Abstract
Mill scale (MS) has a potential to use as an iron source because of its high iron content. MS mainly consists of a mixture of iron oxides, metallic iron and other base metal oxides. MS is formed on the surfaces of steel ingots during continuous casting as a waste material. In this study, the use of MS as an iron source for the production of carbon-free iron containing alloys (unalloyed iron, Fe-Ni, Fe-Cr-Ni, Fe-Cr-Ni-Mo) via a metallothermic reduction process was investigated. Thermodynamic calculations and the experimental studies were performed on the basis of 100 g of MS. The effects of different stoichiometric amounts of MS and aluminum (Al) powders (as reductant) were investigated for the production of unalloyed Fe. While, different amount of metal oxide ratios and their effects on metal recoveries, compositions and microstructure of final alloys were studied during Fe-based alloys production. The highest iron recovery during unalloyed Fe production was obtained as 95.14 % by using 100 g of MS and 100 % stoichiometric Al (28.6 g) containing mixture. In Fe-based alloys production series, the highest metal recovery values were reached up to 95.0 % for Fe, 95.1 % for Ni, 68.3 % for Cr and 77.2 % for Mo, respectively.
Introduction
Mill scale (MS) is a waste material generated during the continuous casting of steel [1]. It is an oxide layer which forms on the surface of the steel ingots during cooling. This layer contains a mixture of iron (II) and iron (III) oxides with high iron content [2]. Generally, about 35–40 kg of MS can be formed per one metric ton of hot rolled steel produced [3]. MS can be a beneficial resource when it is recycled. Annually, 13.5 million metric tons of MS arise in the World [4]. Most of the steel production companies do not reevaluate MS which is arisen during their production routes. When the volume and the quantity of produced MS are considered, this fact brings out the necessity and the interest in finding an economical way to reprocess it [5].
There are several utilization methods of MS as an additive. Such as, MS can be used as a fine aggregate in cement, as an adsorbent of lead ions from aqueous solutions, as an iron oxide source for sintering applications, and as a raw material for the sintering process in the integrated steel plants [1]. MS can be also reused via another application, namely the processing of MS into nano-scale particles. These particles can be used for hydrogen fuel cell, medical imaging and water remediation technologies [6]. Furthermore, MS originating from steelmaking plants can be employed to prepare some iron oxide pigments via specific precursors [3]. It can be also evaluated as an admixture in electromagnetic interference shielding and absorbing building materials [7]. The use of MS as iron source in direct reduction method is a relatively newer method compared to the carbothermal reductive smelting process [2]. Some of the mentioned methods above are in laboratory scale, on the other hand the processes which are available for high-volume production are limited such as filler in road construction, aggregate in cement, iron source for reductive smelting and iron source for direct reduction [1, 2].
Metallothermic reduction is an alternative method for carbothermal reduction for metal production. The main principle is based on the reduction of a metal from its metal oxide by another metal which has a greater affinity to oxygen. Metallothermic reduction is the second subset of metallothermic reactions; the first subset refers to the metallothermic synthesis from elemental reactants and the third subset refers to the synthesis reaction of a compound from the products of two or more metallothermic reduction reactions in the same batch with/without the existence of elemental additions [8]. Metallothermic reactions can be performed in a system reacting in the mode of wave propagation because of heat transfer from hot products to cold reagents after local initiation (ignition) of the process. Metallothermic reduction has many advantages such as simple operation, less process time, high purity carbon-free products etc. It can be applied to produce a wide range of metals and alloys including ferroalloys and intermetallic compounds. Also metallothermic processes has many prominent properties when they are compared with carbothermal reductive smelting processes such as low energy requirement and low cost. In a metallothermic process, the ignition starts reactions and it propagates throughout the reactant mixture to obtain the desired product. However, the disadvantages of the process such as inhomogeneous or unreacted products due to undesirable reaction rates can be overcome by changing some parameters such as ignition temperature, particle size, additives and reaction atmosphere etc. [9, 10, 11 and 12]. Multi-component alloying is widely used in the development of many materials operating under extreme conditions (high temperatures and loads), in particular iron- and nickel-based superalloys [13].
In the present study, it was aimed to produce carbon-free Fe-based alloys via metallothermic reduction starting from a mixture containing MS obtained during continuous steel casting. Due to non-carbon containing reduction, the carbon footprint value of the process is obtained much lower than the commercial production process.
Theoretical background
In a metallothermic process oxygen affinity of a metal is one of the most important parameters. In order to obtain MeI given in eq. (1), MeII must have higher oxygen affinity than that of MeI.
Adiabatic temperature (Tad) and specific heat (S.H.) are the other important thermodynamic parameters to predict the self-sustainability of a metallothermic reaction. If the Tad of reaction is higher than 1527 °C (1800 K), the reaction is in a self-sustainable mode [14].
Specific heat is calculated by dividing the enthalpy of the reaction at 25 °C by the sum of the molecular weights (MW) of the reaction products. The interactions between the reaction products and the specific heat values are given below.
Specific heat<2250 J/g refers to insufficient heat to melt the charge and to separate metal and slag and, the reaction is not self-sustainable,
Specific heat>4500 J/g refers to a violent reaction and may even be explosive, so it may cause to loss of reaction products by scattering,
Specific heat: 2250–4500 J/g refers to a controlled and self-sustaining metallothermic reaction (it is desired.) [14].
Some of the commonly used metallothermic reactions and their specific heat values are given in eqs. (2)–(6), respectively.
The energy values of the given reactions were calculated using the “Reaction” module of FactSageTM 6.4 “The Integrated Thermodynamic Databank System”. The possible phases with amounts of products and the adiabatic temperature of products were simulated using the “Equilib” module of FactSageTM 6.4. This module utilizes the Gibbs energy minimization method [15]. Here, FactPS, FToxide, SGTE (2014), and BINS databases were selected for calculations, and conflicts were collated manually. Thus, the results were given due to the most probable reactions in the selected conditions.
Experimental
MS which was utilized in this study was formed in the continuous steel casting plant of Colakoglu Metalurji and it was of nonoily nature. The other raw materials used in the metallothermic reactions were NiO (99.0 % purity with 45 µm average particle size), Cr2O3 (99.0 % purity with 150 µm average particle size), MoxOy (98.0 % purity with 150 µm average particle size) and Al (96.0 % purity with 200 µm average particle size).
In the experiments, a mixture of mill-scale and Al powders with or without NiO, Cr2O3, MoxOy additions were used in order to produce unalloyed Fe, Fe-Ni, Fe-Ni-Cr, and Fe-Ni-Cr-Mo alloys with selected compositions. The weight of the initial mixtures were depending on the basis of 100 g of MS. The raw materials were dried in a drying oven at 105 °C for 60 min and were mixed by using a turbula mixer for 15 min. All experiments were conducted in a copper crucible having an inner diameter of 40 mm and height of 140 mm. A power supply (called variac) was employed for the ignition of metallothermic reactions. The flowchart of the experimental studies is given in Figure 1.
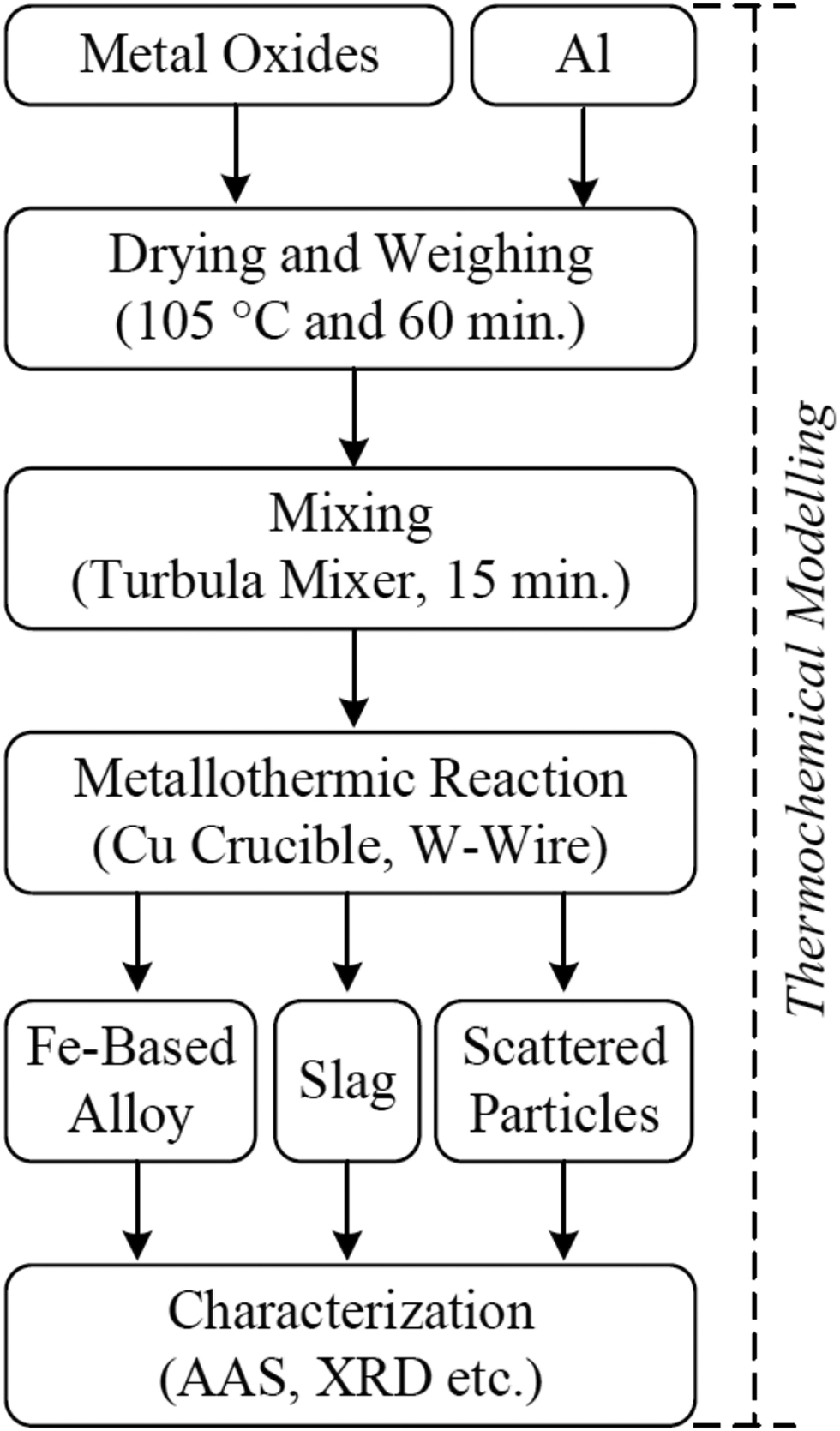
Flowchart of the experimental studies.
Raw materials and products were analyzed by wet chemical analysis due to EPA Method 3050B by using an atomic absorption spectrometer (AAS, Perkin Elmer Analyst 800). The phase compositions of the samples were characterized by X-ray diffractometer (PANalytical PW3040/60, Cu Kα radiation) equipment with X'Pert HighScore+ software and ICDD, ICSD databases.
Micrographs of the selected alloys were taken by using Olympus GX71 optical microscope. Prior to taking the micrographs, metallothermic produced alloys were cut, hot molded, grinded and polished. SiC grinding papers from 320 grit to 2500 grit and 2 µm Al2O3 polishing solution were used for grinding and polishing stages. 2 vol.% HNO3-containing Nital Solution (balance is ethanol) was prepared and used for etching to create contrast on the surfaces of the alloys.
Results and discussion
Thermodynamic simulation results and discussion
Before the experiments, a thermochemical simulation was performed to estimate the effect of precursor compositions on the process. Effect of Al addition to the reactant mixture of MS on the obtained products under air atmosphere was investigated. With increasing Al addition into the green mixture (61.19 g Fe2O3 + 31.63 g FeO + 3.56 g Fe), Fe contents in the liquid alloy slightly increased. Since the adiabatic combustion temperatures of the metallothermic reactions obtained by the high energetic precursor mixtures change between 2227 and 3227 °C, the combustion products (alloy and oxide) would be in liquid-phase state. Thus, the melting temperature difference clearly would result in a very good separation of the multiphase alloy and the slag phase. The systems having appropriate conditions were identified and the experiments were carried out. Figure 2 presents MS reduction simulation, Al addition increases adiabatic temperature to 2830 °C. At this temperature iron is in liquid phase, after 28 g Al addition it also becomes in liquid phase and pollutes the iron structure. Thus, 28 g Al addition was determined 100 % stoichiometric ratio for this process.
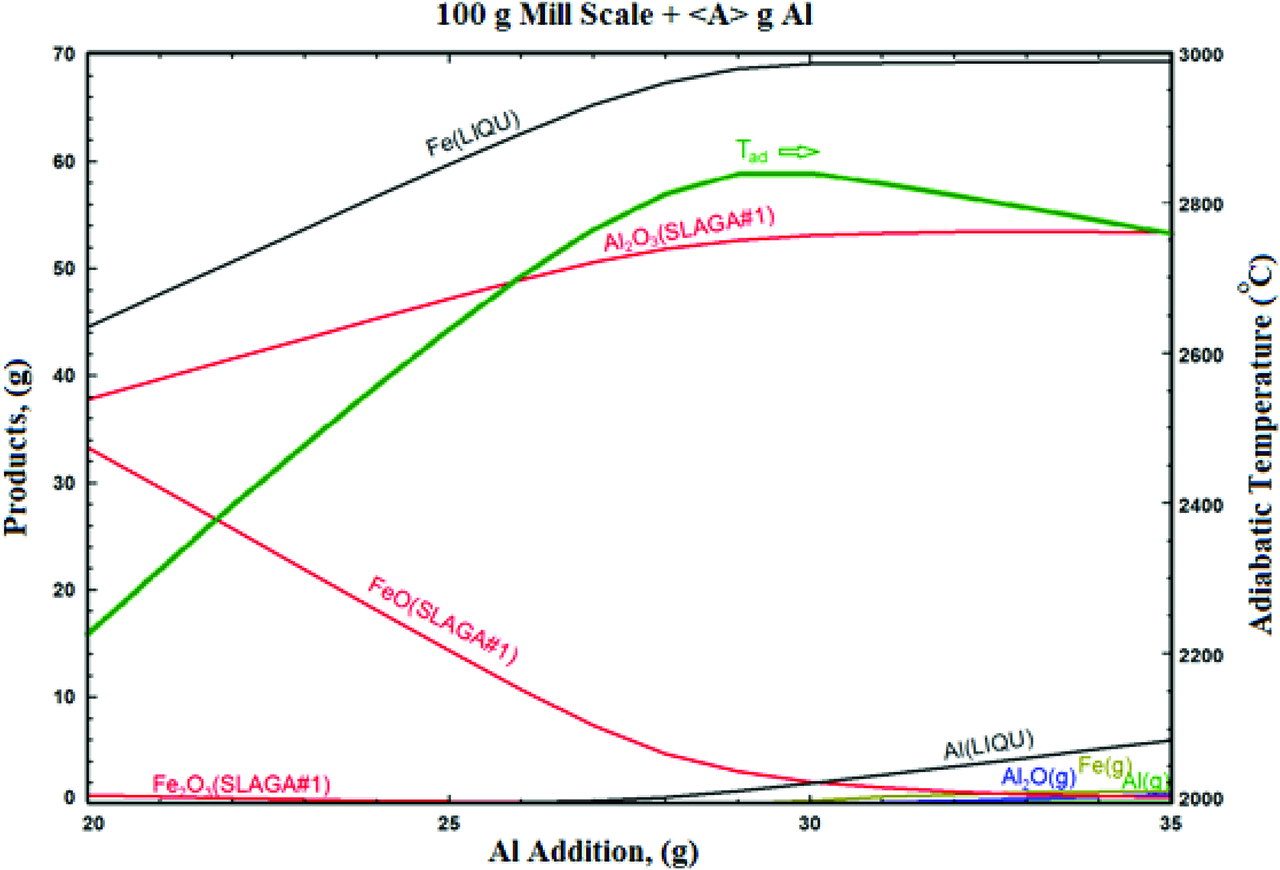
The change of adiabatic temperature with the increase in Al reductant amount on mill scale - Al reactant system.
Figure 3 shows the change of adiabatic temperature and amount of possible products with Al addition. The estimated alloy composition was selected due to the composition range of Grade 316 L stainless steel.
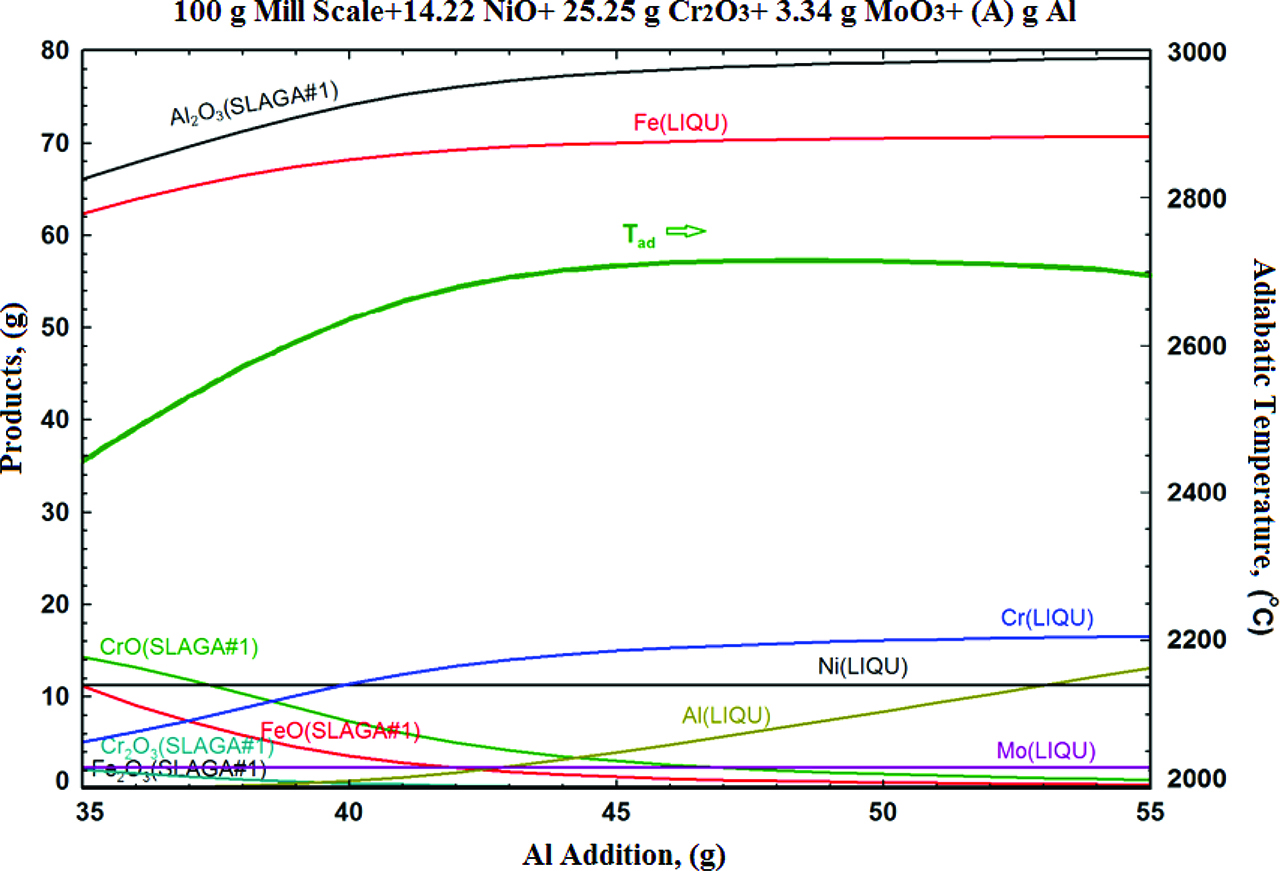
The change of adiabatic temperature with the increase in Al reductant amount on mill scale - NiO - Cr2O3 - MoO3 - Al reactant system.
The adiabatic temperature of the products increases with the increasing in Al addition. Also metal to metal oxide ratio is increased too. However, with Al addition higher than 40 g, the final liquid metal product can contain unreacted Al. This can affect the composition difference between the estimated and produced. The stoichiometric requirement of Al amount was calculated as 42.2 for given example (100 g MS+14.22 g NiO+25.25 g Cr2O3+3.34 g MoO3). At this point adiabatic temperature is 2700 °C which is higher than 1527 °C. Thus, the melting temperature difference clearly will result in a very good separation of the multiphase alloy and the slag phase.
In a carbothermic reduction base process, a carbon containing source is utilized for reduction. For the given example in Figure 3, the stoichiometric amount of carbon is measured as 11.42 g. Reduction reaction and melting of the final product are both endothermic processes. So, totally 390.35 kJ of energy is required due to the total reduction and heating of the final liquid metal product at 1700 °C. Theoretically, 0.95 mol of CO2 gasses having a volume of 21.29 cm3 can be produced. For each 80.21 g of Fe-based produced, 41.85 g of CO2 gasses will be produced. So, 522 kg of CO2 gasses can be produced during the production of 1 metric ton of alloy.
Experimental results and discussion
Table 1 displays the chemical analysis of the MS. XRD pattern of MS was given in Figure 4. According to the figure, MS contains hematite (Fe2O3), magnetite (Fe3O4) and wustite (FeO). Addition to MS, metal oxides and metallic Al powders were also used and their XRD patterns were given in Figure 5.
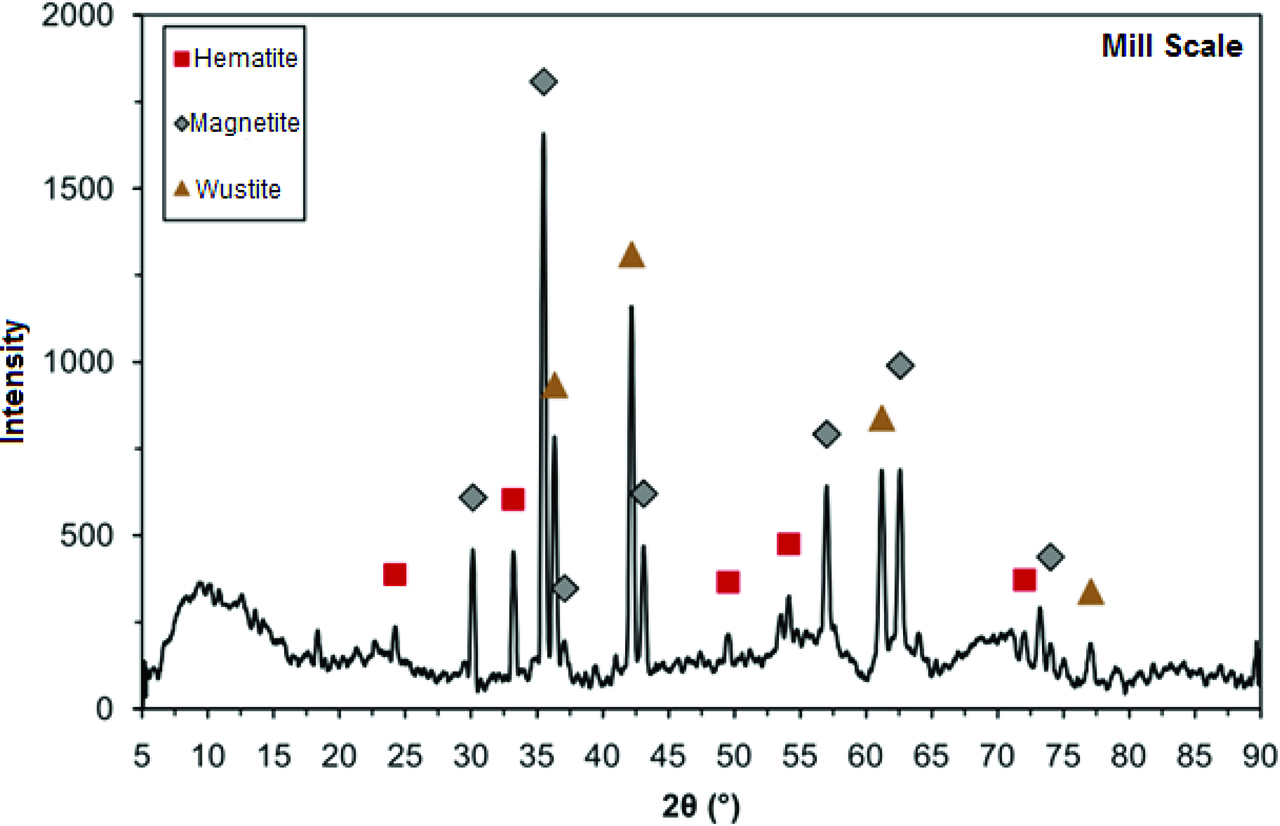
XRD pattern of mill scale.
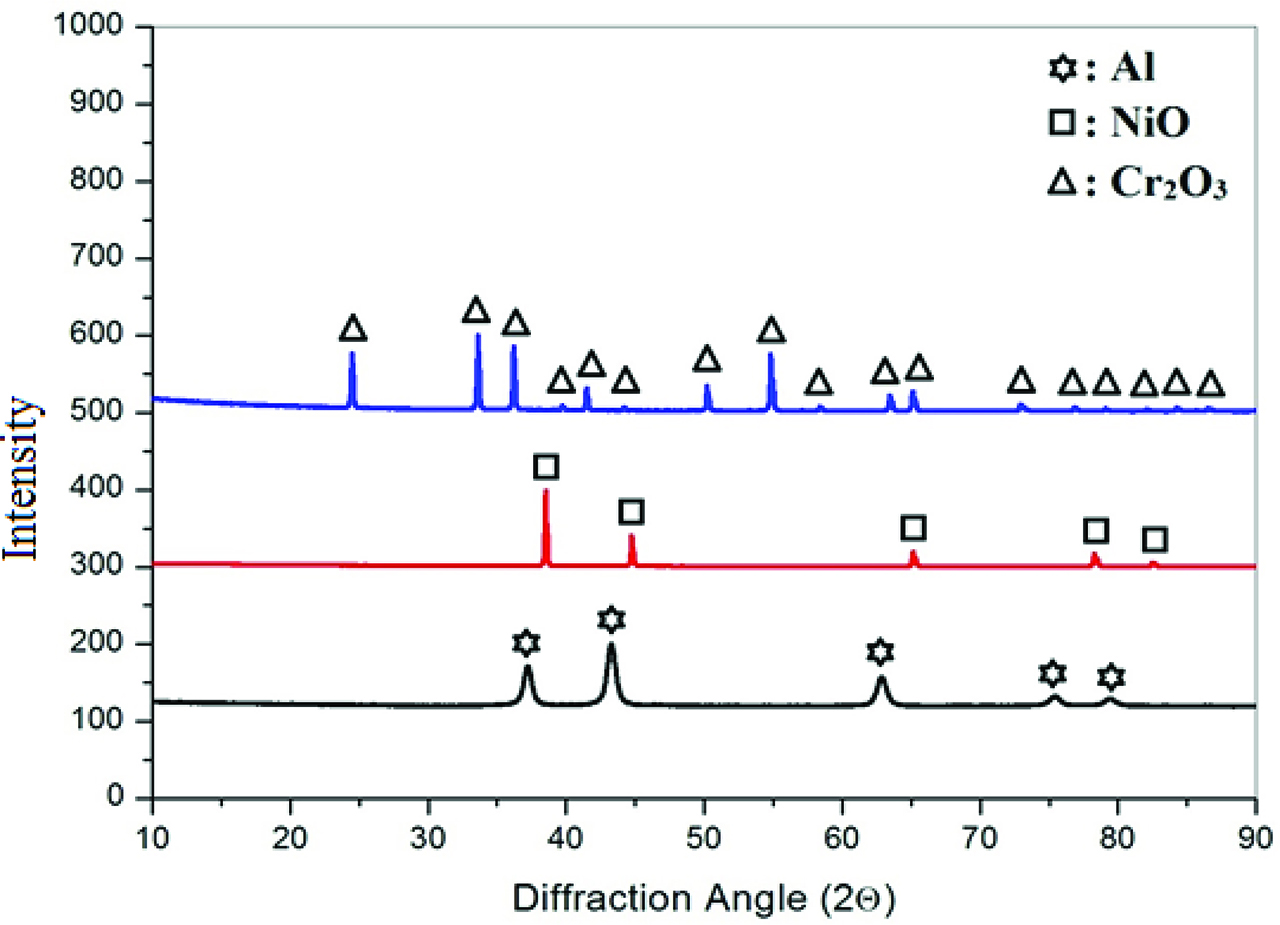
XRD patterns of selected reactants and reductant.
Chemical analysis of mill scale (wt.%).
| Total iron | Fe2+ | Fe3+ | Fe0 | Cu | SiO2 | Mn |
|---|---|---|---|---|---|---|
| 71.04 | 24.59 | 42.80 | 3.65 | 0.75 | 0.39 | 0.14 |
The impurities in NiO are Co3O4, MgO, SiO2, CaO, Na2O, and Fe2O3 with concentrations of 450 ppm Co, 200 ppm Mg, 110 ppm Si, 90 ppm Ca, 80 ppm Fe, and 80 ppm Na. MoxOy contains 56.5 % MoO3, 18.6 % MoO2, 22.9 % Mo4O11, 1.3 % SiO2 and 0.7 % Cu2O by weight, respectively. The mass percent of impurities in Al powders are 1.4 % Fe, 1.2 % Mg, 0.4 % Si, 0.4 % Cu and 0.4 % Zn, respectively.
In order to produce unalloyed Fe, a trial-and-error procedure was utilized. The first initial mixture was contained 100 g of MS and 100 % stoichiometric Al (28.6 g). Metallic recovery value was measured as 95.14 % with a total mass loss of 1.24 %. The mass losses were due to the scattering (from the exothermic reaction) and volatilization (from high adiabatic temperature). In the second mixture, the weight of MS was increased to 110 g with same amount of Al. The metal recovery value was decreased to 92.25 % (while the mass loss occurred as 1.37 %). Then weight of mixture was changed and experiments were repeated with 200 g of MS containing mixtures. The metal recovery values were measured as 92.32 %, and 73.64 %, respectively. While the material losses were as 5.25 %, and 4.66 %, respectively. The highest metal recovery value was obtained with the mixture containing 100 g of MS and the 100 % stoichiometric amount of Al. This starting mixture was utilized for the further Fe containing alloy production experiments. All metal recovery ratios were calculated through eq. (7).
Stainless steel is mainly different from unalloyed steel in terms of the amount of alloying metals (chromium, nickel, molybdenum etc.). Unprotected and unalloyed steel is readily rusted when it is exposed to air and moisture. This iron oxide film (rust) is active and accelerates corrosion by forming more iron oxide and, due to the greater volume of the iron oxide, it tends to flake and fall away. Stainless steels contain sufficient chromium to form a passive film of chromium oxide which prevents further surface corrosion and blocks corrosion from spreading into the metal's internal structure. Chromium oxide film and steel bond very strongly and remain attached to the surface due to the similar size of the steel and chromium oxide [16]. Because of these properties, in the second set of the experiments, productions of Fe-Ni, Fe-Cr-Ni and Fe-Cr-Ni-Mo alloys were investigated by using a mixture of MS and Al with NiO, Cr2O3 and MoxOy.
In second set of the experiments, it was aimed to produce Fe-based alloys between the composition limits of ferronickel and selected stainless steel alloys (Grade 201, 301, 304, 305, 316, and 317). The stainless steel standards were given in Table 2. The list of experimental parameters, weight of the initial mixtures and final products were given in Table 3. NiO addition into the green mixtures decreased the total metal recovery. On the other hand, Cr2O3 addition into the green mixtures decreased the scattered ratio due to the lower exothermicity of Cr2O3 reduction. Metal recovery ratios for the alloying metals in metallothermic Fe-based alloys were shown in Table 4. Fe recovery decreased with the NiO addition into the green mixture but it was ascended with the Cr2O3 addition. The highest metal recoveries were measured as 95.00 % Fe, 95.11 % Ni, 68.25 % Cr and 77.22 % Mo by weight, respectively. The distribution of metallic phases was given in Figure 6. The distribution of metals among the alloy, slag and scattered part were calculated by eq. (8), metallic distributions were calculated due to the chemical compositions and the weight of products distribution ratios of metal in alloy are also known as production efficiency of each metals. The compositions of the final alloys were measured by wet chemical analysis and given in Table 5. Ni contents in the final alloys were obtained within the limits of the standard compositions (estimated values). However, Cr and Mo contents were measured much less than the expected values. Cr contents in the final alloys were obtained between 60.44% and 85.10 % of the estimated alloy compositions, while, Mo composition values were obtained 74.29–87.25 % less than the expected values.
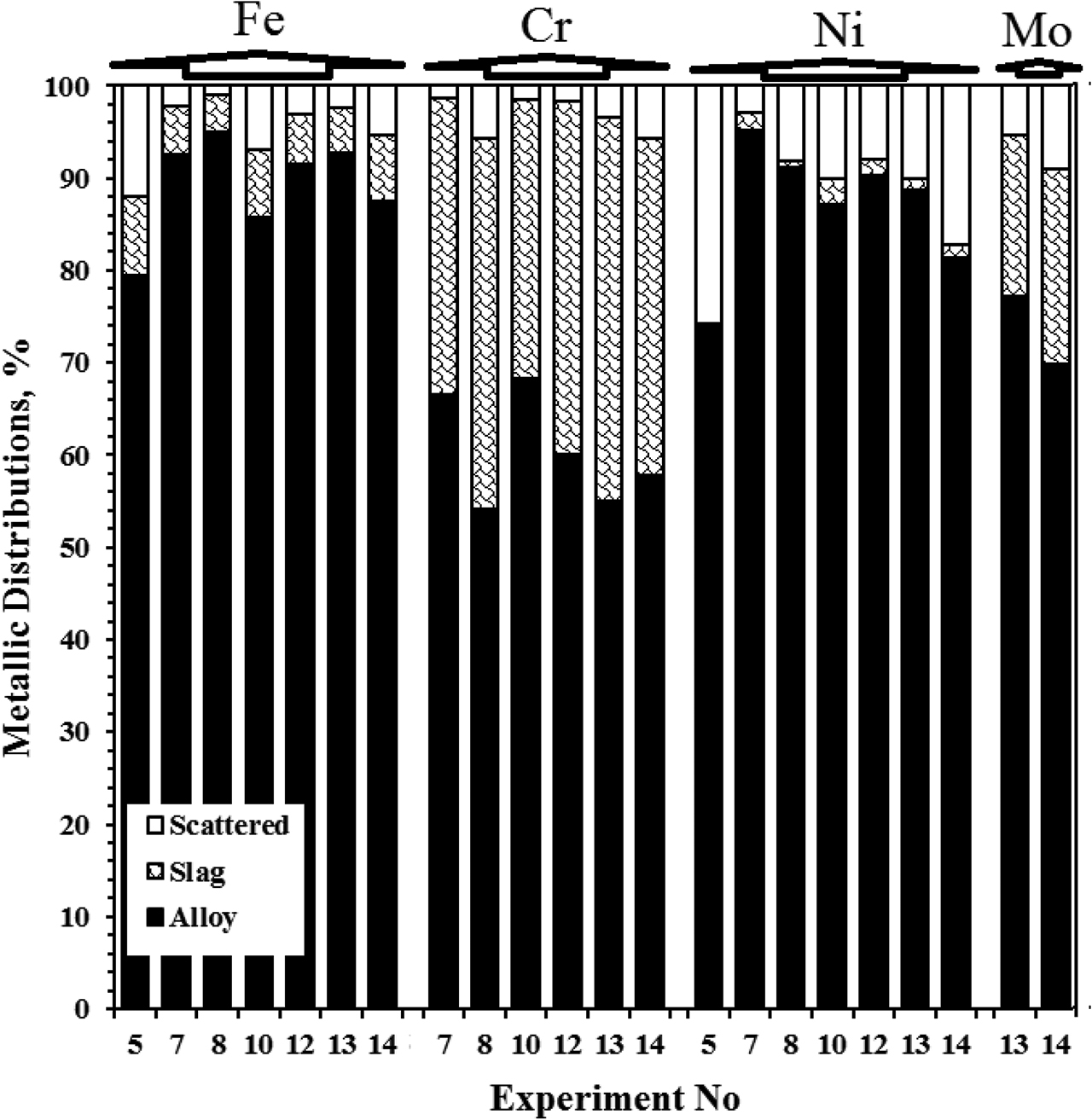
Metal distributions in alloy, slag and scattered parts (Experiment numbers and corresponding experimental conditions are given in Table 5 in detail.).
Stainless steel grades.
| Steel grade | Chemical composition, % | Others | ||||||
|---|---|---|---|---|---|---|---|---|
| C | Mn | Si | Cr | Ni | P | S | ||
| 201 | 0.15 | 5.5–7.5 | 1.0 | 16.0–18.0 | 3.5–5.5 | 0.06 | 0.03 | 0.25 N |
| 301 | 0.15 | 2.0 | 1.0 | 17.5–22.0 | 6.0–8.0 | 0.045 | 0.03 | – |
| 304 | 0.08 | 2.0 | 0.7 | 18.0–20.0 | 8.0–12.0 | 0.045 | 0.03 | 0.10 N |
| 305 | 0.12 | 2.0 | 1.0 | 17.0–19.0 | 10.5–13.0 | 0.045 | 0.03 | – |
| 316 | 0.08 | 2.0 | 1.0 | 16.0–18.0 | 10.0–14.0 | 0.045 | 0.03 | 2.0–3.0 Mo |
| 317 | 0.08 | 2.0 | 1.0 | 18.0–20.0 | 11.0–15.0 | 0.045 | 0.03 | 3.0–4.0 Mo |
Weight of initial mixtures, final products and total metal recovery ratios for metallothermic stainless steel alloys.
| Exp. no | Grade | Initial Mixture, g | Final Products, g | Total Metal Recovery, % | Scattered Ratio, % | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mill Scale | Al | NiO | Cr2O3 | MoO3 | Alloy | Slag | ||||
| 5 | FeNi | 100 | 37.7 | 25.4 | – | – | 73.2 | 68.8 | 78 | 10.9 |
| 7 | 304 S.S. | 100 | 40.8 | 12.5 | 25.9 | – | 88.0 | 86.6 | 89 | 3.0 |
| 8 | 305 S.S. | 100 | 41.8 | 15.4 | 26.6 | – | 89.7 | 80.1 | 88 | 1.7 |
| 10 | 201 S.S. | 100 | 38.0 | 5.7 | 22.5 | – | 80.2 | 85.0 | 88 | 6.8 |
| 12 | 301 S.S. | 100 | 38.8 | 8.3 | 23.2 | – | 81.5 | 88.7 | 87 | 2.4 |
| 13 | 316 S.S. | 100 | 42.2 | 14.2 | 25.2 | 3.3 | 88.5 | 82.3 | 87 | 2.8 |
| 14 | 317 S.S. | 100 | 44.7 | 16.6 | 28.6 | 5.1 | 94.0 | 86.5 | 87 | 4.2 |
Metal recovery ratios for the alloying metals in metallothermic stainless steel alloys (%).
| Exp. no | Grade | Fe | Cr | Ni | Mo |
|---|---|---|---|---|---|
| 5 | FeNi | 79.39 | – | 74.09 | – |
| 7 | 304 S.S. | 92.60 | 66.51 | 95.11 | – |
| 8 | 305 S.S. | 95.00 | 54.21 | 91.20 | – |
| 10 | 201 S.S. | 85.74 | 68.25 | 87.18 | – |
| 12 | 301 S.S. | 91.42 | 60.00 | 90.25 | – |
| 13 | 316 S.S. | 92.75 | 55.06 | 88.69 | 77.22 |
| 14 | 317 S.S. | 87.42 | 57.74 | 81.31 | 69.83 |
Compositions of metallothermic process stainless steel alloys (wt.%).
| Exp. no | Grade | Fe | Cr | Ni | Mo | Al |
|---|---|---|---|---|---|---|
| 5 | FeNi | 77.05 | – | 20.0 | – | 2.9 |
| 7 | 304 S.S. | 74.75 | 13.26 | 10.51 | – | 1.4 |
| 8 | 305 S.S. | 75.24 | 10.89 | 12.18 | – | 1.7 |
| 10 | 201 S.S. | 75.95 | 12.97 | 4.82 | – | 6.2 |
| 12 | 301 S.S. | 79.69 | 11.57 | 7.15 | – | 1.5 |
| 13 | 316 S.S. | 74.45 | 10.62 | 11.07 | 1.9 | 1.8 |
| 14 | 317 S.S. | 66.07 | 11.90 | 11.17 | 2.5 | 8.3 |
Optical microscope micrographs of the selected alloys were shared in Figure 7. Two different phases can be easily seen in Figure 7a. Lighter areas represent α-Fe phase (higher aluminum containing solid-solution), and darker areas represent γ-Fe phase (max 4 % Al dissolved solid-solution). The microstructure images of other alloys (Fe-based alloys) were similar. The darker areas represent Cr-rich α-solid solution, and the lighter areas represent Fe-Ni-rich γ-solid solution. In Figure 7d, there are three different phases that were detected in the microstructure. Cr composition is higher in the darker grey areas, while composition of Al is higher in the lightest grey areas.
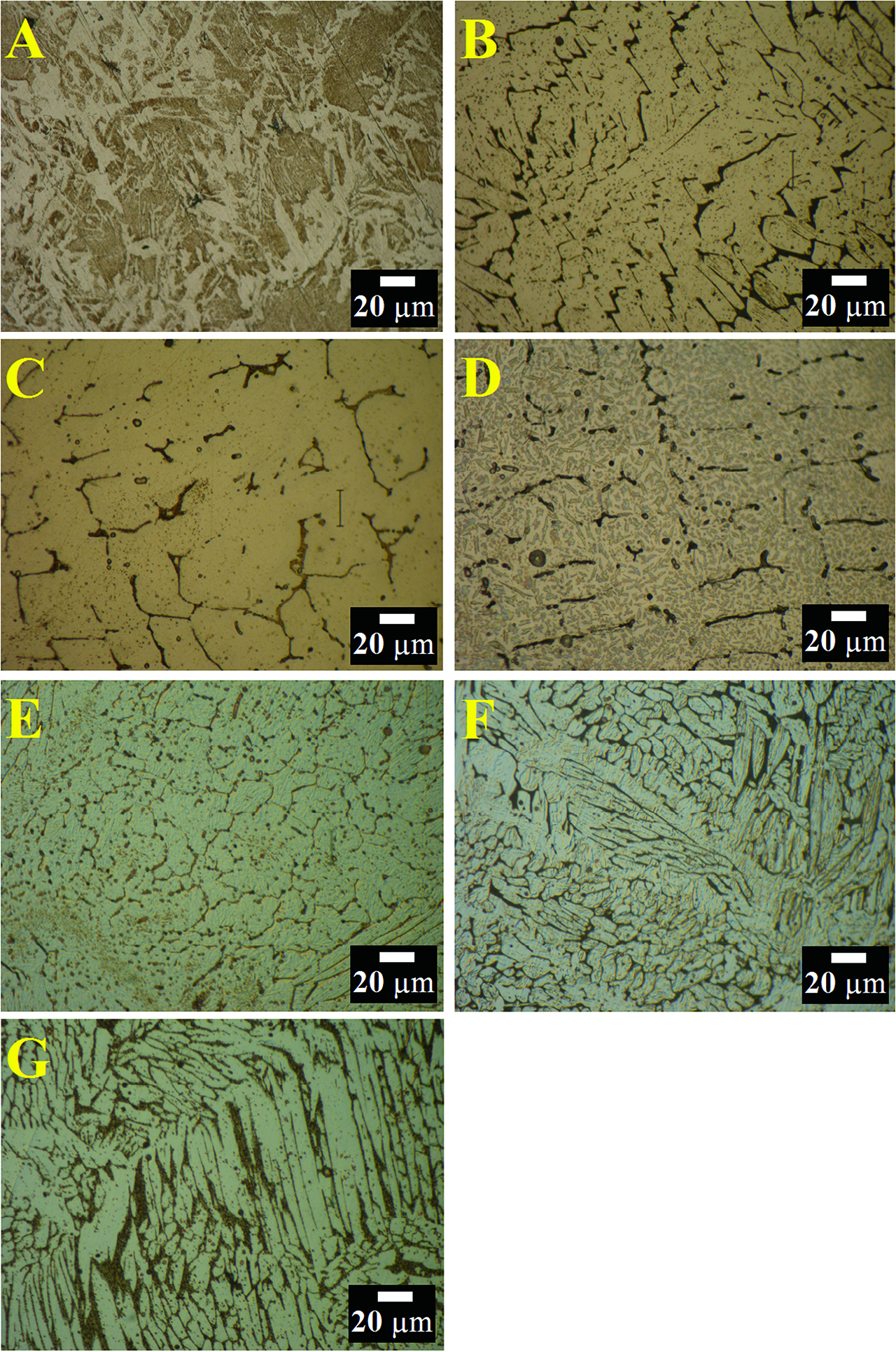
Optical microscope micrographs of the selected Fe-based alloys which were produced by using metallothermic reduction process (A: Unalloyed iron, B: 304, C: 305, D: 201, E: 301, F: 316, G: 317).
Conclusions
In this study, Fe-based alloys were produced via metallothermic reduction process from MS which is formed in steel continuous casting plants. In metallothermic reduction experiments (MS-NiO-Cr2O3-Al system), NiO addition did not have any positive effects on the metal recovery. However NiO+Cr2O3 addition made an increase in the metal recoveries. For the experiments to produce unalloyed iron, the highest total metal recovery was obtained as 95.14 % for the experiment conducted with 100 % stoichiometric MS (100 g) and 100 % stoichiometric Al (28.6 g). Between the experiments to produce Fe-based alloys, the highest metal recovery values were measured as 95.00 % Fe, 95.11 % Ni, 68.25 % Cr and 77.22 % Mo by weight, respectively.
Acknowledgements
The authors are pleased to acknowledge Kağan Benzeşik (ITU) and Selçuk Yeşiltepe (ITU) for assistance in metallography stage.
References
[1] S. Mauthoor, R. Mohee and P. Kowlesser, Waste Manage., 34 (2014) 1800–1805.10.1016/j.wasman.2013.12.014Search in Google Scholar PubMed
[2] O. Yucel, F. Demirci, A. Turan and M. Alkan, High Temp. Mater. Processes, 32 (4) (2013) 405–412.10.1515/htmp-2012-0167Search in Google Scholar
[3] M.A. Legodi and D. De Waal, Dyes Pigm., 74 (1) (2007) 161–168.10.1016/j.dyepig.2006.01.038Search in Google Scholar
[4] S. Cho and J. Lee, Met. Mater. Int., 14 (2) (2008) 193–196.10.3365/met.mat.2008.04.193Search in Google Scholar
[5] M.Z. Ruhiyuddina, C.P. Faizul, D. Murizam and A.R.M. Nazri, Adv. Mat. Res., 626 (2013) 1001–1005.10.4028/www.scientific.net/AMR.626.1001Search in Google Scholar
[6] A.-M. Azad, S. Kesavan and S. Al-Batty, Key Eng. Mater., 380 (2008) 229–255.10.4028/www.scientific.net/KEM.380.229Search in Google Scholar
[7] G. Bantsis, C. Sikalidis, M. Betsiou, T. Yioultsis and T. Xenos, Ceram. Int., 37 (2011) 3535–3545.10.1016/j.ceramint.2011.06.010Search in Google Scholar
[8] A. Turan, Yerli Hammaddelerden Hareketle TiB2 Esaslı İleri Teknoloji Seramiklerin Üretilmesi (PhD Thesis), Istanbul Technical University, Istanbul, Turkey, (2014), 37–54.Search in Google Scholar
[9] A.G. Merzhanov, J. Mater. Chem., 14 (2004) 1779–1786.10.1039/b401358cSearch in Google Scholar
[10] R. Ochoa, A. Flores, J. Torres, J. Guía and R. Muñiz, Can. Metall. Q., 55 (2) (2016) 210–220.10.1080/00084433.2016.1146432Search in Google Scholar
[11] G.F. Tavadze and A.S. Shteinberg, Production of Advanced Materials by Methods of Self-Propagating High-Temperature Synthesis, Springer, Heidelberg, Germany, (2013).10.1007/978-3-642-35205-8Search in Google Scholar
[12] O. Yücel, F.C. Sahin and A. Tekin, High Temp. Mater. Processes, 15 (1–2) (1996) 103–106.10.1515/HTMP.1996.15.1-2.103Search in Google Scholar
[13] V.N. Sanin, V.I. Yukhvid, D.M. Ikornikov, D.E. Andreev, N.V. Sachkova and M.I. Alymov, Dokl. Phys. Chem., 470 (2) (2016) 421–426.10.1134/S001250161610002XSearch in Google Scholar
[14] A. Turan, M. Bugdayci and O. Yucel, High Temp. Mater. Processes, 34 (2) (2015) 185–193.10.1515/htmp-2014-0021Search in Google Scholar
[15] C.W. Bale, et al., Calphad, 33 (2) (2009) 295–311.10.1016/j.calphad.2008.09.009Search in Google Scholar
[16] C.Q. Jessen, Stainless Steel and Corrosion, Damstahl, Denmark (2011).Search in Google Scholar
© 2018 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Research Articles
- Numerical Simulation of the Electron Beam Welding and Post Welding Heat Treatment Coupling Process
- Effect of Ti and Ta on Oxidation Kinetic of Chromia Forming Ni-Base Superalloys in Ar-O2-Based Atmosphere
- Effects of Cerium on the Inclusions and Pitting Corrosion Behavior of 434 Ferritic Stainless Steel
- Critical Assessment of Activities of Structural Units in Fe–Al Binary Melts Based on the Atom and Molecule Coexistence Theory
- A Yield Stress Model for a Solution-Treated Ni-Based Superalloy during Plastic Deformation
- Stress Relaxation Behaviour and Creep Constitutive Equations of SA302Gr.C Low-Alloy Steel
- Effects of Inner Defects on Creep Damage and Crack Initiation for a Brazed Joint
- Experimental and Numerical Investigations on Hot Deformation Behavior and Processing Maps for ASS 304 and ASS 316
- Production of Iron Based Alloys from Mill Scale through Metallothermic Reduction
- Effect of Nb and V on Austenite Grain Growth Behavior of the Cr-Mo-V Steel for Brake Discs
- A Thermodynamic Study of the Reduction of a Limonitic Laterite Ore by Methane
- Electrochemical and Phase Analysis of Si(IV) on Fe Electrode in Molten NaCl-NaF-KCl-SiO2 System
- Characterization of Hot Deformation Behavior for Pure Aluminum Using 3D Processing Maps
- Effect of Chromium Addition on the Cyclic Oxidation Resistance of Pseudo-Binary (Mo,Cr)3 Si Silicide Alloy
- Equiaxed Solidification of 430 Ferritic Stainless Steel Nucleating on Core-Containing Ti
- FE Analysis of Dynamical Recrystallization during the Seamless Tube Extrusion of Semicontinuous Casting Magnesium Alloy and Experimental Verification
- Study on the Reblow Model for Medium-High Carbon Steel Melting by Converter
- Short Communication
- Effect of B2O3 on Slag-Metal Reaction between CaO-Al2O3-Based Mold Flux and High Aluminum Steel
- Review Article
- Computation of the Thermal Residual Stresses in SiC/SiC Composites with Multi-Layered Interphases by Using ANN with the Structure of Random Forest
- Research Articles
- Failure Analysis of the Corroded Water Wall Tube in a 50MW Thermal Power Plant
- CO2 Absorption of Powdered Ba2Fe2O5 with Different Particle Size
- Induced-Pitting Behaviors of MnS Inclusions in Steel
Articles in the same Issue
- Frontmatter
- Research Articles
- Numerical Simulation of the Electron Beam Welding and Post Welding Heat Treatment Coupling Process
- Effect of Ti and Ta on Oxidation Kinetic of Chromia Forming Ni-Base Superalloys in Ar-O2-Based Atmosphere
- Effects of Cerium on the Inclusions and Pitting Corrosion Behavior of 434 Ferritic Stainless Steel
- Critical Assessment of Activities of Structural Units in Fe–Al Binary Melts Based on the Atom and Molecule Coexistence Theory
- A Yield Stress Model for a Solution-Treated Ni-Based Superalloy during Plastic Deformation
- Stress Relaxation Behaviour and Creep Constitutive Equations of SA302Gr.C Low-Alloy Steel
- Effects of Inner Defects on Creep Damage and Crack Initiation for a Brazed Joint
- Experimental and Numerical Investigations on Hot Deformation Behavior and Processing Maps for ASS 304 and ASS 316
- Production of Iron Based Alloys from Mill Scale through Metallothermic Reduction
- Effect of Nb and V on Austenite Grain Growth Behavior of the Cr-Mo-V Steel for Brake Discs
- A Thermodynamic Study of the Reduction of a Limonitic Laterite Ore by Methane
- Electrochemical and Phase Analysis of Si(IV) on Fe Electrode in Molten NaCl-NaF-KCl-SiO2 System
- Characterization of Hot Deformation Behavior for Pure Aluminum Using 3D Processing Maps
- Effect of Chromium Addition on the Cyclic Oxidation Resistance of Pseudo-Binary (Mo,Cr)3 Si Silicide Alloy
- Equiaxed Solidification of 430 Ferritic Stainless Steel Nucleating on Core-Containing Ti
- FE Analysis of Dynamical Recrystallization during the Seamless Tube Extrusion of Semicontinuous Casting Magnesium Alloy and Experimental Verification
- Study on the Reblow Model for Medium-High Carbon Steel Melting by Converter
- Short Communication
- Effect of B2O3 on Slag-Metal Reaction between CaO-Al2O3-Based Mold Flux and High Aluminum Steel
- Review Article
- Computation of the Thermal Residual Stresses in SiC/SiC Composites with Multi-Layered Interphases by Using ANN with the Structure of Random Forest
- Research Articles
- Failure Analysis of the Corroded Water Wall Tube in a 50MW Thermal Power Plant
- CO2 Absorption of Powdered Ba2Fe2O5 with Different Particle Size
- Induced-Pitting Behaviors of MnS Inclusions in Steel

