Abstract
A novel series of meta-diamide compounds incorporating a pyrazole moiety (2a–2v) were designed and synthesized based on cyproflanilide. Their structures were validated through 1H NMR, 13C NMR, and HRMS analyses. These compounds were evaluated for their insecticidal activity against Plutella xylostella, Mythimna separate, Tetranychus cinnabarinus, and Nilaparvata lugens. Most of the title compounds exhibited good activity against N. lugens at 400 mg/L. Compound 2k demonstrated potential for further optimization as an insecticidal lead, thereby extending the application of meta-diamide compounds in the field of sucking mouthparts.
1 Introduction
Meta-diamide insecticides, represented by broflanilide (1; Figure 1), are classified as group 30 insecticides [1–3]. Due to their novel mechanism of action, high efficiency, and lack of cross-resistance with traditional pesticides, meta-diamide insecticides have garnered considerable attention [4–6]. Based on broflanilide, numerous analogous meta-diamide compounds have been extensively modified and synthesized. For example, Lv et al. synthesized cyproflanilide (2; Figure 1) by replacing the methyl group of broflanilide with a cyclopropylmethyl group [7–9]. Furthermore, Zhang et al. synthesized compound 3 (Figure 1) by replacing the methyl group of broflanilide with a cyanomethyl (CNCH2–) group [10]. Both compounds exhibited superior insecticidal activities compared to broflanilide.
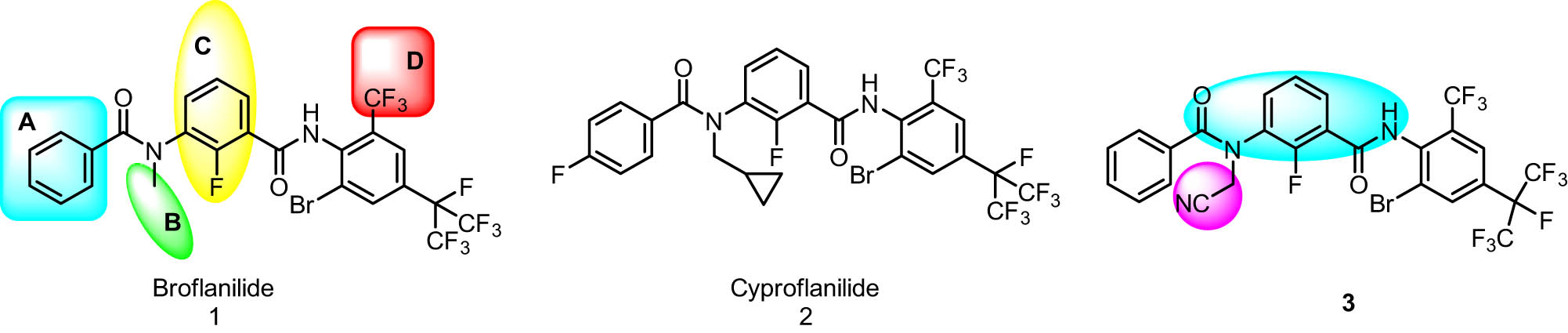
Representative meta-diamide compounds.
The structural modification of meta-diamide insecticides primarily centers around four components: part A, part B, part C, and part D (1, Figure 1) [11–13]. In part A, the active groups are predominantly phenyl or pyridinyl moieties. In part B, alkyl chain substitutions, such as alkyl, alkoxy, and haloalkyl, are generally advantageous for insecticidal activity. Part C entails a substituted benzene with substituents including halogen, alkoxy, and cyano. Part D predominantly focuses on halogen atoms and alkyl groups. Moreover, the activity of meta-diamide insecticides has been mainly against chewing mouthpiece pests, such as lepidopteran pests. Conversely, their activity against sucking mouthparts, such as hemiptera, has been rarely reported.
Pyrazole groups, as a member of the five-membered heterocyclic rings, have garnered increasing attention in the pesticide domain due to their fungicidal, insecticidal, and herbicidal properties [14–18]. In the realm of insecticides, numerous pyrazole derivatives have been developed and commercialized, such as fipronil, fenpyroximate, tebufenpyrad, tolfenpyrad, chlorantraniliprole, cyantraniliprole, cyenopyrafen, and cyetpyrafen (Figure 2). Many of these compounds exhibit effective activity against sucking mouthpart [19–22] pests. For example, cyenopyrafen and cyetpyrafen demonstrate good efficacy in controlling spider mites.
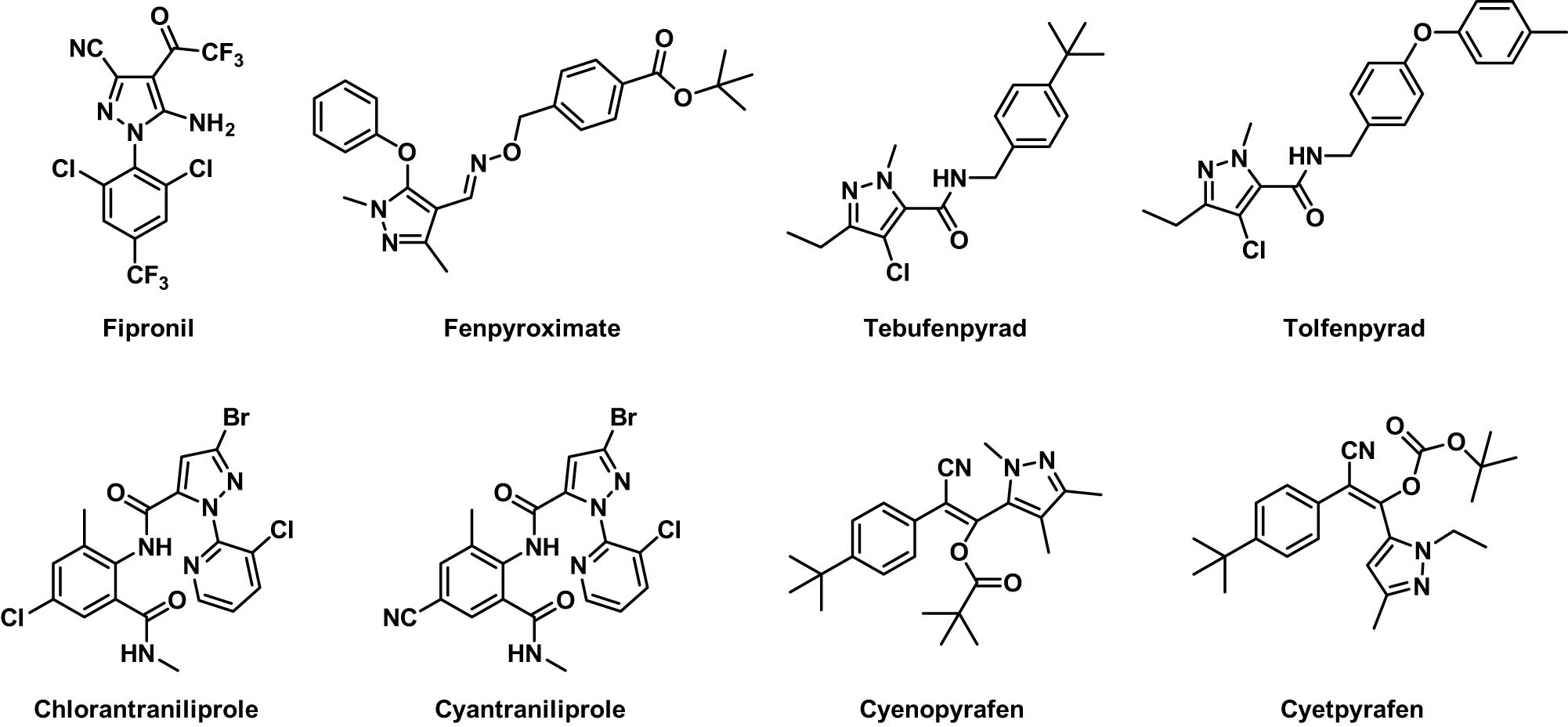
Representative insecticidal compounds.
To facilitate the development of novel meta-diamide compounds with a broader insecticidal spectrum and to build upon our previous research, this study regarded cyproflanilide as the lead compound. Pyrazole was introduced into the structure, and the effects of various substituted anilines on the activity were examined. A series of novel meta-diamide compounds incorporating 1-ethyl-3-methyl-1H-pyrazole-5-yl (easy synthesis and low cost) were designed and synthesized (Figure 3). Their bioactivities against Plutella xylostella, Mythimna separate, Tetranychus cinnabarinus, and Nilaparvata lugens were subsequently evaluated. Notably, most of the title compounds exhibited notable activity against N. lugens at 400 mg/L, significantly surpassing cyproflanilide (0.00% at 400 mg/L). The preliminary structure–activity relationships (SARs) were also discussed. This study indicated that incorporating a pyrazole group could be useful for the application of meta-diamide compounds in the field of sucking mouthpart pests and provides guidance for subsequent research endeavors.
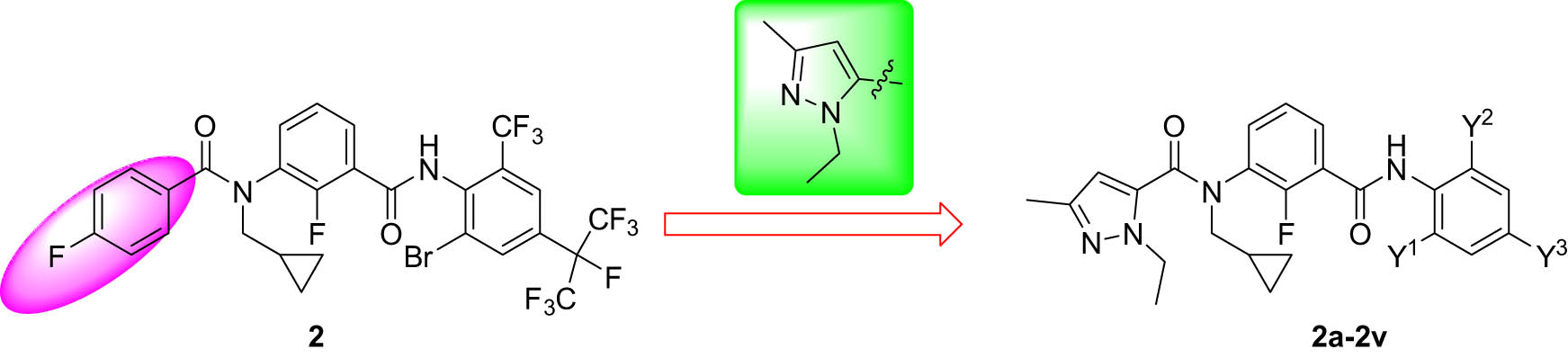
Design strategy employed for the target compounds.
2 Results and discussion
2.1 Synthesis
The synthesis route for compounds 2a–2v is depicted in Scheme 1. Using methyl 3-amino-2-fluorobenzoate (4) as the starting material, we synthesized the key intermediate 3-(N-(cyclopropylmethyl)-1-ethyl-3-methyl-1H-pyrazole-5-carboxamido)-2-fluorobenzoic acid (9) through a series of reactions involving alkylation, amidation, and hydrolysis. Subsequently, intermediate 10 was subjected to further reaction with various anilines to obtain the final products 2a–2v. All the target compounds were characterized and identified through 1H NMR, 13C NMR, and HRMS.
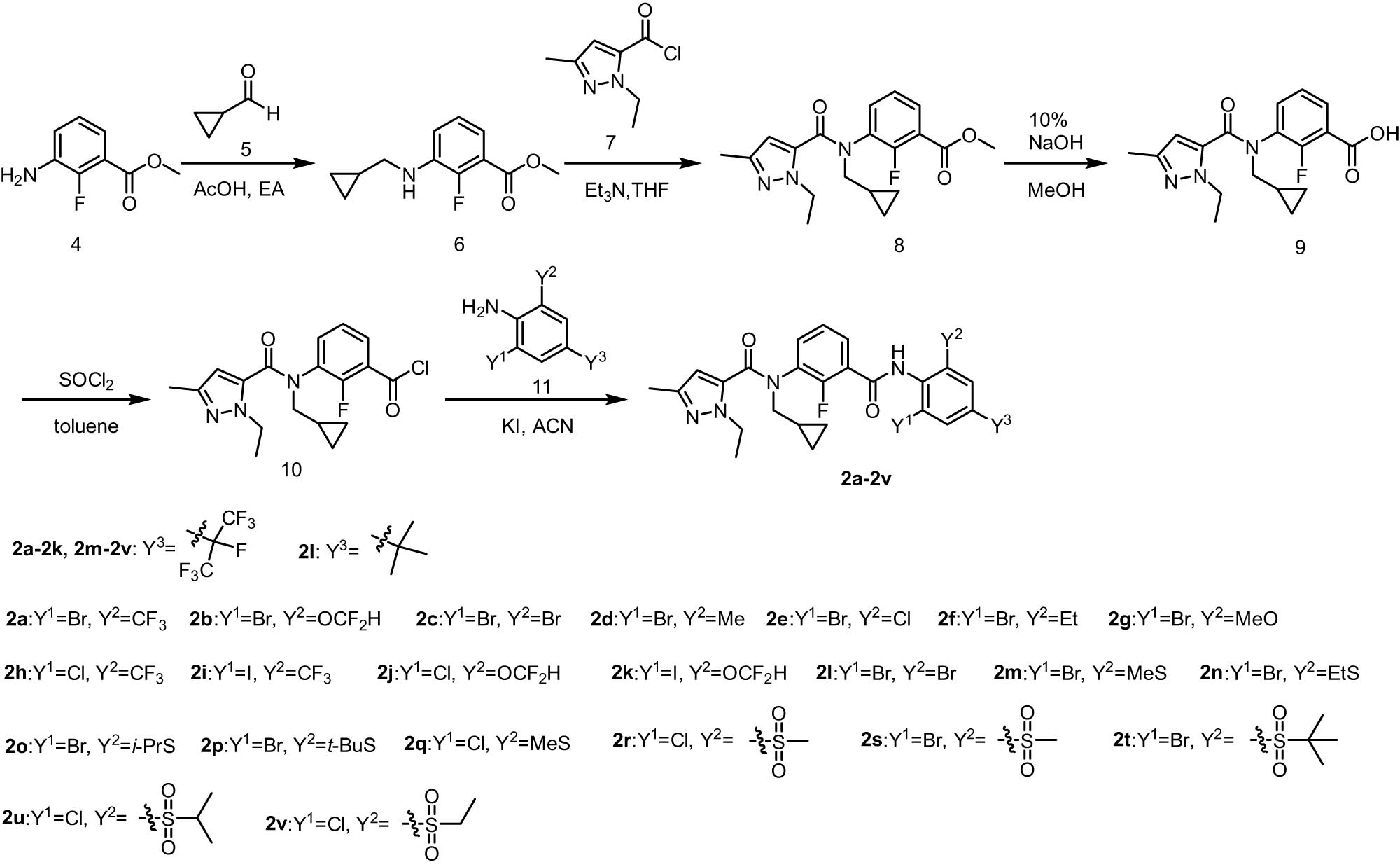
Synthesis route for the target compounds 2a–2v.
2.2 SAR
The insecticidal activity of the target compounds against P. xylostella, M. separate, T. cinnabarinus, and N. lugens was evaluated, with cyproflanilide serving as the control compound. The results are presented in Table 1.
Insecticidal activity of compounds 2a–2v and cyproflanilide
| Compound | Three-day mortality (%, mg/L) | |||||||
|---|---|---|---|---|---|---|---|---|
| P. xylostella | M. separate | T. cinnabarinus | N. lugens | |||||
| 1 | 0.1 | 1 | 0.1 | 100 | 10 | 400 | 100 | |
| 2a | 46.67 | / | 100 | 33.33 | 1.67 | / | 93.33 | 55.56 |
| 2b | 3.33 | / | 86.67 | 20.00 | 61.17 | / | 100 | 83.43 |
| 2c | 0.00 | / | 100 | 13.33 | 0.00 | / | 91.67 | 0.00 |
| 2d | 3.33 | / | 86.67 | 0.00 | 0.00 | / | 93.33 | 0.00 |
| 2e | 0.00 | / | 100 | 0.00 | 3.73 | / | 100 | 0.00 |
| 2f | 0.00 | / | 36.67 | / | 5.41 | / | 67.53 | / |
| 2g | 0.00 | / | 0.00 | / | 0.00 | / | 30.56 | / |
| 2h | 40.00 | / | 93.33 | 3.33 | 0.00 | / | 100 | 26.63 |
| 2i | 100 | 3.33 | 100 | 0.00 | 0.00 | / | 100 | 89.95 |
| 2j | 6.67 | / | 46.67 | / | 2.08 | / | 100 | 46.85 |
| 2k | 100 | 10.00 | 100 | 0.00 | 91.72 | 0.00 | 100 | 98.41 |
| 2l | 0.00 | / | 0.00 | / | 1.75 | / | 15.53 | / |
| 2m | 0.00 | / | 0.00 | / | 5.16 | / | 26.74 | / |
| 2n | 0.00 | / | 0.00 | / | 0.00 | / | 94.12 | 46.67 |
| 2o | 0.00 | / | 0.00 | / | 0.00 | / | 0.00 | / |
| 2p | 0.00 | / | 0.00 | / | 0.00 | / | 0.00 | / |
| 2q | 0.00 | / | 0.00 | / | 0.00 | / | 67.53 | 0.00 |
| 2r | 0.00 | / | 3.33 | / | 0.00 | / | 91.67 | 78.41 |
| 2s | 0.00 | / | 0.00 | / | 0.00 | / | 93.33 | 55.56 |
| 2t | 0.00 | / | 0.00 | / | 0.00 | / | 100 | 95.94 |
| 2u | 0.00 | / | 10.00 | / | 0.00 | / | 91.67 | 38.33 |
| 2v | 10.00 | / | 20.00 | / | 0.00 | / | 100 | 100 |
| Cyproflanilide | 100 | 96.67 | 100 | 100 | 15.24 | / | 0.00 | / |
| Nitenpyram | / | / | / | / | / | / | 100 | 100 |
Note: “/” denotes untested.
For P. xylostella and M. separate, compounds 2a–2v exhibited lower activities compared to cyproflanilide. The preliminary SAR analysis showed that the substituent groups Y1, Y2, and Y3 on the aromatic ring exerted an important effect on the insecticidal activity of the target compounds. Regarding Y1, especially the halogen substitution, I demonstrated superior activity compared to Cl and Br (compounds 2a, 2h, 2i and 2b, 2j, 2k). Regarding Y2, trifluoromethyl and difluoromethoxy groups exhibited enhanced activity compared to other groups. Regarding Y3, heptafluoroisopropyl proved beneficial in maintaining activity (compounds 2c and 2l).
For T. cinnabarinus, only compound 2k exhibited a lethal rate of 91.72% at 100 mg/L, surpassing that of cyproflanilide (15.24% at 100 mg/L). Unfortunately, compound 2k displayed no activity at 10 mg/L.
For N. lugens, most of the title compounds demonstrated good remarkable activity. For example, compounds 2a–2e, 2h–2k, 2n, and 2r–2v demonstrated lethality rates exceeding 90.00% at 400 mg/L, while cyproflanilide exhibited no activity at the same concentration. Moreover, compounds 2k, 2t, and 2v maintained lethality rates of 98.41, 95.94, and 100% at 100 mg/L, respectively. In addition, altering the status of sulfur (sulfide or sulfoxide) influenced the activity. Based on the data of two groups (2m–2v), the activity of sulfoxide was higher than that of sulfide. For example, compounds 2m and 2s demonstrated mortality rates of 26.74 and 93.33% at 400 mg/L, respectively.
Our results indicate that the introduction of pyrazole groups can expand the insecticidal spectrum of meta-diamide compounds to include hemiptera such as N. lugens. Specifically, compound 2k not only exhibited 100% mortality at 1 mg/L against P. xylostella and M. separate but also displayed a 98.41% lethal rate at 100 mg/L against N. lugens. Compound 2k presents promising potential as a lead for the discovery of novel insecticides. Furthermore, our results demonstrate that meta-diamide compounds hold potential for controlling sucking mouthpart pests. Further studies are currently ongoing in our laboratory.
3 Experimental section
3.1 Materials and methods
1H NMR (400 MHz) and 13C NMR (100 MHz) were acquired using a Bruker AV400 spectrometer (Bruker Co., Switzerland) in either DMSO-d 6 or CDCl3 solutions, with tetramethylsilane serving as the internal standard. Chemical shifts (δ) were reported in parts per million (ppm). Mass spectra were generated utilizing the Agilent 1100 LC-MSD-Trap Mass Spectrometer equipped with standard electrospray ionization (ESI) apparatus. Melting points (Mp) were determined using the MP450 melting-point apparatus (Shandong Nanon Instrument Ltd, CITY, China). Flash chromatography was performed using silica gel (200–300 mesh). The crude product was purified by column chromatography using ethyl acetate (EA) and petroleum ether (PE) as the eluent. All solvents and liquid reagents were dried using standard methods and distilled prior to usage.
3.2 Chemical synthesis
3.2.1 Methyl 3-((cyclopropylmethyl)amino)-2-fluorobenzoate (6)
In a 250 mL flask, Zn (5.80 g, 79.39 mmol) and AcOH (7.10 g, 132.32 mmol) were added to methyl 3-amino-2-fluorobenzoate (10.0 g, 66.16 mmol) in EA (100 mL), followed by the addition of compound 5 (4.14 g, 66.16 mmol). The temperature was increased to 60°C for 4 h. Thin-layer chromatography (TLC) indicated the completion of the reaction. Subsequently, the solution was washed with a saturated sodium bicarbonate aqueous solution (100 mL), and the mixture was subjected to extraction using EA (200 mL). The organic layer was washed with saturated brine and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure, and the obtained residue was purified via flash column chromatography using PE and EA as eluents, resulting in 9.68 g (yield: 73.35%) of the target compound in the form of yellow oil. 1H NMR (400 MHz, DMSO-d 6) δ (ppm): 7.08–6.94 (m, 3H), 5.68 (s, 1H), 3.82 (s, 3H), 3.03–2.96 (m, 2H), 1.14–1.04 (m, 1H), 0.49–0.41 (m, 2H), 0.29–0.21 (m, 2H). 13C NMR (100 MHz, DMSO-d 6) δ (ppm): 165.11, 165.08, 151.18, 148.67, 138.46, 138.34, 124.78, 124.74, 118.07, 118.00, 116.59, 116.18, 116.12, 52.56, 47.44, 10.89, 3.90. HRMS (ESI) m/z: Calcd. for C12H14FNO2 [M+H]+ 224.1008, found 224.1077.
3.2.2 Methyl 3-(N-(cyclopropylmethyl)-1-ethyl-3-methyl-1H-pyrazole-5-carboxamido)-2-fluorobenzoate (8)
N,N-diisopropylethylamine (3.73 g, 24.19 mmol) and compound 7 (3.83 g, 22.17 mmol) were added to a solution of compound 6 (4.50 g, 20.16 mmol) in anhydrous tetrahydrofuran (45 mL). Then, the mixture was stirred at 80°C for 6 h. TLC indicated the completion of the reaction. The reaction mixture was subjected to extraction using EA (100 mL) and H2O (80 mL). The organic layer was subsequently washed with saturated brine and then dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure, and the obtained residue was purified via flash column chromatography using PE and EA as eluents, resulting in 6.10 g (yield: 84.20%) of the target compound as a yellow solid. 1H NMR (400 MHz, DMSO-d 6) δ (ppm): 7.85 (t, J = 6.7 Hz, 1H), 7.82–7.77 (m, 1H), 7.38 (t, J = 7.9 Hz, 1H), 5.44 (s, 1H), 4.22 (dd, J = 22.6, 7.8 Hz, 2H), 3.83 (s, 3H), 3.66 (d, J = 74.8 Hz, 2H), 1.93 (s, 3H), 1.33 (t, J = 7.2 Hz, 3H), 0.94 (s, 1H), 0.37 (s, 2H), 0.00 (s, 2H). 13C NMR (100 MHz, DMSO-d 6) δ (ppm): 163.84, 161.70, 158.51, 155.93, 145.63, 135.42, 135.36, 131.71, 125.38, 125.33, 119.51, 119.41, 107.34, 53.00, 45.62, 16.01, 13.38, 9.70, 3.61. HRMS (ESI) m/z: Calcd. for C19H22FN3O3 [M+H]+ 360.1645, found 360.1709.
3.2.3 3-(N-(Cyclopropylmethyl)-1-ethyl-3-methyl-1H-pyrazole-5-carboxamido)-2-fluorobenzoic acid (9)
Compound 8 (6.00 g, 16.70 mmol) was dissolved in methanol (60 mL). Subsequently, 10% sodium hydroxide aqueous solution (2.67 g, 66.80 mmol) was added, and the reaction mixture was stirred at room temperature for 2 h. TLC indicated the completion of the reaction. Upon removal of the solvent by distillation, the crude product was dissolved in H2O (30 mL) and extracted using EA (50 mL). The pH of the aqueous phase was adjusted to 3 by adding 2 M hydrochloric acid, and then extraction was performed using EA (40 mL). The organic layer was subsequently washed with saturated brine, dried over anhydrous sodium sulfate, and evaporated under reduced pressure to obtain 4.42 g (yield: 76.66%) of the target compound as a white solid. 1H NMR (400 MHz, DMSO-d 6) δ (ppm): 13.39 (s, 1H), 7.85 (s, 1H), 7.77–7.71 (m, 1H), 7.34 (d, J = 15.7 Hz, 1H), 5.43 (s, 1H), 4.40–4.08 (m, 2H), 3.66 (d, J = 62.1 Hz, 2H), 1.93 (s, 3H), 1.33 (t, J = 7.2 Hz, 3H), 0.96 (s, 1H), 0.38 (s, 2H), 0.05 (d, J = 43.7 Hz, 2H). 13C NMR (100 MHz, DMSO-d 6) δ (ppm): 163.84, 161.70, 158.51, 155.93, 145.63, 135.42, 135.36, 131.71, 125.38, 125.33, 119.51, 119.41, 107.34, 53.00, 45.62, 16.01, 13.38, 9.70, 3.61. HRMS (ESI) m/z: Calcd. for C18H20FN3O3 [M+H]+ 345.1488, found 346.1550.
3.2.4 3-(N-(Cyclopropylmethyl)-1-ethyl-3-methyl-1H-pyrazole-5-carboxamido)-2-fluorobenzoyl chloride (10)
Thionyl chloride (0.86 g, 7.24 mmol) was added to a solution of compound 9 (0.50 g, 1.45 mmol) in toluene (6 mL). Then, the mixture was heated and refluxed for 2 h. Following the removal of the solvent by distillation, crude product 10 in acetonitrile (3 mL) was utilized for the subsequent step without undergoing additional purification.
3.3 General chemical synthesis of compounds 2a–2v
To 11 (1.45 mmol), KI (0.12 g, 0.73 mmol) in acetonitrile (5 mL) was added 10. The mixture was stirred at 80°C for 8 h. TLC indicated the completion of the reaction. Subsequently, the reaction mixture was diluted with H2O (40 mL) and subjected to extraction using EA (60 mL). The organic layer was subsequently washed with saturated brine, dried over anhydrous sodium sulfate, and evaporated under reduced pressure. The resulting residue was purified via flash column chromatography, utilizing PE and EA as eluents to obtain the target compound.
3.3.1 N-(3-((2-Bromo-4-(perfluoropropan-2-yl)-6-(trifluoromethyl)phenyl)carbamoyl)-2-fluorophenyl)-N-(cyclopropylmethyl)-1-ethyl-3-methyl-1H-pyrazole-5-carboxamide (2a)
Compound 2a was obtained as a yellow solid; yield: 37.32%; Mp: 134.5–135.5°C. 1H NMR (400 MHz, DMSO-d 6) δ (ppm): 10.64 (s, 1H), 8.43 (s, 1H), 7.96 (s, 1H), 7.75–7.63 (m, 2H), 7.42 (t, J = 7.6 Hz, 1H), 5.55 (s, 1H), 4.20 (s, 2H), 3.68 (s, 2H), 1.99 (s, 3H), 1.34 (d, J = 7.1 Hz, 3H), 1.01 (s, 1H), 0.43 (d, J = 7.6 Hz, 2H), 0.08 (s, 2H). 13C NMR (100 MHz, DMSO-d 6) δ (ppm): 163.07, 161.93, 145.71, 139.20, 135.52, 134.62, 133.68, 131.66, 131.35, 129.69, 129.23, 126.93, 126.71, 125.44, 124.29, 123.66, 123.28, 120.93, 107.14, 45.51, 15.97, 13.45, 9.85, 3.72. HRMS (ESI) m/z: Calcd. for C28H22BrF11N4O2 [M+H]+ 735.0750, found 735.0810.
3.3.2 N-(3-((2-Bromo-6-(difluoromethoxy)-4-(perfluoropropan-2-yl)phenyl)carbamoyl)-2-fluorophenyl)-N-(cyclopropylmethyl)-1-ethyl-3-methyl-1H-pyrazole-5-carboxamide (2b)
Compound 2b was obtained as a yellow solid; yield: 52.00%; Mp: 120.0–121.0°C. 1H NMR (400 MHz, DMSO-d 6) δ (ppm): 10.38 (s, 1H), 7.91 (s, 1H), 7.69 (t, J = 6.8 Hz, 2H), 7.55 (s, 1H), 7.40 (t, J = 7.7 Hz, 1H), 7.34 (t, J = 72.0 Hz, 1H), 5.55 (s, 1H), 4.20 (d, J = 12.3 Hz, 2H), 3.68 (d, J = 22.3 Hz, 2H), 1.99 (s, 4H), 1.33 (t, J = 7.2 Hz, 4H), 1.01 (s, 1H), 0.42 (d, J = 7.7 Hz, 2H), 0.10 (s, 2H). 13C NMR (100 MHz, DMSO-d 6) δ (ppm): 162.57, 161.85, 149.27, 135.50, 133.79, 132.72, 129.94, 126.56, 126.35, 126.08, 125.34, 124.40, 124.26, 119.34, 116.75, 116.36, 114.15, 60.22, 45.55, 21.21, 16.02, 14.53, 13.45, 9.84, 3.83. HRMS (ESI) m/z: Calcd. for C28H23BrF10N4O3 [M+H]+ 733.0793, found 733.0859.
3.3.3 N-(Cyclopropylmethyl)-N-(3-((2,6-dibromo-4-(perfluoropropan-2-yl)phenyl)carbamoyl)-2-fluorophenyl)-1-ethyl-3-methyl-1H-pyrazole-5-carboxamide (2c)
Compound 2c was obtained as a white solid; yield: 63.41%; Mp: 110.0–111.0°C. 1H NMR (400 MHz, DMSO-d 6) δ (ppm): 10.58 (s, 1H), 8.04 (s, 2H), 7.79–7.68 (m, 2H), 7.41 (t, J = 7.5 Hz, 1H), 5.55 (s, 1H), 4.20 (s, 3H), 3.74–3.60 (m, 2H), 1.97 (s, 3H), 1.33 (t, J = 7.2 Hz, 4H), 1.02 (s, 1H), 0.43 (d, J = 7.8 Hz, 2H), 0.11 (d, J = 18.7 Hz, 2H). 13C NMR (100 MHz, DMSO-d 6) δ (ppm): 162.19, 161.87, 154.35, 145.74, 139.93, 135.52, 133.70, 129.87, 129.54, 129.44, 127.31, 127.10, 126.33, 125.36, 124.36, 124.23, 121.62, 107.21, 45.56, 16.07, 13.51, 9.85, 3.81. HRMS (ESI) m/z: Calcd. for C27H22Br2F8N4O2 [M+H]+ 747.2942, found 747.0029.
3.3.4 N-(3-((2-Bromo-6-methyl-4-(perfluoropropan-2-yl)phenyl)carbamoyl)-2-fluorophenyl)-N-(cyclopropylmethyl)-1-ethyl-3-methyl-1H-pyrazole-5-carboxamide (2d)
Compound 2d was obtained as a white solid; yield: 70.41%; Mp: 143.0–144.0°C. 1H NMR (400 MHz, DMSO-d 6) δ (ppm): 10.25 (s, 1H), 7.80 (s, 1H), 7.69 (q, J = 6.9 Hz, 3H), 7.39 (t, J = 7.7 Hz, 1H), 5.56 (s, 1H), 4.32–4.12 (m, 2H), 3.77–3.61 (m, 2H), 2.36 (s, 3H), 1.97 (s, 3H), 1.33 (t, J = 7.2 Hz, 3H), 1.02 (s, 1H), 0.43 (d, J = 7.7 Hz, 2H), 0.10 (s, 2H). 13C NMR (100 MHz, DMSO-d 6) δ (ppm): 162.29, 161.88, 154.21, 145.68, 140.84, 139.07, 135.52, 133.39, 129.80, 127.43, 127.31, 127.10, 127.01, 125.80, 125.59, 125.40, 124.92, 124.79, 121.82, 118.96, 107.21, 53.34, 45.54, 19.06, 16.04, 13.49, 9.87, 3.84. HRMS (ESI) m/z: Calcd. for C28H25BrF8N4O2 [M+H]+ 681.1033, found 681.1094.
3.3.5 N-(3-((2-Bromo-6-chloro-4-(perfluoropropan-2-yl)phenyl)carbamoyl)-2-fluorophenyl)-N-(cyclopropylmethyl)-1-ethyl-3-methyl-1H-pyrazole-5-carboxamide (2e)
Compound 2e was obtained as a white solid; yield: 48.41%; Mp: 111.0–112.0°C. 1H NMR (400 MHz, DMSO-d 6) δ (ppm): 10.59 (s, 1H), 8.06–7.91 (m, 2H), 7.72 (t, J = 6.9 Hz, 2H), 7.41 (t, J = 7.1 Hz, 1H), 5.55 (s, 1H), 4.21 (dd, J = 16.2, 7.2 Hz, 2H), 3.78–3.56 (m, 2H), 1.96 (s, 3H), 1.34 (d, J = 7.1 Hz, 3H), 1.01 (s, 1H), 0.42 (d, J = 7.4 Hz, 2H), 0.11 (d, J = 19.4 Hz, 2H). 13C NMR (100 MHz, DMSO-d 6) δ (ppm): 162.30, 161.87, 154.34, 145.71, 138.46, 135.83, 135.50, 133.77, 129.92, 128.97, 128.87, 127.02, 126.80, 126.67, 126.51, 125.40, 124.29, 124.16, 121.59, 118.74, 107.20, 53.34, 45.56, 16.04, 13.49, 9.86, 3.69. HRMS (ESI) m/z: Calcd. for C27H22BrClF8N4O2 [M+H]+ 701.8402, found 701.0557.
3.3.6 N-(3-((2-Bromo-6-ethyl-4-(perfluoropropan-2-yl)phenyl)carbamoyl)-2-fluorophenyl)-N-(cyclopropylmethyl)-1-ethyl-3-methyl-1H-pyrazole-4-carboxamide (2f)
Compound 2f was obtained as a yellow solid; yield: 38.10%; Mp: 127.2–128.0°C. 1H NMR (400 MHz, DMSO-d 6) δ (ppm): 10.28 (s, 1H), 7.82 (s, 1H), 7.70 (t, J = 7.4 Hz, 2H), 7.64 (s, 1H), 7.40 (t, J = 7.7 Hz, 1H), 5.55 (s, 1H), 4.22 (dd, J = 17.7, 10.5 Hz, 2H), 3.70 (d, J = 6.6 Hz, 2H), 2.72 (d, J = 21.7 Hz, 2H), 1.98 (d, J = 9.7 Hz, 4H), 1.33 (t, J = 7.2 Hz, 3H), 1.11 (d, J = 7.5 Hz, 3H), 1.02 (s, 1H), 0.43 (d, J = 7.6 Hz, 2H), 0.12 (d, J = 14.4 Hz, 2H). 13C NMR (100 MHz, DMSO-d 6) δ (ppm): 162.75, 161.91, 154.18, 146.55, 145.64, 138.57, 135.52, 133.26, 129.66, 127.47, 127.36, 126.21, 126.01, 125.78, 125.68, 125.53, 125.42, 125.02, 124.88, 107.19, 53.36, 45.53, 25.77, 16.01, 14.72, 13.48, 9.86, 3.75. HRMS (ESI) m/z: Calcd. for C29H27BrF8N4O2 [M+H]+ 695.4522, found 695.1247.
3.3.7 N-(3-((2-Bromo-6-methoxy-4-(perfluoropropan-2-yl)phenyl)carbamoyl)-2-fluorophenyl)-N-(cyclopropylmethyl)-1-ethyl-3-methyl-1H-pyrazole-4-carboxamide (2g)
Compound 2g was obtained as a yellow solid; yield: 70.10%; Mp: 132.2–133.0°C. 1H NMR (400 MHz, DMSO-d 6) δ (ppm): 10.08 (s, 1H), 7.66 (t, J = 6.8 Hz, 2H), 7.43–7.31 (m, 2H), 7.23 (s, 1H), 5.54 (s, 1H), 4.36–4.15 (m, 2H), 3.90 (s, 3H), 3.68 (d, J = 56.5 Hz, 2H), 1.98 (d, J = 9.9 Hz, 3H), 1.34 (d, J = 7.2 Hz, 3H), 1.00 (s, 1H), 0.42 (d, J = 7.6 Hz, 2H), 0.11 (s, 2H). 13C NMR (100 MHz, DMSO-d 6) δ (ppm): 161.84, 157.48, 154.27, 145.68, 135.50, 134.56, 133.58, 130.11, 127.87, 126.01, 125.80, 125.23, 124.80, 124.66, 121.78, 119.20, 118.26, 108.25, 108.14, 107.32, 57.23, 55.37, 45.57, 16.03, 13.47, 9.84, 3.93, 3.58. HRMS (ESI) m/z: Calcd. for C28H25BrF8N4O3 [M+H]+ 697.4242, found 697.1249.
3.3.8 N-(3-((2-Chloro-4-(perfluoropropan-2-yl)-6-(trifluoromethyl)phenyl)carbamoyl)-2-fluorophenyl)-N-(cyclopropylmethyl)-1-ethyl-3-methyl-1H-pyrazole-5-carboxamide (2h)
Compound 2h was obtained as a yellow solid; yield: 20.10%; Mp: 112.2–112.9°C. 1H NMR (400 MHz, DMSO-d 6) δ (ppm): 10.62 (s, 1H), 8.35 (s, 1H), 7.93 (s, 1H), 7.70 (dt, J = 16.6, 6.9 Hz, 2H), 7.41 (t, J = 7.8 Hz, 1H), 5.55 (s, 1H), 4.27–4.11 (m, 2H), 3.69 (d, J = 6.8 Hz, 2H), 1.97 (s, 3H), 1.32 (t, J = 7.2 Hz, 4H), 1.01 (s, 1H), 0.42 (d, J = 7.4 Hz, 2H), 0.11 (d, J = 19.0 Hz, 2H). 13C NMR (100 MHz, DMSO-d 6) δ (ppm): 163.19, 161.92, 156.86, 154.33, 145.70, 138.11, 137.59, 135.49, 133.78, 131.72, 129.77, 126.84, 126.62, 125.48, 124.14, 123.72, 122.64, 120.99, 118.93, 107.19, 53.43, 45.50, 15.95, 13.43, 9.84, 3.72. HRMS (ESI) m/z: Calcd. for C28H22ClF11N4O2 [M+H]+ 691.9424, found 691.1318.
3.3.9 N-(Cyclopropylmethyl)-1-ethyl-N-(2-fluoro-3-((2-iodo-4-(perfluoropropan-2-yl)-6-(trifluoromethyl)phenyl)carbamoyl)phenyl)-3-methyl-1H-pyrazole-4-carboxamide (2i)
Compound 2i was obtained as a white solid; yield: 33.34%; Mp: 150.1–151.1°C. 1H NMR (400 MHz, DMSO-d 6) δ (ppm): 10.67 (s, 1H), 8.51 (s, 1H), 7.95 (s, 1H), 7.72 (t, J = 7.1 Hz, 2H), 7.43 (t, J = 7.7 Hz, 1H), 5.59 (s, 1H), 4.21 (dd, J = 16.0, 8.1 Hz, 2H), 3.69 (s, 2H), 1.97 (s, 5H), 1.02 (s, 1H), 0.43 (d, J = 7.2 Hz, 2H), 0.11 (d, J = 28.3 Hz, 2H). 13C NMR (100 MHz, DMSO-d 6) δ (ppm): 162.84, 161.94, 145.78, 142.46, 140.37, 135.56, 133.64, 130.25, 129.63, 126.69, 126.48, 125.37, 124.58, 124.45, 123.60, 120.88, 108.24, 107.13, 45.51, 16.00, 13.48, 9.85, 3.72. HRMS (ESI) m/z: Calcd. for C28H22F11IN4O2 [M+H]+ 783.0611, found 783.0667.
3.3.10 N-(3-((2-Chloro-6-(difluoromethoxy)-4-(perfluoropropan-2-yl)phenyl)carbamoyl)-2-fluorophenyl)-N-(cyclopropylmethyl)-1-ethyl-3-methyl-1H-pyrazole-5-carboxamide (2j)
Compound 2j was obtained as a yellow solid; yield: 41.11%; Mp: 116.1–117.0°C. 1H NMR (400 MHz, DMSO-d 6) δ (ppm): 10.40 (s, 1H), 7.82 (s, 1H), 7.68 (q, J = 6.8, 6.1 Hz, 2H), 7.53 (d, J = 8.7 Hz, 1H), 7.45–7.35 (m, 2H), 7.34 (t, J = 72.0, 1H), 5.54 (s, 1H), 4.28–4.12 (m, 2H), 3.68 (d, J = 30.2 Hz, 2H), 1.97 (s, 3H), 1.33 (t, J = 7.2 Hz, 4H), 1.01 (s, 1H), 0.42 (d, J = 7.7 Hz, 2H), 0.10 (s, 2H). 13C NMR (100 MHz, DMSO-d 6) δ (ppm): 162.63, 161.86, 156.86, 154.35, 149.33, 135.48, 133.84, 131.13, 129.99, 126.11, 125.89, 125.39, 124.34, 124.21, 123.82, 123.72, 119.32, 116.72, 115.80, 115.69, 114.13, 107.25, 53.34, 45.55, 16.00, 13.44, 9.84, 3.63. HRMS (ESI) m/z: Calcd. for C28H23ClF10N4O3 [M+H]+ 689.1299, found 689.1357.
3.3.11 N-(Cyclopropylmethyl)-N-(3-((2-(difluoromethoxy)-6-iodo-4-(perfluoropropan-2-yl)phenyl)carbamoyl)-2-fluorophenyl)-1-ethyl-3-methyl-1H-pyrazole-5-carboxamide (2k)
Compound 2k was obtained as a yellow solid; yield: 54.00%; Mp: 166.0–167.0°C. 1H NMR (400 MHz, DMSO-d 6) δ (ppm): 10.35 (s, 1H), 8.02 (s, 1H), 7.69 (t, J = 6.9 Hz, 2H), 7.51 (d, J = 14.7 Hz, 1H), 7.43 (s, 1H), 7.31 (s, 1H), 7.34 t, J = 72.0, 1H), 5.57 (s, 1H), 4.29–4.11 (m, 2H), 3.67 (d, J = 6.9 Hz, 2H), 1.96 (s, 2H), 1.33 (t, J = 7.2 Hz, 4H), 1.01 (s, 1H), 0.43 (d, J = 7.6 Hz, 2H), 0.11 (d, J = 14.3 Hz, 2H). 13C NMR (100 MHz, DMSO-d 6) δ (ppm): 161.87, 154.40, 148.24, 145.73, 135.91, 135.53, 133.73, 132.35, 129.86, 126.95, 126.74, 125.33, 124.59, 124.45, 121.66, 119.35, 116.76, 114.17, 107.17, 104.38, 45.56, 16.06, 14.53, 13.48, 9.82, 3.66. HRMS (ESI) m/z: Calcd. for C28H23F10IN4O3 [M+H]+ 781.0655, found 781.0711.
3.3.12 N-(Cyclopropylmethyl)-N-(3-((2,6-dibromo-4-(tert-butyl)phenyl)carbamoyl)-2-fluorophenyl)-1-ethyl-3-methyl-1H-pyrazole-5-carboxamide (2l)
Compound 2l was obtained as a yellow solid; yield: 70.00%; Mp: 106.1–107.0°C. 1H NMR (400 MHz, DMSO-d 6) δ (ppm): 10.28 (s, 1H), 7.73–7.60 (m, 4H), 7.38 (t, J = 7.5 Hz, 1H), 5.55 (s, 1H), 4.21 (dd, J = 15.1, 7.2 Hz, 2H), 3.79–3.59 (m, 2H), 1.96 (s, 2H), 1.33 (t, J = 7.3 Hz, 13H), 1.01 (p, J = 6.6 Hz, 1H), 0.42 (d, J = 7.6 Hz, 2H), 0.11 (d, J = 12.5 Hz, 2H). 13C NMR (100 MHz, DMSO-d 6) δ (ppm): 162.42, 161.90, 156.79, 154.16, 145.69, 135.55, 133.31, 133.14, 129.80, 129.69, 125.21, 125.09, 124.95, 124.55, 107.23, 60.23, 53.35, 45.56, 35.22, 31.13, 21.24, 16.09, 14.56, 13.54, 9.87, 3.88. HRMS (ESI) m/z: Calcd. for C28H31Br2FN4O2 [M+H]+ 635.3884, found 635.1110.
3.3.13 N-(3-((2-Bromo-6-(methylthio)-4-(perfluoropropan-2-yl)phenyl)carbamoyl)-2-fluorophenyl)-N-(cyclopropylmethyl)-1-ethyl-3-methyl-1H-pyrazole-5-carboxamide (2m)
Compound 2m was obtained as a white solid; yield: 34.00%; Mp: 108.0–108.7°C. 1H NMR (400 MHz, DMSO-d 6) δ (ppm): 10.35 (s, 1H), 7.70 (dd, J = 12.1, 4.6 Hz, 3H), 7.46–7.32 (m, 2H), 5.55 (s, 1H), 4.31–4.13 (m, 2H), 3.77–3.61 (m, 2H), 3.48 (s, 2H), 1.98 (d, J = 11.4 Hz, 4H), 1.34 (d, J = 7.1 Hz, 3H), 1.01 (s, 1H), 0.48–0.38 (m, 2H), 0.12 (d, J = 19.4 Hz, 2H). 13C NMR (100 MHz, DMSO d 6) δ (ppm): 162.33, 161.89, 156.84, 154.30, 145.70, 144.44, 136.35, 135.53, 133.53, 129.84, 126.69, 126.48, 125.58, 125.29, 125.12, 125.01, 124.71, 124.57, 120.58, 107.24, 55.38, 53.34, 45.56, 16.06, 14.65, 13.51, 9.86, 3.70. HRMS (ESI) m/z: Calcd. for C28H25BrF8N4O2S [M+H]+ 713.0753, found 713.0816.
3.3.14 N-(3-((2-Bromo-6-(ethylthio)-4-(perfluoropropan-2-yl)phenyl)carbamoyl)-2-fluorophenyl)-N-(cyclopropylmethyl)-1-ethyl-3-methyl-1H-pyrazole-5-carboxamide (2n)
Compound 2n was obtained as a yellow solid; yield: 27.80%; Mp: 124.4–125.0°C. 1H NMR (400 MHz, DMSO-d 6) δ (ppm): 10.32 (s, 1H), 7.71 (dd, J = 15.8, 9.2 Hz, 3H), 7.47–7.35 (m, 2H), 5.55 (s, 1H), 4.21 (dd, J = 17.5, 7.0 Hz, 2H), 3.68 (d, J = 5.6 Hz, 2H), 3.04 (q, J = 7.3 Hz, 2H), 1.98 (d, J = 9.4 Hz, 3H), 1.33 (t, J = 7.2 Hz, 3H), 1.23 (d, J = 7.3 Hz, 3H), 1.01 (s, 1H), 0.43 (d, J = 7.5 Hz, 2H), 0.12 (d, J = 17.7 Hz, 2H). 13C NMR (100 MHz, DMSO-d 6) δ (ppm): 162.75, 161.91, 154.18, 146.55, 145.64, 138.57, 135.52, 133.26, 129.66, 127.36, 126.21, 126.01, 125.68, 125.53, 125.42, 125.02, 124.88, 121.81, 118.96, 107.19, 53.36, 45.53, 25.77, 16.01, 14.72, 14.53, 13.48, 9.86, 3.75. HRMS (ESI) m/z: Calcd. for C29H27BrF8N4O2S [M+H]+ 727.0910, found 727.0971.
3.3.15 N-(3-((2-Bromo-6-(isopropylthio)-4-(perfluoropropan-2-yl)phenyl)carbamoyl)-2-fluorophenyl)-N-(cyclopropylmethyl)-1-ethyl-3-methyl-1H-pyrazole-5-carboxamide (2o)
Compound 2o was obtained as a yellow solid; yield: 41.11%; Mp: 150.4–151.0°C. 1H NMR (400 MHz, DMSO-d 6) δ (ppm): 10.34 (s, 1H), 7.79 (d, J = 10.1 Hz, 1H), 7.73–7.65 (m, 2H), 7.54 (s, 1H), 7.39 (s, 1H), 5.55 (s, 1H), 4.21 (dd, J = 13.6, 6.3 Hz, 2H), 3.64 (dd, J = 17.6, 11.0 Hz, 4H), 1.97 (s, 3H), 1.34 (d, J = 7.1 Hz, 3H), 1.25 (d, J = 6.6 Hz, 6H), 1.01 (s, 1H), 0.43 (d, J = 7.6 Hz, 2H), 0.12 (d, J = 21.6 Hz, 2H). 13C NMR (100 MHz, DMSO-d 6) δ (ppm): 170.81, 162.26, 161.91, 154.38, 145.66, 135.56, 133.24, 133.18, 129.95, 126.46, 126.37, 125.57, 125.16, 124.40, 124.30, 122.08, 119.22, 118.97, 107.27, 107.21, 60.22, 45.54, 36.52, 22.53, 21.22, 16.08, 14.54, 13.52, 9.86, 3.71. HRMS (ESI) m/z: Calcd. for C30H29BrF8N4O2S [M+H]+ 741.1066, found 741.1125.
3.3.16 N-(3-((2-Bromo-6-(tert-butylthio)-4-(perfluoropropan-2-yl)phenyl)carbamoyl)-2-fluorophenyl)-N-(cyclopropylmethyl)-1-ethyl-3-methyl-1H-pyrazole-5-carboxamide (2p)
Compound 2p was obtained as a yellow solid; yield: 63.33%; Mp: 125.1–126.1°C. 1H NMR (400 MHz, DMSO-d 6) δ (ppm): 8.62 (d, J = 12.0 Hz, 1H), 8.19–8.10 (m, 1H), 7.92 (d, J = 1.8 Hz, 1H), 7.83 (s, 1H), 7.56 (td, J = 7.6, 1.6 Hz, 1H), 7.38 (t, J = 7.9 Hz, 1H), 5.36 (s, 1H), 4.53–4.32 (m, 2H), 3.92 (s, 1H), 3.65 (s, 1H), 1.49 (t, J = 7.2 Hz, 3H), 1.26 (s, 3H), 1.21 (s, 9H), 1.10 (s, 1H), 0.51 (d, J = 7.2 Hz, 2H), 0.18 (d, J = 39.8 Hz, 2H). 13C NMR (100 MHz, DMSO-d 6) δ (ppm): 162.17, 161.82, 156.96, 154.42, 143.92, 136.21, 135.48, 133.83, 133.38, 130.88, 130.76, 129.81, 125.43, 125.21, 125.08, 125.00, 124.74, 124.62, 124.60, 122.02, 121.75, 118.89, 107.15, 48.57, 45.58, 31.08, 29.50, 16.07, 13.53, 9.85, 3.75. HRMS (ESI) m/z: Calcd. for C31H31BrF8N4O2S [M+H]+ 755.1223, found 755.1281.
3.3.17 N-(3-((2-Chloro-6-(methylthio)-4-(perfluoropropan-2-yl)phenyl)carbamoyl)-2-fluorophenyl)-N-(cyclopropylmethyl)-1-ethyl-3-methyl-1H-pyrazole-4-carboxamide (2q)
Compound 2q was obtained as a yellow solid; yield: 41.23%; Mp: 112.1–113.0°C. 1H NMR (400 MHz, DMSO-d 6) δ (ppm): 10.32 (s, 1H), 7.68 (d, J = 6.9 Hz, 2H), 7.61 (s, 1H), 7.46–7.33 (m, 2H), 5.56 (s, 1H), 4.21 (dd, J = 14.4, 7.4 Hz, 2H), 3.68 (d, J = 25.9 Hz, 2H), 2.52 (s, 3H), 1.96 (s, 3H), 1.33 (t, J = 7.2 Hz, 4H), 1.01 (s, 1H), 0.42 (d, J = 7.6 Hz, 2H), 0.10 (s, 2H). 13C NMR (100 MHz, DMSO-d 6) δ (ppm): 162.44, 154.29, 144.45, 135.52, 134.81, 133.60, 129.89, 126.45, 126.24, 125.35, 124.63, 124.49, 122.17, 119.93, 107.26, 53.32, 45.55, 16.04, 14.62, 13.49, 9.86, 3.87. HRMS (ESI) m/z: Calcd. for C28H25ClF8N4O2S [M+H]+ 669.1259, found 669.1319.
3.3.18 N-(3-((2-Chloro-6-(methylsulfonyl)-4-(perfluoropropan-2-yl)phenyl)carbamoyl)-2-fluorophenyl)-N-(cyclopropylmethyl)-1-ethyl-3-methyl-1H-pyrazole-4-carboxamide (2r)
Compound 2r was obtained as a yellow solid; yield: 33.33%; Mp: 133.1–133.7°C. 1H NMR (400 MHz, DMSO-d 6) δ (ppm): 10.63 (s, 1H), 8.39 (s, 1H), 8.15 (s, 1H), 7.74 (t, J = 7.4 Hz, 2H), 7.43 (t, J = 7.8 Hz, 1H), 5.56 (s, 1H), 4.21 (dd, J = 16.5, 7.4 Hz, 2H), 3.75–3.62 (m, 2H), 2.52–2.48 (m, 4H), 1.97 (s, 4H), 1.33 (t, J = 7.2 Hz, 4H), 1.01 (s, 1H), 0.43 (d, J = 7.7 Hz, 2H), 0.11 (d, J = 21.3 Hz, 2H). 13C NMR (100 MHz, DMSO-d 6) δ (ppm): 163.27, 161.91, 156.98, 154.45, 145.74, 142.41, 138.04, 137.73, 135.52, 133.78, 132.52, 129.96, 126.67, 126.45, 125.63, 125.45, 123.96, 121.81, 118.94, 118.67, 107.18, 53.41, 45.54, 43.47, 16.04, 13.48, 9.86, 3.74. HRMS (ESI) m/z: Calcd. for C28H25ClF8N4O4S [M+H]+ 701.1157, found 701.1214.
3.3.19 N-(3-((2-Bromo-6-(methylsulfonyl)-4-(perfluoropropan-2-yl)phenyl)carbamoyl)-2-fluorophenyl)-N-(cyclopropylmethyl)-1-ethyl-3-methyl-1H-pyrazole-5-carboxamide (2s)
Compound 2s was obtained as a yellow solid; yield: 54.12%; Mp: 127.1–127.7°C. 1H NMR (400 MHz, DMSO-d 6) δ (ppm): 10.65 (s, 1 H), 8.51–8.43 (m, 1 H), 8.19 (d, J = 1.6 Hz, 1 H), 7.74 (t, J = 7.4 Hz, 2 H), 7.43 (t, J = 7.7 Hz, 1 H), 5.56 (s, 1 H), 4.20 (dt, J = 13.9, 7.0 Hz, 2 H), 3.70 (d, J = 25.1 Hz, 2 H), 3.36 (s, 3 H), 1.97 (s, 3 H), 1.33 (t, J = 7.2 Hz, 4 H), 1.01 (s, 1 H), 0.43 (d, J = 7.9 Hz, 2 H), 0.11 (d, J = 29.0 Hz, 2 H). 13C NMR (100 MHz, DMSO-d 6) δ (ppm): 163.20, 161.91, 154.46, 145.75, 142.42, 139.27, 135.54, 133.69, 129.92, 129.26, 126.88, 126.66, 126.15, 125.42, 124.22, 121.84, 118.71, 107.15, 53.37, 45.54, 43.46, 16.06, 13.50, 9.85, 3.75. HRMS (ESI) m/z: Calcd. for C28H25BrF8N4O4S [M+H]+ 745.0652, found 745.0711.
3.3.20 N-(3-((2-Bromo-6-(tert-butylsulfonyl)-4-(perfluoropropan-2-yl)phenyl)carbamoyl)-2-fluorophenyl)-N-(cyclopropylmethyl)-1-ethyl-3-methyl-1H-pyrazole-5-carboxamide (2t)
Compound 2t was obtained as a white solid; yield: 29.33%; Mp: 143.21–143.8°C. 1H NMR (400 MHz, DMSO-d 6) δ (ppm): 10.37 (s, 1H), 8.51–8.48 (m, 1H), 8.03 (s, 1H), 7.91–7.88 (m, 1H), 7.84–7.78 (m, 1H), 7.46 (t, J = 7.8 Hz, 1H), 5.48 (s, 1H), 4.35–4.14 (m, 2H), 3.72 (d, J = 97.0 Hz, 2H), 1.97 (s, 3H), 1.34 (t, J = 7.2 Hz, 3H), 1.20 (s, 9H), 0.99 (s, 1H), 0.42 (d, J = 7.5 Hz, 2H), 0.12 (d, J = 51.4 Hz, 2H). 13C NMR (100 MHz, DMSO-d 6) δ (ppm): 166.54, 140.72, 136.37, 136.34, 135.41, 133.81, 133.35, 133.21, 131.15, 129.55, 129.30, 128.56, 128.39, 125.55, 125.33, 62.51, 45.59, 31.61, 22.98, 16.04, 13.51, 9.84, 3.61. HRMS (ESI) m/z: Calcd. for C31H31BrF8N4O4S [M+H]+ 787.1121, found 787.1183.
3.3.21 N-(3-((2-Bromo-6-(isopropylsulfonyl)-4-(perfluoropropan-2-yl)phenyl)carbamoyl)-2-fluorophenyl)-N-(cyclopropylmethyl)-1-ethyl-3-methyl-1H-pyrazole-5-carboxamide (2u)
Compound 2u was obtained as a yellow solid; yield: 63.33%; Mp: 101.2–102.0°C. 1H NMR (400 MHz, DMSO-d 6) δ (ppm): 10.65 (s, 1H), 8.53 (d, J = 1.9 Hz, 1H), 8.10 (s, 1H), 7.75 (t, J = 7.4 Hz, 2H), 7.44 (t, J = 7.8 Hz, 1H), 5.54 (s, 1H), 4.34–4.16 (m, 2H), 3.69 (d, J = 67.2 Hz, 4H), 1.97 (s, 3H), 1.33 (d, J = 7.2 Hz, 3H), 1.14 (d, J = 6.7 Hz, 6H), 1.00 (s, 1H), 0.42 (d, J = 7.4 Hz, 2H), 0.15 (s, 2H). 13C NMR (100 MHz, DMSO-d 6) δ (ppm): 163.21, 161.80, 157.03, 154.52, 145.71, 139.86, 139.16, 135.95, 135.85, 135.45, 133.75, 129.91, 129.80, 127.47, 126.63, 126.42, 125.45, 123.97, 121.54, 118.69, 107.15, 54.25, 45.59, 16.04, 14.67, 13.49, 9.81, 3.71. HRMS (ESI) m/z: Calcd. for C30H29BrF8N4O4S [M+H]+ 773.0965, found 773.1025.
3.3.22 N-(3-((2-Bromo-6-(ethylsulfonyl)-4-(perfluoropropan-2-yl)phenyl)carbamoyl)-2-fluorophenyl)-N-(cyclopropylmethyl)-1-ethyl-3-methyl-1H-pyrazole-5-carboxamide (2v)
Compound 2v was obtained as a yellow solid; yield: 38.99%; Mp: 131.8–132.5°C. 1H NMR (400 MHz, DMSO-d 6) δ (ppm): 10.66 (s, 1H), 8.51 (s, 1H), 8.13 (d, J = 2.1 Hz, 1H), 7.75 (d, J = 7.5 Hz, 2H), 7.45 (d, J = 7.8 Hz, 1H), 5.55 (s, 1H), 4.21 (dd, J = 14.8, 7.5 Hz, 2H), 3.70 (d, J = 36.2 Hz, 2H), 3.43 (s, 2H), 1.97 (s, 3H), 1.34 (d, J = 7.1 Hz, 3H), 1.07 (t, J = 7.3 Hz, 3H), 1.00 (s, 1H), 0.43 (d, J = 7.7 Hz, 2H), 0.11 (d, J = 27.9 Hz, 2H). 13C NMR (100 MHz, DMSO-d 6) δ (ppm): 163.22, 161.88, 145.76, 140.19, 139.64, 135.73, 135.51, 133.70, 131.14, 129.91, 129.63, 127.06, 126.76, 126.55, 125.45, 123.98, 107.08, 49.13, 45.56, 16.06, 13.50, 9.83, 7.17, 3.74. HRMS (ESI) m/z: Calcd. for C29H27BrF8N4O4S [M+H]+ 759.0808, found 759.0863.
3.4 Insecticidal assay
According to statistical protocol, the bioassay was conducted three times at 25 ± 1°C. All compounds were dissolved in DMSO and further diluted with water containing Triton X-100 (0.1 mg/L) to obtain various concentrations for bioassays. Mortality rates were adjusted using Abbott’s method and assessments were conducted based on the dead/alive status. Evaluations were based on a percentage scale (0 indicating no activity and 100 indicating complete eradication). The experiments maintained a 5% error rate.
The insects P. xylostella, M. separate, T. cinnabarinus, and N. lugens evaluated in this study were provided by Green Tech Laboratory. The insecticidal activity of the title compounds against T. cinnabarinus was assessed using the immersion method [23]. Insecticidal activity against P. xylostella and M. separate was assessed using the leaf-dip method [24], while insecticidal activity against N. lugens was evaluated employing the rice seedling immersion method [25].
4 Conclusion
To expand the application of meta-diamide compounds in controlling sucking mouthpart pests, we synthesized 22 novel meta-diamide compounds containing a pyrazole group and validated them via 1H NMR, 13C NMR, and HRMS. Bioassay results indicated that most of the title compounds exhibit notable activity against N. lugens at 400 mg/L. Particularly, compound 2k exhibits beneficial activity against all four insects tested. This underscores the utility of pyrazole groups in the application of meta-diamide compounds in the field of sucking mouthpart pests. Our study revealed that these molecules hold promise as lead molecules for the development of novel insecticidal agents.
-
Funding information: Authors state no funding involved.
-
Author contributions: Jiyong Liu, Huailin Pang, Bin Li and Liang Lv designed the title compounds. Jiyong Liu, Yu Yan and Minghui Wu synthesized the compounds. Juncheng Xiang tested the insecticidal activities.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Katsuta H, Nomura M, Wakita T, Daido H, Kobayashi Y, Kawahara A, et al. Discovery of broflanilide, a novel insecticide. J Pestic Sci. 2019;44:120–8.10.1584/jpestics.D18-088Suche in Google Scholar PubMed PubMed Central
[2] Zhou C, Ji Y, Ren L, Shao X. Photochromic meta-diamides for optical modulation of ligand activity and neuron function. Photochem Photobiol Sci. 2020;19:854–7.10.1039/d0pp00045kSuche in Google Scholar PubMed
[3] Nakao T, Banba S, Hirase K. Comparison between the modes of action of novel meta-diamide and macrocyclic lactone insecticides on the RDL GABA receptor. Pestic Biochem Physiol. 2015;120:101–8.10.1016/j.pestbp.2014.09.011Suche in Google Scholar PubMed
[4] Zhang Z, Liu M, Liu W, Xiang J, Li J, Li Z, et al. Synthesis and fungicidal activities of perfluoropropan-2-yl-based novel quinoline derivatives. Heterocycl Commun. 2019;25(1):91–7. 10.1515/hc-2019-0002.Suche in Google Scholar
[5] Wu D, Liu M, Li Z, Dang M, Liu X, Li J, et al. Synthesis and fungicidal activity of novel imidazo[4, 5-b]pyridine derivatives. Heterocycl Commun. 2024;25(1):8–14. 10.1515/hc-2019-0003.Suche in Google Scholar
[6] Cordova D, Benner EA, Rauh JJ, Sopa JS, Lahm GP, Selby TP, et al. Anthranilic diamides: a new class of insecticides with a novel mode of action, ryanodine receptor activation. Pestic Biochem Physiol. 2006;84(3):196–214.10.1016/j.pestbp.2005.07.005Suche in Google Scholar
[7] Liu JY, Zhou LQ, Xiang JC, Ni JP, Li ZC, Pang HL, et al. The R&D of cyproflanilide. World Pesticide. 2021;43:5.Suche in Google Scholar
[8] Lv L, Liu JY, Xiang JC, Ma WJ, Zhou LQ, Hou S, et al. A meta diamine compound and its preparation method and applications. CN110028423A; 2019.Suche in Google Scholar
[9] Luo CY, Ma WJ, Lv L, Pang HL, Xiang JC, Zhou LQ, et al. Synthesis and insecticidal activity of novel meta-diamide compounds containing cyclopropyl group. Chin J Org Chem. 2020;40:2963.10.6023/cjoc202003025Suche in Google Scholar
[10] Zhang LX, Zhang J, Zhang XH, Gao YX, Wang J, Kang Z. A Benzamide compound and its applications. CN110194726A; 2019.Suche in Google Scholar
[11] Hemavathi SN, Kumar BK, Rai KM. Synthesis and biological screening of some new 2,5-disubstituted 1,3,4-oxadiazoles. Int J Pharm Pharm Sci. 2011;3:110–4.Suche in Google Scholar
[12] Katikireddy R, Kakkerla R, Krishna MP, Durgaiah G, Reddy YN, Satyanarayana M. Synthesis and biological evaluation of (e)-n’-benzylidene-7-methyl-2-propyl-1h-benzo[d] imidazole-5-carbohydrazides as antioxidant, anti-inflammatory and analgesic agents. Heterocycl Commun. 2019;25(1):27–38.10.1515/hc-2019-0009Suche in Google Scholar
[13] Huang PM, Wu MH, Lv L, Zhou LQ, Liu XW, Liu JY. Design, synthesis and insecticidal activities of new meta-diamide compounds containing n-butyl group. Tetrahedron Lett. 2022;96:153743. 10.1016/j.tetlet.2022.153743.Suche in Google Scholar
[14] Crofts AR. The cytochrome bc1 complex: function in the context of structure. Annu Rev Physiol. 2004;66:689–733.10.1146/annurev.physiol.66.032102.150251Suche in Google Scholar PubMed
[15] EiGohary NS, Shaaban MI. Synthesis, antimicrobial, antiquorum-sensing, antitumor and cytotoxic activities of new series of fused [1,3,4]thiadiazoles – ScienceDirect. Eur J Med Chem. 2013;63:185–95.10.1016/j.ejmech.2013.02.010Suche in Google Scholar PubMed
[16] Behzadi SA, Sheikhhosseini E, Ahmadi SA, Ghazanfari D, Akhgar M. Synthesis and characterization of novel biological tetracoumarin derivatives bearing ether moieties. Heterocycl Commun. 2020;26(1):60–7.10.1515/hc-2020-0009Suche in Google Scholar
[17] Carola H, Sozanne S, Hildur P, Tina W. A structural perspective on mechanism and function of the cytochrome bc (1) complex. Results Probl Cell Differ. 2008;45:253–78.10.1007/400_2007_042Suche in Google Scholar PubMed
[18] Bamba F, Jin J, Tai CP, Wang B. Synthesis and biological evaluation of novel 4-oxo-5-cyano thiouracil derivatives as SecA inhibitors. Heterocycl Commun. 2020;26(1):76–83.10.1515/hc-2020-0100Suche in Google Scholar
[19] Lei Z, Wang J, Mao G, Wen Y, Tian Y, Wu H, et al. Glucose positions affect the phloem mobility of glucose–fipronil conjugates. J Agric Food Chem. 2014;62:6065–71.10.1021/jf5010429Suche in Google Scholar PubMed
[20] Hawash M, Eid AM, Jaradat N, Abualhasan M, Mousa A. Synthesis and biological evaluation of benzodioxole derivatives as potential anticancer and antioxidant agents. Heterocycl Commun. 2020;26(1):157–67.10.1515/hc-2020-0105Suche in Google Scholar
[21] Mao M, Li Y, Liu Q, Xiong L, Zhang X, Li Z. Synthesis and biological evaluation of novel n-pyridylpyrazole derivatives containing 1,2,3-triazole moieties. J Pestic Sci. 2015;40:138–42.10.1584/jpestics.D15-020Suche in Google Scholar
[22] Shruthi GT, Sangeetha S, Sumesh E. Design, synthesis and study of antibacterial and antitubercular activity of quinoline hydrazone hybrids. Heterocycl Commun. 2020;26(1):137–47.10.1515/hc-2020-0109Suche in Google Scholar
[23] Huang D, Zheng S, Cheng YX. Design, synthesis and biological evaluation of n-((2-phenyloxazol-4-yl)methyl) pyrimidine carboxamide derivatives as potential fungicidal agents. Heterocycl Commun. 2020;26(1):185–91.10.1515/hc-2020-0117Suche in Google Scholar
[24] Zhang JF, Xu JY, Wang BL, Li YX, Xiong LX, Li YQ, et al. Synthesis and insecticidal activities of novel anthranilic diamides containing acylthiourea and acylurea. J Agric Food Chem. 2012;60:7565–72.10.1021/jf302446cSuche in Google Scholar PubMed
[25] Mu XC, Zhang W, Wang LX, Zhang S, Zhang K, Gao CF, et al. Resistance monitoring and cross-resistance patterns of three rice planthoppers, Nilaparvata lugens, Sogatella furcifera and Laodelphax striatellus to dinotefuran in China. Pestic Biochem Physiol. 2016;5:8.10.1016/j.pestbp.2016.05.004Suche in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Structural simplification of the 3‐nitroimidazo[1,2‐a]pyridine antileishmanial pharmacophore: Design, synthesis, and antileishmanial activity of novel 2,4-disubstituted 5-nitroimidazoles
- Synthesis of a novel water-soluble pyridine dicarboxylate and its application in fluorescence cell imaging
- Synthesis of novel meta-diamide compounds containing pyrazole moiety and their insecticidal evaluation
- Review Articles
- Inorganic nanoparticles promoted synthesis of oxygen-containing heterocycles
- Gold-catalyzed synthesis of small-sized carbo- and heterocyclic compounds: A review
- Synthesis of imidazole derivatives in the last 5 years: An update
- Current progress in the synthesis of imidazoles and their derivatives via the use of green tools
- Synthetic and therapeutic review of triazoles and hybrids
Artikel in diesem Heft
- Research Articles
- Structural simplification of the 3‐nitroimidazo[1,2‐a]pyridine antileishmanial pharmacophore: Design, synthesis, and antileishmanial activity of novel 2,4-disubstituted 5-nitroimidazoles
- Synthesis of a novel water-soluble pyridine dicarboxylate and its application in fluorescence cell imaging
- Synthesis of novel meta-diamide compounds containing pyrazole moiety and their insecticidal evaluation
- Review Articles
- Inorganic nanoparticles promoted synthesis of oxygen-containing heterocycles
- Gold-catalyzed synthesis of small-sized carbo- and heterocyclic compounds: A review
- Synthesis of imidazole derivatives in the last 5 years: An update
- Current progress in the synthesis of imidazoles and their derivatives via the use of green tools
- Synthetic and therapeutic review of triazoles and hybrids


