Abstract
New pyrazoles have been synthesized and evaluated as breast cancer cell growth inhibitors. Condensation of the substituted pyrazole-4-carbaldehyde 1 with acetophenone and chloroacetophenone afforded α, β-unsaturated ketones 2 and 3, respectively. Compounds 2 and 3 were subjected to different reactions using hydrazine hydrate, substituted hydrazine hydrate, hydroxylamine, o-phenylenediamine, malononitrile under different conditions affording 4-substituted pyrazole derivatives 4–28. Structure elucidation of these compounds was conducted using IR, 1H NMR, 13C NMR, mass spectral data and elemental analysis. Antitumor activity of target compounds was tested against MCF-7 cell line (human breast cancer). Compounds 4, 10 and 20 show significant antitumor activity against breast cancer. Docking was performed with protein 1UYK to study the binding mode of the designed compounds.
Introduction
Breast cancer is the most popular malignancy and the leading cause of death in women worldwide [1]. It has been shown that inhibition of the enzyme aromatase is one of the mechanisms of the most antitumor drugs especially in the treatment of breast cancer [2–4]. Heat shock proteins (HSP) are members of the molecular chaperones which play an important role in the folding of a large number of cellular proteins [5, 6]. The proliferative activity of breast cancer cells leads to elevated HSP-90 expression [7, 8]. It has been found that the levels of HSP-90 decreased in patients with clinical and biological response to aromatase inhibitors therapy [9].
A literature survey has revealed the importance of pyrazole derivatives as potent anticancer agents [10–16] including celecoxib (I in Figure 1) that is used in treatment of breast cancer [17–24]. A variety of 1,5-diphenylpyrazole derivatives (Figure 1) have been synthesized and their cytotoxic properties evaluated [25–27]. Chalcone derivatives (Figure 2) exhibit significant biological properties including antiproliferative activities [28–32]. It has been reported that many heterocyclic compounds have a significant antitumor activity when linked to the pyrazole system [33–40].
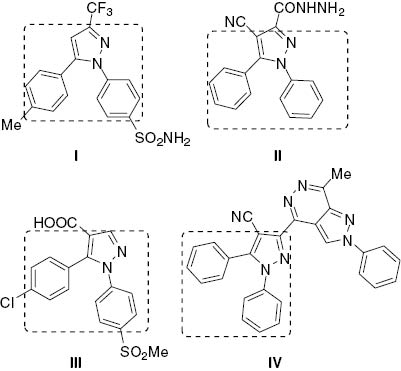
1,5-Diphenylpyrazole derivatives I–IV as anticancer agents.
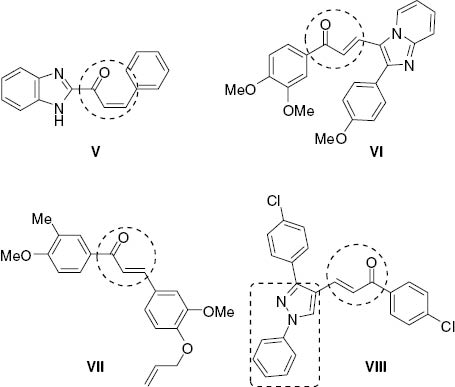
Chalcone derivatives V–VIII with known anticancer activity.
Inspired by these finding, and in order to develop new anticancer therapeutic agents, we were encouraged to integrate a 1,5-diphenylpyrazole moiety as a main scaffold with a chalcone moiety to form new hybrid molecules [41–43].
Results and discussion
Chemistry
The synthetic pathways adopted for the preparation of the target new compounds are illustrated in Schemes 1 and 2. The starting material 1 was obtained via Vilsmeir Haack’s formylation using POCl3 [44, 45]. The α, β-unsaturated carbonyl derivatives 2 and 3 were synthesized via Claisen-Schmidt condensation [46, 47] of compound 1 with a ketone (Scheme 1). The IR spectrum of 3 reveals a C=O band of carbonyl group at 1658 cm-1. The reaction of compounds 2 and 3 with hydrazine hydrate in the presence of acetic acid yielded N-acetylpyrazole derivatives 4 and 5, respectively. A similar reaction conducted in ethanol gave the N-substituted pyrazoles 6 and 7. Moreover, reaction of chalcone 3 with phenylhydrazine afforded compounds 8. 1H NMR spectrum of 4 shows a doublet at 3.85 ppm and a triplet at 5.12 ppm corresponding to the pyrazoline CH2 and CH, respectively. 1H NMR spectrum of 7 shows an exchangeable signal at 7.63 ppm for NH group. The mass spectrum of compound 8 shows a molecular ion peak at m/z 489 corresponding to the molecular formula C31H25ClN4 and a peak at 490 (M++2) due to the presence of the isotopic chlorine atom. Hydroxylamine hydrochloride was also allowed to react with chalcones 2 and 3 to form isoxazoline derivatives 9 and 10, respectively. The 1H NMR spectrum of the compound 9 is characterized by the presence of a doublet at 3.5 ppm for CH2 and a triplet at 5.9 ppm for CH of the isoxazoline ring. Reaction of o-phenylenediamine with chalcones 2 and 3 gave the corresponding 1,5-benzodiazepines 11 and 12. The IR spectrum of 11 shows a significant band at 3350 cm-1 attributed to N-H group and the disappearance of an absorption band assigned to the carbonyl group. 13C NMR spectrum of 12 shows signals at 38.7, 40.4, 121.1, 124.8 ppm corresponding to the benzodiazepine carbon atoms and a signal at 14.2 ppm corresponding to the CH3 group.

The synthesis of 3–12: (a) acetophenone or 4-chloroacetophenone, NaOH, absolute ethanol; (b) NH2NH2, AcOH; (c) R′-NHNH2, absolute ethanol; (d) NH2OH·HCl, KOH; (e) o-phenylenediamine, Et3N, absolute ethanol.
Pyridine-3-carbonitriles and pyran-3-carbonitriles were prepared as shown in Scheme 2. Malononitrile was allowed to react with compounds 2 and 3 in the presence of ammonium acetate to give pyridine-3-carbonitrile derivatives 13 and 14, respectively. A similar reaction conducted in DMF in the presence of piperidine, afforded the corresponding pyran-3-carbonitriles derivatives 15 and 16. Furthermore, reaction of chalcones 2 and 3 with malononitrile in the presence of sodium alkoxide in an alcohol gave the corresponding 2-alkoxypyridine-3-carbonitriles derivatives 17–22. IR spectrum of 13 reveals the presence of a stretching band at 3415 cm-1 attributed to NH2 group. The 1H NMR of 14 displays a signal at 6.27 ppm corresponding to NH2 group while compound 15 shows characteristic 2 signals at 6.56 and 6.83 ppm corresponding to the pyran protons.
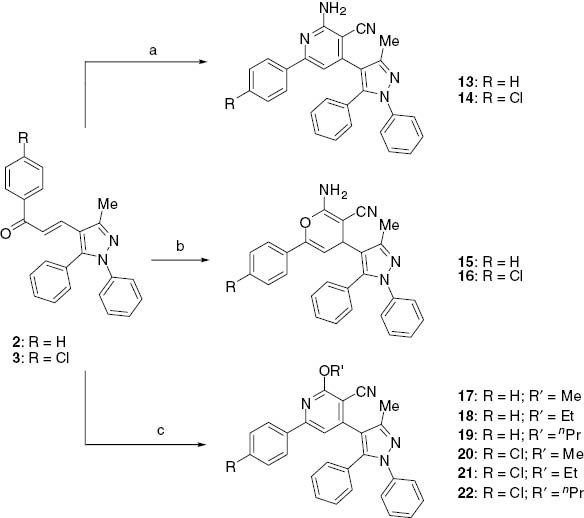
The synthesis of 13–22: (a) malononitrile, ammonium acetate, absolute ethanol; (b) malononitrile, DMF, pyridine; (c) malononitrile, sodium methoxide or sodium ethoxide or sodium propoxide, methanol, ethanol and n-propanol.
Antitumor activity
All synthesized compounds were tested for their cytotoxic activity against MCF-7 (human breast cancer cell line) using the method of Skehan [48, 49]. The results are presented in Table 1. A series of 1,5-diphenylpyrazoles was synthesized and evaluated in vitro for antitumor activity against MFC-7 cell line. Compounds 4, 7 and 20 exhibit strong activity (Table 1). Compounds 5, 8, 11, 13–17 and 22 show moderate activity. Compounds 12 and 18 show weak activity. Compounds 9 and 19 show very weak activity. 1,5-Benzodiazepine derivative 11, pyridine-3-carbonitriles 13 and 14 and pyran derivatives 15 and 16 are moderately active in comparison to the starting compounds 2 and 3. The introduction of a propyl chain in compounds 19, 22 causes reduction in activity in comparison with the other pyridine-3-carbonitriles 17, 18, 20 and 21.
Molecular modeling results of 3–20 with amino acids of the enzyme 1UYK and their biological screening results against breast cancer cell line (MCF-7).
| Comp. no. | Ec of interaction ligand-protein | % inhibition | IC 50b, μg/mL | Comp. no. | Ec of interaction ligand-protein | % inhibition | IC 50b, μg/mL |
|---|---|---|---|---|---|---|---|
| 3 | -40.264 | 33.982 | 13 | -31.271 | 55.288 | ||
| 4 | -34.761 | 78.174a | 11.7 | 14 | -29.997 | 54.672 | |
| 5 | -37.322 | 30.857 | 15 | -42.784 | 48.686 | ||
| 6 | -30.629 | 25.53 | 16 | -33.386 | 40.597 | ||
| 7 | -35.633 | 67.877a | 25.9 | 17 | -30.134 | 43.615 | |
| 8 | -32.328 | 56.28 | 18 | -29.425 | 37.013 | ||
| 9 | -33.727 | 16.848 | 19 | -29.530 | 25.861 | ||
| 10 | -39.095 | 50.244 | 20 | -30.324 | 66.437a | 29.5 | |
| 11 | -29.938 | 58.489 | 21 | -29.072 | 41.855 | ||
| 12 | -35.397 | 38.773 | 22 | -36.324 | 35.488 |
aCompounds showing significant antitumor activity; bIC50 for active antitumor compounds; cenergy (kJ/mol).
Molecular modeling
The molecular operating environment (MOE) [50] based molecular docking was done for the target compounds 3–22 on heat shock proteins (HSP) obtained from the protein data bank (code, 1UYK.pdb) with the help of PharmMapper software [51]. When examining the protein-ligand interaction for the protein molecule 1UYK [52], it was found that the main amino acids involved in binding to the ligand are Asn (51), Asp (93) and Phe (138). The selected complexes calculated as part of this work are shown in Figures 3–6.
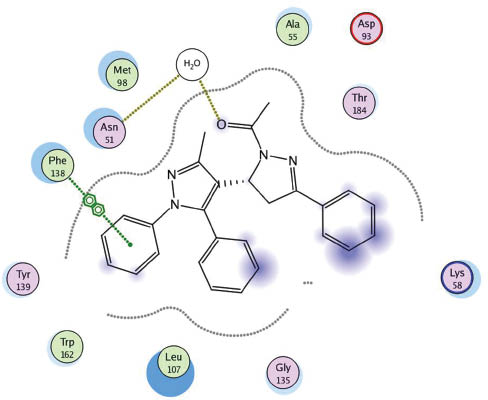
Docking of 4 in 1UYK binding side.
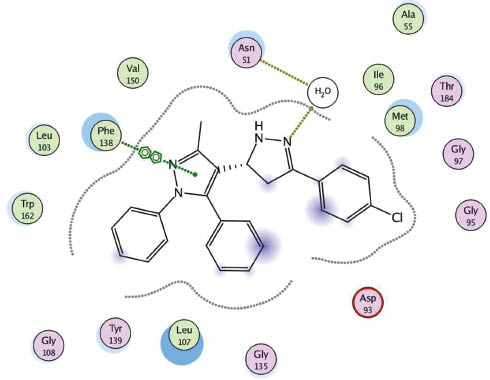
Docking of 7 in 1UYK binding side.
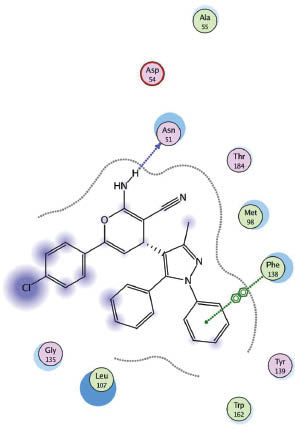
Docking of 16 in 1UYK binding side.
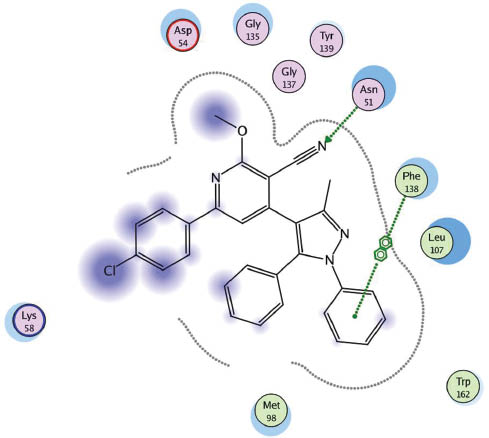
Docking of 20 in 1UYK binding side.
Conclusion
There is a strong correlation between molecular modeling and biological screening results which confirm that the structural modification of the lead structure affects the activity in a predictable manner.
Experimental
Chemistry
Melting points are uncorrected and were recorded in open capillaries on an electro-thermal melting point apparatus. IR spectra were recorded on a Mattson 5000 FT-IR spectrometer in KBr disks at the Faculty of Science, Mansoura University. 1H NMR (200 MHz) and 13C NMR (50 MHz) spectra were obtained on a Gemini Varian spectrometer using TMS as internal standard, at the Micro-analytical Unit of Cairo University. Mass spectral analyses were performed on a JOEL JMS-600H spectrometer at Cairo University. Microanalyses were performed at the Micro-analytical Unit of Cairo University. All reagents were purchased from the Aldrich Chemical Company. Compounds 1, 2 were synthesized according to reported methods [53, 54].
1-(4-Chlorophenyl)-3-[(3-methyl)-1,5-diphenyl-1H-pyrazol-4-yl]prop-2-en-1-one (3)
A mixture of compound 1 (2.6 g, 0.01 mol), chloroacetophenone (0.01 mol) and sodium hydroxide (0.025 mol) in absolute ethanol (20 mL) was heated under reflux for 2 h. The solid obtained was collected by filtration, dried and crystallized from ethanol: light brown solid; yield 85%; mp 155–156°C; IR: 1590 (C=N), 1606 (C=C), 1658 cm-1 (C=O); 1H NMR: δ 2.41 (s, 3H, CH3), 6.8 (d, 1H, J = 16.3 Hz), 7.2 (d, 1H, J = 16.3 Hz), 7.17–7.44 (m, 14H, Ar-H); MS: m/z 399 [M+].
General procedure for the preparation of compounds 4 and 5
A solution of compound 2 or 3 (0.001 mol) and hydrazine hydrate (0.1 mL, 0.002 mol) in glacial acetic acid (15 mL) was heated under reflux for 6–9 h. Cold water was added to the hot mixture and the product extracted using ethyl acetate was crystallized from ethanol.
1-[5-(3-Methyl-1,5-diphenyl-1H-pyrazol-4-yl)-3-phenyl-4,5-dihydro-1H-pyrazol-1-yl]ethan-1-one (4)
Yield 70%; mp 118–120°C; IR: 1645 (C=N), 1675 cm-1 (C=O); 1H NMR: δ 1.92 (s, 3H, CH3), 2.03 (s, 3H, COCH3), 3.85 (d, 2H, CH2 pyrazoline), 5.12 (t, 1H, CH pyrazoline), 7.15–7.57 (m, 15H, Ar-H); MS: m/z 421 [M++1]. Anal. Calcd for C27H24N4O: C, 77.12; H, 5.75; N, 13.32. Found: C, 77.03; H, 5.53; N, 13.12.
1-[3-(4-Chlorophenyl)-5-(3-methyl-1,5-diphenyl-1H-pyrazol-4-yl)-4,5-dihydro-1H-pyrazol-1-yl]ethan-1-one (5)
Yield (67%); mp 122–124°C; IR: 1620 (C=N), 1680 cm-1 (C=O); 1H NMR: δ 1.89 (s, 3H, CH3), 2.32 (s, 3H, COCH3), 3.57 (d, 2H, CH2 pyrazoline), 4.56 (t, 1H, CH pyrazoline), 7.10–7.97 (m, 14H, Ar-H); MS: m/z 455 [M++1], 456 [M++2]. Anal. Calcd for C27H23ClN4O: C, 71.28; H, 5.10; N, 12.30. Found: C, 71.16; H, 5.36; N, 12.04.
Preparation of 3-methyl-1,5-diphenyl-4-[3-(substituted phenyl)-4,5-dihydropyrazol-5-yl)]-1H-pyrazoles 6–8
A mixture of compound 2 or 3 (0.005 mol) with hydrazine hydrate (0.02 mol) or phenylhydrazine (0.005 mol) in absolute ethanol was heated under reflux for a period of time indicated below and then concentrated in vacuo. On cooling the separated solid was filtered and crystallized from ethanol.
3-Methyl-1,5-diphenyl-4-(3-phenyl-4,5-dihydro-1H-pyrazol-5-yl)-1H-pyrazole (6)
Compound 6 was prepared from 2 and hydrazine hydrate, reaction time 6 h: yield 56%; mp 135–137°C; IR: 1599 (C=N), 3305 cm-1 (N-H); 1H NMR: δ 1.8 (s, 3H, CH3), 2.7 (d, 2H, CH2 pyrazoline), 3.3 (t, 1H, CH pyrazoline), 6.7–8 (m, 15H, Ar-H), 7.63 (s, 1H, NH D2O exchangeable); MS: m/z 378 [M+]. Anal. Calcd for C25H22N4: C, 79.34; H, 5.86; N,14.80. Found: C, 79.71; H, 5.66; N, 14.64.
4-[3-(4-Chlorophenyl-4,5-dihydro-1H-pyrazol-5-yl]-3-methyl-1,5-diphenyl-1H-pyrazole (7)
Compound 7 was prepared from 3 and hydrazine hydrate, reaction time 6 h: yield 69%; mp 183–185°C; IR: 1593 (C=N), 3365 cm-1 (N-H); 1H NMR: δ 1.9 (s, 3H, CH3), 2.5 (d, 2H, CH2 pyrazoline), 3.9 (t, 1H, CH pyrazoline), 7.00–8.02 (m, 14H, Ar-H), 7.36 (s, 1H, NH D2O exchangeable); MS: m/z 413 [M++1], 414 [M++2]. Anal. Calcd for C25H21ClN4: C, 72.72; H, 5.13; N, 13.57. Found: C, 72.47; H, 5.37; N, 13.34.
4-[3-(4-Chlorophenyl)-1-phenyl-4,5-dihydro-1H-pyrazol-5-yl]-3-methyl-1,5-diphenyl-1H-pyrazole (8)
Compound 8 was prepared from 3 and phenylhydrazine, reaction time 20 h; yield 63%; mp 110–112°C; IR: 1599 (C=N), 1605 cm-1 (C=C); 1H NMR: δ 11.37 (s, 3H, CH3), 3.35 (d, 2H, CH2 pyrazoline), 5.55 (t, 1H, CH pyrazoline), 6.46–8.02 (m, 19H, Ar-H); MS: m/z 489 [M+1], 490 [M+]. Anal. Calcd for C31H25ClN4: C, 76.14; H, 5.15; N, 11.46. Found: C, 76.34; H, 5.39; N, 11.74.
Preparation of 5-(3-methyl-1,5-diphenyl-1H-pyrazol-4-yl)-3-(substituted phenyl)-4,5-dihydroisoxazoles 9 and 10
A mixture of compound 2 or 3 (0.03 mol), absolute ethanol (15 mL), hydroxylamine hydrochloride (0.2 g, 0.003 mol) and potassium hydroxide (0.2 g, 0.004 mol) was heated under reflux for 10 h. After cooling, the precipitate was filtered and crystallized from ethanol.
5-(3-Methyl-1,5-diphenyl-1H-pyrazol-4-yl)-3-phenyl-4,5-dihydroisoxazole (9)
Yield 60%; mp 103–105°C; 1H NMR: δ 2.5 (s, 3H, CH3), 3.5 (d, 2H, CH2 isoxazoline H-4), 5.9 (t, 1H, CH isoxazoline), 7.03–7.89 (m, 15H, Ar-H); 13C NMR: δ 21.2, 46.6, 80.4, 119.4, 121.2, 123.7, 124.0, 126.7, 129.5, 131.6, 135.0, 136.5, 139.4, 142.7, 144.3, 146.7, 149.4, 153.4, 159.2; MS: m/z 379[M+]. Anal. Calcd for C27H23ClN4O: C, 79.13; H, 5.58; N, 11.07. Found: C, 79.45; H, 5.76; N, 11.37.
3-(4-Chlorophenyl)-5-(3-methyl-1,5-diphenyl-1H-pyrazol-4-yl)-4,5-dihydroisoxazole (10)
Yield 54%; mp 113–115°C; 1H NMR: δ 2.1 (s, 3H, CH3), 3.3(d, 2H, CH2 isoxazoline), 5.2 (t, 1H, CH isoxazoline), 7.01–7.52 (m, 14H, Ar-H); MS: m/z 414 [M++1], 415 [M++2]. Anal. Calcd for C25H20ClN3O: C, 72.55; H, 4.87; N, 10.15. Found: C, 72.20; H, 4.63; N, 10.42.
Preparation of compounds 11 and 12
A mixture of 2 or 3 (2.44 g, 0.01 mol), o-phenylenediamine (1.08 g, 0.01 mol) and triethylamine (3 mL) in absolute ethanol (15 mL) was heated under reflux for 15 h. The reaction mixture was cooled to 0°C and the resultant precipitate was filtered and crystallized from ethanol.
2-(3-Methyl-1,5-diphenyl-1H-pyrazol-4-yl)-4-phenyl-2,3-dihydro-1H-1,5 benzodiazepine (11)
Yield 59%; mp 178–180°C; IR: 1593 (C=N), 3350 cm-1 (N-H); MS: m/z 454 [M+. Anal. Calcd for C31H26N4: C, 81.91; H, 5.77; N, 12.33. Found: C, 81.78; H, 5.47; N, 12.13.
4-(4-Chlorophenyl)-2-(3-methyl-1,5-diphenyl-1H-pyrazol-4-yl)-2,3-dihydro-1H-1,5-benzodiazepine (12)
Yield 63%; mp 186–188°C; IR: 1592 (C=N), 3375 cm-1 (N-H); 13C NMR: δ 14.2, 38.7, 40.4, 115.7, 119.2, 121.1, 124.8, 126.1, 127.7, 128.6, 130.0, 130.1, 135.7, 136.4, 137.7, 138.8, 140.2, 144.9, 146.4, 148.7, 153.2, 165.4; MS: m/z 489 [M++1], 490 [M++2]. Anal. Calcd for C31H25ClN4: C, 76.14; H, 5.15; N, 11.46. Found: C, 76.34; H, 5.37; N, 11.27.
Preparation of compounds 13 and 14
A solution of chalcone 2 or 3 (0.005 mol), malononitrile (0.005 mol) and ammonium acetate (0.04 mol) in ethanol was heated under reflux for 6 h. On cooling, the precipitated solid was filtered, dried and crystallized from ethanol.
2-Amino-4-(3-methyl-1,5-diphenyl-1H-pyrazol-4-yl)-6-phenylpyridine-3-carbonitrile (13)
Yield 64%; mp 131–133°C; IR: 1598 (C=N), 2227 (CN), 3415 cm-1 (NH2); 1H NMR: δ 2.41(s, 3H, CH3), 6.32 (s, 2H, NH2), 7.19 (s, 1H, H-pyridine), 7.31–8.30 (m, 15H, Ar-H); 13C NMR: 19.3, 89.1, 103.4, 116.7, 121.4, 123.7, 124.8, 126.7, 128.5, 130.7, 132.4, 134.6, 136.3, 140.0, 143.8, 150.2, 154.7, 156.3, 163.7; MS: m/z 428 [M++1]. Anal. Calcd for C28H21N5: C, 78.67; H, 4.95; N, 16.38. Found: C, 78.87; H, 4.65; N, 16.63.
2-Amino-6-(4-chlorophenyl)-4-(3-methyl-1,5-diphenyl-1H-pyrazol-4-yl)pyridine-3-carbonitrile (14)
Yield 70%; mp 146–148°C; IR: 1630 (C=N), 2223 (CN), 3350 cm-1 (NH2); 1H NMR: δ 2.34 (s, 3H, CH3), 6.27 (s, 2H, NH2), 7.11 (s, 1H, H-pyridine), 7.29–8.37 (m, 14H, Ar-H); MS: m/z 462 [M++1], 463 (M++2], 3.4%). Anal. Calcd for C28H20ClN5: C, 72.80; H, 4.36; N, 15.16. Found: C, 72.53; H, 4.10; N, 15.42.
Preparation of compounds 15 and 16
A mixture of malononitrile (0.001 mol) compound 2 or 3 (0.001 mol), and a few drops of piperidine in DMF (15 mL) was stirred at room temperature. The precipitated product was washed with DMF and crystallized from ethanol.
2-Amino-4-(3-methyl-1,5-diphenyl-1H-pyrazol-4-yl)-6-phenyl-4H-pyran-3-carbonitrile (15)
Yield 56%; mp 120–122°C; IR: 1593 (C=N), 2230 (CN), 3400 cm-1 (NH2); 1H NMR: δ 2.41(s, 3H, CH3), 6.30 (s, 2H, NH2), 6.56 (d, 1H, J = 3.8 Hz), 6.83 (d, 1H, J = .8 Hz), 7.31–8.30 (m, 15H, Ar-H); MS: m/z 430 [M+. Anal. Calcd for C28H22N4O: C, 78.12; H, 5.15; N, 13.01. Found: C, 78.43; H, 5.36; N, 13.31.
2-Amino-6-(4-chlorophenyl)-4-(3-methyl-1,5-diphenyl-1H-pyrazol-4-yl)-4H-pyran-3-carbonitrile (16)
Yield 59%; mp 126–128°C; IR: 1587 (C=N), 2218 (CN), 3365 cm-1 (NH2); 1H NMR: δ 2.34 (s, 3H, CH3), 6.11 (s, 2H, NH2), 6.34 (d, 1H, J = 3.6 Hz), 6.75 (d, 1H, J = 3.6 Hz), 7.25–8.20 (m, 14H, Ar-H); MS: m/z 464 [M+], 466 [M++2]. Anal. Calcd for C28H21ClN4O: C, 72.33; H, 4.55; N, 12.05. Found: C, 72.63; H, 4.67; N, 12.35.
Preparation of compounds 17–22
A mixture of chalcone 2 or 3 (0.001 mol), malononitrile (0.001 mol) and a solution of sodium alkoxide (15 mL, 0.014 mol of sodium in 100 mL of the appropriate alcohol, namely, methanol, ethanol or n-propanol) was stirred at room temperature for the period of time indicated below. The product was crystallized from ethanol/DMF.
2-Methoxy-4-(3-methyl-1,5-diphenyl-1H-pyrazol-4-yl)-6-phenylpyridine-3-carbonitrile (17)
Compound 17 was prepared from 2 and malononitrile in sodium methoxide and methanol, reaction time 2 h; yield 70%; mp 105–107°C; IR: 1560 (C=N), 2220 cm-1 (CN);1H NMR: δ 2.47 (s, 3H, CH3), 4.27 (s, 3H, OCH3), 7.48 (s, 1H, pyridine-H), 6.73–7.88 (m, 15H, Ar-H); MS: m/z 443 [M++1]. Anal. Calcd for C29H22N4O: C, 78.71; H, 5.01; N, 12.66. Found: C, 78.48; H, 5.39; N, 12.48.
2-Ethoxy-4-(3-methyl-1,5-diphenyl-1H-pyrazol-4-yl)-6-phenylpyridine-3-carbonitrile (18)
Compound 18 was prepared from 2 and malononitrile in sodium ethoxide and ethanol, reaction time 3 h; yield 67%; mp 101–103°C; IR: 1569 (C=N), 2227 cm-1 (CN); 1H NMR: δ 1.36 (t, 3H, O-CH2CH3), 2.01 (s, 3H, CH3), 4.10 (q, 2H, O-CH2CH3), 7.49 (s, 1H, pyridine-H), 7.23–7.85 (m, 15H, Ar-H); MS: m/z 457 [M++1]. Anal. Calcd for C30H24N4O: C, 78.92; H, 5.30; N, 12.27. Found: C, 78.69; H, 5.67; N, 12.42.
4-(3-Methyl-1,5-diphenyl-1H-pyrazol-4-yl)-6-phenyl-2-propoxypyridine-3-carbonitrile (19)
Compound 19 was prepared from 2 and malononitrile in sodium propoxide and n-propanol, reaction time 4 h; yield 64%; mp 138–140°C; IR: 1567 (C=N), 2230 cm-1 (CN); 1H NMR: δ 0.8 (t, 3H, OCH2CH2CH3), 1.89 (m, 2H, OCH2CH2CH3), 2.16 (s, 3H, CH3), 4.56 (t, 2H, OCH2CH2CH3), 7.58 (s, 1H, pyridine-H), 7.13–8.08 (m, 15H, Ar-H); MS: m/z 471 [M++1]. Anal. Calcd for C31H26N4O: C, 79.12; H, 5.57; N, 11.91. Found: C, 79.37; H, 5.32; N, 11.61.
6-(4-Chlorophenyl)-2-methoxy-4-(3-methyl-1,5-diphenyl-1H-pyrazol-4-yl)pyridine-3-carbonitrile (20)
Compound 20 was prepared from 3 and malononitrile in sodium methoxide and methanol, reaction time 1 h; yield 61%; mp 123–125°C; IR: 1570 (C=N), 2219 cm-1 (CN); 1H NMR: δ 2.41 (s, 3H, CH3), 4.34 (s, 3H, OCH3), 7.44 (s, 1H, pyridine-H), 6.88–7.37 (m, 14H, Ar-H); MS: m/z 476 [M+], 478 [M++2]. Anal. Calcd for C29H21ClN4O: C, 73.03; H, 4.44; N, 11.75. Found: C, 73.37; H, 4.28; N, 11.98.
6-(4-Chlorophenyl)-2-ethoxy-4-(3-methyl-1,5-diphenyl-1H-pyrazol-4-yl)pyridine-3-carbonitrile (21)
Compound 21 was prepared from 3 and malononitrile in sodium ethoxide and ethanol, reaction time 2.5 h; yield 66%; mp 140–142°C; IR: 1564 (C=N), 2217 cm-1 (CN); 1H NMR: δ 1.15 (t, 3H, O-CH2CH3), 2.15 (s, 3H, CH3), 4.32 (q, 2H, O-CH2CH3), 7.68 (s, 1H, pyridine-H), 7.11–7.52 (m, 14H, Ar-H); MS: m/z 491 [M++1], [M++2]. Anal. Calcd for C30H23ClN4O: C, 73.39; H, 4.72; N, 11.41. Found: C, 73.70; H, 4.58; N, 11.69.
6-(4-Chlorophenyl)-4-(3-methyl-1,5-diphenyl-1H-pyrazol-4-yl)-2-propoxy pyridine-3-carbonitrile (22)
Compound 22 was prepared from 3 and malononitrile in sodium propoxide and n-propanol, reaction time 6 h; yield 62%; mp 164–166°C; IR: 1561 (C=N), 2224 cm-1 (CN); 1H NMR: δ 0.9 (t, 3H, OCH2CH2CH3), 1.74(m, 2H, OCH2CH2CH3), 2.31 (s, 3H, CH3), 4.46 (t, 2H, OCH2CH2CH3), 7.88 (s, 1H, pyridine-H), 7.10–8.10 (m, 14H, Ar-H); MS: m/z 504 [M+], 506 [M++2]. Anal. Calcd for C31H25ClN4O: C, 73.73; H, 4.99; N, 11.09. Found: C, 73.59; H, 4.69; N, 11.29.
Biology
All synthesized compounds were evaluated for their antitumor activity against human breast cancer cell line (MCF-7) adopting the sulforhodamine B (SRB) assay [48]. All materials were obtained from Sigma Chemical Co. (USA). The cell line was obtained frozen in liquid nitrogen (-180°C) from the American Type Culture Collection. It was maintained by serial sub-culturing in 75 cm2 cell culture flasks (Fisher Scientific, Pittsburgh, PA, USA) at 37°C in atmosphere of 5% CO2 using 10 mL of RPMI-1640 [supplemented with 1% (2 mm) glutamic acid, 10% unheated fetal bovine serum (FBS) 100 μg/mL penicillin and 100 μg/mL streptomycin]. Using 96-well microtiter plates at a concentration of 5×104–105 cell/well in a fresh medium, cells were seeded and left to attach to the plates for 24 h. Treatment with the test compound allowed attachment of cell to the wall of the plate. Monolayer cells were incubated with the compounds for 48 h at 37°C in a humidified incubator with 5% CO2. Cells were fixed with trichloroacetic acid and stained for 30 min with 0.4% (wt/vol) sulforhodamine B (SRB) stain dissolved in 1% acetic acid. Unbound dye was washed with 1% acetic acid and protein bound dye was extracted with Tris EDTA (Meter Tech. Σ 960, USA). The optical density (O.D.) of each well was measured spectrophotometrically at 564 nm with an ELIZA microplate reader (Meter Tech. Σ 960, USA). The percentage of cell survival was calculated as follows: Survival fraction=O.D. (treated cells)/O.D. (control cells). The IC50 values were calculated using different concentrations of the tested compounds. The relation between surviving fraction and compound concentration was plotted to obtain the survival curve (Table 1).
Molecular docking
The docking studies and modeling calculations were done using ‘MOE version 2008.10 release of Chemical Computing Group’s’ which was operated under Windows XP operating system installed on an Intel Pentium IV PC with a 2.8 MHz processor and 512 RAM. The tested compounds were built in 2D using ChemBiooffice suite and geometric optimization was done using Hyperchem, then subjected to docking simulation. The X-ray crystallographic structure of heat shock protein enzyme was obtained from the Protein Data Bank; code ‘1UYK.pdb’.
Acknowledgments
The authors wish to thank Faculty of pharmacy, Mansoura University for using his laboratory equipments and funding this work.
References
[1] Ferlay, J.; Shin, H. R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D. M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer.2010, 127, 2893–2917.10.1002/ijc.25516Search in Google Scholar
[2] Mokbel, K. The evolving role of aromatase inhibitors in breast cancer. Int. J. Clin. Oncol.2002, 7, 279–283.10.1007/s101470200040Search in Google Scholar
[3] Goss, P. E.; Powles, T. J.; Dowsett, M.; Hutchison, G.; Brodie, A. M.; Gazet, J. C. Treatment of advanced postmenopausal breast cancer with an aromatase inhibitor, 4-hydroxyandrostenedione: phase II report. Cancer Res.1986, 46, 4823–4826.Search in Google Scholar
[4] Simpson, E. R. Sources of estrogen and their importance. J. Steroid Biochem. Mol. Biol.2003, 86, 225–230.10.1016/S0960-0760(03)00360-1Search in Google Scholar
[5] Bukau, B.; Weissman, J.; Horwich, A. Molecular chaperones and protein quality control. Cell2006, 125, 443–451.10.1016/j.cell.2006.04.014Search in Google Scholar
[6] Lindquist, S.; Craig, E. A. The heat shock proteins. Ann. Rev. Genet.1988, 22, 631–637.10.1146/annurev.ge.22.120188.003215Search in Google Scholar
[7] Zagouri, F.; Bournakis, E.; Koutsoukos, K.; Papadimitriou, C. A. Heat shock protein 90 (hsp90) expression and breast cancer. Pharmaceuticals (Basel)2012, 5, 1008–1020.10.3390/ph5091008Search in Google Scholar
[8] Che, H. L.; Hong, H. M.; Chang, Y. Y.; Chang, W. W. Inhibition of heat shock protein (Hsp) 27 potentiates the suppressive effect of Hsp90 inhibitors in targeting breast cancer stem-like cells. Biochimie2012, 94, 1382–1389.10.1016/j.biochi.2012.02.034Search in Google Scholar
[9] Yiu, C. C.; Chanplakorn, N.; Chan, M. S.; Loo, W. T.; Chow, L. W.; Toi, M.; Sasano, H. Down-regulation of heat-shock protein 70 (HSP-70) correlated with responsiveness to neoadjuvant aromatase inhibitor therapy in breast cancer patients. Anticancer Res.2010, 30, 3465–3472.10.1016/S1359-6349(10)70160-9Search in Google Scholar
[10] Bouabdallah, I.; M’Barek, L. A.; Zyad, A.; Ramdani, A.; Zidane, I.; Melhaoui, A. New pyrazolic compounds as cytotoxic agents. Nat. Prod. Res.2007, 21, 298–302.10.1080/14786410701192801Search in Google Scholar
[11] Havrylyuk, D.; Zimenkovsky, B.; Vasylenko, O.; Zaprutko, L.; Gzella, A.; Lesyk, R. Synthesis of novel thiazolone-based compounds containing pyrazoline moiety and evaluation of their anticancer activity. Eur. J. Med. Chem.2009, 44, 1396–1404.10.1016/j.ejmech.2008.09.032Search in Google Scholar
[12] Shaharyar, M.; Abdullah, M. M.; Bakht, M. A.; Majeed, J. Pyrazoline bearing benzimidazoles: search for anticancer agent. J. Eur. J. Med. Chem.2010, 45, 114–119.10.1016/j.ejmech.2009.09.032Search in Google Scholar
[13] Perchellet, E. M.; Ward, M. M.; Skaltsounis, A. L.; Kostakis, I. K.; Pouli, N.; Marakos, P.; Perchellet, J. H. Antiproliferative and proapoptotic activities of pyranoxanthenones, pyranothioxanthenones and their pyrazole-fused derivatives in HL-60 cells. Anticancer Res.2006, 26, 2791–2804.Search in Google Scholar
[14] Koca, I.; Ozgur, A.; Coskun, K. A.; Tutar, Y. Synthesis and anticancer activity of acyl thioureas bearing pyrazole moiety. Bioorg. Med. Chem.2013, 21, 3859–3865.10.1016/j.bmc.2013.04.021Search in Google Scholar
[15] Caliskan, B.; Yilmaz, A.; Evren, I.; Menevse, S.; Uludag, O.; Banoglu, E. Synthesis and evaluation of analgesic, anti-inflammatory, and anticancer activities of new pyrazole-3(5)-carboxylic acid derivatives. Med. Chem. Res.2013, 22, 782–793.10.1007/s00044-012-0072-4Search in Google Scholar
[16] Zheng, L. W.; Li, Y.; Ge, D.; Zhao, B. X.; Liu, Y. R.; Lv, H. S.; Ding, J.; Miao, J. Y. Synthesis of novel oxime-containing pyrazole derivatives and discovery of regulators for apoptosis and autophagy in A549 lung cancer cells. Bioorg. Med. Chem. Lett.2010, 20, 4766–4770.10.1016/j.bmcl.2010.06.121Search in Google Scholar
[17] Dang, C. T.; Dannenberg, A. J.; Subbaramaiah, K.; Dickler, M. N.; Moasser, M. M.; Seidman, A. D.; D’Andrea, G. M.; Theodoulou, M.; Panageas, K. S.; Norton, L.; et al. Phase II study of celecoxib and transtuzumab in metastatic breast cancer patients who have progressed after prior transtuzumzb-based treatments. Cancer Res.2004, 10, 4062–4067.10.1158/1078-0432.CCR-03-0463Search in Google Scholar
[18] Canney, P. A.; Machin, M. A.; Curto, J. A feasibility study of the efficacy and tolerability of the combination of exemestane with the COX-2 inhibitor celecoxib in post-menopausal patients with advanced breast cancer. Eur. J. Cancer2006, 42, 2751–2756.10.1016/j.ejca.2006.08.014Search in Google Scholar
[19] Prosperi, R. J.; Robertson, F. M. Cyclooxygenase-2 directly regulates gene expression of P450 Cyp19 aromatase promoter regions pII, pI.3 and pI.7 and estradiol production in human breast tumor cells. Prostag. Oth. Lipid M.2006, 81, 55–70.10.1016/j.prostaglandins.2006.07.003Search in Google Scholar
[20] Singh, B.; Berry, J. A.; Shoher, A.; Lucci, A. COX-2 induces IL-11 production in human breast cancer cells. J. Surg. Res.2006, 131, 267–275.10.1016/j.jss.2005.11.582Search in Google Scholar
[21] Chow, L. W. C.; Yip, A. Y. S.; Loo, W. T. Y.; Toi, M. Evaluation of neoadjuvant inhibition of aromatase activity and signal transduction in breast cancer. Cancer Lett.2008, 262, 232–238.10.1016/j.canlet.2007.12.003Search in Google Scholar
[22] Chow, L. W.; Cheng, C. W.; Wong, J. L.; Toi, M. Serum lipid profiles in patients receiving endocrine treatment for breast cancer–the results from the Celecoxib Anti-Aromatase Neoadjuvant (CAAN) Trial. Biomed. Pharmacother.2005, 59, 302–305.10.1016/S0753-3322(05)80051-4Search in Google Scholar
[23] Brodie, A. M. Aromatase, its inhibitors and their usein breast cancer treatment. Pharmacol. Ther.1993, 60, 501–515.10.1016/0163-7258(93)90033-ASearch in Google Scholar
[24] Miller, W. L. Molecular biology of steroid hormone synthesis. Endocr. Rev.1988, 9, 295–318.10.1210/edrv-9-3-295Search in Google Scholar
[25] Insuasty, B.; Tigreros, A.; Orozco, F.; Quiroga, J.; Abonia, R.; Nogueras, M. Sanchez, A.; Cobo, J. Synthesis of novel pyrazolic analogues of chalcones and their 3-aryl-4-(3-aryl-4,5-dihydro-1H-pyrazol-5-yl)-1-phenyl-1H-pyrazole derivatives as potential antitumor agents. Bioorg. Med. Chem.2010, 18, 4965–4974.10.1016/j.bmc.2010.06.013Search in Google Scholar
[26] Farag, A. M.; Ali, A. K.; El-Debss, M. A.; Mayhoub, A. S.; Amr, A. E.; Abdel-Hafez, N. A.; Abdulla, M. M. Design, synthesis and structure activity relationship study of novel pyrazole-based heterocycles as potential antitumor agents. Eur. J. Med. Chem.2010, 45, 5887–5898.10.1016/j.ejmech.2010.09.054Search in Google Scholar
[27] Zhang, D.; Wang, G.; Zhao, G.; Xu, W.; Huo, L. Synthesis and cytotoxic activity of novel 3-(1H-indol-3-yl)-1H-pyrazole-5-carbohydrazide derivatives. Eur. J. Med. Chem.2011, 46, 5868–5877.10.1016/j.ejmech.2011.09.049Search in Google Scholar
[28] Modzelewska, A.; Catherine, P.; Geetha, A. and Nancy, E. Anticancer activities of novel chalcone and bis-chalcone derivatives, Bioorg. Med. Chem. Lett.2006, 14, 3491–3495.10.1016/j.bmc.2006.01.003Search in Google Scholar
[29] Ngameni, B.; Kuete, V.; Ambassa, P.; Justin, k.; Marlyse, M. L.; Tchoukoua, A.; Roy, R.; Ngadjui, B. T.; Tetsuya, M. Synthesis and evaluation of anticancer activity of O-allylchalcone derivatives. Med. Chem.2013, 3, 233–237.10.4172/2161-0444.1000144Search in Google Scholar
[30] Juvale, K.; Pape, V. F.; Wiese, M. Investigation of chalcones and benzochal-cones as inhibitors of breast cancer resistance protein. Bioorg. Med. Chem.2012, 20, 346–355.10.1016/j.bmc.2011.10.074Search in Google Scholar
[31] Kamal, A.; Reddy, J. S.; Ramaiah, M. J.; Dastagiri, D.; Bharathi, E. V.; Prem Sagar, M. V. Synthesis and biological evaluation of imidazopyridine/pyrimidine-chalcone derivatives as potential anticancer agents. Med. Chem. Commun.2010, 1, 355–360.10.1039/c0md00116cSearch in Google Scholar
[32] Nofal, Z. M.; Soliman, E. A.; Abd El-karim, S. S.; El-zahar, M. I.; Srour, A. M.; Sethumadhavan, S.; Maher, T. J. Novel benimidazole derivatives as expected anticancer agents. Acta Poloniae Pharm. Drug Res.2011, 68, 519–534.Search in Google Scholar
[33] Thabit, M. G.; Atta, S. A.; Nasr, M. N. Synthesis and biological evaluation of new 3-(4-subsituted phenyl)aminoquinoxaline derivatives as anticancer agents. Heterocycl. Commun.2015, 21, 25–35.10.1515/hc-2014-0081Search in Google Scholar
[34] Farag, A. M., Mayhoub, A. S.; Eldebss, M. A.; Amr, A. E.; Ali, A.K.; Abdel-Hafez, N. A.; Abdulla, M. M. Synthesis and structure-activity relationship studies of pyrazole-based heterocycles as antitumor agents. Arch. Pharm. Chem. Life Sci.2010, 343, 384–396.10.1002/ardp.200900176Search in Google Scholar
[35] Balbi, A.; Anzaldi, M. Macciò, C.; Aiello, C.; Mazzei, M.; Gangemib, R.; Castagnola, P. Miele, M.; Rosanod, C.; Viale, M. Synthesis and biological evaluation of novel pyrazole derivatives with anticancer activity. Eur. J. Med. Chem.2011, 46, 5293–5309.10.1016/j.ejmech.2011.08.014Search in Google Scholar
[36] Nitulescu, G. M.; Draghici, C.; Missir, A. V. Synthesis of new pyrazole derivatives and their anticancer evaluation. Eur. J. Med. Chem.2010, 45, 4914–4919.10.1016/j.ejmech.2010.07.064Search in Google Scholar
[37] Moa, W. Y.; Liang, Y. J.; Gu, Y. C.; Fu, L. W.; He, H. W. Synthesis and cytotoxicity of 8-cyano-3-substitutedalkyl-5-methyl-4-methylene-7-methoxy-3,4-dihydropyrido[4,3-d]pyrimidines. Bioorg. Med. Chem. Lett.2011, 21, 5975–5977.10.1016/j.bmcl.2011.07.067Search in Google Scholar
[38] Ghorab, M. M.; El-Gazzar, M. G.; Alsaid; M. S. Synthesis, characterization and anti-breast cancer activity of new 4-aminoantipyrine-based heterocycles. Int. J. Mol. Sci.2014, 15, 7539–7553.10.3390/ijms15057539Search in Google Scholar
[39] Ansari, F. L.; Iftikhar, F.; Ul-Haq, I.; Mirza, B.; Baseer, M.; Rashid, U. Solid-phase synthesis and biological evaluation of a parallel library of 2,3-dihydro-1,5-benzothiazepines. Bioorg. Med. Chem.2008, 16, 7691–7697.10.1016/j.bmc.2008.07.009Search in Google Scholar
[40] Bariwal, J. B.; Upadhyay, K. D.; Manvar, A. T.; Trivedi, J. C.; Singh, J. S.; Jain, K. S.; Shah, A. K. 1,5-Benzothiazepine, a versatile pharmacophore: a review. Eur. J. Med. Chem.2008, 43, 2279–2290.10.1016/j.ejmech.2008.05.035Search in Google Scholar
[41] Viegas, J. C.; Danuello, A.; Silva, B. V.; Barreiro, E. J.; Fraga, C. A. Molecularhybridization: a useful tool in the design of new drug prototypes. Curr. Med. Chem.2007, 14, 1829–1852.10.2174/092986707781058805Search in Google Scholar
[42] Walsh, J. J.; Bell, A. Hybrid drugs for malaria. Curr. Pharm. Des.2009, 15, 2970–2985.10.2174/138161209789058183Search in Google Scholar
[43] Anand, N.; Singh, P.; Sharma, A.; Tiwari, S.; Singh, V.; Singh, D. K. Synthesis and evaluation of small libraries of triazolylmethoxy chalcones, flavanones and 2-aminopyrimidines as inhibitors of mycobacterial FAS-II and PknG. Bioorg. Med. Chem.2012, 20, 5150–5163.10.1016/j.bmc.2012.07.009Search in Google Scholar
[44] Yoneda, F.; Tsukuda, K.; Kawazoe, M.; Sone, A. Synthesis and properties of 1-benzothiopyrano[2,3-o]-pyrimidine-2,4-(3H)-diones (10-thia-5-deazaflavins). J. Heterocycl. Chem.1981, 18, 1329–1333.10.1002/jhet.5570180711Search in Google Scholar
[45] Chan, W. L.; Ho, D. D.; Lau, C. P.; Wat, K. H.; Kong, Y. C.; Cheng, K. F.; Wong, T. T.; Chan, T. Y. Structure function relationship study of yuehchukene. I. Anti-implantation and estrogenic activities of substituted yuehchukene derivatives. Eur. J. Med. Chem.1991, 26, 387–390.10.1016/0223-5234(91)90099-9Search in Google Scholar
[46] Pool, W. O.; Harwood, H. J.; Ralston, A. W. 2-Alkyl benzimidazoles as derivatives for identification of aliphatic acids. J. Amer. Chem. Soc.1937, 1, 178–179.10.1021/ja01280a044Search in Google Scholar
[47] Albogami, A. S.; Karama, U.; Mousa, A. A.; khan, M. Simple and efficient one step synthesis of functionalized flavanones and chalcones. Orient. J. Chem.2012, 28, 619–626.10.13005/ojc/280201Search in Google Scholar
[48] Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J. T.; Bokesch, H.; Kenney, S.; Boyd, M. R. New colorimetric cytotoxicity assay for anticancer drug screening. Natl. Cancer Inst.1990, 82, 1107–1112.10.1093/jnci/82.13.1107Search in Google Scholar
[49] Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc.2006, 1, 1112–1116.10.1038/nprot.2006.179Search in Google Scholar
[50] Molecular Operating Environment (MOE), 2013.08; Chemical Computing Group Inc., 1010 Sherbooke St. West, Suite #910, Montreal, QC, Canada, H3A 2R7, 2015.Search in Google Scholar
[51] Liu, X.; Ouyang, S.; Yu, B.; Huang, K.; Liu, Y.; Gong, J.; Zheng, S.; Li, Z.; Li, H.; Jiang, H. PharmMapper Server: a web server for potential drug target identification via pharmacophore mapping approach. Nucleic Acids Res.2010, 38, 609–614.10.1093/nar/gkq300Search in Google Scholar
[52] Wright, L.; Barril, X.; Dymock, B.; Sheridan, L.; Surgenor, A.; Beswick, M.; Drysdale, M.; Collier, A.; Massey, A.; Davies, N.; et al. Structure-activity relationships in purine-based inhibitor binding to HSP90 isoforms. Chem. Biol.2004, 11, 775–785.10.1016/j.chembiol.2004.03.033Search in Google Scholar
[53] Genin, M. J.; Biles, C.; Keiser, B. J.; Poppe, S. M.; Swaney, S. M.; Tarpley, W. G.; Yagi, Y.; Romero, D. L. Novel 1,5-diphenylpyrazole non nucleoside HIV-1 reverse transcriptase inhibitors with enhanced activity versus the delavirdine-resistant P236L mutant: lead identification and SAR of 3- and 4-subsituted derivatives. J. Med. Chem.2000, 43, 1034–1039.10.1021/jm990383fSearch in Google Scholar
[54] Finar, I. L.; Manning, M. The Preparation and Some Reactions of 4-Formyl-1-phenylpyrazoles. J. Chem. Soc.1961, 2737–2741.10.1039/jr9610002733Search in Google Scholar
©2015 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Preliminary Communications
- Triphenylphosphine catalyzed domino reaction of dialkyl acetylenedicarboxylate with 3-aryl- 2-benzoylcyclopropane-1,1-dicarbonitrile
- Synthesis of colletotrichumine A
- Research Articles
- Synthesis of the spiroacetal fragments of spirofungins A and B, antibiotics isolated from Streptomyces violaceusniger Tü 4113
- Efficient synthesis and fungicidal activities of strobilurin analogues containing benzofuro [3,2-d]-1,2,4-triazolo[1,5-a]pyrimidinone side chains
- Synthesis of 2-amino-6,7,8,9-tetrahydro-6-phenethyl-3H-pyrimido[4,5-e][1,4]diazepin-4(5H)-one: a model for a potential pyrimido[4,5-e][1,4]diazepine-based folate anti-tumor agent
- Cascade assembling of pyrazolin-5-ones and benzylidenemalononitriles: the facile and efficient approach to medicinally relevant spirocyclopropylpyrazolone scaffold
- Synthesis, characterization and bioactivity of novel 5,6-dihydropyrrolo[3,4-c]pyrazol-4- (1H)one derivatives
- Molecular modeling and synthesis of new 1,5-diphenylpyrazoles as breast cancer cell growth inhibitors
- An efficient synthesis of 11-aryl-10-oxo-7,8,10,11-tetrahydro-1H-[1,2,3]triazolo [4′,5′:3,4]benzo[1,2-b][1,6]naphthyridine derivatives under catalyst-free conditions
- Mechanochemical synthesis of 2,2-difluoro-4, 6-bis(β-styryl)-1,3,2-dioxaborines and their use in cyanide ion sensing
- Visible-light-mediated radical aryltrichloromethylation of N-arylacrylamides for the synthesis of trichloromethyl-containing oxindoles
- A stereolibrary of conformationally restricted amino acids based on pyrrolidinyl/piperidinyloxazole motifs
- Synthesis of [1,3]thiazolo[3,2-b][1,2,4]triazol-7-ium and [1,2,4]triazolo[5,1-b][1,3]thiazin-4-ium salts via regioselective electrophilic cyclization of 3-[(2-alken-1-yl)sulfanyl]-4H-1,2,4-triazoles
Articles in the same Issue
- Frontmatter
- Preliminary Communications
- Triphenylphosphine catalyzed domino reaction of dialkyl acetylenedicarboxylate with 3-aryl- 2-benzoylcyclopropane-1,1-dicarbonitrile
- Synthesis of colletotrichumine A
- Research Articles
- Synthesis of the spiroacetal fragments of spirofungins A and B, antibiotics isolated from Streptomyces violaceusniger Tü 4113
- Efficient synthesis and fungicidal activities of strobilurin analogues containing benzofuro [3,2-d]-1,2,4-triazolo[1,5-a]pyrimidinone side chains
- Synthesis of 2-amino-6,7,8,9-tetrahydro-6-phenethyl-3H-pyrimido[4,5-e][1,4]diazepin-4(5H)-one: a model for a potential pyrimido[4,5-e][1,4]diazepine-based folate anti-tumor agent
- Cascade assembling of pyrazolin-5-ones and benzylidenemalononitriles: the facile and efficient approach to medicinally relevant spirocyclopropylpyrazolone scaffold
- Synthesis, characterization and bioactivity of novel 5,6-dihydropyrrolo[3,4-c]pyrazol-4- (1H)one derivatives
- Molecular modeling and synthesis of new 1,5-diphenylpyrazoles as breast cancer cell growth inhibitors
- An efficient synthesis of 11-aryl-10-oxo-7,8,10,11-tetrahydro-1H-[1,2,3]triazolo [4′,5′:3,4]benzo[1,2-b][1,6]naphthyridine derivatives under catalyst-free conditions
- Mechanochemical synthesis of 2,2-difluoro-4, 6-bis(β-styryl)-1,3,2-dioxaborines and their use in cyanide ion sensing
- Visible-light-mediated radical aryltrichloromethylation of N-arylacrylamides for the synthesis of trichloromethyl-containing oxindoles
- A stereolibrary of conformationally restricted amino acids based on pyrrolidinyl/piperidinyloxazole motifs
- Synthesis of [1,3]thiazolo[3,2-b][1,2,4]triazol-7-ium and [1,2,4]triazolo[5,1-b][1,3]thiazin-4-ium salts via regioselective electrophilic cyclization of 3-[(2-alken-1-yl)sulfanyl]-4H-1,2,4-triazoles

