Abstract
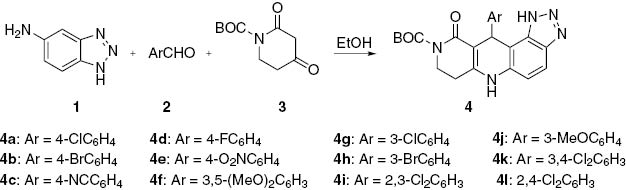
A three-component reaction of an aromatic aldehyde, 1H-benzo[d][1,2,3]triazol-5-amine and tert-butyl 2,4-dioxopiperidine-1-carboxylate in refluxing EtOH under catalyst-free conditions furnishes the title compounds in high yields.
Introduction
Naphthyridines are an important class of heterocycles with significant pharmacological and biological activities, especially antibacterial activity [1–5]. Many triazoles are also bioactive [6]. To the best of our knowledge, only one example of a triazolobenzonaphthyridine has been described in the literature concerning the synthesis and potential application as an antimicrobial agent [7]. As a continuation of our research devoted to the development of new methods for preparing heterocycles via multi-component reactions [8–12], we now report the synthesis of a series of substituted triazolobenzonaphthyridines 4 by a three-component reaction of an aromatic aldehyde, 1H-benzo[d][1,2,3]triazol-5-amine and tert-butyl 2,4-dioxopiperidine-1-carboxylate (Scheme 1).
Results and discussion
The required benzotriazolamine 1 was prepared in a two-step procedure starting with commercially available 4-nitrobenzene-1,2-diamine as described in the literature [13]. Subsequently, the amine 1 was allowed to react with equimolar amounts of aromatic aldehyde 2 and tert-butyl 2,4-dioxopiperidine-1-carboxylate (3) in ethanol under reflux conditions. This reaction produced the desired product 4 in high yield (Scheme 1).
Synthesis of product 4a from the amine 1, 4-chlorobenzaldehyde (2a) and tert-butoxycarbonyl (BOC) derivative 3 was used as a model reaction for optimization. Several reaction parameters including temperature, catalysts and solvents were varied. Only trace amount of 4a was detected by TLC for the reaction conducted at room temperature. The reaction could not be catalyzed by bases (5 mol%), such as DBU, Et3N, piperidine and NaHCO3 using a variety of solvents, including alcohols, N,N-dimethylformamide, acetonitrile, dioxane and tetrahydrofuran. The highest yield of 4a of 92% was obtained for the reaction conducted in ethanol without any additive under reflux conditions. After cooling, the resulting precipitate of 4a was filtered. By using this simple workup, compound 4a was obtained in an analytically pure form. Crystallization was not necessary. The remaining products 4b–l were synthesized in high yields under similar conditions. As can be seen from Scheme 1, benzaldehydes substituted with an electron-withdrawing or electron-donating group can successfully be used. The steric hindrance of an ortho-substitution does not hinder the product formation, as evidenced by synthesis of 4i and 4l in the respective yields of 94% and 91%. All products were characterized by IR, 1H NMR and HRMS.
The mechanism of the formation of 4 is suggested in Scheme 2. As can be seen, the initial Knoevenagel condensation product 5 undergoes Michael addition with 1. The resultant adduct 6 undergoes an intramolecular cyclization to 7 which is a direct precursor to 4. The observed regioselectivity for the addition reaction of 1 is apparently a result of a higher reactivity of the position 4 in comparison to the position 6. More specifically, the electron density at position 4 is greater than that at position 6 because the position 4 is ortho to the amino group and α to the nitrogen atom of the adjacent triazole.

Conclusion
A mild, facile, efficient and environmentally benign method was developed for the synthesis of 1H-[1,2,3]triazolo[4′,5′:3,4]benzo[1,2-b][1,6]naphthyridine-9(6H)-carboxylate derivatives 4 under catalyst-free conditions.
Experimental
Melting points were determined in open capillaries and are uncorrected. IR spectra were recorded on a Tensor 27 spectrometer in KBr pellets. 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were taken in DMSO-d6 with Me4Si as internal standard using a Bruker-400 spectrometer. HR-MS analyses were carried out using a Bruker-micro-TOF-Q-MS analyzer. 1H-Benzo[d][1,2,3]triazol-5-amine (2) was prepared by a two-step procedure from the 4-nitrobenzene-1,2-diamine according to the literature [13].
General procedure for the synthesis of compounds 4
A dry flask (50 mL) was charged with 1H-benzo[d][1,2,3]triazol-5-amine (1, 134 mg, 1.0 mmol), aromatic aldehyde 2 (1.0 mmol), tert-butyl 2,4-dioxopiperidine-1-carboxylate (3, 213 mg, 1.0 mmol), and EtOH (10.0 mL). The reaction mixture was stirred at 80°C for 6–10 h until the starting material 1 was consumed as monitored by TLC. Product 4 was obtained directly by simple filtration after cooling the mixture to room temperature.
tert-Butyl 11-(4-chlorophenyl)-10-oxo-7,8,10,11-tetrahydro-1H-[1, 2,3]triazolo [4′,5′:3,4]benzo[1,2-b][1,6]naphthyridine-9(6H)-carboxylate (4a)
This compound was obtained in 92% yield (0.415 g) as a pale yellow solid; mp 234–236°C; IR: ν 3423, 3281, 2974, 2889, 2824, 1735, 1639, 1546, 1519, 1492, 1467, 1369, 1321, 1247, 1212, 1158, 1138, 1050, 1013, 945, 850, 810, 779 cm-1; 1H NMR: δH 1.41 (s, 9H, 3CH3), 2.64–2.68 (m, 1H, CH), 2.76–2.84 (m, 1H, CH), 3.47–3.52 (m, 1H, CH), 3.99–4.02 (m, 1H, CH), 5.55 (s, 1H, CH), 7.10 (s, 1H, ArH), 7.24 (d, J = 8.4 Hz, 2H, ArH), 7.37 (d, J = 8.4 Hz, 2H, ArH), 7.81 (s, 1H, ArH), 9.96 (s, 1H, NH), 15.55 (s, 1H, NH); 13C NMR: δC 164.5, 162.8, 152.9, 149.1, 145.8, 142.3, 135.3, 131.3, 129.8, 128.5, 118.3, 115.2, 105.3, 100.2, 81.5, 42.6, 36.9, 28.2, 26.8. ESI-HR-MS. Calcd for C23H21ClN5O3 [M-H]-: m/z 450.1332. Found: m/z 450.1324.
tert-Butyl 11-(4-bromophenyl)-10-oxo-7,8,10,11-tetrahydro-1H-[1, 2,3]triazolo[4′,5′:3,4] benzo[1,2-b][1,6]naphthyridine-9(6H)-carboxylate (4b)
This compound was obtained in 90% yield (0.446 g) as a pale yellow solid; mp 231–232°C; IR: ν 3420, 3283, 2981, 2934, 2888, 1736, 1636, 1546, 1492, 1467, 1398, 1320, 1248, 1208, 1136, 1073, 1051, 989, 946, 849, 836, 781, 748 cm-1; 1H NMR: δH 1.41 (s, 9H, 3CH3), 2.63–2.67 (m, 1H, CH), 2.76–2.84 (m, 1H, CH), 3.49–3.53 (m, 1H, CH), 3.98–4.01 (m, 1H, CH), 5.56 (s, 1H, CH), 7.11 (d, J = 8.0 Hz, 1H, ArH), 7.31 (d, J = 8.4 Hz, 2H, ArH), 7.38 (d, J = 8.4 Hz, 2H, ArH), 7.77 (s, 1H, ArH), 9.94 (s, 1H, NH), 15.52 (s, 1H, NH); 13C NMR: δC 164.5, 162.0, 152.9, 151.8, 149.1, 148.5, 146.3, 131.4, 130.8, 130.2, 119.8, 118.1, 115.5, 100.0, 81.6, 42.6, 37.0, 28.2, 26.8. ESI-HR-MS. Calcd for C23H21BrN5O3 [M-H]-: m/z 494.0827. Found: m/z 494.0836.
tert-Butyl 11-(4-cyanophenyl)-10-oxo-7,8,10,11-tetrahydro-1H-[1,2,3]triazolo[4′,5′:3,4] benzo[1,2-b][1,6]naphthyridine-9(6H)-carboxylate (4c)
This compound was obtained in 93% yield (0.411 g) as a pale yellow solid; mp 235–237°C; IR: ν 3412, 3292, 2980, 2932, 2884, 2229, 1724, 1683, 1620, 1546, 1492, 1463, 1396, 1328, 1248, 1215, 1139, 1053, 967, 942, 851, 813, 779, 749 cm-1; 1H NMR: δH 1.41 (s, 9H, 3CH3), 2.66–2.70 (m, 1H, CH), 2.77–2.85 (m, 1H, CH), 3.49–3.53 (m, 1H, CH), 3.97–4.01 (m, 1H, CH), 5.65 (s, 1H, CH), 7.13 (s, 1H, ArH), 7.55 (d, J = 8.4 Hz, 2H, ArH), 7.68 (d, J = 8.4 Hz, 2H, ArH), 7.83 (s, 1H, ArH), 10.02 (s, 1H, NH), 15.54 (s, 1H, NH); 13C NMR: δC 164.5, 162.8, 152.8, 152.1, 149.5, 142.1, 134.6, 132.6, 129.0, 124.0, 119.3, 116.2, 109.5, 105.2, 99.4, 81.6, 42.6, 37.8, 28.2, 26.8. ESI-HR-MS. Calcd for C24H21N6O3 [M-H]-: m/z 441.1674. Found: m/z 441.1670.
tert-Butyl 11-(4-fluorophenyl)-10-oxo-7,8,10,11-tetrahydro-1H-[1,2,3]triazolo[4′,5′:3,4] benzo[1,2-b][1,6]naphthyridine-9(6H)-carboxylate (4d)
This compound was obtained in 87% yield (0.379 g) as a pale yellow solid; mp 227–229°C; IR: ν 3416, 3366, 2978, 2875, 1733, 1652, 1552, 1491, 1428, 1389, 1369, 1278, 1216, 1135, 1022, 1008, 970, 932, 890, 848, 783, 749 cm-1; 1H NMR: δH 1.41 (s, 9H, 3CH3), 2.65–2.69 (m, 1H, CH), 2.76–2.85 (m, 1H, CH), 3.46–3.53 (m, 1H, CH), 3.99–4.02 (m, 1H, CH), 5.58 (s, 1H, CH), 6.97–7.03 (m, 2H, ArH), 7.11 (d, J = 6.4 Hz, 1H, ArH), 7.37–7.40 (m, 2H, ArH), 7.78 (s, 1H, ArH), 9.93 (s, 1H, NH), 15.54 (s, 1H, NH); 13C NMR: δC 164.6, 162.8, 161.1 (d, J(F-C) = 241 Hz), 152.9, 149.0, 143.2, 142.0, 135.2, 132.6, 129.7 (d, J(F-C) = 8 Hz), 118.3, 115.2 (d, J(F-C) = 21 Hz), 106.0, 100.3, 81.5, 42.6, 36.7, 28.2, 26.8. ESI-HR-MS. Calcd for C23H21FN5O3 [M-H]-: m/z 434.1628. Found: m/z 434.1644.
tert-Butyl 11-(4-nitrophenyl)-10-oxo-7,8,10,11-tetrahydro-1H-[1,2,3]triazolo[4′,5′:3,4] benzo[1,2-b][1,6]naphthyridine-9(6H)-carboxylate (4e)
This compound was obtained in 92% yield (0.425 g) as a yellow solid; mp 239–241°C; IR: ν 3420, 3301, 2977, 2932, 2880, 1724, 1620, 1605, 1545, 1514, 1492, 1463, 1394, 1324, 1287, 1213, 1138, 1053, 966, 942, 827, 816, 785, 722 cm-1; 1H NMR: δH 1.40 (s, 9H, 3CH3), 2.66–2.70 (m, 1H, CH), 2.78–2.86 (m, 1H, CH), 3.49–3.56 (m, 1H, CH), 3.97–4.01 (m, 1H, CH), 5.70 (s, 1H, CH), 7.11 (d, J = 8.8 Hz, 1H, ArH), 7.62 (d, J = 8.4 Hz, 2H, ArH), 7.86 (d, J = 8.8 Hz, 1H, ArH), 8.08 (d, J = 8.4 Hz, 2H, ArH), 10.08 (s, 1H, NH), 15.56 (s, 1H, NH); 13C NMR: δC 164.3, 162.8, 152.7, 149.6, 143.9, 134.0, 132.5, 131.5, 128.9, 127.2, 118.3, 114.9, 111.0, 98.8, 81.6, 42.9, 36.5, 28.2, 26.9. ESI-HR MS. Calcd for C23H21N6O5 [M-H]-: m/z 461.1573. Found: m/z 461.1564.
tert-Butyl 11-(3,5-dimethoxyphenyl)-10-oxo-7,8,10,11-tetrahydro-1H-[1,2,3]triazolo [4′,5′:3,4]benzo[1,2-b][1,6]naphthyridine-9(6H)-carboxylate (4f)
This compound was obtained in 90% yield (0.429 g) as a gray solid; mp 209–210°C; IR: ν 3416, 3296, 2997, 2934, 2885, 2835, 1710, 1622, 1595, 1547, 1489, 1430, 1396, 1371, 1339, 1226, 1205, 1161, 1062, 1047, 992, 802, 780 cm-1; 1H NMR: δH 1.42 (s, 9H, 3CH3), 2.67–2.71 (m, 1H, CH), 2.75–2.84 (m, 1H, CH), 3.48–3.54 (m, 1H, CH), 3.64 (s, 6H, 2CH3O), 3.99–4.03 (m, 1H, CH), 5.45 (s, 1H, CH), 6.23 (s, 1H, ArH), 6.54 (s, 2H, ArH), 7.06 (d, J = 8.8 Hz, 1H, ArH), 7.80 (d, J = 8.8 Hz, 1H, ArH), 9.86 (s, 1H, NH), 15.52 (s, 1H, NH); 13C NMR: δC 164.6, 160.5, 152.9, 149.1, 142.2, 135.3, 132.6, 118.1, 115.1, 110.2, 106.4, 105.9, 100.3, 97.8, 81.5, 56.5, 42.7, 37.5, 28.2, 26.7. ESI-HR-MS. Calcd for C25H26N5O5 [M-H]-: m/z 476.1933. Found: m/z 476.1932.
tert-Butyl 11-(3-chlorophenyl)-10-oxo-7,8,10,11-tetrahydro-1H-[1,2,3]triazolo[4′,5′:3,4] benzo[1,2-b][1,6]naphthyridine-9(6H)-carboxylate (4g)
This compound was obtained in 87% yield (0.392 g) as a pale yellow solid; mp 220–221°C; IR: ν 3416, 3215, 2973, 2887, 1717, 1600, 1545, 1489, 1420, 1397, 1336, 1252, 1211, 1142, 1051, 992, 949, 893, 777 cm-1; 1H NMR: δH 1.41 (s, 9H, 3CH3), 2.67–2.71 (m, 1H, CH), 2.76–2.84 (m, 1H, CH), 3.48–3.54 (m, 1H, CH), 3.98–4.01 (m, 1H, CH), 5.54 (s, 1H, CH), 7.08–7.14 (m, 2H, ArH), 7.19–7.23 (m, 2H, ArH), 7.48 (s, 1H, ArH), 7.84 (d, J = 6.8 Hz, 1H, ArH), 9.99 (s, 1H, NH), 15.57 (s, 1H, NH); 13C NMR: δC 164.6, 162.8, 152.8, 149.3, 142.1, 135.5, 133.1, 132.6, 130.6, 127.7, 126.6, 121.9, 118.4, 115.3, 105.4, 100.1, 81.6, 42.6, 37.3, 28.2, 26.8. ESI-HR-MS. Calcd for C23H21ClN5O3 [M-H]-: m/z 450.1332. Found: m/z 450.1326.
tert-Butyl 11-(3-bromophenyl)-10-oxo-7,8,10,11-tetrahydro-1H-[1, 2,3]triazolo[4′,5′:3,4] benzo[1,2-b][1,6]naphthyridine-9(6H)-carboxylate (4h)
This compound was obtained in 89% yield (0.441 g) as a pale yellow solid; mp 214–216°C; IR: ν 3443, 3297, 2972, 2923, 2886, 1716, 1624, 1599, 1567, 1545, 1488, 1421, 1398, 1335, 1252, 1224, 1158, 1142, 1050, 992, 891, 776 cm-1; 1H NMR: δH 1.42 (s, 9H, 3CH3), 2.66–2.71 (m, 1H, CH), 2.75–2.83 (m, 1H, CH), 3.49–3.54 (m, 1H, CH), 3.97–4.01 (m, 1H, CH), 5.52 (s, 1H, CH), 7.09 (d, J = 8.8 Hz, 1H, ArH), 7.13–7.17 (m, 1H, ArH), 7.27 (s, 2H, ArH), 7.63 (s, 1H, ArH), 7.84 (d, J = 8.8 Hz, 1H, ArH), 9.99 (s, 1H, NH), 15.57 (s, 1H, NH); 13C NMR: δC 164.5, 162.8, 152.8, 149.3, 142.2, 135.5, 132.6, 131.0, 130.6, 129.6, 127.0, 121.9, 118.5, 115.2, 105.2, 100.1, 81.6, 42.6, 37.3, 28.2, 26.8. ESI-HR-MS. Calcd for C23H21BrN5O3 [M-H]-: m/z 494.0827. Found: m/z 494.0834.
tert-Butyl 11-(2,3-dichlorophenyl)-10-oxo-7,8,10,11-tetrahydro-1H-[1,2,3]triazolo [4′,5′:3,4]benzo[1,2-b][1,6]naphthyridine-9(6H)-carboxylate (4i)
This compound was obtained in 94% yield (0.456 g) as a brown solid; mp 231–232°C; IR: ν 3367, 3173, 2977, 2904, 2831, 1735, 1646, 1605, 1551, 1486, 1423, 1394, 1306, 1280, 1216, 1136, 1050, 964, 943, 842, 773 cm-1; 1H NMR: δH 1.40 (s, 9H, 3CH3), 2.60–2.64 (m, 1H, CH), 2.75–2.83 (m, 1H, CH), 3.43–3.47 (m, 1H, CH), 3.97–4.01 (m, 1H, CH), 6.22 (s, 1H, CH), 7.18 (s, 1H, ArH), 7.33 (d, J = 6.8 Hz, 1H, ArH), 7.42 (s, 1H, ArH), 7.58 (s, 1H, ArH), 7.84 (s, 1H, ArH), 9.91 (s, 1H, NH), 15.52 (s, 1H, NH); 13C NMR: δC 164.4, 162.8, 152.6, 149.5, 147.7, 143.4, 136.5, 132.5, 131.2, 128.5, 127.9, 118.3, 114.9, 110.9, 105.9, 99.1, 81.5, 42.6, 37.6, 28.2, 26.9. ESI-HR-MS. Calcd for C23H20Cl2N5O3 [M-H]-: m/z 484.0942. Found: m/z 484.0940.
tert-Butyl 11-(3-methoxyphenyl)-10-oxo-7,8,10,11-tetrahydro-1H-[1, 2,3]triazolo[4′,5′:3,4] benzo[1,2-b][1,6]naphthyridine-9(6H)-carboxylate (4j)
This compound was obtained in 86% yield (0.384 g) as a pale yellow solid; mp 207–208°C; IR: ν 3416, 3366, 2977, 2874, 2839, 1740, 1645, 1585, 1494, 1459, 1388, 1304, 1265, 1212, 1154, 1079, 1021, 962, 880, 838, 769 cm-1; 1H NMR: δH 1.42 (s, 9H, 3CH3), 2.66–2.70 (m, 1H, CH), 2.76–2.84 (m, 1H, CH), 3.47–3.53 (m, 1H, CH), 3.67 (s, 3H, CH3O), 3.99–4.03 (m, 1H, CH), 5.50 (s, 1H, CH), 6.64–6.66 (m, 1H, ArH), 6.89 (d, J = 7.6 Hz, 1H, ArH), 7.01 (s, 1H, ArH), 7.06–7.11 (m, 2H, ArH), 7.80 (d, J = 8.8 Hz, 1H, ArH), 9.92 (s, 1H, NH), 15.54 (s, 1H, NH); 13C NMR: δC 164.6, 159.5, 152.3, 148.9, 148.3, 142.2, 135.3, 132.6, 129.7, 120.0, 118.1, 115.1, 114.2, 111.5, 105.9, 100.45, 81.5, 56.5, 42.6, 37.4, 28.2, 26.8. ESI-HR-MS. Calcd for C24H24N5O4 [M-H]-: m/z 446.1828. Found: m/z 446.1839.
tert-Butyl 11-(3,4-dichlorophenyl)-10-oxo-7,8,10,11-tetrahydro-1H-[1,2,3]triazolo [4′,5′:3,4]benzo[1,2-b][1,6]naphthyridine-9 (6H)-carboxylate (4k)
This compound was obtained in 92% yield (0.455 g) as a brown solid; mp 222–224°C; IR: ν 3420, 3367, 2987, 2906, 2870, 2833, 1737, 1651, 1551, 1487, 1426, 1394, 1305, 1279, 1139, 1076, 1031, 944, 901, 847, 830, 781 cm-1; 1H NMR: δH 1.41 (s, 9H, 3CH3), 2.64–2.70 (m, 1H, CH), 2.75–2.83 (m, 1H, CH), 3.50–3.55 (m, 1H, CH), 3.96–4.99 (m, 1H, CH), 5.53 (s, 1H, CH), 7.09 (d, J = 8.8 Hz, 1H, ArH), 7.21–7.23 (m, 1H, ArH), 7.45 (d, J = 8.4 Hz, 1H, ArH), 7.67 (s, 1H, ArH), 7.86 (d, J = 8.8 Hz, 1H, ArH), 10.03 (s, 1H, NH), 15.57 (s, 1H, NH); 13C NMR: δC 164.5, 162.8, 152.8, 149.2, 147.5, 142.2, 135.4, 132.5, 131.0, 129.8, 129.4, 128.3, 118.7, 115.2, 104.6, 99.9, 81.6, 42.6, 37.0, 28.2, 26.7. ESI-HR-MS. Calcd for C23H20Cl2N5O3 [M-H]-: m/z 484.0942. Found: m/z 484.0939.
tert-Butyl 11-(2,4-dichlorophenyl)-10-oxo-7,8,10,11-tetrahydro-1H-[1,2,3]triazolo [4′,5′:3,4]benzo[1,2-b][1,6]naphthyridine-9(6H)-carboxylate (4l)
This compound was obtained in 91% yield (0.441 g) as a pale yellow solid; mp 227–228°C; IR: ν 3420, 3377, 2979, 2883, 2834, 1728, 1650, 1604, 1586, 1555, 1489, 1425, 1393, 1323, 1281, 1216, 1134, 1051, 1006, 998, 943, 892, 844, 770 cm-1; 1H NMR: δH 1.40 (s, 9H, 3CH3), 2.58–2.63 (m, 1H, CH), 2.74–2.82 (m, 1H, CH), 3.45–3.51 (m, 1H, CH), 3.97–4.00 (m, 1H, CH), 6.12 (s, 1H, CH), 7.15 (d, J = 6.8 Hz, 1H, ArH), 7.26–7.35 (m, 2H, ArH), 7.46 (s, 1H, ArH), 7.82 (s, 1H, ArH), 9.89 (s, 1H, NH), 15.52 (s, 1H, NH); 13C NMR: δC 164.5, 162.8, 154.7, 152.8, 149.4, 146.5, 142.2, 135.5, 132.6, 129.1, 123.9, 118.8, 115.2, 110.9, 104.4, 99.6, 81.6, 42.6, 37.7, 28.2, 26.8. ESI-HR-MS. Calcd for C23H20Cl2N5O3 [M-H]-: m/z 484.0942. Found: m/z 484.0942.
Acknowledgments
We are grateful to the Major Natural Science Foundation of Jiangsu Province (14KJA150004), a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions for financial support.
References
[1] Mogilaiah, K.; Reddy, C. T.; Reddy, N. Efficient of synthesis and antibacterial activity of 2-(4-cinnamoylphenylamino)-3-(3-trifluoromethylphenyl)-1,8- naphthyridines. Indian J. Heterocycl. Chem. 2014, 23, 267–270.Suche in Google Scholar
[2] Surivet, J. P.; Zumbrunn, C.; Rueedi, G.; Hubschwerlen, C.; Bur, D.; Bruyere, T.; Locher, H.; Ritz, D.; Keck, W.; Seiler, P. Design, synthesis, and characterization of novel tetrahydropyran-based bacterial topoisomerase inhibitors with potent anti-Gram-positive activity. J. Med. Chem.2013, 56, 7396–7415.10.1021/jm400963ySuche in Google Scholar PubMed
[3] Surivet, J. P.; Lange, R.; Hubschwerlen, C.; Keck, W.; Specklin, J. L.; Ritz, D.; Bur, D.; Locher, H.; Seiler, P.; Strasser, D. S. Structure-guided design, synthesis and biological evaluation of novel DNA ligase inhibitors with in vitro and in vivo anti-staphylococcal activity. Bioorg. Med. Chem. Lett.2012, 22, 6705–6711.10.1016/j.bmcl.2012.08.094Suche in Google Scholar PubMed
[4] Varadi, L.; Gray, M.; Groundwater, P. W.; Hall, A. J.; James, A. L.; Orenga, S.; Perry, J. D.; Anderson, R. J. Synthesis and evaluation of fluorogenic 2-amino-1,8-naphthyridine derivatives for the detection of bacteria. Org. Biomol. Chem.2012, 10, 2578–2589.10.1039/c2ob06986eSuche in Google Scholar PubMed
[5] Gootz, T. D.; Zaniewski, R.; Haskell, S.; Schmieder, B.; Tankovic, J.; Girard, D.; Courvalin, P.; Polzer, R. J. Activity of the new fluoroquinolone trovafloxacin (CP-99,219) against DNA gyrase and topoisomerase IV mutants of Streptococcus pneumoniae selected in vitro. Antimicrob. Agents Chemother.1996, 40, 2691–2697.10.1128/AAC.40.12.2691Suche in Google Scholar PubMed PubMed Central
[6] Flori, N.; Funakoshi, N.; Duny, Y.; Valats, J. C.; Bismuth, M.; Christophorou, D.; Daurès, J. P.; Blanc, P. Pegylated interferon-α2a and ribavirin versus pegylated interferon-α2b and ribavirin in chronic hepatitis C: a meta-analysis. Drugs2013, 73, 263–277.10.1007/s40265-013-0027-1Suche in Google Scholar PubMed
[7] Bishnoi, A.; Tiwari, A. K.; Singh, S.; Sethi, A.; Tripathi, C. M.; Banerjee, B. Synthesis, characterization, and biological evaluation of novel thiazole and pyrazole derivatives of quinoline-4-carboxylic acid as potential antimicrobial agents. Med. Chem. Res. 2013, 22, 3527–3535.10.1007/s00044-012-0333-2Suche in Google Scholar
[8] Li, C.; Zhang, W. T.; Wang, X. S. Domino synthesis of fused hexacyclic imidazoquinolinoacridinones catalyzed by CuI/L-proline. Tetrahedron2014, 70, 8919–8924.10.1016/j.tet.2014.09.084Suche in Google Scholar
[9] Wang, W.; Jiang, H.; Zhang, M. M.; Wang, X. S. Iodine-catalyzed synthesis of cyclopenta[c]quinoline derivatives via imino Diels-Alder reaction. J. Heterocycl. Chem.2014, 51, 830–834.10.1002/jhet.1670Suche in Google Scholar
[10] Chen, D. S.; Li, Y. L.; Liu, Yun; Wang, X. S. Synthesis of bis-benzoquinoline derivatives catalyzed by iodine via ring-opening of furan. Tetrahedron2013, 69, 7045–7050.10.1016/j.tet.2013.06.042Suche in Google Scholar
[11] Du, B. X.; Zhao, B.; Cai, G.; Li, Y. L.; Wang, X. S. Mild and efficient one-pot three-component synthesis of benzopyrimidoquinoline-tetralone derivatives in ionic liquids. J. Chem. Res.2012, 36, 453–456.10.3184/174751912X13384724679874Suche in Google Scholar
[12] Wang, W.; Yin, M. Y.; Zhang, M. M.; Wang, X. S. Iodine-catalysed synthesis of thiopyrano[3,4-c]quinoline derivatives via imino-Diels-Alder reaction. J. Chem. Res.2012, 36, 318–321.10.3184/174751912X13352797144052Suche in Google Scholar
[13] Wasik, R.; Lebska, M.; Felczak, K.; Poznanski, J.; Shugar, D. relative role of halogen bonds and hydrophobic interactions in inhibition of human protein kinase CK2α by tetrabromobenzotriazole and some C(5)-substituted analogues. J. Phys. Chem. B.2010, 114, 10601–10611.10.1021/jp102848ySuche in Google Scholar PubMed
©2015 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Frontmatter
- Preliminary Communications
- Triphenylphosphine catalyzed domino reaction of dialkyl acetylenedicarboxylate with 3-aryl- 2-benzoylcyclopropane-1,1-dicarbonitrile
- Synthesis of colletotrichumine A
- Research Articles
- Synthesis of the spiroacetal fragments of spirofungins A and B, antibiotics isolated from Streptomyces violaceusniger Tü 4113
- Efficient synthesis and fungicidal activities of strobilurin analogues containing benzofuro [3,2-d]-1,2,4-triazolo[1,5-a]pyrimidinone side chains
- Synthesis of 2-amino-6,7,8,9-tetrahydro-6-phenethyl-3H-pyrimido[4,5-e][1,4]diazepin-4(5H)-one: a model for a potential pyrimido[4,5-e][1,4]diazepine-based folate anti-tumor agent
- Cascade assembling of pyrazolin-5-ones and benzylidenemalononitriles: the facile and efficient approach to medicinally relevant spirocyclopropylpyrazolone scaffold
- Synthesis, characterization and bioactivity of novel 5,6-dihydropyrrolo[3,4-c]pyrazol-4- (1H)one derivatives
- Molecular modeling and synthesis of new 1,5-diphenylpyrazoles as breast cancer cell growth inhibitors
- An efficient synthesis of 11-aryl-10-oxo-7,8,10,11-tetrahydro-1H-[1,2,3]triazolo [4′,5′:3,4]benzo[1,2-b][1,6]naphthyridine derivatives under catalyst-free conditions
- Mechanochemical synthesis of 2,2-difluoro-4, 6-bis(β-styryl)-1,3,2-dioxaborines and their use in cyanide ion sensing
- Visible-light-mediated radical aryltrichloromethylation of N-arylacrylamides for the synthesis of trichloromethyl-containing oxindoles
- A stereolibrary of conformationally restricted amino acids based on pyrrolidinyl/piperidinyloxazole motifs
- Synthesis of [1,3]thiazolo[3,2-b][1,2,4]triazol-7-ium and [1,2,4]triazolo[5,1-b][1,3]thiazin-4-ium salts via regioselective electrophilic cyclization of 3-[(2-alken-1-yl)sulfanyl]-4H-1,2,4-triazoles
Artikel in diesem Heft
- Frontmatter
- Preliminary Communications
- Triphenylphosphine catalyzed domino reaction of dialkyl acetylenedicarboxylate with 3-aryl- 2-benzoylcyclopropane-1,1-dicarbonitrile
- Synthesis of colletotrichumine A
- Research Articles
- Synthesis of the spiroacetal fragments of spirofungins A and B, antibiotics isolated from Streptomyces violaceusniger Tü 4113
- Efficient synthesis and fungicidal activities of strobilurin analogues containing benzofuro [3,2-d]-1,2,4-triazolo[1,5-a]pyrimidinone side chains
- Synthesis of 2-amino-6,7,8,9-tetrahydro-6-phenethyl-3H-pyrimido[4,5-e][1,4]diazepin-4(5H)-one: a model for a potential pyrimido[4,5-e][1,4]diazepine-based folate anti-tumor agent
- Cascade assembling of pyrazolin-5-ones and benzylidenemalononitriles: the facile and efficient approach to medicinally relevant spirocyclopropylpyrazolone scaffold
- Synthesis, characterization and bioactivity of novel 5,6-dihydropyrrolo[3,4-c]pyrazol-4- (1H)one derivatives
- Molecular modeling and synthesis of new 1,5-diphenylpyrazoles as breast cancer cell growth inhibitors
- An efficient synthesis of 11-aryl-10-oxo-7,8,10,11-tetrahydro-1H-[1,2,3]triazolo [4′,5′:3,4]benzo[1,2-b][1,6]naphthyridine derivatives under catalyst-free conditions
- Mechanochemical synthesis of 2,2-difluoro-4, 6-bis(β-styryl)-1,3,2-dioxaborines and their use in cyanide ion sensing
- Visible-light-mediated radical aryltrichloromethylation of N-arylacrylamides for the synthesis of trichloromethyl-containing oxindoles
- A stereolibrary of conformationally restricted amino acids based on pyrrolidinyl/piperidinyloxazole motifs
- Synthesis of [1,3]thiazolo[3,2-b][1,2,4]triazol-7-ium and [1,2,4]triazolo[5,1-b][1,3]thiazin-4-ium salts via regioselective electrophilic cyclization of 3-[(2-alken-1-yl)sulfanyl]-4H-1,2,4-triazoles

