Abstract
In this article, polyacrylonitrile (PAN) and two kinds of graphene oxide (GO) with different structural properties are blended in dimethyl sulfoxide (DMSO) according to a certain ratio to prepare stable-spinning solution. One kind of GO is purchased, and we call it GO1. The other is a kind of self-made GO with liquid crystal property in solution, which is called GO2. The effects of two kinds of GO on the rheological properties of PAN/GO spinning solution (15 wt%) were studied. The results show that the viscous activation energy of the liquid crystal GO2/PAN blend solution is significantly higher than that of the nonliquid crystal GO1/PAN blend solution. Moreover, the liquid crystal system is more obviously affected by temperature. The structural viscosity index of the liquid crystalline GO2/PAN solution is obviously lower than that of the GO1/PAN solution. It indicates that the existence of liquid crystallinity is beneficial to the spinning of the solution. In the low-frequency region, the addition of GO1 makes the solution more elastic, and the addition of GO2 makes the solution more viscous.
1 Introduction
The orientation of the molecules in the polyacrylonitrile (PAN) carbon fiber precursor along the fiber axis largely determines the orientation of the graphite-like layers formed during the carbonization process, which is the most critical factor determining the mechanical properties of carbon fibers [1,2]. However, during the preoxidation process, the thermal motion of the molecules will produce deorientation. Therefore, how to increase the degree of orientation of the original filament and reduce the deorientation during the preoxidation process is of great significance to improve the performance of carbon fibers. Rigid molecular materials have good thermal stability, are easily oriented at high temperatures, and can maintain this orientation structure, such as carbon nanotubes (CNT). Therefore, adding rigid molecular materials is beneficial to limit the deorientation of PAN molecules due to thermal motion. Research on CNT to improve PAN carbon fiber has achieved certain results [3], but this material also has certain defects: CNT has a tubular structure, which may have poor compatibility with graphite-like laminate.
At the same time, graphene oxide (GO) is a two-dimensional (2D) layered structure, which induces PAN molecules to align along the fiber axis during the spinning process. Moreover, it contains oxygen-containing functional groups on its surface, which can be well dispersed in organic solvents [4]. With the formation of graphite-like structures in the carbonization process of PAN, GO will be reduced to graphene. Therefore, it may become an additive to improve the structure and properties of PAN carbon fiber. Spinning is one of the core elements to improve the quality of the raw silk. The rheological properties of the spinning solution directly affect its spinnability, which determines the structure and properties of the finished fiber. Therefore, the effects of various factors on the rheological behavior of PAN/GO blending solution were studied. Therefore, the optimal spinning dope preparation and spinning process parameters will play an important role in the preparation of high-performance PAN precursors and carbon fibers [5]. The effect of carbon materials on the rheological properties of PAN spinning solution has been partially studied [6,7,8]. However, few people have studied the rheological properties of liquid crystalline spinning solution after GO with liquid crystalline properties is added to PAN and nonliquid crystalline spinning solution. In this study, the rheological properties of two kinds of GO/PAN spinning solution were investigated through both steady-state and dynamic rheological measurements.
The addition of an appropriate amount of GO reduces the apparent viscosity of GO/PAN spinning solution and increases the structural viscosity index, flow activation energy, and mechanical loss factor of GO/PAN spinning solution. Liquid crystalline GO2/PAN spinning solution has a better spinnability than nonliquid crystalline GO1/PAN spinning solution.
2 Materials and methods
2.1 Materials
PAN with molecules weight of 1.3 × 105 g·mol−1 is provided by Sinopek Shanhai Petrochemical Co., Ltd. GO1 was purchased from Hangzhou Gaoxitech Co., Ltd. GO2 was prepared from purified expandable graphite by a modified Hummers method [9]. Deionized water (H2O) was self-made by Donghua University.
2.2 Preparation of PAN/GO/DMSO solution
GO was dispersed in DMSO by ultrasonic comminutor with ultrasonic power of 200 W uniformly. The GO solution was exfoliated by the ultrasonic treatment for 2 h in 5s–5s mode. The PAN powder was dried in a vacuum oven at 70°C for 48 h. Then, the PAN powder and GO/DMSO dispersion solution were added in the three-necked flask with the predetermined proportion. The mass fraction of GO1 in PAN was 0.2, 0.5, and 1%, respectively. The uniform and stable spinning solution was prepared. When the mass fraction of GO2 in PAN was 3 and 4%, the spinning solution shows liquid crystalline properties. The solid content of both solutions is 15 wt%. The three-necked flask with PAN/GO/DMSO dispersion solution was placed in an oil bath, heated to 70°C, and stirred at high speed in nitrogen atmosphere for 2 h. After PAN was completely dissolved, the spinning solution was filtered to remove impurities in the solution. Finally, it was placed in an oven at 70°C for 48 h to obtain a well-mixed spinning dope.
2.3 Characterization
ARES-RFS-type (TA, USA) advanced rotary rheometer was used to test the rheological properties of PAN/GO/DMSO spinning dope. To obtain the relationship between the viscosity of the sample and the shear frequency, a steady-state rheological test was performed on different samples from 0.1 to 100 s−1 at 70°C. To obtain the relationship curves of loss modulus, storage modulus, and shear frequency, a dynamic rheological test was carried out for different samples from 0.1 to 100 Hz under the condition of 70°C and 10% strain.
3 Results and discussion
3.1 GO properties
The results of element content analysis of GO1 and GO2 are shown in Table 1. Since the oxygen-containing functional groups on GO are mainly epoxy, carbonyl, carboxyl, and hydroxyl, the main elements are C, H, and O. It can be seen from the table that GO2 has a higher O element content than GO1, indicating that GO2 contains more functional groups than GO1. It indicates that the dispersion of GO2 in water and organic solvents is better than that of GO1, which will play a positive role in the preparation of spinning solution with a good dispersion.
Elemental analysis of two different GOs
| Element | C (%) | H (%) | O (%) |
|---|---|---|---|
| Content (GO1) | 49.23 ± 0.73 | 3.70 ± 0.11 | 47.07 ± 0.84 |
| Content (GO2) | 43.90 ± 0.60 | 2.88 ± 0.09 | 53.22 ± 0.69 |
The properties of GO are closely related to the structure. Figure 1(a) and (b) is the AFM images of GO1 and GO2, respectively. In the atomic force microscope (AFM) photo, the depth of the color corresponds to the height of the GO sheet. Moreover, the brighter the color, the higher the height. It can be seen from the figure that the thickness of the GO1 sheet is about 4.18 nm, which is equivalent to the thickness of three to four layers of GO stacked together; the thickness of the GO2 sheet is about 8 nm, which is about seven to eight layers of GO that are superimposed on the thickness. Different lamellar structures may make GO have different chemical properties [10]. The main reason for the stacking of GO sheets is that the GO is easy to shrink and agglomerate together when the solvent evaporates at high temperature, which leads to the stacking of sheets and the increase in thickness. In addition, it can be seen from the height map that the lamella surface of GO2 is smoother and flatter than GO1, indicating that the lamella thickness of GO2 is more uniform.

The AFM image of GO: (a) GO1 and (b) GO2.
Raman spectroscopy was applied to study the carbon structure of GO1 and GO2, as shown in Figure 2. The Raman spectrum shows that GO1 has two characteristic peaks at 1,327 and 1,587 cm−1, respectively. The disorder induced D-band at 1,327 cm−1 was first named by Tuinstra and Koenig in 1970, and the intrinsic Raman mode at 1,587 cm−1 was called G-band [11]. The D-band belongs caused by a breathing mode-of-point phonons of A1g symmetry of the defects involved in the sp3-hybridized carbon bonds such as hydroxyl and/or epoxide bonds [12], and the 2D band (∼26,741 cm−1), which is much sensitive to stacking of graphene sheets [13] are observable in the spectra of GO2. However, the G-band (1,587 cm−1) represents the E2g phonon vibration mode of the central point of the Brillouin zone, which belongs to the in-plane stretching vibration of sp2 hybrid carbon atoms on the ring and chain [14]. I d/I g represents the strength ratio of D-band and G-band, which is used to represent the disorder degree of graphite material. It can be calculated from the figure that the I d/I g value of GO2 is 0.92, which is significantly less than 1.04 of GO1. It indicates that the disorder degree of GO1 is higher than that of GO2. In addition, the existence of 2D peak indicates that there are fewer layers of GO2.

The Raman spectra of (a) GO1 and (b) GO2.
3.2 Micromorphology
Figure 3(a) shows the TEM images of GO1 and GO2 under the same magnification. It can be seen from Figure 3 that the GO flakes and the irregular flake boundaries of GO. At the same time, it can be seen that the GO flakes present a wrinkled tulle shape that is different from the copper mesh carbon film structure. The reason for this structure is due to the repulsion of many oxygen-containing functional groups between the GO layers. However, due to the tetrahedral sp3 hybridization, the carbon atoms are connected to –OH, which is not thermodynamically stable for 2D crystals. The fluctuations in thermodynamics will destroy the long-range ordered structure of the crystal [15]. Figure 3(b) shows the SEM image of GO1 and GO2, with different magnifications. As the types and numbers of functional groups on GO increase, GO exhibits corrugated folds. This is different from the smooth, flat, stacked structure of natural graphite. By comparing the SEM images of GO1 and GO2, it can be clearly seen that GO2 exhibits a larger GO sheet size at a relatively small magnification. It indicates that the sheet area of GO2 is larger than that of GO1. This is very beneficial for the formation of GO liquid crystals [16].

TEM (a) and SEM (b) images of GO1 and GO2.
3.3 Liquid crystal performance
High-concentration dispersions of fully soluble material yield lyotropic, nematic, liquid-crystalline aqueous suspensions due to the reorganization of the GO sheets in the liquid [17]. When its dispersion in the solvent is higher than a critical value, liquid crystals will be formed. According to the cross-polarized photos, it can be directly confirmed whether the GO/DMSO dispersion liquid has liquid crystals and liquid crystal types. Figure 4, when the GO1 concentration is 2 mg·mL−1 (the mass fraction fm is 1%), the visual field is completely black. It indicates that the dispersion does not have any birefringence and is in a disordered phase. As the concentration increases to 8 mg·mL−1 (the mass fraction fm is 4%), there is still no birefringence in the solution. When the GO2 concentration is 2 mg·mL−1, there are obvious bright spots. As the GO2 concentration continues to increase, light and dark stripes begin to appear in the field of view, and the stripes become more dense and compact, the color becomes more and more abundant, and the birefringence phenomenon is obvious. It shows the formation of ordered regions in the dispersion, and it can be inferred that there is a large anisotropic structure in the dispersion system. The increase in GO2 concentration makes the dispersion change from disorder to order until a stable liquid crystal is formed. In addition, it can be seen from the picture that as the concentration increases, the previous bright spots merge with each other to form a bright area, and a more obvious filamentous texture appears in the system, which is one of the typical signs of nematic liquid crystals [16]. It can be inferred that the dispersion finally formed a stable nematic liquid crystal.

The orthogonal polarization photos of GO1/DMSO solution (a–d) and GO2/DMSO solution (e–h).
Figure 5 is a cross-polarized photo of the PAN/GO2/DMSO spinning dope. The GO2 content is 3 and 4 wt%. It can be seen from the figure that the spinning dope also has a good liquid crystallinity. The field of view is full of light and dark stripes, and the stripes are compact and dense, with rich colors and obvious birefringence. It shows that an ordered structure is formed in the spinning dope. In this way, continuous fibers can be spun from the liquid-crystalline spinning dope. Because the internal structure of the spinning dope is arranged in an orderly manner, it is extremely beneficial to increase the orientation and crystallinity of the composite fiber in the subsequent molding process, which provides the possibility to produce a high-performance PAN precursor.

Photographs of orthogonal polarization of GO2/PAN/DMSO spinning dope with different concentrations. (a) 3%, (b) 3%.
3.4 Steady-state rheological properties
Graphene steady-state rheology is used to study the relationship between viscosity and shear rate. Figure 6 is a graph showing the relationship between apparent viscosity and shear rate of PAN/GO/DMSO-blended solutions with different mass fractions of GO at 70°C. It can be seen from Figure 6 that the viscosity of PAN composite solution decreases with the increase of shear rate. It presents a typical “shear thinning” phenomenon, which is a typical performance of non-Newtonian fluid [18]. The reason of polymer fluid shear thinning is the destruction of entanglement between the macromolecular chains. As the shear rate increases, the entanglement points between the polymers in the solution are destroyed, and the decrease in the concentration of entanglement points correspondingly reduces the viscosity. In addition, when the shear rate increases, because the stress in the chain segments between the entanglement points is too late to relax, the orientation of the chain segments in the flow field will increase. The segment orientation effect will reduce the drag force between the macromolecular chains. When the degree of orientation reaches an extreme value, the viscosity of the solution reaches the lowest value [19]. However, the PAN content in the blend solution is relatively large. Due to the influence of shear stress on the curling state of PAN macromolecules, the conformation of the molecule changes slowly at low shear rates. As the relaxation time is shortened, the system exhibits a Newtonian-like fluid properties. With the increase of the shear rate, the macromolecular chains are oriented and have no time to relax; hence, the system exhibits non-Newtonian properties at this time. However, due to the interaction between PAN itself, PAN and solvent, PAN and GO, as well as between GO and solvent, physical crosslinking is formed. The blended solution, as a transient network system, makes the entanglement points in a dynamic equilibrium of continuous dispersion and reunion [20]. The polymer solution can be oriented under the action of shearing. And, it is of great significance to maintain this oriented structure. Because the obtained orientation during spinning can not only increase the final orientation of the fiber but also play a preorientation role for the subsequent stretching orientation of the fiber.

The apparent viscosity of (a) PAN/GO1/DMSO and (b) PAN/GO2/DMSO blend solutions with different GO content changes with shear rate.
According to the power law equation τ = Kγn, the relationship between shear stress and rate of solution is characterized by taking logarithm from both sides [21].
It can be clearly seen from Figure 7 that the slope of the straight line represents the non-Newtonian index of the fluid. Table 2 lists the non-Newtonian index values. The values are all less than 1, indicating that all fluids are pseudoplastic fluid properties.

lg τ–lg γ curve of PAN/GO/DMSO blend solution.
Non-Newtonian index of blend solutions with different GO contents
| GO types | GO1 | GO2 | |||
|---|---|---|---|---|---|
| Content (wt%) | 0.2 | 0.5 | 1 | 3 | 4 |
| n | 0.886 | 0.864 | 0.828 | 0.896 | 0.916 |
The viscosity of the polymer solution changes with temperature in accordance with the Arrhenius equation [22]:
In the equation (3), A – physical property constant; Eη – viscous flow activation energy; R – molar gas constant, 8.314 J·(mol·K)−1; and T – thermodynamic temperature, K.
According to the relationship between the viscosity of PAN/GO/DMSO-blended solutions with different GO contents and temperature in Figure 8(a), the shear rate is fixed, and the relationship curve of ln η α versus T –1 is drawn (Figure 8(b)). It is linearly fitted, and its slope is the viscous flow activation energy. Table 3 lists the obtained viscous flow activation energy.
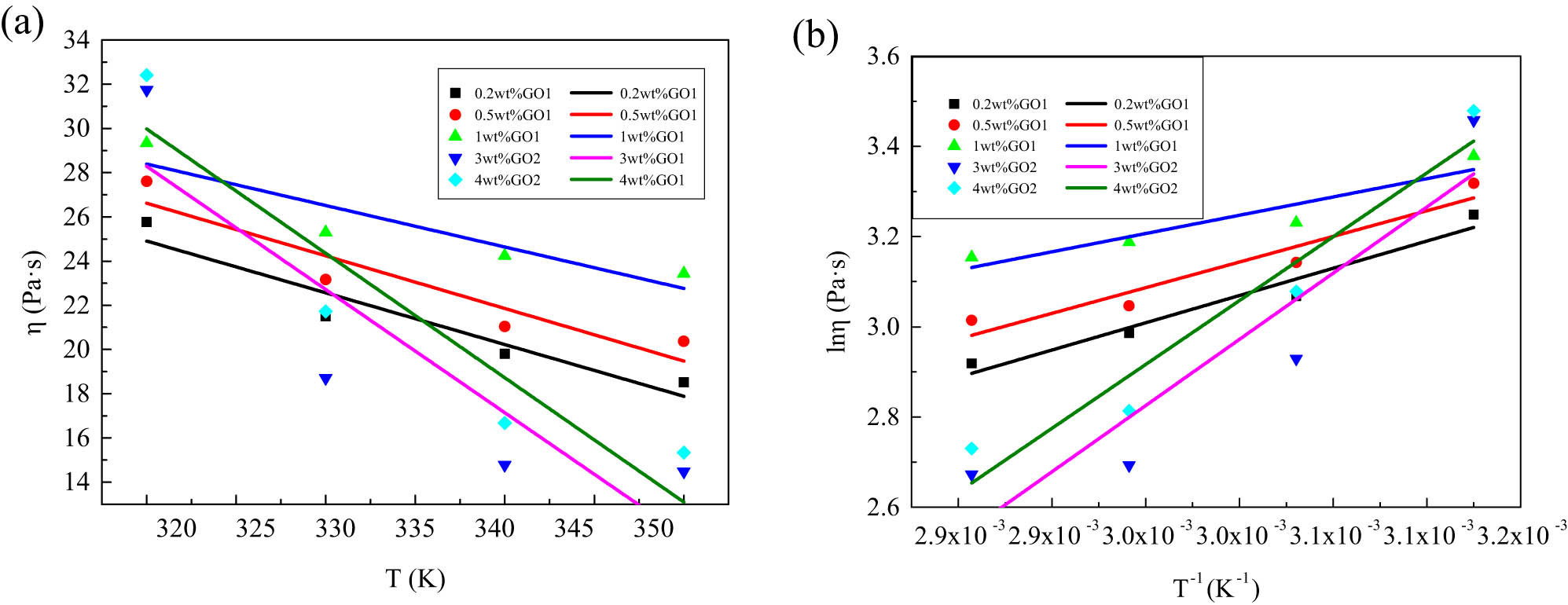
(a) The apparent viscosity–temperature curve (γ = 1 s−1) and (b) the ln η ∼ T −1 curve (γ = 1 s−1) of blended solutions with different GO contents.
Viscous activation energy of PAN/GO/DMSO blend solution (γ = 1 s−1)
| GO types | GO1 | GO2 | |||
|---|---|---|---|---|---|
| Content (wt%) | 0.2 | 0.5 | 1 | 3 | 4 |
| ∆η | 3.95 | 4.61 | 5.53 | 3.11 | 3.60 |
The activation energy of viscous flow reflects the sensitivity of the polymer rheological behavior to temperature. The smaller the value, the apparent viscosity is not sensitive to temperature changes. It can be seen from Table 3 that as the GO concentration increases, the viscous flow activation energy decreases accordingly. The activation energy of viscous flow of PAN/GO2/DMSO blend solution is obviously higher than that of PAN/GO1/DMSO blend solution, indicating that the liquid crystal system is more obviously affected by temperature. Therefore, during the spinning process, the PAN/GO2/DMSO blend solution especially needs to keep the temperature of the spinning process system stable.
The structural viscosity index Δη is an important parameter that characterizes the degree of structure of the solution, which is closely related to the dissolution process. In the non-Newtonian zone, the structural viscosity index Δη of pseudoplastic fluid is greater than 0. The larger the value, the higher the structure of the composite solution. According to relevant studies, the spinnability of the spinning dope is closely related to the structural viscosity index, and the smaller the degree of structure, the better the spinnability of the dope [23]. According to the formula (equation (4)) of structural viscosity index:
Plot lg η vs γ 0.5, as shown in Figure 9. According to its slope, the structural viscosity index can be obtained. The results are listed in Table 4. It can be found that as the GO concentration in the blended solution increases, the structural viscosity index also gradually becomes bigger. This shows that as the content of GO increases, the degree of structuring becomes higher, and its spinnability becomes worse. The main reason is that the increase of rigid GO in the solution induces the orientation of PAN molecules. When subjected to external forces, the interface between GO and PAN will slip, resulting in a reduction in the proportion of instantaneous entanglement between macromolecular chains, thereby, improving spinning the degree of structuring of the original solution. However, the structural viscosity index of the liquid crystalline GO2/PAN solution is significantly lower than that of the GO1/PAN solution, indicating that the liquid crystallinity is beneficial to the spinning of the solution.

lg η ∼ γ 0.5 curves of PAN/GO/DMSO solutions with different contents.
Structural viscosity index of PAN/GO1/DMSO blend solutions with different GO contents
| Solid content (%) | GO content | ∆Eη (kJ·mol−1) |
|---|---|---|
| 15 | 0.2% GO1 | 9.996 |
| 15 | 0.5% GO1 | 9.429 |
| 15 | 1% GO1 | 6.724 |
| 15 | 3% GO2 | 24.395 |
| 15 | 4% GO2 | 23.450 |
3.5 Dynamic rheological properties
Compared with steady-state flow behavior, the dynamic rheological behavior of PAN spinning dope is measured under a certain frequency and alternating stress. It reflects the viscosity change of the composite solution under different stresses. It can be used to judge the physical stability of the solution. To judge the physical stability of the solution. PAN-concentrated solution has both viscosity and elasticity. This viscoelasticity will produce viscous flow and elastic deformation under the action of tension and shear. Among them, the loss modulus G″ is used to reflect the size of the viscous force in the system. The quantity G′ characterizes the strength of the elastic structure of the spinning dope. The process will not cause damage to the structure of the material itself, and the linear viscoelastic response of the polymer material is very sensitive to the change of the morphological structure. Therefore, the dynamic rheology is considered to be an effective method to characterize the morphological structure of polymers [24,25].
As shown in Figure 10, when the temperature remains constant, the G′ and G″ of PAN/GO/DMSO blend solutions with different GO contents gradually increase. In the scanning range of 0.1–100 rad·s−1, the phenomenon of G″ > G′ was observed, which indicated that the viscosity is more prominent than the elasticity in the blended solution and the viscosity is mainly system. At the same time, it can be seen that under the same alternating stress, G′ and G″ increase continuously with the increase of GO. Moreover, the viscous force and elastic network structure in the composite dope system are improved.

(a) G′ ∼ ω, G″ ∼ ω curves of PAN/GO1/DMSO and (b) PAN/GO2/DMSO solutions.
From the point of view of spinnability, the problem of spinnability is essentially a rheological problem of uniaxial stretching. For the stretch flow of viscoelastic fluid, when the stored elastic energy density exceeds a certain critical value, it will break fluid flow. In the spinning process, the elasticity of the spinning fluid cannot be too large; otherwise, it will cause disadvantages to the processing.
Loss tangent tan δ is also called the loss factor or internal friction, which is the ratio of loss modulus to storage modulus. It represents the tangent of the phase difference between the strain and the stress period of the viscoelastic material under the action of alternating force. The formula is as follows [26]:
Figure 11 shows that the samples exhibited viscous behavior (tan δ > 1) in both the low-frequency and high-frequency regions. The GO/PAN solution still has certain flow properties after adding GO. In the low-frequency region, with the increase of GO1 mass fraction, tan δ gradually decreases. This means that the addition of GO1 makes the solution more elastic. As the mass fraction of GO2 increases, tan δ gradually increases, indicating that the addition of GO2 makes the solution more viscous. The change of the added mass fraction of GO in the high-frequency region has little effect on tan δ. The tan δ decreases continuously with the increase of ω. This phenomenon is mainly due to the increase of the elastic network structure in the system with the increase of the specific gravity of the viscosity. When the mixed solution exhibits elastic properties, the spinning dope exhibits a serious extrusion swelling phenomenon in the spinneret hole. Therefore, it is necessary to control the elastic properties of the spinning dope during the spinning process, and the results show that PAN/GO/DMSO-mixed solution is mainly viscous under appropriate shear rate. It shows that the solution spinnability is high, which has a positive effect on wet spinning [27].

tan δ ∼ ω curves of blended solutions with different GO contents.
In summary, the viscosity of GO1/PAN copolymer gradually decreased with the increase of GO1 content. However, due to the unique rigid sheet structure of GO1, it will induce the orientation of PAN molecules when it is added to PAN. The interface between GO and PAN will slip when subjected to external force, which reduces the viscosity of the system [20]. In contrast, when GO is added to the PAN solution, GO may act as a lubricating point, which is more conducive to the movement of molecular chains. Moreover, the weakening of the intermolecular force of PAN increases the intermolecular distance and decreases the concentration of entangled nodes. Therefore, the viscosity of the system decreases and the fluidity increases. With the increase of GO2 content, the viscosity of GO2/PAN copolymer gradually increases. The formation of liquid crystal solution makes the ordered PAN molecules increase, which increases the solution viscosity.
4 Conclusion
The rheological behavior of nonliquid crystal PAN/GO1 and liquid crystal PAN/GO2 spinning solution were studied. The results are as follows:
Both the blend solutions belong to non-Newtonian fluid. The activation energy of viscous flow of liquid crystal PAN/GO2-blended solution is obviously higher than that of nonliquid crystal PAN/GO1-blended solution. This shows that the liquid crystal system is more obviously affected by temperature.
With the concentration of GO in the blended solution increases, its structural viscosity index gradually increases and the spinnability becomes worse. However, the structural viscosity index of the liquid crystal GO2/PAN solution is significantly lower than that of the nonliquid crystal GO1/PAN solution. The liquid crystallinity is beneficial to the spinning of the solution.
In the low-frequency region, tan δ gradually decreases with the GO1 mass fraction increasing. This means that the nonliquid crystal solution tends to be more elastic with the addition of GO1. However, tan δ increased with the increase of GO2 mass fraction. This indicates that the addition of GO2 makes the liquid crystal solution more prone to viscous system. The change of the added mass fraction of GO in the high-frequency region has little effect on tan δ. As the shear rate increases, the loss tangent gradually decreases.
Acknowledgements
I would like to express my gratitude to all those who helped me during the writing of this thesis. My deepest gratitude goes first and foremost to Professor Zhou Yumuhuo, my supervisor, for his constant encouragement and guidance. He has walked me through all the stages of the writing of this thesis. Without his consistent and illuminating instruction, this thesis could not have reached its present form. Second, I would like to express my heartfelt gratitude to Wangcong, who helped me a lot in the past three years. Last my thanks would go to my beloved family for their loving considerations and great confidence in me all through these years. I also owe my sincere gratitude to my friends and my fellow classmates who gave me their help and time in listening to me and helping me work out my problems during the difficult course of the thesis.
-
Funding information: This study was supported by Central Military Commission [grant numbers: 17-163-13-ZT-009-040].
-
Author contributions: Baihua Liu, Cong Wang,Muhuo Yu propose concepts; Baihua Liu, Cong Wang participated in the design of the study; Cong Wang collected field data; Baihua Liu carried out the date analyses; Baihua Liu drafted the manuscript; Muhuo Yu coordinated of the study and provide funds. All authors gave final approval for publication.
-
Conflicts of interest: Authors state no conflict of interest.
References
[1] Xu, Q., L. Xu, W. Cao, and S. Wu. A study on the orientation structure and mechanical properties of polyacrylonitrile precursors. Polymers for Advanced Technologies, Vol. 16, 2005, pp. 642–645.10.1002/pat.625Search in Google Scholar
[2] Edie, D. D. The effect of processing on the structure and properties of carbon fibers. Carbon, Vol. 36, 1998, pp. 345–362.10.1016/S0008-6223(97)00185-1Search in Google Scholar
[3] Chae, H. G., T. V. Sreekumar, T. Uchida, and S. Kumar. A comparison of reinforcement efficiency of various types of carbon nanotubes in polyacrylonitrile fiber. Polymer, Vol. 46, 2005, pp. 10925–10935.10.1016/j.polymer.2005.08.092Search in Google Scholar
[4] Fryczkowska, B. and L. Przywara. The application of composite GO/PAN membranes for removing surfactants from laundry wastewater. Desalin Water Treat, Vol. 157, 2019, pp. 259–265.10.5004/dwt.2019.23880Search in Google Scholar
[5] Hu, H., X. B. Wang, J. Wang, W. Li, F. Liu, Z. Han, et al. Preparation and properties of graphene nanosheets–polystyrene nanocomposites via in situ emulsion polymerization. Chemical Physics Letters, Vol. 484, 2010, pp. 247–253.10.1016/j.cplett.2009.11.024Search in Google Scholar
[6] Wang, B., J. Li, H. Wang, J. Jiang, and Y. Liu. Rheological behavior of spinning dope of multiwalled carbon nanotube/polyacrylonitrile composites. Macromolecular Symposia, Vol. 216, 2004, pp. 189–194.10.1002/masy.200451218Search in Google Scholar
[7] Li, F. M., Y. Zheng, and B. Wang. A barley UDP-glucosyltransferase inactivates nivalenol and provides Fusarium Head Blight resistance in transgenic wheat. Materials Science Forum, Vol. 898, 2017, pp. 2187–2197.10.1093/jxb/erx109Search in Google Scholar PubMed PubMed Central
[8] Ki-Yong, L., K. Min-Young, I. Son-Ki. Deactivation by coke deposition on the HZSM-5 catalysts in the methanol-to-hydrocarbon conversion. Journal of Physics & Chemistry of Solids, Vol. 73, No. 12, 2012, pp. 1542–1545.10.1016/j.jpcs.2012.09.005Search in Google Scholar
[9] Hummers, W. S. and R. E. Offeman. Preparation of graphitic oxide. Journal of the American Chemical Society, Vol. 80, 1958, id. 1339.10.1021/ja01539a017Search in Google Scholar
[10] Akhavan, O. The effect of heat treatment on formation of graphene thin films from graphene oxide nanosheets. Carbon, Vol. 48, 2010, pp. 509–519.10.1016/j.carbon.2009.09.069Search in Google Scholar
[11] Schedin, F., E. Lidorikis, A. Lombardo, V. G. Kravets, and A. C. Ferrari. Surface-enhanced Raman spectroscopy of graphene. ACS Nano, Vol. 4, 2010, pp. 5617–5626.10.1021/nn1010842Search in Google Scholar
[12] Jerng, S. K., S. Y. Dong, and J. H. Lee. Graphitic carbon growth on crystalline and amorphous oxide substrates using molecular beam epitaxy. Nanoscale Research Letters, Vol. 6, 2011, id. 565.10.1186/1556-276X-6-565Search in Google Scholar
[13] Malard, L. M., M. A. Pimenta, G. Dresselhaus, and M. S. Dresselhaus. Raman spectroscopy in graphene. Physics Reports, Vol. 473, 2009, pp. 51–87.10.1016/j.physrep.2009.02.003Search in Google Scholar
[14] Zhang, Y., X. Xu, J. Xu, and L. Zhang. Dynamic viscoelastic behavior of triple helical Lentinan in water: Effects of concentration and molecular weight. Polymer, Vol. 48, 2007, pp. 6681–6690.10.1016/j.polymer.2007.09.005Search in Google Scholar
[15] Kumar, P., U. N. Maiti, K. E. Lee, and S. O. Kim. Rheological properties of graphene oxide liquid crystal. Carbon, Vol. 80, 2014, pp. 453–461.10.1016/j.carbon.2014.08.085Search in Google Scholar
[16] Kudin, K. N., B. Ozbas, H. C. Schniepp, R. K. Prud“Homme”, I. A. Aksay, and R. Car. Raman spectra of graphite oxide and functionalized graphene sheets. Nano Letters, Vol. 8, 2008, pp. 36–41.10.1021/nl071822ySearch in Google Scholar
[17] Aboutalebi, S. H., M. M. Gudarzi, Q. B. Zheng, and J. K. Kim. Spontaneous formation of liquid crystals in ultralarge graphene oxide dispersions. Advanced Functional Materials, Vol. 21, 2011, pp. 2978–2988.10.1002/adfm.201100448Search in Google Scholar
[18] Bajaj, P., S. H. Bhrami, K. Sen, and T. V. Sreekumar. Thermal and rheological behavior of acrylonitrile-carboxylic acid copolymers and their metal salt complexes. Journal of Applied Polymer Science, Vol. 74, 1999, pp. 567–582.10.1002/(SICI)1097-4628(19991017)74:3<567::AID-APP11>3.0.CO;2-6Search in Google Scholar
[19] Mukhamedzhanova, M. Y., N. Y. Shirshova, and G. Khamrakulov. Rheological properties of concentrated solutions of ternary acrylonitrile copolymers. Fibre Chemistry, Vol. 32, 2000, pp. 340–343.10.1007/BF02360639Search in Google Scholar
[20] Zhang, W. L., B. J. Park, and H. J. Choi. Colloidal graphene oxide/polyaniline nanocomposite and its electrorheology. Chemical Communications, Vol. 46, 2010, pp. 5596–5598.10.1039/c0cc00557fSearch in Google Scholar
[21] Tanner, R. I. and R. S. Rivlin. Engineering rheology. Journal of Applied Mechanics, Vol. 54, No. 2, 1985.10.1115/1.3173055Search in Google Scholar
[22] Chowdhury, S., G. Gao, and J. F. Kadla. Investigating the viscoelasticity of lignin-polyacrylonitrile blends using dynamic rheology. Abstract Papers of American Chemical Society, Vol. 2012, 2012, p. 243.Search in Google Scholar
[23] Du, W., H. Chen, Y. Xu, and D. Pan. Constant rotational rheological behaviors of the PAN/DMSO/nonsolvent systems. Journal of Applied Polymer Science, Vol. 114, 2009, pp. 598–602.10.1002/app.30568Search in Google Scholar
[24] Byel’nikevich, N. G., V. P. Sklizkova, V. V. Kudryavtsev, M. M. Koton, S. Y. Frenkel, and Z. V. Gerashchenko. Effect of the thermodynamic quality of solvent on the viscosity properties of concentrated solutions of poly(4,4′-oxydiphenylene)pyromellitamic acid. Polymer Science USSR, Vol. 30, 1988, pp. 1153–1157.10.1016/0032-3950(88)90343-7Search in Google Scholar
[25] Tan, L., J. Pan, and A. Wan. Shear and extensional rheology of polyacrylonitrile solution: effect of ultrahigh molecular weight polyacrylonitrile. Colloid and Polymer Science, Vol. 290, 2012, pp. 289–295.10.1007/s00396-011-2546-1Search in Google Scholar
[26] Du, W. F. and H. F. Chen. Study on spinnability of UHMW/PAN dope in DMSO. Materials Science Forum, Vol. 789, 2014, pp. 255–258.10.4028/www.scientific.net/MSF.789.255Search in Google Scholar
[27] Larson, R. G. The structure and rheology of complex fluids. New York: Oxford university press, 1999.Search in Google Scholar
© 2021 Baihua Liu et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Fused deposition modeling of poly(ether ether ketone) scaffolds
- Investigation of the microstructure evolution in TP347HFG austenitic steel at 700°C and its characterization method
- Hot deformation behavior and processing maps of 9Cr3W3Co oxide dispersion-strengthened steel
- Evolution of physicochemical properties of quick lime at converter-smelting temperature
- Influence of phase distribution of converter slag microzones on the occurrence of P
- Investigation on ultrasonic assisted friction stir welding of aluminum/steel dissimilar alloys
- Analysis of oxide scale thickness and pores position of HCM12A steel in supercritical water
- Behavior of MnS inclusions during homogenization process in low-alloyed steel FAS3420H
- Preparation and cutting performance of nano-scaled Al2O3-coated micro-textured cutting tool prepared by atomic layer deposition
- Prediction of hot metal temperature based on data mining
- Effect of TiO2 content in slag on Ti content in molten steel
- Performance evaluation of titanium-based metal nitride coatings and die lifetime prediction in a cold extrusion process
- Effect of different drilling techniques on high-cycle fatigue behavior of nickel-based single-crystal superalloy with film cooling hole
- Effect of CO2 injection into blast furnace tuyeres on the pulverized coal combustion
- Microstructure and properties of Co–Al porous intermetallics fabricated by thermal explosion reaction
- Evolution regularity of temperature field of active heat insulation roadway considering thermal insulation spraying and grouting: A case study of Zhujidong Coal Mine, China
- Evolution of reduction process from tungsten oxide to ultrafine tungsten powder via hydrogen
- A thermodynamic assessment of precipitation, growth, and control of MnS inclusion in U75V heavy rail steel
- Effect of basicity on the reduction swelling properties of iron ore briquettes
- Effect of Cr and Al alloying on the oxidation resistance of a Ti5Si3-incorporated MoSiBTiC alloy
- Microstructure and mechanical properties of 2060 Al–Li alloy welded by alternating current cold metal transfer with high-frequency pulse current
- Effects of composition and strain rate on hot ductility of Cr–Mo-alloy steel in the two-phase region
- Effect of K and Na on reduction swelling performance of oxidized roasted briquettes
- Dephosphorization mechanism and phase change in the reduction of converter slag
- Parametric investigation and optimization for CO2 laser cladding of AlFeCoCrNiCu powder on AISI 316
- Optimization of heat transfer and pressure drop of the channel flow with baffle
- Quantitative analysis of microstructure and mechanical properties of Nb–V microalloyed high-strength seismic reinforcement with different Nb additions
- Visualization of the damage evolution for Ti–3Al–2Mo–2Zr alloy during a uniaxial tensile process using a microvoids proliferation damage model
- Research on high-temperature mechanical properties of wellhead and downhole tool steel in offshore multi-round thermal recovery
- Dephosphorization behavior of reduced iron and the properties of high-P-containing slag
- Jet characteristics of CO2–O2 mixed injection using a dual-parameter oxygen lance nozzle for different smelting periods
- Effects of ball milling on powder particle boundaries and properties of ODS copper
- Heat transfer behavior in ultrahigh-speed continuous casting mold
- Solidification microstructure characteristics of Cu–Pb alloy by ECP treatment
- Luminescence properties of Eu2+ and Sm3+ co-doped in KBaPO4
- Research on high-temperature oxidation resistance, hot forming ability, and microstructure of Al–Si–Cu coating for 22MnB5 steel
- The differential analysis for temperature distribution diagnostics of arc current-carrying region in sheet slanting tungsten electrode inert gas welding with the electrostatic probe
- Reactions at the molten flux-weld pool interface in submerged arc welding
- The effect of liquid crystalline graphene oxide compared with non-liquid crystalline graphene oxide on the rheological properties of polyacrylonitrile solution
- Study on manganese volatilization behavior of Fe–Mn–C–Al twinning-induced plasticity steel
- Physical modeling of bubble behaviors in molten steel under high pressure
- Rapid Communication
- The new concept of thermal barrier coatings with Pt + Pd/Zr/Hf-modified aluminide bond coat and ceramic layer formed by PS-PVD method
- Topical Issue on Science and Technology of Solar Energy
- Solution growth of chalcopyrite Cu(In1−xGax)Se2 single crystals for high open-circuit voltage photovoltaic device
- Copper-based kesterite thin films for photoelectrochemical water splitting
Articles in the same Issue
- Research Articles
- Fused deposition modeling of poly(ether ether ketone) scaffolds
- Investigation of the microstructure evolution in TP347HFG austenitic steel at 700°C and its characterization method
- Hot deformation behavior and processing maps of 9Cr3W3Co oxide dispersion-strengthened steel
- Evolution of physicochemical properties of quick lime at converter-smelting temperature
- Influence of phase distribution of converter slag microzones on the occurrence of P
- Investigation on ultrasonic assisted friction stir welding of aluminum/steel dissimilar alloys
- Analysis of oxide scale thickness and pores position of HCM12A steel in supercritical water
- Behavior of MnS inclusions during homogenization process in low-alloyed steel FAS3420H
- Preparation and cutting performance of nano-scaled Al2O3-coated micro-textured cutting tool prepared by atomic layer deposition
- Prediction of hot metal temperature based on data mining
- Effect of TiO2 content in slag on Ti content in molten steel
- Performance evaluation of titanium-based metal nitride coatings and die lifetime prediction in a cold extrusion process
- Effect of different drilling techniques on high-cycle fatigue behavior of nickel-based single-crystal superalloy with film cooling hole
- Effect of CO2 injection into blast furnace tuyeres on the pulverized coal combustion
- Microstructure and properties of Co–Al porous intermetallics fabricated by thermal explosion reaction
- Evolution regularity of temperature field of active heat insulation roadway considering thermal insulation spraying and grouting: A case study of Zhujidong Coal Mine, China
- Evolution of reduction process from tungsten oxide to ultrafine tungsten powder via hydrogen
- A thermodynamic assessment of precipitation, growth, and control of MnS inclusion in U75V heavy rail steel
- Effect of basicity on the reduction swelling properties of iron ore briquettes
- Effect of Cr and Al alloying on the oxidation resistance of a Ti5Si3-incorporated MoSiBTiC alloy
- Microstructure and mechanical properties of 2060 Al–Li alloy welded by alternating current cold metal transfer with high-frequency pulse current
- Effects of composition and strain rate on hot ductility of Cr–Mo-alloy steel in the two-phase region
- Effect of K and Na on reduction swelling performance of oxidized roasted briquettes
- Dephosphorization mechanism and phase change in the reduction of converter slag
- Parametric investigation and optimization for CO2 laser cladding of AlFeCoCrNiCu powder on AISI 316
- Optimization of heat transfer and pressure drop of the channel flow with baffle
- Quantitative analysis of microstructure and mechanical properties of Nb–V microalloyed high-strength seismic reinforcement with different Nb additions
- Visualization of the damage evolution for Ti–3Al–2Mo–2Zr alloy during a uniaxial tensile process using a microvoids proliferation damage model
- Research on high-temperature mechanical properties of wellhead and downhole tool steel in offshore multi-round thermal recovery
- Dephosphorization behavior of reduced iron and the properties of high-P-containing slag
- Jet characteristics of CO2–O2 mixed injection using a dual-parameter oxygen lance nozzle for different smelting periods
- Effects of ball milling on powder particle boundaries and properties of ODS copper
- Heat transfer behavior in ultrahigh-speed continuous casting mold
- Solidification microstructure characteristics of Cu–Pb alloy by ECP treatment
- Luminescence properties of Eu2+ and Sm3+ co-doped in KBaPO4
- Research on high-temperature oxidation resistance, hot forming ability, and microstructure of Al–Si–Cu coating for 22MnB5 steel
- The differential analysis for temperature distribution diagnostics of arc current-carrying region in sheet slanting tungsten electrode inert gas welding with the electrostatic probe
- Reactions at the molten flux-weld pool interface in submerged arc welding
- The effect of liquid crystalline graphene oxide compared with non-liquid crystalline graphene oxide on the rheological properties of polyacrylonitrile solution
- Study on manganese volatilization behavior of Fe–Mn–C–Al twinning-induced plasticity steel
- Physical modeling of bubble behaviors in molten steel under high pressure
- Rapid Communication
- The new concept of thermal barrier coatings with Pt + Pd/Zr/Hf-modified aluminide bond coat and ceramic layer formed by PS-PVD method
- Topical Issue on Science and Technology of Solar Energy
- Solution growth of chalcopyrite Cu(In1−xGax)Se2 single crystals for high open-circuit voltage photovoltaic device
- Copper-based kesterite thin films for photoelectrochemical water splitting

