Abstract
Since many of the U.S. Food and Drug Administration (FDA)-approved medications contain oxygen-containing heterocyclic molecules, they have been discovered to be quite important. Moreover, over the past 10 years, the field of reusable nanocatalysts has expanded quickly. Therefore, the development of nanotechnology has led to a wide range of applications for nanocatalysis in the synthesis of heterocyclic molecules. The domains of organic chemistry and pharmaceuticals have recently shown a great deal of interest in nanocatalyzed organic processes. Such nanocatalysts enable non-toxic, simpler, environmentally friendly, and more affordable synthetic processes that give only the most desirable compounds in higher quantities and provide simple catalyst separation. As a result of their efficient methods for separating catalysts and products, nanocatalysts were chosen over other catalysts for the synthesis of heterocyclic compounds. This review emphasized the preparation of nanocatalysts, synthetic approaches, and recycling studies of highly excited catalytic systems employed for the synthesis of oxygen-containing heterocyclic compounds.
1 Introduction
In this modern day, the need to provide environmentally acceptable methods for the synthesis and use of various types of catalysts is growing to prevent pollution [1]. Magnetic material-based nanocatalysts can be readily recovered from obtained products and reused. Therefore, all of the functionalized organic materials grafted to magnetic nanoparticles (MNPs) might be taken into consideration for their basic magnetic properties, excellent biodegradability, and bio-environment suitability [2]. Materials that have at least one dimension in the nanometer range (1–100 nm) are referred to as nanomaterials. With the recent significant advancement, nanotechnology has found wide-ranging applications in fields such as biotechnology, biology and medicine, carbon nanotubes, therapeutics, automobiles, wastewater management, food and nutraceuticals, environmental applications, fabrics, and textiles [3,4,5,6,7,8,9,10,11]. The development of both complex and straightforward spectrophotometric and robotic instrumentation has also made a large range of approaches available for the characterization of nanomaterials. Nuclear magnetic resonance (NMR), UV–visible spectroscopy, X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), etc., can be used to characterize the size of nanoparticles (NPs). Vibrating sample magnetometry and Mossbauer spectroscopy can be used to assess the magnetic behavior of NPs. The liquid NMR and the Brunauer, Emmett, and Teller approach can be used to measure the NPs’ surface properties. XRD, X-ray photoelectron spectroscopy, inductively coupled plasma mass spectrometry, and NMR can be used to determine the chemical composition of NPs and TEM, high-resolution TEM, and atomic force microscopy can be used to characterize their shape [12]. Because of their extremely small size and high surface-to-volume ratio, which cause changes in their chemical and physical properties when compared to bulk materials with similar chemical composition, such as mechanical and biological properties, optical absorption, superior catalytic activity, melting point, and thermal and electrical conductivity, nanostructure catalysts have been crucial in the synthesis of organic materials [13,14,15]. In this review, we are systematically presenting the synthesis of different oxygen-containing heterocyclic compounds using recyclable inorganic NPs.
2 Multicomponent synthesis of heterocycles of different sizes and ring system containing an oxygen atom
2.1 Three-membered rings
In 2020, Mohamed and his co-workers designed a novel and efficient nanocatalyst, Nitmtaa@N-Thiourea dioxide (TUD)-1, for the epoxidation of cyclohexene 1 to cyclohexene oxide 2 under ambient conditions [16]. The reaction is carried out in the presence of meta-chloroperoxybenzoic acid (m-CPBA) as an oxidant and CH2Cl2/CH3CN (1:1 v/v) as a solvent. In addition, the catalyst Nitmtaa@N-TUD-1 demonstrated great stability over the course of four cycles, maintaining a strong quasi-constant catalytic activity. Atomic absorption spectroscopy (AAS) measurements of leaching supported the highly stable nature of the synthesized nanocatalyst. Mechanistically, the rapid adsorption of m-CPBA onto the catalyst Nitmtaa@N-TUD-1 initiates the reaction. Furthermore, m-CPBA is deprotonated and nickel-bonded activated-m-CPBA (NiII-OOAr, A) is generated. The bond O–O in the intermediate NiII-OOAr would heterotically cleave to generate the active NiIVoxo (B), with the change in color from brown to yellow along with a proton transfer that would help the formation of bi-product meta-chlorobenzoic acid. Following this interaction with cyclohexene, the electrophilic nickel-oxo intermediate NiIV-oxo transfers the oxygen atom to the cyclohexene double bond. The desired product, cyclohexene oxide 2, is generated by the cleavage of the intermediate (C), the oxygen atom transfer from NiIV-oxo to the cyclohexene occurs via a concerted transition state (D), and the catalyst Nitmtaa@N-TUD-1 is recovered (Scheme 1).
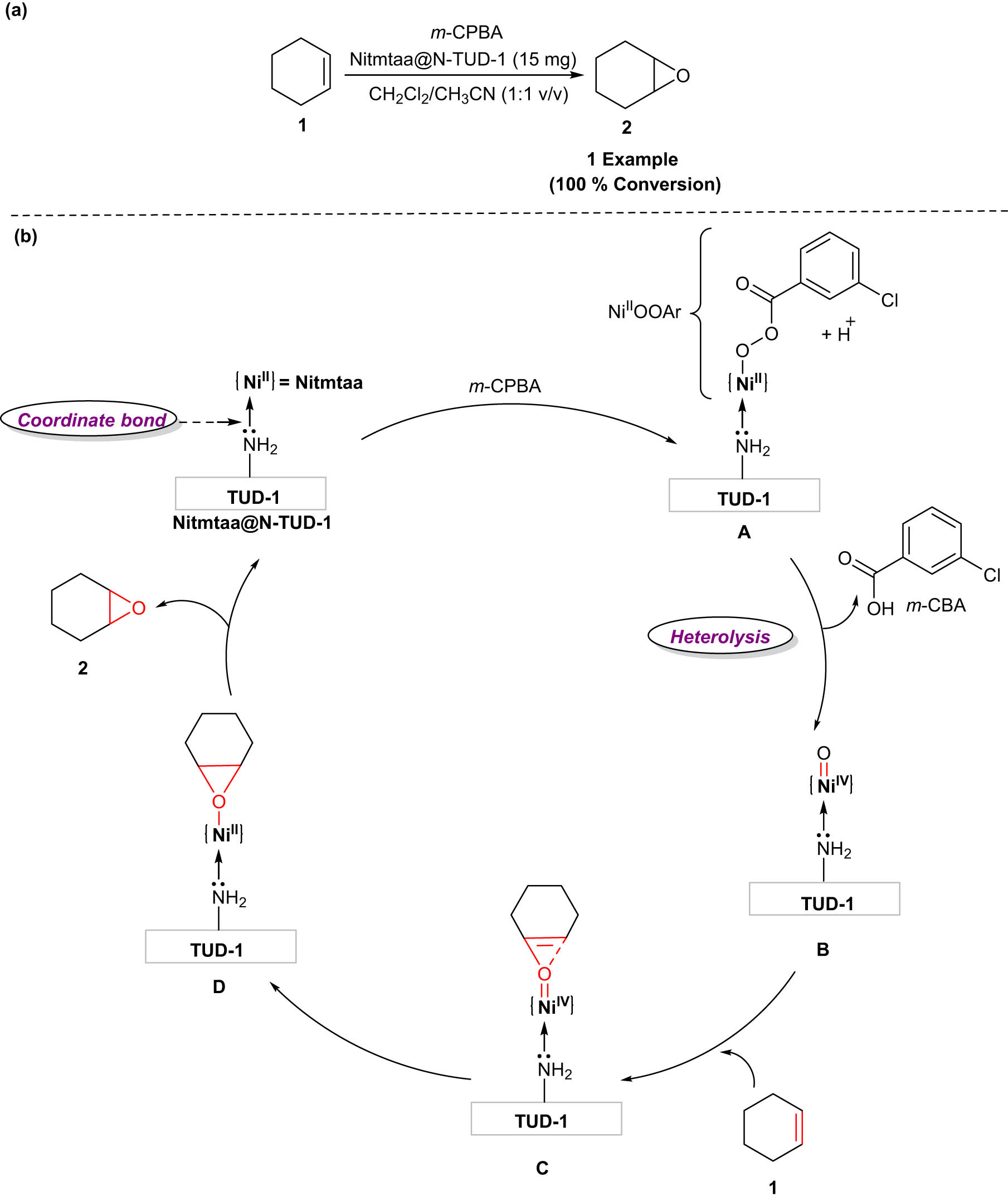
The epoxidation of cyclohexene employing Nitmtaa@N-TUD-1 nanocatalyst. (a) Method review; (b) Plausible Mechanism.
2.2 Five-membered rings
2.2.1 Two-component reactions
CuO NPs catalyzed the synthesis of poly-substituted furans 5 via oxidative C–H/C–H functionalization was developed in 2016 [17]. In this reaction, CuO NPs were used as reusable catalysts in direct functionalization of α,β-unsaturated carbonyl compounds 3 through conjugate addition in aqueous ethanol. Through the oxidative reaction of 1,3-diphenyl-prop-2-ene-1-one 3 and acetylacetone 4 in aqueous ethanol, Payra et al. explored a 3,4-dicabonyl-substituted furan derivative 5 using CuO NPs as a catalyst and tertiary butyl hydroperoxide (TBHP) as an oxidizing agent. This method afforded the products in greater isolated yields (70–91%) with faster reaction time. Moreover, the recyclability and homogenous nature of CuO NP catalyst and use of aqueous ethanol as a green solvent made the protocol environment benign (Scheme 2).
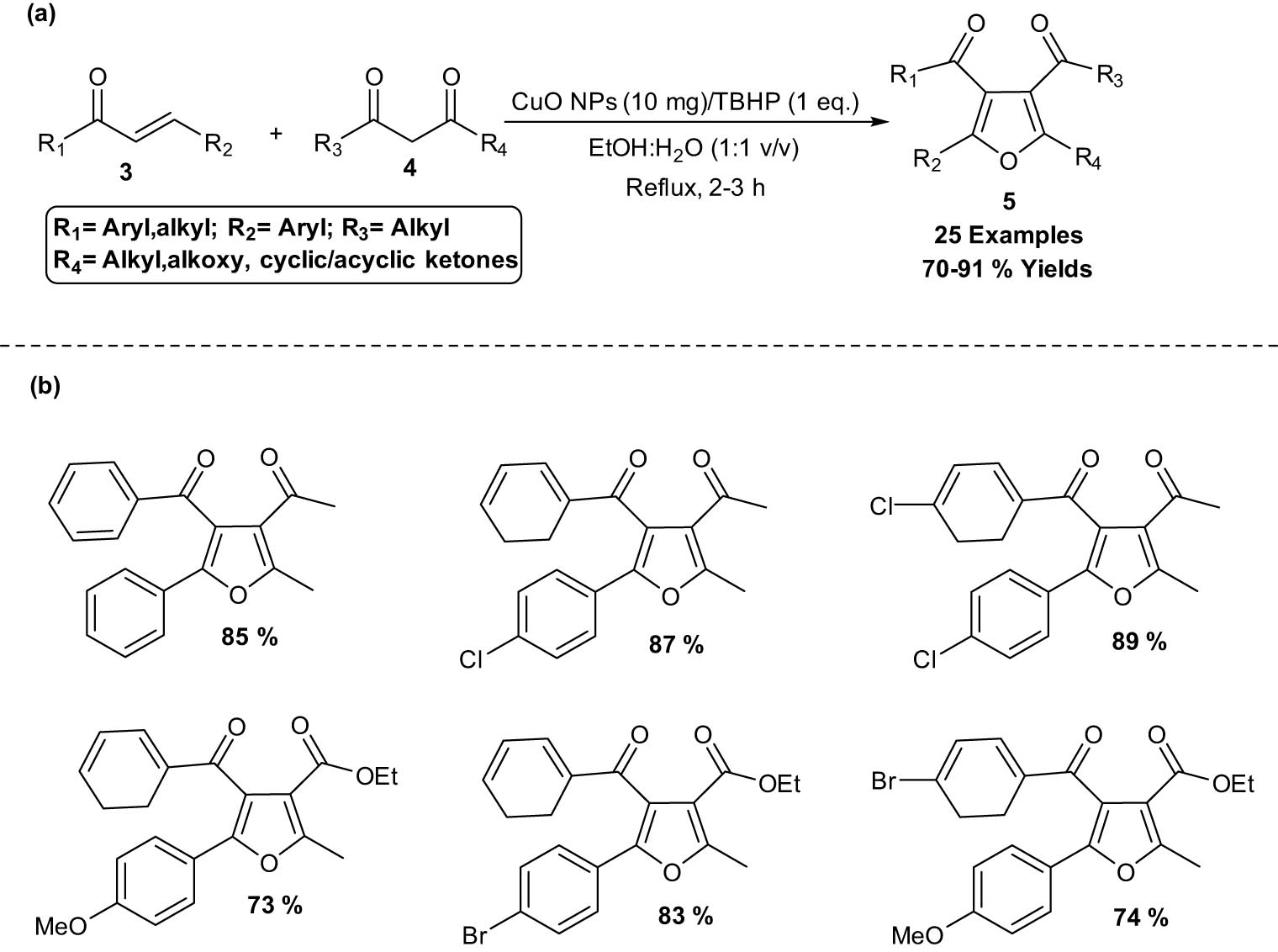
CuO NPs catalyzed the synthesis of poly-substituted furans. a) Method overview; (b) representative examples.
Ji et al. reported a highly efficient protocol for the synthesis of benzofurans 8 via one-pot two-component reaction of o-iodophenols 6 with phenylacetylene 7. The reaction occurs in the presence of palladium NPs supported on N,O-dual-doped hierarchical porous carbon through Sonogashira cross-coupling followed by cyclization of o-halogenated phenols 6 with terminal alkynes 7 under copper- and ligand-free conditions (Scheme 3). The catalyst has great stability and is simple to recover for further usage. The approach is an environmentally friendly way to create biologically useful heterocyclic compounds [18].
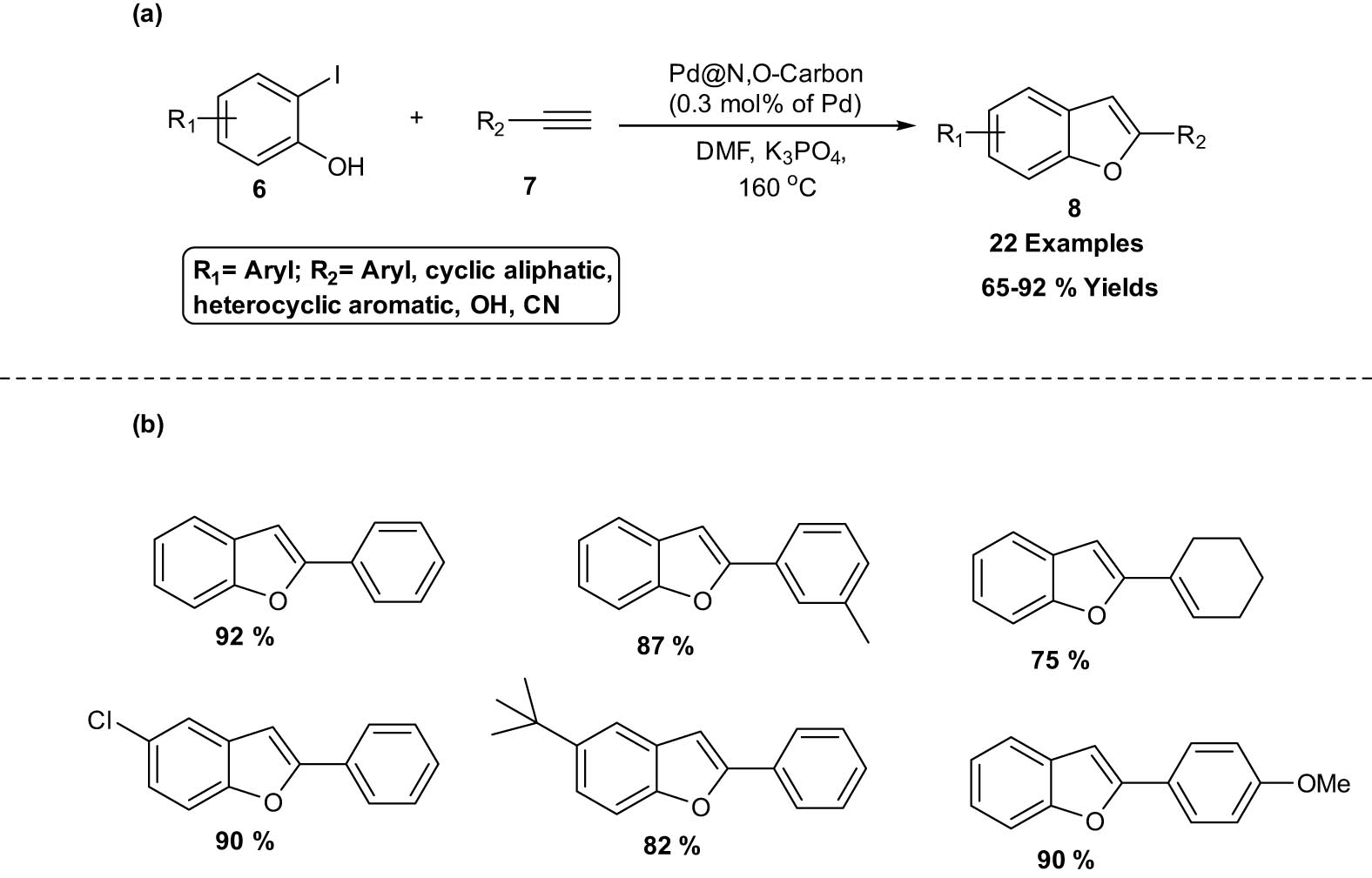
Synthesis of benzofurans employing Pd@N,O-carbon. (a) Method overview; (b) representative examples.
2.2.2 Three-component reactions
A one-pot three-component reaction between isocyanides 11, secondary amines 9, and an electron-poor 2-hydroxybenzaldehyde derivative 10 for the synthesis of benzo[b]furan derivatives 12 using silica NPs from rice husk ash as a green catalyst at ambient temperature was reported in 2014 [19]. Due to greater electrophilicity of the carbonyl groups of electron-withdrawing 2-hydroxybenzaldehyde derivatives relative to carbonyl groups of electron-releasing 2-hydroxybenzaldehyde derivatives, the electron-withdrawing 2-hydroxybenzaldehyde derivatives have more preference as a suitable starting material over electron-releasing derivatives (Scheme 4).
![Scheme 4
Three-component synthesis of benzo[b]furan derivatives using silica NPs. (a) Method overview; (b) representative examples.](/document/doi/10.1515/hc-2022-0171/asset/graphic/j_hc-2022-0171_fig_004.jpg)
Three-component synthesis of benzo[b]furan derivatives using silica NPs. (a) Method overview; (b) representative examples.
According to the possible mechanism of this reaction (Scheme 5), the intermediate A would be produced by the condensation of 2-hydroxybenzaldehyde derivative 10 and secondary amine 9, which would then react with the alkyl isocyanide 11 to produce intermediate B. The cyclization of the ionic intermediate B would produce benzofuran, followed by tautomerization of intermediate C afforded the desired product, benzo[b]furan derivatives 12.
![Scheme 5
Mechanism for the formation of benzo[b]furan derivatives using silica NPs.](/document/doi/10.1515/hc-2022-0171/asset/graphic/j_hc-2022-0171_fig_005.jpg)
Mechanism for the formation of benzo[b]furan derivatives using silica NPs.
In 2018, a facile synthesis of trans-dihydroindeno[1,2-b]furans 16 was developed via one-pot three-component reaction between N-[2-(aryl)-2-oxoethyl)]pyridinium bromide 13 with 1,3-indandione 14 and an aryl glyoxal 15 in the presence of nano-γ-Fe2O3-quinuclidine based as a catalyst in an aqueous medium [20]. The advantages of this approach include fast setup, avoidance of toxic catalysts and organic solvents, reusability of the catalyst, little catalyst loading, quick reaction times, and simple product separation (Scheme 6).
![Scheme 6
Synthesis of trans-dihydroindeno[1,2-b]furans with γ-Fe2O3-quinuclidine NPs. (a) Method overview; (b) representative examples.](/document/doi/10.1515/hc-2022-0171/asset/graphic/j_hc-2022-0171_fig_006.jpg)
Synthesis of trans-dihydroindeno[1,2-b]furans with γ-Fe2O3-quinuclidine NPs. (a) Method overview; (b) representative examples.
Shirzaei et al. reported a green approach for the synthesis of furan derivatives using thiocarbohydrazide doped iron nanoparticles as catalyst. This three components reaction of dialkyl acetylenedicarboxylate 18 with aromatic aldehydes 10 and aromatic amines 17 in the presence of the catalyst was carried out in EtOH giving high yields of the product (Scheme 7). The general route for the preparation of nanocatalyst, Fe3O4@SiO2@ionic liquid (IL) as a heterogeneous acidic catalyst, is shown in Scheme 8. The catalyst’s capacity to be reused up to five times without experiencing any desired decrease in efficacy is by far its most notable attribute. As a result, this catalyst offers several advantages over additional non-magnetic catalysts [1].
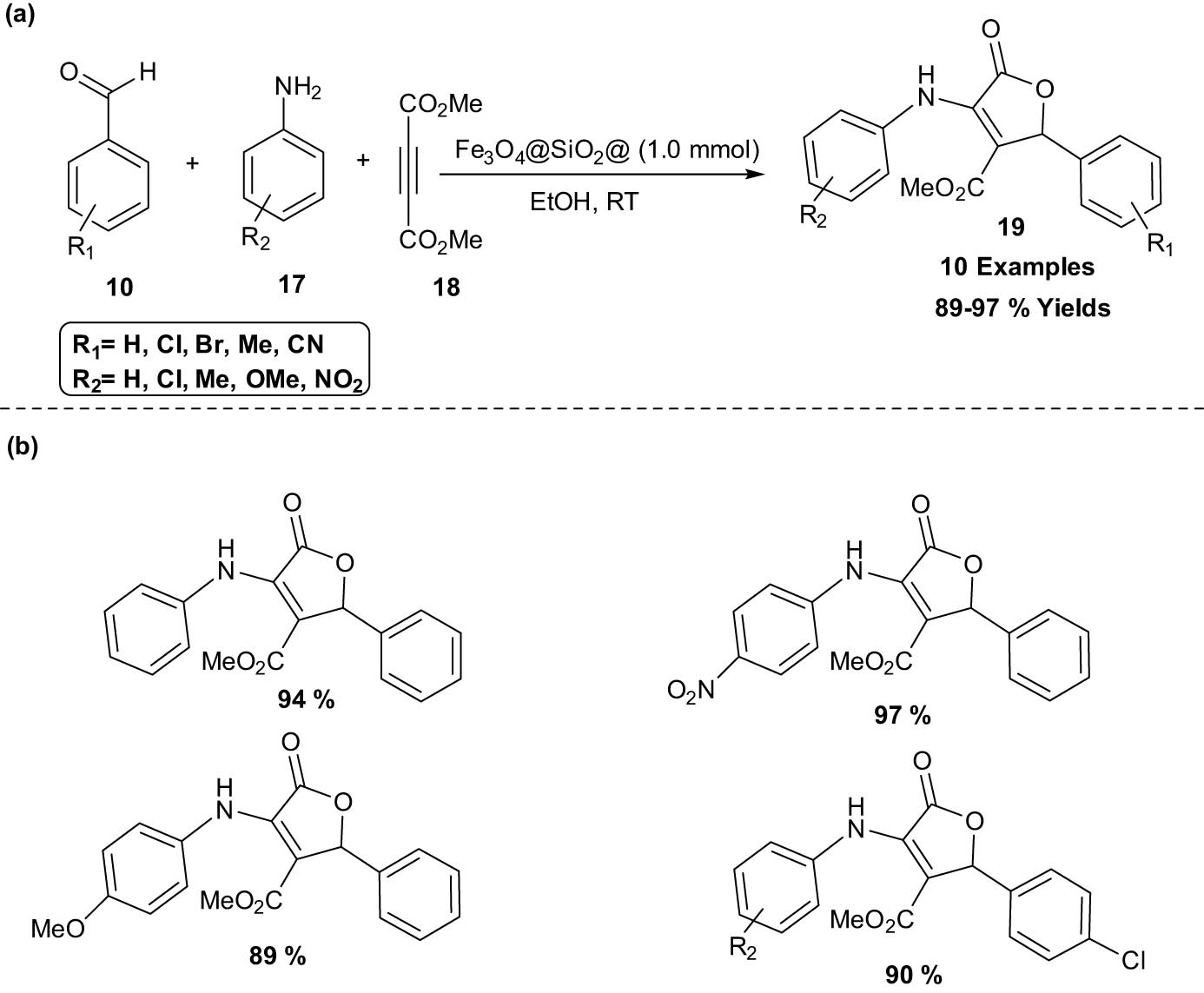
Synthesis of furan derivatives catalyzed by Fe3O4@SiO2@IL. (a) Method overview; (b) representative examples.
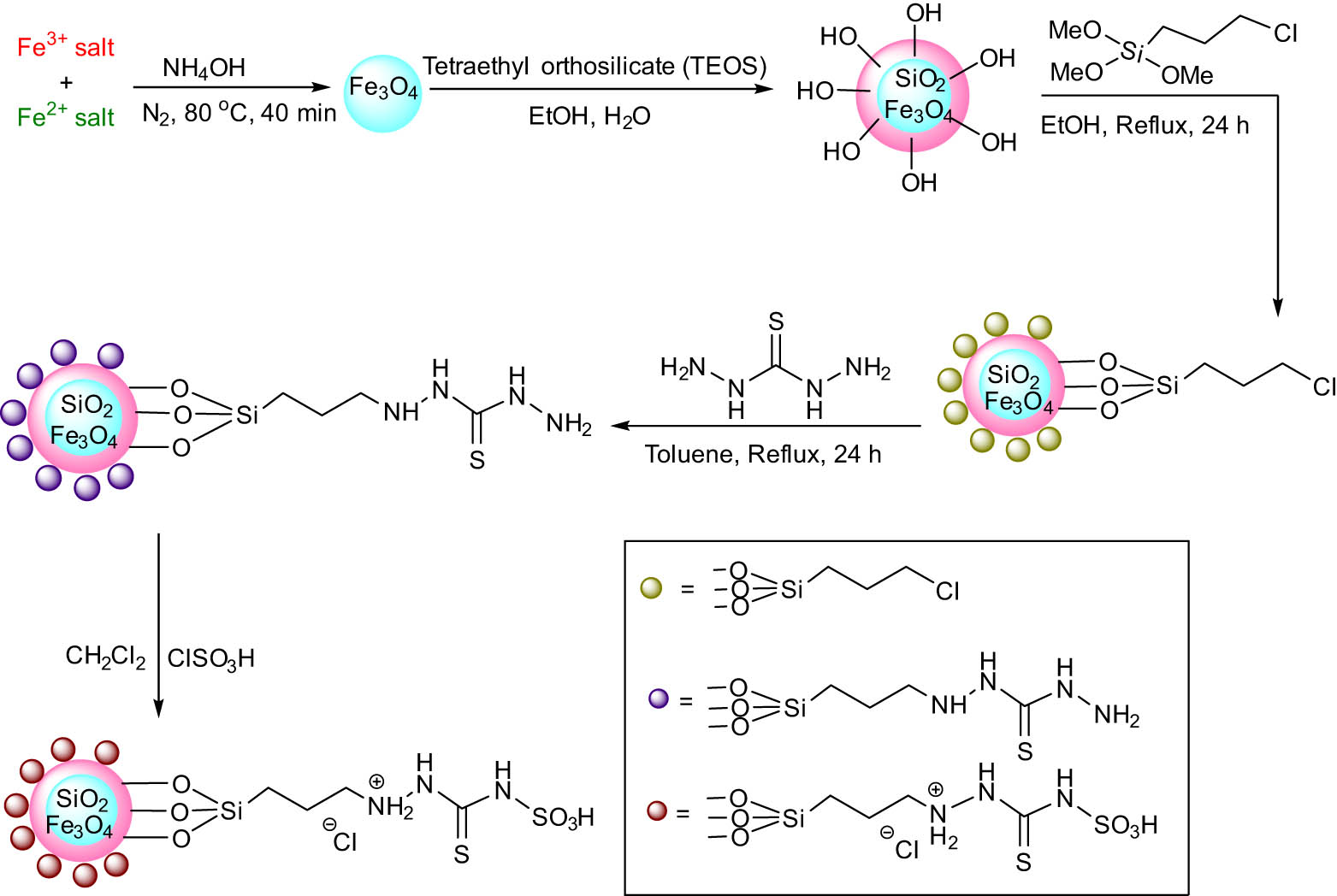
General route for the synthesis of Fe3O4@SiO2@IL.
According to the plausible mechanism of the reaction, MNP catalyst promotes the condensation of the carbonyl group between intermediates A and B to produce the intermediate C. The catalyst further activates the carbonyl group of intermediate C, followed by nucleophilic attack through Michael addition, producing intermediate D. Finally, the removal of H+ terminated the reaction, generating the desired product 19 (Scheme 9). In another study, 4-carboxybenzyl sulfamic acid-functionalized Fe3O4 NPs were also used as a novel catalyst in this reaction [2].
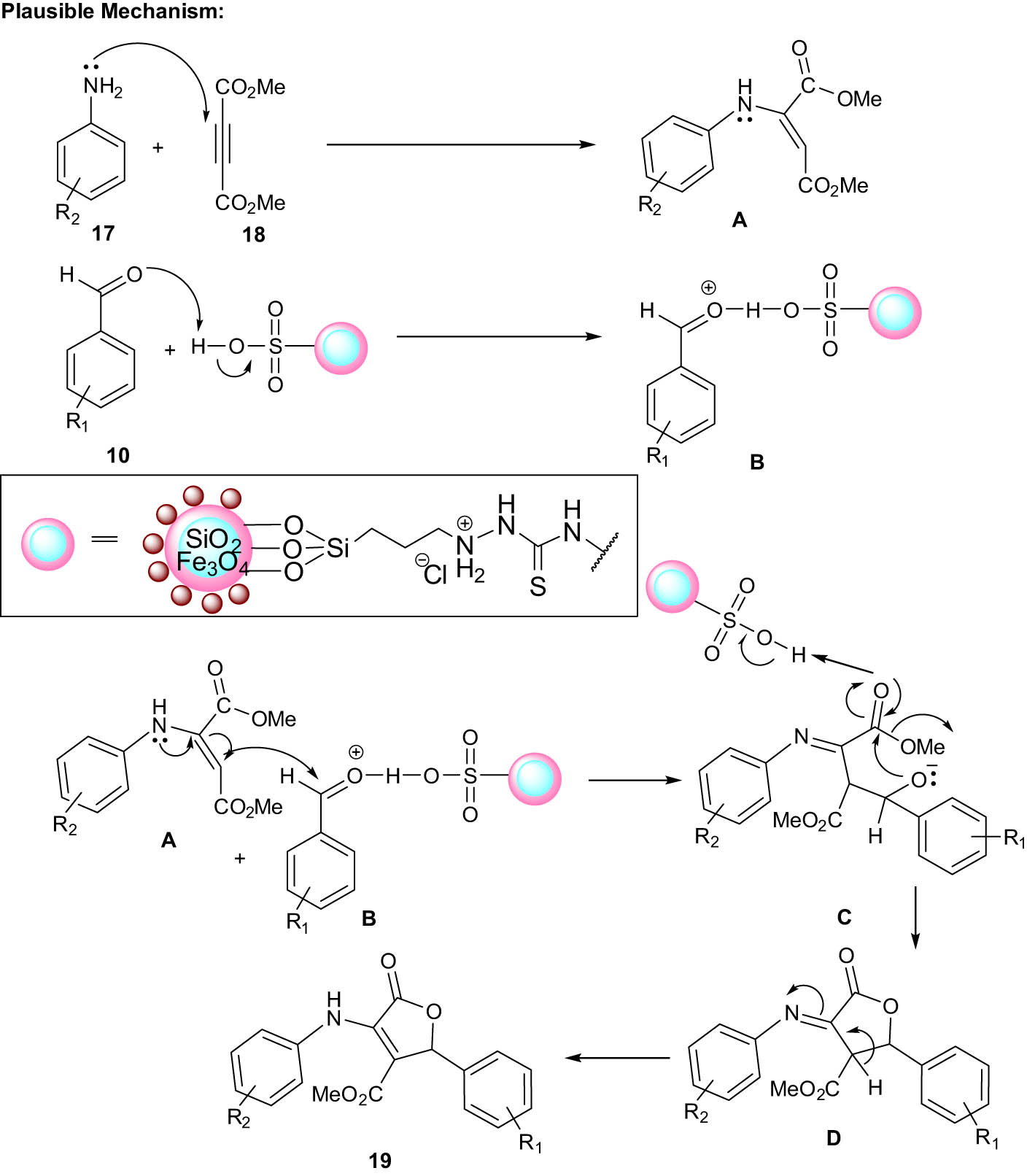
Proposed mechanism for the synthesis of furans catalyzed by {Fe3O4@SiO2@IL}.
Feng and his co-workers, in 2021, slightly modified the previous work in Scheme 7 for the synthesis of furan derivatives [21]. In this work, they used sulfamic acid 2-aminobenzothiazole-6-carboxylic acid (SA-ABTCA)-functionalized Fe3O4 as nanocatalyst for the synthesis of novel 3,4,5-trisubstituted furan-2(5H)-ones derivatives 21 via one-pot reaction of aryl aldehydes 10, 4-amino pyridine 20, and dimethylacetylenedicarboxylate 18. The average particle diameter determined by the Scherrer equation afforded the crystalline size for SA-ABTCA Fe3O4 NPs to be about 21 nm. Moreover, some compounds also exhibited good antibacterial activity and can be further promoted as powerful antibacterial agents. The antibacterial active compounds have higher effectiveness against Bacillus subtilis but exert moderate activity against both gram-positive and gram-negative bacteria. This green nanocatalytic protocol for the synthesis of the desired compound offers clean production in a brief amount of time and has strong reversibility, making the strategy more feasible and affordable for researchers (Scheme 10).
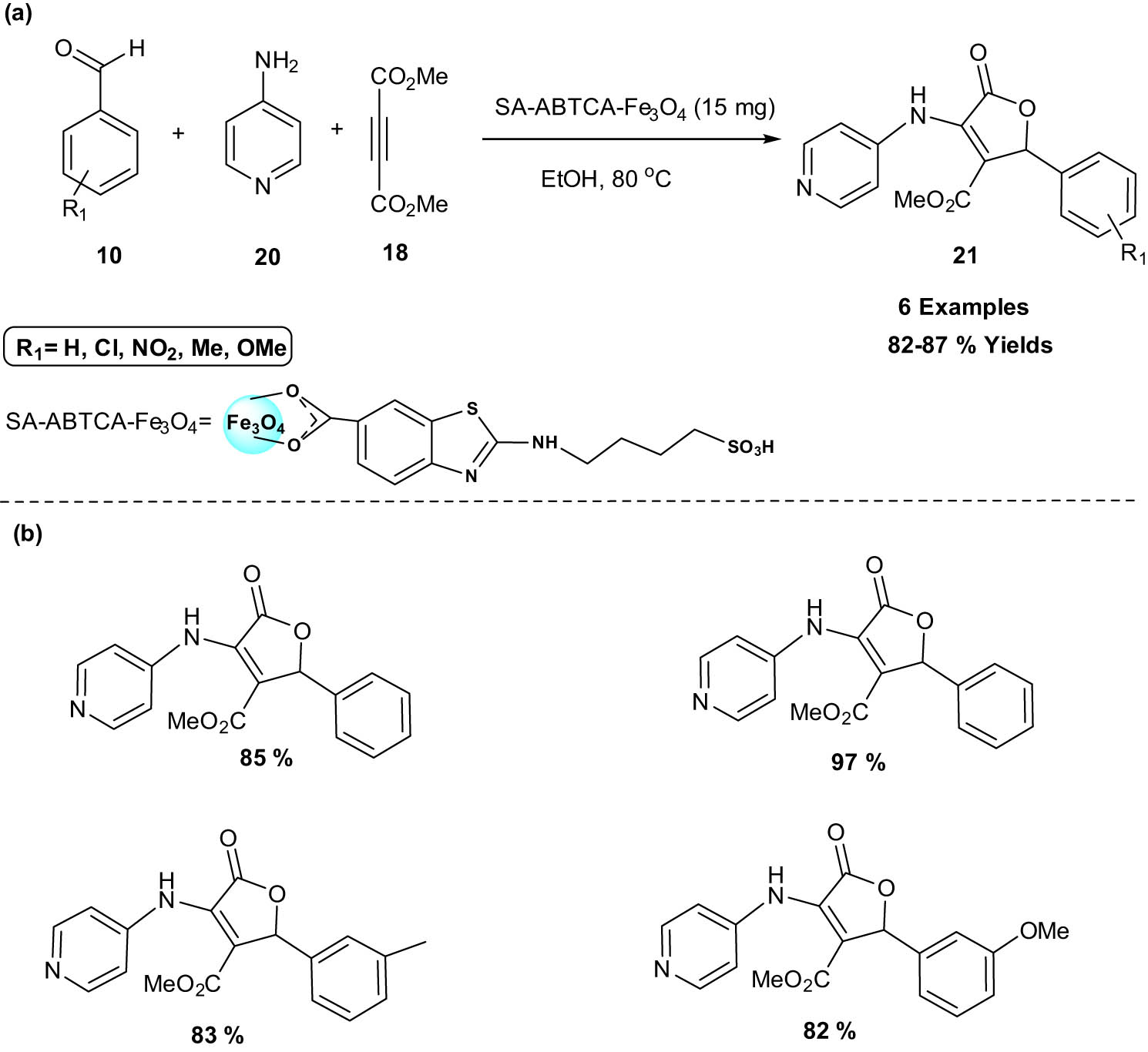
SA-ABTCA-Fe3O4 catalyzed synthesis of novel 3,4,5-trisubstituted furans. (a) Method overview; (b) representative examples.
Wang et al. developed an effective carbonylation of cinnamyl chloride 22 and phenylacetylene 11 with CO2 23 to afford 4-dihydronaphtho[2,3-c]furan-1(3H)-one 24 using α-cyclodextrin (α-CD) doping dendritic fibrous nano-silica (DFNS)-supported gold NPs as a catalyst [22]. It was discovered that the DFNS/α-CD/Au nanostructures could be nominated because of their efficient and distinctive catalytic behavior during the synthesis of the desired compound (Scheme 11).
![Scheme 11
Synthesis of 4-dihydronaphtho[2,3-c]furan-1(3H)-ones in the presence of DFNS/α-CD/Au NPs. (a) Method overview; (b) representative examples.](/document/doi/10.1515/hc-2022-0171/asset/graphic/j_hc-2022-0171_fig_011.jpg)
Synthesis of 4-dihydronaphtho[2,3-c]furan-1(3H)-ones in the presence of DFNS/α-CD/Au NPs. (a) Method overview; (b) representative examples.
2.3 Six-membered rings
2.3.1 Two-component reactions
A high atom economy and efficient approach to synthesize a xanthone 25 skeleton via highly efficient copper-based magnetically recoverable nanocatalyzed coupling of 2-substituted benzaldehydes 10 with phenol derivatives 6 was developed by Cintia and his co-workers [23]. The catalyst is easily recovered by means of an external magnet and reused again as a catalyst for further reaction without significant loss of catalytic activity. Moreover, the technology offers an interesting protocol for the production of a small library of xanthones 25 in very good yield and is compatible with a number of functional groups. Better yields of the cyclization products were obtained with regard to the electronic characteristics of the substituents when electron-donating groups were present on the aromatic ring of the phenols (Scheme 12).
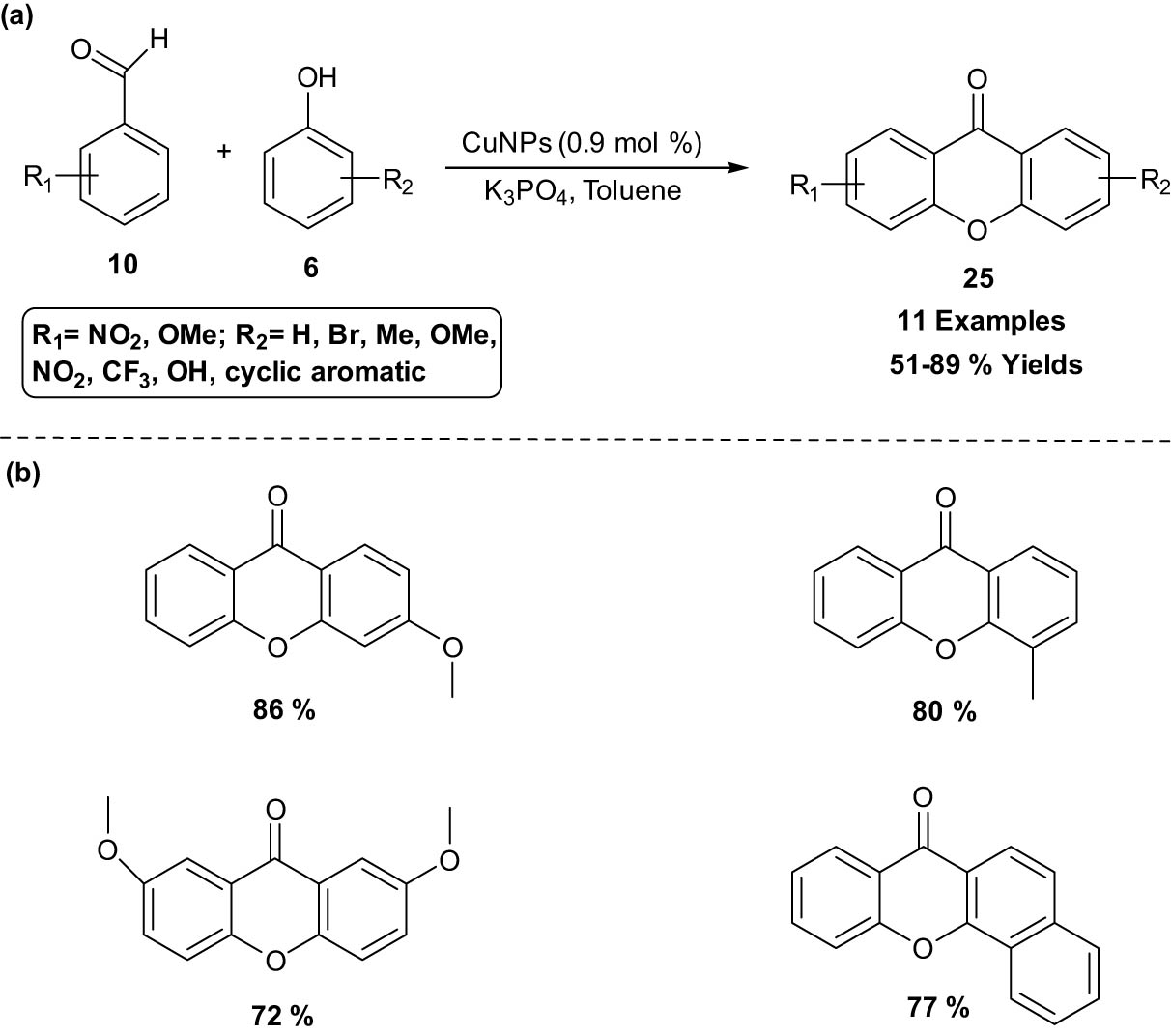
Synthesis of xanthone skeleton using Cu nanoparticle (CuNP) catalyst. (a) Method overview; (b) representative examples.
Anshu and his co-workers reported a highly efficient, chemoselective synthesis of pyrano[2,3-c:6,5-c′]dipyrazol]-2-ones 28 in water via one-pot reaction of carbonyl compounds 26 with 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one 27 (1:2 ratio) in the presence of Ag NPs decked graphene oxide (GO) composite as a catalyst (Scheme 13). This technique demonstrates the high selectivity of pyranodipyrazolones 28 over arylmethylene bispyrazolols and arylmethylenepyrazolones. Due to the loss of two water molecules, the method has a high atom economy and is environmentally friendly. Furthermore, the catalyst was easily recoverable and could be recycled at least seven times without suffering a significant reduction in catalytic activity [24].
![Scheme 13
The chemo-selective synthesis of pyrano[2,3-c:6,5-c′]dipyrazol]-2-ones. (a) Method overview; (b) representative examples.](/document/doi/10.1515/hc-2022-0171/asset/graphic/j_hc-2022-0171_fig_013.jpg)
The chemo-selective synthesis of pyrano[2,3-c:6,5-c′]dipyrazol]-2-ones. (a) Method overview; (b) representative examples.
The mechanism of this reaction involves electrophilic activation of benzaldehyde 26 by coordinating with the Lewis acid sites of Ag NPs/GO (Ag NPs/GO) composite. Then, Knoevenagel condensation of activated benzaldehyde A with pyrazolone 27 formed Knoevenagel adduct B. Furthermore, activation of adduct B by the catalyst through coordination with oxygen facilitates the Michael addition of a second pyrazolone 27 unit forming hydroxyl derivate C. Finally, successive cyclization afforded the desired product 28 with the loss of water molecule and released the catalyst for the next cycle (Scheme 14).
![Scheme 14
Plausible mechanism for synthesis of pyrano[2,3-c:6,5-c’]dipyrazol]-2-ones.](/document/doi/10.1515/hc-2022-0171/asset/graphic/j_hc-2022-0171_fig_014.jpg)
Plausible mechanism for synthesis of pyrano[2,3-c:6,5-c’]dipyrazol]-2-ones.
A highly efficient and recyclable CoNP@SBA-15 (Co nanoparticles@santa barbara amorphous-15) nanocatalyst supported an aqueous and environmentally friendly approach for the synthesis of 1,8-dioxo-octahydroxanthenes 30 through the reaction of dimedone 29 with aromatic aldehydes 10 (2:1 ratio) was developed by Rajabi et al. [25]. In this protocol, high yields, minimal catalyst loading, quick reaction times, and simple workup offered the benefits of employing this heterogeneous catalytic system. Additionally, the catalyst was successfully reused 10 times in a row without significantly losing any catalytic activity. The catalyst with a pore size of 3.6 nm had a surface area of 448 m2 g−1 and 0.77 mL g−1 mesoporous pore volume (Scheme 15).
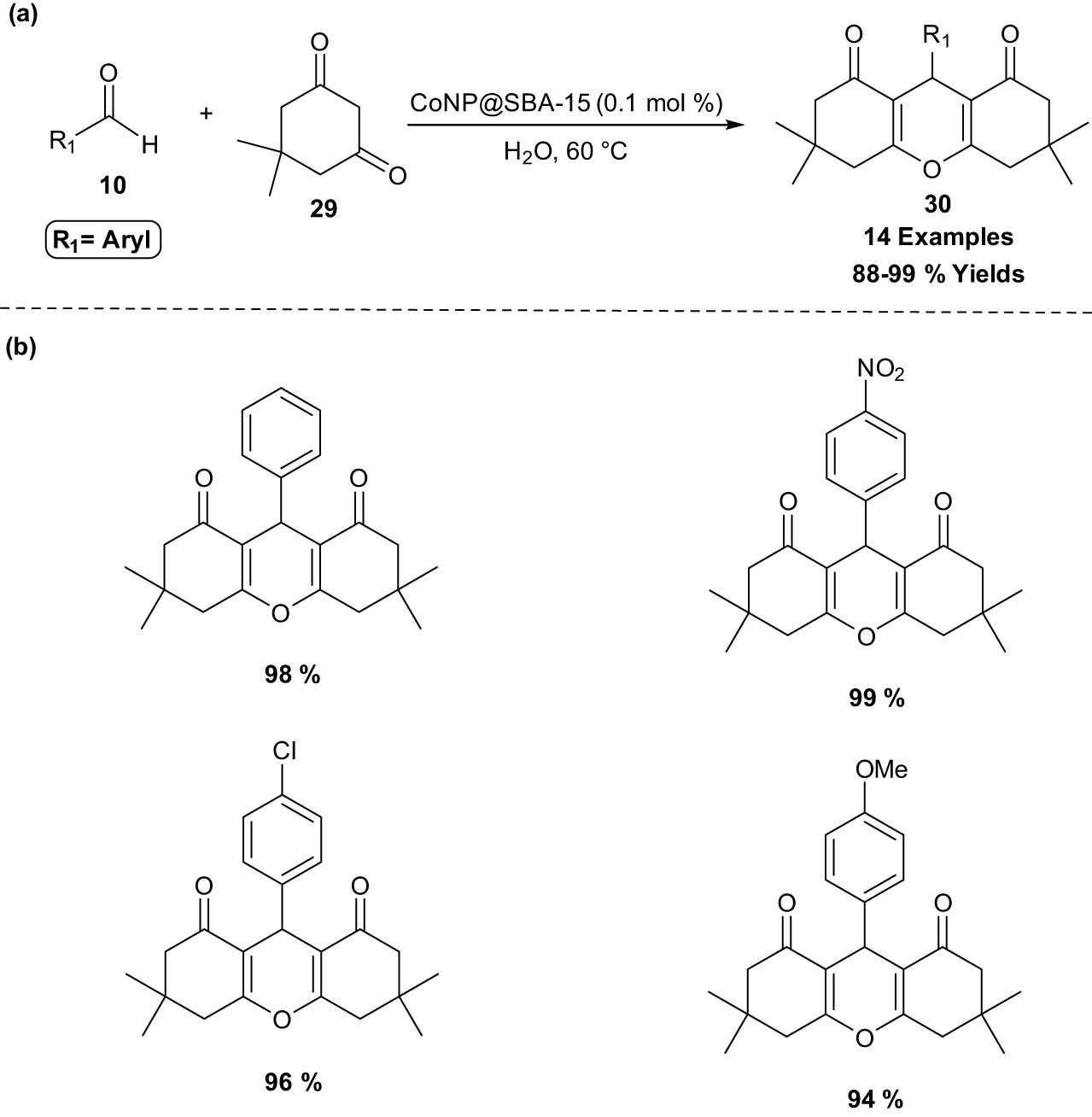
Synthesis of 1,8-dioxo-octahydroxanthenes in the presence of CoNP@SBA-15. (a) Method overview; (b) representative examples.
An efficient, selective, rapid, and eco-friendly microwave-assisted direct synthesis of xanthones 32 via intermolecular catalytic coupling of salicylaldehydes 10 and 1,2-dihaloarenes 31 using green nanopalladium-supported catalyst (PdNPs) was developed in 2019 (Scheme 16). The reaction proceeds with high regioselectivity and excellent yields, and the catalyst can be recycled up to four times without significant loss of its catalytic activity [26].
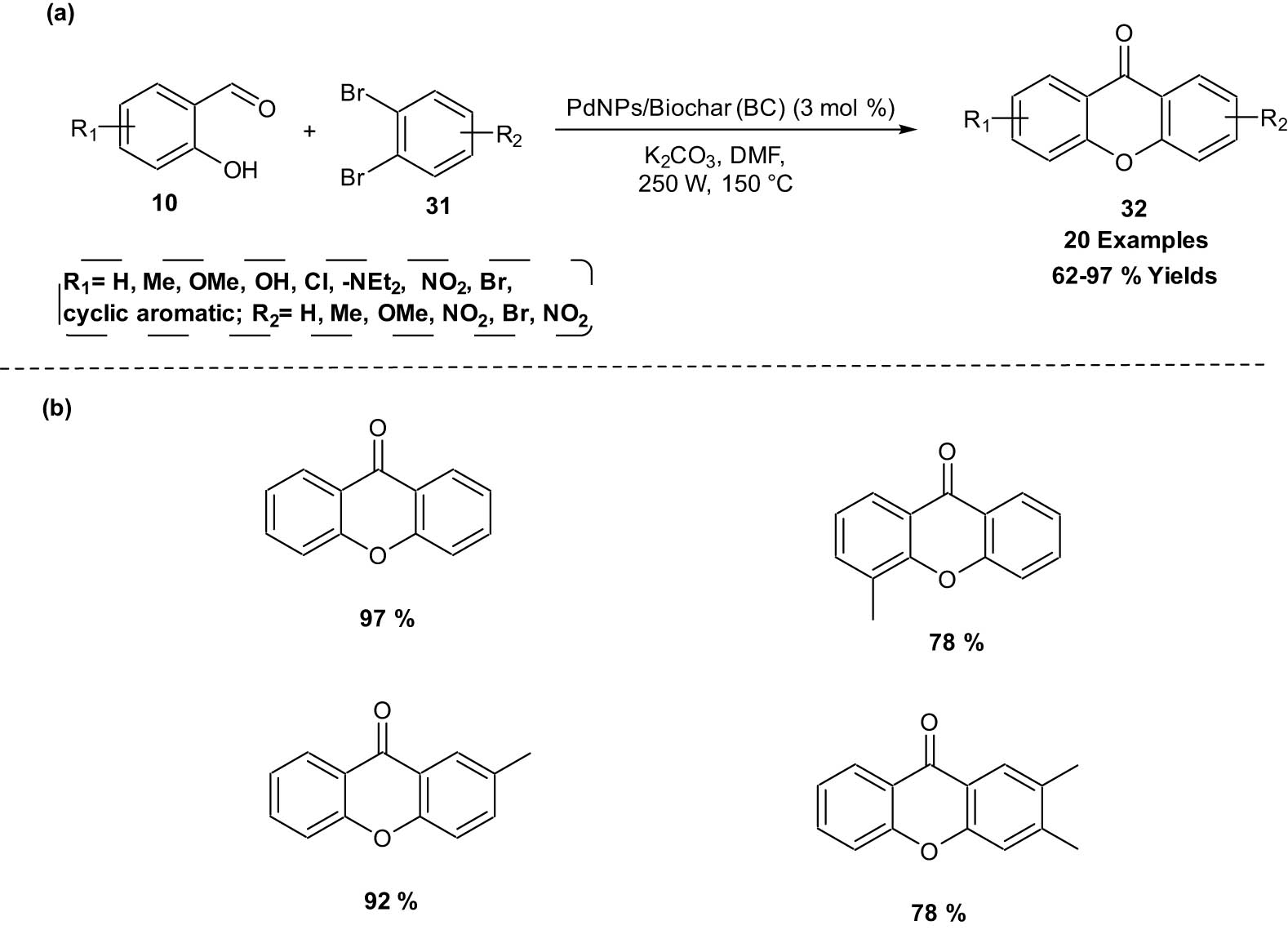
Microwave-assisted synthesis of xanthones using a green nanopalladium-supported catalyst. (a) Method overview; (b) representative examples.
Mechanistically, the oxidative addition of 1,2-dihaloarene 31 with palladium (O) catalyst formed aryl-palladium(ii) intermediate A. Then, salicylate 10, generated under basic conditions by displacing the halide, reacts with intermediate A to form aryl(aryloxy) palladium(ii) intermediate B. Consequently, intermediate B undergoes a C–H activation through the C–H bond of the aldehyde to afford cyclic palladium intermediate C. Finally, the desired xanthone 32 is formed by the β-elimination of C and an intramolecular nucleophilic substitution (Scheme 17).
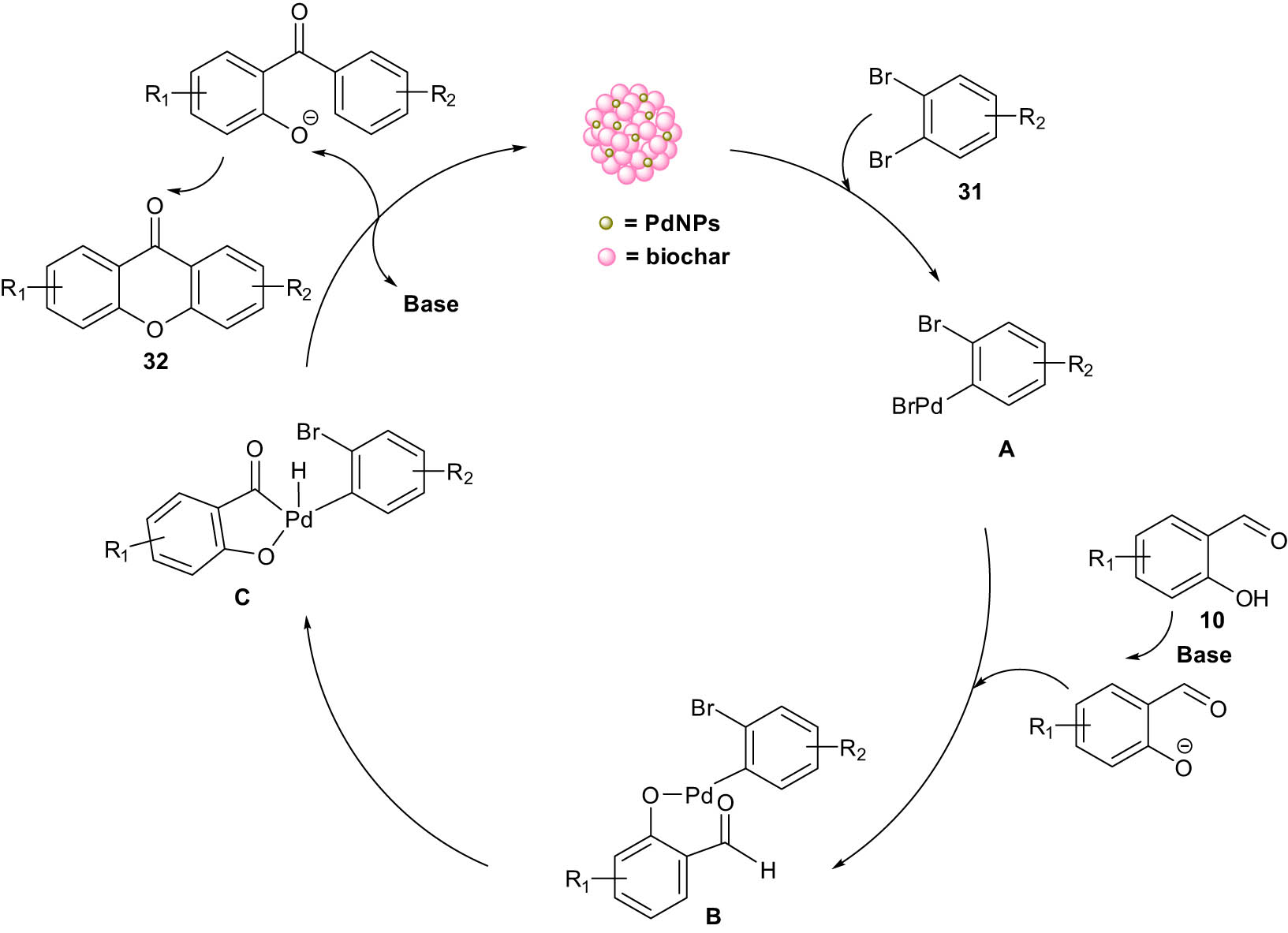
Proposed catalytic cycle for the PdNP-catalyzed direct synthesis of xanthones.
2.3.2 Three-component reactions
Nezhad et al. [27] explored a silica-coated Fe3O4@SiO2 MNP-catalyzed synthesis of 2-amino-4H-chromene-3-carbonitrile derivatives 35 under mild, green, and heterogeneous conditions through one-pot three-component coupling reaction between indole derivatives 33, salicylaldehyde 10, and malononitrile 34 (Scheme 18). The synthesis of MNPs supported l-cysteine (LCMNP) is shown in Scheme 19.
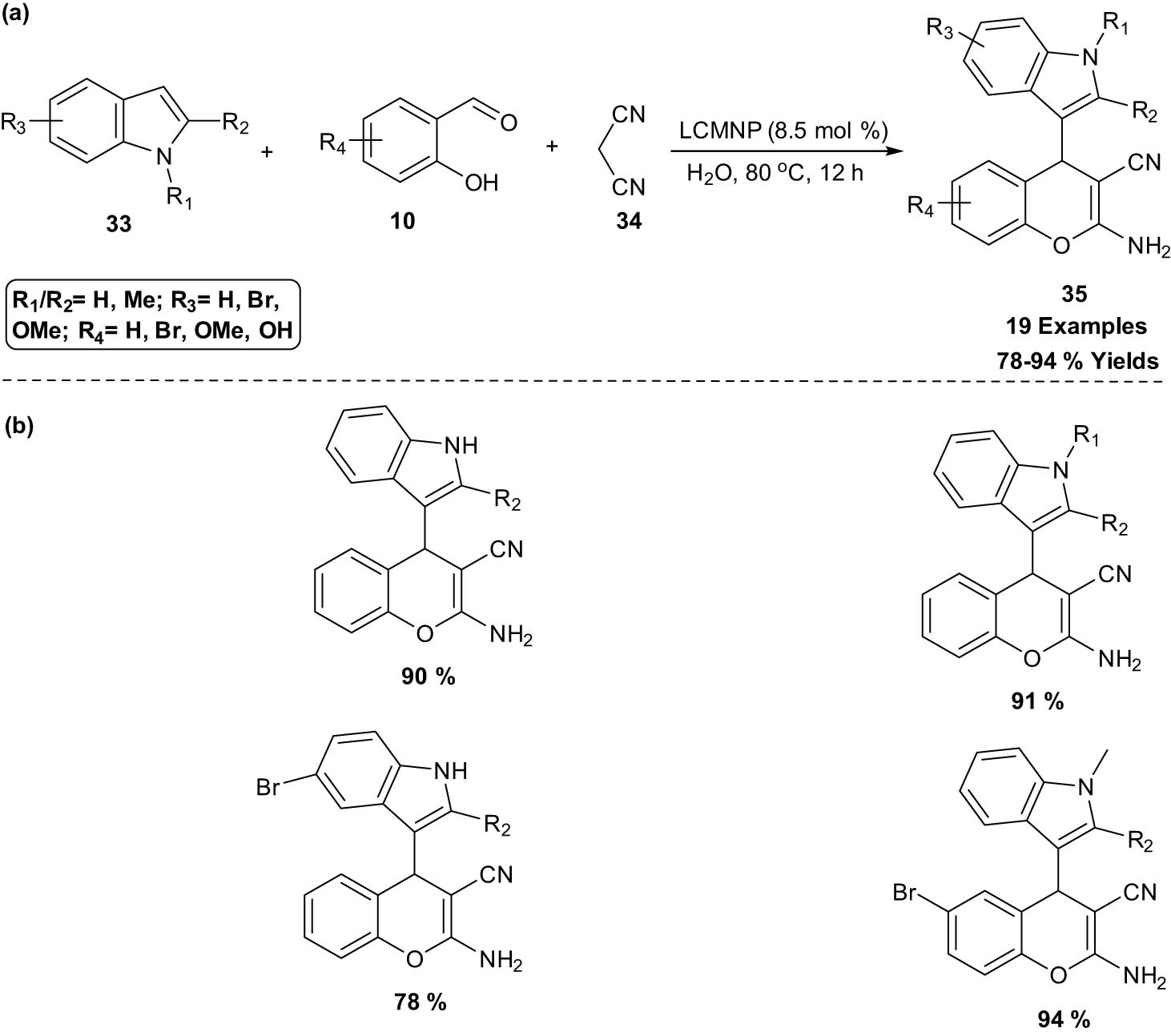
Synthesis of 2-amino-4H-chromene-3-carbonitriles using LCMNP. (a) Method overview; (b) representative examples.
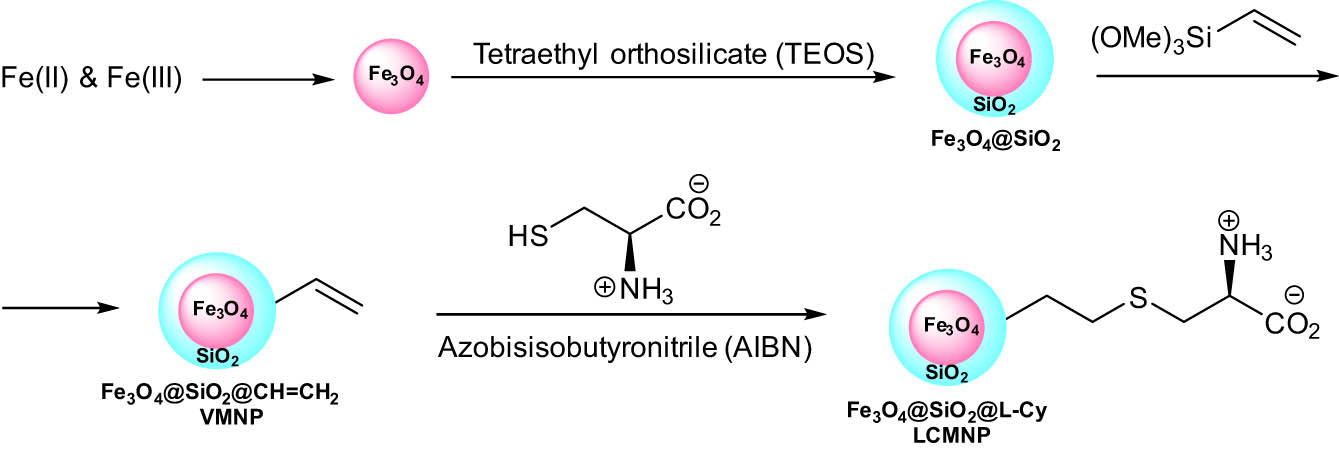
Synthesis of MNPs supported l-cysteine.
The designed technique allowed for the reuse of this magnetic recyclable organ catalyst system seven times without the need for any modifications to its catalytic activity. These NPs have an average size of 10 nm according to dynamic light scattering analysis, with a spherical shape displayed in TEM images of the NPs.
A facile one-pot ultrasound-assisted efficient synthesis of benzo[g]chromenes 37 using Fe3O4/polyethylene glycol (PEG) core/shell NP was developed in 2016 [28]. The reaction proceeds through one-pot three-component condensation of aldehyde 10 (1 mmol), malononitrile 34 (1.5 mmol), and 2-hydroxy-1,4-naphthoquinone 36 (1 mmol) in ethanol with 12 mg of nano-Fe3O4/PEG. The significant benefits of this protocol include simple setup, rapid reaction times, easy workup, catalyst recycling, little catalyst loading, and the effective and efficient use of ultrasonic irradiation (Scheme 20). After completion of the reaction, the catalyst was separated by an external magnetic field and can be reused five times with a slight reduction in the product yields on each reuse (run 1, 95%; run 2, 95%; run 3, 94%; run 4, 94%; run 5, 93%).
![Scheme 20
Fe3O4/PEG core/shell catalyzed synthesis of benzo[g]chromenes. (a) Method overview; (b) representative examples.](/document/doi/10.1515/hc-2022-0171/asset/graphic/j_hc-2022-0171_fig_020.jpg)
Fe3O4/PEG core/shell catalyzed synthesis of benzo[g]chromenes. (a) Method overview; (b) representative examples.
Davood and Masoumeh developed an efficient, green, and reusable thiourea dioxide‐grafted γ‐Fe2O3/hydroxyapatite (HAp) MNP for the synthesis of pyranopyridine derivatives 39 in high yields. The reaction proceeds through one‐pot three‐component reactions between aldehydes 10, malononitrile 34, and 3‐cyano‐6‐hydroxy‐4‐methyl‐pyridin‐2(1H)‐one 38 under mild and solvent‐free conditions (Scheme 21). The catalyst is easily recyclable in a magnetic field and can be used five times in a row without noticeably losing any substantial catalytic activity. From the study of field‐emission (FE‐SEM) and TEM, the NPs display a regularly spherical morphology with an average diameter of about 80 nm [29].
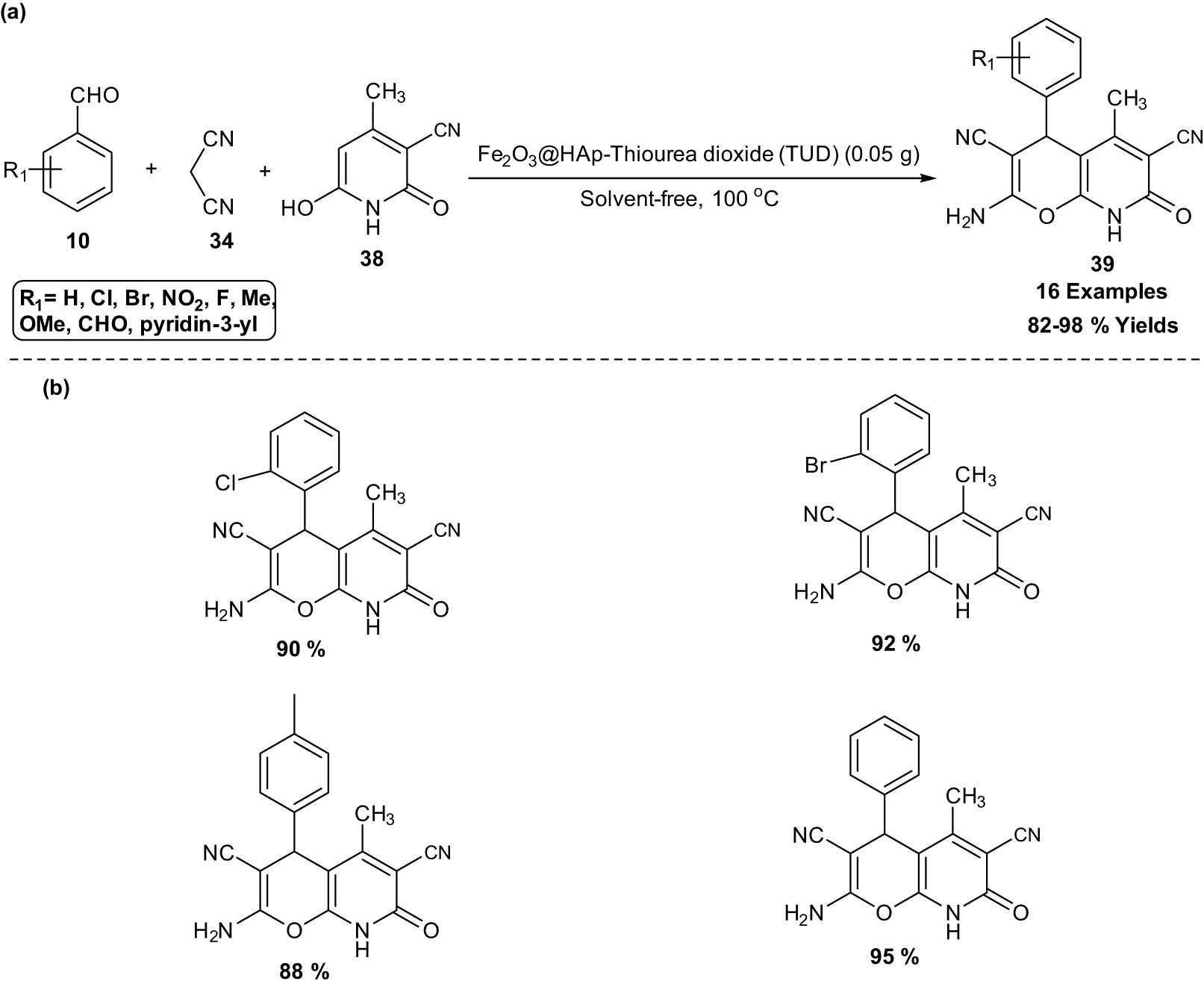
Thiourea dioxide‐grafted γ‐Fe2O3/HAp catalyzed synthesis of pyranopyridine derivatives. (a) Method overview; (b) representative examples.
Green and magnetic recyclable formamidine sulfonic acid-functionalized Fe3O4@SiO2 as an efficient and hydrogen bonding catalyst for the synthesis of pyrano[2,3-d] pyrimidinone derivatives 41 via one-pot three-component condensation reaction of benzaldehyde 10 (1 mmol), malononitrile 34 (1.2 mmol), and barbituric acid 40 (1 mmol) in water at room temperature was employed by Ghandi et al. [30]. The catalyst could be employed repeatedly without a noticeable decrease in its activity, and Fourier transform infrared (FT-IR) observations show no substantial changes in the structure of the catalyst before and after the reaction. This reaction protocol has a short reaction time, high yield, simplicity of product isolation, clean reaction profile, and environmental benignity (Scheme 22).
![Scheme 22
One-pot three-component synthesis of pyrano[2,3-d] pyrimidinone derivatives. (a) Method overview; (b) representative examples.](/document/doi/10.1515/hc-2022-0171/asset/graphic/j_hc-2022-0171_fig_022.jpg)
One-pot three-component synthesis of pyrano[2,3-d] pyrimidinone derivatives. (a) Method overview; (b) representative examples.
A practical three-component reaction between 4-hydroxycoumarin 43, malononitrile 34/ethylcyanoacetate 42 and arylaldehydes 10 for the synthesis of 4-aryl-substituted pyranofuzed coumarins 44 using recyclable molybdenum oxide nanocatalyst (MoO3 NPs) was described by Yaghoub and his co-workers (Scheme 23). The SEM image analysis of MoO3 NPs shows a diameter of about 40 nm of the nanocatalyst without any amorphous or other kinds of crystallized phase particles. The catalyst can be recovered and reused over six successive runs in comparable yields with that obtained from freshly prepared catalysts. The TEM and XRD patterns of the reused catalyst clearly displayed that no notable agglomeration or changes in the crystalline phase had taken place through each run [31].
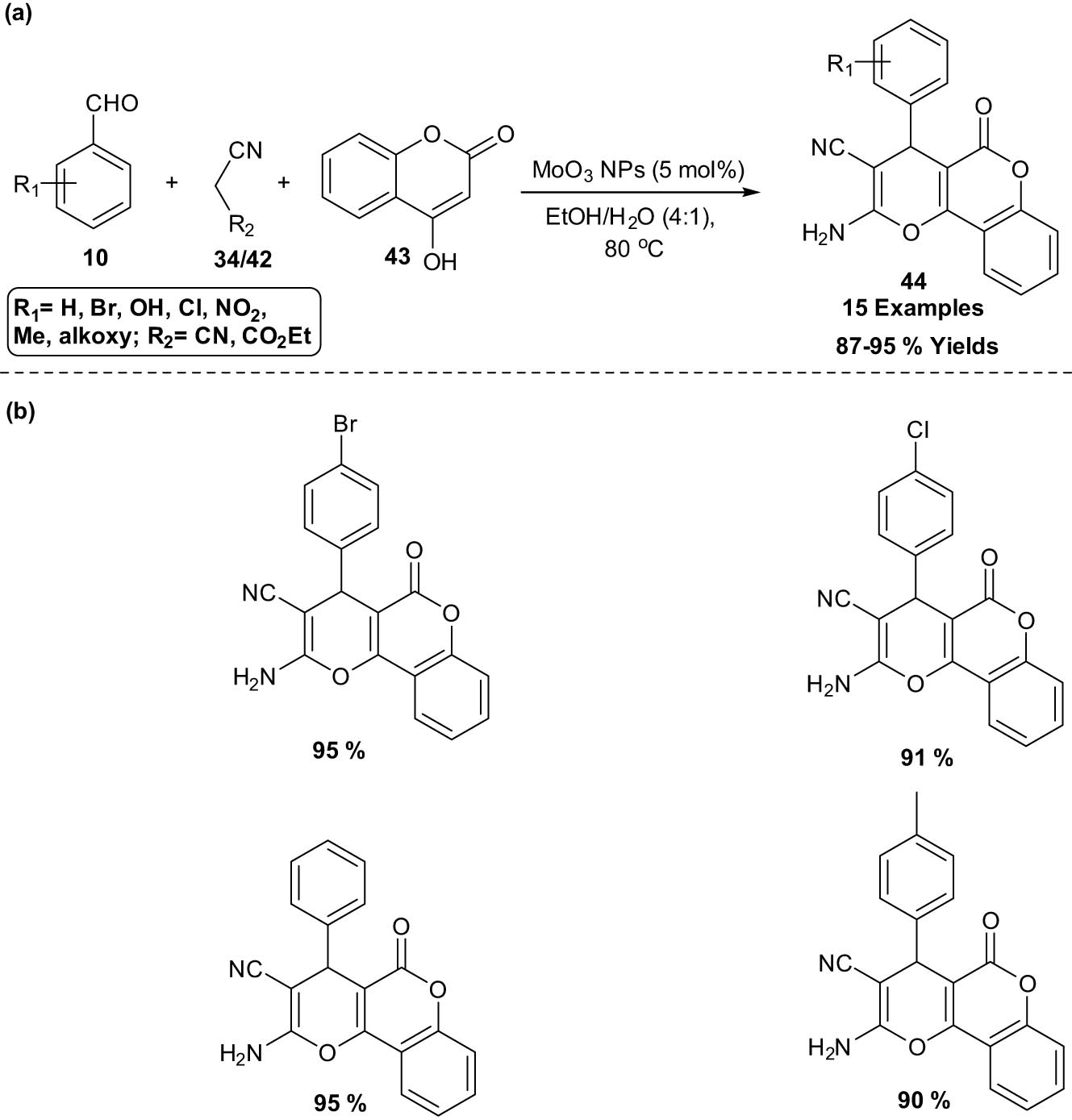
MoO3 NPs catalyzed the synthesis of 4-aryl-substituted pyranofuzed coumarins. (a) Method overview; (b) representative examples.
Sadjadi et al. [32] reported a heterogeneous nanocatalyst Ag@cyclodextrin nanosponge (CDNS)-santa barbara amorphous‐15 (SBA‐15) promoted three‐component reaction of benzaldehydes 10, 4‐hydroxycoumarin 43, and urea 44 or thiourea 45 under ultrasonic irradiation to afford benzopyranopyrimidines 46. The catalyst can be recovered after the reaction and reused for up to four reaction runs with slight Ag (0) leaching and loss of catalytic activity (Scheme 24).
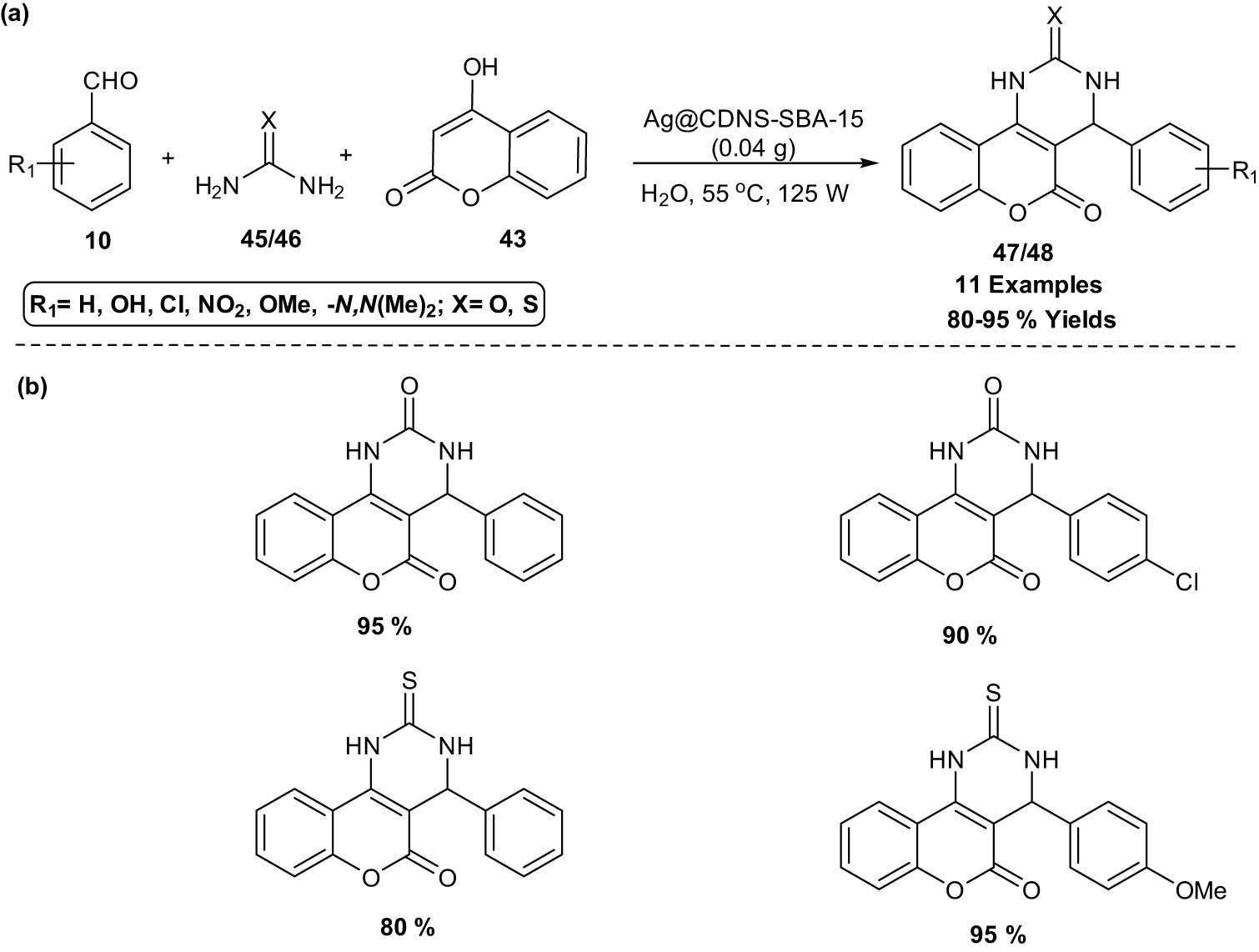
Ag@CDNS-SBA‐15 promoted the synthesis of benzopyranopyrimidines. (a) Method overview; (b) representative examples.
According to the proposed mechanism (Scheme 25), activation of aldehyde 10 by the catalyst (compound A) followed by Knoevenagel condensation reaction with urea 45/thiourea 46 formed intermediate B. Then, the reaction of intermediate B with 4-hydroxycumarin 43 afforded intermediate C. Finally, the cyclization of intermediate C with the loss of a water molecule formed the desired final product 47/48.
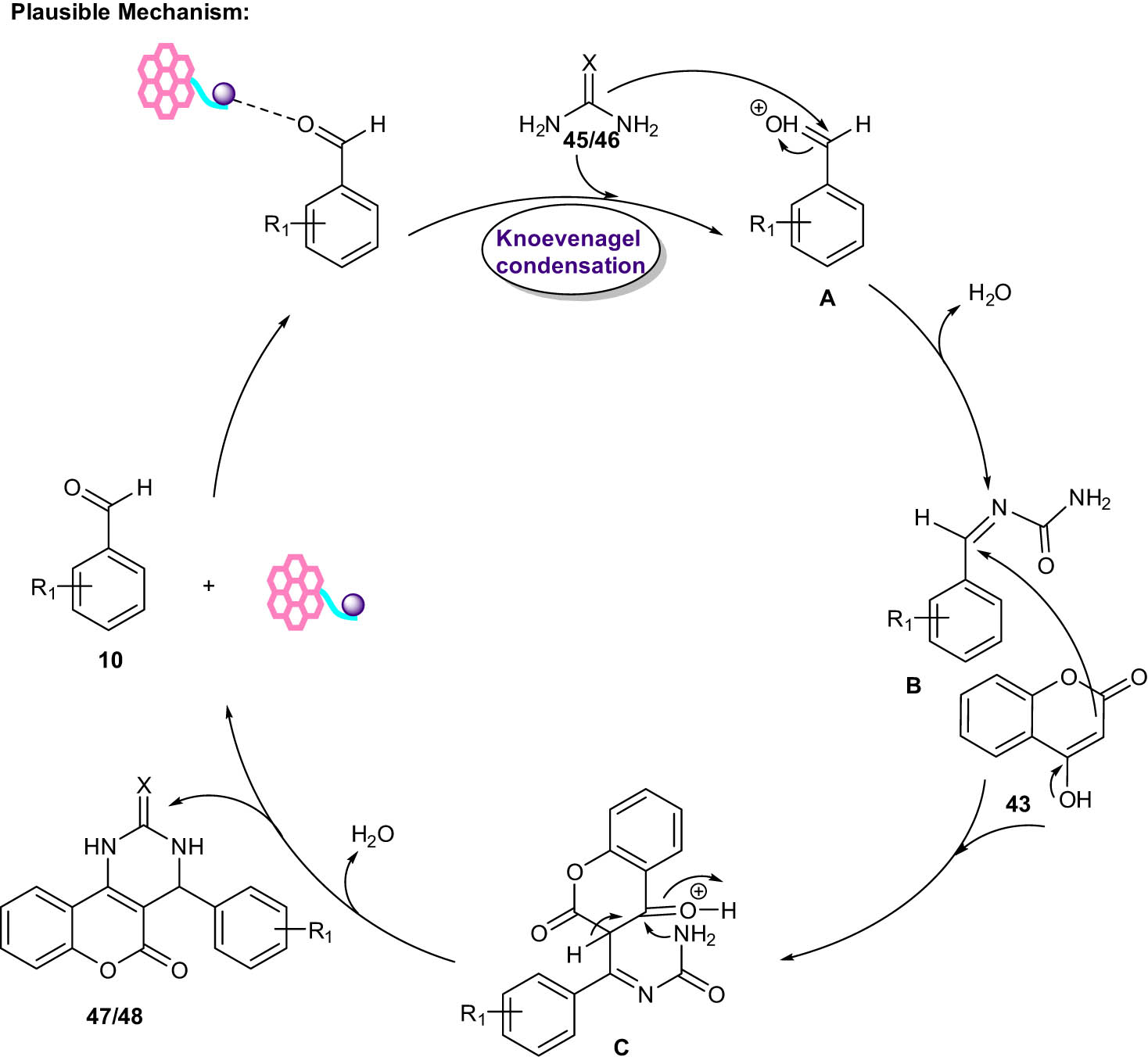
Plausible mechanism for synthesis of benzopyranopyrimidines.
Highly efficient and magnetically retrievable amine-functionalized SiO2@Fe3O4 NP-catalyzed green and mechanochemical one-pot multicomponent synthesis of bioactive 2‑amino‑4H‑benzo[b]pyrans 49 was explored in 2020 (Scheme 26). The reaction is an environmentally benign reaction achieved by simply grinding substituted aromatic aldehydes 10, dimedone 29, and malononitrile 34 at room temperature under solvent and waste-free conditions with good yields, high purity, milder reaction conditions, and short reaction time.
![Scheme 26
SiO2@Fe3O4 catalyzed synthesis of 2‑amino‑4H‑benzo[b]pyrans. (a) Method overview; (b) representative examples.](/document/doi/10.1515/hc-2022-0171/asset/graphic/j_hc-2022-0171_fig_026.jpg)
SiO2@Fe3O4 catalyzed synthesis of 2‑amino‑4H‑benzo[b]pyrans. (a) Method overview; (b) representative examples.
Furthermore, the proposed catalyst has a number of significant properties, including broad functional group tolerance, enhanced yield, and recyclability [33]. A schematic illustration for the formation of NH2@SiO2@Fe3O4 MNPs is shown in Scheme 27.
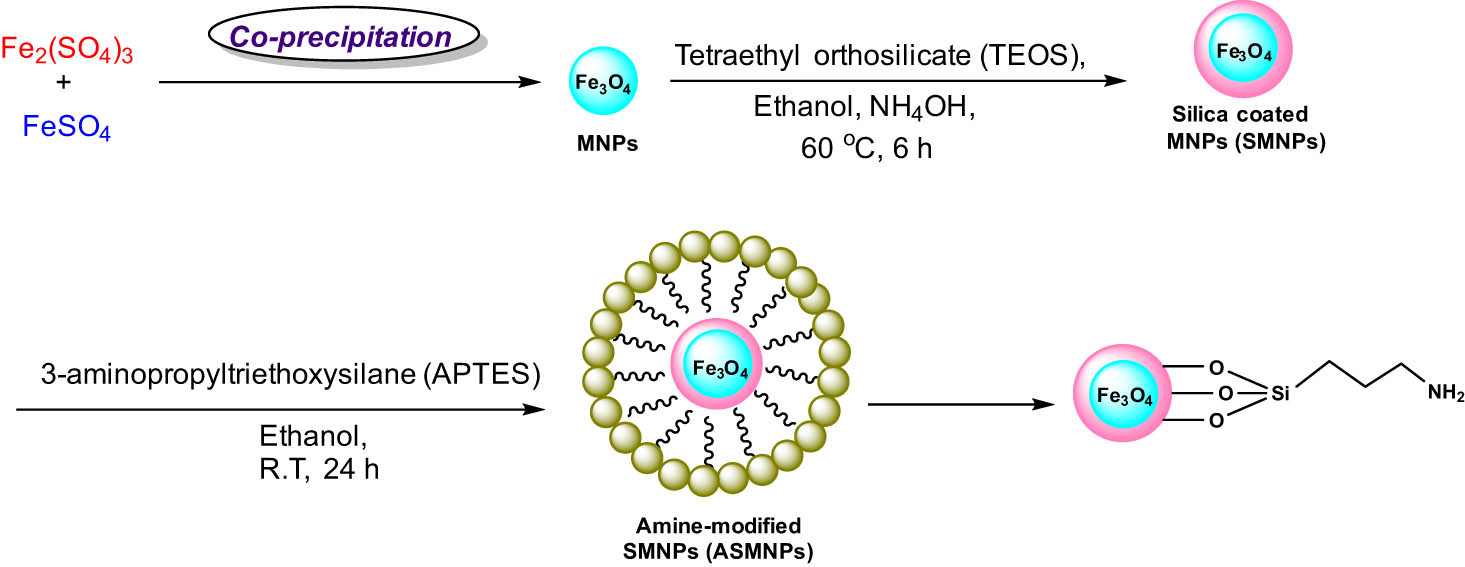
Synthesis of NH2@SiO2@Fe3O4 MNPs.
The mechanism of the reaction is driven specifically by the basic amino sites of the catalyst. Initially, Knoevenagel condensation between malononitrile 34 and aromatic aldehyde 10 formed the arylidiene malononitrile A, followed by Michael’s addition of dimedone 29 to arylidiene malononitrile A to form the intermediate B. Finally, intramolecular cyclization and protonation to the intermediate B results in the formation of the desired products 49, and the catalyst is regenerated in the reaction mixture (Scheme 28). In another study, a magnetic nanocatalyst Fe3O4@SiO2-imidazole (imid)-H3PMo12O4 NPs (PMA n ) was used for this reaction under ultrasonic irradiation or reflux conditions by Preeti and his co-workers [34].
![Scheme 28
Mechanism of synthesis of 2‑amino‑4H‑benzo[b]pyrans.](/document/doi/10.1515/hc-2022-0171/asset/graphic/j_hc-2022-0171_fig_028.jpg)
Mechanism of synthesis of 2‑amino‑4H‑benzo[b]pyrans.
An eco-friendly novel Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs nanocatalyst for the synthesis of chromene-annulated heterocycles via one-pot three-component reaction of aromatic aldehydes 10, various phenols (2-hydroxynaphthalene-1,4-dione 36/resorcinol 51/β-naphthol 50), and malononitrile 34 in ethanol under reflux conditions was explored in 2021. This method represents a novel and significant advancement in the synthesis of several chromene-annulated heterocycles with no usage of column chromatography, simple techniques, good yields, and magnetic recoverability of the catalyst (Scheme 29). The higher activity of this nanocatalyst due to its small size between 26 and 45 nm leads to the dispersion and diffusion of the nanocatalyst in the reaction mixture and can be rapidly taken out from the mixture with an external magnet. Furthermore, it can be reused directly in seven sequential runs without any loss in activity after washing with ethanol and drying it (Scheme 29) [35].
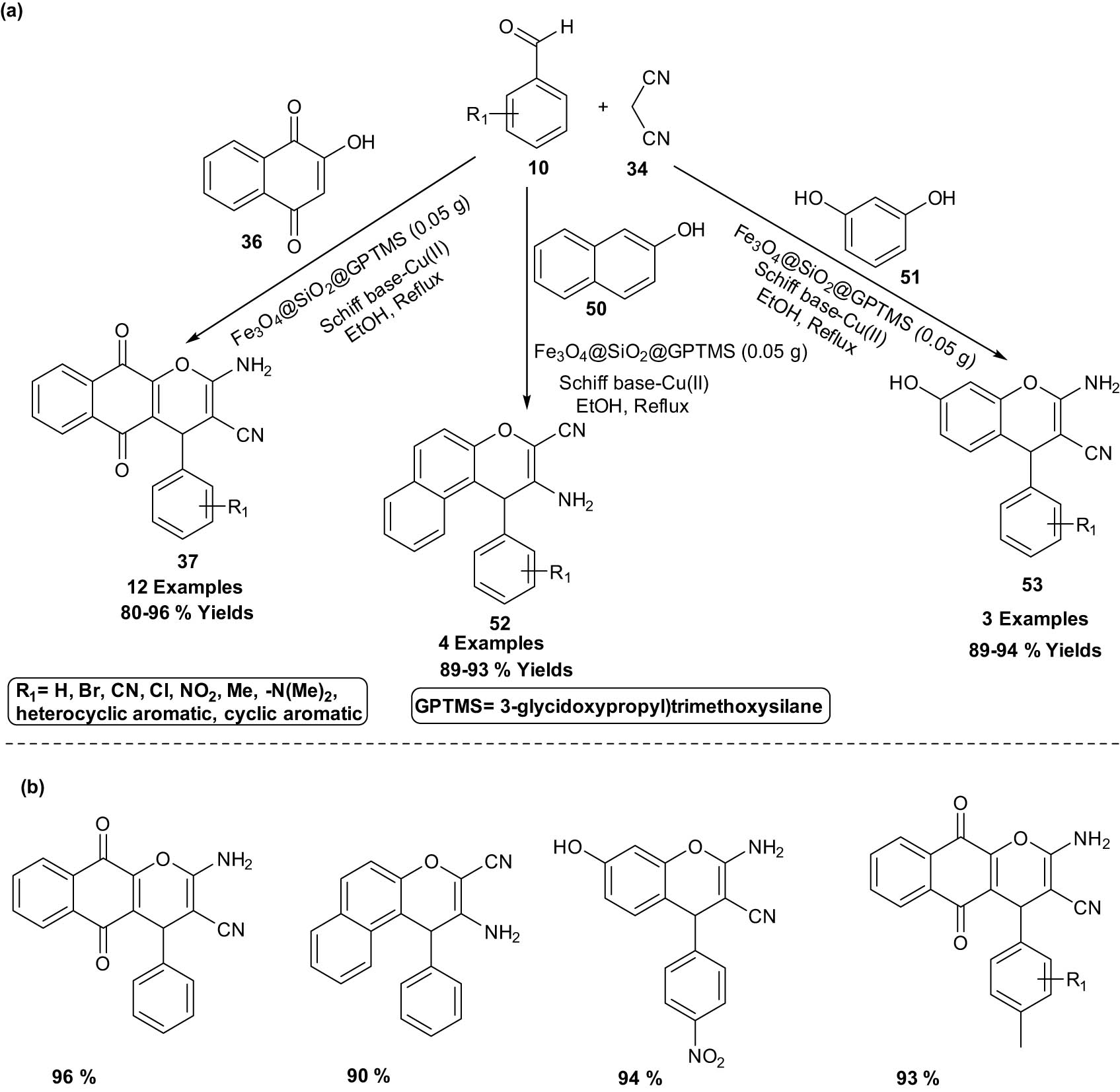
Fe3O4@SiO2 MNPs catalyzed the synthesis of chromene-annulated heterocycles. (a) Method overview; (b) representative examples.
According to the plausible mechanism of this reaction, Knoevenagel condensation reaction between aldehydes 10 and malononitrile 34 in the presence of the Schiff base complex of copper coated on epoxy modified Fe3O4@SiO2 MNPs nanocatalyst as a Lewis acid formed Knoevenagel product C. Then, the intermediate E is produced through Michael’s addition of 2-hydroxynaphthalene-1,4-dionein D with Knoevenagel product C. Finally, intramolecular nucleophilic cyclization of intermediate F, formed by enolization of intermediate E afforded the 2-amino-4H-chromene derivatives 37/52/53. Then, the nanocatalyst was removed by a magnetic field from the reaction mixture for further use (Scheme 30).
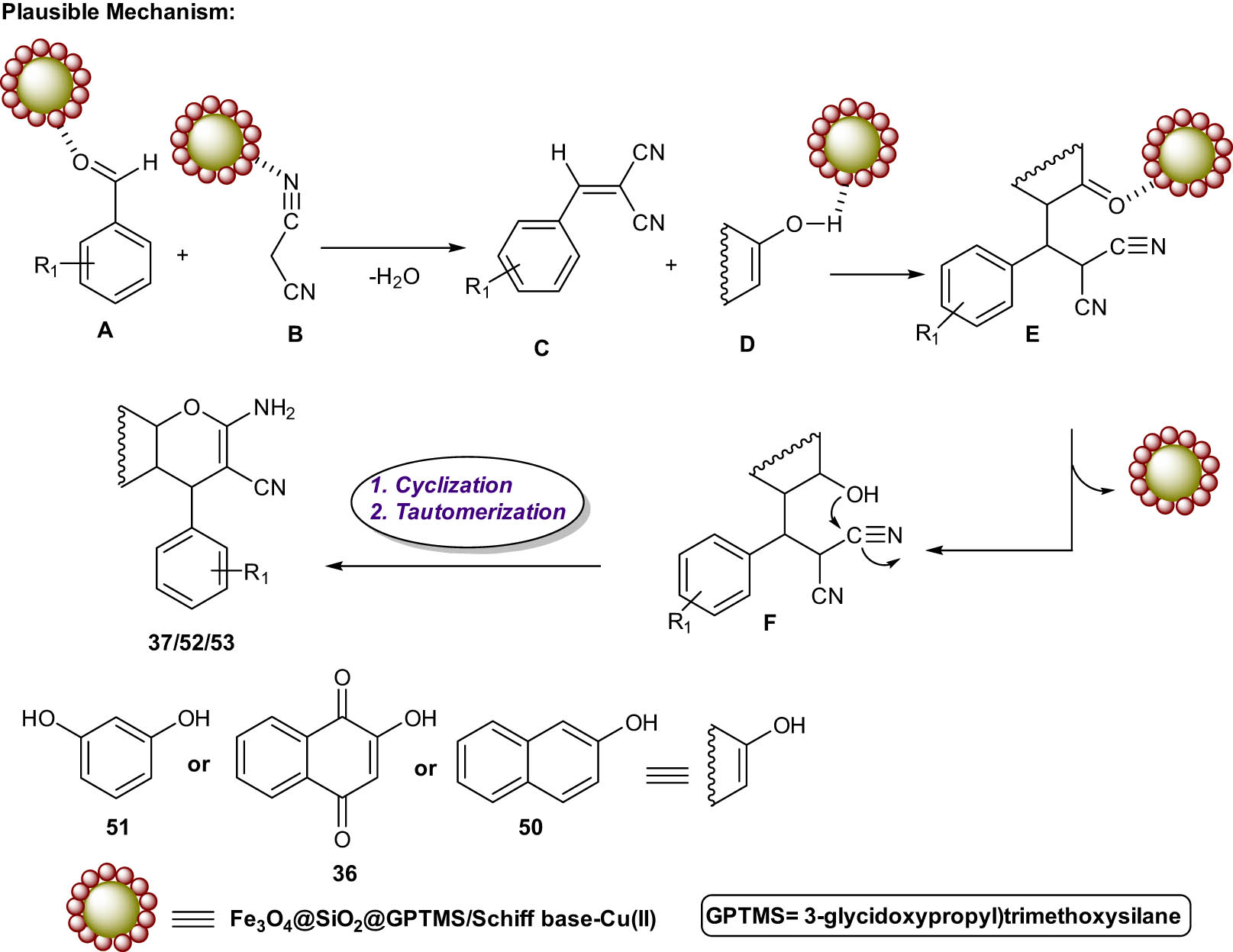
Plausible mechanism for the synthesis of chromene-annulated heterocycles.
2.3.3 Four-component reactions
Zahra and Mohammad explored a facile one-pot, four-component condensation reaction of ethyl-3-oxobutanoate 4, hydrazine hydrate 54, aromatic aldehydes 10, and barbituric acid 40 employing an efficient Cu-immobilized mesoporous silica NP, [Cu2+ @MSNs-(CO2 −)2] as a catalyst for the synthesis of pyrazolopyranopyrimidines 55 in water. The catalyst was characterized by using nitrogen adsorption–desorption analysis, SEM, small angle powder XRD, thermal analyses (TGA and DTA), FT-IR studies, SEM, TEM, and EDX. The catalyst can be easily recovered from the reaction mixture at the end and reused numerous times without any loss in its catalytic activity (Scheme 31). The present protocol offered a relatively short reaction time, ease of product isolation, efficiency, generality, and high yield of products [36].
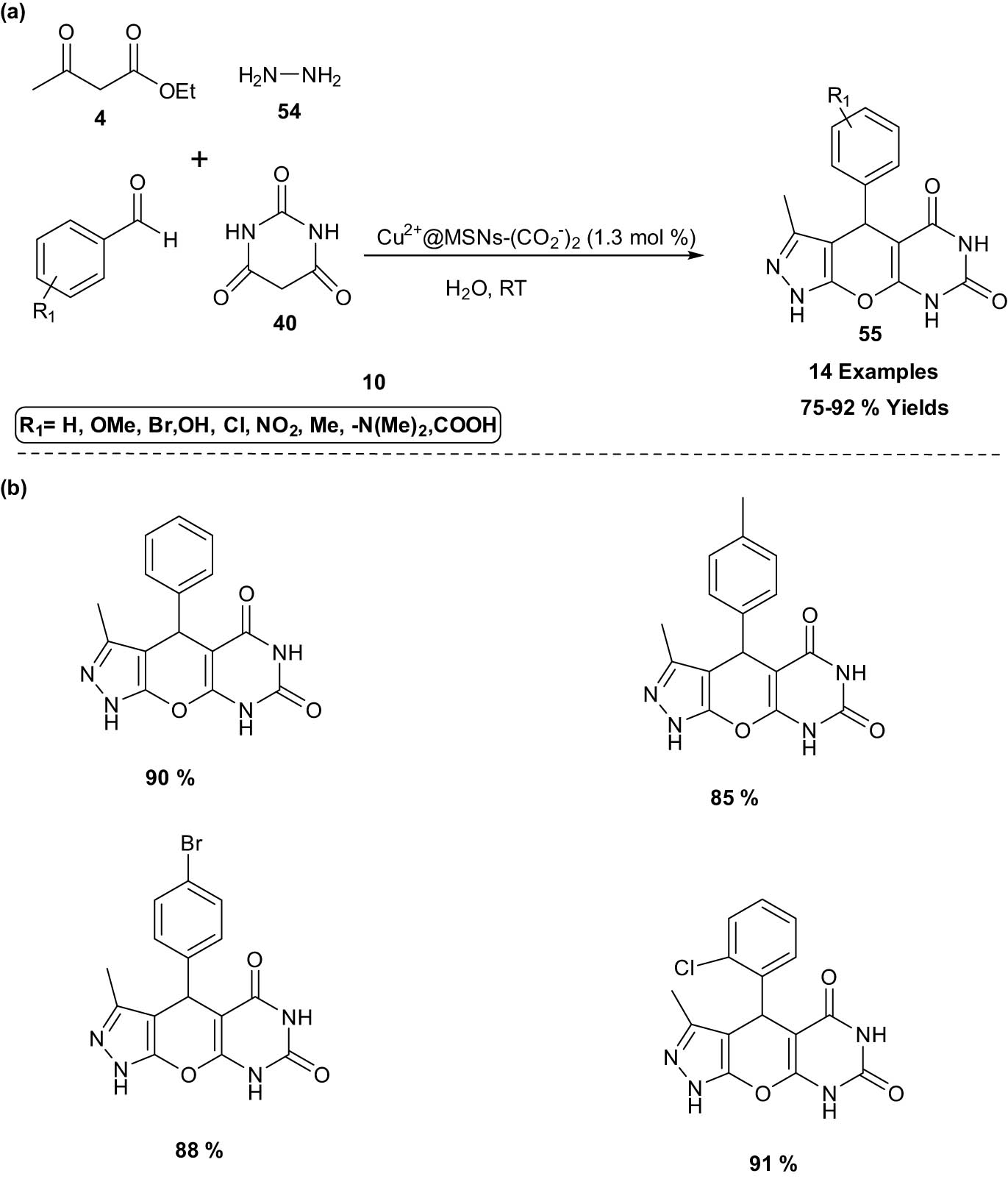
Cu-immobilized mesoporous silica NP synthesis of pyrazolopyranopyrimidines. (a) Method overview; (b) representative examples.
3 Conclusion
The present review focuses on the synthesis of oxygen-containing heterocyclic compounds employing recyclable nanocatalysts for the past ten years. Various non-toxic, simpler, environmentally friendly, and more affordable multicomponent synthetic approaches have successfully synthesized heterocycles of different sizes and ring systems containing oxygen atoms using various recyclable reusable nanocatalysts. The oxygen-containing heterocycles are a significant family of molecules in organic chemistry, mostly due to their wide range of biological roles and natural abundance. Well-known examples of promising medicinal substances are natural and semi-synthetic oxygen heterocyclic compounds, such as lovastatin (hypolipidemic), digoxin (treating CHF), cyclosporine-A (immunosuppressive), and taxol.
Acknowledgments
Ms. Thangjam Linda Devi is grateful to DST, Govt. of India, for INSPIRE-SRF fellowship (IF:200322).
-
Funding information: Authors state no funding involved.
-
Author contributions: Among the authors in the list, Thangjam Linda Devi designed this article and also wrote the manuscript. Dr. Kongbrailatpam Gayatri Sharma and Professor Okram Mukherjee Singh provided technical guidance and overall modifications of the article.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Shirzaei M, Mollashahi E, Maghsoodlou MT, Lashkari M. Novel synthesis of silica-coated magnetic nanoparticles based on acidic ionic liquid, as a highly efficient catalyst for three component system leads to furans derivatives. J Saudi Chem Soc. 2020;24:216–22.10.1016/j.jscs.2020.01.001Search in Google Scholar
[2] Khodaei MM, Alizadeh A, Haghipour M. Supported 4-carboxybenzyl sulfamic acid on magnetic nanoparticles as a recoverable and recyclable catalyst for synthesis of 3,4,5-trisubstituted furan-2(5H)-one derivatives. J Organomet Chem. 2018;870:58–67.10.1016/j.jorganchem.2018.06.012Search in Google Scholar
[3] West JL, Halas NJ. Applications of nanotechnology to biotechnology commentary. Curr Opin Biotechnol. 2000;11:215–7.10.1016/S0958-1669(00)00082-3Search in Google Scholar
[4] Salata OV. Applications of nanoparticles in biology and medicine. J Nanobiotechnol. 2004;2:1–6.10.1186/1477-3155-2-3Search in Google Scholar PubMed PubMed Central
[5] O’Connell MJ. Carbon nanotubes: properties and applications. 1st edn. Boca Raton: CRC Press; 2006.Search in Google Scholar
[6] Sanna V, Sechi M. Therapeutic potential of targeted nanoparticles and perspective on nanotherapies. ACS Med Chem Lett. 2020;11:1069–73.10.1021/acsmedchemlett.0c00075Search in Google Scholar PubMed PubMed Central
[7] Asmatulu R, Nguyen P, Asmatulu E. Chapter 5. Nanotechnology safety. Amsterdam, The Netherlands: Elsevier; 2013. p. 57–72. Nanotechnology Safety in the Automotive Industry.10.1016/B978-0-444-59438-9.00005-9Search in Google Scholar
[8] Qu X, Alvarez PJJ, Li Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013;47:3931–46.10.1016/j.watres.2012.09.058Search in Google Scholar PubMed
[9] Sozer N, Kokini JL. Nanotechnology and its applications in the food sector. Trends Biotechnol. 2009;27:82–9.10.1016/j.tibtech.2008.10.010Search in Google Scholar PubMed
[10] Mauter MS, Elimelech M. Environmental applications of carbon-based nanomaterials. Environ Sci Technol. 2008;42:5843–59.10.1021/es8006904Search in Google Scholar PubMed
[11] Yetisen AK, Qu H, Manbachi A, Butt H, Dokmeci MR, Hinestroza JP, et al. Nanotechnology in textiles. ACS Nano. 2016;10:3042–68.10.1021/acsnano.5b08176Search in Google Scholar PubMed
[12] Dhameliya TM, Donga HA, Vaghela PV, Panchal BG, Sureja DK, Bodiwala KB, et al. A decennary update on applications of metal nanoparticles (MNPs) in the synthesis of nitrogen and oxygen-containing heterocyclic scaffolds. RSC Adv. 2020;10:32740–820.10.1039/D0RA02272ASearch in Google Scholar
[13] Cao G, Wang Y. Nanostructures and nanomaterials: synthesis, properties, and applications. Vol. 2. 2nd edn. London: Imperial College Press; 2004. p. 510.1142/9781860945960Search in Google Scholar
[14] Klaine SJ, Alvarez PJ, Batley GE, Fernandes TF, Handy RD, Lyon DY, et al. Nanomaterials in the environment: behaviour, fate, bioavailability, and effects. Environ Toxicol Chem. 2008;27:1825–51.10.1897/08-090.1Search in Google Scholar PubMed
[15] Sharma KG. Inorganic nanoparticles promoted synthesis of heterocycles. Phys Sci Rev. 2022. 10.1515/psr-2022-0129 Search in Google Scholar
[16] Abboud M, Sahlabji T, Eissa M, Bel-Hadj-Tahar R, Mubarak AT, Al-Zaqri N, et al. Nickel(II)dibenzotetramethyltetraaza[14]annulene complex immobilized on amino-functionalized TUD-1: an efficient catalyst for immediate and quantitative epoxidation of cyclohexene under ambient conditions. New J Chem. 2020;44:20137–47.10.1039/D0NJ03822ASearch in Google Scholar
[17] Payra S, Saha A, Guchhaitb S, Banerjeea S. Direct CuO nanoparticles catalyzed synthesis of poly-substituted furans via oxidative C-H/C-H functionalization in aqueous medium. RSC Adv. 2016;6:33462–7.10.1039/C6RA04181GSearch in Google Scholar
[18] Ji G, Duan Y, Zhang S, Yang Y. Synthesis of benzofurans from terminal alkynes and iodophenols catalyzed by recyclable palladium nanoparticles supported on N,O-dual doped hierarchical porous carbon under copper- and ligand-free conditions. Catal Today. 2019;330:101–8.10.1016/j.cattod.2018.04.036Search in Google Scholar
[19] Ramazani A, Rouhani M, Joo SW. Silica nanoparticles from rice husk ash: a green catalyst for the one-pot three-component synthesis of benzo[b]furan derivatives. Adv Mat Res. 2014;875-877:202–7.10.4028/www.scientific.net/AMR.875-877.202Search in Google Scholar
[20] Vatanchian R, Mosslemin MH, Tabatabaee M, Sheibani A. Synthesis of trans-dihydroindeno[1,2-b]furans via nano γ-Fe2O3-quinuclidine-based catalyst in an aqueous medium. J Chem Res. 2018;42:598–600.10.3184/174751918X15411641337056Search in Google Scholar
[21] Hao F, Wang X, Mohammadnia M. Preparation and characterization of a novel magnetic nano catalyst for synthesis and antibacterial activities of novel furan-2(5H)-ones derivatives. Polycycl Aromat Compd. 2022;42:4255–69.10.1080/10406638.2021.1887298Search in Google Scholar
[22] Wang Z, Li X, Feng L, Liu B, Shamsa F. DFNS/α-CD/Au as a nano-catalyst for interpolation of CO2 into aryl alkynes followed by SN2 coupling with allylic chlorides. Catal Lett. 2021;151:1911–22.10.1007/s10562-020-03451-1Search in Google Scholar
[23] Menendez CA, Nador F, Radivoy G, Gerbino DC. One-step synthesis of xanthones catalyzed by a highly efficient copper-based magnetically recoverable nano-catalyst. Org Lett. 2014;16:2846–9.10.1021/ol500964eSearch in Google Scholar PubMed
[24] Dandia A, Gupta SL, Indora A, Saini P, Parewa V, Rathore KS. Ag NPs decked GO composite as a competent and reusable catalyst for ‘ON WATER’ chemoselective synthesis of pyrano[2,3-c:6,5-c’]dipyrazol]-2-ones. Tetrahedron Lett. 2017;58:1170–5.10.1016/j.tetlet.2017.02.014Search in Google Scholar
[25] Rajabi F, Dios MP, Abdollahi M, Luque R. Aqueous synthesis of 1,8-dioxo-octahydroxanthenes using supported cobalt nanoparticles as a highly efficient and recyclable nano-catalyst. Catal Commun. 2019;120:95–100.10.1016/j.catcom.2018.10.004Search in Google Scholar
[26] Steingruber HS, Mendioroz P, Diez AS, Gerbino DC. A green nanopalladium-supported catalyst for the microwave assisted direct synthesis of xanthones. Synth. 2019;51:A–J.10.1055/s-0039-1691069Search in Google Scholar
[27] Nezhad AK, Nourisefata M, Panahi F. l-cysteine functionalized magnetic nanoparticles (LCMNP): a novel magnetically separable organocatalyst for one-pot synthesis of 2- amino-4H-chromene-3-carbonitriles in water. Org Biomol Chem. 2015;13:7772–9.10.1039/C5OB01030FSearch in Google Scholar
[28] Ghomi JS, Eshteghal F, Alavi HS. A facile one-pot ultrasound assisted for an efficient synthesis of benzo[g] chromenes using Fe3O4/polyethylene glycol (PEG) core/shell nanoparticles. Ultrason Sonochem. 2016;33:99–105.10.1016/j.ultsonch.2016.04.025Search in Google Scholar PubMed
[29] Azarifar D, Ghaemi M. Thiourea dioxide‐grafted γ‐Fe2O3/HAp magnetic nanoparticles: efficient, green and reusable nano-catalyst for synthesis of pyranopyridine derivatives. Appl Organomet Chem. 2017;31:e3834.10.1002/aoc.3834Search in Google Scholar
[30] Ghandi L, Miraki MK, Radfar I, Yazdani E, Heydari A. Formamidinesulfinic acid-functionalized Fe3O4@SiO2 as a green and magnetic recyclable catalyst for synthesis of pyrano[2,3-d] pyrimidinone derivatives. ChemistrySelect. 2018;3:1787–92.10.1002/slct.201702887Search in Google Scholar
[31] Pourshojaei Y, Jadidi MH, Eskandari K, Foroumadi A, Asadipour A. An eco-friendly synthesis of 4-aryl-substituted pyranofuzed coumarins as potential pharmacological active heterocycles using molybdenum oxide nanoparticles as an effective and recyclable catalyst. Res Chem Intermed. 2018;44:4195–212.10.1007/s11164-018-3363-7Search in Google Scholar
[32] Sadjadi S, Heravi MM, Malmir M. Bio‐assisted synthesized Ag(0) nanoparticles immobilized on SBA‐15/cyclodextrin nanosponge adduct: efficient heterogeneous catalyst for the ultrasonic‐assisted synthesis of benzopyranopyrimidines. Appl Organomet Chem. 2018;32:e4286.10.1002/aoc.4286Search in Google Scholar
[33] Singh P, Yadav P, Mishra A, Satish K, Awasthi SK. Green and mechanochemical one-pot multicomponent synthesis of bioactive 2-amino-4H-benzo[b]pyrans via highly efficient amine functionalized SiO2@Fe3O4 nanoparticles. ACS Omega. 2020;5:4223–32.10.1021/acsomega.9b04117Search in Google Scholar PubMed PubMed Central
[34] Esmaeilpour M, Javidi J, Dehghania F, Dodejib FN. A green one‐pot three‐component synthesis of tetrahydrobenzo [b]pyran and 3,4‐dihydropyrano[c]chromene derivatives using Fe3O4@SiO2-imid-PMAn magnetic nanocatalystunder ultrasonic irradiationor reflux conditions. RSC Adv. 2015;5:26625–33.10.1039/C5RA01021GSearch in Google Scholar
[35] Rezayati S, Ramazani A, Sajjadifar S, Aghahosseini H, Rezaei A. Design of a schiff base complex of copper coated on epoxy modified core-shell MNPs as an environmentally friendly and novel catalyst for the one-pot synthesis of various chromene annulated heterocycles. ACS Omega. 2021;6:25608–22.10.1021/acsomega.1c03672Search in Google Scholar PubMed PubMed Central
[36] Nasresfahani Z, Kassaee MZ. Cu-immobilized mesoporous silica nanoparticles [Cu2+ @MSNs-(CO2¯)2] as an efficient nano-catalyst for one-pot synthesis of pyrazolopyranopyrimidines in water. ChemistrySelect. 2017;15:9637–41.Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Structural simplification of the 3‐nitroimidazo[1,2‐a]pyridine antileishmanial pharmacophore: Design, synthesis, and antileishmanial activity of novel 2,4-disubstituted 5-nitroimidazoles
- Synthesis of a novel water-soluble pyridine dicarboxylate and its application in fluorescence cell imaging
- Synthesis of novel meta-diamide compounds containing pyrazole moiety and their insecticidal evaluation
- Review Articles
- Inorganic nanoparticles promoted synthesis of oxygen-containing heterocycles
- Gold-catalyzed synthesis of small-sized carbo- and heterocyclic compounds: A review
- Synthesis of imidazole derivatives in the last 5 years: An update
- Current progress in the synthesis of imidazoles and their derivatives via the use of green tools
- Synthetic and therapeutic review of triazoles and hybrids
Articles in the same Issue
- Research Articles
- Structural simplification of the 3‐nitroimidazo[1,2‐a]pyridine antileishmanial pharmacophore: Design, synthesis, and antileishmanial activity of novel 2,4-disubstituted 5-nitroimidazoles
- Synthesis of a novel water-soluble pyridine dicarboxylate and its application in fluorescence cell imaging
- Synthesis of novel meta-diamide compounds containing pyrazole moiety and their insecticidal evaluation
- Review Articles
- Inorganic nanoparticles promoted synthesis of oxygen-containing heterocycles
- Gold-catalyzed synthesis of small-sized carbo- and heterocyclic compounds: A review
- Synthesis of imidazole derivatives in the last 5 years: An update
- Current progress in the synthesis of imidazoles and their derivatives via the use of green tools
- Synthetic and therapeutic review of triazoles and hybrids

