Abstract
Imidazoles have a unique position in heterocyclic chemistry as these constitute the basic framework of several bio-molecules. Thus, increasing research is being carried out on the synthesis of imidazoles and their derivatives, mainly because of the application of imidazoles in pharmaceutical and medicinal research. Keeping sustainability in mind, researchers are developing synthetic pathways for the synthesis of imidazoles and their derivatives by employing techniques involving green tools, thus leading to sustainable pathways. In this review, we aim to compile such synthetic methodologies involving green tools for the synthesis of imidazoles. The review will cover the synthetic reactions that involve green tools such as microwave irradiation, ultrasound irradiation, and ball milling. We aim to highlight the scope and relevance of such green tools in today’s synthetic research. Through this review, we wish to contribute towards the synthesis of imidazoles that serve as a useful class of heterocyclic compounds involved in the development of pharmaceutically active molecules. We sincerely hope that this review will serve as a relevant guide for future sustainable research in the synthesis of imidazoles and their derivatives.
1 Introduction
Imidazoles are an integral part of heterocyclic chemistry and form the core structure of many biologically active compounds like histidine, biotin, and histamine [1]. Imidazoles exhibit a range of biological activities such as anti-allergic, anti-inflammatory, antitumor, antiprotozoal, antiparasitic, antibacterial, anti-ulcerative, and antidiabetic [2,3,4,5,6,7,8,9,10]. Imidazoles are thus starting materials for a range of biologically and industrially applicable molecules.
Several conventional methods of imidazole synthesis have been reported in the literature [11,12,13,14,15,16]. A few groups have implemented an inherent greener strategy for the synthesis of nitrogen-based heterocycles [17,18,19,20,21,22,23,24,25,26,27,28,29,30]. In the last few decades, green chemistry has become a popular choice in organic synthesis as environmental sustainability is the necessity of the day. Thus, synthetic chemists all around the globe are trying to develop alternative sustainable pathways to conventional modes. This has paved the way for better and more in-depth research on imidazole synthesis via green tools like microwave heating, ultrasound irradiation, ball milling, use of nonhazardous solvent, and/or ionic liquids as reaction medium to name a few. These tools usually involve high yields, low waste, short reaction times, low energy requirement, and often minimize the use of organic solvents in synthesis, thus making the protocols greener in nature.
As a part of our contribution towards the development of greener methods of heterocycle synthesis, in this review, we show the impact of these green tools for the sustainable synthesis of imidazoles and their derivatives. We produce a compilation that includes most of the recent examples of imidazole synthesis via the use of these green tools. The review comprises three subsections, namely, the synthesis of imidazoles via microwave irradiation, the synthesis of imidazoles via ultrasound irradiation, and the synthesis of imidazoles via ball milling. We create a platform that can be used as a standard piece of reference by future scientists working on sustainable methods of heterocycle synthesis.
2 Synthesis of imidazoles via microwave irradiation
Microwave irradiation is a green alternative to conventional (thermal) heating and not only accelerates the reaction, leading to high yields in short reaction times but also improves the product selectivity. Microwave heating results in mass production at a low cost and is energy-saving; it also provides a sustainable pathway. Since the last couple of decades, microwave irradiation has been used as an efficient tool for the synthesis of imidazoles [31].
In 2004, Wolkenberg and his co-workers reported the synthesis of 2,4,5-trisubstituted imidazoles (3) via the reaction between 1, 2-diketones (1) and aldehydes (2) in the presence of ammonium acetate under microwave irradiation (Scheme 1) [32]. The reactions were fast and quite high-yielding, leading to the formation of a library of substituted imidazoles.
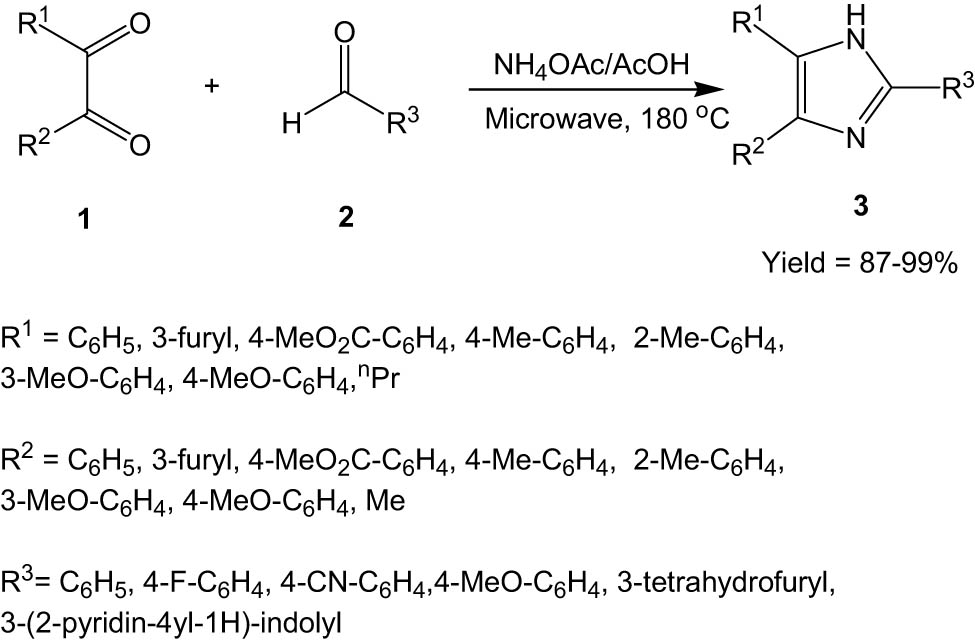
Synthesis of imidazoles from 1,2-diketones and aldehydes under microwave irradiation.
Several diversely substituted 1,2-diketones were compatible with the methodology. Aldehydes with both electron-donating and electron-withdrawing groups participated quite well in the reaction. To demonstrate the versatility and applicability of the protocol, the authors extended the methodology to synthesize Lepidiline B and Trifenagrel, both of which are biologically active imidazole-based molecules.
Kauhaluoma and his group developed a microwave-assisted methodology of disubstituted imidazole synthesis via 1,3 dipolar cycloaddition reaction between p-toluenesulfonylmethyl isocyanide (5), and imines immobilized on polymer support (4) (Scheme 2) [33]. The pure product (7) was separated from the resin support by treatment with trifluoroacetic acid. The main advantage of the method is the formation of imidazole products with high yields and purity. One shortcoming of the protocol is the presence of resin appendage (4-hydroxy-2-methoxyphenyl) as a fixed 5-substituent in the imidazole product (7).
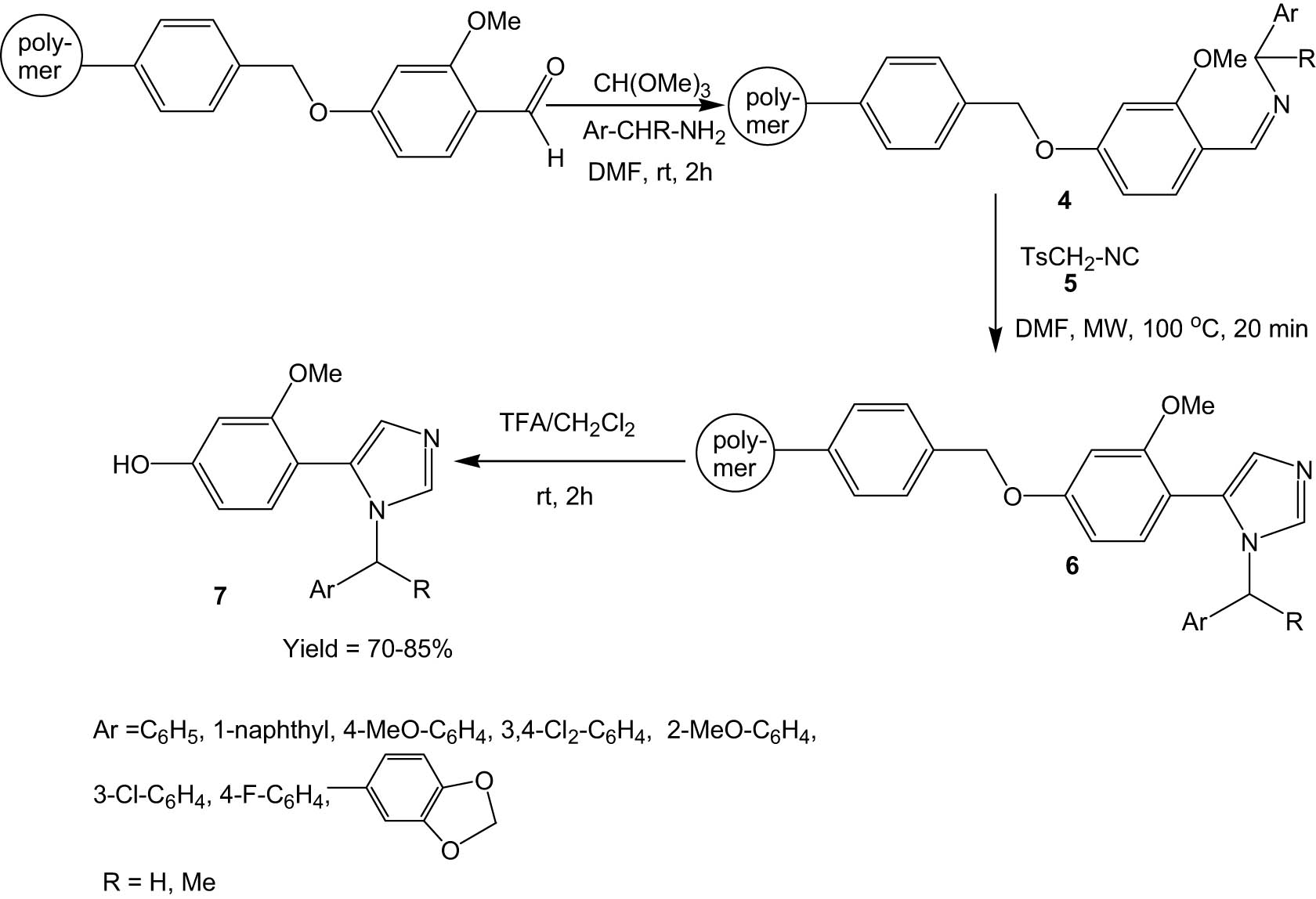
Microwave-assisted synthesis of imidazoles from p-toluenesulfonylmethyl isocyanide and imines immobilized on polymer support.
Heravi et al. synthesized trisubstituted imidazole derivatives (10) through a solvent-free microwave-assisted protocol involving the reaction between 1,2 diketones (8a) or 2-hydroxyketones (8b), aldehydes (9), and ammonium acetate supported on NaHSO4–silica (Scheme 3) [34]. The use of either silica or NaHSO4 as a solid support resulted in lower yields than silica and NaHSO4 combined.
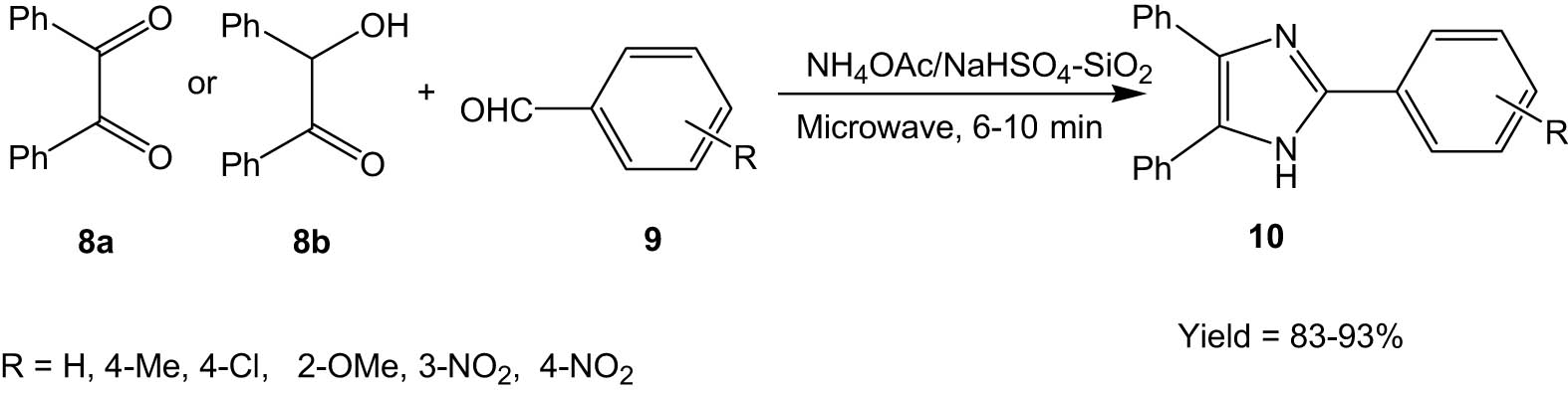
Solid-phase synthesis of imidazoles under microwave irradiation.
The reaction avoids the use of any solvent and even proceeds with a non-aqueous workup, making it truly a green methodology. The method offers an eco-friendly and economical approach to the synthesis of imidazoles in short reaction times with high yields.
A solvent-free microwave-assisted synthesis of imidazolines and imidazoles was reported by Hoz and his co-workers in 2006 [35]. The method involves the synthesis of 2-substituted imidazolines (13) via cyclization of nitriles (11) with ethylene diamines (12) in the presence of sulphur (Scheme 4). Imidazolines were oxidized to the corresponding imidazoles by oxidation with MagtrieveTM (a chromium dioxide-based oxidant) under microwave irradiation. The authors chose MagtrieveTM, as it is an environmentally benign oxidant and can be separated magnetically and reused. Five of the imidazoline and imidazole products were characterized by X-ray crystallography, which showed that the molecules contained helical chains of N–H⋯N hydrogen bonds.
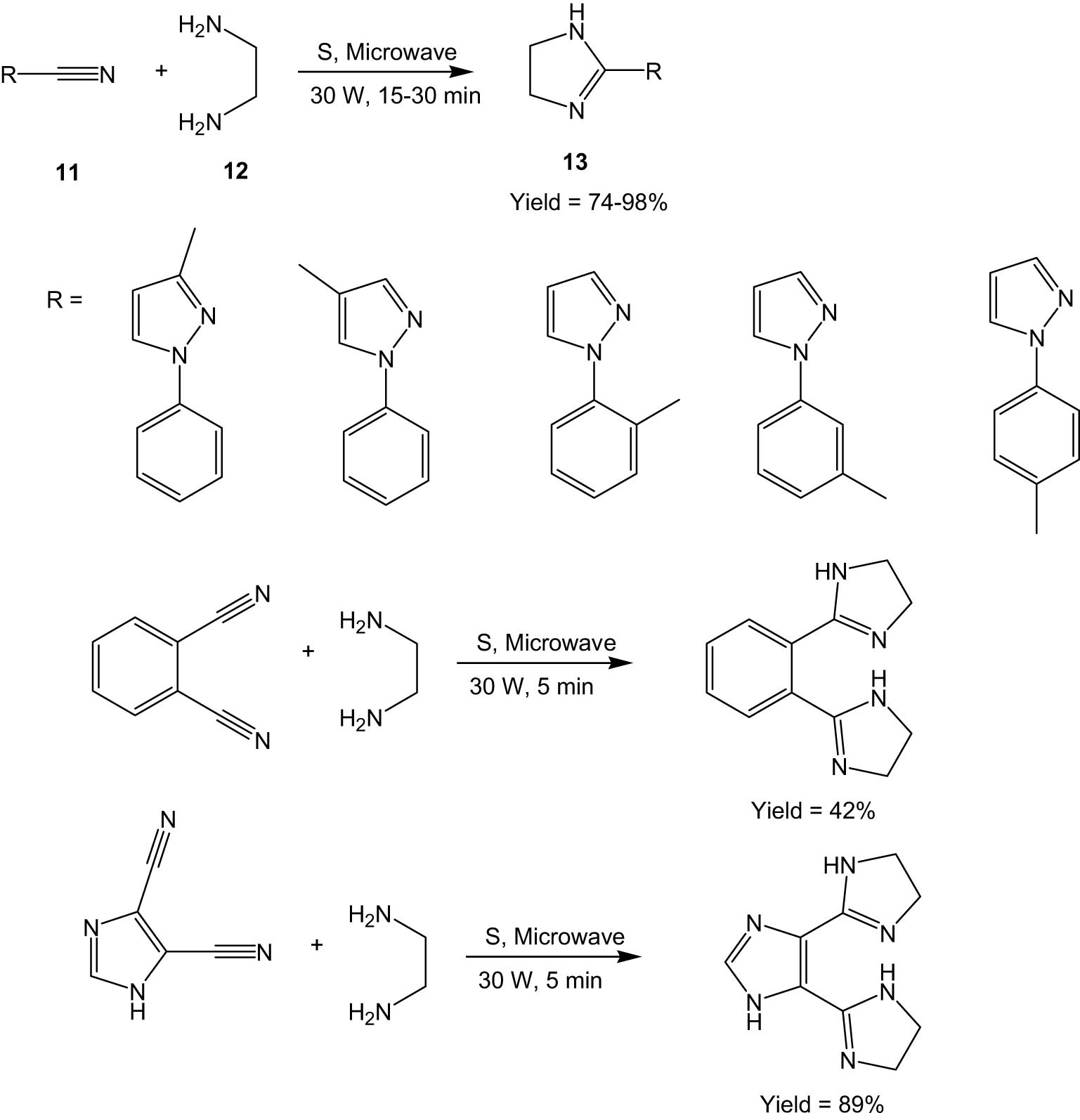
Microwave-assisted synthesis of imidazolines.
Safari and his group prepared an antimony chloride catalyst supported on silica based on a previously reported literature method (Scheme 5) [36] and used this catalyst for the synthesis of substituted imidazole derivatives under microwave irradiation without the use of any organic solvent (Scheme 6) [37]. The method involved the reaction between 1,2-diketones (16), aldehydes (17), ammonium acetate, and/or amines in the presence of SbCl3/SiO2 as a catalyst leading to the formation of substituted imidazoles (18/19). The authors also carried out the reaction under conventional heating, and it was observed that under microwave irradiation, the yields were much better in shorter reaction times than in conventional heating.
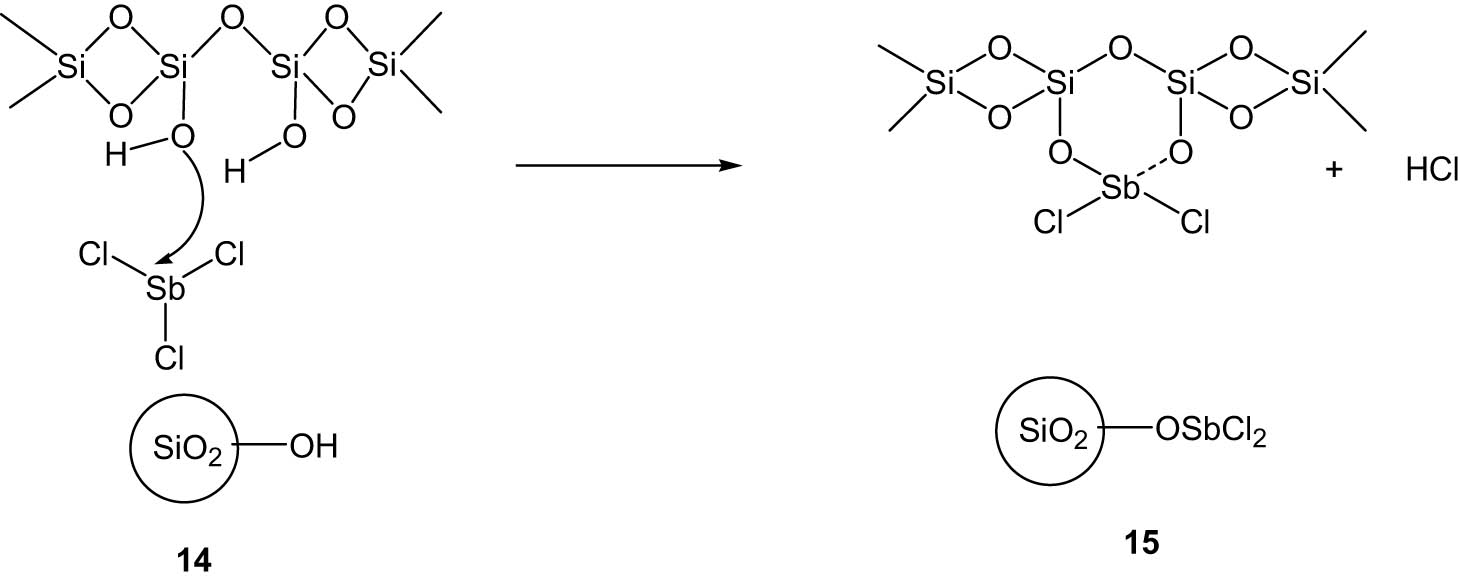
Formation of the SbCl3–SiO2 catalyst.
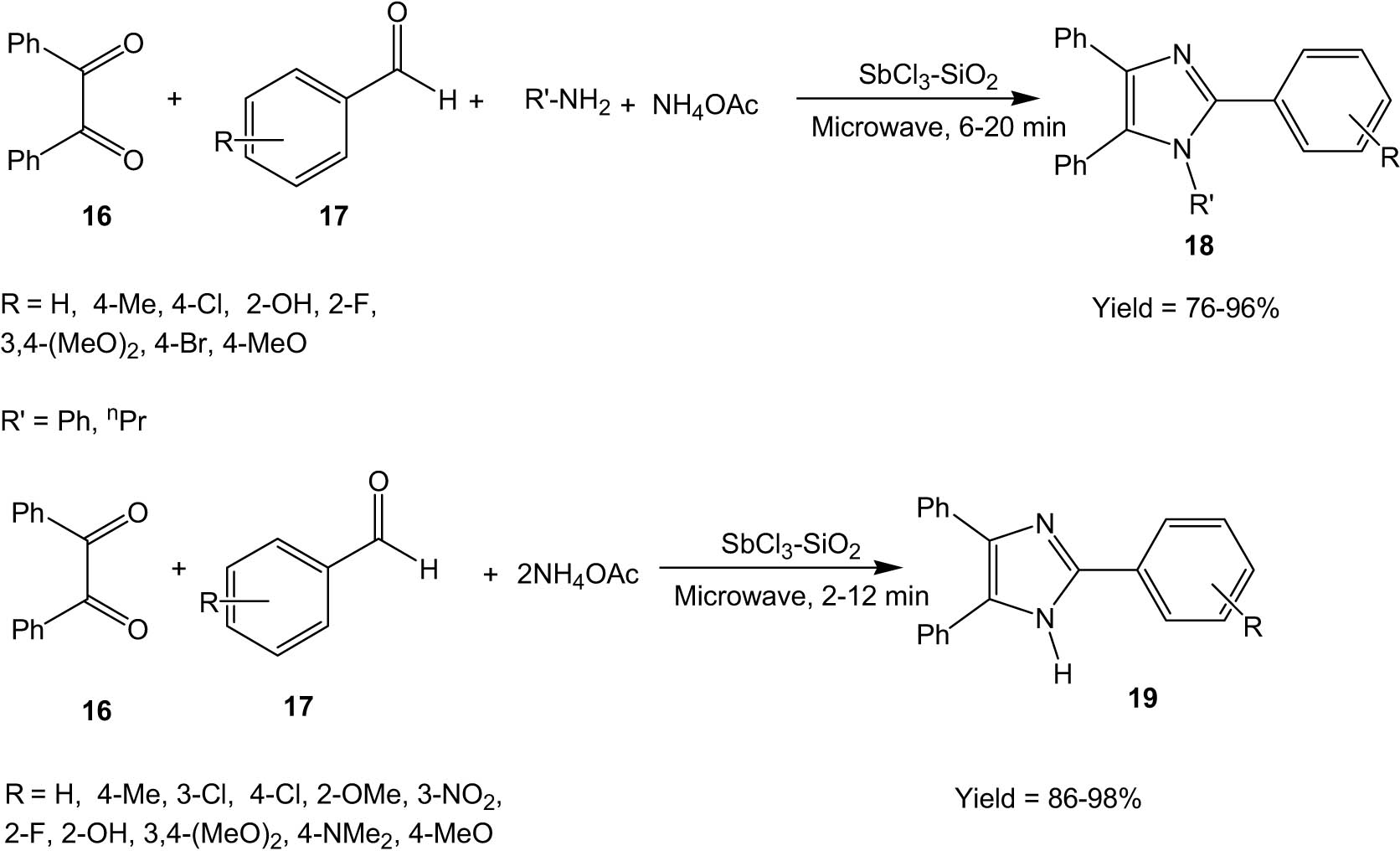
SbCl3–SiO2 catalysed synthesis of imidazoles under microwave irradiation.
SbCl3 is quite toxic, and thus the immobilization of SbCl3 on the silica support resulted in a relatively safer use of the chemical. Post reaction, the supported SbCl3 catalyst could be easily separated by filtration and reused for successive runs without appreciable loss of activity. The methodology is thus environmentally and economically sustainable.
Desai and his group developed a green microwave-assisted alternative for the synthesis of quinoline-based imidazoles that are biologically important [38]. The method involves the Perkin condensation reaction between 2-chloroquinoline-3-carbaldehyde (20), hippuric acid (21), and acetic anhydride in the presence of sodium acetate under microwave irradiation. The resultant 4-((2-chloroquinolin-3-yl)methylene)-2-phenyloxazol-5(4H)-one (22) is then treated with N-aminoarylcarboxamides (23) under microwave irradiation to produce the desired quinoline-based imidazoles (24) (Scheme 7) in significantly high yields within a few minutes.

Synthesis of quinoline-based imidazoles under microwave irradiation.
The authors compared conventional methods of heating to microwave irradiation, wherein microwave irradiation was found to be much better in terms of yield, reaction times, in addition to environmental sustainability. Five of these imidazoles showed high antimicrobial activity.
Abdel-Hameed and his co-workers reported an operationally simple, environmentally friendly method for the synthesis of imidazole derivatives via the Strecker reaction between benzoyl cyanide (27/32), aromatic aldehydes (26/31), and 2-aminoimidazole-4,5-dicarbonitrile (25)/3-amino-1,2,4-triazole (30) under microwave irradiation [39]. When 2-aminoimidazole-4,5-dicarbonitrile is used, 1H-imidazo[1,2-a]imidazole derivatives (28/29) are formed (Scheme 8) while the use of 3-amino-1,2,4-triazole resulted in imidazo[2,1-c][1,2,4]triazole derivatives (33/34) (Scheme 9). The authors also proposed a mechanism for the reaction that proceeds via the formation of a Schiffs base (Scheme 10).
![Scheme 8
Synthesis of imidazo[1,2-a]imidazole derivatives under microwave irradiation.](/document/doi/10.1515/hc-2022-0175/asset/graphic/j_hc-2022-0175_fig_008.jpg)
Synthesis of imidazo[1,2-a]imidazole derivatives under microwave irradiation.
![Scheme 9
Synthesis of imidazo[2,1-c][1,2,4]triazole derivatives under microwave irradiation.](/document/doi/10.1515/hc-2022-0175/asset/graphic/j_hc-2022-0175_fig_009.jpg)
Synthesis of imidazo[2,1-c][1,2,4]triazole derivatives under microwave irradiation.
![Scheme 10
Plausible mechanism for the synthesis of imidazo[1,2-a]imidazole derivatives and imidazo[2,1-c][1,2,4]triazole derivatives under microwave irradiation.](/document/doi/10.1515/hc-2022-0175/asset/graphic/j_hc-2022-0175_fig_010.jpg)
Plausible mechanism for the synthesis of imidazo[1,2-a]imidazole derivatives and imidazo[2,1-c][1,2,4]triazole derivatives under microwave irradiation.
In most of the previously reported methods of imidazole derivative synthesis via the Strecker reaction, a toxic cyanide source like trimethylsilyl cyanide, cyanamide, or cyanohydrin is used. Abdel-Hameed and his group eliminated the use of toxic cyanide sources, thus making the protocol environmentally benign. In addition, microwave heating led to increased reaction rates in short reaction times with high yields and low waste generation. The process is thus a sustainable alternative for the synthesis of triazole-based imidazole derivatives via the Strecker reaction.
Very recently, in 2022, Marjani and his group developed a green method of preparation of Cr2O3 nanoparticles from Zingiber officinal (Ginger) extract. The resultant Cr2O3 nanocatalyst was characterized by scanning electron microscopy (SEM), TEM, XRD, FT-IR, and VSM techniques.
The authors employed this Cr2O3 nanocatalyst for the synthesis of imidazole derivatives (43) via the reaction between aldehydes (42), benzil (41), and ammonium acetate under microwave irradiation with water as solvent (Scheme 11) [40]. The reaction proceeds in a few minutes, giving high yields of products. Differently substituted aryl aldehydes were compatible with the methodology. However, the presence of electron-withdrawing groups like nitro in the aryl aldehyde part required more time to produce the corresponding product. The nanocatalyst could be recycled for six successive runs without significant loss of activity.
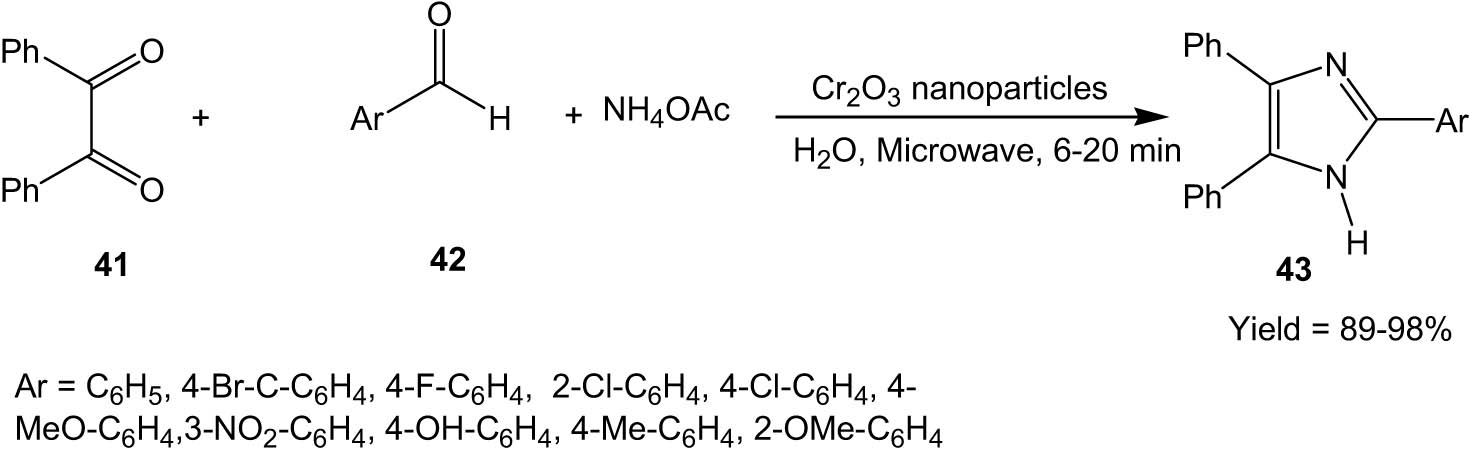
Cr2O3 nanoparticles catalysed the synthesis of imidazoles from 1,2-diketones and aldehydes under microwave irradiation.
Nagarapu and his co-workers developed an efficient protocol for the synthesis of tetrasubstituted imidazoles via the reaction of benzil/benzoin (44), benzaldehyde (45), amine (46), and ammonium acetate in the presence of potassium dodecatungstocobaltate trihydrate (K5CoW12O40·3H2O) as a catalyst (Scheme 12) [41]. The methodology could proceed both under microwave irradiation and classical heating. The reaction resulted in the formation of tetrasubstituted imidazoles in high yields under solvent-free conditions.
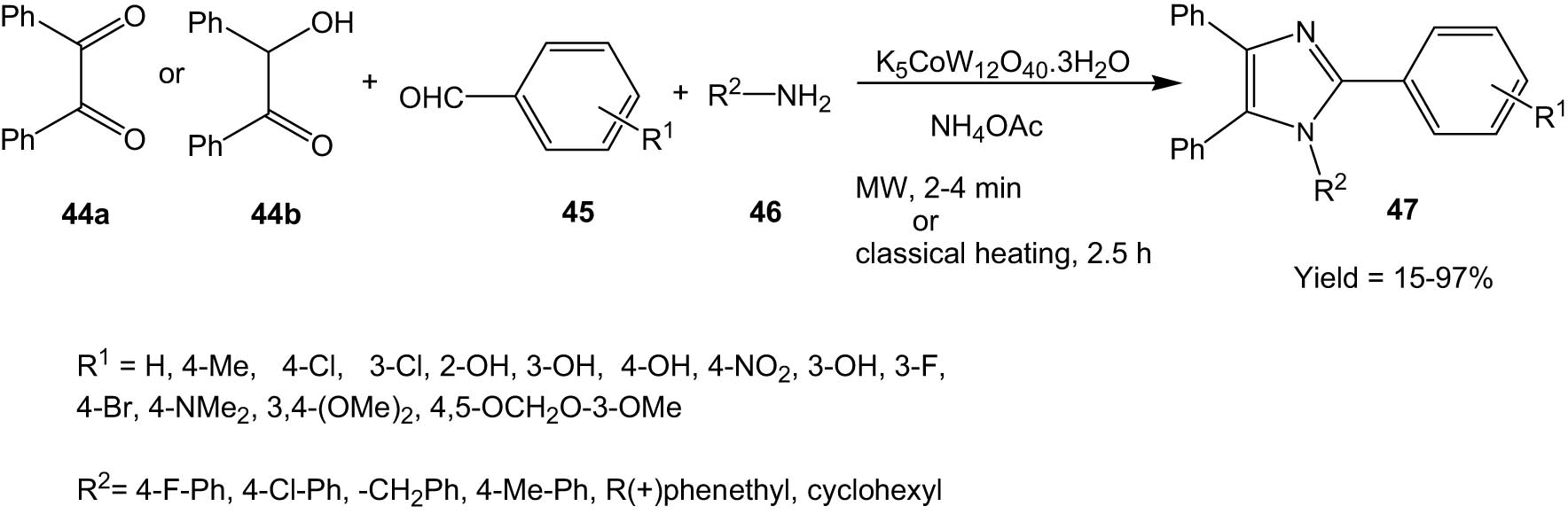
Potassium dodecatungstocobaltate trihydrate-catalysed synthesis of imidazoles under microwave irradiation.
The catalyst could be recovered and reused for six successive runs without appreciable loss in catalytic activity.
3 Synthesis of imidazoles via ultrasound irradiation
Ultrasound irradiation is a convenient method for carrying out reactions that are difficult to proceed under thermal heating. The main advantages of ultrasound irradiation are a great reduction in reaction time, operational simplicity, and increased purity of products with fewer by-products, thus minimizing waste, making workup procedures easy, and conserving energy. Ultrasound irradiation is thus a suitable alternative to conventional heating and serves as a sustainable tool in the synthesis of organic molecules [42,43,44].
In 2010, Cheng and his group employed an ionic liquid, 1-ethyl-3-methylimidazole acetate, as a catalyst for the one-pot synthesis of 2-aryl-4,5-diphenyl imidazoles (50) from the three-component reaction between benzyl (48), aromatic aldehydes (49), and ammonium acetate under ultrasonic irradiation at room temperature (Scheme 13) [45].
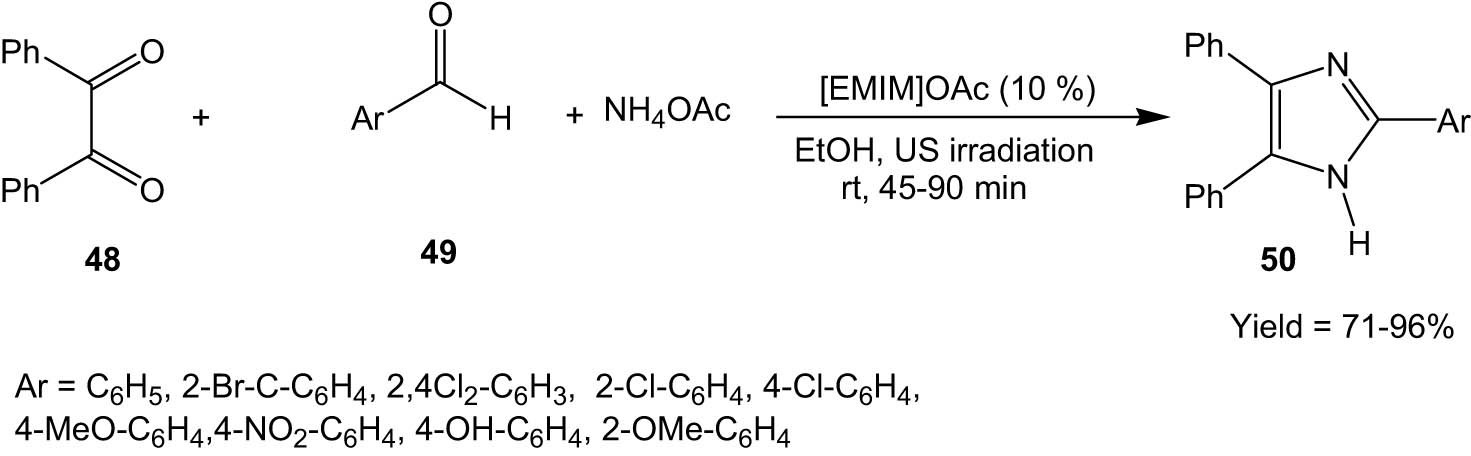
Ionic liquid-catalysed synthesis of imidazoles from 1,2-diketones and aldehydes under ultrasonic irradiation.
The ionic liquid, 1-ethyl-3-methylimidazole acetate [EMIM]OAc, was found to be crucial for the reaction to proceed. The effect of ultrasonic irradiation was also studied by comparing the reaction yields with and without ultrasonic irradiation. The reaction yields without ultrasound irradiation (with high-speed stirring) were extremely low, which showed that ultrasound irradiation was indispensable for the reaction. The authors attributed this to the phenomenon of cavitation that is generated by ultrasound, which led to the enhancement of the reaction. The protocol is an important development in sustainable synthesis as it proceeded at room temperature under mild conditions, followed by an easy workup procedure.
Safari et al. synthesized nanocrystalline magnesium aluminate spinel (MgAl2O4) and used it as a catalyst for the synthesis of tetrasubstituted imidazole derivatives (54) via multicomponent coupling of 1, 2-diketone (51), aldehyde (52), amine (53), and ammonium acetate under ultrasound irradiation. A variety of aldehydes containing either electron-donating or electron-withdrawing functionalities participated in the reaction quite well, leading to high yields of the product imidazole (Scheme 14) [46].
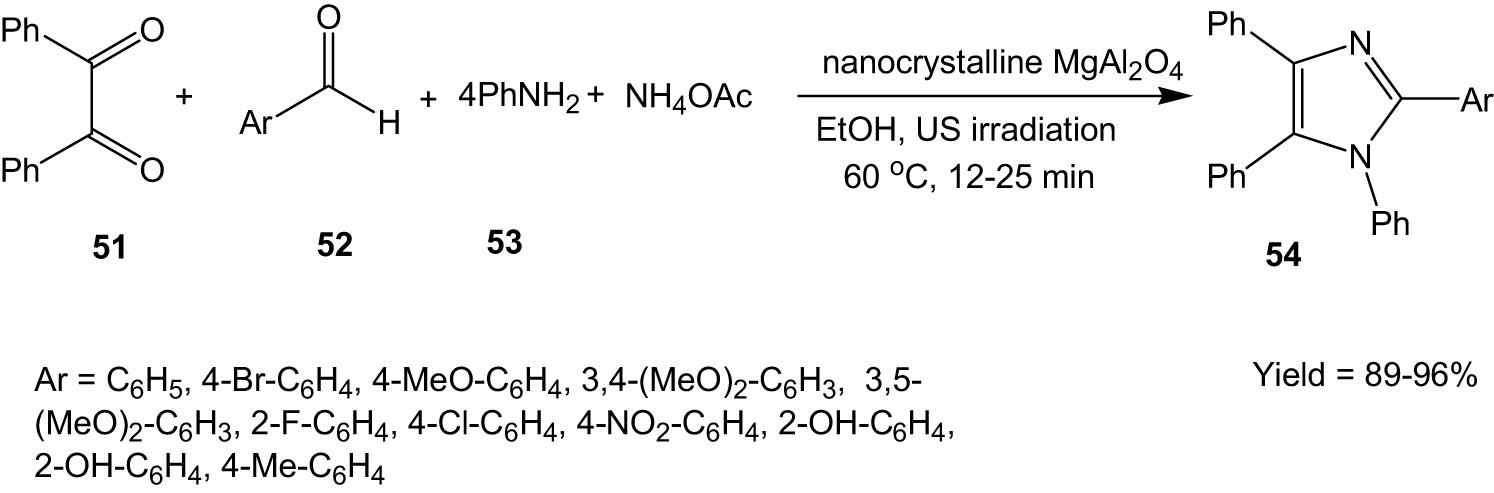
MgAl2O4-catalysed synthesis of imidazoles from 1,2-diketones and aldehydes under ultrasonic irradiation.
The authors proposed a plausible mechanism that involves the formation of a diamine intermediate followed by a nucleophilic attack of the diamine to the carbonyl of benzil. Finally, cyclization and dehydration led to the product imidazole.
In 2016, Esmaeilpour and co-workers developed dendrimer-encapsulated phosphotungstic acid nanoparticles supported on nanosilica (Dendrimer-PWAn) [47]. This nanocatalyst was characterized by X-ray diffraction, thermogravimetric analysis, TEM, SEM, DLS, FT-IR, N2 adsorption–desorption isotherm analysis, UV-vis, and elemental analysis.
In the following year, this catalyst was employed by the same group for the synthesis of imidazole derivatives (58/61) through a one-pot condensation reaction under ultrasonic irradiation (Scheme 15) [48]. The authors compared the reaction under ultrasonic irradiation with the reaction under conventional heating in solvent-free conditions and it was observed that the reaction under ultrasound irradiation gave comparable yields in shorter reaction times. A wide range of aromatic aldehydes participated in the reaction leading to high yields of the product imidazole. However, aldehydes containing electron-withdrawing groups required shorter reaction times than aldehydes with electron-donating groups. The Dendrimer PWAn catalyst could be recycled at least six times without appreciable loss in catalytic activity. Effective reusability of the catalyst made the protocol economically sustainable.
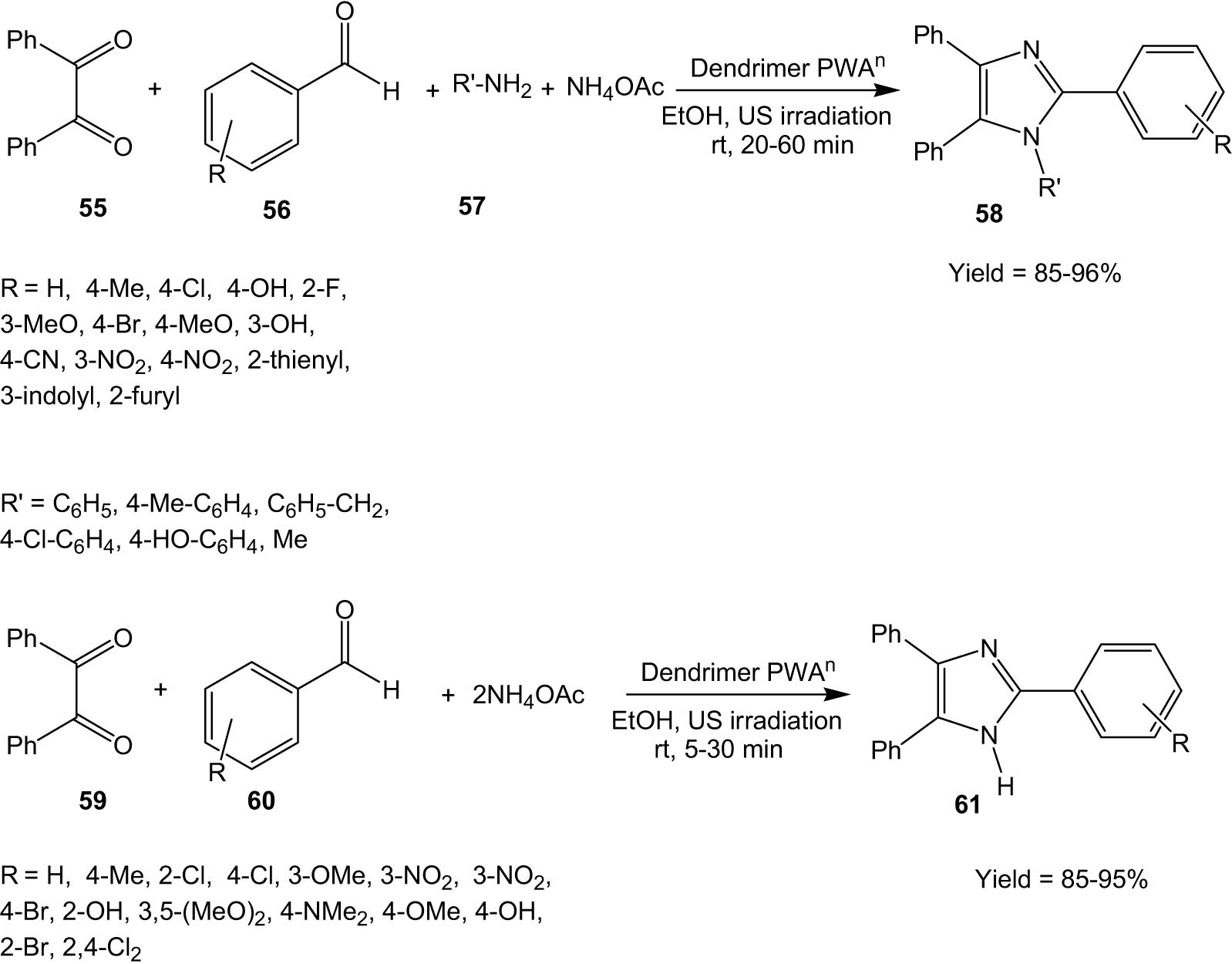
Dendrimer PWAn-catalysed synthesis of imidazoles under ultrasonic irradiation.
Sharma and his co-workers synthesized hollow magnetic spheres functionalized with sulphamic acid groups (HMS-SA catalyst) and employed this as an efficient catalyst for the synthesis of pharmaceutically important imidazole derivatives (Scheme 16) [49]. Benzaldehydes (63) were made to react with 1,2-diketones (benzil/anisil) (62) in the presence of ammonium acetate and the HMS-SA catalyst under ultrasonic irradiation to yield the corresponding imidazole derivatives (64).
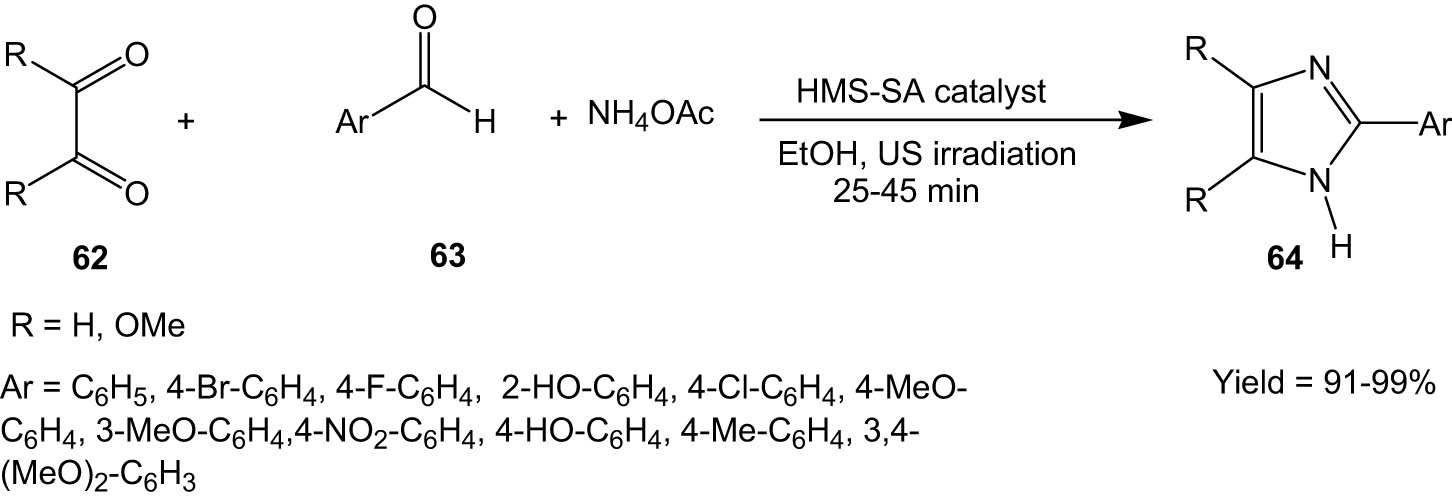
HMS-SA-catalysed synthesis of imidazoles from 1,2-diketones and aldehydes under ultrasonic irradiation.
The reaction methodology is characterized by short reaction times, clean reactions, high selectivity, low waste generation, and conservation of energy via ultrasonic irradiation. The catalyst could be recovered magnetically and reused for nine runs with negligible loss in activity, thus adding to the sustainable features of the protocol.
In 2020, Manafi and his group developed a graphene oxide-based nanocatalyst, which was characterized by FT-IR, transmission electron microscopy, SEM, and atomic force microscopy. The catalyst was used for the synthesis of benzimidazoles (67) from aldehydes (65) and 1,2-benzenediamine (66) under ultrasonic irradiation under solvent-free conditions (Scheme 17) [50]. The reaction proceeded under thermal conditions as well.
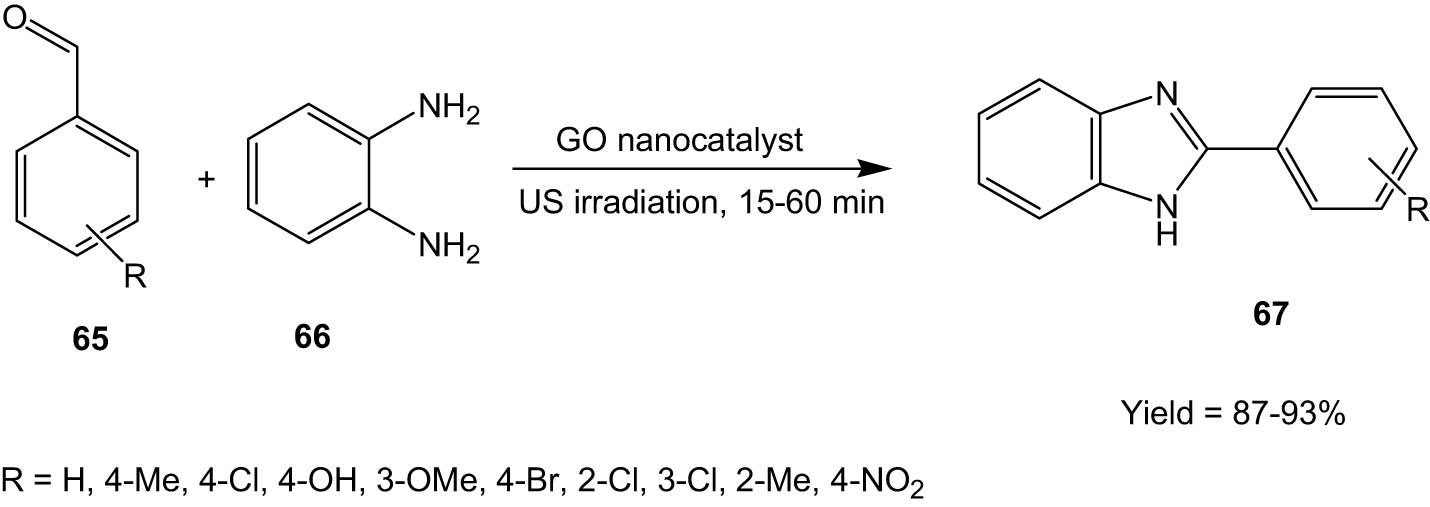
Graphene oxide nanocatalyst-catalysed synthesis of benzimidazoles aldehydes and 1,2-benzenediamine under ultrasonic irradiation.
The methodology has several advantages: short reaction times, high yields, reusability of the catalyst, and most importantly, solvent-free protocol that resulted in a reduction of environmental waste.
4 Synthesis of imidazoles via ball milling
Solvent-free synthetic organic reactions are viewed as environmentally benign pathways as these avoid the use of toxic organic solvents and are highly relevant in academia and industries in order to maintain sustainability. Ball milling serves as the most important tool among solvent-free reaction strategies. In the ball-milling method, the substrates and reagents are put inside a container containing grinding balls, which are shaken at a very high speed. The high-speed results in the formation of an amorphous mixture that promotes chemical reactions. In the last decade, ball milling has gained importance as a green tool for the synthesis of organic frameworks in an environmentally friendly manner [51,52,53,54].
In 2015, Singh, Jang, and co-workers employed ionic liquid-coated ZnO nanoparticles for solvent-free synthesis of disubstituted benzimidazoles (70) via the reaction between o-phenylenediamine (68) with aromatic aldehydes (69) using the ball-milling technique (Scheme 18) [55].
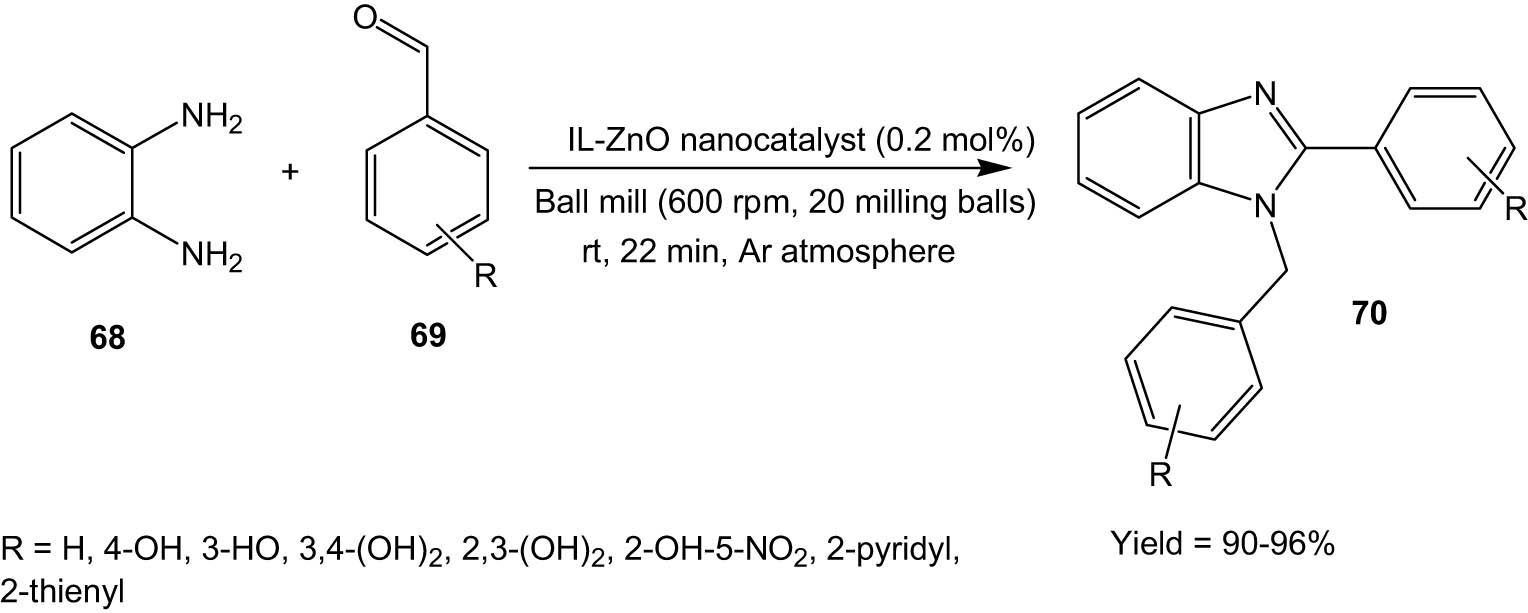
IL-ZnO nanocatalyst-catalysed synthesis of benzimidazoles under ball milling.
The methodology showed high yields of product, high turnover number, and high FT-IR frequency of the catalyst. The ball-milling technique helped avoid the use of toxic solvents, thus making the protocol environmentally benign. The reaction could also be reproduced at a multi-gram scale, which is an advantage for application in industries. The catalyst could be recycled six times without significant loss in catalytic activity.
Hagar and his group developed a ball-milling method for the synthesis of benzimidazoles (73) from the reaction between o-phenylene diamine (71) and organic electrophilic substrates (72) (Scheme 19) [56]. The reaction proceeded smoothly without any solvent or catalyst, thereby promoting sustainability. Several substituted aldehydes and carboxylic acids participated in the reaction to produce the corresponding benzimidazole derivatives in high yields.
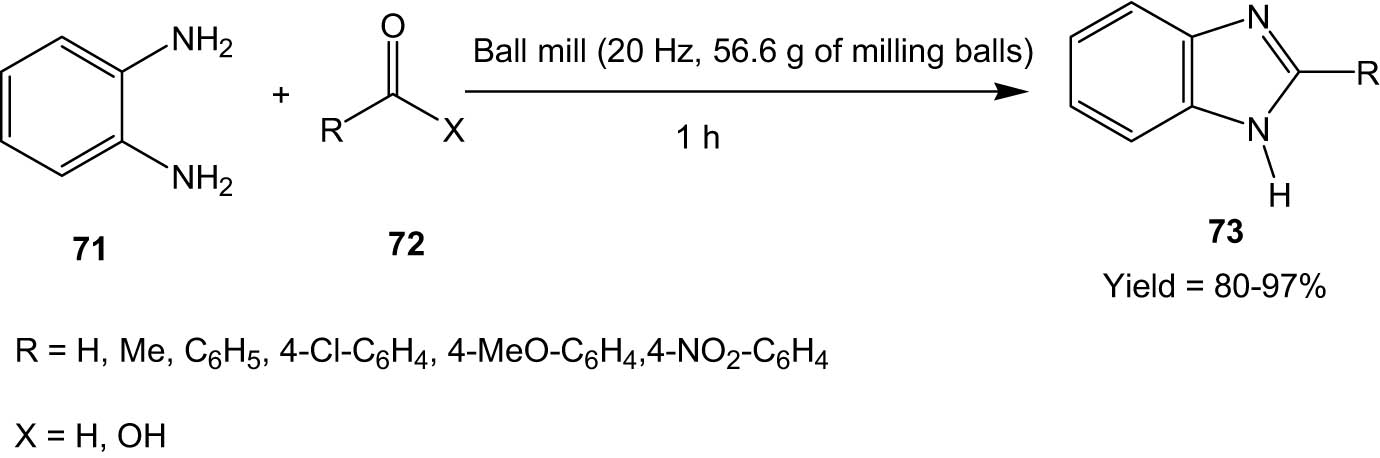
Solvent and catalyst-free synthesis of benzimidazoles under ball milling.
The authors also extended the protocol to reactions between o-phenylene diamine and urea and thiourea to produce benzimidazol-2-one and benzimidazol-2-thione, respectively. Benzimidazol-2-thione is also produced when o-phenylene diamine is made to react with ammonium thiocyanate under identical ball-milling conditions. The reaction protocol is thus quite versatile, leading to a range of important organic moieties.
Mal and his group used IBX (2-iodoxybenzoic acid) as an oxidant for the conversion of substituted benzyl alcohols (74) into the corresponding benzaldehydes (75), which then reacted with o-phenylene diamine (76) under ball milling to produce the corresponding benzimidazole derivatives (77) (Scheme 20) [57]. Benzyl alcohols with alkyl and halide substitutions participated in the reaction to produce the corresponding substituted benzimidazoles in reasonably high yields. The method involves solvent and catalyst-free one-pot synthesis of benzimidazoles, which makes the protocol environment-friendly.
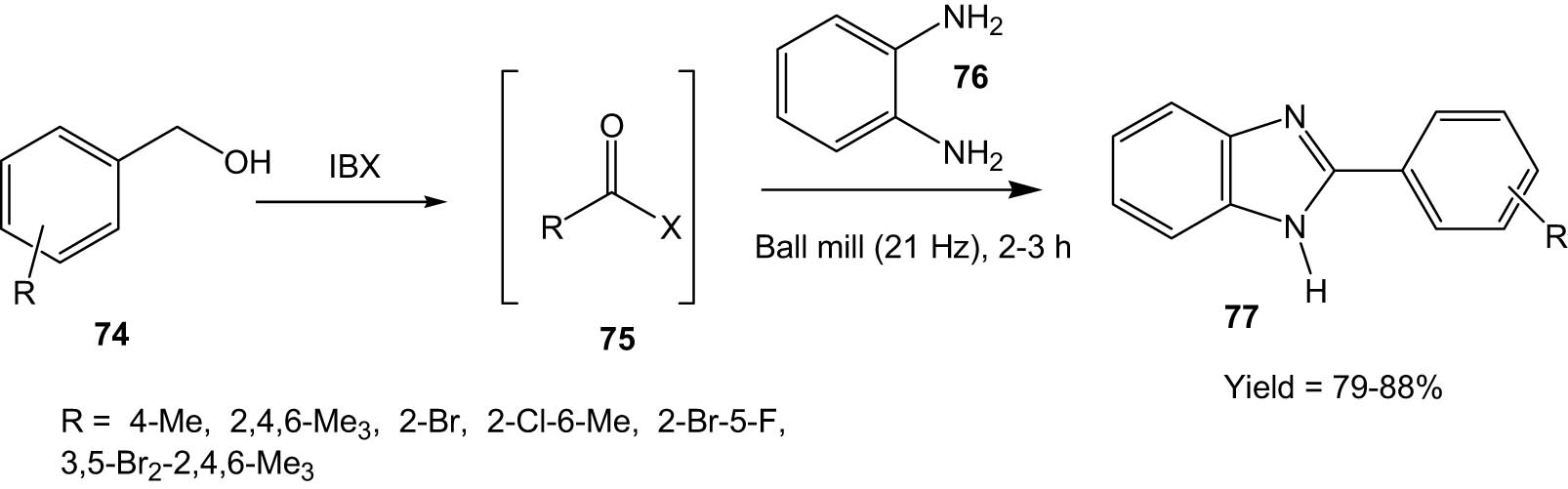
One-pot synthesis of benzimidazoles from benzyl alcohols and o-phenylene diamine under ball milling.
5 Conclusions
Imidazole and its derivatives are important components in academic research laboratories and industries. Imidazoles constitute an important part of heterocyclic chemistry, and thus, development in the field of imidazole synthesis leads to a growth in heterocycle-based synthetic chemistry. In this review, we attempted to highlight the use and relevance of green tools like microwave irradiation, ultrasound irradiation, and ball milling for the synthesis of various imidazoles, benzimidazoles, and their derivatives.
Although the implementation of green tools in organic synthesis has a number of advantages, like low energy consumption, higher yields in shorter reaction time, and more sustainable reactions employing tandem one-pot and solvent-free processes, there are a few disadvantages as well. The major challenges associated with microwave- and ultrasound-irradiated reactions are the low chance of time-dependent monitoring and difficulty in scale-up reactions. Ball milling has its disadvantages of being very noisy and also poses contamination risks due to the occurrence of wear from the balls. All the green tool implemented protocols are expensive in nature overall, as a separate apparatus is required, which is yet another challenge. However, since green tools improve the sustainability of organic synthesis to a great extent, it should be the aim of future research scientists to eliminate or minimize the challenges associated with them.
Research is a dynamic process, and as such, research on sustainable methods of imidazole synthesis is constantly being updated. We tried to compile a few recent methodologies based on green tool-implemented imidazole synthesis, and we earnestly hope that this compilation will be useful for future research chemists who wish to work in the field of sustainable heterocycle synthesis.
-
Funding information: Authors state no funding involved.
-
Author contributions: Both D.S. and C. M. have contributed in writing and reviewing the manuscript. Both the authors have read and approved the final manuscript.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
References
[1] Laufer SA, Zimmermann W, Ruff KJ. Tetrasubstituted imidazole inhibitors of cytokine release: probing substituents in the N-1 position. J Med Chem. 2004 Nov;47(25):6311–25.10.1021/jm0496584Search in Google Scholar PubMed
[2] Misono M. Unique acid catalysis of heteropoly compounds (heteropolyoxometalates) in the solid state. Chem Commun. 2001 Jun;1141–52.10.1039/b102573mSearch in Google Scholar
[3] Black JW, Durant GJ, Emmett JC, Ganellin CR. Sulphurmethyleneisosterism in the development of metiamide, a new histamine H2-receptor antagonist. Nature. 1974 Mar;248:65–7.10.1038/248065a0Search in Google Scholar PubMed
[4] Ucucu U, Karaburun NG, Iskdag I. Synthesis and analgesic activity of some 1-benzyl-2-substituted-4,5-diphenyl-1H-imidazole derivatives. Farmaco. 2001 Apr;56(4):285–90.10.1016/S0014-827X(01)01076-XSearch in Google Scholar PubMed
[5] Katritzky AR, Scriven EFV, editors. Comprehensive Heterocyclic Chemistry II. Vol. 3. Oxford: Pergamon; 1996.Search in Google Scholar
[6] Sennequier N, Wolan D, Stuehr D. Antifungal imidazoles block assembly of inducible no synthase into an active dimer. J Biol Chem. 1999 Jan;274(2):930–8.10.1074/jbc.274.2.930Search in Google Scholar PubMed
[7] Brogden RN, Heel RC, Speight TM, Avery GS. Metronidazole in anaerobic infections: a review of its activity, pharmacokinetics and therapeutic use. Drugs. 1978 Nov;16(5):387–417.10.2165/00003495-197816050-00002Search in Google Scholar PubMed
[8] Niwano Y, Seo A, Kanai K, Hamaguchi H, Uchida K, Yamaguchi H. Therapeutic efficacy of lanoconazole, a new imidazole antimycotic agent, for experimental cutaneous candidiasis in guinea pigs. Antimicrob Agents Chemother. 1994 Sep;38(9):2204–6.10.1128/AAC.38.9.2204Search in Google Scholar PubMed PubMed Central
[9] Botta M, Corelli F, Gasparrini F, Messina F, Mugnaini C. Chiral Azole Derivatives. 4. Enantiomers of bifonazole and related antifungal agents: synthesis, configuration assignment, and biological evaluation. J Org Chem. 2000 Jun;65(15):4736–9.10.1021/jo991937pSearch in Google Scholar PubMed
[10] Jain R, Vangapandu S, Jain M, Kaur N, Singh S, Singh PP. Antimalarial activities of ring-substituted bioimidazoles. Bioorg Med Chem Lett. 2002 Jul;12(13):1701–4.10.1016/S0960-894X(02)00289-5Search in Google Scholar
[11] Antherden LM. Bentley and driver’s. Textbook of Pharmaceutical Chemistry. 8th edn. London: Oxford University Press; 2007.Search in Google Scholar
[12] Wang Z, editor. Comprehensive organic name reactions and reagents. New York: John Wiley and Sons, Inc; 2009.10.1002/9780470638859Search in Google Scholar
[13] Matsuda K, Yanagisawa I, Isomura Y, Mase T, Shibanuma T. One pot preparation of 1-substituted imidazole-2-thione from isothiocyanate and amino acetal. Synth Commun. 1997 Mar;27(20):3565–71.10.1080/00397919708007078Search in Google Scholar
[14] Shaabani A, Maleki A, Behnam M. Tandem oxidation process using ceric ammonium nitrate: three-component synthesis of trisubstituted imidazoles under aerobic oxidation conditions. Synth Commun. 2008 Jun;39(1):102–10.10.1080/00397910802369661Search in Google Scholar
[15] Sheth AH, Prajapati PM, Shah YR, Sen DJ. Synthesis of bispyrimido imidazole fused ring heterocyclic adduct with urea of mannich base for CNS depression. Int J Drug Dev Res. 2009 Sep–Dec;1(1):75–80.Search in Google Scholar
[16] Molina P, López-Leonardo C, Llamas-Botía J, Foces-Foces C, Llamas-Saiz AL. Preparation of Highly Functionalized Imidazoles from α-Azidoacetonitrile: X-ray Crystal Structure of 4-Methoxy-2-methylamino-5-methylthiocarbamoylimidazole. Synthesis. 1995;4:449–52.10.1055/s-1995-3925Search in Google Scholar
[17] Padilla-Martínez II, Cruz A, García-Báez EV, Rosales-Hernández MC, Wejebe JEM. N-substitution reactions of 2-aminobenzimidazoles to access pharmacophores. Curr Org Synth. 2023;20(2):177–219.10.2174/1570179419666220310124223Search in Google Scholar PubMed
[18] Mueller Jr LG, Keller TM, Fleming FF. One-pot syntheses of substituted oxazoles and imidazoles from the isocyanide asmic. J Org Chem. 2023 Jan;88(2):909–16.10.1021/acs.joc.2c02290Search in Google Scholar PubMed
[19] Chinta B, Satyadev TNVSS, Adilakshmi GV. Zn(OAc)2•2H2O-catalyzed one-pot synthesis of divergently substituted imidazoles. Curr Chem Lett. 2023;12(1):175–84.10.5267/j.ccl.2022.8.007Search in Google Scholar
[20] Frantz DE, Morency L, Soheili A, Murry JA, Grabowski EJJ, Tillyer RD. Synthesis of Substituted Imidazoles via Organocatalysis. Org Lett. 2004 Feb;6(5):843–6.10.1021/ol0498803Search in Google Scholar PubMed
[21] Aziizi N, Manochehri Z, Nahayi A, Torkashvand S. A facile one-pot synthesis of tetrasubstituted imidazoles catalyzed by eutectic mixture stabilized ferrofluid. J Mol Liq. 2014;196:153–8.10.1016/j.molliq.2014.03.013Search in Google Scholar
[22] Das B, Bhunia N, Lingaiah MA. Simple and efficient metal-free synthesis of tetrasubstituted pyrroles byiodine-catalyzed four-component coupling reaction of aldehydes, amines,dialkyl acetylenedicarboxylates, and nitromethane. Synthesis. 2011;21:3471–74.10.1055/s-0030-1260228Search in Google Scholar
[23] Marama L, Tanaka F. Mannich reactions of carbohydrate derivatives with ketones to afford polyoxy-functionalized piperidines. Org Lett. 2019 Jan;21(4):1165–9.10.1021/acs.orglett.9b00105Search in Google Scholar PubMed
[24] Marama L, Tanaka F. Switching Electrophile intermediates to nucleophiles: Michael and Oxa-Diels-Alder reactions to afford polyoxy-functionalized piperidine derivatives with tetrasubstituted carbon. Org Lett. 2020 Mar;22(7):2751–5.10.1021/acs.orglett.0c00735Search in Google Scholar PubMed
[25] Wang X, DeFilippis RA, Weldemichael T, Gunaganti N, Tran P, Leung YK, et al. An imidazo[1,2-a]pyridine-pyridine derivative potently inhibits FLT3-ITD and FLT3-ITD secondary mutants, including gilteritinib-resistant FLT3-ITD/F691L. Eur J Med Chem. 2024 Jan;264:115977.10.1016/j.ejmech.2023.115977Search in Google Scholar PubMed
[26] Azizi N, Dado N, Amiri AK. Highly efficient one-pot synthesis of trisubstituted imidazoles under catalyst-free conditions. Can J Chem. 2012;90:195–8.10.1139/v11-141Search in Google Scholar
[27] Little TL, Webber SE. A simple and practical synthesis of 2-aminoimidazoles. J Org Chem. 1994 Dec;59(24):7299–305.10.1021/jo00103a021Search in Google Scholar
[28] Murthy SN, Madhav B, Nageswar YVD. DABCO as a mild and efficient catalytic system for the synthesis of highly substituted imidazoles via multicomponent condensation strategy. Tetrahedron Lett. 2010 Oct;51(40):5252–7.10.1016/j.tetlet.2010.07.128Search in Google Scholar
[29] Patel G, Dewangan DK, Bhakat N, Banerjee S. Green approaches for the synthesis of poly-functionalized imidazole derivatives: A comprehensive review. Curr Res in Green and Sustain Chem. 2021;4:100175.10.1016/j.crgsc.2021.100175Search in Google Scholar
[30] Yu X-L, Fan YH, Zheng XN, Gao JF, Zhuang LG, Yu YL, Xi JH, Zhang DW. , Synthesis of imidazole-based molecules under ultrasonic irradiation approaches. Molecules. 2023 Jun;28(12):4845.10.3390/molecules28124845Search in Google Scholar PubMed PubMed Central
[31] Gupta GK, Rani N, Kumar V. Microwave assisted synthesis of imidazoles – a review. Mini Rev Org Chem. 2012;9(3):270–84.10.2174/1570193X11209030270Search in Google Scholar
[32] Wolkenberg SE, Wisnoski DD, Leister WH, Wang Y, Zhao Z, Lindsley CW. Efficient synthesis of imidazoles from aldehydes and 1,2-diketones using microwave irradiation. Org Lett. 2004 Mar;6(9):1453–6.10.1021/ol049682bSearch in Google Scholar PubMed
[33] Samanta SK, Kylanlahti I, Kauhaluoma JY. Microwave-assisted synthesis of imidazoles: Reaction of p-toluenesulfonylmethyl isocyanide and polymer-bound imines. Bioorg Med Chem Lett. 2005 Jun;15(16):3717–9.10.1016/j.bmcl.2005.05.066Search in Google Scholar PubMed
[34] Oskooie HA, Alimohammadi Z, Heravi MM. Microwave-assisted solid-phase synthesis of 2,4,5-triaryl imidazoles in solventless system: an improved protocol. Heteroat Chem. 2006 Oct;17(7):699–702.10.1002/hc.20237Search in Google Scholar
[35] Hoz ADL, Ortiz AD, Mateo MDC, Moral M, Moreno A, Elguero J, et al. Microwave assisted synthesis and crystal structures of 2-imidazolines and imidazoles. Tetrahedron. 2006 Jun;62(25):5868–74.10.1016/j.tet.2006.04.020Search in Google Scholar
[36] Darabi HR, Aghapoor K, Mohsenzadeh F, Taala F, Asadol-lahnejad N, Badiei A. Silica-supported antimony(III) chloride as highly effective and reusable heterogeneous catalyst for the synthesis of quinoxalines. Catal Lett. 2009 Sep;133(1–2):84–9.10.1007/s10562-009-0161-2Search in Google Scholar
[37] Safari J, Naseh S, Zarnegar Z, Akbar Z. Applications of microwave technology to rapid synthesis of substituted imidazoles on silica-supported SbCl3 as an efficient heterogeneous catalyst. J Taibah Univ Sci. 2014 Apr;8(4):323–30.10.1016/j.jtusci.2014.01.007Search in Google Scholar
[38] Desai NC, Maheta AS, Rajpara KM, Joshi VV, Vaghani HV, Satodiya HM. Green synthesis of novel quinoline based imidazole derivatives and evaluation of their antimicrobial activity. J Saudi Chem Soc. 2014 Dec;18(6):963–71.10.1016/j.jscs.2011.11.021Search in Google Scholar
[39] Sadek KU, Abdel-Hameed AM, Abdelnabi HA, Meleigy Y. An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating. Green Process Synth. 2019;8:297–301.10.1515/gps-2018-0093Search in Google Scholar
[40] Kafi-Ahmadi L, Khademinia S, Marjani AP, Nozad E. Microwave-assisted preparation of polysubstitutedimidazoles using Zingiber extract synthesized green Cr2O3 nanoparticles. Sci Rep. 2022 Nov;12:19942.10.1038/s41598-022-24364-6Search in Google Scholar PubMed PubMed Central
[41] Nagarapu L, Apuri S, Kantevari S. Potassium dodecatugstocobaltate trihydrate (K5CoW12O40·3H2O):A mild and efficient reusable catalyst for the one-pot synthesis of1,2,4,5-tetrasubstituted imidazoles under conventional heating and microwave irradiation. J Mol Catal A Chem. 2007 Apr;266:(1–2):104–8.10.1016/j.molcata.2006.10.056Search in Google Scholar
[42] Mason TJ, Peters D. Practical sonochemistry. 2nd edn. London: Ellis Horwood; 2002.10.1533/9781782420620Search in Google Scholar
[43] Zang H, Wang M, Cheng BW, Song J. Ultrasound-promoted synthesis of oximes catalyzed by a basic ionic liquid [bmIm]OH. Ultrason Sonochem. 2009 Mar;16(3):301–3.10.1016/j.ultsonch.2008.09.003Search in Google Scholar PubMed
[44] Khanvilkar V, Dasgupta S. Recent advances in ultrasound assisted synthesis of 2,4,5-trisubstituted imidazoles: A comparative study of effective catalytic systems. Int J Curr Sci Res Rev. 2022 Nov;5(11):4237–46.10.47191/ijcsrr/V5-i11-22Search in Google Scholar
[45] Zang H, Su Q, Mo Y, Cheng BW, Jun S. Ionic liquid [EMIM]OAc under ultrasonic irradiation towards the first synthesis of trisubstituted imidazoles. Ultrason Sonochem. 2010 Jun;18(5):749–51.10.1016/j.ultsonch.2010.01.015Search in Google Scholar PubMed
[46] Safari J, Gandomi-Ravandi S, Akbari Z. Sonochemical synthesis of 1,2,4,5-tetrasubstituted imidazoles using nanocrystalline MgAl2O4 as an effective catalyst. J Adv Res. 2013 Nov;4(6):509–14.10.1016/j.jare.2012.09.001Search in Google Scholar PubMed PubMed Central
[47] Esmaeilpour M, Javidi J, Dehghani F. Preparation, characterization and catalytic activity of dendrimer-encapsulated phosphotungstic acid nanoparticles immobilized on nanosilica for the synthesis of 2H-indazolo[2,1-b]phthalazine-triones under solvent-free or sonochemical conditions. J Iran Chem Soc. 2016 Apr;13(4):695–714.10.1007/s13738-015-0782-xSearch in Google Scholar
[48] Esmaeilpour M, Javidi J, Dehghani F, Saeed Z. One-pot synthesis of multisubstitutedimidazolescatalyzed by Dendrimer-PWAn nanoparticles under solvent-free conditions and ultrasonic cirradiation. Res Chem Intermed. 2017 Jan;43(1):163–85.10.1007/s11164-016-2613-9Search in Google Scholar
[49] Arora G, Gupta R, Yadav P, Dixit R, Srivastava A, Sharma RK. Ultrasonically-mediated one-pot synthesis of substituted imidazoles via sulfamic acid functionalized hollow magnetically retrievable solid-acid catalyst. Curr Res Green Sustain Chem. 2021;4:100050–7.10.1016/j.crgsc.2020.100050Search in Google Scholar
[50] Karami AY, Manafi M, Ghodrati K, Khajavi R, Hojjati M. Nanoparticles supported graphene oxide and its application as an efficient and recyclable nanocatalyst in the synthesis of imidazole derivatives in ultrasound solvent‑free condition. Int Nano Lett. 2020 Jun;10(2):89–95.10.1007/s40089-020-00297-8Search in Google Scholar
[51] Stolle A, Szuppa T, Leonhardt SES, Ondruschka B. Ball milling in organic synthesis: solutions and challenges. Chem Soc Rev. 2011 Mar;40:2317–29.10.1039/c0cs00195cSearch in Google Scholar PubMed
[52] Ferguson M, Giri N, Huang X, Apperley D, James SL. One-pot two-step mechanochemical synthesis: ligand and complex preparation without isolating intermediates. Green Chem. 2014 Dec;16:1374–82.10.1039/C3GC42141DSearch in Google Scholar
[53] Cinčić D, Brekalo I, Kaitner B. Effect of atmosphere on solid-state amine–aldehyde condensations: gas-phase catalysts for solid-state transformations. Chem Commun. 2012 Oct;48:11683–5.10.1039/c2cc36357gSearch in Google Scholar PubMed
[54] Rothenberg G, Downie AP, Raston CL, Scott JL. Understanding Solid/Solid Organic Reactions. J Am Chem Soc. 2001 Aug;123(36):8701–8.10.1021/ja0034388Search in Google Scholar PubMed
[55] Sharma H, Kaur N, Singh N, Jang DO. Synergetic catalytic effect of ionic liquids and ZnO nanoparticles on the selective synthesis of 1,2-disubstituted benzimidazoles using a ball-milling technique. Green Chem. 2015 Jun;17:4263–70.10.1039/C5GC00536ASearch in Google Scholar
[56] EL-Sayed TH, Aboelnaga A, Hagar M. Ball milling assisted solvent and catalyst free synthesis of benzimidazoles and their derivatives. Molecules. 2016 Sep;21(9):1111–8.10.3390/molecules21091111Search in Google Scholar PubMed PubMed Central
[57] Achar TK, Maiti S, Mal P. IBX works efficiently under solvent free conditions in ball milling. RSC Adv. 2014 Feb;4:12834–9.10.1039/C4RA00415ASearch in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Structural simplification of the 3‐nitroimidazo[1,2‐a]pyridine antileishmanial pharmacophore: Design, synthesis, and antileishmanial activity of novel 2,4-disubstituted 5-nitroimidazoles
- Synthesis of a novel water-soluble pyridine dicarboxylate and its application in fluorescence cell imaging
- Synthesis of novel meta-diamide compounds containing pyrazole moiety and their insecticidal evaluation
- Review Articles
- Inorganic nanoparticles promoted synthesis of oxygen-containing heterocycles
- Gold-catalyzed synthesis of small-sized carbo- and heterocyclic compounds: A review
- Synthesis of imidazole derivatives in the last 5 years: An update
- Current progress in the synthesis of imidazoles and their derivatives via the use of green tools
- Synthetic and therapeutic review of triazoles and hybrids
Articles in the same Issue
- Research Articles
- Structural simplification of the 3‐nitroimidazo[1,2‐a]pyridine antileishmanial pharmacophore: Design, synthesis, and antileishmanial activity of novel 2,4-disubstituted 5-nitroimidazoles
- Synthesis of a novel water-soluble pyridine dicarboxylate and its application in fluorescence cell imaging
- Synthesis of novel meta-diamide compounds containing pyrazole moiety and their insecticidal evaluation
- Review Articles
- Inorganic nanoparticles promoted synthesis of oxygen-containing heterocycles
- Gold-catalyzed synthesis of small-sized carbo- and heterocyclic compounds: A review
- Synthesis of imidazole derivatives in the last 5 years: An update
- Current progress in the synthesis of imidazoles and their derivatives via the use of green tools
- Synthetic and therapeutic review of triazoles and hybrids

