A preliminary systematic review and meta-analysis on the effects of heart rate variability biofeedback on heart rate variability and respiration of athletes
Abstract
To date, there is no quantitative review examining the influence of heart rate variability biofeedback (HRV BFB) on the athlete population. Such an undertaking may provide valuable information on the autonomic and respiration responses of athletes when performing HRV BFB. Thus, purpose of this preliminary systematic review and meta-analysis on the effects of HRV BFB on HRV and respiration of athletes. Searches of Springerlink, SportDiscus, Web of Science, PROQUEST Academic Research Library, Google Scholar, and ScienceDirect were conducted for studies that met the following criteria: (1) experimental studies involving athletes that underwent randomized control trial; (2) availability of HRV BFB as a treatment compared with a control (CON)/placebo (PLA); (3) any pre and post HRV variable and/or breathing frequency as dependent variable/s; and, (4) peer-reviewed articles written in English. Four out of 660 studies involving 115 athletes (25 females and 90 males) ages 16–30 years old were assessed in this review. Preliminary findings suggest the promising ability of HRV BFB to improve respiratory mechanics in athlete population. More work is needed to determine the autonomic modulatory effect of HRV BFB in athletes.
Introduction
Cardiac rhythm is controlled by the autonomic nervous system (ANS) through the parasympathetic nervous system (PNS) and sympathetic nervous system (SNS) [1], [2], [3], [4]. PNS and SNS operate via the sinoatrial node (SA node) which is mainly responsible for increasing or decreasing heart rate [5]. The interaction of PNS and SNS can be assessed using a non-invasive and reliable method called heart rate variability (HRV) [1, 4]. HRV refers to fluctuations between heartbeats that represent sinus node depolarizations in the QRS complexes of the electrocardiogram (ECG). The QRS intervals, specifically the distance between R to R intervals, are computed to derive time, frequency, and non-linear domains of HRV [4, 6, 7]. Depressed HRV reflect sympathetic overactivation and is linked to various clinical and psychological diseases [8], [9], [10]. On the other hand, the presence of high HRV is believed to represent homeostasis and resilience to stress [9, 11, 12].
Over the last decade, interventions aimed at increasing HRV, with the goal of improving health, have received notable attention. Among these is HRV biofeedback (HRV BFB), a non-invasive intervention utilizing paced respiration assisted by visual feedback [9, 13], [14], [15], [16]. HRV BFB was first documented in a clinical facility in Russia [17]. A typical HRV BFB set-up consists of heart rate (HR) and respiration sensors linked to a computer screen with breathing pacer and provides real-time values of HR and respiratory rate (RR). HRV BFB use resonance frequency (RF) which presents oscillatory episodes: (a) a 0-degree phase shift between HR and respiration; and, (b) a 180-degree phase relationship between HR and blood pressure (BP) [18], [19], [20]. Additionally, researchers also discovered peak gas exchange and oxygen saturation at RF [21, 22].
Autonomic responses from HRV BFB have been linked to various physiological mechanisms [9, 14]. Firstly, HRV BFB is believed to enhance baroreflex sensitivity [14, 23]. The baroreflex system (BRS) plays a critical role in regulating BP that protects the body from acute blood pressure shifts [14, 20]. The BRS operates in a closed loop system wherein baroreceptors react to shifts in BP and increase or decrease HR [20]. Briefly, elevation in BP reduces HR, while BP depression increases HR. Oppositely, increases in HR elevate BP, while decreases in HR elevates BP. The baroreceptors in the BRS are located in the heart and aortic arch that send chemical and mechanical information to the nucleus of the solitary tract [5]. The nucleus of the solitary tract is connected to other regulatory centers in the medulla wherein SNS outflow to the heart and blood vessels are controlled. These reactions present a mechanical delay of about five seconds due to inertia and vascular plasticity. During HRV BFB, the amplitude of HR oscillations is maximized and stimulates baroreflex response [13, 18], [19], [20]. With constant HRV BFB practice, improved baroreflex function can be achieved over time [14]. Another possible mechanism in HRV BFB is the vagal afferent pathway stimulation [9, 14]. HRV BFB promotes the activity of subdiaphragmatic vagal afferents and enhance the vagal braking system responsible for immediate control of HR and BP. It has also been postulated that HRV BFB strengthens the parasympathetic vagal efferent pathway through accentuated antagonism, a physiological response that inhibits tonic sympathetic activation from abrupt parasympathetic stimulation under normal physiological conditions in rest and exercise [3, 14, 24]. Similarly, enhancement of cholinergic anti-inflammatory pathway (CAP) may also be present in HRV BFB [25, 26]. During HRV BFB, vagal activation in CAP releases acetylcholine which reduces inflammatory activity in macrophages, thereby diminishing pathogenesis [27, 28].
As the majority of literature reviews in HRV BFB has been conducted on healthy populations or people with chronic conditions, there remains a paucity of systematic literature on the effects of HRV BFB on autonomic indices in athletes. This information may be helpful in understanding the autonomic and respiratory responses of athlete population with HRV BFB training. Thus, the purpose of this study was to conduct preliminary systematic review and meta-analysis on the effects of HRV BFB on HRV and respiration of athletes.
Materials and methods
Search strategy and inclusion criteria
Literature search was administered between July 1st to December 20th 2017 using the search term “heart rate variability biofeedback” AND (athletes OR athletic population OR sport OR performance OR sport performance) in electronic databases (Springerlink, SportDiscus, Web of Science, PROQUEST Academic Research Library, Google Scholar, and ScienceDirect) adhering to the PRISMA guidelines [29, 30]. A manual search in the reference section of relevant articles were performed to include additional studies for assessment. Studies met all the following inclusion criteria: (1) experimental studies that involved random group allocation of athletes; (2) availability of HRV BFB as a treatment group compared with a control (CON)/placebo (PLA); (3) any pre and post HRV parameter and/or breathing frequency as dependent variable/s; and, (4) peer-reviewed articles written in English.
Coding of studies
Literature search and selection of studies was conducted by a single investigator (JP) with studies coded and organized in an Excel spreadsheet. Data extraction was evaluated by a second investigator (YSC). Articles included in the systematic review were encoded by author/s and year of publication, sample size information, intervention, measured HRV parameters, and results. Risk of bias in a study was also assessed by both investigators using the eight-point Consolidated Standards of Reporting Trials (CONSORT) statement [31]. Each item in the CONSORT statement is answerable by 0 (absent or inadequately described) or 1 (explicitly described and present). A study with a score of 0–2 is regarded as having a high risk of bias, 3–5 with medium risk of bias, and 6–8 considered as having low risk of bias [31]. Any disagreement presented in data extraction and CONSORT output was settled by a consensus between the first and second investigator. Personal correspondence to the author/s of an included study for any clarification was also administered.
Meta-analysis
Meta-analysis was carried out if at least two studies provided sufficient data to compute for effect sizes (ES). The natural logarithm of low frequency HRV (lnLF), high frequency HRV (lnHF) and total power (lnTP) were utilised as HRV markers for analysis [4, 32, 33]. LF (0.04–0.15 Hz) is a marker of parasympathetic and sympathetic activity, while HF (0.15–0.40 Hz) depicts parasympathetic activity. Total power (0.04–0.40 Hz) reflects a global marker of autonomic modulation. Additionally, normalized units of LF and HF were also used for HRV analysis [4, 34]. Respiration was examined via breathing frequency. The mean difference and change in standard deviation (SD) from baseline to post-measures were computed in all the studies. Change in SD was derived based on imputed standard deviation method with correlation coefficient set at 0.40 [35], [36], [37]. Meta-analysis was conducted in a free software (Review Manager version 5.3). The standard mean difference was used to interpret ES as small=0.20, moderate=0.50, or large=0.80 [38, 39]. Heterogeneity was evaluated using I 2 [40]. The I 2 represents the percentage of between-study variance due to heterogeneity vs. chance based on 0% (no heterogeneity) −100% (high heterogeneity) scale. Visual inspection of a funnel plot was utilised to examine potential publication bias [41]. Lastly, data at resting conditions were utilised in meta-analysis.
Results
Literature search
A total of 656 potential articles and four identified articles from reference lists were included in the database. Removal of duplicates (n=90) led to initial screening of 570 articles on the basis of article title and abstract. After initial screening, 556 articles were eliminated by JP. Then, full articles of 14 studies were assessed for eligibility. An article was then excluded after failing to meet any of the items mentioned in the above inclusion criteria. In addition, the study of Paul and Garg [42] was excluded as it posted the same HRV and respiration values with earlier published study (Paul et al., 2012) [43]. Four studies were eventually included in the systematic review, while three studies qualified for meta-analysis. Figure 1 displays the flow chart and selection process for the systematic review and meta-analysis.
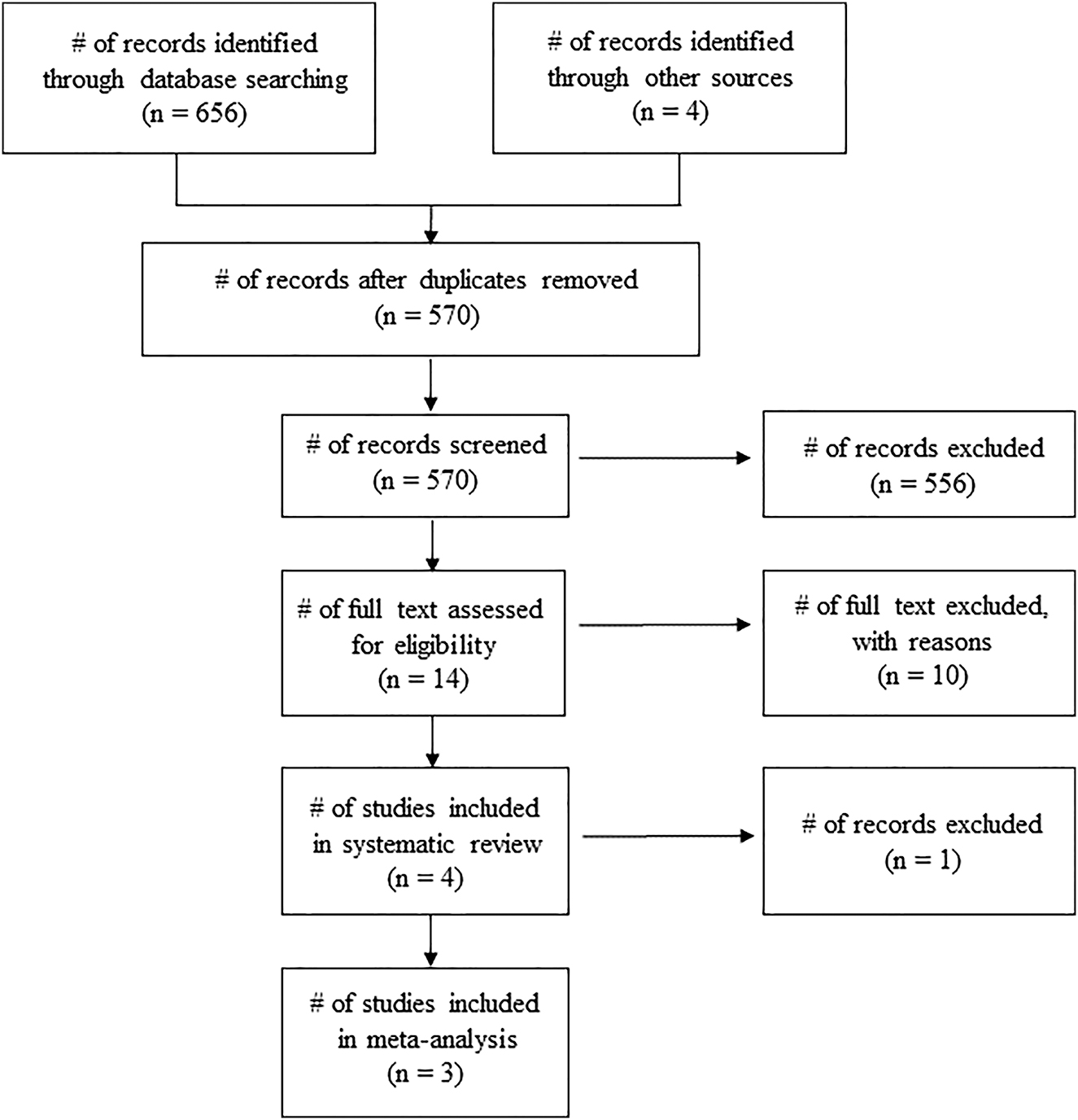
Flow diagram of search Process.
Risk of bias in the study is displayed in Table 1. Two studies scored 5 points [43, 44] while two studies scored 3 points [45, 46].
CONSORT scores of HRV BFB studies included in systematic review.
| Were the groups comparable at baseline on key characteristics? | Did the study include a true control group (randomised participants – not a comparison group)? | Was the randomisation procedure adequately described and carried out? | Did the study report a power calculation and was the study adequately powered to detect intervention effects? | Were the assessors blinded to treatment allocation at baseline and post-test? | Did at least 80% of participants complete follow up assessments? | Did the study analyses account for potential differences at baseline? | Did the study report effect sizes? | Total | |
|---|---|---|---|---|---|---|---|---|---|
| Choudhary et al. 2016 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 3 |
| Dziembowska et al. 2016 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 3 |
| Paul et al. 2012 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 5 |
| Rusciano et al. 2017 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 5 |
Experimental protocols
Participants in the four studies involved 115 athletes (25 females and 90 males) which comprised 28 male and 13 female basketball athletes, 50 male football athletes, 12 male and 12 female track and field athletes with ages ranging from 16 to 30 years old.
All four studies included comparison of HRV BFB and CON [44], [45], [46], while only one study [43] compared HRV BFB and PLA. CON involved regular sport training in all studies. Paul et al. [43] implemented 10 consecutive days of HRV BFB with each session lasting for 20 min. Additionally, motivational videos were used in PLA. Dziembowska et al. [46] administered HRV BFB for 10 20-min sessions within 3 weeks. Rusciano et al. [44] facilitated 15 sessions of HRV BFB lasting 30 min/session for twice a week. Choudhary et al. [45] conducted 10 formal sessions of HRV BFB alongside with two 20 min daily HRV BFB practice at convenience for 10 weeks.
Different physiological parameters were identified from the studies above. Four studies included LF as a parameter for comparison [43, 44, 46]. Two studies utilized high frequency (HF) and total HRV for assessment [43, 46]. One study compared LF/HF output [45]. Respiration rate (RR) was differentiated in two studies [43, 44]. The characteristics of studies are presented in Table 2.
HRV BFB and physiology of athletes.
| Authors | Participants | Intervention | Outcome |
|---|---|---|---|
| Choudhary et al. 2016 | 18–25 yr old male (n=12) and female (n=12) university, state, and national level long distance runners age: 22.5 ± 1.72 yrs, height: 172 ± 7.95 cm weight: 55.6 ± 7.52 kg | HRV BFB: 10-week HRV BFB; once a week formal HRV BFB; 2 × 20-min/day home practice; regular sport training | LF/HF in HRV BFB: post<pre |
| LF/HF in CON: post↔pre | |||
| CON: regular sport training | |||
| Dziembowska et al. 2016 | 16–22 yr old male basketball and football players with at least 3 yr experience | HRV BFB (n=20): Ten 20-min HRV BFB in 3 weeks | HRV BFB: post LF, HF, total HRV>pre LF, HF, total HRV |
| CON (n=21): regular sport training | CON: post LF, HF, total HRV↔pre LF, HF, total HRV | ||
| Paul et al. 2012 | 18–28 yr old male (n=17) and female (n=13) university, state, and national basketball athletes age: 21.7 ± 2.71 yrs | HRV BFB (males: n=8; females: n=2): 10 consecutive days of 20-min HRV BFB; regular sport training | LF: HRV BFB>CON; HRV BFB>PLA; |
| HF: HRV BFB>CON; HRV BFB>PLA; | |||
| PLA (males: n=2; females: n=8) motivational video clips for 10 days at 10 min/day; regular sport training | Total HRV: HRV BFB>CON; HRV BFB>PLA; | ||
| CON (males: n=7; females: n=3): regular sport training only | RR: HRV BFB<CON; HRV BFB<PLA; | ||
| Rusciano et al. 2017 | 20 male professional football players age: 30.4 ± 4.1 yrs; height: 182 ± 55.9 cm; weight: 79.0 ± 6.3 kg | HRV BFB: Fifteen 30-min biofeedback feedback sessions (2×/week); | LF: HRV BFB>CON |
| 4th–9th session: HRV BFB + SCL + EMG + hand temperature 10th - 15th session: HRV BFB + math tasks + hyperventilation + videos of matches won/lost | RR: HRV BFB<CON | ||
| Regular sport training | |||
| CON: regular sport training |
-
HRV BFB, heart rate variability biofeedback; PLA, placebo; CON, control; LF, low frequency; HF, high frequency; RR, respiration rate.
HRV BFB vs. CON
lnLF
There was no significant difference in lnLF between HRV BFB and CON, ES=0.12 [−0.39, 0.62], Z=0.65, p<0.05 (Figure 2).
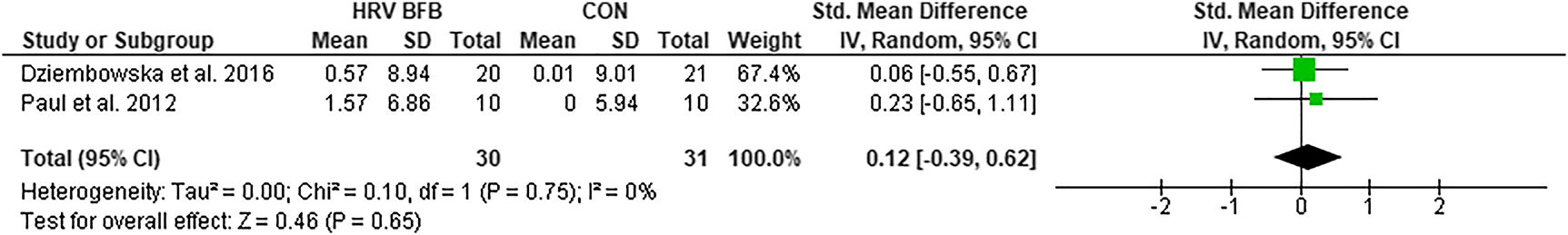
Forest plot of lnLF in HRV BFB vs CON.
LFnu
The LFnu in HRV BFB was significantly higher compared to CON, ES=0.46 [0.02, 0.91], Z=2.05, p<0.05 (Figure 3).
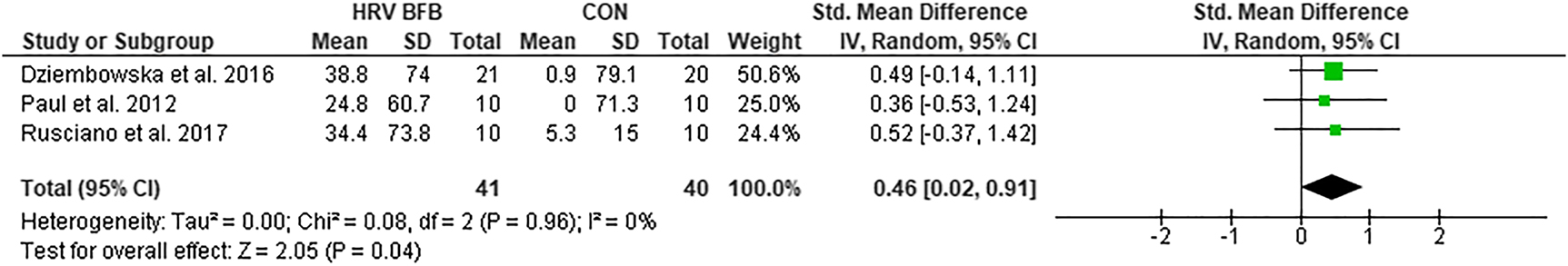
Forest plot of LFnu in HRV BFB vs CON.
lnHF
The lnHF was not significantly different in HRV BFB and CON, ES=−0.04 [−0.55, 0.46], Z=0.05, p>0.05 (Figure 4).
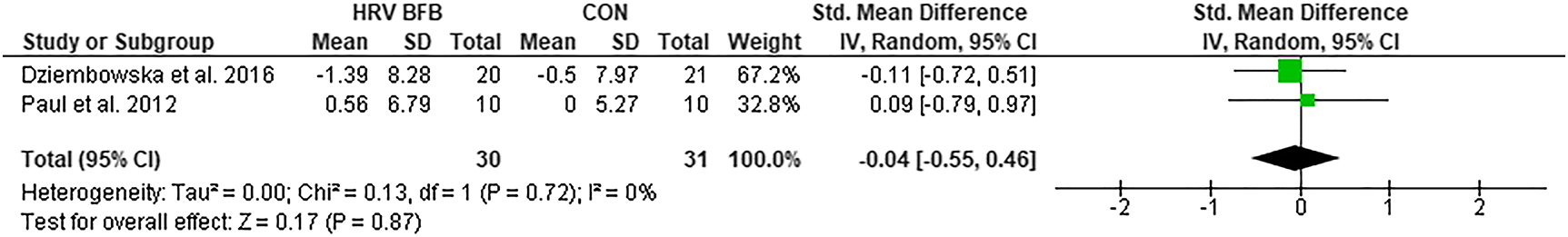
Forest plot of lnHF in HRV BFB vs CON.
HFnu
Meta-analysis of HFnu between HRV BFB and CON exhibited lower HFnu in HRV BFB than CON, ES=−0.78 [−1.31, −0.25], Z=2.88, p<0.01 (Figure 5).
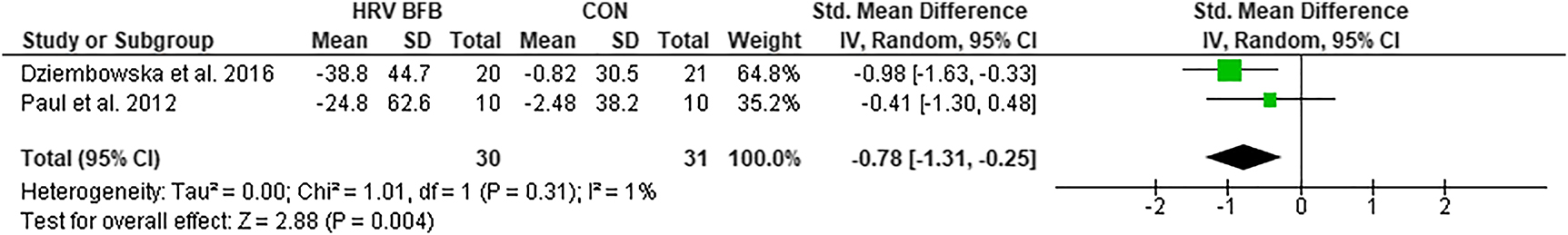
Forest plot of HFnu in HRV BFB vs CON.
lnTP
There was no significant difference in lnTP in HRV BFB and CON, ES=−0.36 [−1.20, 0.48] Z=0.84, p>0.05 (Figure 6).
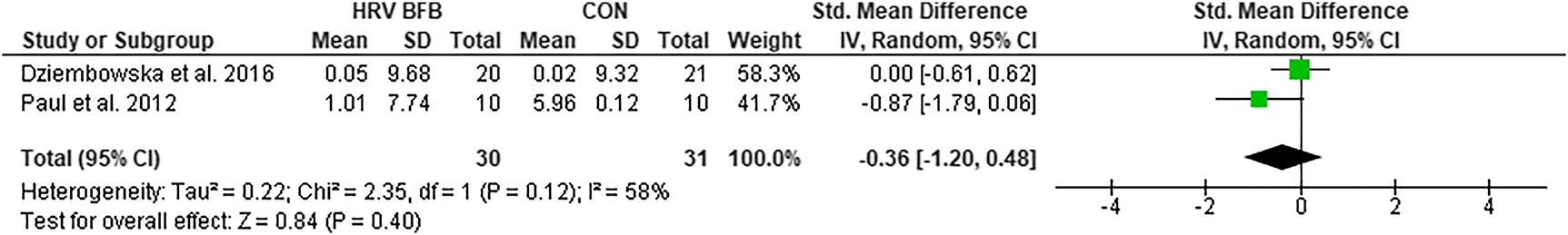
Forest plot of lnTP in HRV BFB vs CON.
RR
Meta-analysis of RR between HRV BFB and CON posted significant reductions in RR of HRV BFB than CON, ES=−4.30 [−5.53, −3.08], Z=6.90, p<0.01 (Figure 7).
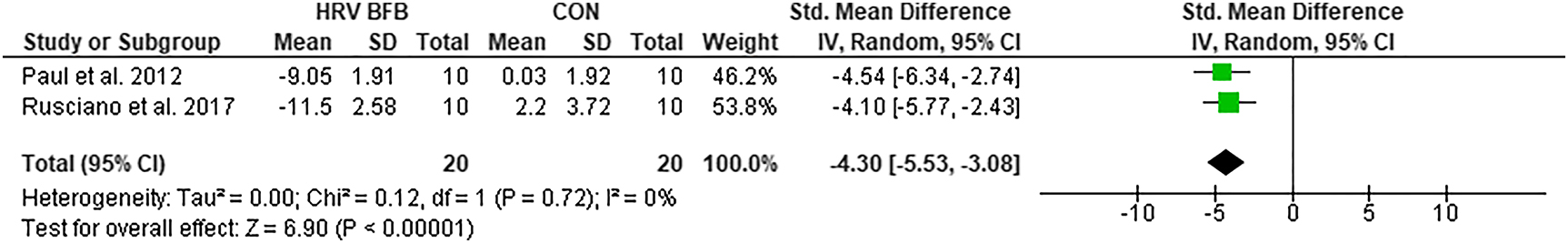
Forest plot of RR in HRV BFB vs CON.
The funnel plots of HRV and respiration measures in the meta-analyses are demonstrated in Figure 8. The HRV values and respiration in HRV BFB and CON are displayed in Table 3.
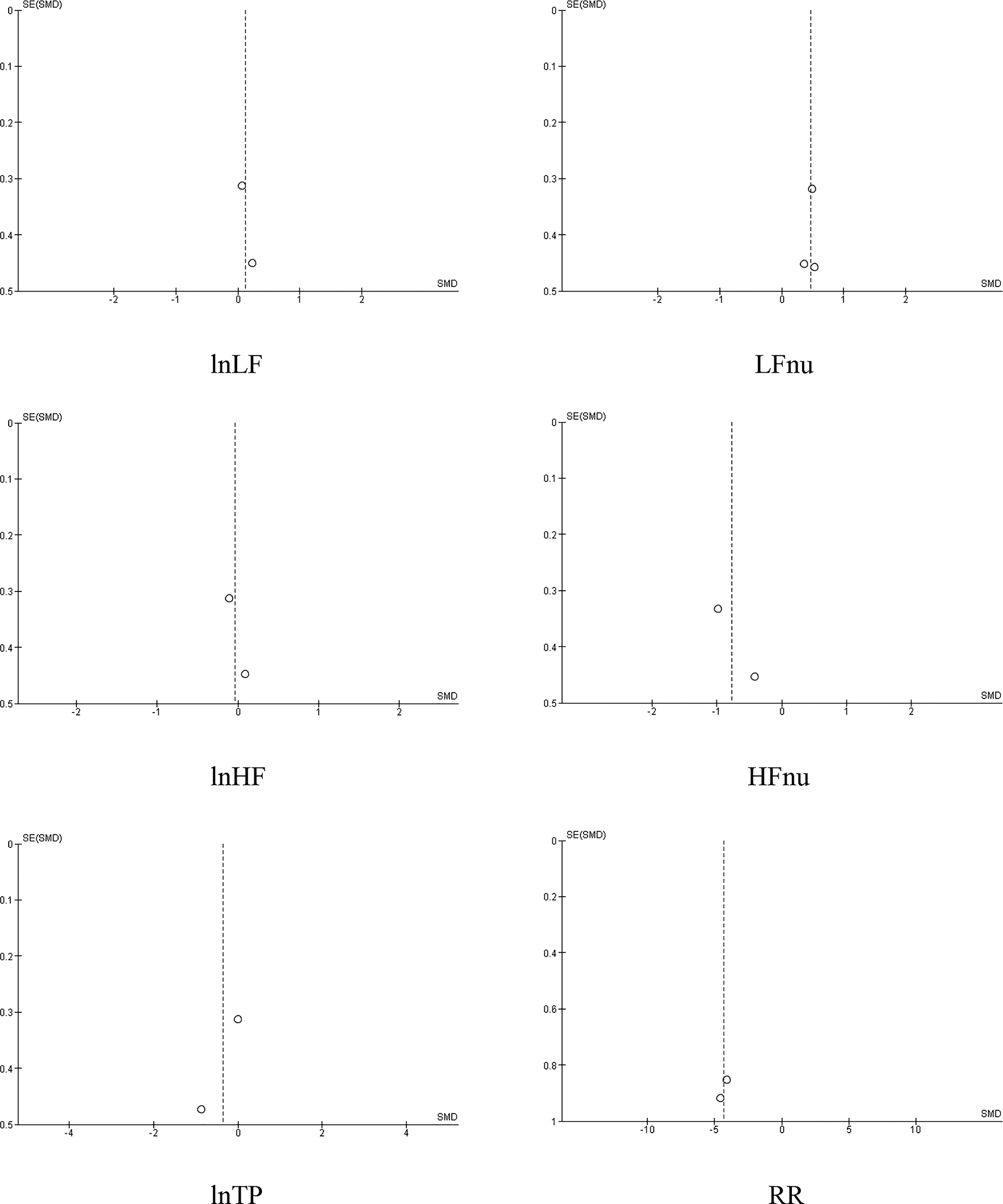
Funnel plots of HRV indices and RR in HRV BFB vs CON.
Heart rate variability and respiration in CON and HRV BFB.
| HRV | HRV BFB | CON | ||||
|---|---|---|---|---|---|---|
| n | Pre | Post | n | Pre | Post | |
| lnLF, ms2 | ||||||
| Dziembowska et al. 2016 | 20 | 8.47 ± 8.08 | 9.04 ± 8.25 | 21 | 8.26 ± 8.27 | 8.27 ± 8.18 |
| Paul et al. 2012 | 10 | 5.54 ± 5.39 | 7.11 ± 6.92 | 10 | 5.50 ± 5.43 | 5.50 ± 5.42 |
| LFnu | ||||||
| Dziembowska et al. 2016 | 20 | 47.8 ± 51.3 | 86.6 ± 77.6 | 21 | 62.6 ± 72.4 | 63.5 ± 72.0 |
| Paul et al. 2012 | 10 | 39.7 ± 32.1 | 64.6 ± 65.9 | 10 | 65.2 ± 65.1 | 65.2 ± 65.0 |
| Rusciano et al. 2017 | 10 | 52.2 ± 11.3 | 60.1 ± 11.9 | 10 | 49.0 ± 15.7 | 54.3 ± 10.4 |
| lnHF, ms2 | ||||||
| Dziembowska et al. 2016 | 20 | 8.56 ± 8.03 | 7.17 ± 7.00 | 21 | 7.74 ± 7.31 | 7.71 ± 7.24 |
| Paul et al. 2012 | 10 | 5.96 ± 6.14 | 6.51 ± 6.26 | 10 | 4.88 ± 4.80 | 4.88 ± 4.80 |
| HFnu | ||||||
| Dziembowska et al. 2016 | 20 | 52.2 ± 48.7 | 13.4 ± 22.4 | 21 | 36.4 ± 27.6 | 36.5 ± 28.0 |
| Paul et al. 2012 | 10 | 60.3 ± 67.9 | 35.4 ± 34.1 | 10 | 37.4 ± 34.9 | 34.4 ± 35.0 |
| lnTP, ms2 | ||||||
| Dziembowska et al. 2016 | 20 | 9.38 ± 8.99 | 9.43 ± 8.68 | 21 | 8.90 ± 8.50 | 8.92 ± 8.52 |
| Paul et al. 2012 | 10 | 6.70 ± 6.71 | 7.71 ± 7.39 | 10 | 6.12 ± 5.96 | 6.12 ± 5.96 |
| RR (breaths/min) | ||||||
| Paul et al. 2012 | 10 | 15.3 ± 2.00 | 6.25 ± 0.25 | 10 | 14.6 ± 1.77 | 14.6 ± 1.73 |
| Rusciano et al. 2017 | 10 | 17.1 ± 2.80 | 5.60 ± 0.90 | 10 | 16.7 ± 3.00 | 18.9 ± 3.70 |
-
lnLF, log-transformed low frequency HRV; LFnu, normalized low frequency; lnHF, log-transformed high frequency HRV; HFnu, normalized high frequency; lnTP, log-transformed; total power HRV; RR, respiration rate.
Discussion
The purpose of this novel study was to conduct a systematic review and meta-analysis on the effect of HRV BFB on physiological indices among athletes. Meta-analyses revealed the following outcomes comparing HRV BFB and CON: (1) HRV BFB reduced breathing rate compared to CON (ES = −4.34; large); (2) Greater LFnu in HRV BFB than CON (ES=0.46; moderate); (3) relatively, HRV BFB posted lower HFnu than CON (ES=−0.78; moderate).
In this review, HRV BFB demonstrated reduction in breathing frequency compared to CON. This is supported by increased LFnu seen in HRV BFB. HRV BFB practice facilitates respiratory homeostasis by decreasing chemoreceptor activation [22, 47, 48]. This in turn increases arterial oxygen saturation, and reduces breathing frequency [21, 22, 48]. The lower breathing frequency attained with HRV BFB may be crucial to reduction of psychophysiological stressors of athletes, thereby improving performance (Paul and Garg, 2012) [42]. Thus, HRV BFB can serve as a promising intervention to improving the respiration mechanics of athletes at resting condition.
Another finding in this review is the non-enhancement in baroreflex function with HRV BFB. At resting conditions, LF represents baroreceptor activity from PNS, SNS, and blood pressure regulation from PNS [2, 4, 5, 6, 7, 49]. HRV BFB activates resonance in the cardiovascular system and creates oscillatory vagal outflow coinciding with the baroreflex function [2, 13, 16, 21, 50]. This resonance produces large increases in HR amplitudes and ‘exercises’ the baroreflex [16, 20, 21]. Findings revealed non-differences in lnLF, lnHF and lnTP between HRV BFB and CON. Therefore, the results of these HRV indices under HRV BFB are linked to no enhancement in baroreflex function. Possible factors contributing to non-significant results in autonomic markers supporting baroreflex function are ambiguous. More studies are needed to elucidate information on the mechanism of baroreflex under HRV BFB in athlete population.
This review also posted non-alteration in modulating the cardiac vagal tone after HRV BFB among athletes. The cardiac vagal tone characterises the contribution of PNS in cardiac regulation [51]. The lnHF was utilised as an index of vagal modulation in this review [4, 32]. Results revealed non-significant lnHF in HRV BFB and CON. The scarcity in literature constrained the researchers to conduct additional analysis that can identify possible variables that led to non-improvement in lnHF. Further investigation utilising HRV BFB in athlete population should be carried out to demonstrate vagal influence of HRV BFB in athletes.
Adaptations in respiratory sinus arrhythmia (RSA) with HRV BFB in athlete population is unclear [4, 5, 10, 33, 52]. RSA is a cyclical change in HR synchronized with respiration, resulting to vagal discharge in the medulla. Specifically, RSA accelerates and slows down HR during inhalation and expiration respectively. Inhalation inhibits the vagal outflow from the cardiovascular center and speeds up HR. Conversely, exhalation facilitates vagal outflow by acetylcholine release. HRV BFB is believed to increase RSA [13, 21, 51, 53, 54]. Although the change in LFnu and decreased breathing frequency may suggest RSA shift from HF to LF, additional HRV and HR indices (maximal HR and minimum HR) in future HRV BFB studies can allow the robust interpretation of RSA in HRV BFB.
From a methodological perspective, statistical inferences from this preliminary review are less worthwhile due to small sample sizes. In relation to this, the small number of studies lack power to reasonably interpret heterogeneity and publication bias [55]. Additionally, subgroup analyses for potential covariates (e.g. age, level of ability, HRV BFB duration, gender) crucial for understanding autonomic function with HRV BFB were not determined. Also, utilising a common performance marker (e.g. cardiovascular endurance) and relate it to HRV adaptations with HRV BFB was not achieved. In regard to HRV markers in meta-analyses, normalized LF and HF do not reflect unique physiological occurrences within the ANS [34]. As such, LFnu and HFnu were only used to depict breathing dominance within HRV frequency band. Other time-domain HRV parameters that may be helpful for determining autonomic phenomena with HRV BFB in athletes were not available [56, 57]. Despite these limitations, this study has a noteworthy strength in that it is the first meta-analysis on the topic of HRV BFB in athletes.
In conclusion, the application of HRV BFB suggests enhancement of respiratory mechanics in athlete population. More studies are needed to identify the effect of HRV BFB on autonomic modulation among athletes at the resting condition.
Acknowledgments
The authors would like to thank Dr. Fredric Shaffer for his valuable insights to the results of this review.
-
Research funding: None declared.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: Authors state no conflict of interest.
-
Informed consent: Not applicable.
-
Ethical approval: Not applicable.
References
1. Bernston, GG, Bigger, JTJr., Eckberg, DL, Grossman, P, Kaufmann, PG, Malik, M, et al.. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology 1997;34:623–48.10.1111/j.1469-8986.1997.tb02140.xSuche in Google Scholar PubMed
2. McCraty, R, Shaffer, F. Heart rate variability: new perspectives on physiological mechanisms, assessment of self-regulatory capacity, and health risk. Glob Adv Health Med 2015;4:46–61.10.7453/gahmj.2014.073Suche in Google Scholar PubMed PubMed Central
3. Olshansky, B, Sabbah, HN, Hauptman, PJ, Colucci, WS. Parasympathetic nervous system and heart failure: pathophysiology and potential implications for therapy. Circulation 2008;118:863–71.10.1161/CIRCULATIONAHA.107.760405Suche in Google Scholar PubMed
4. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 1996;93:1043–65.10.1161/01.CIR.93.5.1043Suche in Google Scholar
5. Shaffer, F, McCraty, R, Zerr, CL. A healthy heart is not a metronome: an integrative review of the heart’s anatomy and heart rate variability. Front Psychol 2014;5:1040.10.3389/fpsyg.2014.01040Suche in Google Scholar PubMed PubMed Central
6. Nunan, D, Sandercock, GR, Brodie, DA. A quantitative systematic review of normal values for short-term heart rate variability in healthy adults. Pacing Clin Electrophysiol 2010;33:1407–17.10.1111/j.1540-8159.2010.02841.xSuche in Google Scholar PubMed
7. Reyes del Paso, GA, Langewitz, W, Mulder, LJ, van Roon, A, Duschek, S. The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: a review with emphasis on a reanalysis of previous studies. Psychophysiology 2013;50:477–87.10.1111/psyp.12027Suche in Google Scholar PubMed
8. Gang, Y, Malik, M. Heart rate variability analysis in general medicine. Indian Pacing Electrophysiol J 2003;3:34–40.Suche in Google Scholar
9. Gevirtz, R. The promise of heart rate variability biofeedback: evidence-based applications. Biofeedback 2013;4:110–20.10.5298/1081-5937-41.3.01Suche in Google Scholar
10. Prinsloo, GE, Rauch, HG, Derman, WE. A brief review and clinical application of heart rate variability biofeedback in sports, exercise, and rehabilitation medicine. Physician Sportsmed 2014;42:88–99.10.3810/psm.2014.05.2061Suche in Google Scholar PubMed
11. Porges, SW. The Polyvagal Theory: phylogenetic contributions to social behavior. Physiol Behav 2003;79:503–13.10.1016/S0031-9384(03)00156-2Suche in Google Scholar
12. Thayer, JF, Sternberg, E. Beyond heart rate variability: vagal regulation of allostatic systems. Ann N Y Acad Sci 2006;1088:361–72.10.1196/annals.1366.014Suche in Google Scholar PubMed
13. Lehrer, P, Eddie, D. Dynamic processes in regulation and some implications for biofeedback and biobehavioral interventions. Appl Psychophysiol Biofeedback 2013;38:143–55.10.1007/s10484-013-9217-6Suche in Google Scholar PubMed PubMed Central
14. Lehrer, PM, Gevirtz, R. Heart rate variability biofeedback: how and why does it work?. Front Psychol 2014;5:756.10.3389/fpsyg.2014.00756Suche in Google Scholar PubMed PubMed Central
15. Lehrer, P, Vaschillo, E. The future of heart rate variability biofeedback. Biofeedback 2008;36:11–14.Suche in Google Scholar
16. Lehrer, PM, Vaschillo, E, Vaschillo, B. Resonant frequency biofeedback training to increase cardiac variability: rationale and manual for training. Appl Psychophysiol Biofeedback 2000;25:177–91.10.1023/A:1009554825745Suche in Google Scholar PubMed
17. Chernigovskaia, NV, Vaschillo, EG, Rusanovskiĭ, VV, Kashkarova, OE. Instrumental autogenic training of the mechanisms of regulation of the cardiovascular system functions in the treatment of patients with neuroses. Zh Nevropatol Psikhiatr Im S S Korsakova 1990;90:24–8.Suche in Google Scholar
18. Vaschillo, E, Lehrer, P, Rishe, N, Konstantinov, M. Heart rate variability biofeedback as a method for assessing baroreflex function: a preliminary study of resonance in the cardiovascular system. Appl Psychophysiol Biofeedback 2002;27:1–27.10.1023/A:1014587304314Suche in Google Scholar
19. Vaschillo, E, Vaschillo, B, Lehrer, P. Heartbeat synchronizes with respiratory rhythm only under specific circumstances. Chest 2004;126:1385–6.10.1016/S0012-3692(15)31329-5Suche in Google Scholar
20. Vaschillo, EG, Vaschillo, B, Lehrer, PM. Characteristics of resonance in heart rate variability stimulated by biofeedback. Appl Psychophysiol Biofeedback 2006;31:129–42.10.1007/s10484-006-9009-3Suche in Google Scholar PubMed
21. Lehrer, PM, Vaschillo, E, Vaschillo, B, Lu, SE, Eckberg, DL, Edelberg, R, et al.. Heart rate variability biofeedback increases baroreflex gain and peak expiratory flow. Psychosom Med 2003;65:796–805.10.1097/01.PSY.0000089200.81962.19Suche in Google Scholar
22. Lehrer, PM, Vaschillo, E, Vaschillo, B, Lu, SE, Scardella, A, Siddique, M, et al.. Biofeedback treatment for asthma. Chest 2004;126:352–61.10.1378/chest.126.2.352Suche in Google Scholar PubMed
23. Fonoberova, M, Mezić, I, Buckman, JF, Fonoberov, V, Mezić, A, Vaschillo, EG, et al.. A computational physiology approach to personalized treatment models: the beneficial effects of slow breathing on the human cardiovascular system. Am J Physiol Heart Circ Physiol 2014;307:H1073–91.10.1152/ajpheart.01011.2013Suche in Google Scholar PubMed PubMed Central
24. Hobson, AR, Furlong, PL, Aziz, Q. Oesophageal afferent pathway sensitivity in non-erosive reflux disease. Neuro Gastroenterol Motil 2008;20:877–83.10.1111/j.1365-2982.2008.01122.xSuche in Google Scholar PubMed
25. Tracey, KJ. The inflammatory reflex. Nature 2002;420:853–9.10.1038/nature01321Suche in Google Scholar PubMed
26. Tracey, KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest 2007;117:289–96.10.1172/JCI30555Suche in Google Scholar PubMed PubMed Central
27. Bernik, TR, Friedman, SG, Ochani, M, DiRaimo, R, Ulloa, L, Yang, H, et al.. Pharmacological stimulation of the cholinergic antiinflammatory pathway. J Exp Med 2002;195:781–8.10.1084/jem.20011714Suche in Google Scholar PubMed PubMed Central
28. Borovikova, LV, Ivanova, S, Zhang, M, Yang, H, Botchkina, GI, Watkins, LR, et al.. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000;405:458–62.10.1038/35013070Suche in Google Scholar PubMed
29. Jiménez Morgan, S, Molina Mora, JA. Effect of heart rate variability biofeedback on sport performance, a systematic review. Appl Psychophysiol Biofeedback 2017;42:235–45.10.1007/s10484-017-9364-2Suche in Google Scholar PubMed
30. Moher, D, Liberati, A, Tetzlaff, J, Altman, DG. Preferred reporting Items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Med 2009;6: e1000097.10.1371/journal.pmed.1000097Suche in Google Scholar PubMed PubMed Central
31. Moher, D, Schulz, KF, Altman, DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 2001;357:1191–4.10.1016/S0140-6736(00)04337-3Suche in Google Scholar
32. Maheshwari, A, Norbi, FL, Soliman, EZ, Adabag, S, Whitsel, EA, Alonso, A, et al.. Low heart rate variability in a 2-minute electrocardiogram recording is associated with an increased risk of sudden cardiac death in the general population: the atherosclerosis risk in communities study. PLoS One 2016;11: e0161648.10.1371/journal.pone.0161648Suche in Google Scholar PubMed PubMed Central
33. Shaffer, F, Ginsberg, JP. An overview of heart rate variability metrics and norms. Front Public Health 2017;5:258.10.3389/fpubh.2017.00258Suche in Google Scholar PubMed PubMed Central
34. Burr, RL. Interpretation of normalized spectral heart rate variability indices in sleep research: a critical review. Sleep 2007;30:913–19.10.1093/sleep/30.7.913Suche in Google Scholar PubMed PubMed Central
35. Becker, BJ. Synthesizing standardized mean change measures. Br J Math Stat Psychol 1988;41:257–78.10.1111/j.2044-8317.1988.tb00901.xSuche in Google Scholar
36. Furukawa, TA, Barbui, C, Cipriani, A, Brambilla, P, Watanabe, N. Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol 2006;59:7–10.10.1016/j.jclinepi.2005.06.006Suche in Google Scholar PubMed
37. Gu, S, Shi, J, Tang, Z, Sawhney, M, Hu, H, Shi, L, et al.. Comparison of glucose lowering effect of metformin and acarbose in type 2 diabetes mellitus: a meta-analysis. PLoS One 2015;10: e0126704.10.1371/journal.pone.0126704Suche in Google Scholar PubMed PubMed Central
38. Cohen, J. Statistical power analysis for the behavioural sciences, 2nd ed. Hillsdale, NJ: Erlbaum; 1988. p. 75–144.Suche in Google Scholar
39. Durlak, JA. How to select, calculate, and interpret effect sizes. J Pediatr Psychol 2009;34:917–28.10.1093/jpepsy/jsp004Suche in Google Scholar PubMed
40. Higgins, JPT, Thompson, SG, Deeks, JJ, Altman, DG. Measuring inconsistencies in meta- analyses. BMJ 2003;327:557–60.10.1136/bmj.327.7414.557Suche in Google Scholar PubMed PubMed Central
41. Sterne, JA, Egger, M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol 2001;54:1046–55.10.1016/S0895-4356(01)00377-8Suche in Google Scholar
42. Paul, M, Garg, K. The effect of heart rate variability biofeedback on performance psychology of basketball players. Appl Psychophysiol Biofeedback 2012;37:131–44.10.1007/s10484-012-9185-2Suche in Google Scholar PubMed
43. Paul, M, Garg, K, Sandhu, JS. Role of biofeedback in optimizing psychomotor performance. Asian J Sports Med 2012;3:29–40.10.5812/asjsm.34722Suche in Google Scholar PubMed PubMed Central
44. Rusciano, A, Corradini, G, Stoianov, I. Neuroplus biofeedback improves attention, resilience and injury prevention in elite soccer players. Psychophysiology 2017;54:916–26.10.1111/psyp.12847Suche in Google Scholar PubMed
45. Choudhary, R, Triveti, V, Choudhary, SG. Effect of heart rate variability biofeedback training on the performance of track athletes. Int J Ther Rehabil Res 2016;5:166–74.10.5455/ijtrr.000000159Suche in Google Scholar
46. Dziembowska, I, Izdebski, P, Rasmus, A, Brudny, J, Grzelczak, M, Cysewski, P. Effects of heart rate variability biofeedback on EEG alpha asymmetry and anxiety symptoms in male athletes: a pilot study. Appl Psychophysiol Biofeedback 2016;41:141–50.10.1007/s10484-015-9319-4Suche in Google Scholar PubMed
47. Bernardi, l, Gabutti, A, Porta, C, Spicuzza, L. Slow breathing reduces chemoreflex response to hypoxia and hypercapnia, and increase baroreflex sensitivity. J Hypertens 2001;19:2221–9.10.1097/00004872-200112000-00016Suche in Google Scholar PubMed
48. Bernardi, L, Spadacini, G, Bellwon, J, Hajric, R, Roskamm, H, Frey, AW. Effect of breathing rate on oxygen saturation and exercise performance in chronic heart failure. Lancet 1998;351:1308–11.10.1016/S0140-6736(97)10341-5Suche in Google Scholar PubMed
49. Goldstein, DS, Bentho, O, Park, MY, Sharabi, Y. Low-frequency power of heart rate variability is not a measure of cardiac sympathetic tone but may be a measure of modulation of cardiac autonomic outflows by baroreflexes. Exp Physiol 2011;96:1255–61.10.1113/expphysiol.2010.056259Suche in Google Scholar PubMed PubMed Central
50. Ahmed, AK, Harness, JB, Mearns, AJ. Respiratory control of heart rate. Eur J Appl Physiol 1982;50:95–104.10.1007/BF00952248Suche in Google Scholar
51. Laborde, S, Mosley, E, Thayer, JF. Heart rate variability and cardiac vagal tone in psychophysiological research – recommendations for experimental planning, data analysis, and data reporting. Front Psychol 2017;8:213.10.3389/fpsyg.2017.00213Suche in Google Scholar PubMed PubMed Central
52. Eckberg, DL, Eckberg, MJ. Human sinus node responses to repetitive, ramped, carotid baroreceptor stimuli. Am J Physiol 1982;242:H638–44.10.1152/ajpheart.1982.242.4.H638Suche in Google Scholar PubMed
53. Julien, C. The enigma of Mayer waves: facts and models. Cardiovasc Res 2006;70:12–21.10.1016/j.cardiores.2005.11.008Suche in Google Scholar PubMed
54. Russo, MA, Santarelli, DM, O’Rourke, D. The physiological effects of slow breathing in healthy human. Breathe 2017;13:298–309.10.1183/20734735.009817Suche in Google Scholar PubMed PubMed Central
55. Jackson, D, Turner, R. Power analysis for random-effects meta-analysis. Res Synth Methods 2017;8:290–302.10.1002/jrsm.1240Suche in Google Scholar PubMed PubMed Central
56. Buchheit, M. Monitoring training status with HR measures: do all roads lead to Rome?. Front Physiol 2014;5:73.10.3389/fphys.2014.00073Suche in Google Scholar PubMed PubMed Central
57. Saboul, D, Pialoux, V, Hautier, C. The impact of breathing on HRV measurements: implications for the longitudinal follow-up of athletes. Eur J Sport Sci 2013;13:534–42.10.1080/17461391.2013.767947Suche in Google Scholar PubMed
© 2021 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Reviews
- A preliminary systematic review and meta-analysis on the effects of heart rate variability biofeedback on heart rate variability and respiration of athletes
- The potential positive epigenetic effects of various mind-body therapies (MBTs): a narrative review
- The effect of Chlorella vulgaris on obesity related metabolic disorders: a systematic review of randomized controlled trials
- Biological and medicinal application of Cucumis sativus Linn. – review of current status with future possibilities
- The effect of the herbal medicine on severity of cyclic mastalgia: a systematic review and meta-analysis
- Research Articles
- Chemical compositions, antibacterial, antifungal and cytotoxic effects of Alhagi mannifera five extracts
- Anticonvulsive and anti-epileptogenesis effects of Echinacea purpurea root extract, an involvement of CB2 receptor
- Fermented maize slurry (Ogi) and its supernatant (Omidun) mitigate elevated intraocular pressure by modulating BDNF expression and glial plasticity in the retina-gut axis of glaucomatous rats
- Levetiracetam exposure during prenatal and postnatal period induces cognitive decline in rat offsprings, not completely prevented by Bacopa monnieri
- Antibiofilm action of Persea americana glycolic extract over Acinetobacter baumannii and absence of toxicity in Galleria mellonella
- Validation of Unani concept of Abadāl-i-Adwiya (drug substitution) by physicochemical standardization and hepatoprotective activity of Aristolochia rotunda Linn. and its substitute Curcuma Zedoaria Rosc. in albino Wistar rats
- Cinnamon oil as a co-chemotherapy agent through inhibition of cell migration and MMP-9 expression on 4T1 cells
- Assessment of biochemical changes in normal and diabetic rats treated by phenolic enriched extracts of Juglans regia L. barks
- Influence of Clerodendrum volubile leaf extract on doxorubicin-induced toxicity and inhibition of carbonyl reductase mediated metabolism
- Quantification of anacardic acid, the toxic component in raw and purified samples of Semecarpus anacardium L. by Siddha purification processes
- Molecular docking and molecular dynamics approach to identify potential compounds in Huperzia squarrosa for treating Alzheimer’s disease
- Evaluation of ethanol extracts from three species of Artocarpus as natural gastroprotective agents: in vivo and histopathological studies
- Attenuation of cisplatin induced myelosuppression by methanol extract of Cedrus deodara in Wistar rats
- Acute and sub-acute toxicity assessment of the standardized extract of Sanguisorba minor in vivo
- Efficacy of lettuce seed syrup on insomnia in patients with breast cancer: a pilot double blind randomized placebo controlled clinical trial
- The effect of aromatherapy with rose essential oil on apparent anxiety in patients with myocardial infarction
- Effect of Jyoti-Trataka on intraocular pressure, autonomic control, and blood glucose in diabetic patients with high-tension primary open-angle glaucoma: a randomized-controlled trial
- Efficacy of Ḥammām-i-yābis (dry bath) in metabolic syndrome: a single arm, open-labelled clinical trial
- Effect of Ḥijāma (wet cupping), Dalk (massage) and Bukhūr (medicated steam) in amelioration of Waja al-Zahr (non-specific low back pain) – an open prospective clinical trial
- Effect of yoga on cardiovascular functions and psychological aspects of people on public service-related work: an exploratory study
Artikel in diesem Heft
- Frontmatter
- Reviews
- A preliminary systematic review and meta-analysis on the effects of heart rate variability biofeedback on heart rate variability and respiration of athletes
- The potential positive epigenetic effects of various mind-body therapies (MBTs): a narrative review
- The effect of Chlorella vulgaris on obesity related metabolic disorders: a systematic review of randomized controlled trials
- Biological and medicinal application of Cucumis sativus Linn. – review of current status with future possibilities
- The effect of the herbal medicine on severity of cyclic mastalgia: a systematic review and meta-analysis
- Research Articles
- Chemical compositions, antibacterial, antifungal and cytotoxic effects of Alhagi mannifera five extracts
- Anticonvulsive and anti-epileptogenesis effects of Echinacea purpurea root extract, an involvement of CB2 receptor
- Fermented maize slurry (Ogi) and its supernatant (Omidun) mitigate elevated intraocular pressure by modulating BDNF expression and glial plasticity in the retina-gut axis of glaucomatous rats
- Levetiracetam exposure during prenatal and postnatal period induces cognitive decline in rat offsprings, not completely prevented by Bacopa monnieri
- Antibiofilm action of Persea americana glycolic extract over Acinetobacter baumannii and absence of toxicity in Galleria mellonella
- Validation of Unani concept of Abadāl-i-Adwiya (drug substitution) by physicochemical standardization and hepatoprotective activity of Aristolochia rotunda Linn. and its substitute Curcuma Zedoaria Rosc. in albino Wistar rats
- Cinnamon oil as a co-chemotherapy agent through inhibition of cell migration and MMP-9 expression on 4T1 cells
- Assessment of biochemical changes in normal and diabetic rats treated by phenolic enriched extracts of Juglans regia L. barks
- Influence of Clerodendrum volubile leaf extract on doxorubicin-induced toxicity and inhibition of carbonyl reductase mediated metabolism
- Quantification of anacardic acid, the toxic component in raw and purified samples of Semecarpus anacardium L. by Siddha purification processes
- Molecular docking and molecular dynamics approach to identify potential compounds in Huperzia squarrosa for treating Alzheimer’s disease
- Evaluation of ethanol extracts from three species of Artocarpus as natural gastroprotective agents: in vivo and histopathological studies
- Attenuation of cisplatin induced myelosuppression by methanol extract of Cedrus deodara in Wistar rats
- Acute and sub-acute toxicity assessment of the standardized extract of Sanguisorba minor in vivo
- Efficacy of lettuce seed syrup on insomnia in patients with breast cancer: a pilot double blind randomized placebo controlled clinical trial
- The effect of aromatherapy with rose essential oil on apparent anxiety in patients with myocardial infarction
- Effect of Jyoti-Trataka on intraocular pressure, autonomic control, and blood glucose in diabetic patients with high-tension primary open-angle glaucoma: a randomized-controlled trial
- Efficacy of Ḥammām-i-yābis (dry bath) in metabolic syndrome: a single arm, open-labelled clinical trial
- Effect of Ḥijāma (wet cupping), Dalk (massage) and Bukhūr (medicated steam) in amelioration of Waja al-Zahr (non-specific low back pain) – an open prospective clinical trial
- Effect of yoga on cardiovascular functions and psychological aspects of people on public service-related work: an exploratory study


