Abstract
KBaPO4:Eu2+/Sm3+ phosphor was synthesized via the high-temperature solid-phase reaction method. The structural properties, surface morphology, and optical properties of the synthesized samples were obtained by the X-ray diffraction (XRD) analysis, scanning electron microscopy (SEM), and fluorescence measurements. The XRD patterns indicate that the crystal structure of KBaPO4 has not been changed by co-doped with two rare-earth ions. Under the excitation of 400 nm, the Eu2+ and Sm3+ co-doped KBaPO4 phosphors showed typical emission peaks at 430 (blue), 562 (yellow), and 600 nm (red). Meanwhile, with the increase of the Sm3+ content, the photoluminescent (PL) emission intensity of Sm3+ increased until the content reaches 0.12. However, the PL intensity of Eu2+ ions gradually decreased, which indicated that there was a possible energy transfer between the two ions. Therefore, the obtained results indicated that Eu2+/Sm3+ co-doped KBaPO4 is a promising phosphor for the use in white light-emitting diodes with near ultraviolet chips.
1 Introduction
Recently, white light-emitting diodes (W-LEDs) become promising light sources because of their properties such as high energy saving, long service life, high material stability, and environmental protection [1,2,3,4]. Currently, the most common W-LEDs are phosphor-converted due to their high luminous efficiency, superior color uniformity, and long-serving time [5,6,7,8]. But, this type of W-LEDs still has drawbacks, such as high color temperature, which are attributed to the scarcity of red emission.
Rare earth (RE) ions have been applied widely in solid-state lightings and displays. RE ions in orthophosphates host emit photons efficiently under the excitation by near ultraviolet (N-UV) light due to their unique electronic structure [9,10,11,12,13]. Therefore, the RE ions doped in phosphors have attracted the focus of many researchers. The 5d electrons of rare-earth ions such as Eu2+, Sm3+, Dy3+, and Tb3+ play a significant role in the phosphors owing to the electron transitions [9,14–16]. Among these rare-earth ions, Eu2+ ions are used as a sensitizer in many phosphors owing to their excellent absorptivity in the UV region, which could enhance the luminescent intensity excited by UV light [15–18].
ABPO4 is a large family of orthophosphates with various structures, which depend on the radii ratio of A and B [19,20]. ABPO4:Eu2+ phosphors with tetrahedral structure have excellent characteristics, for instance, good optical properties, high thermal stability, and large bandgap, for example, KZnPO4, RbBaPO4, and KSrPO4 [21–23]. Sun et al. found that LiBaPO4:Eu2+ has higher stability and good optical performance [24]. Qiao et al. reported that K2BaCa(PO4)2:Eu2+ showed thermally stabled luminescence [25]. Generally, the excellent color temperature of an ABPO4-based phosphor could be achieved by co-doping two or more different RE ions [26]. The Sm3+ ions whose photoluminescent (PL) emission is in the red wavelength region could act as a sensitizer in many phosphors [27,28]. The phosphor synthesized by Tb3+ and Ce3+ co-doped in BaY(BO3)3, which emit green light, was evaluated earlier [29]. Dy3+/Sm3+ co-doped Lu3Ga5O12 emitted blue, yellow, and orange light, which indicated that co-doped RE ions in the phosphor could change the color temperature of the synthesized samples [30]. Jia et al. found the low color temperature of Ce3+/Eu2+ co-doped Ca4(PO4)2O phosphor, and the sample emits warm white light [31]. So far, different combinations of RE ions co-doped in the host were reported in the previous contributions. However, the studies about the optical properties of ABPO4:Eu2+, Sm3+ have not been discussed in detail yet.
In our work, we synthesized a series of KBaPO4:Eu2+, Sm3+ (KBP:Eu2+, Sm3+) samples via the solid-state reaction method. The formation conditions and structural and luminescent properties of KBaPO4:Eu2+, Sm3+ phosphors under the N-UV illumination were investigated. The Eu2+ ions in the KBP host emit blue light, and the doped of Sm3+ ions can adjust the emission color of doped phosphor. The emission color of KBP:Eu2+, Sm3+ phosphor can be adjusted by the change of Eu2+/Sm3+ ratio.
2 Experiment
2.1 Sample preparation
The raw materials K2CO3, BaCO3, NH4H2PO4, Eu2O3, and Sm2O3 of analytical grade were purchased from Aladdin Reagent Co. Ltd. The raw materials were accurately weighed according to the designed composition, and the mixture was ground in an agate mortar for 20 min. The batch was transferred into a crucible with a lid to minimize the losses due to evaporation during synthesis. First, the reaction materials were sintered at 1,000°C for 6 h in the air and cooled naturally in a muffle furnace. Then, the synthesized samples were ground into powder. Second, the compounds were heated up to 1,200°C for 4 h in crucibles under a reducing gas mixture (3% H2/97% N2). At last, a series of samples were taken out of the tubular furnace and reground for characterization. The flow diagram is shown in Figure 1.

The flow diagram of the preparation steps of KBaPO4 co-doped Eu2+ and Sm3+.
The models of the used muffle furnace and the tube furnace were KSL-1700X and GSL-1700X. They were manufactured by Hefei Kejing Material Technology Co. Ltd. The precise balance FA1104 was made from Shanghai Liangping INSTRUMENT & METER CO. Ltd. The corundum boats were bought in the Tangshan Jidong Chemical Porcelain Factory.
2.2 Characterization
The X-ray diffraction (XRD) patterns of the KBP:Eu2+/Sm3+ samples were recorded with the use of an XRD-6000 device (Cu Kα; Shimadzu, Japan). The XRD patterns were collected in the 2θ range from 15–70° with a step of 0.02°. The morphologies of the KBP:Eu2+/Sm3+ were identified by scanning electron microscopy (SEM; JSM-7800F, JEOL, Japan). The luminescence properties, including the excitation and emission spectra, were characterized by a fluorescence spectrophotometer (HITACH F-7000, Japan) with a 150 W Xenon lamp. All measurements were carried out in the air at 20°C.
3 Results and discussion
3.1 XRD and SEM analysis
Figure 2 shows the XRD patterns of KBa(1−x−y)PO4:xEu2+, ySm3+(x = 0, 0.1%; y = 0, 0.05, 0.09, 0.12, 0.15) samples. The diffraction peaks in the XRD patterns are well-matched with the standard data for the KBaPO4 phase and previous study [32]. There are no foreign peaks observed, and it means that the co-doping ions have not changed the crystal phase of the KBaPO4 host. It confirms that the samples are of high phase purity. The introduction of Eu2+ or Sm3+ causes little changes in the host structure in such a small proportion. Moreover, the XRD patterns of the K2CO3, BaCO3, and NH4H2PO4 reaction materials don’t exhibit any consistency with diffraction peaks in Figure 2, and it indicates that the raw materials are fully reacted.

XRD patterns of KBaPO4:xEu2+, ySm3+.
Figure 3 shows the structure elements of KBaPO4, which has a typical tridymite-type structure. In orthophosphates which belong to tridymite structure mostly, four O ions coordinate with one P atom and four BaO4 tetrahedrons were connected with one PO4 tetrahedron [20]. In the synthesized samples, Eu2+and Sm3+ ions are substituted by the Ba2+ ions in the structure of KBaPO4 due to the closer ion radii. Therefore, the XRD patterns of the samples are mainly consistent with the standard data card JCPDS No. 73-1405 (Ba2+ ion [1.47 Å, CN = 9], Eu2+ ion [1.30 Å, CN = 9], Sm3+ ion [1.324 Å, CN = 6]) [33,34]. However, the valence states of Sm3+ and Ba2+ ions are different, and the electron-hole (v−) in host KBaPO4 will come into being and reach charge balance (Sm3+ v− → Ba2+) [35].

The structure fragments of KBaPO4.
Figure 4 exhibits three SEM images of KBP:0.001Eu2+, ySm3+ (y = 0(a), 0.12(b), 0.15(c)), which shows the dense morphology of the samples. It is found that the representative phosphors consist of irregular grains with a particle size of 5–15 μm. It was concluded that the small portions of Eu2+ and Sm3+ hardly change the structure and particle morphology of KBaPO4-based phosphor from the results of XRD patterns and SEM images. However, the particle size of compound (KBP:Eu2+, 0.15Sm3+) (c) is slightly bigger than that of the other two compounds, and it suggests the aggregation in (c) compound with the creation of larger grains with the Sm3+ content y = 0.15.
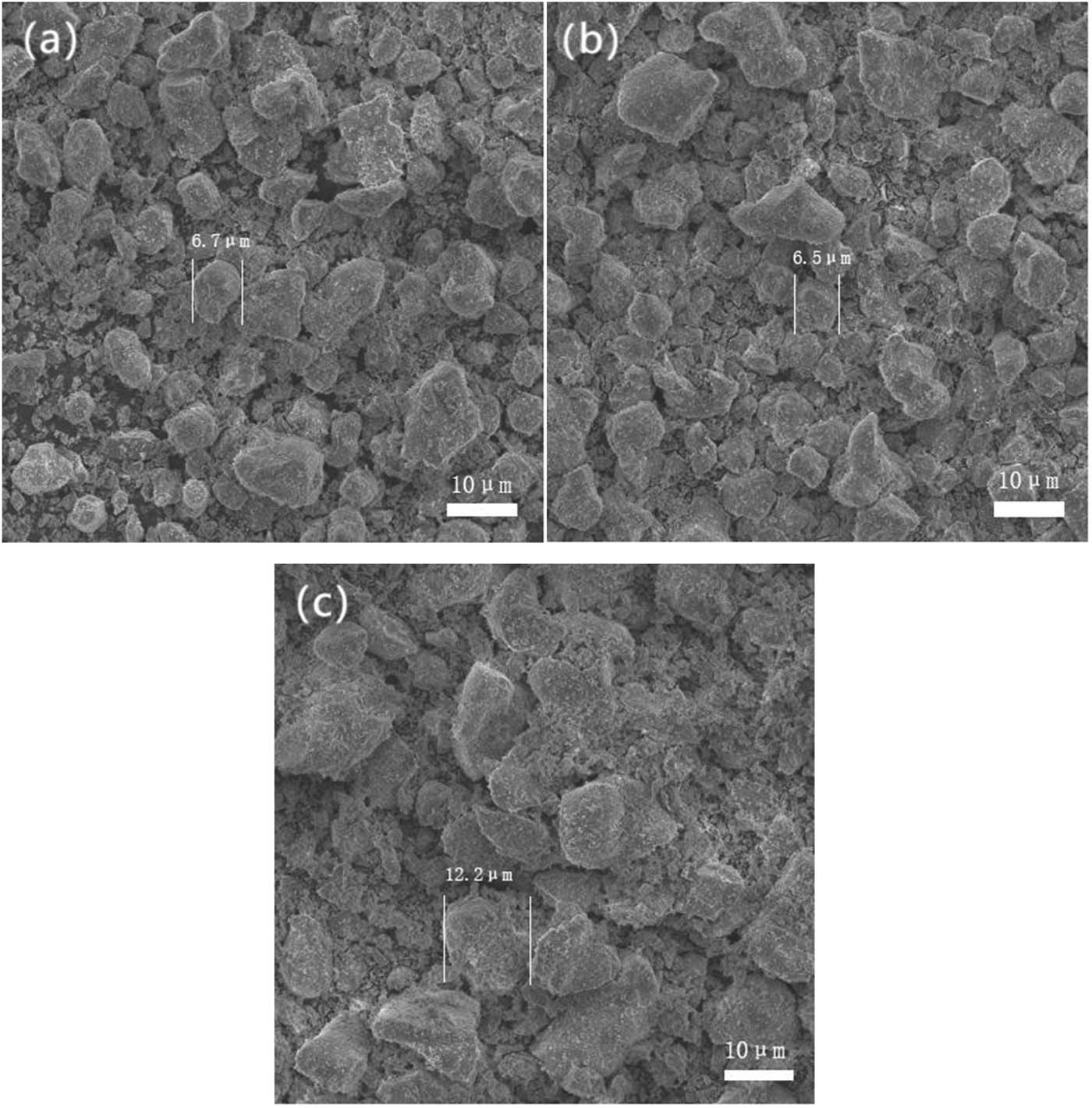
SEM images of KBaPO4:0.001Eu2+, ySm3+ (y = 0(a), 0.12(b),0.15(c)).
3.2 PL spectra of KBaPO4:Eu2+, Sm3+ phosphors
The PLE (λ em = 430 nm) and PL (λ ex = 350 nm) spectra of KBP:0.1% Eu2+ are shown in Figure 5a. Figure 5a exhibits the PLE and PL spectra of the KBP:0.1% Eu2+. The photoluminescent excitation spectra monitored at 430 nm exhibit a broadband in the N-UV region with the maximum at 350 nm. It indicates a typical excitation spectrum of Eu2+ ions [36–38]. Figure 5b shows the photoluminescence spectrum of a series of samples (KBP:xEu2+) under the 350 nm excitation wavelength. The PL peak of Eu2+ ions is located at 431 nm, which corresponded to the transition of 4f65d1 → 4f7 [39–41]. Among three samples, the PL peak of KBP:0.1% Eu2+ is the strongest, and the 0.1% content of Eu2+ is optimal. Meanwhile, the photoluminescence spectrum indicates that the synthesized samples can be excited by N-UV light, and the co-doped KBaPO4:Eu2+, Sm3+ can be a potential LED phosphor excited by N-UV chips.

(a) PLE and PL spectra of KBP:0.1% Eu2+ sample and (b) photoluminescence emission spectra of KBP:xEu2+ (x = 0.05–0.15%) phosphors (λ em = 430 nm, λ ex = 350 nm).
Figure 6 displays the PLE and PL spectra of the KBP:0.12Sm3+ sample. The excitation spectrum consists of several sharp peaks in the range from 300 to 500 nm ascribed to the characteristic bands of Sm3+ ion. The excitation peaks located at 348, 375, and 402 nm ( the strongest one), as monitored at 600 nm, are assigned with 6H5/2 → 4F9/2, 6H5/2 → 4K11/2, and 6H5/2 → 4P5/2, respectively, which indicated that KBaPO4:Sm3+ phosphor can be used in LED structures because the excitation is possible in the N-UV region.

PLE and PL spectra of KBP:0.12Sm3+ phosphors (λ em = 600 nm, λ ex = 400 nm).
The PL spectrum of KBaPO4:0.12Sm3+ is shown in Figure 6. The emission spectrum contains several strong peaks due to the electron transitions of Sm3+ ions. The strongest emission component at 600 nm corresponds to the 4G5/2 → 6H7/2, which belongs to the characteristic 4f → 4f transitions of RE ions [42,43]. The PL spectrum of KBaPO4:0.12Sm3+ embodies orange or reddish-orange wavelength usually used to compensate red component in the emission of phosphor due to its stronger red emission. Therefore, the host phosphor doped Sm3+ ions were usually employed in W-LED.
Figure 7 shows the PL and PLE spectra of KBP:0.12Sm3+ (Figure 7a) and KBP:0.1% Eu2+ (Figure 7b). Figure 7a shows the excitation spectrum in the range of 320–470 nm and a series of PL peaks at 562, 600, and 648 nm monitored by 400 nm. Figure 7b displays a wide emission spectrum of Eu2+ ions in the blue light region, in which the PL peak is located at 430 nm. Figure 7c shows the excitation of Sm3+ and emission spectra of Eu2+ in the KBP host. As seen in Figure 7, there exists an overlap between the two spectra of the RE ions, and it indicates a possibility for the energy transfer from Eu2+ to Sm3+ in the KBP host.

PL and PLE spectra of (a) KBP:0.12Sm3+, (b) KBP:0.1% Eu2+, and (c) the PL of Eu2+ and PLE of Sm3+ in KBP.
Figure 8a shows the photoluminescence spectra of a series of KBP: 0.1% Eu2+, ySm3+. As excited at 400 nm, the photoluminescence spectra of KBP:Eu2+/Sm3+ include the typical peaks at 430, 562, 600, and 648 nm. The shoulders at 430 nm are assigned to the characteristic electron transition of Eu2+ ions, which is located in the blue region. The peak at 600 nm is congruous with the strongest emission line of Sm3+ (6H5/2 → 6P3/2). The PL spectra reveal that the spectral emission intensity is controlled by the concentration of Sm3+ ions (y). As the concentration of Sm3+ ions increased, the emission intensities gradually increased. The intensities of Sm3+ peaks are the largest at y = 0.12. At a higher concentration of Eu2+, the emission intensities began to decrease due to the quenching effect. Meanwhile, the luminescent intensity of Eu2+ decreased by degrees with the raising of Sm3+ content. The emission intensity of Eu2+ in single-doped KBP is greater than the intensity of Eu2+ in co-doped KBP:Eu2+/Sm3+, as evident in Figure 8(b), and it further confirms the possible existence of the energy transfer between these two RE ions.

(a) PL spectrum of KBP:0.1% Eu2+/ySm3+ phosphors excited by 400 nm and (b) photoluminescence emission spectrum of KBP:0.1% Eu2+/ySm3+ phosphors (y = 0, 0.06, 0.09, 0.12, 0.15).
Moreover, the emission of KBP:Eu2+, Sm3+ includes blue, yellow, and red components. The color of emission from KBP phosphor relied on the co-doping proportion of Eu2+ and Sm3+ ions mainly. Therefore, KBP:xEu2+, ySm3+ could become a potential W-LED phosphor.
4 Conclusion
In this study, the KBaPO4:xEu2+, ySm3+ compounds were synthesized via the high-temperature solid-state reaction method. The XRD and SEM results indicated that the synthesized samples remain in the pure phase state after the co-doping by two rare-earth ions. The blue light of Eu2+ ions and red light of Sm3+ emitted by the synthesized samples can be simultaneously observed under the 400 nm excitation (N-UV), and it classifies KBaPO4:Eu2+, Sm3+ among potential phosphors for use in W-LED structures. The optimum concentration of Eu2+ is 0.001 and Sm3+ is 0.12. However, the intensity emission of Eu2+ ions was reduced by increasing Sm3+ concentration. Meanwhile, the overlap exists between the emission/excitation spectra of two RE ions. In summary, there is a possible energy transfer between Sm3+ and Eu2+.
Acknowledgements
We are grateful for the support from the Scientific Research Projects of Liaoning Provincial Department of Education (No. J2020055).
-
Funding information: This work was financially supported by the Scientific Research Projects of Liaoning Provincial Department of Education (No. J2020055).
-
Author contributions: Yingwei Xu and Tingting Zhang completed the experiments and drew the figures. Ailing Zou and Li Zheng proposed the problem, pointed research direction, and reveiwed the manuscirpt. Ailing Zou wrote the manuscript. All authors contributed to the article and approved the submitted version.
-
Conflict of interest: Authors state no conflict of interest.
References
[1] Deng, H., Z. Zhao, J. Wang, Z. Hei, M. Li, H. M. Noh, et al. Photoluminescence properties of a new orange-red emitting Sm3-doped Y2Mo4O15 phosphor. Journal of Solid State Chemistry, Vol. 228, 2015, pp. 110–116.10.1016/j.jssc.2015.04.023Search in Google Scholar
[2] Du, P., X. Huang, and J. S. Yu. Facile synthesis of bifunctional Eu3-activated NaBiF4, red-emitting nanoparticles for simultaneous white light-emitting diodes and field emission displays. Chemical Engineering Journal, Vol. 337, 2018, pp. 91–100.10.1016/j.cej.2017.12.063Search in Google Scholar
[3] Li, K., and R. Van Deun. Color tuning from greenish-yellow to orange-red in thermal-stable KBaY(MoO4)3:Dy3+, Eu3+ phosphors via energy transfer for UV W-LEDs. Applied Electronic Materials, Vol. 2, 2020, pp. 1735–1744.10.1021/acsaelm.0c00307Search in Google Scholar
[4] Xie, R. J., and N. Hirosaki. Silicon-based oxynitride and nitride phosphors for white LEDs: a review. Science and Technology of Advanced Materials, Vol. 8, 2007, pp. 588–600.10.1016/j.stam.2007.08.005Search in Google Scholar
[5] Ray, S., P. Tadge, S. Dutta, T. M. Chen, G. B. Nair, and S. J. Dhoble. Synthesis, luminescence and application of BaKYSi2O7 Eu2a new blue-emitting phosphor for near-UV white-light LED. Ceramics International, Vol. 44, 2018, pp. 8334–8343.10.1016/j.ceramint.2018.02.022Search in Google Scholar
[6] Xia, Z., Y. Zhang, M. S. Molokeev, and V. V. Atuchin. Structural and luminescence properties of yellow-emitting NaScSi2O6:Eu2+ phosphors: Eu2+ site preference analysis and generation of red emission by codoping Mn2+ for white-light-emitting diode applications. Journal of Physical Chemistry C, Vol. 117, 2013, pp. 20847–20854.10.1021/jp4062225Search in Google Scholar
[7] Ji, H., Z. Huang, Z. Xia, M. S. Molokeev, V. V. Atuchin, M. Fang, et al. New yellow-emitting whitlockite-type structure Sr1.75Ca1.25(PO4)2:Eu2+ phosphor for near-UV pumped white light-emitting devices. Inorganic Chemistry, Vol. 53, 2014, pp. 5129–5135.10.1021/ic500230vSearch in Google Scholar PubMed
[8] Leaño Jr, J. L., S. Y. Lin, A. Lazarowska, S. Mahlik, M. Grinberg, C. Liang, et al. Green light-excitable Ce-doped nitridomagnesoaluminate Sr[Mg2Al2N4] phosphor for white light-emitting diodes. Chemistry of Materials, Vol. 28 , 2016, pp. 6822–6825.10.1021/acs.chemmater.6b03442Search in Google Scholar
[9] Qin, X., X. Liu, W. Huang, M. Bettinelli, and X. Liu. Lanthanide-activated phosphors based on 4f-5d optical transitions: theoretical and experimental aspects. Chemical Reviews, Vol. 117, 2017, pp. 4488–4527.10.1021/acs.chemrev.6b00691Search in Google Scholar PubMed
[10] Atuchin, V. V., A. S. Aleksandrovsky, O. D. Chimitova, T. A. Gavrilova, A. S. Krylov, M. S. Molokeev, et al. Synthesis and spectroscopic properties of monoclinic α-Eu2(MoO4)3. Journal of Physical Chemistry C, Vol. 118, 2014, pp. 15404–15411.10.1021/jp5040739Search in Google Scholar
[11] Ji, H., Z. Huang, Z. Xia, M. S. Molokeev, V. V. Atuchin, M. Fang, et al. Discovery of new solid solution phosphors via cation substitution-dependent phase transition in M3(PO4)2:Eu2+(M = Ca/Sr/Ba) quasi-binary sets. Journal of Physical Chemistry C, Vol. 119, 2015, pp. 2038–2045.10.1021/jp509743rSearch in Google Scholar
[12] Ji, H., L. Wang, M. S. Molokeev, N. Hirosaki, R. Xie, Z. Huang, et al. Structure evolution and photoluminescence of Lu3(Al,Mg)2(Al,Si)3O12:Ce3+phosphors: new yellow-color converters for blue LED-driven solid state lighting. Journal of Materials Chemistry C: Materials for Optical and Electronic Devices, Vol. 4, 2016, pp. 6855–6863.10.1039/C6TC00966BSearch in Google Scholar
[13] Lim, C. S., A. Aleksandrovsky, M. Molokeev, A. Oreshonkov, and V. Atuchin. The modulated structure and frequency upconversion properties of CaLa2(MoO4)4:Ho3+/Yb3+phosphors prepared by microwave synthesis. Physical Chemistry Chemical Physics, Vol. 17, 2015, pp. 19278–19287.10.1039/C5CP03054DSearch in Google Scholar PubMed
[14] Zhang, C., T. Uchikoshi, R. J. Xie, L. Liu, Y. Cho, Y. Sakka, et al. Tunable luminescence properties and energy transfer of single-phase Ca4(PO4)2O: Dy3 Eu2multi-color phosphors for warm white light. Journal of Materials Science, Vol. 53, 2018, pp. 6414–6423.10.1007/s10853-017-1947-zSearch in Google Scholar
[15] Zhang, C., T. Uchikoshi, R. J. Xie, L. Liu, Y. Cho, Y. Sakka, et al. Reduced thermal degradation of the red-emitting Sr2Si5N8: Eu2phosphor via thermal treatment in nitrogen. Journal of Materials Chemistry C: Materials for Optical and Electronic Devices, Vol. 3, 2015, pp. 7642–7651.10.1039/C5TC01575HSearch in Google Scholar
[16] Dorenbos, P. Energy of the first 4f7 → 4f65d transition of Eu2+in inorganic compounds. Journal of Luminescence, Vol. 104, 2003, pp. 239–260.10.1016/S0022-2313(03)00078-4Search in Google Scholar
[17] Zhao, C., Z. Xia, and M. Li. Eu2-activated full color orthophosphate phosphors for warm white light-emitting diodes. RSC Advances, Vol. 4, 2014, pp. 33114–33119.10.1039/C4RA04452ESearch in Google Scholar
[18] He, L., Z. Song, X. Jia, Z. Xia, and Q. Liu. Control of luminescence in Eu2+-doped orthosilicate-orthophosphate phosphors by chainlike polyhedra and electronic structures. Inorganic Chemistry, Vol. 57, No. 2, 2018 Jan 16, pp. 609–616. 10.1021/acs.inorgchem.7b02431.Search in Google Scholar PubMed
[19] Zhang, S. Y., Y. L. Huang, and H. J. Seo. The spectroscopy and structural sites of Eu2ions doped KCaPO4 phosphor. Journal of the Electrochemical Society, Vol. 157, 2010, pp. 261–266.10.1149/1.3429887Search in Google Scholar
[20] Lin, C. C., Z. R. Xiao, G. Y. Guo, T. S. Chan, and R. S. Liu. Versatile phosphate phosphors ABPO4 in white light-emitting diodes: collocated characteristic analysis and theoretical calculations. Journal of the American Chemical Society, Vol. 132, 2010, pp. 3020–3028.10.1021/ja9092456Search in Google Scholar PubMed
[21] Gou, J., J. Wang, S. Ye, and B. Yu. Luminescence and microstructural features of Dy3-activated KZnPO4 phosphors. Materials Research Bulletin, Vol. 70, 2015, pp. 827–831.10.1016/j.materresbull.2015.06.011Search in Google Scholar
[22] Chen, J., W. Zhao, J. Zhong, L. Lan, J. Wang, and N. Wang. Synthesis and luminescence properties of Ce3-doped RbBaPO4. Ceramics International, Vol. 40, 2014, pp. 15241–15248.10.1016/j.ceramint.2014.06.120Search in Google Scholar
[23] Zhang, Z. W., A. J. Song, S. T. Song, J. P. Zhang, W. G. Zhang, and D. J. Wang. Synthesis and luminescence properties of novel KSrPO4: Dy3+ phosphor. Journal of Alloys and Compounds, Vol. 629, 2015, pp. 32–35.10.1016/j.jallcom.2014.12.066Search in Google Scholar
[24] Sun, J. Y., X. Y. Zhang, Z. G. Xia, and H. Du. Luminescent properties of LiBaPO4:RE (RE = Eu2+, Tb3+, Sm3+) phosphors for white light-emitting diodes. Journal of Applied Physics, Vol. 111, 2012, pp. 13101–13107.10.1063/1.3673331Search in Google Scholar
[25] Qiao, J., L. Ning, M. S. Molokeev, Y. C. Chuang, Q. Liu, and Z. Xia. Eu2+ site preferences in the mixed cation K2BaCa(PO4)2 and thermally stable luminescence. Journal of the American Chemical Society, Vol. 140, 2018, pp. 9730–9736.10.1021/jacs.8b06021Search in Google Scholar PubMed
[26] Zheng, J., S. Wu, G. Chen, S. Dang, Y. Zhuang, Z. Guo, et al. Blue-emitting Ca5(PO4)3Cl: Eu2phosphor for near-UV pumped light emitting diodes: electronic structures, luminescence properties and LED fabrications. Journal of Alloys and Compounds, Vol. 663, 2016, pp. 332–339. 10.1016/j.jallcom.2015.12.054.Search in Google Scholar
[27] Huang, Y. L., W. F. Kai, Y. G. Cao, K. Jang, H. S. Lee, K. Ilgon, et al. Spectroscopic and structural studies of Sm2doped orthophosphate KSrPO4 crystal. Journal of Applied Physics, Vol. 103, 2008, pp. 53501–53507.10.1063/1.2890152Search in Google Scholar
[28] Wang, G. J., D. J. Pan, T. Xu, G. X. Xiang, Z. J. Zhang, and H. T. Hintzen. Photoluminescence properties and energy level of RE (RE = Pr, Sm, Tb, Er, Dy) in Y4Si2O7N2. Journal of Alloys and Compounds, Vol. 708, 2017, pp. 154–161.10.1016/j.jallcom.2017.02.298Search in Google Scholar
[29] Cheng, Z., J. Yu, Y. Zhang, and N. Zou. Luminescence and energy transfer mechanism of α-Ba3Y(BO3)3:Ce3+,Tb3+. Journal of Luminescence, Vol. 42, 2017, pp. 1004–1009.10.1016/j.jlumin.2017.08.041Search in Google Scholar
[30] Pamuluri, H., M. Rathaiah, K. Linganna, C. K. Jayasankar, V. Lavin, and V. Venkatramu. Role of Dy3+→ Sm3+ energy transfer in the tuning of warm to cold white light emission in Dy3+/Sm3+co-doped Lu3Ga5O12 nano-garnets. New Journal of Chemistry, Vol. 42, 2018, id. 1260.10.1039/C7NJ04034BSearch in Google Scholar
[31] Jia, Y., R. Pang, H. Li, W. Sun, J. Fu, L. Jiang, et al. Single-phased white-light-emitting Ca4(PO4)2O: Ce3+, Eu2+phosphors based on energy transfer. Dalton, Vol. 44, 2015, pp. 11399–114071.10.1039/C5DT01018GSearch in Google Scholar PubMed
[32] Wei, D., Y. Huang, S. Zhang, Y. M. Yu, and H. J. Seo. Luminescence spectroscopy of Ce3-doped ABaPO4 (A = Li, Na, K) phosphors. Applied Physics B: Lasers and Optics, Vol. 108, 2012, pp. 447–453.10.1007/s00340-012-4969-xSearch in Google Scholar
[33] Zhang, H., D. Yuan, X. Mi, X. Zhang, Z. Bai, X. Liu, et al. Crystal structure and luminescence properties of Na2MMg(PO4)2:Eu2(M = Ca/Sr/Ba) phosphors. Journal of Alloys and Compounds, Vol. 798, 2019, pp. 119–128.10.1016/j.jallcom.2019.05.178Search in Google Scholar
[34] Gao, H. and P. Li. Luminescence and energy transfer of white emitting phosphor Ba3Ce(PO4)3: Dy3. Optik, Vol. 170, 2018, pp. 272–277.10.1016/j.ijleo.2018.04.061Search in Google Scholar
[35] Cao, R., X. Wang, X. Ouyang, Y. Jiao, Y. Li, H. Wan, et al. Thermally stable orange-red emitting Ba2SiO4:Sm3+phosphor: Synthesis and luminescence properties. Journal of Luminescence, Vol. 224, 2020, id. 117292.10.1016/j.jlumin.2020.117292Search in Google Scholar
[36] Xia, Z., Y. Zhang, M. S. Molokeev, V. V. Atuchin, and Y. Luo. Linear structural evolution induced tunable photoluminescence in clinopyroxene solid-solution phosphors. Scientific Reports, Vol. 3, 2013, id. 3310.10.1038/srep03310Search in Google Scholar PubMed PubMed Central
[37] Wang, Z., Z. Xia, M. S. Molokeev, V. V. Atuchin, and Q. L. Liu. Blue-shift of Eu2+emission in (Ba,Sr)3Lu(PO4)3:Eu2+eulytite solid-solution phosphors resulting from release of neighbouring-cation-induced stress. Dalton Transactions, Vol. 43, 2014, pp. 16800–16804.10.1039/C4DT02319FSearch in Google Scholar PubMed
[38] Ji, H., Z. Huang, Z. Xia, Y. Xie, M. S. Molokeev, and V. V. Atuchin. Facile solution-precipitation assisted synthesis and luminescence property of greenish-yellow emitting Ca6Ba(PO4)4O:Eu2+ phosphor. Materials Research Bulletin, Vol. 75, 2016, pp. 233–238.10.1016/j.materresbull.2015.11.055Search in Google Scholar
[39] Ji, H., Z. Huang, Z. Xia, M. S. Molokeev, V. V. Atuchin, and S. Huang. Cation substitution dependent bimodal photoluminescence in whitlockite structural Ca3−xSrx(PO4)2:Eu2(0 ≤ x ≤ 2) solid solution phosphors. Inorganic Chemistry, Vol. 53, 2014, pp. 11119–11124.10.1021/ic501679fSearch in Google Scholar PubMed
[40] Li, G., C. C. Lin, W. T. Chen, M. S. Molokeev, V. V. Atuchin, C. Y. Chiang, et al. Photoluminescence tuning via cation substitution in oxonitridosilicate phosphors: DFT calculations, different site occupations, and luminescence mechanisms. Chemistry of Materials, Vol. 26, 2014, pp. 2991–3001.10.1021/cm500844vSearch in Google Scholar
[41] Ji, H., Z. Huang, Z. Xia, M. S. Molokeev, M. Chen, V. V. Atuchin, et al. Phase transformation in Ca3(PO4)2:Eu2+via the controlled quenching and increased Eu2+ content: identification of new cyan-emitting α-Ca3(PO4)2:Eu2+ phosphor. Journal of the American Chemical Society, Vol. 98, No. 10, 2015, pp. 3280–3284.10.1111/jace.13787Search in Google Scholar
[42] Atuchin, V. V., A. S. Aleksandrovsky, O. D. Chimitova, C. P. Diao, T. A. Gavrilova, V. G. Kesler, et al. Electronic structure of β-RbSm(MoO4)2 and chemical bonding in molybdates. Dalton Transactions, Vol. 44, 2015, pp. 1805–1815.10.1039/C4DT03203ASearch in Google Scholar
[43] Atuchin, V. V., A. S. Aleksandrovsky, M. S. Molokeev, A. S. Krylov, A. S. Oreshonkov, and D. Zhou. Structural and spectroscopic properties of self-activated monoclinic molybdate BaSm2(MoO4)4. Journal of Alloys and Compounds, Vol. 729, 2017, pp. 843–849.10.1016/j.jallcom.2017.07.259Search in Google Scholar
© 2021 Yingwei Xu et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Fused deposition modeling of poly(ether ether ketone) scaffolds
- Investigation of the microstructure evolution in TP347HFG austenitic steel at 700°C and its characterization method
- Hot deformation behavior and processing maps of 9Cr3W3Co oxide dispersion-strengthened steel
- Evolution of physicochemical properties of quick lime at converter-smelting temperature
- Influence of phase distribution of converter slag microzones on the occurrence of P
- Investigation on ultrasonic assisted friction stir welding of aluminum/steel dissimilar alloys
- Analysis of oxide scale thickness and pores position of HCM12A steel in supercritical water
- Behavior of MnS inclusions during homogenization process in low-alloyed steel FAS3420H
- Preparation and cutting performance of nano-scaled Al2O3-coated micro-textured cutting tool prepared by atomic layer deposition
- Prediction of hot metal temperature based on data mining
- Effect of TiO2 content in slag on Ti content in molten steel
- Performance evaluation of titanium-based metal nitride coatings and die lifetime prediction in a cold extrusion process
- Effect of different drilling techniques on high-cycle fatigue behavior of nickel-based single-crystal superalloy with film cooling hole
- Effect of CO2 injection into blast furnace tuyeres on the pulverized coal combustion
- Microstructure and properties of Co–Al porous intermetallics fabricated by thermal explosion reaction
- Evolution regularity of temperature field of active heat insulation roadway considering thermal insulation spraying and grouting: A case study of Zhujidong Coal Mine, China
- Evolution of reduction process from tungsten oxide to ultrafine tungsten powder via hydrogen
- A thermodynamic assessment of precipitation, growth, and control of MnS inclusion in U75V heavy rail steel
- Effect of basicity on the reduction swelling properties of iron ore briquettes
- Effect of Cr and Al alloying on the oxidation resistance of a Ti5Si3-incorporated MoSiBTiC alloy
- Microstructure and mechanical properties of 2060 Al–Li alloy welded by alternating current cold metal transfer with high-frequency pulse current
- Effects of composition and strain rate on hot ductility of Cr–Mo-alloy steel in the two-phase region
- Effect of K and Na on reduction swelling performance of oxidized roasted briquettes
- Dephosphorization mechanism and phase change in the reduction of converter slag
- Parametric investigation and optimization for CO2 laser cladding of AlFeCoCrNiCu powder on AISI 316
- Optimization of heat transfer and pressure drop of the channel flow with baffle
- Quantitative analysis of microstructure and mechanical properties of Nb–V microalloyed high-strength seismic reinforcement with different Nb additions
- Visualization of the damage evolution for Ti–3Al–2Mo–2Zr alloy during a uniaxial tensile process using a microvoids proliferation damage model
- Research on high-temperature mechanical properties of wellhead and downhole tool steel in offshore multi-round thermal recovery
- Dephosphorization behavior of reduced iron and the properties of high-P-containing slag
- Jet characteristics of CO2–O2 mixed injection using a dual-parameter oxygen lance nozzle for different smelting periods
- Effects of ball milling on powder particle boundaries and properties of ODS copper
- Heat transfer behavior in ultrahigh-speed continuous casting mold
- Solidification microstructure characteristics of Cu–Pb alloy by ECP treatment
- Luminescence properties of Eu2+ and Sm3+ co-doped in KBaPO4
- Research on high-temperature oxidation resistance, hot forming ability, and microstructure of Al–Si–Cu coating for 22MnB5 steel
- The differential analysis for temperature distribution diagnostics of arc current-carrying region in sheet slanting tungsten electrode inert gas welding with the electrostatic probe
- Reactions at the molten flux-weld pool interface in submerged arc welding
- The effect of liquid crystalline graphene oxide compared with non-liquid crystalline graphene oxide on the rheological properties of polyacrylonitrile solution
- Study on manganese volatilization behavior of Fe–Mn–C–Al twinning-induced plasticity steel
- Physical modeling of bubble behaviors in molten steel under high pressure
- Rapid Communication
- The new concept of thermal barrier coatings with Pt + Pd/Zr/Hf-modified aluminide bond coat and ceramic layer formed by PS-PVD method
- Topical Issue on Science and Technology of Solar Energy
- Solution growth of chalcopyrite Cu(In1−xGax)Se2 single crystals for high open-circuit voltage photovoltaic device
- Copper-based kesterite thin films for photoelectrochemical water splitting
Articles in the same Issue
- Research Articles
- Fused deposition modeling of poly(ether ether ketone) scaffolds
- Investigation of the microstructure evolution in TP347HFG austenitic steel at 700°C and its characterization method
- Hot deformation behavior and processing maps of 9Cr3W3Co oxide dispersion-strengthened steel
- Evolution of physicochemical properties of quick lime at converter-smelting temperature
- Influence of phase distribution of converter slag microzones on the occurrence of P
- Investigation on ultrasonic assisted friction stir welding of aluminum/steel dissimilar alloys
- Analysis of oxide scale thickness and pores position of HCM12A steel in supercritical water
- Behavior of MnS inclusions during homogenization process in low-alloyed steel FAS3420H
- Preparation and cutting performance of nano-scaled Al2O3-coated micro-textured cutting tool prepared by atomic layer deposition
- Prediction of hot metal temperature based on data mining
- Effect of TiO2 content in slag on Ti content in molten steel
- Performance evaluation of titanium-based metal nitride coatings and die lifetime prediction in a cold extrusion process
- Effect of different drilling techniques on high-cycle fatigue behavior of nickel-based single-crystal superalloy with film cooling hole
- Effect of CO2 injection into blast furnace tuyeres on the pulverized coal combustion
- Microstructure and properties of Co–Al porous intermetallics fabricated by thermal explosion reaction
- Evolution regularity of temperature field of active heat insulation roadway considering thermal insulation spraying and grouting: A case study of Zhujidong Coal Mine, China
- Evolution of reduction process from tungsten oxide to ultrafine tungsten powder via hydrogen
- A thermodynamic assessment of precipitation, growth, and control of MnS inclusion in U75V heavy rail steel
- Effect of basicity on the reduction swelling properties of iron ore briquettes
- Effect of Cr and Al alloying on the oxidation resistance of a Ti5Si3-incorporated MoSiBTiC alloy
- Microstructure and mechanical properties of 2060 Al–Li alloy welded by alternating current cold metal transfer with high-frequency pulse current
- Effects of composition and strain rate on hot ductility of Cr–Mo-alloy steel in the two-phase region
- Effect of K and Na on reduction swelling performance of oxidized roasted briquettes
- Dephosphorization mechanism and phase change in the reduction of converter slag
- Parametric investigation and optimization for CO2 laser cladding of AlFeCoCrNiCu powder on AISI 316
- Optimization of heat transfer and pressure drop of the channel flow with baffle
- Quantitative analysis of microstructure and mechanical properties of Nb–V microalloyed high-strength seismic reinforcement with different Nb additions
- Visualization of the damage evolution for Ti–3Al–2Mo–2Zr alloy during a uniaxial tensile process using a microvoids proliferation damage model
- Research on high-temperature mechanical properties of wellhead and downhole tool steel in offshore multi-round thermal recovery
- Dephosphorization behavior of reduced iron and the properties of high-P-containing slag
- Jet characteristics of CO2–O2 mixed injection using a dual-parameter oxygen lance nozzle for different smelting periods
- Effects of ball milling on powder particle boundaries and properties of ODS copper
- Heat transfer behavior in ultrahigh-speed continuous casting mold
- Solidification microstructure characteristics of Cu–Pb alloy by ECP treatment
- Luminescence properties of Eu2+ and Sm3+ co-doped in KBaPO4
- Research on high-temperature oxidation resistance, hot forming ability, and microstructure of Al–Si–Cu coating for 22MnB5 steel
- The differential analysis for temperature distribution diagnostics of arc current-carrying region in sheet slanting tungsten electrode inert gas welding with the electrostatic probe
- Reactions at the molten flux-weld pool interface in submerged arc welding
- The effect of liquid crystalline graphene oxide compared with non-liquid crystalline graphene oxide on the rheological properties of polyacrylonitrile solution
- Study on manganese volatilization behavior of Fe–Mn–C–Al twinning-induced plasticity steel
- Physical modeling of bubble behaviors in molten steel under high pressure
- Rapid Communication
- The new concept of thermal barrier coatings with Pt + Pd/Zr/Hf-modified aluminide bond coat and ceramic layer formed by PS-PVD method
- Topical Issue on Science and Technology of Solar Energy
- Solution growth of chalcopyrite Cu(In1−xGax)Se2 single crystals for high open-circuit voltage photovoltaic device
- Copper-based kesterite thin films for photoelectrochemical water splitting

