Abstract
The influence mechanism of basicity on the reduction swelling index (RSI) of iron ore briquettes was investigated using the SEM analysis and Factsage 7.3 thermodynamic calculations based on the addition of pure CaO to Bayan Obo iron concentrate. The results revealed that the solid solution of Ca2+ in the FeO lattice increased with the basicity of the briquettes, whereas the diffusion channels of Fe2+ ions increased during the reduction process from FeO to Fe and resulted in the formation of a great number of slender and anisotropic iron whiskers, which consequently increased the RSI. Furthermore, the melting point of the slag phase decreased as the CaO content increased; this reduced its ability to resist the reduction swelling of iron oxides. When the basicity was increased from 0.3 to 0.8, the RSI reached a maximum of 69.85%. However, due to the saturated solid solution of Ca2+ in FeO lattice, as the basicity further increased from 0.8 to 1.2, excess CaO melting into the slag phase promoted the precipitation of spinel minerals with high melting points and difficult reduction properties. Thus, the diffusion of Fe2+ and the growth of the iron whiskers were hindered, and the RSI was reduced.
1 Introduction
Iron ore pellets are the key burden materials for blast furnace iron making due to their high grade and strength, uniform particle size, and convenient transportation and storage. Coupled with sinter, they are the main components of blast furnace charge structures [1,2]. The appropriate combination of pellets and sinter not only improves the permeability of the blast furnace charge column and promotes stable blast furnace operation but also ensures the appropriate use of different types of iron ore concentrate and optimizes the burden structure to reduce the iron-making cost in blast furnaces [3,4]. However, compared to high-basicity sinter, acid iron ore pellets possess high reduction swelling indices (RSIs). In practice, the RSI exceeding 20% causes various problems such as permeability in the blast furnace, column decline, and abnormal distribution of the gas flow [5,6]. The reduction expansion of pellets refers to a phenomenon in which a series of physical and chemical reactions occur when pellets come into contact with reducing gas after entering the blast furnace, which causes the pellets to become larger in volume. The main factors affecting the reduction swelling rate of pellets are CaO content, SiO2 content, reduction temperature, and atmosphere. The main theories that lead to reduction and swelling of pellets are (1) gas pressure theory, (2) carbon deposition swelling theory, (3) crystal change theory of iron oxide, and (4) iron whisker theory. Thus, domestic and foreign scholars have conducted numerous studies on the RSI of iron ore pellets. Li et al. [7] investigated the effect of alkali metals on the reduction swelling properties of acid pellets, arguing that alkali metals react with gangue minerals and iron oxides to form a slag phase that deteriorates the RSI of iron ore pellets. Mohanty et al. [8] studied the effect of the roasting temperature on the reduction swelling properties of iron ore pellets and suggested that the roasting time and temperature affect the porosity of iron ore pellets in such a way that a larger porosity causes a more severe RSI. Dwarapudi et al. [9,10] investigated the effect of basicity and MgO on the microstructure and RSI of hematite pellets. They concluded that the RSI of iron ore pellets is relatively high when the basicity is 0.6 and that the increase in the MgO content can lead to the formation of a high-melting-point slag phase and suppress the RSI. Coetsee et al. [11] and Wang et al. [12] studied the effect of reducing atmosphere and gangue composition on the RSI of iron ore pellets and suggested that an increase in the H2 content in the reducing atmosphere or an increase in the MgO and SiO2 content in the iron ore pellets assists in decreasing the RSI.
Previous studies have illustrated that the RSI can be decreased by increasing the basicity of iron ore pellets to an appropriate level. Nevertheless, few in-depth studies have focused on the influence mechanism of basicity on RSI, and thus, the changes in the occurrence state of CaO in iron oxides during the reduction process as well as the effect of CaO on the growth of iron whiskers and the mineralogical composition of iron ore pellets need to be systematically investigated. Therefore, this study investigates the effect of basicity on the RSI of iron ore briquettes using Bayan Obo iron concentrate. This not only provides a theoretical basis for improving the reduction swelling properties of iron ore pellets but also provides guidance regarding the production practices of fluxed pellets of Bayan Obo iron ore concentrate, which is of great significance for the efficient and appropriate exploitation and use of special mineral resources in China.
2 Experimental materials and research program
2.1 Experimental materials
The raw materials used in the experiment were Bayan Obo iron concentrate and pure CaO. By varying the ratios of pure CaO to Bayan Obo iron concentrate, the basicity of the briquettes was changed.
The chemical composition of Bayan Obo iron concentrate is presented in Table 1, where the TFe, CaO, and SiO2 contents are 63.00, 1.58, and 5.28%, respectively. Due to the presence of K, Na, and F, briquettes composed of Bayan Obo iron concentrate often possess a high RSI, which may be detrimental to steel quality and have severe effects on iron making in blast furnaces. This is a problem that has been plaguing the development of iron ore briquette production.
Chemical composition of Bayan Obo iron concentrate (wt%)
| Chemical composition | TFe | FeO | CaO | SiO2 | MgO | F | K2O | Na2O | Al2O3 | S |
|---|---|---|---|---|---|---|---|---|---|---|
| Content | 63.00 | 27.00 | 1.58 | 5.28 | 0.83 | 0.52 | 0.14 | 0.23 | 0.50 | 0.8 |
2.2 Research program
Aiming at reducing the RSI of Bayan Obo iron ore concentrate briquettes, experiments were planned to test the RSI of iron ore briquettes with different basicity values before and after reduction and to detect changes in the structure and composition. To investigate the effect of basicity on the RSI of iron ore briquettes, a combination of X-ray diffraction (XRD) and scanning electron microscopy (SEM) was used. The experimental plan is presented in Table 2.
The addition of CaO to briquettes with different basicity
| Briquettes experimental scheme no. | Basicity (R) | CaO | Bayan Obo iron concentrate |
|---|---|---|---|
| Ratio (wt%) | Ratio (wt%) | ||
| #1 | 0.3 | 0 | 100.00 |
| #2 | 0.4 | 0.54 | 99.46 |
| #3 | 0.6 | 1.60 | 98.40 |
| #4 | 0.8 | 2.64 | 97.37 |
| #5 | 1.0 | 3.70 | 96.36 |
| #6 | 1.2 | 4.73 | 95.37 |
Six briquette specimens with basicity of 0.3, 0.4, 0.6, 0.8, 1.0, and 1.2 were prepared by adding pure CaO to Bayan Obo iron ore concentrate, with the increasing CaO content from 0 to 4.37%, respectively.
3 Experimental procedure and methods
The experimental process included the preparation, reduction, and testing of iron ore briquettes. The morphology and the microstructure of the iron ore briquettes before and after reduction were analyzed using XRD and SEM.
3.1 Preparation of iron ore briquettes
The Bayan Obo iron ore concentrate with a particle size of 0.074 mm or less was dried at 100 ± 10°C for 3 h. The pure CaO was also dried for use. The basicity of the iron ore briquettes was adjusted according to the experimental program. The reaction sample mixture was prepared according to Table 2 in a mixer for 5 h, followed by a 2 h drying cycle. A manual briquetting machine was used to prepare the briquettes (4 g each) via the application of 15 ± 1 MPa of pressure for 2 min. The briquette is pressed into a cylindrical shape with a diameter of 20 mm and a height of 10 mm. The resulting briquettes were placed in a muffle furnace with a temperature ramp protocol of 10°C/min until 200°C, followed by a 30 min hold. Next, the temperature was increased at a rate of 8°C/min with a 30 min hold at 900°C. Finally, the temperature was increased to 1,250°C with a 30 min temperature hold to complete the roasting process. This followed by cooling along with the furnace after roasting in an oxidizing atmosphere. After cooling, the briquettes that exhibited no cracks or physical defects were selected for the reduction experiments. The experimental process of preparation, roasting, and reduction of briquettes is shown in Figure 1.

Experimental conditions diagram.

Schematic diagram of experimental devices for the reduction swelling experiment (1, gas cylinder; 2, flow meter; 3, blender; 4, reduction furnace; 5, specimens; 6, thermocouple; 7, gas inlet; 8, gas outlet; 9, reduction tube; 10, experimental vessels).
3.2 The reduction process of iron ore briquettes
The reduction equipment comprised a reduction tube, reduction furnace, and specimen vessel. The experiment devices are shown in Figure 2.
Reduction procedure
The briquette specimens were placed in a vessel, the vessel was placed in a reduction tube, and the reduction tube was placed in an electric furnace for roasting. When the temperature exceeded 100°C, inert gas (N2) was injected. The standard state flow rate was 5 L/min, and the heating rate was <10°C/min. When the temperature in the center of the reduction tube reached 900°C, the nitrogen flow rate was increased to 15 L/min, and the temperature was maintained at 900°C for 30 min under isothermal conditions of 900 ± 10°C. Next, and after 30 min of heat insulation, CO gas with a standard state flow rate of 15 L/min was injected to replace nitrogen for a reduction period of 1 h. The reduction gas was cut off after the 1 h reduction, and inert gas (N2) was injected into the reduction tube at a flow rate of 5 L/min while the specimen cooled along with the furnace to a final temperature below 100°C. The impurity composition in the reducing gas used in the experiment was H2 ≤ 0.2%, O2 ≤ 0.1%, and H2O ≤ 0.2%. The standard flow rate of the reducing gas was maintained within a range of 15 ± 1 L/min throughout the experiments. The RSI was calculated according to the following equation:
where V 0 and V 1 are the volumes of the briquette specimens before and after reduction (mL), respectively.
3.3 Test method for the RSI of iron ore briquettes
The immersion method was employed to test the briquette volumes before and after reduction according to the Chinese standard GB/T13240-91. The test equipment included an absorber, a thermometer, a beaker holder, a large beaker, a balance, a wire basket, and a fishing line. Ion exchange water was placed in a beaker. According to the testing procedure, a thermometer was used to measure the water temperature before the test to check the water density at the corresponding temperature, and the reading was recorded with an accuracy of four decimal places. The wire basket was dipped into the water and then oscillated up and down to eliminate air bubbles. The briquette specimens were placed in the water for more than 20 min. Then, a pipette was used to remove the bubbles adhering to the specimens, and the beaker was fixed to ensure the accuracy of the experimental readings. The balance reading was recorded as m 1. To reduce the effect of impurities in the water on the experimental results, ion exchange water was used in this experiment. Moreover, the water was renewed after testing each specimen. The briquette specimens were gently removed from the wire basket, and the absorber was used to absorb the residual water on the specimen surface. Each briquette specimen was immediately weighed, and the weighing result was recorded as m 2. The weighing paper was replaced after each weighing. The volume of each briquette specimen was calculated using the following equation [13]:
Here,
4 Results and discussion
4.1 Effect of basicity on the cold compressive strength of iron ore briquettes
The effect of basicity on the cold compressive strength of briquettes is shown in Figure 3. It is shown in Figure 3 that in the range of basicity from 0.2 to 0.8, as the basicity increases, the cold compressive strength of the briquettes gradually increases and reaches the maximum when the basicity is 0.8. This is mainly due to the increase in basicity, which increases the liquid content and promotes the strengthening of the briquettes. However, with the further increase of basicity, a more liquid phase is produced in the briquettes, which destroys the stability of the overall structure of hematite. Due to the high strength of hematite, the internal stress caused by the shrinkage of the center of the briquettes during the cooling process is larger, resulting in a lot of micro cracks, which reduces the cold compressive strength of the briquettes.

Cold compressive strength variation of the iron ore briquettes with different basicity values.
4.2 Effect of basicity on the RSI of iron ore briquettes
The relationship between the RSI and basicity of the iron ore briquettes is shown in Figure 4. When the basicity is 0.3, the RSI is greater than 20%. This is mainly due to the particularity of the Bayan Obo iron ore concentrate. The Bayan Obo iron ore concentrate is a special symbiotic ore containing K, Na, and F at the same time, and the presence of alkali metals will cause abnormal swelling of briquettes.

RSI variation of the iron ore briquettes with different basicity values.
As the basicity increased from 0.3 to 1.2, the RSI of the iron ore briquettes first increased and then decreased. The maximum RSI of 69.85% was attained when the basicity was 0.8, indicating catastrophic swelling that would significantly affect blast furnace iron making. However, when the basicity of the iron ore briquettes is >0.8, CaO addition may play a role in suppressing the RSI.
4.3 Effect of basicity on the macrostructure of iron ore briquettes after reduction
The RSI of iron ore briquettes is related to gangue composition and the ability of the slag phase to withstand reduction stress [14,15]. A high-melting-point slag phase is not easy to soften and is able to maintain high strength during the reduction process, which can effectively suppress reduction swelling. In contrast, a low-melting-point slag phase may be detrimental to the RSI [16,17,18].
The macrostructure of the iron ore briquettes after reduction is shown in Figure 5. When the basicity was 0.3, the specimen exhibited a number of evenly distributed minor cracks, and the briquette strength after reduction was relatively high. As the basicity increased, the cracks gradually expanded in volume and quantity, indicating an increasing RSI. The reduction swelling was the most severe when the basicity was 0.8, and the specimen surface appeared sparse and porous and the specimen had extremely low strength, indicating catastrophic reduction swelling. When the basicity was further increased, the cracks on the specimen surface diminished along with a reduced degree of cracking. When the basicity was 1.2, only minor cracks were present on the specimen surface, the specimen strength increased, and the RSI was suppressed. The diameter of the reduced briquettes is about 20 mm, and the volume of briquettes changes due to different basicity values, which leads to the change in the briquettes RSI.

Images of briquette specimens with different basicity values reduced at 900°C for 1 h. (a)–(f) Briquette specimens #1 (0.2), #2 (0.4), #3 (0.6), #4 (0.8), #5 (1.0), and #6 (1.2).
4.4 Effect of basicity on the mineralogical composition and structure of iron ore briquettes
The briquette specimens were coarsely ground, finely ground, and then polished, and their mineralogical structures were observed using an ore microscope. According to the principle of different gray image levels, the mineral composition of briquettes with different basicity was tested using a mineral phase microscope. The mineralogical composition and the structure of the briquettes are presented in Table 3 and Figure 6, respectively.
Mineralogical composition of roasted briquette specimens (Vol%)
| Basicity (R) | Hematite | Magnetite | Silicate slag phase | Porosity factor |
|---|---|---|---|---|
| 0.3 | 40 | Little | 15 | 45 |
| 0.4 | 48 | Little | 12 | 40 |
| 0.6 | 41 | Little | 17 | 42 |
| 0.8 | 46 | Little | 17 | 37 |
| 1.0 | 44 | Little | 18 | 38 |
| 1.2 | 53 | Little | 22 | 25 |
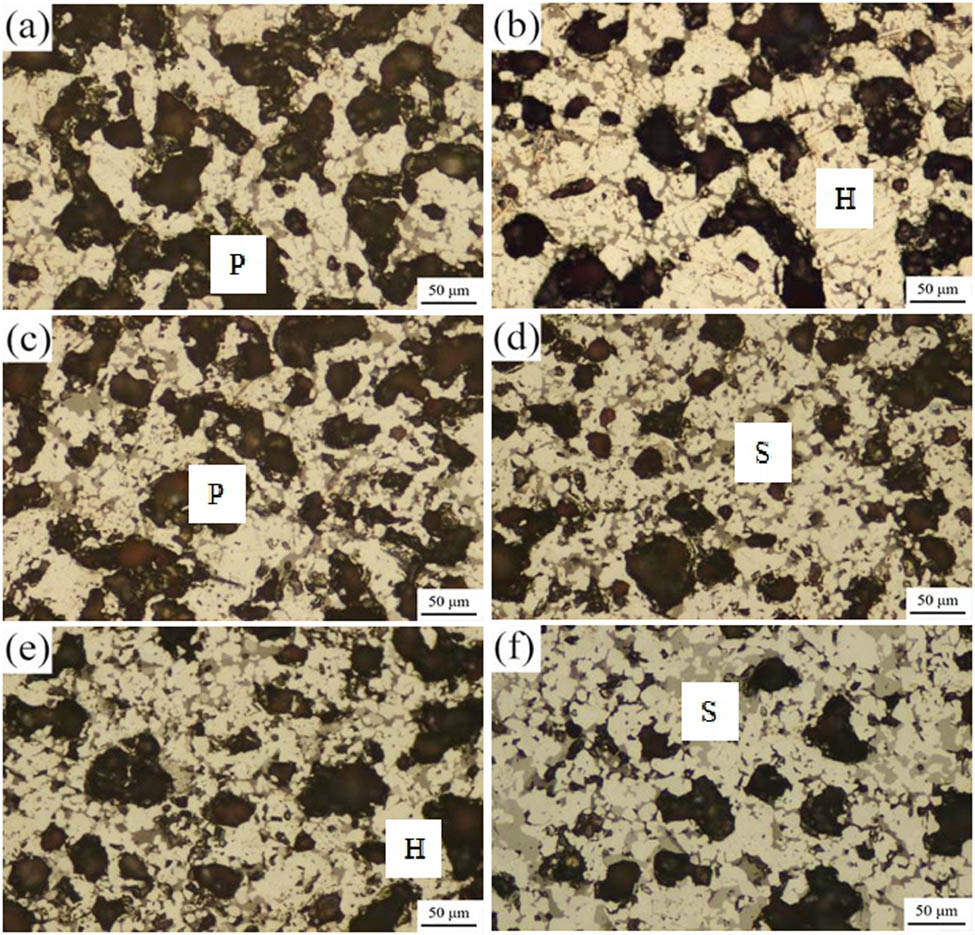
Mineralogical microstructure photographs of roasted briquette specimens at different basicity values (H, hematite phase; S, silicate slag phase; P, porosity). (a)–(f) Briquette specimens #1 (0.2), #2 (0.4), #3 (0.6), #4 (0.8), #5 (1.0), and #6 (1.2).
The mineralogical structure of the briquette specimens mainly comprised hematite and silicate slag phases (including a low-melting-point silicate slag phase and high-melting-point spinel group of minerals dispersed in the slag phase), with the sparse magnetite phase. As the CaO content increased, the silicate slag phase increased and the hematite crystalline growth was suppressed. In addition, the particles were dispersed and the number of pores decreased. According to the theory regarding the reduction swelling of briquettes, the RSI of iron ore briquettes is positively correlated with the reduction degree: the greater the reduction degree, the greater the RSI. As the porosity decreased, the contact area of the gas–solid phase reaction and the reduction rate decreased, inhibiting the RSI of the briquettes. However, the RSI of the briquettes is also related to other factors, such as the crystalline swelling of iron oxides in the reduction process and the ability of the silicate slag phase to suppress the RSI, with the latter being an interactional effect of multiple factors that requires specific analysis.
4.5 Effect of basicity on the micromorphology of iron ore briquettes after reduction
The micromorphology of the iron ore briquettes after reduction at different basicity values was observed using SEM (Figure 7).

SEM-EDS images of briquettes with different basicity values after reduction. (a)–(f) Briquette specimens #1 (0.2), #2 (0.4), #3 (0.6), #4 (0.8), #5 (1.0), and #6 (1.2).
As shown in Figure 7, when the basicity was 0.3, the iron crystal of the reduced specimen comprised large particles with a small number of minor iron whiskers. Moreover, the specimen strength after reduction was high and the RSI was 23.77%, a relatively low value. Owing to the low basicity, low CaO content, and small low-melting-point slag phase content of the roasted iron ore briquettes, the briquette strength was mainly derived from the coarse hematite crystalline growth [19,20,21], which demonstrates poor reducibility, a slow reduction rate, and low RSI. As the basicity of the iron ore briquettes increased, the granular iron crystals and iron whiskers intertwined to form an iron base in the reduced specimens. Simultaneously, the number of iron whiskers increased, the morphology coarsened, and the iron base structure turned sparse. Consequently, the RSI was increased. When the basicity increases to 0.8, the iron base of the reduced specimens almost entirely appeared as interwoven fibrous iron whiskers in a sparse structure, and the RSI reached 69.85%, which was mainly due to the increase in the basicity and the CaO content. The solid solution of Ca2+ in the FeO lattice increased during the reduction process and the FeO lattice distortion deteriorated [22,23], resulting in a number of Fe2+ diffusion channels and accelerating the growth of iron whiskers. When the basicity was 0.8, the solid solution of Ca2+ in the FeO lattice became saturated [24], and the RSI of the iron ore briquettes reached its maximum. As basicity was further increased, the excess CaO melted into the slag phase, promoting the precipitation of a high-melting-point and difficult-to-reduce spinel group minerals containing Mg, Al, and Fe from the slag phase (Table 6). These high-melting point minerals diffused in the slag phase, which increased the melting point of the slag phase and inhibited the growth and development of iron whiskers [25,26]. Moreover, in the briquette specimens with basicity of 1.0 and 1.2, the reduced iron base mainly comprised granular iron crystals with only a small amount of minor iron whiskers, and the RSI significantly decreased. When the basicity was 1.2, the RSI decreased to a minimum, and the reduced iron base was relatively intact. In addition, the specimen maintained a high strength, and the RSI was suppressed. Therefore, when the basicity was >0.8, the formation of high-melting-point spinel-group minerals increased, and the RSI of the iron ore briquettes significantly decreased. Thus, the basicity of Bayan Obo iron ore concentrate fluxed briquettes should be maintained above 1.0.
The effect of basicity on the solid solution of Ca2+ in the FeO lattice was also calculated. According to the binary phase diagram of FeO n –CaO, the saturated solid solubility of CaO in FeO at 900°C was 5.5% [27]. The chemical composition of the iron ore briquettes at the reduction stage from iron oxide to FeO can be deduced from the components of the iron ore briquette and the Fe equilibrium during the reduction process (Table 4).
Chemical composition of iron ore briquettes reduced to FeO calculated on the basis of Fe equilibrium (wt%)
| Briquette experimental scheme no. | TFe | FeO | CaO | SiO2 | MgO | F | K2O | Na2O | Al2O3 | P | R | CaOsolution |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | 63.00 | 81.00 | 2.61 | 8.74 | 1.37 | 0.86 | 0.23 | 0.38 | 0.83 | 0.13 | 0.3 | 2.11 |
| #2 | 62.66 | 80.56 | 3.42 | 8.50 | 1.33 | 0.84 | 0.23 | 0.37 | 0.81 | 0.13 | 0.4 | 2.76 |
| #3 | 61.99 | 79.70 | 4.90 | 8.10 | 1.26 | 0.79 | 0.22 | 0.36 | 0.76 | 0.12 | 0.6 | 3.91 |
| #4 | 61.34 | 78.86 | 6.30 | 7.85 | 1.20 | 0.77 | 0.21 | 0.33 | 0.74 | 0.12 | 0.8 | 4.97 |
| #5 | 60.71 | 78.06 | 7.63 | 7.44 | 1.17 | 0.73 | 0.19 | 0.32 | 0.70 | 0.12 | 1.0 | 5.96 |
| #6 | 60.08 | 77.25 | 8.80 | 7.29 | 1.12 | 0.71 | 0.14 | 0.31 | 0.68 | 0.11 | 1.2 | 6.80 |
During the stage in which the iron oxide reduces to FeO, assuming that the CaO in the iron ore briquettes has an equal probability of contacting each component for mineralization, the content of CaO that contacts and dissolves into FeO can be calculated based on the FeO content in the iron ore briquette. That is, it is equal to the content of FeO in the iron ore briquette multiplied by the total content of CaO. For example, in briquette specimen #1, the solid solution of CaO in FeO was CaOsolution = 81.00% × 2.61% × 100 = 2.11%. The content of the CaO dissolved in other specimens can be similarly calculated. Table 4 presents that the solid solution of CaO in FeO increases with the basicity of the iron ore briquettes. When the basicity of the iron ore briquettes was 0.8, and the solid solubility was 4.97%, which is close to the saturated solid solubility of 5.5%. When the basicity increased to 1.0, the solid solubility was 5.96%, which is >5.5% and represents a supersaturated state. Therefore, when the basicity exceeded 0.8, the excess CaO melted into the slag phase, promoting the precipitation of high-melting-point and difficult-to-reduce spinel-group minerals containing Mg, Al, and Fe from the slag (Table 6). Indeed, the calculation of solid solubility is based on the assumption that the CaO in the iron ore briquettes has an equal probability of contacting each component for mineralization, the accuracy of which is subject to the further analytical demonstration but is of practical significance for understanding the effect of basicity on the solid solution of Ca2+ in the FeO lattice.
The composition and the content of the slag phase and the equilibrium mineralogical composition in the iron ore briquettes with different basicity values were calculated at the roasting temperature of 1,250°C using the Ftoxid database of the Equilib module in the Factsage 7.3 thermodynamic software. The results are presented in Tables 5 and 6. During the calculation, the atmosphere settings included a partial oxygen pressure of 21 kPa and a partial nitrogen pressure of 79 kPa.
Equilibrium slag phase composition and content of briquettes with different basicity and CaO content (1,250°C)
| Basicity (R) | Slag phase content (wt%) | Slag phase composition (wt%) | Slag phase basicity (R) | Slag phase melting point (°C) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al2O3 | SiO2 | CaO | FeO | Fe2O3 | MgO | K2O + Na2O | M x F | Sum | ||||
| 0.3 | 12.16 | 0.86 | 40.84 | 12.24 | 2.60 | 16.96 | 6.39 | 5.97 | 14.14 | 100 | 0.30 | 910 |
| 0.4 | 12.76 | 1.00 | 38.86 | 15.65 | 2.36 | 16.97 | 6.11 | 5.49 | 13.55 | 100 | 0.40 | 910 |
| 0.6 | 14.04 | 1.09 | 35.22 | 21.37 | 1.97 | 17.48 | 5.53 | 4.93 | 12.40 | 100 | 0.61 | 882 |
| 0.8 | 15.48 | 1.10 | 31.82 | 25.88 | 1.71 | 18.80 | 5.00 | 4.33 | 11.36 | 100 | 0.81 | 830 |
| 1.0 | 17.01 | 1.01 | 28.83 | 29.60 | 1.55 | 21.12 | 3.60 | 3.90 | 10.39 | 100 | 1.03 | 833 |
| 1.2 | 18.70 | 0.92 | 26.09 | 32.31 | 1.46 | 23.63 | 2.59 | 3.48 | 9.52 | 100 | 1.24 | 831 |
Note: M x F stands for fluoride, which mainly refers to the general term including MgF2, FeF2, FeF3, AlF3, SiF4, CaF2, and other fluorides.
Equilibrium mineralogical composition of briquettes with different basicity and CaO content (wt%; 1,250°C)
| Basicity (R) | Slag phase | Spinel minerals containing elements of Mg, Al, and TFe | Hematite | Sum |
|---|---|---|---|---|
| 0.3 | 12.16 | 0.00 | 87.84 | 100 |
| 0.4 | 12.76 | 0.00 | 87.24 | 100 |
| 0.6 | 14.04 | 0.00 | 85.96 | 100 |
| 0.8 | 15.48 | 0.00 | 84.52 | 100 |
| 1.0 | 17.01 | 1.66 | 81.33 | 100 |
| 1.2 | 18.70 | 2.94 | 78.36 | 100 |
The thermodynamic calculations show that when the basicity of the briquettes increased from 0.3 to 0.8, the production of the slag phase in the iron ore briquettes continued to increase. The slag phase was mainly composed of SiO2, CaO, Fe2O3, and M x F. When the CaO content increased, the SiO2 content decreased, or the CaO and Fe2O3 contents increased. Simultaneously, the melting point of the slag phase decreased, and the flowability was reinforced. On the one hand, the slag phase was easy to fill with hematite particles during the high-temperature roasting process, which implies that the contact conditions for iron ore particles deteriorated, hindering and sabotaging the crystalline consolidation among grains and reducing the crystalline consolidation strength of the iron ore briquettes [28,29]. On the other hand, since the contraction coefficient of the slag phase differed from that of the hematite grains during the condensation process of iron ore briquettes, the volumetric shrinkage of the cooling slag phase was intense and could easily cause consolidated crystal crushing and the formation of pores. As a result, the porosity of the iron ore briquettes increased, accelerating the reduction reaction rate and increasing the reduction degree and RSI, consequently affecting the reduction swelling properties.
Table 5 presents that the melting point of the slag phase first decreased and then increased with the increasing basicity. When the basicity was 0.8, the melting point of the slag phase reached a minimum at 830°C, which played a significant role in promoting reduction swelling and lead to a maximum RSI at 69.85%. The melting point of the slag phase slightly increases as the basicity further increased. In other words, the effect of the low-melting-point slag phase in promoting reduction swelling no longer increased with the basicity. When the basicity was further increased from 0.8 to 1.2, the Al2O3 content in the slag phase decreased and the MgO content significantly decreased (Table 5). Table 6 presents that the high-melting-point and difficult-to-reduce spinel-group minerals precipitated from the iron ore briquettes and diffused in the slag phase. On the one hand, this impeded the hematite reduction process and decreased the reduction degree of the iron ore briquettes. On the other hand, it enhanced the ability of the slag phase to resist reduction swelling and inhibited the growth of iron whiskers, leading to a decrease in the RSI of the iron ore briquettes.
4.6 XRD analysis of iron ore briquettes before and after reduction
When the basicity was 0.8, the RSI of the iron ore briquettes was at a maximum of 69.85%, representing catastrophic swelling. Therefore, the reduction swelling mechanism of iron ore briquettes at this basicity needs to be researched in depth. The briquette specimens were analyzed before and after reduction using XRD.
Magnetite is the main component of Bayan Obo iron concentrate, and the main component of the specimens after oxidizing and during roasting was hematite. As shown in Figure 8, the main component of the reduced specimens was metal Fe, and almost no calcium ferrite or silicate was detected.

XRD spectra of iron ore briquettes before and after reduction. The left-hand image shows the briquette specimens before reduction, and the right-hand image shows the briquette after reduction.
4.7 SEM analysis of iron ore briquettes before and after reduction
The microstructural characteristics of the iron ore briquettes at a basicity of 0.8 were analyzed using SEM before and after reduction.
SEM analysis of iron ore briquettes before reduction
The SEM analysis of the iron ore briquette specimens before the reduction with the distribution of elements is shown in Figure 9.
Figure 9(a), (b), and (e) reveal that Fe and O were widely distributed and overlapped in large areas, mainly in hematite grains. Figure 9(a), (c), and (f) reveal that the distributions of Ca and Si greatly overlapped and were mosaic-distributed with Fe, mainly in the silicate slag phase containing more Ca and less Mg. The iron oxides of the roasted iron ore briquettes were mainly hematite, and the distributions of Fe and Ca barely overlapped. That is, a small quantity of calcium ferrite was present in the roasted iron ore briquettes. This is because the reaction capacity of SiO2 to CaO was higher than that of Fe2O3. The main reaction product was the silicate slag phase containing Ca, and calcium ferrite can be formed only if additional CaO is added.
SEM analysis of iron ore briquettes after reduction

Distribution of the main elements of briquette specimens before reduction at basicity of 0.8. (a) SEM-EDS image of briquette, (b) Fe-KA, (c) Ca-KA, (d) Mg-KA, (e) O-KA, and (f) Si-KA.

Distribution of main elements of briquette specimens after reduction at basicity of 0.8. (a) SEM-EDS image of briquette, (b) Fe-KA, (c) Ca-KA, (d) Mg-KA, (e) O-KA, and (f) Si-KA. (b) Fe-KA, (c) Ca-KA, (d) Mg-KA, (e) O-KA, and (f) Si-KA.
The briquette specimens were analyzed after reduction using SEM to investigate the distribution characteristics of the elements on the surfaces of the specimens, as shown in Figure 10.
Figure 10(a) shows the SEM image of the reduced specimen at a basicity of 0.8. This figure shows that the iron base was dominated by a large number of anisotropic iron whiskers. The distribution characteristics of the elements reveal that the distributions of Fe and iron whiskers were highly consistent. In addition, the Ca and Mg contents were relatively low, and their distributions greatly overlapped with Si and O and were mosaic distributed with Fe. That is, a silicate slag phase containing Ca and Mg mainly formed, and the slag phase content of the silicate was low and diffused around the iron whiskers. A large number of anisotropic iron whiskers were observed to be the main cause of the catastrophic reduction swelling properties of the iron ore briquettes.
5 Conclusions
In this study, different proportions of pure CaO were added to Bayan Obo iron ore concentrate to vary the basicity of iron ore briquettes. A number of related experiments, including the preparation, roasting, testing, and analysis of the briquettes, were conducted to investigate the influence of the RSI of the oxidized briquettes. The main findings are as follows:
As the basicity of the iron ore briquettes increased, the RSI of the briquettes first increased and then decreased. When the basicity was 0.8, the RSI attained a maximum of 69.85%, indicating catastrophic reduction swelling. When the basicity was increased beyond 0.8, the RSI of the iron ore briquettes significantly decreased with the increasing basicity. This indicates that an increase in CaO decreases the reduction swelling properties of iron ore briquettes.
The main reason for the catastrophic swelling of the iron ore briquettes was the formation and anisotropic growth of iron whiskers as a reduction product. As the basicity of the iron ore briquettes increased from 0.3 to 0.8, the amount of solid Ca2+ content dissolved into FeO gradually increased and then reached saturation at a basicity of 0.8. This played a significant role in promoting the lattice distortion of FeO and the growth of iron whiskers. The iron whiskers afforded conspicuous anisotropic growth characteristics. Simultaneously, the melting point of the slag phase decreased with the increasing basicity, and the inhibition effect on the growth of iron whiskers was weakened. This deteriorated the reduction swelling properties in the iron ore briquettes.
Due to the dissolubility of the additional solid Ca2+ in the FeO lattice after saturation, its role in promoting the growth of iron whiskers was no longer strengthened when the basicity increased from 0.8 to 1.2. In contrast, excess CaO melted into the slag phase, promoting the precipitation of high-melting-point and difficult-to-reduce spinel-group minerals containing Mg, Al, and Fe. This enhanced the ability of the slag phase to resist the reduction swelling of iron oxides, suppressed the growth of iron whiskers, and improved the reduction swelling properties of the iron ore briquettes.
Acknowledgments
The authors gratefully acknowledge financial support from the institution of National Nature Science Foundations of China (51364030).
-
Funding information: This work was supported by the National Natural Science Foundation of China (51364030).
-
Author contributions: Guo-Cheng Zhang: analysed the results and wrote the paper; Guo-Ping Luo: assisted in revising the paper; Peng-Fei Jia: supervised the experiments; Yi-Ci Wang: provided help in calculation; Yi-Fan Chai: carried out the experimental work. All authors have read and agreed to the published version of the manuscript.
-
Conflict of interest: No conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication.
-
Data availability statement: All authors can confirm that all data used in this article can be published the Journal “High Temperature Materials and Processes.”
References
[1] Qin, G. L. , K. Wu , H. S. Liu , and J. Q. Tian . Effect and mechanism of calcination temperature on reduction swelling rate of low silica magnesium pellets. The Chinese Journal of Nonferrous Metals, Vol. 25, No. 10, 2015, pp. 2906–2911 (in Chinese).Search in Google Scholar
[2] Luo, G. P. , A. K. Liu , and Y. B. Wang . Effect of MgO on RSI of pellets containing K, Na, and F. Iron & Steel, Vol. 50, No. 6, 2015, pp. 36–39 (in Chinese).Search in Google Scholar
[3] Gao, Q. J. , F. M. Shen , G. Wei , X. Jiang , and H. Y. Zheng . Effects of MgO containing additive on low-temperature metallurgical properties of oxidized pellet. Journal of Iron and Steel Research International, Vol. 20, No. 7, 2013, pp. 25–28.10.1016/S1006-706X(13)60121-1Search in Google Scholar
[4] Cui, Z. X. , X. H. Fan , T. Jiang , Z. X. Kuang , R. H. Chen , and F. H. Shu . A study on the reduction-swelling performance of Brazilian Carajas hematite pellets. Iron & Steel, Vol. 50, No. 6, 2015, pp. 36–41 (in Chinese).Search in Google Scholar
[5] Silva, F. R. , L. R. Lemos , P. de Freitas Nogueira , and M. Bressan . Effect of ternary basicity of iron ore-fluxed pellets on melting and softening properties in a blast furnace. Metallurgical and Materials Transactions, Vol. 52, No. 1, 2021, pp. 69–76.10.1007/s11663-020-01917-6Search in Google Scholar
[6] Dwarapudi, S. and M. Ranjan . Influence of oxide and silicate melt phases on the RDI of iron ore pellets suitable for shaft furnace of direct reduction process. ISIJ International, Vol. 50, No. 11, 2010, pp. 1581–1589.10.2355/isijinternational.50.1581Search in Google Scholar
[7] Li, J. , H. F. An , W. X. Liu , A. M. Yang , and M. S. Chu . Effect of basicity on metallurgical properties of magnesium fluxed pellets. Journal of Iron and Steel Research International, Vol. 27, No. 3, 2020, pp. 239–247.10.1007/s42243-019-00307-wSearch in Google Scholar
[8] Mohanty, M. K. , S. Mishra , B. Mishra , and S. Sarkar . Effect of basicity on the reduction behavior of iron ore pellets. Arabian Journal for Science and Engineering, Vol. 43, No. 11, 2018, pp. 5989–5998.10.1007/s13369-018-3107-4Search in Google Scholar
[9] Dwarapudi, S. , T. K. Ghosh , A. Shankar , V. Tathavadkar , D. Bhattacharjee , and R. Venugopal . Effect of pellet basicity and MgO content on the quality and microstructure of hematite pellets. International Journal of Mineral Processing, Vol. 99, No. 1–4, 2011, pp. 43–53.10.1016/j.minpro.2011.03.004Search in Google Scholar
[10] Dwarapudi, S. , T. K. Ghosh , V. Tathavadkar , M. B. Denys , D. Bhattacharjee , and R. Venugopal . Effect of MgO in the form of magnesite on the quality and microstructure of hematite pellets. International Journal of Mineral Processing, Vol. 112–113, 2012, pp. 55–62.10.1016/j.minpro.2012.06.006Search in Google Scholar
[11] Coetsee, T. , P. C. Pistorius , and E. E. de Villiers . Rate-determining steps for the reduction in magnetite-coal pellets. Minerals Engineering, Vol. 15, No. 11, 2002, pp. 919–929.10.1016/S0892-6875(02)00120-6Search in Google Scholar
[12] Wang, Z. C. , M. S. Chu , Y. Tang , and X. X. Xue . Effects of reducing atmosphere and gangue composition on reduction swelling of oxidized pellets, Changsha. Journal of Northeastern University, Vol. 33, No. 1, 2012, pp. 95–97. (in Chinese).Search in Google Scholar
[13] Qiu, G. Z. , T. Jiang , K. Q. Fa , D. Q. Zhu , and D. Z. Wang . Interfacial characterizations of iron ore concentrates affected by binders. Powder Technology, Vol. 139, No. 1, 2004, pp. 1–6.10.1016/j.powtec.2003.10.001Search in Google Scholar
[14] Li, G. H. , Z. K. Tang , Y. B. Zhang , Z. X. Cui , and T. Jiang . Reduction swelling behaviour of haematite/magnetite agglomerates with addition of MgO and CaO. Ironmaking & Steelmaking, Vol. 37, No. 6, 2010, pp. 393–397.10.1179/030192310X12690127076352Search in Google Scholar
[15] Xu, B. , T. Hou , X. L. Chen , Q. Li , T. Jiang , and P. Li . Effect of dolomite on reduction swelling property of iron ore pellets. Journal of Central South University, Vol. 20, No. 10, 2013, pp. 2806–2810.10.1007/s11771-013-1800-8Search in Google Scholar
[16] Dwarapudi, S. , T. K. Ghosh , A. Shankar , V. Tathavadkar , D. Bhattacharjee , and R. Venugopal . Effect of pyroxenite flux on the quality and microstructure of hematite pellets. International Journal of Mineral Processing, Vol. 96, No. 1, 2010, pp. 45–53.10.1016/j.minpro.2010.06.002Search in Google Scholar
[17] Srinivas, D. , S. Chandra , P. Indrajit , M. Kapil , R. P. Atanu , and C. Ujjal , et al. Effect of fluxing agents on the quality and microstructure of hematite pellets. International Journal of Metallurgical Engineering, Vol. 6, No. 1, 2017, pp. 13–20.Search in Google Scholar
[18] Wang, H. T. and H. Y. Sohn . Effects of reducing gas on swelling and iron whisker formation during the reduction of iron oxide compact. Steel Research International, Vol. 83, No. 9, 2012, pp. 903–909.10.1002/srin.201200054Search in Google Scholar
[19] Xu, R. S. , J. L. Zhang , H. B. Zuo , K. X. Jiao , Z. W. Hu , and X. D. Xing . Mechanisms of swelling of iron ore oxidized pellets in high reduction potential atmosphere. Journal of Iron and Steel Research International, Vol. 22, No. 1, 2015, pp. 1–8.10.1016/S1006-706X(15)60001-2Search in Google Scholar
[20] Tasuku, F. and S. BAN-YA . Swelling of iron ore pellets during reduction. Transactions of the Iron and Steel Institute of Japan, Vol. 9, No. 2, 2020, pp. 137–147.10.2355/isijinternational1966.9.137Search in Google Scholar
[21] Zhang, J. L. , Z. Y. Wang , X. D. Xing , and Z. J. Liu . Effect of aluminum oxide on the compressive strength of pellets. International Journal of Minerals Metallurgy and Materials, Vol. 21, No. 4, 2014, pp. 339–344.10.1007/s12613-014-0914-9Search in Google Scholar
[22] Qing, G. , K. Wu , Y. Tian , G. An , X. Yuan , and D. Xu , et al. Effect of the firing temperature and the added MgO on the reduction swelling index of the pellet with low SiO2 content. Ironmaking & Steelmaking, Vol. 45, No. 1, 2018, pp. 83–89.10.1080/03019233.2016.1242248Search in Google Scholar
[23] Wang, H. T. and H. Y. Sohn . Effect of CaO and SiO2 on swelling and iron whisker formation during the reduction of iron oxide compact. Ironmaking and Steelmaking, Vol. 38, No. 6, 2011, pp. 447–452.10.1179/1743281211Y.0000000022Search in Google Scholar
[24] Hayashi, M. , B. Cai , and M. Susa . Dominant factor affecting reducibility of calcio-wüstite originating from silico-ferrite of calcium and aluminum: fundamentals of high temperature processes. ISIJ International, Vol. 60, No. 12, 2020, pp. 2649–2658.10.2355/isijinternational.ISIJINT-2020-140Search in Google Scholar
[25] Feng, J. X. , Y. Zhang , H. W. Zheng , X. Y. Xie , and C. Zhang . Drying and preheating processes of iron ore pellets in a traveling grate. International Journal of Minerals, Metallurgy, and Materials, Vol. 17, No. 5, 2010, pp. 535–540.10.1007/s12613-010-0354-0Search in Google Scholar
[26] Guo, H. , X. Jiang , F. M. Shen , H. Y. Zheng , Q. J. Gao , and X. Zhang . Influence of SiO2 on the compressive strength and reduction-melting of pellets. Metals, Vol. 9, No. 8, 2019, id. 852.10.3390/met9080852Search in Google Scholar
[27] Wang, Z. C. , M. S. Chu , Z. G. Liu , Z. Y. Chen , and X. X. Xue . Effects of temperature and atmosphere on pellets reduction swelling index. Journal of Iron and Steel Research International, Vol. 19, No. 10, 2012, pp. 7–12.10.1016/S1006-706X(12)60144-7Search in Google Scholar
[28] Han, G. H. , D. Zhang , Y. F. Huang , and T. Jiang . Swelling behavior of hot preheated pellets in reduction roasting process. Journal of Central South University, Vol. 23, No. 11, 2016, pp. 2792–2799.10.1007/s11771-016-3342-3Search in Google Scholar
[29] Umadevi, T. , P. Kumar , N. F. Lobo , M. Prabhu , P. C. Mahapatra , and M. Ranjan . Influence of pellet basicity (CaO/SiO2) on iron ore pellet properties and Microstructure. ISIJ International, Vol. 51, No. 1, 2011, pp. 14–20.10.2355/isijinternational.51.14Search in Google Scholar
© 2021 Guo-Cheng Zhang et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Fused deposition modeling of poly(ether ether ketone) scaffolds
- Investigation of the microstructure evolution in TP347HFG austenitic steel at 700°C and its characterization method
- Hot deformation behavior and processing maps of 9Cr3W3Co oxide dispersion-strengthened steel
- Evolution of physicochemical properties of quick lime at converter-smelting temperature
- Influence of phase distribution of converter slag microzones on the occurrence of P
- Investigation on ultrasonic assisted friction stir welding of aluminum/steel dissimilar alloys
- Analysis of oxide scale thickness and pores position of HCM12A steel in supercritical water
- Behavior of MnS inclusions during homogenization process in low-alloyed steel FAS3420H
- Preparation and cutting performance of nano-scaled Al2O3-coated micro-textured cutting tool prepared by atomic layer deposition
- Prediction of hot metal temperature based on data mining
- Effect of TiO2 content in slag on Ti content in molten steel
- Performance evaluation of titanium-based metal nitride coatings and die lifetime prediction in a cold extrusion process
- Effect of different drilling techniques on high-cycle fatigue behavior of nickel-based single-crystal superalloy with film cooling hole
- Effect of CO2 injection into blast furnace tuyeres on the pulverized coal combustion
- Microstructure and properties of Co–Al porous intermetallics fabricated by thermal explosion reaction
- Evolution regularity of temperature field of active heat insulation roadway considering thermal insulation spraying and grouting: A case study of Zhujidong Coal Mine, China
- Evolution of reduction process from tungsten oxide to ultrafine tungsten powder via hydrogen
- A thermodynamic assessment of precipitation, growth, and control of MnS inclusion in U75V heavy rail steel
- Effect of basicity on the reduction swelling properties of iron ore briquettes
- Effect of Cr and Al alloying on the oxidation resistance of a Ti5Si3-incorporated MoSiBTiC alloy
- Microstructure and mechanical properties of 2060 Al–Li alloy welded by alternating current cold metal transfer with high-frequency pulse current
- Effects of composition and strain rate on hot ductility of Cr–Mo-alloy steel in the two-phase region
- Effect of K and Na on reduction swelling performance of oxidized roasted briquettes
- Dephosphorization mechanism and phase change in the reduction of converter slag
- Parametric investigation and optimization for CO2 laser cladding of AlFeCoCrNiCu powder on AISI 316
- Optimization of heat transfer and pressure drop of the channel flow with baffle
- Quantitative analysis of microstructure and mechanical properties of Nb–V microalloyed high-strength seismic reinforcement with different Nb additions
- Visualization of the damage evolution for Ti–3Al–2Mo–2Zr alloy during a uniaxial tensile process using a microvoids proliferation damage model
- Research on high-temperature mechanical properties of wellhead and downhole tool steel in offshore multi-round thermal recovery
- Dephosphorization behavior of reduced iron and the properties of high-P-containing slag
- Jet characteristics of CO2–O2 mixed injection using a dual-parameter oxygen lance nozzle for different smelting periods
- Effects of ball milling on powder particle boundaries and properties of ODS copper
- Heat transfer behavior in ultrahigh-speed continuous casting mold
- Solidification microstructure characteristics of Cu–Pb alloy by ECP treatment
- Luminescence properties of Eu2+ and Sm3+ co-doped in KBaPO4
- Research on high-temperature oxidation resistance, hot forming ability, and microstructure of Al–Si–Cu coating for 22MnB5 steel
- The differential analysis for temperature distribution diagnostics of arc current-carrying region in sheet slanting tungsten electrode inert gas welding with the electrostatic probe
- Reactions at the molten flux-weld pool interface in submerged arc welding
- The effect of liquid crystalline graphene oxide compared with non-liquid crystalline graphene oxide on the rheological properties of polyacrylonitrile solution
- Study on manganese volatilization behavior of Fe–Mn–C–Al twinning-induced plasticity steel
- Physical modeling of bubble behaviors in molten steel under high pressure
- Rapid Communication
- The new concept of thermal barrier coatings with Pt + Pd/Zr/Hf-modified aluminide bond coat and ceramic layer formed by PS-PVD method
- Topical Issue on Science and Technology of Solar Energy
- Solution growth of chalcopyrite Cu(In1−xGax)Se2 single crystals for high open-circuit voltage photovoltaic device
- Copper-based kesterite thin films for photoelectrochemical water splitting
Articles in the same Issue
- Research Articles
- Fused deposition modeling of poly(ether ether ketone) scaffolds
- Investigation of the microstructure evolution in TP347HFG austenitic steel at 700°C and its characterization method
- Hot deformation behavior and processing maps of 9Cr3W3Co oxide dispersion-strengthened steel
- Evolution of physicochemical properties of quick lime at converter-smelting temperature
- Influence of phase distribution of converter slag microzones on the occurrence of P
- Investigation on ultrasonic assisted friction stir welding of aluminum/steel dissimilar alloys
- Analysis of oxide scale thickness and pores position of HCM12A steel in supercritical water
- Behavior of MnS inclusions during homogenization process in low-alloyed steel FAS3420H
- Preparation and cutting performance of nano-scaled Al2O3-coated micro-textured cutting tool prepared by atomic layer deposition
- Prediction of hot metal temperature based on data mining
- Effect of TiO2 content in slag on Ti content in molten steel
- Performance evaluation of titanium-based metal nitride coatings and die lifetime prediction in a cold extrusion process
- Effect of different drilling techniques on high-cycle fatigue behavior of nickel-based single-crystal superalloy with film cooling hole
- Effect of CO2 injection into blast furnace tuyeres on the pulverized coal combustion
- Microstructure and properties of Co–Al porous intermetallics fabricated by thermal explosion reaction
- Evolution regularity of temperature field of active heat insulation roadway considering thermal insulation spraying and grouting: A case study of Zhujidong Coal Mine, China
- Evolution of reduction process from tungsten oxide to ultrafine tungsten powder via hydrogen
- A thermodynamic assessment of precipitation, growth, and control of MnS inclusion in U75V heavy rail steel
- Effect of basicity on the reduction swelling properties of iron ore briquettes
- Effect of Cr and Al alloying on the oxidation resistance of a Ti5Si3-incorporated MoSiBTiC alloy
- Microstructure and mechanical properties of 2060 Al–Li alloy welded by alternating current cold metal transfer with high-frequency pulse current
- Effects of composition and strain rate on hot ductility of Cr–Mo-alloy steel in the two-phase region
- Effect of K and Na on reduction swelling performance of oxidized roasted briquettes
- Dephosphorization mechanism and phase change in the reduction of converter slag
- Parametric investigation and optimization for CO2 laser cladding of AlFeCoCrNiCu powder on AISI 316
- Optimization of heat transfer and pressure drop of the channel flow with baffle
- Quantitative analysis of microstructure and mechanical properties of Nb–V microalloyed high-strength seismic reinforcement with different Nb additions
- Visualization of the damage evolution for Ti–3Al–2Mo–2Zr alloy during a uniaxial tensile process using a microvoids proliferation damage model
- Research on high-temperature mechanical properties of wellhead and downhole tool steel in offshore multi-round thermal recovery
- Dephosphorization behavior of reduced iron and the properties of high-P-containing slag
- Jet characteristics of CO2–O2 mixed injection using a dual-parameter oxygen lance nozzle for different smelting periods
- Effects of ball milling on powder particle boundaries and properties of ODS copper
- Heat transfer behavior in ultrahigh-speed continuous casting mold
- Solidification microstructure characteristics of Cu–Pb alloy by ECP treatment
- Luminescence properties of Eu2+ and Sm3+ co-doped in KBaPO4
- Research on high-temperature oxidation resistance, hot forming ability, and microstructure of Al–Si–Cu coating for 22MnB5 steel
- The differential analysis for temperature distribution diagnostics of arc current-carrying region in sheet slanting tungsten electrode inert gas welding with the electrostatic probe
- Reactions at the molten flux-weld pool interface in submerged arc welding
- The effect of liquid crystalline graphene oxide compared with non-liquid crystalline graphene oxide on the rheological properties of polyacrylonitrile solution
- Study on manganese volatilization behavior of Fe–Mn–C–Al twinning-induced plasticity steel
- Physical modeling of bubble behaviors in molten steel under high pressure
- Rapid Communication
- The new concept of thermal barrier coatings with Pt + Pd/Zr/Hf-modified aluminide bond coat and ceramic layer formed by PS-PVD method
- Topical Issue on Science and Technology of Solar Energy
- Solution growth of chalcopyrite Cu(In1−xGax)Se2 single crystals for high open-circuit voltage photovoltaic device
- Copper-based kesterite thin films for photoelectrochemical water splitting

