and generating mechanism of large precipitates in H13 tool steel. China Metallurgy, Vol. 26, No. 4, 2016, pp. 32–37.Search in Google Scholar
[40] Zhang, Z., D. Delagnes, and G. Bernhart. Microstructure evolution of hot-work tool steels during tempering and definition of a kinetic law based on hardness measurements. Materials Science Engineering A, Vol. 380, 2004, pp. 222–230.10.1016/j.msea.2004.03.067Search in Google Scholar
[41] Huang, S., R. Wu, W. Li, N. Min, and X. Li. Fluctuations of properties of Cr-Mo-V hot work die steels by artificial increment of vanadium. Materials Today Communications, Vol. 33, 2022, id. 105024.10.1016/j.mtcomm.2022.105024Search in Google Scholar
[42] Kang, J., X. Sun, Z. Li, and Q. Yong. Precipitation and strengthening behaviors of MC type carbides in a Ti-V low carbon steel during tempering process. Journal of Iron and Steel Research, Vol. 27, No. 8, 2015, pp. 50–54.Search in Google Scholar
[43] Wang, Z., X. Sun, Z. Yang, Q. Yong, C. Zhang, Z. Li, et al. Carbide precipitation in austenite of a Ti-Mo-containing low-carbon steel during stress relaxation. Materials Science & Engineering A, Vol. 573, 2013, pp. 84–91.10.1016/j.msea.2013.02.056Search in Google Scholar
[44] Zhang, Z., X. Sun, Z. Wang, Z. Li, Q. Yong, and C. Zhang. Carbide precipitation in austenite of Nb-Mo-bearing low-carbon steel during stress relaxation. Materials Letters, Vol. 159, 2015, pp. 249–252.10.1016/j.matlet.2015.06.111Search in Google Scholar
[45] Han, L., Z. Liu, L. Yu, Z. Ma, Y. Huang, Y. Liu, et al. Effect of WC nanoparticles on the thermal stability and mechanical performance of dispersion-reinforced Cu composites. Scripta Materialia, Vol. 222, 2023, id. 115030.10.1016/j.scriptamat.2022.115030Search in Google Scholar
[46] Zhao, X., B. Wang, D. Sun, C. Li, L. Han, and J. Gu. Effect of pre-existing VC carbides on nitriding and wear behavior of hot-work die steel. Applied Surface Science, Vol. 486, 2019, pp. 179–186.10.1016/j.apsusc.2019.04.270Search in Google Scholar
[47] Li, M., Y. Wang, Y. Rong, Y. Hu, and M. Lu. Influence of precipitation on dynamic fracture toughness of 10Ni3MnCuAl die steel. Journal of Shanghai Jiao Tong University, Vol. 2, 1998, pp. 71–74.Search in Google Scholar
[48] Ghost, G. and G. B. Olson. Precipitation of paraequilibrium cementite: Experiments, and thermodynamic and kinetic modeling. Acta Materialia, Vol. 50, 2002, pp. 2099–2119.10.1016/S1359-6454(02)00054-XSearch in Google Scholar
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Endpoint carbon content and temperature prediction model in BOF steelmaking based on posterior probability and intra-cluster feature weight online dynamic feature selection
- Thermal conductivity of lunar regolith simulant using a thermal microscope
- Multiobjective optimization of EDM machining parameters of TIB2 ceramic materials using regression and gray relational analysis
- Research on the magnesium reduction process by integrated calcination in vacuum
- Microstructure stability and softening resistance of a novel Cr-Mo-V hot work die steel
- Effect of bonding temperature on tensile behaviors and toughening mechanism of W/(Ti/Ta/Ti) multilayer composites
- Exploring the selective enrichment of vanadium–titanium magnetite concentrate through metallization reduction roasting under the action of additives
- Effect of solid solution rare earth (La, Ce, Y) on the mechanical properties of α-Fe
- Impact of variable thermal conductivity on couple-stress Casson fluid flow through a microchannel with catalytic cubic reactions
- Effects of hydrothermal carbonization process parameters on phase composition and the microstructure of corn stalk hydrochars
- Wide temperature range protection performance of Zr–Ta–B–Si–C ceramic coating under cyclic oxidation and ablation environments
- Influence of laser power on mechanical and microstructural behavior of Nd: YAG laser welding of Incoloy alloy 800
- Aspects of thermal radiation for the second law analysis of magnetized Darcy–Forchheimer movement of Maxwell nanomaterials with Arrhenius energy effects
- Use of artificial neural network for optimization of irreversibility analysis in radiative Cross nanofluid flow past an inclined surface with convective boundary conditions
- The interface structure and mechanical properties of Ti/Al dissimilar metals friction stir lap welding
- Significance of micropores for the removal of hydrogen sulfide from oxygen-free gas streams by activated carbon
- Experimental and mechanistic studies of gradient pore polymer electrolyte fuel cells
- Microstructure and high-temperature oxidation behaviour of AISI 304L stainless steel welds produced by gas tungsten arc welding using the Ar–N2–H2 shielding gas
- Mathematical investigation of Fe3O4–Cu/blood hybrid nanofluid flow in stenotic arteries with magnetic and thermal interactions: Duality and stability analysis
- Influence on hexagonal closed structure and mechanical properties of outer heat treatment cycle and plasma arc transfer Ti54Al23Si8Ni5XNb+Ta coating for Mg alloy by selective laser melting process
- Effect of rare-earth yttrium doping on the microstructure and texture of hot-rolled non-oriented electrical steel
- Study on the rheological behavior and microstructure evolution of isothermal compression of high-chromium cast steel
- Topical Issue on Conference on Materials, Manufacturing Processes and Devices - Part II
- Effects of heat treatment on microstructure and properties of CrVNiAlCu high-entropy alloy
- Enhanced bioactivity and degradation behavior of zinc via micro-arc anodization for biomedical applications
- Study on the parameters optimization and the microstructure of spot welding joints of 304 stainless steel
- Research on rotating magnetic field–assisted HRFSW 6061-T6 thin plate
- Efficient preparation and evaluation of dry gas sealed spiral grooves
- Special Issue on A Deep Dive into Machining and Welding Advancements - Part II
- Microwave hybrid process-based fabrication of super duplex stainless steel joints using nickel and stainless steel filler materials
- Special Issue on Polymer and Composite Materials and Graphene and Novel Nanomaterials - Part II
- Low-temperature corrosion performance of laser cladded WB-Co coatings in acidic environment
- Special Issue for the conference AMEM2025
- Effect of thermal effect on lattice transformation and physical properties of white marble
Articles in the same Issue
- Research Articles
- Endpoint carbon content and temperature prediction model in BOF steelmaking based on posterior probability and intra-cluster feature weight online dynamic feature selection
- Thermal conductivity of lunar regolith simulant using a thermal microscope
- Multiobjective optimization of EDM machining parameters of TIB2 ceramic materials using regression and gray relational analysis
- Research on the magnesium reduction process by integrated calcination in vacuum
- Microstructure stability and softening resistance of a novel Cr-Mo-V hot work die steel
- Effect of bonding temperature on tensile behaviors and toughening mechanism of W/(Ti/Ta/Ti) multilayer composites
- Exploring the selective enrichment of vanadium–titanium magnetite concentrate through metallization reduction roasting under the action of additives
- Effect of solid solution rare earth (La, Ce, Y) on the mechanical properties of α-Fe
- Impact of variable thermal conductivity on couple-stress Casson fluid flow through a microchannel with catalytic cubic reactions
- Effects of hydrothermal carbonization process parameters on phase composition and the microstructure of corn stalk hydrochars
- Wide temperature range protection performance of Zr–Ta–B–Si–C ceramic coating under cyclic oxidation and ablation environments
- Influence of laser power on mechanical and microstructural behavior of Nd: YAG laser welding of Incoloy alloy 800
- Aspects of thermal radiation for the second law analysis of magnetized Darcy–Forchheimer movement of Maxwell nanomaterials with Arrhenius energy effects
- Use of artificial neural network for optimization of irreversibility analysis in radiative Cross nanofluid flow past an inclined surface with convective boundary conditions
- The interface structure and mechanical properties of Ti/Al dissimilar metals friction stir lap welding
- Significance of micropores for the removal of hydrogen sulfide from oxygen-free gas streams by activated carbon
- Experimental and mechanistic studies of gradient pore polymer electrolyte fuel cells
- Microstructure and high-temperature oxidation behaviour of AISI 304L stainless steel welds produced by gas tungsten arc welding using the Ar–N2–H2 shielding gas
- Mathematical investigation of Fe3O4–Cu/blood hybrid nanofluid flow in stenotic arteries with magnetic and thermal interactions: Duality and stability analysis
- Influence on hexagonal closed structure and mechanical properties of outer heat treatment cycle and plasma arc transfer Ti54Al23Si8Ni5XNb+Ta coating for Mg alloy by selective laser melting process
- Effect of rare-earth yttrium doping on the microstructure and texture of hot-rolled non-oriented electrical steel
- Study on the rheological behavior and microstructure evolution of isothermal compression of high-chromium cast steel
- Topical Issue on Conference on Materials, Manufacturing Processes and Devices - Part II
- Effects of heat treatment on microstructure and properties of CrVNiAlCu high-entropy alloy
- Enhanced bioactivity and degradation behavior of zinc via micro-arc anodization for biomedical applications
- Study on the parameters optimization and the microstructure of spot welding joints of 304 stainless steel
- Research on rotating magnetic field–assisted HRFSW 6061-T6 thin plate
- Efficient preparation and evaluation of dry gas sealed spiral grooves
- Special Issue on A Deep Dive into Machining and Welding Advancements - Part II
- Microwave hybrid process-based fabrication of super duplex stainless steel joints using nickel and stainless steel filler materials
- Special Issue on Polymer and Composite Materials and Graphene and Novel Nanomaterials - Part II
- Low-temperature corrosion performance of laser cladded WB-Co coatings in acidic environment
- Special Issue for the conference AMEM2025
- Effect of thermal effect on lattice transformation and physical properties of white marble

