Abstract
A short synthesis of colletotrichumine A, an oxindole-pyrazine alkaloid isolated from the pathogenic fungus Colletotrichum capsici, is described.
The fungus Colletotrichum capsici is an economically damaging plant pathogen with a range of hosts including vegetables, legumes, cereals and various tree fruits [1]. The fungus causes the often devastating anthracnose in chili, one of the most important crops in the tropics [2].
The ethyl acetate extract of C. capsici was recently shown to contain colletotrichumine A (1), a structurally unique alkaloid comprising an oxindole fused to trimethylpyrazine moiety (Figure 1) [3]. Glume blotch in wheat is caused by Septoria nodorum, a pathogenic fungus that produces septorine (2), which is a pyrazine that causes a decoupling action on wheat mitochondria and likely contributes to the pathogenicity of the fungus [4]. Thus, we set out to determine if colletotrichumine A (1) plays a role in the pathogenicity of C. capsici and as such, set out to synthesize this natural product to provide a sufficient quantity for biological evaluation.
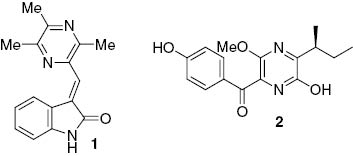
The fungal pyrazines colletotrichumine A (1) and septorine (2).
A classic Knoevenagel approach [5] was used to construct colletotrichumine A (1 in Figure 1 and Scheme 1). Oxidation of the known alcohol 3 [6] with manganese dioxide gave 3,5,6-trimethylpyrazine-2-carbaldehyde (4) using the literature protocol [7]. The Knoevenagel condensation of 4 with 2-oxindole gave exclusively the desired regioisomer 1, as determined by NOE studies (Scheme 1). The spectroscopic data of synthetic 1 were identical in all aspects to the natural product (Table 1).

Synthesis of colletotrichumine A (1).
NMR data for synthetic and authentic colletotrichumine A (1).
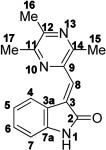 | ||||
|---|---|---|---|---|
| Atom No. | Synthetic 1 (DMSO-d6) | Colletotrichumine A (DMSO-d6) [3] | ||
| δH (400 MHz) | δC (100 MHz) | δH (400 MHz) | δC (100 MHz) | |
| 1 | 10.65, s | 10.64, s | ||
| 2 | – | 169.0 | – | 169.0 |
| 3 | – | 130.0 | – | 130.1 |
| 3a | – | 121.3 | – | 121.4 |
| 4 | 8.60, br d (J 7.7) | 127.2 | 8.58, br d (J 7.7) | 127.3 |
| 5 | 6.98, br t (J 7.6) | 121.1 | 6.96, br t (J 7.7) | 121.2 |
| 6 | 7.28, br t (J 7.7) | 130.9 | 7.27, br t (J 7.2) | 130.9 |
| 7 | 6.88, br d (J 7.7) | 109.7 | 6.87, br d (J 7.7) | 109.8 |
| 7a | – | 143.6 | – | 143.6 |
| 8 | 7.66, s | 129.2 | 7.64, s | 129.3 |
| 9 | – | 143.3 | – | 143.3 |
| 11 | – | 151.9 | – | 151.9 |
| 12 | – | 149.1 | – | 149.1 |
| 14 | – | 150.8 | – | 150.8 |
| 15 | 2.63, s | 20.9 | 2.61, s | 21.0 |
| 16 | 2.54, s | 21.6 | 2.53, s | 21.7 |
| 17 | 2.60, s | 21.2 | 2.59, s | 21.2 |
In summary, a straightforward synthesis of the oxindole-pyrazine alkaloid colletotrichumine A is reported. Biological evaluation of colletotrichumine A is in progress, the results of which will hopefully determine if this heteroaromatic compound plays a role in the pathogenicity of C. capsici.
Experimental
Commercially available reagents were used throughout without purification. Anhydrous solvents were used as supplied. All reactions were routinely carried out in oven-dried glassware under a nitrogen atmosphere. Analytical thin layer chromatography was performed using silica plates and compounds were visualized at 254 nm and/or 360 nm ultraviolet irradiation followed by staining with either alkaline permanganate or ethanolic vanillin solution. Melting points were recorded on an electrothermal melting point apparatus and are uncorrected. NMR spectra were recorded at 400 MHz for 1H nuclei and 100 MHz for 13C nuclei. Assignments were made with the aid of NOESY and HMBC experiments. High resolution mass spectra were obtained by electrospray ionization in positive ion mode at a nominal accelerating voltage of 70 eV on a microTOF mass spectrometer.
3,5,6-Trimethylpyrazine-2-carbaldehyde (4)
To a solution of 2-hydroxymethyl-3,5,6-trimethylpyrazine (3) [6] (40 mg 0.26 mmol) in ethanol (3 mL) was added manganese dioxide (1 g, 11.5 mmol) and the mixture was stirred for 1 h at room temperature, filtered and the solid washed with ethanol. The filtrate was concentrated in vacuo to yield compound 4 (39 mg, 0.26 mmol, 98%) as a colorless solid; mp 75–77°C; 1H NMR: (CDCl3): δH 10.16 (1 H, s, CHO), 2.80 (3 H, s, Me), 2.60 (3 H, s, Me), 2.46 (3 H, s, Me); 13C NMR (CDCl3): δC 194.6 (C=O), 155.5 (C), 151.8 (C), 150.2 (C), 141.9 (C), 22.4 (Me), 21.5 (Me), 18.5 (Me); 1H NMR data consistent with the literature [6].
Colletotrichumine A (1)
A solution of 3,5,6-trimethylpyrazine-2-carbaldehyde (4) (40 mg, 0.26 mmol), 2-oxindole (35 mg, 0.26 mmol) and piperidine (0.02 mL) in EtOH (3 mL) was heated to reflux for 3 h. Upon cooling to room temperature, the mixture was concentrated in vacuo, diluted in ethyl acetate (30 mL), washed with brine (20 mL), dried (Na2SO4), filtered and concentrated in vacuo. The crude solid was purified by flash chromatography on silica gel (30:70 EtOAc/CH2Cl2) to yield compound 1 (18 mg, 0.07 mmol, 45%) as an orange solid; mp 255–257°C (lit [3] mp not stated); IR (neat): νmax 3300, 1701, 1605, 1460, 1405, 1362, 1321, 747 cm-1; For NMR data, see Table 1. ESI-HRMS. Calcd for [C16H15N3O + Na]+: m/z 288.1113. Found: m/z 288.1114.
Acknowledgments
The University of Auckland is thanked for the award of a doctoral scholarship (K.V.G).
References
[1] Bailey, J. A.; Jeger, M. J. Colletotrichum: Biology, Pathology and Control. Commonwealth Mycological Institute: Wallingford, UK, 1992.10.1079/9780851987569.0000Search in Google Scholar
[2] Pakdeevaraporn, P.; Wasee, S.; Taylor, P. W. J.; Mongkolporn, O. Inheritance of resistance to anthracnose caused by Colletotrichum capsici in Capsicum. Plant Breeding2005, 124, 206–208.10.1111/j.1439-0523.2004.01065.xSearch in Google Scholar
[3] Hu, Z.; Wang, J.; Bi, X.; Zhang, J.; Xue, Y.; Yang, Y.; Luo, Z.; Yao, G.; Zhang, Y. Colletotrichumine A, a novel indole-pyrazine alkaloid with an unprecedented C16N3-type skeleton from cultures of Colletotrichum capsici.Tetrahedron Lett. 2014, 55, 6093–6095.10.1016/j.tetlet.2014.09.041Search in Google Scholar
[4] Devys, M.; Barbier, M.; Kollmann, A.; Bousquet, J.-F. Septorine and N-methoxyseptorine, substituted pyrazines from the fungus Septoria nodorumBerk. Tetrahedron Lett. 1982, 23, 5409–5412.10.1016/0040-4039(82)80143-3Search in Google Scholar
[5] Ziarani, G. M.; Gholamzadeh, P.; Lashgari, N.; Hajiabbasi, P. Oxindole as starting material in organic synthesis. ARKIVOC2013, 470–535.10.3998/ark.5550190.p008.074Search in Google Scholar
[6] Deng, L.; Guo, X.; Zhai, L.; Song, Y.; Chen, H.; Zhan, P.; Wu, J.; Liu, X. Ligustrazine derivatives. Part 4. Design, synthesis, and biological evaluation of novel ligustrazine-based stilbene derivatives as potential cardiovascular agents. Chem. Biol. Drug. Des.2012, 79, 731–739.10.1111/j.1747-0285.2012.01332.xSearch in Google Scholar
[7] Sun, Y.; Jiang, J.; Zhang, Z.; Yu, P.; Wang, L.; Xu, C.; Liu, W.; Wang, Y. Antioxidative and thrombolytic TMP nitrone for treatment of ischemic stroke. Bioorg. Med. Chem.2008, 16, 8868–8874.10.1016/j.bmc.2008.08.075Search in Google Scholar
©2015 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Preliminary Communications
- Triphenylphosphine catalyzed domino reaction of dialkyl acetylenedicarboxylate with 3-aryl- 2-benzoylcyclopropane-1,1-dicarbonitrile
- Synthesis of colletotrichumine A
- Research Articles
- Synthesis of the spiroacetal fragments of spirofungins A and B, antibiotics isolated from Streptomyces violaceusniger Tü 4113
- Efficient synthesis and fungicidal activities of strobilurin analogues containing benzofuro [3,2-d]-1,2,4-triazolo[1,5-a]pyrimidinone side chains
- Synthesis of 2-amino-6,7,8,9-tetrahydro-6-phenethyl-3H-pyrimido[4,5-e][1,4]diazepin-4(5H)-one: a model for a potential pyrimido[4,5-e][1,4]diazepine-based folate anti-tumor agent
- Cascade assembling of pyrazolin-5-ones and benzylidenemalononitriles: the facile and efficient approach to medicinally relevant spirocyclopropylpyrazolone scaffold
- Synthesis, characterization and bioactivity of novel 5,6-dihydropyrrolo[3,4-c]pyrazol-4- (1H)one derivatives
- Molecular modeling and synthesis of new 1,5-diphenylpyrazoles as breast cancer cell growth inhibitors
- An efficient synthesis of 11-aryl-10-oxo-7,8,10,11-tetrahydro-1H-[1,2,3]triazolo [4′,5′:3,4]benzo[1,2-b][1,6]naphthyridine derivatives under catalyst-free conditions
- Mechanochemical synthesis of 2,2-difluoro-4, 6-bis(β-styryl)-1,3,2-dioxaborines and their use in cyanide ion sensing
- Visible-light-mediated radical aryltrichloromethylation of N-arylacrylamides for the synthesis of trichloromethyl-containing oxindoles
- A stereolibrary of conformationally restricted amino acids based on pyrrolidinyl/piperidinyloxazole motifs
- Synthesis of [1,3]thiazolo[3,2-b][1,2,4]triazol-7-ium and [1,2,4]triazolo[5,1-b][1,3]thiazin-4-ium salts via regioselective electrophilic cyclization of 3-[(2-alken-1-yl)sulfanyl]-4H-1,2,4-triazoles
Articles in the same Issue
- Frontmatter
- Preliminary Communications
- Triphenylphosphine catalyzed domino reaction of dialkyl acetylenedicarboxylate with 3-aryl- 2-benzoylcyclopropane-1,1-dicarbonitrile
- Synthesis of colletotrichumine A
- Research Articles
- Synthesis of the spiroacetal fragments of spirofungins A and B, antibiotics isolated from Streptomyces violaceusniger Tü 4113
- Efficient synthesis and fungicidal activities of strobilurin analogues containing benzofuro [3,2-d]-1,2,4-triazolo[1,5-a]pyrimidinone side chains
- Synthesis of 2-amino-6,7,8,9-tetrahydro-6-phenethyl-3H-pyrimido[4,5-e][1,4]diazepin-4(5H)-one: a model for a potential pyrimido[4,5-e][1,4]diazepine-based folate anti-tumor agent
- Cascade assembling of pyrazolin-5-ones and benzylidenemalononitriles: the facile and efficient approach to medicinally relevant spirocyclopropylpyrazolone scaffold
- Synthesis, characterization and bioactivity of novel 5,6-dihydropyrrolo[3,4-c]pyrazol-4- (1H)one derivatives
- Molecular modeling and synthesis of new 1,5-diphenylpyrazoles as breast cancer cell growth inhibitors
- An efficient synthesis of 11-aryl-10-oxo-7,8,10,11-tetrahydro-1H-[1,2,3]triazolo [4′,5′:3,4]benzo[1,2-b][1,6]naphthyridine derivatives under catalyst-free conditions
- Mechanochemical synthesis of 2,2-difluoro-4, 6-bis(β-styryl)-1,3,2-dioxaborines and their use in cyanide ion sensing
- Visible-light-mediated radical aryltrichloromethylation of N-arylacrylamides for the synthesis of trichloromethyl-containing oxindoles
- A stereolibrary of conformationally restricted amino acids based on pyrrolidinyl/piperidinyloxazole motifs
- Synthesis of [1,3]thiazolo[3,2-b][1,2,4]triazol-7-ium and [1,2,4]triazolo[5,1-b][1,3]thiazin-4-ium salts via regioselective electrophilic cyclization of 3-[(2-alken-1-yl)sulfanyl]-4H-1,2,4-triazoles

