Abstract
The recovery of nickel from the oxidic nickeliferous laterite ores is receiving increasing attention due to the difficulty of recovering this metal from the sulphide ore deposits. One possible solution is to selectively reduce the nickel oxide in the ore, which could then be upgraded by, for example, magnetic concentration. In this article, a thermodynamic study was performed on the reduction of a limonitic laterite ore by methane. Methane was selected as the reducing agent as it has a lower environmental impact than carbon due to the reduced carbon dioxide emissions. The effects of temperatures and methane additions on the nickel recovery and nickel grade were investigated. High recoveries of over 95 % were predicted, but the grades were limited to about 2.5 % due to the formation of magnetite. The thermodynamic simulations for reduction by methane were in agreement with the experimental results in the literature for other reducing agents, reflecting the fact that the nickel oxide in the limonitic ore is relatively unstable. Thus, high recoveries could be achieved irrespective of the reducing agent involved.
Introduction
Limonitic laterite ore
The increasing difficulty of mining the nickel sulphide ore deposits has resulted in the nickeliferous laterite ores becoming the primary source of nickel worldwide [1, 2]. Despite being present in small concentrations in the sulphide ores, the nickel in the pentlandite ((Fe,Ni)8S9) can be concentrated using conventional flotation techniques. These ores also contain cobalt and other precious metals that can often be extracted. The contained sulphur can be combusted as fuel in the roasting process. This is not the case with the oxidic laterite ores, where the nickel is in solid solution in the host rock, which makes it impossible to upgrade. The oxidic laterite ores do not contain any precious metals or sulphur and the co-existing cobalt is difficult to recover. Furthermore, the process of recovering nickel from these ores may result in greater environmental issues than for the sulphide ores [3].
A nickeliferous laterite ore deposit is commonly comprised of two nickel-containing layers: limonite and saprolite. Both layers are formed at the surface through the weathering of ultramafic rock. For the purpose of this article, the investigation will be limited to the reduction of the nickel oxide in the limonitic ores. In the limonitic layer, the nickel is present in solid solution with iron in the mineral goethite (FeO·OH). The limonitic ores are primarily treated by hydrometallurgical techniques, such as high pressure acid leaching (HPAL), due to their low nickel (1 to 1.6 %), high iron (>40 %) and high water contents. This process is expensive since a large amount of acid is consumed.
It would be of interest to develop a less costly and a more environmentally friendly method of extracting nickel from these laterite ores. It has been proposed that the use of either heap leaching or nitric acid leaching could reduce the processing costs [4, 5]. However, it was also noted that these processes have not yet reached the capacity required for commercial application. Another option is to selectively reduce the nickel oxide in the limonitic ore, producing a ferronickel alloy with a high nickel content. This alloy could be further concentrated by, for example, magnetic separation, thereby reducing the cost of energy and acid consumptions. Research is being conducted on the selective reduction of nickel oxide using various reducing agents, such as coal, carbon monoxide, hydrogen and their mixtures.
Carbon is the most commonly employed reducing agent in pyrometallurgical processes owing to its ample supply and high energy density, which also allows it to be used as an energy source. Unfortunately, the use of carbon-based reagents, such as coal, generates adverse environmental impacts through the emission of carbon dioxide as well as organics, sulphur and ash. In order to mitigate these environmental effects, the use of alternative reductants, including other metals, hydrogen and methane (CH4), should be considered. It is feasible to reduce the nickel oxide in the limonitic ores using metals that are more reactive than nickel, but this method is limited due to the high cost. The use of hydrogen in lieu of carbon replaces the carbon dioxide emission with water vapour. The disadvantages of using hydrogen arise from the cost of manufacturing it from either water or methane. Methane is an alternative reductant that has been largely ignored due to it being both expensive and difficult to obtain [6, 7, 8]. However, methane has become more accessible and its utilization provides a significant reduction in the negative environmental effects of the process. As an example, a fifty percent reduction in carbon dioxide emission can be achieved for the production of an equal amount of metal if methane is employed instead of carbon-based reagents, such as coke and coal, according to the following reactions:
The purpose of this work is to study the selective reduction of the limonitic laterite ores by methane to produce a ferronickel alloy that can be further concentrated to increase its nickel content. This is achieved by developing a thermodynamic model of the process based on pre-existing models for reduction by carbon and hydrogen as well as activity coefficient data obtained from previous works [9, 10, 11, 12, 13]. In this work, the model has been used to analyse the effects of temperature and the amount of reductant on the nickel recovery, the nickel grade (of the ferroalloy), the behaviours of the gases, the behaviours of iron- and nickel-containing species, and the efficiencies of the reducing agents.
Previous thermodynamic studies
Previous thermodynamic studies on the reduction of laterite ores have focused on the use of either carbon, carbon monoxide or hydrogen as the reducing agents [9, 14, 15, 16, 17, 18, 19, 20, 21]. There has been little to no information available on reduction by methane. The studies, which utilized other reductants, are in agreement that it is possible to produce a ferronickel with high grade at low temperature, although it is impossible to form a completely pure nickel product through selective reduction. This is due to the fact that as the conditions become more reducing, the nickel recovery increases along with the amount of metallic iron, which reduces the nickel grade in the alloy. In addition to the co-reduction of the iron oxides, the studies also demonstrated that there might be several limitations to the kinetics of the reduction reaction when it proceeded below 600 °C. Only one article has examined the thermodynamics of utilizing methane as a reducing agent and pointed out its economic advantage over hydrogen [22]. The same study showed that the reduction behaviour at 600 to 800 °C exhibits similar characteristics as when carbon monoxide is employed as the reductant. Although the nickel grade in the product was not provided, a high nickel recovery was indicated.
Experimental studies
Experimental studies that have been conducted on the reduction of nickeliferous laterite ore by carbon, carbon monoxide or hydrogen are in general agreement with the predictions made by previous thermodynamic studies [20, 23, 24, 25, 26, 27, 28, 29]. In all of the experimental works, it was found that nickel always occurred with iron as a ferronickel alloy. It was also shown that the nickel recovery increased with temperature in contrast with the decreasing nickel grade. It is important to note that most of the experimental studies have been conducted on saprolitic ores. On the other hand, the limonitic ores are used in many of the thermodynamic studies since they are simpler to model than the saprolitic ores. Some of the experimental studies reported that the optimal temperature for the selective reduction of the limonitic laterite ore was 600 °C. It has been generally agreed that due to the different mineralogy of the ores, the limonite should be treated separately from the saprolite.
Similar to the thermodynamic studies, there have been limited experimental works on the gaseous reduction of the limonitic laterite ore by methane. In a thermogravimetric study on the reduction of nickel oxide by methane gas at 600 to 750 °C, it was shown that increasing the reduction temperature and the CH4 to Ar ratio in the gas atmosphere resulted in an increased rate of reaction [30]. The reduction reaction of the nickel oxide reached completion in approximately 15 minutes under an atmosphere with 20 % methane at a temperature of 660 °C. In addition to observing the rate of reaction, the authors also proposed that the gaseous by-product, which consisted of a mixture of H2 and CO, could be utilized in the synthesis of hydrocarbons. This increases the economic benefit of using methane as a reductant.
There have been several other experimental works that examined the use of methane or hydrogen-enriched methane in the reduction of various metal oxides. As an example, the use of methane in the reduction of cobalt oxide has been found to give rise to carbon deposition that impedes diffusion [31]. This has also been reported by other authors studying the use of carbon monoxide as a reducing agent [23, 29] as well as those who examined the reduction of various metal oxides by methane-containing gas [8].
Equilibrium considerations
As mentioned previously, nickel oxide in the limonitic ore is present in solid solution in the goethite. Dehydroxylation of the nickel-containing goethite occurs under oxidizing conditions between 250 and 350 °C. The goethite will be converted to hematite as follows:
while under reducing conditions, magnetite will form as follows:
Subsequently, at higher temperatures the nickel ferrite decomposes to nickel oxide and magnetite as follows:
for 0<x<1. The reactions for the overall reduction of the iron and nickel oxides by methane in the limonitic ore are as follows:
and the overall reaction for the reduction of the nickel ferrite to nickel and iron is as follows:
In an actual reduction process, the methane decomposes to carbon and hydrogen. Carbon monoxide is also produced as the reduction reaction proceeds. As such, reduction of the metal oxides can be due to carbon, hydrogen or carbon monoxide. The hematite in the dehydroxylated ore will initially be reduced to magnetite (Fe3O4), which has the potential of being reduced further to wüstite (FeO) and ultimately, metallic iron (Fe) given sufficient reduction potential and temperature. Consequently, the reduction of the nickel and iron oxides in the ore can be described by the following reactions:
where R is carbon, hydrogen or carbon monoxide.
The utilization efficiency of the reducing agent is an important parameter in the reduction process. If methane (CH4) is used in a reduction process, the utilization efficiencies of both carbon and hydrogen can be determined. The conversion of hydrogen to water vapour is considered as 100 % efficient. The conversion of carbon to carbon monoxide is considered as 50 % efficient, while the conversion of carbon to carbon dioxide is considered as 100 % efficient. The utilization efficiencies (η) of carbon and hydrogen are defined as follow:
In many of the previous thermodynamics studies, the models assumed the ideal behaviour of the species and did not take into account the activity coefficients. In the present research, the non-ideal behaviour of some of the species was accommodated by including activity coefficients for the species existing in the monoxide and spinel [11], as well as for those existing in the alloy [13]. A description of the equilibrium model has been given previously for application to the sulphidation of a limonitic laterite ore [32], the solid-state reduction of a limonitic laterite ore by carbon [17], by carbon monoxide gas [18] and by hydrogen gas [15]. In the present paper, the model made by Elliott et al. [9] has been developed to cover the gaseous reduction of the limonitic ore by methane. Consequently, only a summary of the relevant features of the model is provided here along with any necessary changes to adjust the model for reduction by methane. The calculation of the multi-component equilibrium composition was performed using the Gibbs-free energy minimization method [33] through the Equilibrium Module of HSC Chemistry® 6.1. The limonitic ore was assumed to be comprised of eight elements: Mg, Si, Al, Fe, Ni, Co, H and O. These elements plus carbon were inputted into the Equilibrium Composition calculations program of HSC Chemistry® 6.1, which then generated a list of 268 possible species. However, most of these generated species are not stable under the conditions of the gaseous reduction process and therefore only the 31 species shown in Table 1 were used in the model. The selected species were classified into four phases: gases, oxides (including monoxide (FeO-NiO-CoO) and spinel (Fe3O4-NiFe2O4-CoFe2O4)), ferronickel alloy (Fe-Ni-Co) and carbon. The methane addition is represented in terms of kilomoles of methane per 100 kg of ore.
Phases (in bold) and species utilized in the equilibrium calculations for the reduction of the limonitic laterite ore by methane.
| Gases | Oxides | Oxides | Oxides | Oxides | Alloy |
|---|---|---|---|---|---|
| O2 | CoO | FeOOH | MgFe2O4 | NiO | Fe |
| H2 | 2CoO·SiO2 | 2FeO·SiO2 | MgO | SiO2 | Ni |
| H2O | FeO | FeSiO3 | MgSiO3 | NiFe2O4 | Co |
| CO | Fe2O3 | Fe2SiO4 | Mg2SiO4 | Fe3O4 | Carbon |
| CO2 | Al2O3 | Fe2MgO4 | 2NiO·SiO2 | CoFe2O4 | C |
| CH4 | Al2O3·SiO2 |
Table 2 shows the composition of the limonitic ore used in the modelling of the gaseous reduction process. The limonitic ore contains a very high amount of iron oxide (Fe2O3) with low amounts of magnesia (MgO) and silica (SiO2).
The ore composition that was used in the modelling of the reduction of the limonitic laterite ore by methane (wt%).
| CoO | Fe2O3 | MgO | NiO | SiO2 | Al2O3 |
|---|---|---|---|---|---|
| 0.05 % | 92.02 % | 0.46 % | 1.62 % | 2.65 % | 3.20 % |
Results and discussion
Figures 1(A) and 1(B) show the nickel recovery and the nickel grade of the ferronickel for the limonitic ore as a function of temperature and methane addition. Reduction was observed to begin at a temperature of about 400 °C. This temperature decreased only slightly with increasing methane addition. At this temperature, the minimum methane addition for reduction was about 0.065 kmol/100 kg of ore as some methane was utilized to reduce hematite to magnetite and magnetite to wüstite. This minimum methane addition increased with increasing reduction temperature as more of the methane was utilized to reduce the iron oxides. At low methane additions, there was a maximum in the nickel recovery with increasing temperature as some reoxidation of the nickel could occur. With regards to the nickel grade, the grade was high at low methane additions and low temperatures, reflecting the relative instability of nickel oxide in comparison to iron oxide. For example, for a methane addition of about 0.125 kmol/100 kg of ore, the nickel grade was in the range of about 80 to 90 % from about 400 to 1200 °C. As the temperature increased, the amount of methane required to achieve this grade increased as a greater proportion of the methane was used for the reduction of the iron oxides. The nickel grade was about 90 % at about 400 °C, where nickel began to form, and again, this temperature decreased only slightly with increasing methane addition. The temperature and methane addition ranges where these high grades occurred were relatively narrow. As the temperature and the methane additions increased, the grade decreased rapidly as an increased amount of metallic iron was formed. Consequently, at high temperatures and methane additions, the nickel grade began to level off at about 10 %.
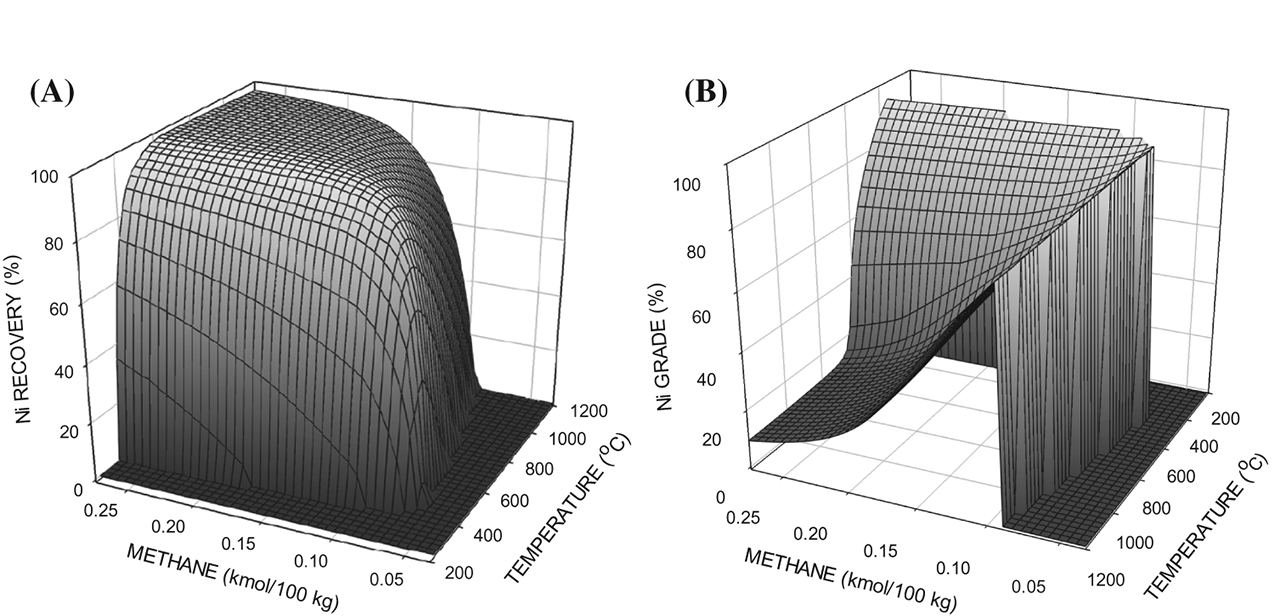
The effects of methane additions and temperatures on the nickel recovery (A) and the nickel grade (B) for the limonitic ore.
For the treatment of the laterite ores, it would be of interest to reduce all the nickel oxide and some of the iron oxide in the solid state to produce a high grade ferronickel alloy and then further concentrate the ferronickel by magnetic separation. Additionally, it would be desirable to obtain a high nickel grade concentrate with high nickel recoveries. Also, the conditions required to avoid magnetite formation need to be determined, as the magnetite will dilute the concentrate. Therefore, the stoichiometric methane requirement for the reduction of the nickel oxide to metallic nickel and the hematite to wüstite was considered, as given by the following reactions:
For the limonitic ore, this stoichiometric requirement corresponds to 0.199 kmol of CH4/100 kg of ore.
Figure 2 shows the nickel and iron grades and the nickel and iron recoveries as a function of temperature for the stoichiometric carbon addition for the nickeliferous limonitic ore. It could be seen that nickel metal began to form at about 400 °C and simultaneously metallic iron was also produced. This is in agreement with many other researchers who found that nickel and iron were coreduced [14, 23, 27, 34]. This was in large part due to the reduced activity of the iron in the nickel. Since nickel oxide is a relatively unstable oxide in comparison to wüstite, the amount of metallic nickel increased very rapidly with temperature, while the amount of metallic iron increased more slowly. At about 400 °C, the nickel grade reached a maximum value of about 90 % with a nickel recovery of about 65 %. Subsequently, the amount of metallic iron increased more quickly and consequently, the nickel grade decreased rapidly. At about 600 °C, the nickel recovery had reached a maximum value of about 99 %, but only about 2 % metallic iron had been recovered and thus, the nickel grade remained high at about 55 %. After about 600 °C, the nickel recovery decreased slightly, which could be due to reoxidation. However, the iron recovery increased more quickly than previously and therefore the nickel grade continued to drop before beginning to level off at about 25 % at 800 °C. Similarly, both the iron grade and recovery began to level off at about 75 % and 6 %, respectively. As a consequence, at the relatively high reduction temperature of 1000 °C, the nickel recovery remained high at 98 %, the metallic iron recovery was very low at about 7 % and therefore the nickel grade remained at 22 %.

The effect of temperature on the nickel and iron grades and nickel and iron recoveries for 0.199 kmol of CH4/100 kg of the limonitic ore.
Figure 3 shows the behaviour of the nickel-containing species as a function of temperature for the stoichiometric methane addition. At room temperature, nickel ferrite was the most stable nickel-containing species but as the temperature increased, it decomposed and nickel oxide began to form according to reaction (5). However, by about 350 °C this nickel oxide was being reduced and thus at higher temperatures, further decomposition of the nickel ferrite resulted in metallic nickel rather than nickel oxide as given by reaction (8). As mentioned previously, the nickel recovery reached a maximum of about 99 % at about 600 °C, then decreased slightly due to reoxidation into the wüstite phase.

The effect of temperature on the equilibrium amounts of the nickel-containing species for 0.199 kmol of CH4/100 kg of the limonitic ore.
Figure 4 shows the behaviour of the iron-containing species as a function of temperature for the stoichiometric methane addition. Also included is the behaviour of carbon. At room temperature, the major iron oxide-containing species was magnetite with some hematite. As the temperature increased, the amount of wüstite increased, the amounts of magnetite and hematite decreased and the amount of iron silicate increased. This coincided with a decrease in the amount of carbon, which was generated by the decomposition of methane. The carbon was consumed slowly from room temperature, then more quickly above about 500 °C. This corresponded to an increase in the amount of metallic iron. The carbon disappeared completely at about 625 °C, where a number of changes occurred. Above 625 °C, the amounts of metallic iron and wüstite increased more slowly, while the amount of magnetite decreased more slowly. Furthermore, the amount of the iron silicates decreased above this temperature.

The effect of temperature on the equilibrium amounts of the iron-containing species for 0.199 kmol of CH4/100 kg of the limonitic ore.
Figure 5 shows the equilibrium gas composition as a function of temperature for the stoichiometric methane addition and also included is the behaviour of carbon. At room temperature, the gas phase consisted of methane, water vapour, and a small amount of carbon dioxide. As the temperature increased to about 300 °C, the amounts of water vapour and methane decreased slightly. The amount of carbon dioxide increased and hydrogen began to form as the carbon was consumed. Above 300 °C, the amounts of methane, water vapour and carbon decreased rapidly, while the amounts of carbon dioxide and hydrogen continued to increase. Carbon monoxide began to form at about 400 °C and the amount increased rapidly. At 625 °C, all the carbon had been consumed and there were dramatic changes in the gas composition. The amount of water vapour stopped declining and began to increase. The rate of formation of carbon monoxide decreased with increasing temperature. The amount of carbon dioxide began to level off and then decreased above about 700 °C. There was still some methane present up to about 700 °C. The decomposition of this methane resulted in an increasing amount of hydrogen until about 700 °C, thereafter it decreased.

The effect of temperature on the equilibrium amounts of the gaseous species and carbon for 0.199 kmol of CH4/100 kg of the limonitic ore.
Figure 6 shows the effect of the nickel oxide contents of the ore in the range of 0.5 to 3 % on the nickel grade and recovery at a temperature of 600 °C and a methane addition of 0.199 kmol/100 kg of ore. The nickel grade continually increased from about 47.5 to 54 %. The rate of increase of the nickel recovery slowed down with increasing nickel oxide content and eventually levelled off. For the range of nickel oxide contents investigated, the nickel recovery increased from 96.25 to 99.10 %.

The effect of nickel oxide content of the ore on nickel grade and recovery for 0.199 kmol of CH4/100 kg of the limonitic ore at 600 °C.
Figure 7 shows the effect of iron oxide (Fe2O3) content in the range of 75 to 95 % on the nickel grade and recovery for a methane addition of 0.199 kmol at 600 °C. The nickel grade increased slowly at low iron oxide contents and then more rapidly at higher iron oxide contents. The nickel recovery remained relatively constant at 98 % when the iron oxide contents were below 93 %. At higher iron oxide contents, the nickel recovery declined to just below 93 %. As the iron oxide contents increased, more of the reducing agent was utilized in the reduction of hematite to magnetite and thus, less nickel oxide was reduced and the recovery decreased. At the same time, less metallic iron was produced and therefore the nickel grade increased.

The effect of iron oxide content of the ore on nickel grade and recovery for 0.199 kmol of CH4 for the limonitic ore at 600 °C.
Figures 8(A) and (B) display the hydrogen and carbon utilization efficiencies. The hydrogen efficiency was close to 100 % at low methane additions for all temperatures. As the amount of methane increased, the hydrogen efficiency first rapidly decreased and then levelled out at approximately 50 % at 1200 °C. With regards to temperature, when the temperature was below 400 °C, the hydrogen efficiency approached 30 %. The efficiency of hydrogen demonstrated a sudden decrease for moderate to high methane additions at 600 °C. This was due to the formation of hydrogen gas from the reaction of carbon with water vapour under the aforementioned conditions. Similarly, the carbon efficiency reached a maximum of 100 % at low methane additions over the whole temperature range. When a moderate to high amount of methane was added and the temperature was above 600 °C, the carbon efficiency decreased slowly from 100 % and reached a constant value of about 70 % at 1200 °C. This behaviour was attributed to the reaction of carbon dioxide with carbon to produce carbon monoxide. As the temperature decreased, the carbon efficiency decreased rapidly and reached about 5 % for 0.275 kmol of CH4/100 kg of ore and a temperature of 200 °C.

The effects of methane additions and temperatures on the hydrogen utilization efficiency (A) and carbon utilization efficiency (B) for the limonitic ore.
Figure 9 shows the equilibrium amounts of deposited carbon under varying methane additions and temperatures for the limonitic ore. The amount of deposited carbon was highest under high methane additions and low temperatures, for example when 0.275 kmol of CH4/100 kg of ore was added at approximately 500 °C. Under these conditions, about 0.090 kmol of carbon was deposited. For lower methane additions, the amount of deposited carbon decreased rapidly to zero. The same behaviour was observed when the temperature increased. The addition of a small amount of methane led to the complete conversion of the methane into carbon dioxide and water vapour. This conversion occurred over a relatively low temperature range, where there was an insufficient amount of methane for carbon deposition. At high temperatures, all carbon was used up in the reduction reactions to produce metallic nickel and metallic iron in the ferronickel alloy. It is also important to note that iron carbide did not form during the reduction of the limonitic ore under any conditions.

The effects of methane additions and temperatures on the amount of deposited carbon for the limonitic ore.
In order to produce a magnetic concentrate, it is important to minimize the amount of magnetite. Figure 10 shows the effects of methane additions and temperatures on the equilibrium amount of magnetite for the limonitic ore. At low methane additions, the amount of magnetite increased across the whole temperature range. Also, at low temperatures, the amount of magnetite increased across the whole methane addition range. With increasing methane additions and temperatures, the amount of magnetite decreased as it was reduced to wüstite. For typical reducing conditions (above 600 °C), the amount of magnetite was high at about 0.220 kmol and on cooling, the amount of magnetite increased even further to about 0.330 kmol.

The effects of methane additions and temperatures on the equilibrium amount of magnetite for the limonitic ore.
Figure 11 shows the nickel grade of the concentrate, which not only contains the ferronickel alloy, but also magnetite. A nickel grade of 2.5 % was achieved at high methane additions and temperatures, which corresponded to the lowest magnetite amount in Figure 10. Decreasing temperatures or methane additions rapidly lowered the grade of the concentrate due to the increased formation of magnetite. These thermodynamic predictions demonstrate that for the limonitic ores, it is difficult to produce a high grade concentrate. This is due to the high iron content of the ore that leads to the high magnetite content of the concentrate. In order to increase the grade, the amount of magnetite could be minimized by quenching or cooling under controlled atmospheres.

The effects of methane additions and temperatures on the nickel grade of the magnetite-containing concentrate.
Comparison with experimental results
Only a few experimental studies have been performed on the reduction of limonitic laterite ores [25, 35, 36]. In these studies, pure hydrogen, carbon monoxide, or coal was used as the reductants. The maximum nickel recoveries reported are summarized in Table 3. These results showed that the nickel recoveries were in the range of 90 to 100 % at temperatures between 550 and 1250 °C. This is in agreement with the thermodynamic predictions for methane. The instability of the nickel oxide in the limonitic ore resulted in the high recoveries of nickel irrespective of the reducing agent. The nickel grades were only reported for reduction by coal in the temperature range of 1000 to 1250 °C. In these tests, sulphur or sulphur-containing compounds were added and this resulted in grades of about 4 to 5 %, which were higher than the theoretical value of about 2.5 %. These additives promoted selective reduction of nickel and facilitated particle agglomeration.
Summary of experimental results on the reduction of limonitic ores by hydrogen, carbon-based reductants, or a combination of the two types of reducing agents. Maximum nickel recoveries and the corresponding reducing conditions are given.
| Ore type | Reductant | Temperature (°C) | Ni Grade (%) | Ni recovery (%) | Reference |
|---|---|---|---|---|---|
| Manuran | H2 | 850 | N/A | 95 | [25] |
| Manuran | CO followed by H2 | 550 | N/A | 95 | [25] |
| Manuran | CO2/CO | 600 | N/A | ~100 | [25] |
| Limonite | Coal | 1250 | 5.1 | 98.79 | [36] |
| Limonite | Bituminous Coal | 1000 | 4 | 93.2 | [35] |
Conclusions
A thermodynamic model was developed using the Equilibrium program of HSC Chemistry® 6.1 to investigate the reduction behaviours of the nickeliferous limonitic laterite ores, with methane as the reducing agent. Metallic nickel began to form concurrently with iron at about 400 °C and a methane addition of about 0.199 kmol/100 kg of ore. The recovery was low but the grade was very high at low methane additions and temperatures, reflecting the relative instability of nickel oxide in comparison to wüstite. The recovery increased rapidly with both temperature and methane additions, while the grade decreased.
For the stoichiometric methane addition of 0.199 kmol of CH4/100 kg and at a temperature just below 400 °C, the nickel recovery was only 65 % but the grade was 90 %. When the temperature increased to 600 °C, the nickel recovery was about 99 % and the grade was still high at 55 %. As the temperature continued to increase, the recovery remained high but the grade decreased rapidly due to the increased formation of metallic iron. The methane was totally decomposed by about 700 °C and the carbon was consumed by about 650 °C. The amount of hydrogen increased up to about 700 °C and then decreased.
The effect of ore composition was examined at a temperature of 600 °C by varying the nickel oxide and iron oxide contents of the ore. The nickel oxide content was varied in the range of 0.5 to 3.0 %. Both the nickel recovery and the nickel grade increased along with the nickel oxide content. The iron oxide content was varied from 75 to 95 %. The nickel recovery decreased with increasing iron oxide content, while the nickel grade increased. The increase in the nickel grade was due to the consumption of methane for converting hematite into magnetite instead of metallic iron.
The hydrogen efficiency was in the range of approximately 20 to 50 % for nickel recoveries close to 100 % and nickel grades of about 10 %. Meanwhile, the carbon efficiency was constant at about 70 % under the same conditions. The carbon efficiency dropped to zero when the temperature was lower than 600 °C, indicating that carbon was not optimally used in nickel extraction in this temperature range. Consequently, the nickel recovery also decreased and eventually reached zero as the temperature decreased from 600 °C.
Carbon deposition occurred at low temperatures and high methane additions, where there was no reduction of metal oxides. A significant amount of magnetite formed for all temperatures and methane additions due to the high hematite content of the ore. Consequently, this limited the nickel grade of the concentrate to about 2.5 %. The amount of magnetite also increased with decreasing methane additions or temperatures, which resulted in the grade decreasing even further. The comparison with the experimental results available in the literature showed that the measured nickel recoveries were in agreement with the thermodynamic predictions. However, the reported nickel grades in the experiments were higher than the thermodynamic predictions because of the addition of sulphur, which promoted selective reduction of nickel oxide.
Acknowledgments
The authors thank the Natural Sciences and Engineering Research Council of Canada (NSERC) for supporting this research.
References
[1] A.D. Dalvi, W.G. Bacon and R.C. Osborne, PDAC 2004 International Conference Trade Show and Investors Exchange, Prospectors and Developers Association of Canada, Toronto (2004).Search in Google Scholar
[2] G.M. Mudd and S.M. Jowitt, Econ. Geol., 109 (2014) 1813–1841.10.2113/econgeo.109.7.1813Search in Google Scholar
[3] G.M. Mudd, Proceedings of 48th Annual Conference of Metallurgists, Canadian Metallurgical Society, Sudbury (2009), pp. 1–10.Search in Google Scholar
[4] A. Mitchell, Processing of Nickel Ores and Concentrates 2015, May 13–14, 2015, Minerals Engineering International, Falmouth (2015).Search in Google Scholar
[5] A. Oxley and N. Barcza, Miner. Eng., 54 (2013) 2–13.10.1016/j.mineng.2013.02.012Search in Google Scholar
[6] A. Domsa, L. Szabo, Z. Spirchez and A. Palfavi, Chapter 1 in Modern Developments in Powder Metallurgy edited by H.H. Hausner, Metal Powder Industries Federation and the Metallurgical Society of AIME, Springer US (1966), pp. 3–14.10.1007/978-1-4684-7706-1_1Search in Google Scholar
[7] D. Ghosh, A.K. Roy and A. Ghosh, Trans. Iron Steel Inst. Jpn., 26 (1986) 186–193.10.2355/isijinternational1966.26.186Search in Google Scholar
[8] O. Ostrovski and G. Zhang, AIChE J., 52 (2006) 300–310.10.1002/aic.10628Search in Google Scholar
[9] R. Elliott, C.A. Pickles, J. Forster and J. Miner, Mater. Charact. Eng., 4 (2016) 320–346.10.4236/jmmce.2016.46028Search in Google Scholar
[10] K.-C. Hsieh and Y.A. Chang, Metall. Trans. B, 17 (1986) 133–146.10.1007/BF02670826Search in Google Scholar
[11] A.D. Pelton, H. Schmalzried and J. Sticher, J. Phys. Chem. Solids, 40 (1979) 1103–1122.10.1016/0022-3697(79)90146-XSearch in Google Scholar
[12] Y. Shirane, S. Nabika, S. Sakamoto and I. Nakashima, Int. J. Miner. Process., 19 (1987) 237–251.10.1016/0301-7516(87)90044-5Search in Google Scholar
[13] L.J. Swartzendruber, V.P. Itkin and C.B. Alcock, J. Phase Equilibria, 12 (1991) 288–312.10.1007/BF02649918Search in Google Scholar
[14] J.H. Canterford and A.G. Turnbull, Proceedings of the Australasian Institute of Mining and Metallurgy, Australasian Institute of Mining and Metallurgy (1980), pp. 43–51.Search in Google Scholar
[15] R. Elliott and C.A. Pickles, High Temp. Mater. Process, 36 (2015) 835–846.10.1515/htmp-2015-0208Search in Google Scholar
[16] C.J. Hallet, Proceedings of Nickel-Cobalt 97 International Symposium Pyrometallurgical Fundamentals and Process Development, Sudbury (1997), pp. 299–312.Search in Google Scholar
[17] C.A. Pickles, J. Forster and R. Elliott, Miner. Eng., 65 (2014) 33–40.10.1016/j.mineng.2014.05.006Search in Google Scholar
[18] C.A. Pickles and R. Elliott, Miner. Process. Extr. Metall., 124 (2015) 208–216.10.1179/1743285515Y.0000000009Search in Google Scholar
[19] M.A. Rhamdhani, P.C. Hayes and E. Jak, Miner. Process. Extr. Metall. Trans. Inst. Min. Metall. C, 118 (2009) 146–155.10.1179/174328509X431409Search in Google Scholar
[20] T. Utigard and R.A. Bergman, Metall. Trans. B, 23 (1992) 271–275.10.1007/BF02660871Search in Google Scholar
[21] M. Valix, W.H. Cheung, P.D. Dan and G.A. Foulds, Proceedings of the 23rd Australasian Conference on Chemical Engineering, Australasian Institute of Mining and Metallurgy, Adelaide (1995), pp. 142–146.Search in Google Scholar
[22] S. Mohanty, S.K. Roy and P.K. Sen, Metall. Mater. Trans. B, 39 (2008) 639–642.10.1007/s11663-008-9185-zSearch in Google Scholar
[23] O. Antola, L. Holappa and P. Paschen, Miner. Process. Extr. Metall. Rev., 15 (1995) 169–179.10.1080/08827509508914195Search in Google Scholar
[24] A. Cores, A. Formoso, M. Larrea and J. Ortiz, Ironmak. Steelmak., 16 (1989) 446–449.Search in Google Scholar
[25] J.E. De Graaf, Hydrometallurgy, 5 (1979) 47–65.10.1016/0304-386X(79)90027-6Search in Google Scholar
[26] G.B. Itao and N.M. Anacleto, Mindanao Forum, 24 (2011) 25–45.Search in Google Scholar
[27] M. Kawahara, J.M. Toguri and R.A. Bergman, Metall. Trans. B, 19 (1988) 181–186.10.1007/BF02654202Search in Google Scholar
[28] T.A. Utigard, G. Plascencia, T. Marin, A.E.M. Warner, J. Liu, A. Vahed and M. Muinonen, Can. Metall. Q., 44 (2005) 421–428.10.1179/cmq.2005.44.3.421Search in Google Scholar
[29] M. Valix and W.H. Cheung, Miner. Eng., 15 (2002) 607–612.10.1016/S0892-6875(02)00068-7Search in Google Scholar
[30] H. Rashidi, H.A. Ebrahim and B. Dabir, Thermochim. Acta, 561 (2013) 41–48.10.1016/j.tca.2013.03.014Search in Google Scholar
[31] B. Khoshandam, R.V. Kumar and E. Jamshidi, Metall. Mater. Trans. B, 35 (2004) 825–828.10.1007/s11663-004-0076-7Search in Google Scholar
[32] C.T. Harris, J.G. Peacey and C.A. Pickles, Miner. Eng., 54 (2013) 21–31.10.1016/j.mineng.2013.02.016Search in Google Scholar
[33] A. Roine, HSC Chemistry 6.0 – User’s Guide, Outokumpu Research Oy, Poir, Finland (2006).Search in Google Scholar
[34] D.R. Swinbourne, Miner. Process. Extr. Metall., 123 (2014) 127–140.10.1179/1743285514Y.0000000056Search in Google Scholar
[35] R. Elliott, F. Rodrigues, C.A. Pickles and J.G. Peacey, Can. Metall. Q., 54 (2015) 395–405.10.1179/1879139515Y.0000000009Search in Google Scholar
[36] Q. Li, Y. Cui, D. Zhu, J. Pan, H. Zhang and G. Zheng, XXV International Mineral Processing Congress (IMPC) 2010 Proceedings, International Mineral Processing Congress, Brisbane (2010), pp. 1549–1556.Search in Google Scholar
© 2018 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Research Articles
- Numerical Simulation of the Electron Beam Welding and Post Welding Heat Treatment Coupling Process
- Effect of Ti and Ta on Oxidation Kinetic of Chromia Forming Ni-Base Superalloys in Ar-O2-Based Atmosphere
- Effects of Cerium on the Inclusions and Pitting Corrosion Behavior of 434 Ferritic Stainless Steel
- Critical Assessment of Activities of Structural Units in Fe–Al Binary Melts Based on the Atom and Molecule Coexistence Theory
- A Yield Stress Model for a Solution-Treated Ni-Based Superalloy during Plastic Deformation
- Stress Relaxation Behaviour and Creep Constitutive Equations of SA302Gr.C Low-Alloy Steel
- Effects of Inner Defects on Creep Damage and Crack Initiation for a Brazed Joint
- Experimental and Numerical Investigations on Hot Deformation Behavior and Processing Maps for ASS 304 and ASS 316
- Production of Iron Based Alloys from Mill Scale through Metallothermic Reduction
- Effect of Nb and V on Austenite Grain Growth Behavior of the Cr-Mo-V Steel for Brake Discs
- A Thermodynamic Study of the Reduction of a Limonitic Laterite Ore by Methane
- Electrochemical and Phase Analysis of Si(IV) on Fe Electrode in Molten NaCl-NaF-KCl-SiO2 System
- Characterization of Hot Deformation Behavior for Pure Aluminum Using 3D Processing Maps
- Effect of Chromium Addition on the Cyclic Oxidation Resistance of Pseudo-Binary (Mo,Cr)3 Si Silicide Alloy
- Equiaxed Solidification of 430 Ferritic Stainless Steel Nucleating on Core-Containing Ti
- FE Analysis of Dynamical Recrystallization during the Seamless Tube Extrusion of Semicontinuous Casting Magnesium Alloy and Experimental Verification
- Study on the Reblow Model for Medium-High Carbon Steel Melting by Converter
- Short Communication
- Effect of B2O3 on Slag-Metal Reaction between CaO-Al2O3-Based Mold Flux and High Aluminum Steel
- Review Article
- Computation of the Thermal Residual Stresses in SiC/SiC Composites with Multi-Layered Interphases by Using ANN with the Structure of Random Forest
- Research Articles
- Failure Analysis of the Corroded Water Wall Tube in a 50MW Thermal Power Plant
- CO2 Absorption of Powdered Ba2Fe2O5 with Different Particle Size
- Induced-Pitting Behaviors of MnS Inclusions in Steel
Articles in the same Issue
- Frontmatter
- Research Articles
- Numerical Simulation of the Electron Beam Welding and Post Welding Heat Treatment Coupling Process
- Effect of Ti and Ta on Oxidation Kinetic of Chromia Forming Ni-Base Superalloys in Ar-O2-Based Atmosphere
- Effects of Cerium on the Inclusions and Pitting Corrosion Behavior of 434 Ferritic Stainless Steel
- Critical Assessment of Activities of Structural Units in Fe–Al Binary Melts Based on the Atom and Molecule Coexistence Theory
- A Yield Stress Model for a Solution-Treated Ni-Based Superalloy during Plastic Deformation
- Stress Relaxation Behaviour and Creep Constitutive Equations of SA302Gr.C Low-Alloy Steel
- Effects of Inner Defects on Creep Damage and Crack Initiation for a Brazed Joint
- Experimental and Numerical Investigations on Hot Deformation Behavior and Processing Maps for ASS 304 and ASS 316
- Production of Iron Based Alloys from Mill Scale through Metallothermic Reduction
- Effect of Nb and V on Austenite Grain Growth Behavior of the Cr-Mo-V Steel for Brake Discs
- A Thermodynamic Study of the Reduction of a Limonitic Laterite Ore by Methane
- Electrochemical and Phase Analysis of Si(IV) on Fe Electrode in Molten NaCl-NaF-KCl-SiO2 System
- Characterization of Hot Deformation Behavior for Pure Aluminum Using 3D Processing Maps
- Effect of Chromium Addition on the Cyclic Oxidation Resistance of Pseudo-Binary (Mo,Cr)3 Si Silicide Alloy
- Equiaxed Solidification of 430 Ferritic Stainless Steel Nucleating on Core-Containing Ti
- FE Analysis of Dynamical Recrystallization during the Seamless Tube Extrusion of Semicontinuous Casting Magnesium Alloy and Experimental Verification
- Study on the Reblow Model for Medium-High Carbon Steel Melting by Converter
- Short Communication
- Effect of B2O3 on Slag-Metal Reaction between CaO-Al2O3-Based Mold Flux and High Aluminum Steel
- Review Article
- Computation of the Thermal Residual Stresses in SiC/SiC Composites with Multi-Layered Interphases by Using ANN with the Structure of Random Forest
- Research Articles
- Failure Analysis of the Corroded Water Wall Tube in a 50MW Thermal Power Plant
- CO2 Absorption of Powdered Ba2Fe2O5 with Different Particle Size
- Induced-Pitting Behaviors of MnS Inclusions in Steel

