Abstract
In order to study the effect of composite clays on the mechanical properties, water absorption and salt tolerance of a hydrogel, a poly(acrylic acid-co-acrylamide)/bentonite/kaolin composite hydrogel was prepared. Acrylic acid and acrylamide have been used as water absorbent monomers. N,N′-methylene bisacrylamide was used as a crosslinking agent while potassium persulfate was used as an initiator. The water preserving capability, repeated water absorption, salt resistance and the mechanical properties of the composite hydrogel are analyzed and discussed. The results show that a small quantity of bentonite can increase the storage modulus of the composite hydrogel, whereas the excess clay had an unfavorable effect on the mechanical strength of the composite hydrogel. Both bentonite and kaolin significantly improved the water preserving capability, repeated water absorption and salt resistance of the composite hydrogel. Optimum values for the amounts of bentonite and kaolin were found to be 10% and 5%, respectively.
1 Introduction
Super absorbent polymers (SAP) are a new type of functional polymer material which have a three-dimensional network structure and a strong water absorbing capability. These have widely been used in different areas. Some of the applications of SAPs include personal care products (1), agricultural water retaining agents (2), (3), flame retardants for self-ignition of coal and sulfide ore (4), packaging materials (5), oil extraction (6), (7), (8), (9), (10), heavy metal absorbents (11), (12), and drug release carriers (13), (14). Poly(acrylic acid) hydrogels have are used widely due to their high water absorption and low cost. However, this kind of SAP usually has low salt resistance and slow water absorption rate. Additionally, after water absorption it shows inferior gel strength, dispersion and elastic properties (15). These drawbacks have highly restricted the product quality and the application areas of this kind of SAP. To improve the properties of such SAP material, researchers have used methods such as the formation of interpenetrating networks (15) and compounding with inorganic clays (14). Such methods help to improve the mechanical properties of the hydrogels. Adding inorganic clay is a relatively effective method to improve the properties of this type of hydrogel (16), (17), (18), (19). Montmorillonite (20), attapulgite (21), (22), kaolin (23) and mica (24) have commonly been used to modify high water absorbing hydrogel. The results have shown that the addition of appropriate amounts of inorganic clays could improve the mechanical strength and water absorbing capability of the hydrogel (14). Kabiri et al. prepared a composite hydrogel by the addition of kaolin. The hydrogel rapidly absorbed water and had high strength (25). Zhang et al. prepared a hydrogel using acrylic acid, 2-acrylamide-2-methylpropyl sodium sulfonate and modified montmorillonite (26). The gel prepared by these researchers showed improved salt resistance and strength. Xia et al. synthesized a clay-polymer hydrogel by in-situ polymerization of poly(N-isopropylacrylamide) (NIPAAM) in an aqueous solution of Na-montmorillonite layered silicates (Na-MLS) (27). The gel strength was characterized by a parallel plate rheometer. The results showed that after an initial decrease, the gel strength increased with an increase in Na-MLS concentration. In 2003, Zhang et al. used an inverse suspension method to synthesize a poly(acrylic acid)/kaolin composite hydrogel which had a high water absorbing capability (28). Their research showed that in comparison to a gel without kaolin, the addition of 5–10% kaolin in the composite hydrogel increased its water absorbing ratio by 20% and the water preserving capability by 25%. Qiu et al. first modified montmorillonite by adding carboxymethyl cellulose and then co-polymerized it with acrylic acid (29). Their results showed that the addition of modified montmorillonite not only increased the water absorbing ratio, but also improved the water preserving capability of the gel.
Bentonite is a clay mineral with montmorillonite as the major component. Its 2:1 crystal structure consists of two layers of oxygen-silicon tetrahedron with one layer of alumina octahedral sheet in between. This clay has good expansion, absorption and caking properties (30). Kaolin is a type of clay with kaolinite as the major component. It has relatively high plasticity and caking properties (23). It is easily dispersed in water and has good fire resistance. Much research has recently reported composite hydrogels prepared with bentonite and kaolin (25), (26), (27), (28), (29), (30). Studies have shown that bentonite and kaolin produce different effects on composite hydrogels. Kaolin significantly increases the tensile strength and mechanical properties of composite gels (23), while bentonite significantly increases their water absorption capacity (30). To date, most studies have focused on the effects of one specific type of clay on the performance of gels. However, it is unclear how the performance of a hydrogel changes when two different types of clay are added simultaneously and whether two different types of gels can be synergistically applied to good effect.
Therefore, the present study used bentonite and kaolin as the inorganic filling materials, acrylic acid and acrylamide as the water absorbent monomer, N,N′-methylene bisacrylamide (MBA) as the crosslinking agent and potassium persulfate (KPS) as the initiator to prepare a bentonite/kaolin/acrylic acid-co-acrylamide composite hydrogel. The impacts of concentrations of initiator, crosslinking agent, kaolin and bentonite on the water absorption ability of the hydrogel have been studied. Further, the impacts of the neutralization degree of acrylic acid and those of the reaction temperature on the ability of hydrogel to absorb water were also studied. The thermal stability, water preserving capability, repeated water absorption and salt resistance of composite hydrogel were analyzed. The micro-morphology and the chemical structure of the gel were also investigated. The results helped in providing significant technical support for the engineering applications of this type of hydrogel.
2 Experimental
2.1 Materials
Acrylic acid (chemically pure, Kermel Chemreagent, Co., Ltd., Tianjin, China) was distilled under reduced pressure before use. Acrylamide (chemically pure, Fuchen Chemical Co., Ltd., Tianjin, China) was used as purchased. Bentonite (chemically pure, Trial Four Hervey Chemical Co., Ltd., Shanghai, China) and kaolin (chemically pure, Fuchen Chemical Co., Ltd., Tianjin, China) were milled through a 300-mesh screen. These were then dried at 105°C for 20 h prior to use. Potassium persulfate (KPS, analytical grade, Kermel Chemreagent, Co., Ltd., Tianjin, China) was recrystallized from water. N,N′-methylene bisacrylamide (MBA, chemically pure, Shanghai Chemical Reagent Factory, Shanghai, China) was used as purchased. Sodium hydroxide (analytical grade), surfactant (Span 60, sorbitan monostearate), sodium chloride (analytical grade), calcium chloride (analytical grade) and aluminium chloride (analytical grade) were purchased from Tianjin Kermel Chemical Reagent Co., Ltd., Tianjin, China and were used as purchased.
2.2 Preparation of the hydrogel
The experimental conditions for the preparation of composite hydrogels are shown in Table 1. A number of samples with different amounts of initiator, crosslinking agent, acrylamide, kaolin and bentonite were prepared at different reaction temperatures with varying neutralization degrees of acrylic acid by the following procedure. Acrylic acid was titrated at room temperature by using a 25 wt% solution of NaOH. The solution was continuously stirred during the addition of NaOH. The stirring was continued until the neutralization degree of the acrylic acid reached a constant value. The neutralized acrylic acid, acrylamide, kaolin, bentonite and surfactant (Span 60) were successively added in a 500 ml four-neck flask, which was equipped with a magnetic stirrer, a thermometer and a nitrogen line. Under nitrogen atmosphere, the crosslinking agent (MBA) and initiator (KPS) were added to the above mixture solution. The mixed solution was stirred at room temperature for 30 min and then, was heated slowly to a constant final value while being stirred. The temperature was maintained at this final value for 1 h. After the completion of the reaction, the product was washed several times with deionized water, cut and dried in an oven at 120°C until a dry powder with a constant weight was obtained. The powder was ground and screened through a 100-mesh sieve.
Experimental program of composite hydrogels.
| No. | AA (%) | AM (%) | Bentonite (%) | Kaolin (%) | MBA (%) | KPS (%) | Neutralization degree (%) | T (°C) |
|---|---|---|---|---|---|---|---|---|
| H-1 | 100 | 50 | 0 | 0 | 0.10 | 0.25 | 70 | 80 |
| H-2 | 100 | 50 | 5 | 0 | 0.10 | 0.25 | 70 | 80 |
| H-3 | 100 | 50 | 10 | 0 | 0.10 | 0.25 | 70 | 80 |
| H-4 | 100 | 50 | 15 | 0 | 0.10 | 0.25 | 70 | 80 |
| H-5 | 100 | 50 | 20 | 0 | 0.10 | 0.25 | 70 | 80 |
| H-6 | 100 | 50 | 0 | 5 | 0.10 | 0.25 | 70 | 80 |
| H-7 | 100 | 50 | 0 | 10 | 0.10 | 0.25 | 70 | 80 |
| H-8 | 100 | 50 | 0 | 15 | 0.10 | 0.25 | 70 | 80 |
| H-9 | 100 | 50 | 0 | 20 | 0.10 | 0.25 | 70 | 80 |
| H-10 | 100 | 50 | 5 | 5 | 0.05 | 0.25 | 70 | 80 |
| H-11 | 100 | 50 | 5 | 5 | 0.10 | 0.25 | 70 | 80 |
| H-12 | 100 | 50 | 5 | 5 | 0.15 | 0.25 | 70 | 80 |
| H-13 | 100 | 50 | 5 | 5 | 0.20 | 0.25 | 70 | 80 |
| H-14 | 100 | 50 | 5 | 5 | 0.25 | 0.25 | 70 | 80 |
| H-15 | 100 | 50 | 5 | 5 | 0.30 | 0.25 | 70 | 80 |
| H-16 | 100 | 50 | 5 | 5 | 0.10 | 0.10 | 70 | 80 |
| H-17 | 100 | 50 | 5 | 5 | 0.10 | 0.15 | 70 | 80 |
| H-18 | 100 | 50 | 5 | 5 | 0.10 | 0.20 | 70 | 80 |
| H-19 | 100 | 50 | 5 | 5 | 0.10 | 0.30 | 70 | 80 |
| H-20 | 100 | 50 | 5 | 5 | 0.10 | 0.35 | 70 | 80 |
| H-21 | 100 | 50 | 5 | 5 | 0.10 | 0.25 | 65 | 80 |
| H-22 | 100 | 50 | 5 | 5 | 0.10 | 0.25 | 75 | 80 |
| H-23 | 100 | 50 | 5 | 5 | 0.10 | 0.25 | 80 | 80 |
| H-24 | 100 | 50 | 5 | 5 | 0.10 | 0.25 | 85 | 80 |
| H-25 | 100 | 35 | 5 | 5 | 0.10 | 0.25 | 70 | 80 |
| H-26 | 100 | 40 | 5 | 5 | 0.10 | 0.25 | 70 | 80 |
| H-27 | 100 | 45 | 5 | 5 | 0.10 | 0.25 | 70 | 80 |
| H-28 | 100 | 55 | 5 | 5 | 0.10 | 0.25 | 70 | 80 |
| H-29 | 100 | 50 | 10 | 5 | 0.10 | 0.25 | 70 | 80 |
| H-30 | 100 | 50 | 15 | 5 | 0.10 | 0.25 | 70 | 80 |
| H-31 | 100 | 50 | 20 | 5 | 0.10 | 0.25 | 70 | 80 |
| H-32 | 100 | 50 | 5 | 10 | 0.10 | 0.25 | 70 | 80 |
| H-33 | 100 | 50 | 5 | 15 | 0.10 | 0.25 | 70 | 80 |
| H-34 | 100 | 50 | 5 | 20 | 0.10 | 0.25 | 70 | 80 |
| H-35 | 100 | 50 | 5 | 5 | 0.10 | 0.25 | 70 | 65 |
| H-36 | 100 | 50 | 5 | 5 | 0.10 | 0.25 | 70 | 70 |
| H-37 | 100 | 50 | 5 | 5 | 0.10 | 0.25 | 70 | 75 |
| H-38 | 100 | 50 | 5 | 5 | 0.10 | 0.25 | 70 | 85 |
| H-39 | 100 | 50 | 5 | 5 | 0.10 | 0.25 | 70 | 90 |
The concentration of each component was based on acrylic acid.
In the experiment (Table 1), the specific mass ratio of acrylamide over acrylic acid is 35–50%; the specific mass ratio of added kaolin over acrylic acid is 0–20%; the specific mass ratio of bentonite over acrylic acid is 0–20%; the specific mass ratio of added MBA over acrylic acid is 0.05–0.3%; the specific mass ratio of added KPS over acrylic acid is 0.1–0.35%.
2.3 Testing and characterization of hydrogel
2.3.1 Water absorption (Q) of hydrogel
A total of 0.2 g of the prepared dry gel powder was weighed and transferred to a nylon bag. The bag was immersed in deionized water (pH=6.7) and was taken out after a certain time. When the equilibrium swelling was reached, the residual water was filtered and removed using a 100-mesh standard sieve. After the swelling, the mass of the hydrogel was determined. The water absorption ratio (Q, g/g) can be calculated according to the following equation:
where W2 is the weight of the dry gel (in g) while W1 is the weight (in g) of the gel after water absorption.
2.3.2 FTIR analysis of the hydrogel
The hydrogel was dried and ground to a powder form. A sample was prepared using a KBr tablet and was analyzed by using a TENSOR27 FTIR spectrometer (Bruker, Karlsruhe, German).
2.3.3 Water preserving capability of the composite hydrogel
A total of 8–10 mg of the composite hydrogel was taken and its water preserving capability was measured using thermogravimetric analysis (TGA). Temperature was increased from 25°C to 150°C at a rate of 3°C/min to measure the residual weight of the hydrogel at different temperatures.
2.3.4 TGA measurement of the dry gel
A total of 8–10 mg of the dry composite hydrogel was taken. thermal gravity analysis (TG)/differential thermal gravity (DTG) testing was conducted using an STA409C131F thermogravimetric analyzer (TGA, NETZSCH Company, Bavarian State, Germany) in a temperature range of 30°C–600°C. The heating rate was kept constant at 5°C.
2.3.5 Scanning electron microscopy (SEM) analysis of the composite hydrogel
The hydrogel was freeze-dried in a vacuum freeze drier (starting conditions: –40°C, atmospheric pressure, 48 h. After 48 h: –70°C, 10 Pa, 48 h). The freeze-dried gel sample was broken in a liquid nitrogen environment. Its fracture section was observed by using SEM (Quanta 250, FEI Company, Hillsboro, USA).
2.3.6 X-ray diffraction (XRD) characterization of the composite hydrogel
The freeze-dried gel sample was ground into a fine powder. The dried powder was analyzed by using an X-ray diffractometer (Rigaku DMAX-2000, Tokyo, Japan), which used a copper target ray to get the diffraction spectra. The conditions set were: Cu Ka emission wavelength λ=1.54056 Å, scanning speed 1°/min, 2θ scanning range 1–15°.
2.3.7 Repeated water absorption analysis of the composite hydrogel
A certain amount of the composite hydrogel was immersed in deionized water at room temperature. The gel was kept immersed in deionized water until it reached equilibrium water absorption. The water saturated gel sample was dried at 90°C until the weight achieved a constant value. Then the dried sample was immersed in deionized water at room temperature until it reached swelling equilibrium. The “swelling-de-swelling-swelling” cycle was repeated multiple times. For studying the repeated water absorption of the composite hydrogel, an index called the gel water absorption recovery ratio (r) has been introduced in the current study. The water absorption recovery ratio (r) is defined as: r (dried “n” times)=water absorptivity after drying “n” times/initial water absorptivity.
2.3.8 Rheology testing of composite hydrogel
The saturated hydrogels were analyzed using a rheology meter (Anton Paar, Ashland, VA, USA, Phisical MCR 302). The water saturated composite hydrogel was placed on the rheometer test bench and the rheological factors such as G′, G and tan δ were tested. The clamp was a parallel plate rotor with a diameter of 50 mm. The clipper seam was 0.5 mm. The deformation was 2% and the frequency was 0.1 Hz (26).
3 Results and discussion
3.1 Effect of different factors on water absorption of hydrogel
The effect of concentration of initiator on the Q value of hydrogel is shown in Figure 1A. It is seen that as the amount of initiator increased, the hydrogel’s Q value initially increased and then started to decrease. The hydrogel had the highest Q value at an initiator concentration of 0.25%. When the amount of initiator is low, the corresponding amount of the formed radicals in the reaction system is low. Under such conditions, the networking reactions among chemical species are not conducive to form the three-dimensional network of water absorbent resin. However, with an increase in the amount of the initiator, a large amount of monomer radicals are generated. The reaction is sped up and hence, the Q value of the hydrogel is increased. It should be noted that an excess amount of initiator will increase the network density among the materials and hence, will lead to smaller pores and decreased Q value for the hydrogel (20).
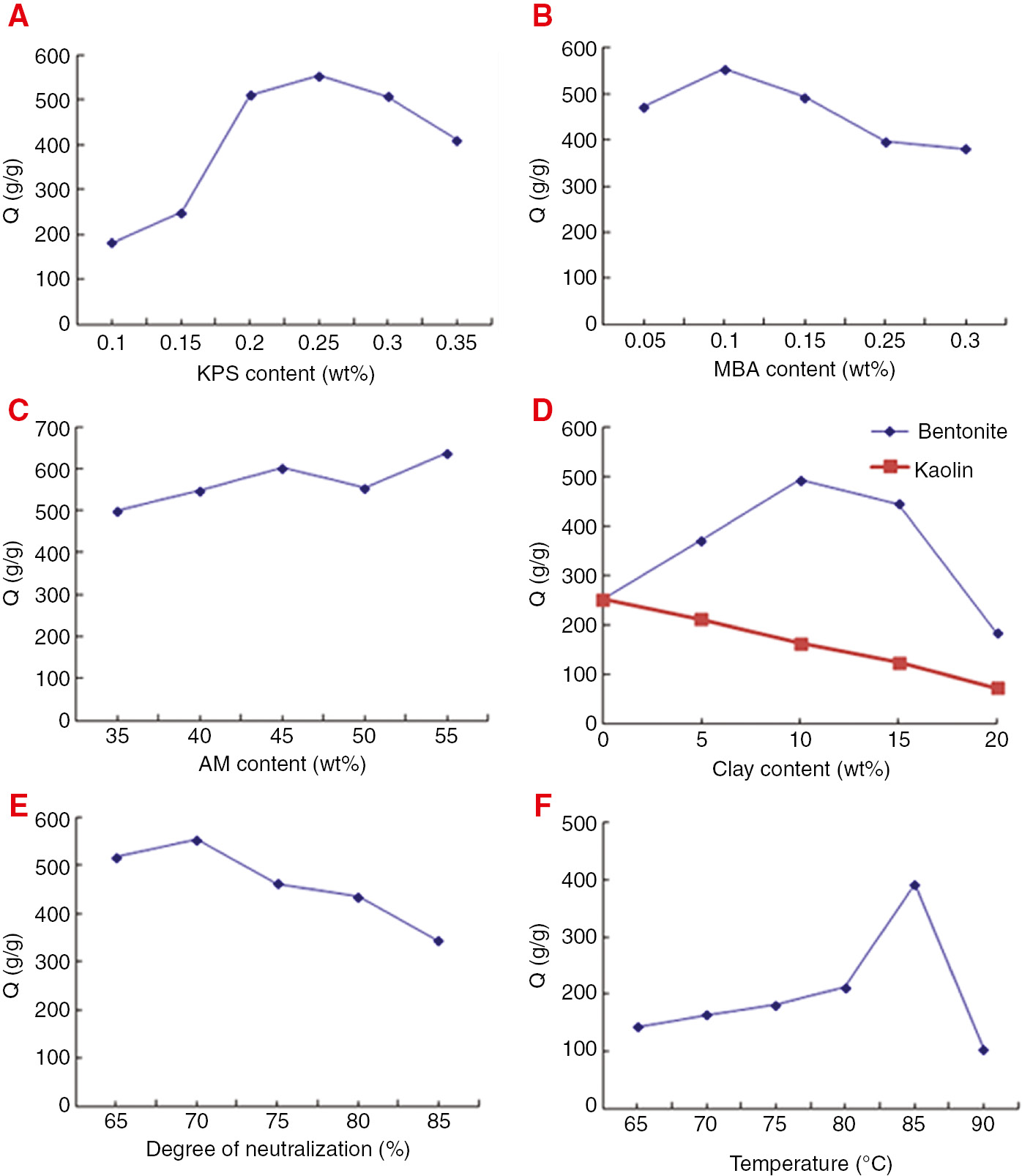
Effect of KPS (A), MBA (B), AM (C), clay (D), degree of neutralization (E) and tempareture (F) on water absorbency of hydrogels.
The effect of the concentration of crosslinking agent on the hydrogel’s Q value is shown in Figure 1B. It is seen that as the amount of MBA increased, the Q value of the hydrogel initially increased and then started to decrease. The hydrogel showed the highest Q value for a crosslinking agent concentration of 0.1%. When the amount of crosslinking agent is low, the networking degree of the hydrogel is too low to form a three-dimensional network. Due to this reason, a polymer which has relatively high water solubility is formed. However, the strength and the water absorption ability of the hydrogel are low. When the amount of crosslinking agent is high, the network density increases. An increased network density leads to an excessive dense network and hence, lowers the water absorption ability of the hydrogel.
The effect of the concentration of the acrylamide on the Q value of the hydrogel is shown in Figure 1C. It is seen that with an increase in the amount of the acrylamide, the hydrogel’s Q value gradually increased. The hydrogel had the highest Q value at an acrylamide concentration of 55%. This because that the ratio hydrophilic polymer/inorganic compounds increases as AM is added (31).
The effect of the concentration of clay on the Q value of the hydrogel is shown in Figure 1D. It is seen that with an increase in the amount of bentonite, the Q value of the hydrogel initially increased and then started to decrease. The hydrogel had the highest Q value at a bentonite concentration of 10%. As described in a previous study, bentonite in a network acts as an additional network point (31). At suitable bentonite concentrations, the crosslinking density of the superabsorbent composite either does not change or changes only slightly, giving superabsorbent composite enough space to absorb and hold water molecules. However, when the bentonite is present in excess, the networking degree becomes too high. Water penetration into the bulk becomes difficult and therefore, is only absorbed on the surface. Due to this reason, the Q value of the hydrogel is decreased (32).
The effect of kaolin on hydrogel’s Q value is also shown in Figure 1D. It is seen that as the amount of kaolin increased, the Q value of the hydrogel gradually decreased. With an increase in the kaolin content, the physical crosslinking nodes of the hydrogel are increased. The space network becomes too dense. Meanwhile, the Q value of kaolin itself is relatively low. Both of these reasons lead to a lower Q value of the composite hydrogel.
Table 2 summarized the effects of two clays on the Q value of the hydrogel. The results showed that the H-29 hydrogel showed the highest Q values in both the deionized water and the 0.9 wt% NaCl solution for a bentonite and kaolin concentrations of 10% and 5%, respectively. The Q values were found to be 494 g/g and 95 g/g, respectively.
Effect of two kinds of clay on the water absorption of hydrogels.
| No. | Bentonite (%) | Kaolin (%) | Q1 (in deionized water) | Q2 (in 0.9 wt% NaCl solution) |
|---|---|---|---|---|
| H-2 | 5 | 0 | 372 | 64 |
| H-6 | 0 | 5 | 230 | 56 |
| H-10 | 5 | 5 | 335 | 60 |
| H-29 | 10 | 5 | 490 | 125 |
| H-30 | 15 | 5 | 445 | 79 |
| H-31 | 20 | 5 | 184 | 62 |
| H-32 | 5 | 10 | 164 | 58 |
| H-33 | 5 | 15 | 126 | 51 |
| H-34 | 5 | 20 | 74 | 48 |
The effect of the neutralization degree of the acrylic acid on the hydrogel’s Q value is shown in Figure 1E. It is seen that for a neutralization degree of 70% or lower of the acrylic acid, the proportion of the functional groups present on the polymer chains became appropriate in number. Due to the complementary and coordinative effects among the functional groups, the hydrogel’s Q value increased. However, when the neutralization degree of the acrylic acid was too large in value, the soluble components in the hydrogel increased and it became soluble in deionized water. This ultimately resulted in a gradual drop in the Q value of the hydrogel.
The effect of reaction temperature on the Q value is shown in Figure 1F. It is seen that as the temperature increased, the Q value of the hydrogel initially increased and then started to decrease. The hydrogel had the highest Q value at a temperature of 85°C. At low temperatures, the system had a low initiator decomposition rate which led to low network density. Part of the polymer chain was water soluble and the viscosity of the system was large, resulting in a lower Q value for the gel. At very high reaction temperature, the decomposition rate of initiator was high resulting in a higher crosslinking density. Higher crosslinking density resulted in a denser network structure and a smaller water absorption space. So the Q value of the hydrogel decreased.
3.2 Fourier transform infrared (FTIR) analysis
Figure 2 shows the FTIR spectra of bentonite and kaolin. The peaks at 3617 cm−1, 3554 cm−1 and 3419 cm−1 correspond to the stretching vibration of different hydroxyl groups while the peaks in the range of 900–1000 cm−1 correspond to the Si-O stretching vibration.
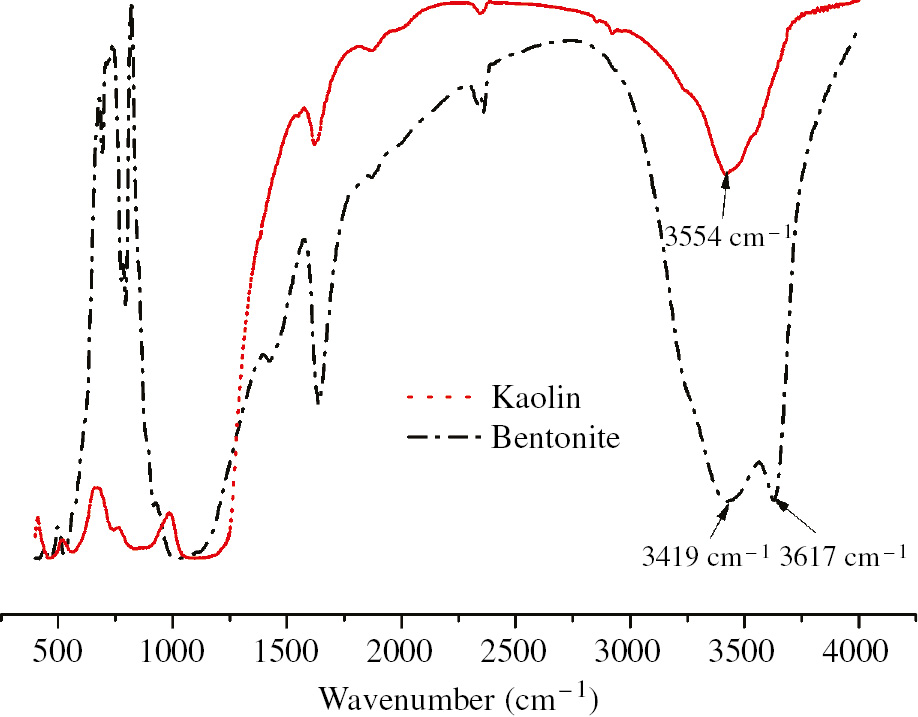
FTIR of bentonite and kaolin.
Figure 3 shows the FTIR spectra of H-2, H-6 and H-10. The peak at 3439 cm−1 corresponds to the stretching vibration of N-H bond in acrylamide unit. The peak at 2945 cm−1 corresponds to the stretching vibration of -CH- in the acrylic acid unit. The peak at 1721 cm−1 corresponds to the υ(C=O) of the carboxylate unit of acrylic acid. The peak at 1668 cm−1 corresponds to the C=O stretching vibration in acrylamide unit. The peak at 1454 cm−1 corresponds to the C-N stretching vibration. The peak at 1404 cm−1 corresponds to the symmetric stretching vibration of carbonyl group in -COO-. The peak at 1175 cm−1 corresponds to the -CO-O- stretching vibration in acrylic acid. The peak at 1010 cm−1 corresponds to the relatively weak Si-O stretching vibration in bentonite and kaolin. The FTIR spectra of the composite hydrogel show all the characteristic peaks, which included -COOH (or -COONa), -CONH-, -CH- and Si-O. In addition, compared to Figure 8, the absorption peaks at 3698 cm−1, 3621 cm−1 and 3549 cm−1 (corresponding to the -OH on the surface of bentonite and kaolin) were significantly weakened or disappeared (see Figure 3). It is suggested that the graft copolymerization between -OH groups on clay (bentonite or kaolin) and monomers (acrylic acid or acrylamide) takes place during the reaction. The synthesis process of gel is shown in Scheme 1.
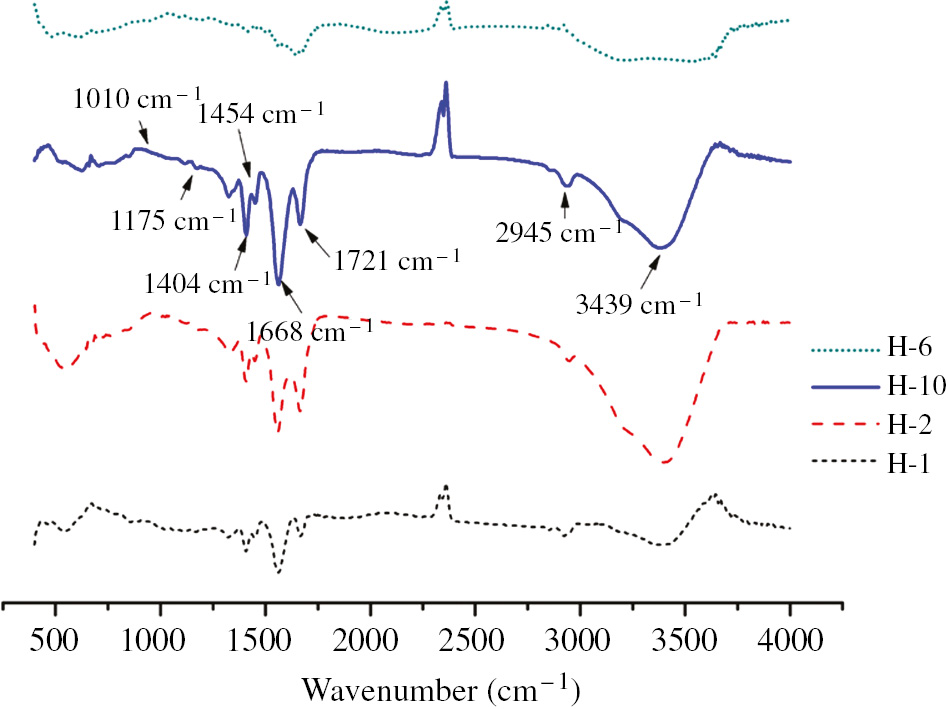
FTIR of hydrogels.
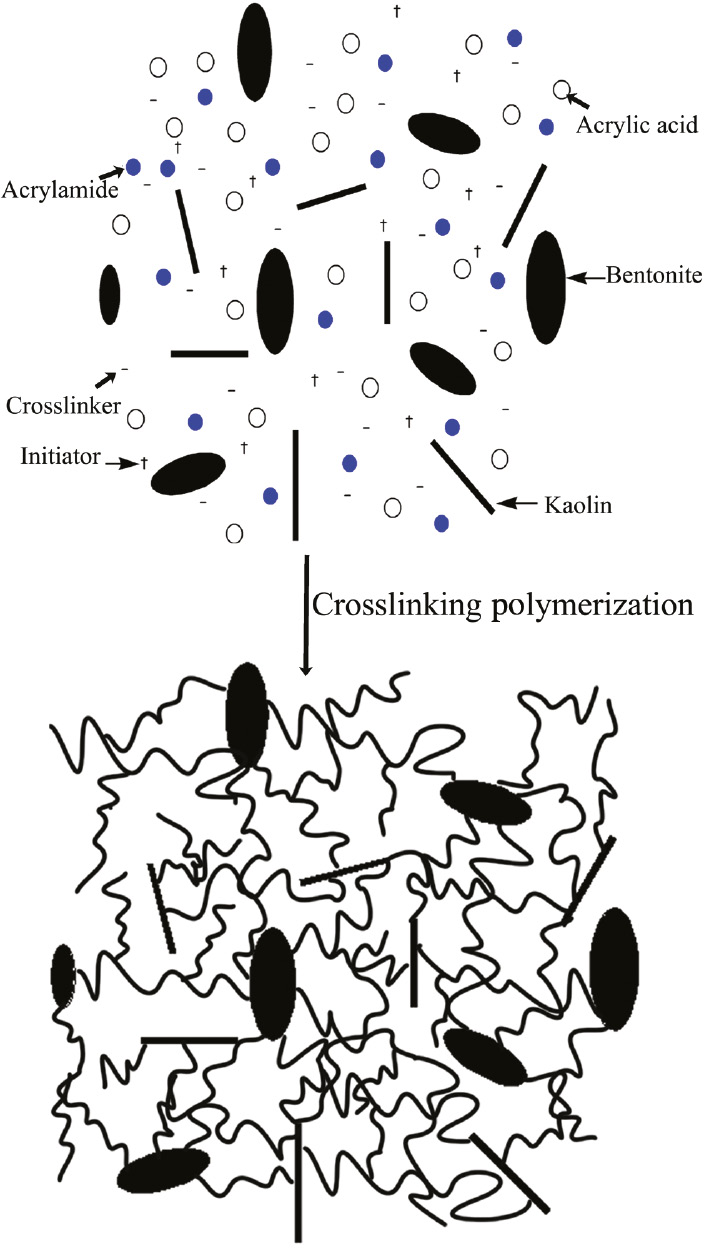
Proposed preparation mechanism for composite hydrogel.
3.3 XRD analysis
To study the dispersion of clay lamellae in hydrogel, XRD analysis of dry hydrogel with different proportions of raw materials was conducted (see Figure 4). It was seen that the diffraction peaks of bentonite and kaolin appeared at 2θ=7.0° which corresponded to the interlamellar spacing of the clay. The diffraction peaks of H-2, H-6 and H-10 composite hydrogel disappeared at 2θ=7.0° which indicated that the clay had been exfoliated and had uniformly been dispersed in the gel. Meanwhile, when the clay amount was relatively high, the diffraction peaks of H-10 gel were not obvious, which indicated that even a relatively high amount of clay could well be dispersed in hydrogel.
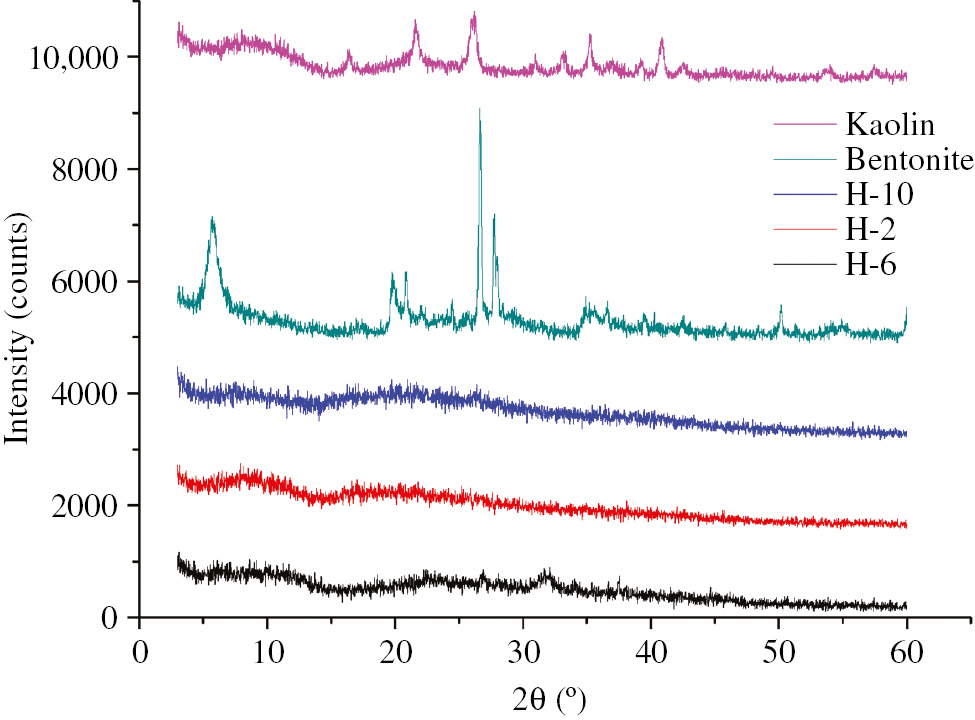
XRD of bentonite, kaolin and hydrogels.
3.4 Micro-morphology analysis
Figure 5 shows the micro-structure of different freeze-dried composite hydrogels by SEM. It is seen that the section of H-1 (AA-AM gel) was relatively compact and had uneven structure. There are a lot of pores on the section of the clay composite hydrogel. The size and the shape of the pores changed with a change of the clay content. When the added amount of bentonite or kaolin reached a value of 5%, the section of H-2 and H-6 gels obtained relatively large pore structure. However, there was a significant morphological difference between the two clay composite gels. The hole diameter of the H-2 gel was significantly larger than that of the H-6 gel. This was due to the reason that kaolin, as a network point, plays a more important role than bentonite in the formation of the composite. In comparison to H-2 (5% bentonite) and H-6 (5% kaolin) gels, addition of 10% bentonite and 5% kaolin increased the hole number of H-29 and decreased its pore size. It was due to the reason that the clay acts as a physical crosslinking agent in the hydrogel. With an increase in clay content, crosslinking density of gel increased, causing an increase in the number of holes and a corresponding decrease in the pore size. These structures favored water absorption ratio of the gel, which is consistent with the water absorption results of the gel (as shown previously in Table 2). With an increase in the clay content, the section of H-30 composite hydrogel transformed from a pore structure to a lamellar structure. Haraguchi et al. proposed that the existence of the nano-clay affected the sublimation of ice during freeze-drying, which enables the gel to form layered pore structure (33). However, the section of H-33 gel was not a lamellar structure, but an uneven structure covered with some pores. This was mainly due to the reason that H-33 gel contained relatively larger amount of kaolin, which played an important role in physical crosslinking, leading to a denser structure with smaller pores.
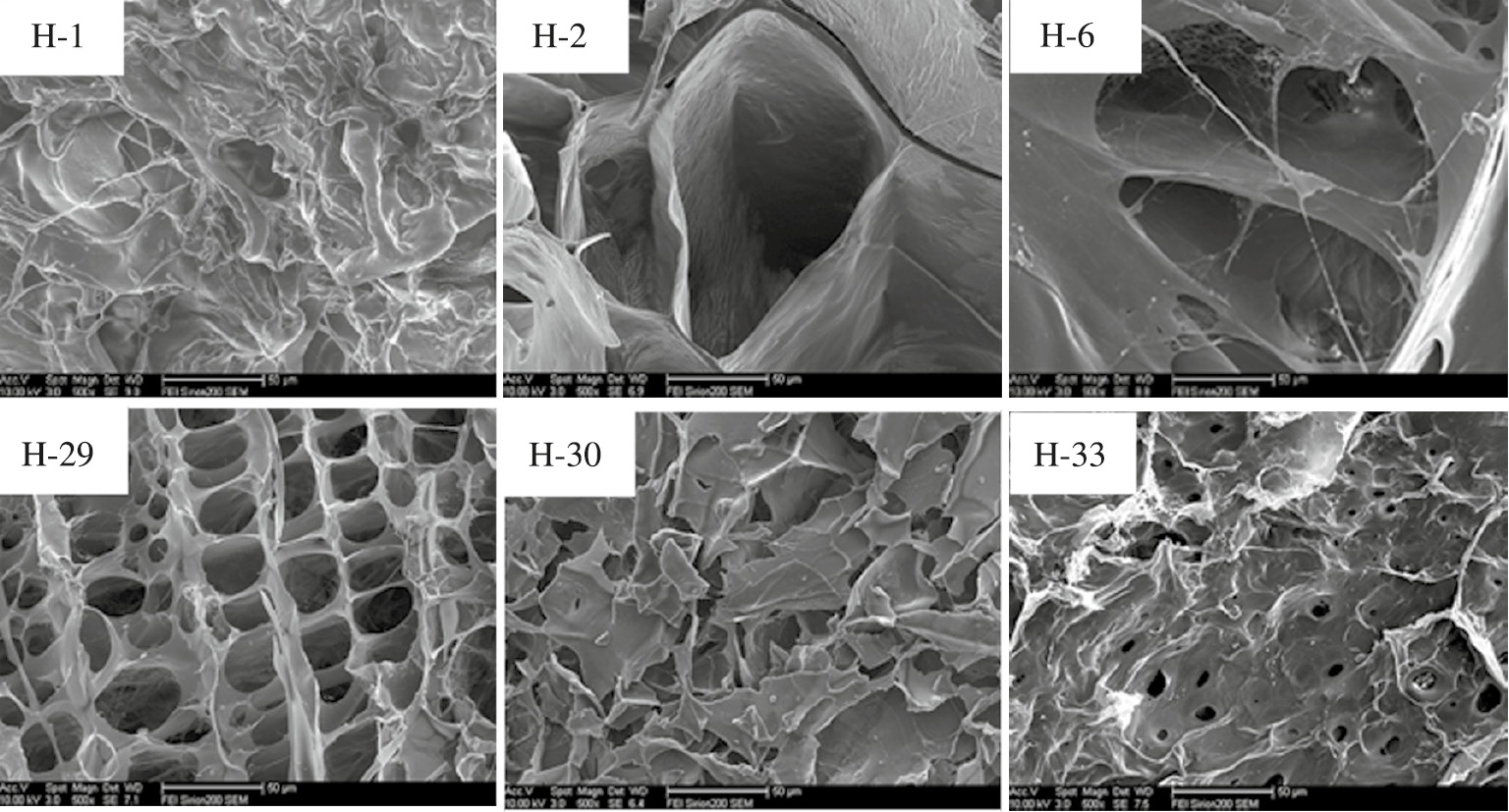
Scanning electron micrographs of hydrogels.
3.5 Thermal stability analysis
The thermogravimetric analysis results of H-1, H-4, H-8 and H-29 hydrogels are shown in Figure 6A. The results showed that the addition of kaolin and bentonite increased the thermal stability of the composite hydrogel. At 600°C, the residual weight percent of H-1 gel (without the addition of clay) was 33.6%. The residual weight percent of H-4 gel (with 15% of bentonite) was 40.2%. The residual weight percent of H-8 gel (with 15% of kaolin) was 40.7% and the residual weight percent of H-29 gel (with 15% of bentonite and 5% of kaolin) was 48.2%. These results indicated that the two clays of bentonite and kaolin are conducive to improve the thermal stability of the materials. Thermal stability improvement for a superabsorbent composite based on clay-poly(sodium acrylate) has previously been reported in the literature (31), (32).
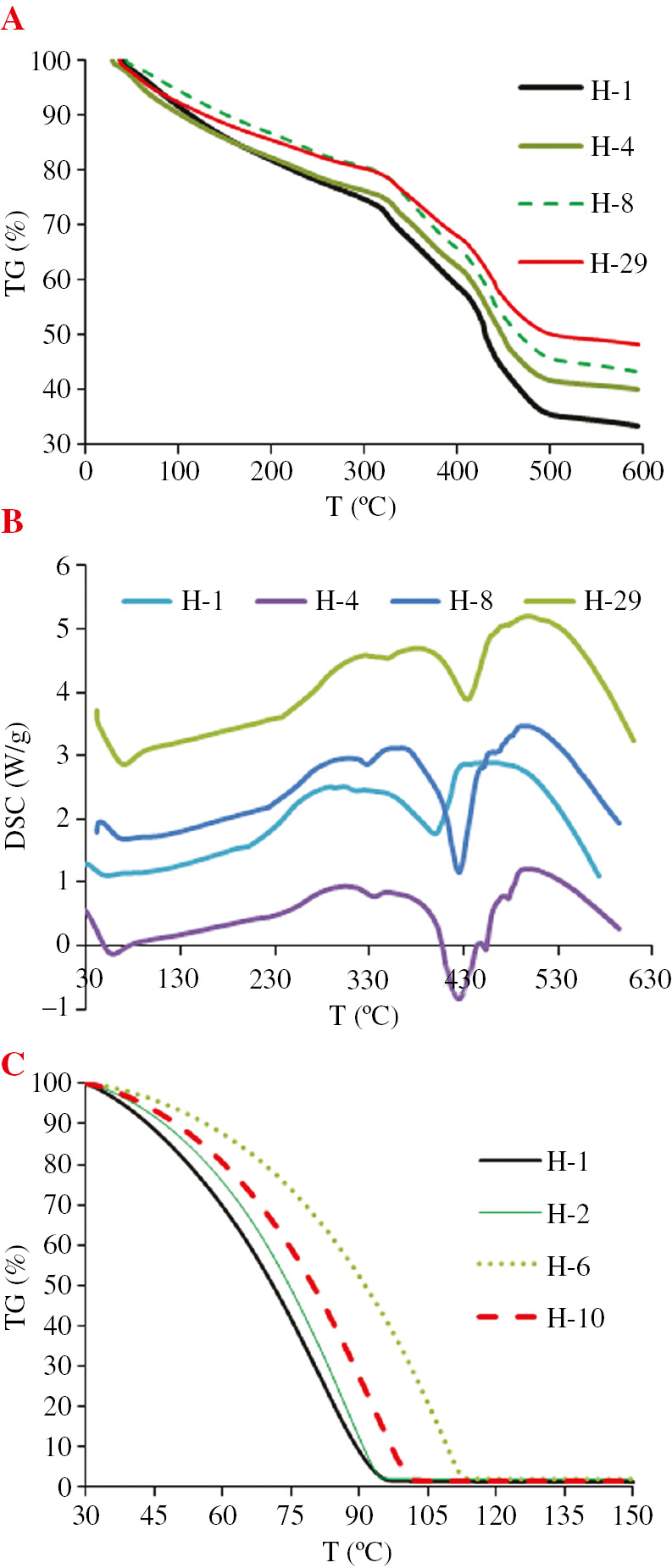
Thermal stability and water retention of hydrogels.
The differential scanning calorimetry (DSC) analysis of the composite hydrogel shown in Figure 6B demonstrates that the endothermic processes of the gel from 30°C to 250°C are caused by the volatilization of free and bound water. As the temperature continues to increase, an exothermic conversion of the gel begins, which signals the thermal decomposition of the gel. The exothermic onset temperatures of clay-containing composite gels were significantly higher than those of single-clay hydrogels. This indicates that the addition two clays is able to significantly enhance the thermal stability of gels because of the shielding effects of clay, which obstruct the transfer of heat and prevent the loss of low-molecular-weight compounds formed by thermal decomposition.
3.6 Water preserving capability of composite hydrogel
Figure 6C showed the water preserving capability of water-saturated gel with the change of temperature. The results showed that with an increase in temperature, the water preserving capability of the hydrogel gradually decreased. Among all gels, the H-1 gel showed the fastest while the H-6 gel showed the slowest water loss rate. Table 3 showed that the water preserving capability of H-1 gel at 80°C was only 30.2%. The water preserving capability of H-2 gel (with 5% of bentonite) was 37.8% which was significantly higher than the H-1 hydrogel. More important is that this H-6 hydrogel “keeps” some water at temperatures above 100°C. This indicated that bentonite and kaolin could significantly improve the water preserving capability of hydrogel. Furthermore, kaolin showed a more obvious water preserving effect.
Water preserving capability of composite hydrogels.
| No. | H-1 | H-2 | H-6 | H-10 |
|---|---|---|---|---|
| Water preserving capability of composite hydrogels at 80°C (%) | 30.2 | 37.8 | 67.5 | 49.9 |
3.7 Repeated water absorption of composite hydrogel
Figure 7 showed the degree of water absorption recovery for different composite hydrogels after the process of “swelling-de-swelling-swelling”. The results showed that after the addition of kaolin or bentonite, the water absorption recovery ratio of the composite hydrogel significantly increased compared to the AA-AM gels. After four cycles of “swelling-de-swelling-swelling” process, the water absorption recovery ratio of H-1 gel (AA-AM gel) was only 52%. The water absorption recovery ratio of H-2 gel (with 5% of bentonite) was 73%. The H-29 gel (with 10% of bentonite and 5% of kaolin) showed relatively high water absorption recovery ratio which was found to be 79%. This indicated that appropriate amounts of bentonite and kaolin were helpful in improving the repeated water absorption property of the composite hydrogel.
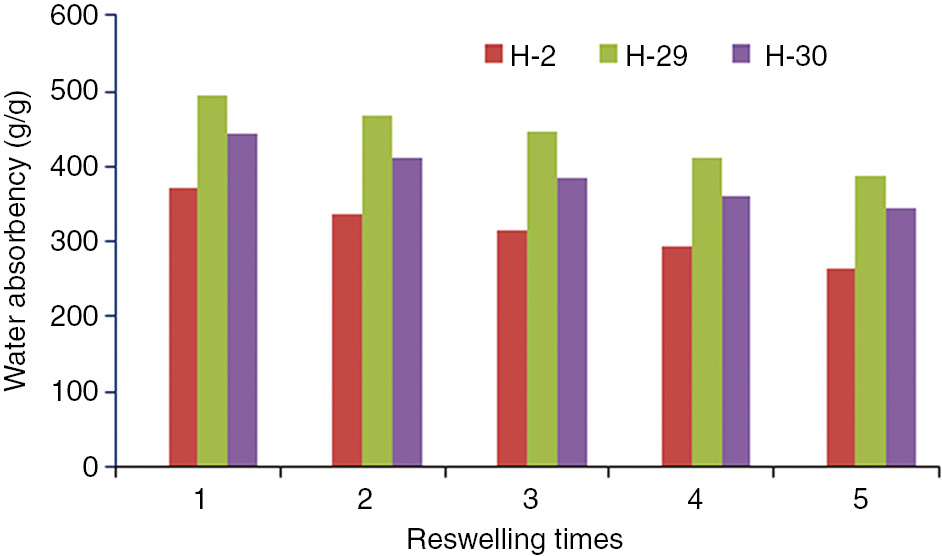
Reswelling of hydrogels.
3.8 Rheological properties of composite hydrogel
Figure 8 shows the effect of bentonite and kaolin on storage modulus of the water-swollen form of the samples versus angular frequency. The storage modulus values showed an increasing trend with respect to the frequency change. When 5% of bentonite was added, the storage modulus of H-2 hydrogel reached a maximum in all the samples, which was higher than that of the reference hydrogel (H-1). However, when 5% kaolin was added or when both kaolin and bentonite were added, the storage modulus of the hydrogels (H-6, H-10, H-29, H-30) decreased and attained a value which was lower than that of the reference hydrogel (H-1). The storage modulus of the composite hydrogel decreased with an increase of clay content. This is because, smaller quantities of clay played the role of crosslinking sites in gel, resulting in a denser and more stable networking structure, which increases the crosslink density and storage modulus of the gel. However, higher clay content could cause an increase of viscosity in the reaction mixture, thus decreasing the probability of collisions between the monomers and the crosslinker which could initiate the growth of the polymer chain. Hence, at higher clay content, inhibiting role of clay to the polymerization progress dominates and leads to a decreased crosslink density, which in turn decreases the storage modulus (34).
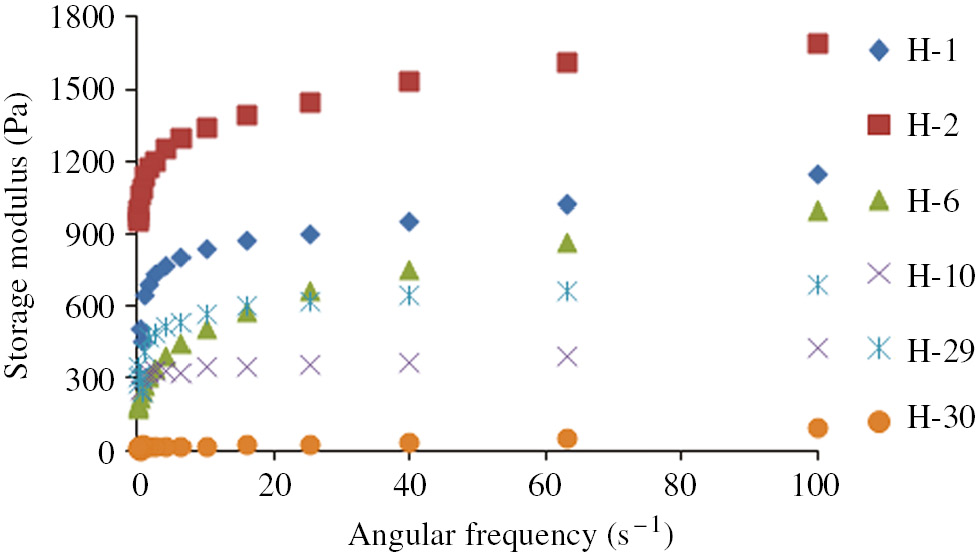
Storage modulus of hydrogels.
3.9 Salt resistance of composite hydrogel
SAPs are usually used in salt containing environments. The water absorption of hydrogel is significantly affected by the salt. Therefore, the salt resistance is an important indicator to measure the effectiveness of SAP materials. To characterize the salt resistance of the composite hydrogel, a non-dimensional factor (f) was introduced in the present study. This non-dimensional factor (f) is defined as the ratio of water absorption ratio of hydrogel in NaCl solution to the water absorption ratio of hydrogel in deionized water. Table 4 showed f values of H-1, H-2, H-6, H-10, and H-29 hydrogel samples in different concentrations of NaCl solutions. The results showed that the f values of these five hydrogels followed the order: f(H-1)<f(H-2)<f(H-6)<f(H-10)<f(H-29). Bentonite and kaolin were introduced to the composite hydrogels which are not sensitive to salt. From Table 4, we might conclude that the presence of kaolin is the crucial factor (compare H-1 with H-6), while the effect of bentonite is marginal (compare H-1 with H-2, or H-6, H-10 and H-29). Qi et al. also suggested a similar conclusion (29).
Salt resistance of composite hydrogels.
| No. | Bentonite (%) | Kaolin (%) | f1 (in 0.02 wt% NaCl solution) | f2 (in 0.9 wt% NaCl solution) |
|---|---|---|---|---|
| H-1 | 0 | 0 | 0.51 | 0.15 |
| H-2 | 5 | 0 | 0.55 | 0.17 |
| H-6 | 0 | 5 | 0.58 | 0.24 |
| H-10 | 5 | 5 | 0.59 | 0.25 |
| H-29 | 10 | 5 | 0.62 | 0.26 |
3.10 The effect of salt solution on the water absorption of composite hydrogel
Figure 9 shows the effect of different salt solutions on the Q value of H-29 hydrogel. The Q value of the hydrogel decreased with an increase in the concentration of the salt solution. The Q value decreased more obviously when a high oxidative state salt solution (e.g. CaCl2) was used. According to the Flory equation, the ionic strength of the solution depends on both the concentration and the charge of each individual ion. In fact the presence of ions in the solution decreases the osmotic pressure difference, the driving force for swelling, between the gel and the solution. The existence of the high oxidative state cation resulted in ion aggregation in the hydrogel’s interior (Ca and Al ions are known to form compact “complexes” with sodium polyacrylate). It led to the increase of the network density inside the hydrogel, which resulted in a significant reduction of Q value of the hydrogel in high oxidative state salt solution.
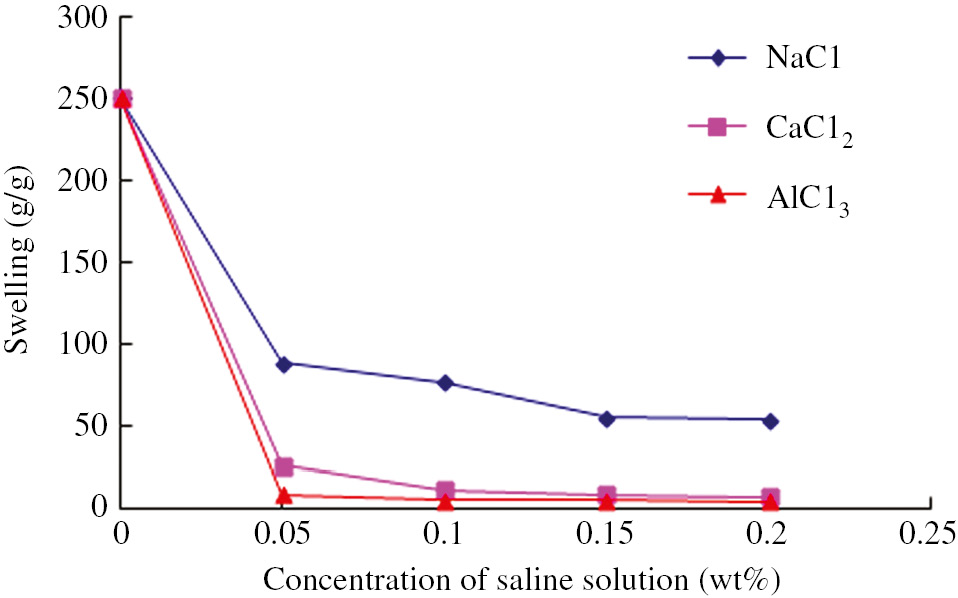
Water absorbency of hydrogel in NaCl, MgCl2 and AlCl3 aqueous solutions with various concentions.
4 Conclusions
Bentonite, kaolin, acrylic acid and acrylamide have been used as raw materials while N,N′-methylene bisacrylamide has been used as a crosslinking agent to prepare a novel bentonite/kaolin/acrylic acid-acrylamide composite hydrogel. Appropriate amounts of bentonite and kaolin could lead to an increase of pores on the hydrogel sections, while the excess amount of clay led to a lamellar structure on the sections. The mixed use of bentonite and kaolin could effectively improve the thermal stability, water preserving capability and repeated water absorption of the composite hydrogel. A comprehensive comparison showed that the H-29 composite hydrogel containing 10% bentonite and 5% kaolin exhibited higher water absorption rates (494 g/g), higher repeated water absorbency (79%), improved salt resistance (f=0.62), and lower production costs as compared with gels that only contain 15% bentonite (H-4 gel) or 15% kaolin (H-8 gel). These results show that the addition of a mixture of two different clays to form hydrogels enhances the overall performance of the gels, which may encourage their widespread use in the fields of agriculture, gardening, and fire control.
Acknowledgments
This study was supported by the funds coming from National Natural Science Foundation of China (51304027, 51674038), China Postdoctoral Science Foundation (2014M560567 and 2015T80730), Shandong Province Science and Technology Development Plan (2014GSF120012), State Key Program of Coal Joint Funds of National Natural Science Foundation of China (No. 51134020, No. U1261205) and Key Technology Projects for Preventing Major Accidents of National Security State Administration of Work Safety. Wei-Min Cheng and Yan-Yun Zhao designed the experiments; Xing-Teng Yu, Zun-Xiang Hu and Ming-Yue Wu contributed to the synthesis of bentonite/kaolin/acrylic acid-co-acrylamide composite hydrogels; Xiang-Ming Hu interpreted the results and discussion, and also contributed to the writing of the paper.
References
1. Kamat M, Malkani R. Disposable diapers: A hygienic alternative. Indian J Pediatr. 2003;70(11):879–81.10.1007/BF02730591Search in Google Scholar
2. Karada E, Saraydin D, Caldiran Y, Güven O. Swelling studies of copolymeric acrylamide/crotonic acid hydrogels as carriers for agricultural uses. Polym Adv Technol. 2000;11(2):59–68.10.1002/(SICI)1099-1581(200002)11:2<59::AID-PAT937>3.0.CO;2-ZSearch in Google Scholar
3. Chu M, Zhu SQ, Li HM, Huang ZB, Li SQ. Synthesis of poly(acrylic acid)/sodium humate superabsorbent composite for agricultural use. J Appl Polym Sci. 2006;102(6):5137–43.10.1002/app.24661Search in Google Scholar
4. Deng J, Yang Y, Tang K. Preparation and thermosensitive property of linear P (NIPA-co-SA) hydrogels. J China Coal Soc. 2014;39(1):154–60.Search in Google Scholar
5. Kuswandi B, Jayus, Restyana A, Abdullah A, Heng LY, Ahmad M. A novel colorimetric food package label for fish spoilage based on polyaniline film. Food Control. 2012;25(1):184–9.10.1016/j.foodcont.2011.10.008Search in Google Scholar
6. Alsabagh AM, Abdou MI, Khalil AA, Ahmed HE, Aboulrous AA. Investigation of some locally water-soluble natural polymers as circulation loss control agents during oil fields drilling. Egypt J Petrol. 2014;23(1):27–34.10.1016/j.ejpe.2014.02.005Search in Google Scholar
7. Tongwa P, Nygaard R, Bai BJ. Evaluation of a nanocomposite hydrogel for water shut-off in enhanced oil recovery applications: design, synthesis, and characterization. J App Polym Sci. 2013;128(1):787–94.10.1002/app.38258Search in Google Scholar
8. Tongwa P, Nygaard R, Blue A, Bai B. Evaluation of potential fracture-sealing materials for remediating CO2 leakage pathways during CO2 sequestration. J Int J Greenh Gas Con. 2013;18:128–38.10.1016/j.ijggc.2013.06.017Search in Google Scholar
9. Tongwa P, Bai BJ. Degradable nanocomposite preformed particle gel for chemical enhanced oil recovery applications. J Petrol Sci Eng. 2014;124:35–45.10.1016/j.petrol.2014.10.011Search in Google Scholar
10. Tongwa P, Bai BJ. A more superior preformed particle gel with potential application for conformance control in mature oil fields. J Petrol Explor Prod Technol. 2015;5(2):201–10.10.1007/s13202-014-0136-8Search in Google Scholar
11. Wang L, Zhang JP, Wang AQ. Removal of methylene blue from aqueous solution using chitosan-g-poly(acrylic acid)/montmorillonite superadsorbent nanocomposite. Colloid Surface A. 2008;322(1-3):47–53.10.1016/j.colsurfa.2008.02.019Search in Google Scholar
12. Kaşgöz H, Durmuş A, Kaşgöz A. Enhanced swelling and adsorption properties of AAm-AMPSNa/clay hydrogel nanocomposites for heavy metal ion removal. Polym Adv Technol. 2008;19(3):213–20.10.1002/pat.999Search in Google Scholar
13. Gupta P, Vermani K, Garg S. Hydrogels: from controlled release to pH-responsive drug delivery. Drug Discov Today. 2002;7(10):569–79.10.1016/S1359-6446(02)02255-9Search in Google Scholar
14. Chang C, Duan B, Cai J, Zhang L. Superabsorbent hydrogels based on cellulose for smart swelling and controllable delivery. Eur Polym J. 2010;46(1):92–100.10.1016/j.eurpolymj.2009.04.033Search in Google Scholar
15. Kabiri K, Omidian H, Zohuriaan-Mehr MJ, Doroudiani S. Superabsorbent hydrogel composites and nanocomposites: a review. Polym Composite. 2011;32(2):277–89.10.1002/pc.21046Search in Google Scholar
16. Tanaka Y, Kuwabara R, Gong JP, Kurokawa T, Gong JP, Osada Y. Determination of fracture energy of high strength double network hydrogels. J Phys Chem B. 2005;109(23):11559–62.10.1021/jp0500790Search in Google Scholar
17. Su XF, Zhang G, Xu K, Wang JH, Song CL, Wang PX. The effect of MMT/modified MMT on the structure and performance of the superabsorbent composite. Polym Bull. 2008;60(1):69–78.10.1007/s00289-007-0843-0Search in Google Scholar
18. Kabiri K, Mirzadeh H, Zohuriaan-Mehr MJ. Chitosan-modified nanoclay-poly(AMPS) nanocomposite hydrogels with improved gel strength. Polym Int. 2009; 58(11):1252–9.10.1002/pi.2652Search in Google Scholar
19. Kabiri K, Mirzadeh H, Zohuriaan-Mehr MJ. Chitosan modified MMT-poly(AMPS) nanocomposite hydrogel: Heating effect on swelling and rheological behavior. J Appl Polym Sci. 2010;116(5):2548–56.10.1002/app.31727Search in Google Scholar
20. HaraguchiK, Takehisa T. Nanocomposite hydrogels: A unique organie-inorganic network structure with extraordinary mechanical, optical, and swelling/de-swelling properties. Adv Mater. 2002;14(16):1120–4.10.1002/1521-4095(20020816)14:16<1120::AID-ADMA1120>3.0.CO;2-9Search in Google Scholar
21. Li A, Wang AQ, Chen JM. Studies on poly(acrylic acid)/attapulgite superabsorbent composite. I. Synthesis and characterization. J Appl Polym Sci. 2004;92(3):1596–603.10.1002/app.20104Search in Google Scholar
22. Li A, Wang AQ, Chen JM. Studies on poly(acrylic acid)/attapulgite superabsorbent composites. II. Swelling behaviors of superabsorbent composites in saline solutions and hydrophilic solvent-water mixtures. J Appl Polym Sci. 2004;94(5):1869–76.10.1002/app.20850Search in Google Scholar
23. Karadağ E, Topç F, Kundakci S, Üzüm ÖB. Novel composite sorbent AAm/MA hydrogels containing starch and kaolin for water sorption and dye uptake. B Mater Sci. 2014;37(7):1637–46.10.1007/s12034-014-0723-9Search in Google Scholar
24. Lee WF, Tsao KT. Effect of intercalant content of mica on the various properties for the charged nanocomposite poly(N-isopropyl acrylamide) hydrogels. J Appl Polym Sci. 2007;104(4):2277–87.10.1002/app.25689Search in Google Scholar
25. Kabiri K, Zohuriaan-Mehr MJ. Superabsorbent hydrogel composites. Polym Adv Technol. 2003;14(6):438–44.10.1002/pat.356Search in Google Scholar
26. Zhang J, Sun MW, Zhang L, Xie XM, Yang Y. Improvement of salt resistance and gel strength of super absorbent resin. Petrochem Technol. 2002;31(12):994–7.Search in Google Scholar
27. Xia X, Yih J, D’Souza NA, Hu Z. Swelling and mechanical behavior of poly (N-isopropylacrylamide)/Na-montmorillonite layered silieates composite hydrogels. Polymer. 2003;44(11):3389–93.10.1016/S0032-3861(03)00228-3Search in Google Scholar
28. Zhang XH, Cui BJ, Cui YD, Synthesis and characterization of poly(sodium acrylate)/kaolin superabsorbent composite. Fine Chem. 2003;20(6):584–8.Search in Google Scholar
29. Qiu HX, Yu JG. Polyacrylate/(carboxymethylcellulose modified montmorillonite) superabsorbent nanocomposite: preparation and water absorbency. J App Polym Sci. 2008;107(1):118–23.10.1002/app.26261Search in Google Scholar
30. Hussam-Aldeen K, Mohammad T, Yomen A. Preparation of a clay based superabsorbent polymer composite of copolymer poly(acrylate-co-acrylamide) with bentonite via microwave radiation. ACS Appl Mater Inter. 2013;4(4):145–50.Search in Google Scholar
31. Li A, Wang AQ. Synthesis and properties of clay-based superabsorbent composite. Eur Polym J. 2005;41(7):1630–7.10.1016/j.eurpolymj.2005.01.028Search in Google Scholar
32. Ramazani-Harandy MJ, Zohuriaan-Mehr MJ, Ershad-Langroudi A, Yousefi AA, Kabiri K. Rheological determination of the swollen gel strength of superabsorbent polymer hydrogels. Polym Test. 2006;25(4):470–4.10.1016/j.polymertesting.2006.01.011Search in Google Scholar
33. Haraguchi K, Matsuda K. Spontaneous formation of characteristic layered morphologies in porous nanocomposites prepared from nanocomposite hydrogels. Chem Mat. 2005;17(5):931–4.10.1021/cm048093xSearch in Google Scholar
34. Kabiri K, Hesarian S, Zohuriaan-Mehr MJ, Jamshidi A, Boohendi H, Pourheravi MR, Hashemi SA, Omidian H, Fathollahi S. Superabsorbent polymer composites: does clay always improve properties? J Mater Sci. 2011;46(20):6718–25.10.1007/s10853-011-5627-0Search in Google Scholar
©2017 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- In this Issue
- Editorial
- Innovations in polymers and composite materials
- Full length articles
- Preparation and properties of chemically reduced graphene oxide/copolymer-polyamide nanocomposites
- Synthesis and properties of well-defined carbazole-containing fluorescent star polymers of different arms
- The effect of high-current pulsed electron beam modification on the surface wetting property of polyamide 6
- Synthesis and application of waterborne polyurethane fluorescent composite
- Medicated structural PVP/PEG composites fabricated using coaxial electrospinning
- Research of the thermal aging mechanism of polycarbonate and polyester film
- Damage indication of 2′, 7′-dichlorofluorescein for epoxy polymer and the effect of water on its damage indicating ability
- Synthesis and characterization of thermosensitive and polarity-sensitive fluorescent PNIPAM-coated gold nanoparticles
- Comparative study of crystallization and lamellae orientation of isotactic polypropylene by rapid heat cycle molding and conventional injection molding
- Determination of deformation of a highly oriented polymer under three-point bending using finite element analysis
- Kinetic studies on the cure reaction of hydroxyl-terminated polybutadiene based polyurethane with variable catalysts by differential scanning calorimetry
- Preparation and swelling properties of poly(acrylic acid-co-acrylamide) composite hydrogels
Articles in the same Issue
- Frontmatter
- In this Issue
- Editorial
- Innovations in polymers and composite materials
- Full length articles
- Preparation and properties of chemically reduced graphene oxide/copolymer-polyamide nanocomposites
- Synthesis and properties of well-defined carbazole-containing fluorescent star polymers of different arms
- The effect of high-current pulsed electron beam modification on the surface wetting property of polyamide 6
- Synthesis and application of waterborne polyurethane fluorescent composite
- Medicated structural PVP/PEG composites fabricated using coaxial electrospinning
- Research of the thermal aging mechanism of polycarbonate and polyester film
- Damage indication of 2′, 7′-dichlorofluorescein for epoxy polymer and the effect of water on its damage indicating ability
- Synthesis and characterization of thermosensitive and polarity-sensitive fluorescent PNIPAM-coated gold nanoparticles
- Comparative study of crystallization and lamellae orientation of isotactic polypropylene by rapid heat cycle molding and conventional injection molding
- Determination of deformation of a highly oriented polymer under three-point bending using finite element analysis
- Kinetic studies on the cure reaction of hydroxyl-terminated polybutadiene based polyurethane with variable catalysts by differential scanning calorimetry
- Preparation and swelling properties of poly(acrylic acid-co-acrylamide) composite hydrogels

