Abstract
A stereolibrary of conformationally restricted oxazole-containing amino acids, namely all isomers of 5–pyrrolydinyl- and 5-piperidinyloxazole-4-carboxylic acids, were designed and synthesized in three steps by the reaction of the corresponding N-Boc-protected amino acids and ethyl isocyanoacetate. These natural products-inspired amino acids are valuable building blocks for the synthesis of peptidomimetics and potential lead compounds for drug discovery.
Introduction
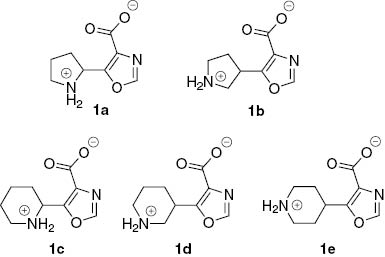
Providing drug discovery with novel potential biologically active compounds remains one of the most practically important tasks of synthetic organic chemistry. Many therapeutic areas benefit from molecules derived from or inspired by natural products [1–5]. Since many natural compounds violate rules of lead-likeness and have limited metabolic stability, their synthetic counterparts, which are based on similar structural motifs might be advantageous as the starting points for medicinal chemistry. Being guided by these principles, we were intrigued by the structures of oxazole-containing peptides, which were discovered among marine products in the late 1980s and have attracted much attention since then [6–9]. Design and synthesis of building blocks inspired by oxazole peptidomimetics, which comply with criteria of lead-likeness have been mentioned in the literature (Figure 1) [10–13]. In this work, we aimed at mounting such structural motifs onto conformationally restricted cores, which can provide diverse spatial arrangement of the functional groups. This approach leads to the sets of compounds which are referred to as “stereolibraries” (mini-libraries of isomers and/or homologues which differ only by the relative position of the functional groups in space, while the molecular topology remains the same) [14–19]. Herein we report synthesis of amino acid 1a–e stereolibrary, where the structural motif is inspired by oxazole peptidomimetics. It should be noted that prior 2014, derivatives of amino acids 1a and 1e were mentioned only in patents [20, 21]. Recently, synthesis of N-Boc-protected amino acid 1a was also described [22, 23], and it was shown that its derivatives are promising asymmetric organo- and metallocatalysts. Analysis of the values of predicted physico-chemical parameters of the simplest model derivatives of 1a–e (i.e. N-acetyl-N′-methylamide) [24] (Table 1) shows that the structures of these building blocks comply with the concept of the lead-oriented synthesis [25] and leave much room for the design of lead-like libraries.
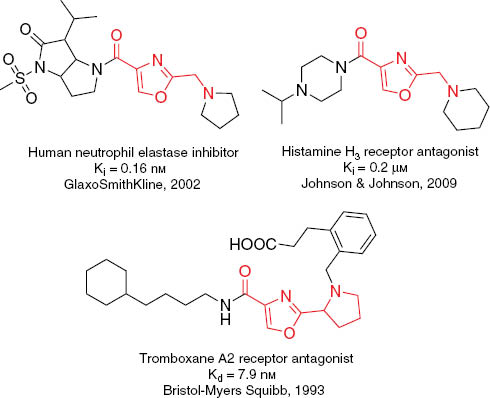
Biologically active derivatives of lead-oriented building blocks inspired by oxazole peptidomimetics.
Predicted physico-chemical parameters of N-acetyl-N′-methylamide derivatives of amino acids 1a–e.
| Parameter | Value range |
|---|---|
| Molecular weight (MW) | 237…251 |
| Hydrophilicity (cLogP) | –1.47…–0.63 |
| H-bond acceptors | 3 |
| H-bond donors | 1 |
| Total polar surface area (TPSA, Å2) | 75.4 |
| Rotatable bonds number (RotB) | 2 |
| Fraction of sp3 carbons (Fsp3) | 0.54…0.58 |
Results and discussion
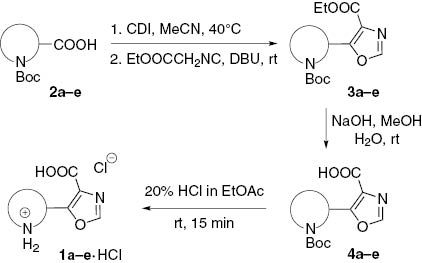
For the construction of the oxazole ring in the molecules 1a–e, the reaction of activated carboxylic acid derivatives with ethyl isocyanoacetate was used. Various methods for the co-activation of aliphatic carboxylic acids in this transformation were described in the literature, most of them including formation of chloro anhydrides [26–29], anhydrides [30–36] or azides [22, 37, 38]. We turned our attention to the method mentioned recently by Heiser and co-workers [39], which uses activation of the hydrocinnamic acid with CDI, followed by the reaction of the intermediate N-acylimidazolide with ethyl isocyanoacetate in the presence of DBU. The method worked well with all the N-Boc-protected amino acids 2a–e, resulting in the formation of oxazoles 3a–e in 77–98% yields (Table 2).
Synthesis of amino acids 1a–e (as hydrochlorides).
| Starting compound | Yield, % | ||
|---|---|---|---|
| 3 | 4 | 1·HCl | |
| rac-N-Boc-proline (2a) | 86 | 86 | 87 |
| rac-N-Boc-β-proline (2b) | 77 | 81 | 73 |
| rac-N-Boc-pipecolic acid (2c) | 83 | 85 | 98 |
| rac-N-Boc-nipecotic acid (2d) | 98 | 88 | 95 |
| N-Boc-isonipecotic acid (2e) | 93 | 86 | 98 |
Alkaline hydrolysis of the ester moiety in 3a–e proceeded without a problem, and the corresponding carboxylic acids 4a–e were obtained in 81–88% yields. On the contrary, the removal of the Boc protective groups in 4a–e required some additional efforts. Standard procedures including prolonged reaction with TFA – CH2Cl2 or HCl – EtOAc led to partial decomposition of the products, especially with oxazoles 4a and 4c derived from α-amino acids. It was found, however, that short-time treatment of neat 4 with concentrated HCl in EtOAc upon vigorous stirring led to the formation of the target amino acids 1a–e as hydrochlorides in good to excellent yields (73–98%). The pure crystalline compounds 1a–e are stable and could be stored at least for a month; however, partial decomposition was observed for some samples if the excessive HCl was not removed thoroughly.
Obviously, the method can be applied for the preparation of optically pure compounds 1b and 1d, if the corresponding optically pure Boc-amino acids 2b and 2d available from commercial sources are used as the starting materials. In the case of amino acids 1a and 1c, racemization is possible at the oxazole formation step. Experiments with both (R)- and (S)-2a showed that this is the case for the derivatives of α-amino acids. For this reason, we recommend using DPPA instead of CDI for the activation of the carboxylic acid moiety in the molecules of 2a and 2c as described recently by Kamal and co-workers [22].
Conclusions
A stereolibrary of conformationally restricted oxazole-containing amino acids was designed and synthesized in only three steps starting from easily available materials. These natural product-inspired compounds fit well the restrictions imposed by the lead-oriented synthesis and can be used as starting points in drug discovery programs; they are also of interest in the search of unusual secondary structure elements when incorporated into peptidomimetics.
Experimental
The solvents were purified according to standard procedures. Racemic compounds 2a–e were used for the synthesis. Analytical TLC was performed using Polychrom SI F254 plates. Column chromatography was performed using Kieselgel Merck 60 (230–400 mesh) as the stationary phase. 1H and 13C NMR spectra were recorded on a Varian Gemini 2000 spectrometer (at 400 MHz for protons and 100 MHz for carbon-13). Elemental analysis was conducted at the Laboratory of Organic Analysis, Institute of Organic Chemistry, National Academy of Sciences of Ukraine. Mass spectra were recorded on an Agilent 1200 LCMSD SL instrument [electrospray ionization (ESI)] and Agilent 1100 LCMS instrument [chemical ionization (APCI)].
General procedure for the preparation of 3
To a solution of N-Boc-protected amino acid 2 (0.140 mol) in CH3CN (250 mL), CDI (27.31 g, 0.168 mol) was added. The mixture was stirred at 40°C for 1 h, then cooled and treated with methyl isocyanoacetate (17.5 g, 0.154 mol) and DBU (23.5 g, 0.154 mol) and then stirred at room temperature overnight. The solution was concentrated under reduced pressure to half a volume, and 10% aqueous citric acid (600 mL) was added. The mixture was extracted with EtOAc (4×150 mL). The combined organic phases were washed with water (2×150 mL), 10% aqueous citric acid (150 mL), and brine (150 mL), then dried over Na2SO4 and concentrated in vacuo to give 3.
Ethyl 5-(1-(tert-butoxycarbonyl)pyrrolidin-2-yl)oxazole-4-carboxylate (3a)
This compound was obtained as a 3:1 mixture of rotamers; yield 86%; yellowish solid; mp 54–55°C; 1H NMR (CDCl3): δ 7.76 (s, 0.75H), 7.72 (s, 0.25H), 5.61–5.49 (m, 1H), 4.38 (q, J = 7.2 Hz, 2H), 3.64–3.53 (m, 1.5H), 3.52–3.43 (m, 0.5H), 2.42–2.29 (m, 1H), 2.12–2.01 (m, 0.75H), 2.01–1.88 (m, 2.25H), 1.38 (t, J = 7.1 Hz, 3H), 1.23 (s, 9H); 13C NMR (CDCl3): δ 161.2, 159.7, 153.2, 148.3, 126.2, 79.4, 60.8, 52.1, 46.2, 32.3, 27.7, 23.5, 14.0; ESI-MS: m/z 333 (Mna+). Anal. Calcd for C15H22N2O5: C, 58.05; H, 7.15; N, 9.03. Found: C, 58.31; H, 6.89; N, 9.25.
Ethyl 5-(1-(tert-butoxycarbonyl)pyrrolidin-3-yl)oxazole-4-carboxylate (3b)
This compound was obtained as a 1:1 mixture of rotamers; yield 77%; yellowish oil; 1H NMR (CDCl3): δ 7.79 (s, 1H), 4.38 (q, J = 7.0 Hz, 2H), 4.29–4.17 (m, 1H), 3.80–3.70 (m, 1H), 3.68–3.61 (m, 0.5H), 3.61–3.53 (m, 0.5H), 3.52–3.36 (m, 2H), 2.30–2.20 (m, 1H), 2.19–2.09 (m, 1H), 1.46 (s, 4.5H), 1.44 (s, 4.5H), 1.39 (t, J = 7.1 Hz, 3H); 13C NMR (CDCl3): δ 161.3, 158.0 and 157.9, 153.8, 148.8, 127.0, 79.1, 60.7, 49.1 and 48.7, 45.2 and 44.9, 35.2 and 34.4, 30.2 and 29.3, 28.0, 13.8; ESI-MS: m/z 333 (MNa+), 255 (MH+–C4H8), 211 (MH+–C4H8–CO2), 165. Anal. Calcd for C15H22N2O5: C, 58.05; H, 7.15; N, 9.03. Found: C, 57.73; H, 7.23; N, 8.68.
Ethyl 5-(1-(tert-butoxycarbonyl)piperidin-2-yl)oxazole-4-carboxylate (3c)
Yield 83%; yellowish oil; 1H NMR (CDCl3): δ 7.79 (s, 1H), 5.97 (dd, J = 5.5, 2.8 Hz, 1H), 4.45–4.31 (m, 2H), 4.09 (d, J = 11.2 Hz, 1H), 3.26 (td, J = 13.0, 3.6 Hz, 1H), 2.05–1.85 (m, 2H), 1.83–1.73 (m, 1H), 1.72–1.64 (m, 1H), 1.60–1.50 (m, 1H), 1.47–1.43 (m, 1H), 1.39 (t, J = 7.1 Hz, 3H), 1.36 (s, 9H); 13C NMR (CDCl3): δ 160.9, 160.2, 154.6, 148.5, 125.9, 79.7, 60.8, 46.9, 41.2, 28.9, 27.8, 24.3, 19.3, 13.9; ESI-MS: m/z 347 (MNa+), 225 (MH+–C4H8–CO2), 179. Anal. Calcd for C16H24N2O5: C, 59.24; H, 7.46; N, 8.64. Found: C, 59.07; H, 7.80; N, 8.48.
Ethyl 5-(1-(tert-butoxycarbonyl)piperidin-3-yl)oxazole-4-carboxylate (3d)
Yield 98%; white solid; mp 70–71°C; 1H NMR (CDCl3): δ 7.77 (s, 1H), 4.39 (q, J = 7.1 Hz, 2H), 4.02 (br s, 1H), 4.02 (d, J = 12.7 Hz, 1H), 3.62 (td, J = 10.5, 5.3 Hz, 1H), 3.08 (br s, 1H), 2.93–2.85 (m, 1H), 2.08–1.98 (m, 1H), 1.85–1.68 (s, 2H), 1.66–1.52 (m, 1H), 1.45 (s, 9H), 1.40 (t, J = 7.1 Hz, 3H); 13C NMR (CDCl3): δ 161.3, 159.7, 154.0, 148.6, 126.5, 79.4, 60.8, 46.9 and 45.8, 44.1 and 43.1, 33.7, 28.4, 28.0, 24.0, 13.9; ESI-MS: m/z 347 (MNa+), 225 (MH+–C4H8–CO2), 179. Anal. Calcd for C16H24N2O5: C, 59.24; H, 7.46; N, 8.64. Found: C, 59.57; H, 7.18; N, 8.55.
Ethyl 5-(1-(tert-butoxycarbonyl)piperidin-4-yl)oxazole-4-carboxylate (3e)
Yield 93%; light yellowish solid; mp 72–73°C; 1H NMR (CDCl3) δ 7.77 (s, 1H), 4.39 (q, J = 7.1 Hz, 2H), 4.22 (d, J = 12.1 Hz, 2H), 3.65 (tt, J = 11.7, 3.9 Hz, 1H), 2.84 (t, J = 12.0 Hz, 2H), 1.84 (dd, J = 13.1, 3.0 Hz, 2H), 1.81–1.69 (m, 2H), 1.48 (s, 9H), 1.44–1.38 (m, 3H); 13C NMR: (CDCl3): δ 161.6, 161.3, 154.2, 148.5, 125.7, 79.3, 60.8, 43.5, 43.1, 33.5, 29.2, 28.1, 14.0; ESI-MS: m/z 347 (MNa+), 225 (MH+–C4H8–CO2), 179. Anal. Calcd for C16H24N2O5: C, 59.24; H, 7.46; N, 8.64. Found: C, 59.28; H, 7.07; N, 8.94.
General procedure for the preparation of 4
To a solution of 3 (0.05 mol) in MeOH (300 mL), a pre-cooled solution of NaOH (0.15 mol) in water (12 mL) was added in one portion. The mixture was stirred at room temperature overnight (monitored by TLC), then concentrated in vacuo to 70–80 mL (below 35°C), diluted with water (100 mL) and washed with CH2Cl2 (2×50 mL). The aqueous phase was acidified with 15% citric acid (200–220 mL) and extracted with EtOAc (3×150 mL). The combined organic phases were washed with brine (150 mL), dried over Na2SO4 and concentrated in vacuo to give 5.
5-(1-(tert-Butoxycarbonyl)pyrrolidin-2-yl)oxazole-4-carboxylic acid (4a)
This compound was obtained as a 3: 2 mixture of rotamers; yield 86%; white solid; mp 158–160°C; 1H NMR (CDCl3): δ 7.84 (s, 1H), 5.55 (br s, 0.6H), 5.48 (br s, 0.4H), 3.70–3.47 (br m, 2H), 2.49–2.28 (br m, 1H), 2.21–1.91 (br m, 3H), 1.44 (s, 3.6H), 1.27 (s, 5.4H), COOH is exchanged with D2O; 13C NMR (CDCl3): δ 164.7 and 163.6, 160.7 and 158.4, 154.4 and 153.5, 149.0 and 148.7, 126.9 and 125.8, 80.5 and 79.8, 52.1, 46.6 and 46.3, 32.3 and 31.4, 28.0 and 27.7, 24.1 and 23.5; ESI-MS: m/z 305 (MNa+). Anal. Calcd for C13H18N2O5: C, 55.31; H, 6.43; N, 9.92. Found: C, 55.05; H, 6.52; N, 10.10.
5-(1-(tert-Butoxycarbonyl)pyrrolidin-3-yl)oxazole-4-carboxylic acid (4b)
Yield 81%; yellowish oil; 1H NMR (CDCl3): δ 7.88 (s, 1H), 4.17–4.34 (m, 1H), 3.71–3.89 (m, 1H), 3.54–3.68 (m, 1H), 3.37–3.51 (m, 2H), 2.24–2.32 (m, 2H), 1.46 (s, 9H), COOH is exchanged with HDO; 13C NMR (CDCl3): δ 163.6, 158.4 and 158.3, 154.3, 149.4, 126.9, 79.7, 49.2 and 48.7, 45.3 and 45.0, 35.1 and 34.4, 30.0 and 29.3, 28.0; ESI-MS: m/z 305 (MNa+). Anal. Calcd for C13H18N2O5: C, 55.31; H, 6.43; N, 9.92. Found: C, 55.47; H, 6.81; N, 9.67.
5-(1-(tert-Butoxycarbonyl)piperidin-2-yl)oxazole-4-carboxylic acid (4c)
Yield 85%; white powder; mp 164–166°C; 1H NMR (CDCl3): δ 9.13 (br s, 1H), 7.87 (s, 1H), 5.98 (dd, J = 5.6, 2.9 Hz, 1H), 4.10 (d, J = 11.6 Hz, 1H), 3.26 (td, J = 13.0, 3.4 Hz, 1H), 2.06–1.89 (m, 2H), 1.80 (d, J = 12.4 Hz, 1H), 1.75–1.64 (m, 1H), 1.64–1.43 (m, 2H), 1.38 (s, 9H); 13C NMR (CDCl3): δ 164.0, 160.6, 154.9, 149.0, 125.7, 80.2, 46.9, 41.2, 28.9, 27. 9, 24.3, 19.3; ESI-MS: m/z 319 (MNa+), 263 (MNa+–C4H8). Anal. Calcd for C14H20N2O5: C, 56.75; H, 6.80; N, 9.45. Found: C, 56.36; H, 7.13; N, 9.27.
5-(1-(tert-Butoxycarbonyl)piperidin-3-yl)oxazole-4-carboxylic acid (4d)
Yield 88%; white amorphous solid; 1H NMR (CDCl3): δ 10.66 (br s, 1H), 7.87 (s, 1H), 4.08 (br s, 1H), 4.01 (d, J = 12.7 Hz, 1H), 3.66 (ddd, J = 13.8, 10.4, 3.6 Hz, 1H), 3.09 (br s, 1H), 2.90 (td, J = 11.1, 2.5 Hz, 1H), 2.09–1.99 (m, 1H), 1.84–1.69 (m, 2H), 1.65–1.52 (m, 1H), 1.44 (s, 9H); 13C NMR (CDCl3): δ 164.2, 160.5, 154.3, 149.0, 126.2, 79.8, 47.0 and 45.7, 44.0 and 43.2, 33.6, 28.4, 28.1, 24.0; ESI-MS: m/z 319 (MNa+). Anal. Calcd for C14H20N2O5: C, 56.75; H, 6.80; N, 9.45. Found: C, 57.03; H, 6.53; N, 9.39.
5-(1-(tert-Butoxycarbonyl)piperidin-4-yl)oxazole-4-carboxylic acid (4e)
Yield 86%; white solid; mp 180–182°C; 1H NMR (CDCl3): δ 7.84 (s, 1H), 4.23 (d, J = 12.0 Hz, 2H), 3.68 (ddd, J = 11.8, 7.9, 3.8 Hz, 1H), 2.87 (t, J = 11.9 Hz, 2H), 1.87 (dd, J = 13.3, 2.8 Hz, 2H), 1.83–1.70 (m, 2H), 1.49 (s, 9H), COOH is exchanged with D2O; 13C NMR (CDCl3): δ 164.3, 162.0, 154.4, 149.0, 125.4, 79.7, 43.2, 33.4, 29.2, 28.1; ESI-MS: m/z 319 (MNa+). Anal. Calcd for C14H20N2O5: C, 56.75; H, 6.80; N, 9.45. Found: C, 56.47; H, 7.00; N, 9.48.
General procedure for the preparation of 1·HCl
To the finely powdered compound 4 (3.37 mmol), a pre-cooled saturated solution of HCl in EtOAc (~20%) was added. The resultant slurry was vigorously stirred at room temperature for 15 min (if the clots are formed at this stage, it is recommended to split them with a spatula). CAUTION! Violent gas emission! The mixture was filtered, and the precipitate was washed thoroughly with EtOAc (15 mL) and acetone (15 mL). The product was dried in vacuo thoroughly to give 1·HCl. The complete structures of these products are given in Table 1.
5-(Pyrrolidin-2-yl)oxazole-4-carboxylic acid, hydrochloride (1a·HCl)
Yield 87%; white crystals; mp 163–165°C; 1H NMR (D2O) δ 8.28 (s, 1H), 5.41 (t, J = 8.2 Hz, 1H), 5.41 (t, J = 7.2 Hz, 2H), 2.56–2.13 (m, 4H); 13C NMR (D2O) δ 165.7, 154.4, 153.1, 132.1, 55.6, 48.2, 30.6, 26.0; ESI-MS: m/z 183 (MH+). Anal. Calcd for C8H11ClN2O3: C, 43.95; H, 5.07; Cl, 16.22; N, 12.81. Found: C, 44.15; H, 5.44; Cl, 16.36; N, 12.72.
5-(Pyrrolidin-3-yl)oxazole-4-carboxylic acid, hydrochloride (1b·HCl)
Yield 73%; white solid; mp 168–170°C; 1H NMR (DMSO-d6): δ 9.71 (s, 2H), 8.42 (s, 1H), 4.29–4.12 (m, 1H), 3.62–3.50 (m, 1H), 3.41–3.32 (m, 1H), 3.29 (m, 1H), 3.23–3.17 (m, 1H), 2.39–2.27 (m, 1H), 2.13–2.01 (m, 1H), 1H is exchanged with D2O; 13C NMR (DMSO-d6): δ 162.5, 155.6, 150.6, 127.7, 47.2, 44.5, 34.3, 29.1; ESI-MS: m/z 183 (MH+), 139 (MH+–CO2). Anal. Calcd for C8H11ClN2O3: C, 43.95; H, 5.07; Cl, 16.22; N, 12.81. Found: C, 43.68; H, 5.1; Cl, 16.46; N, 12.50.
5-(Piperidin-2-yl)oxazole-4-carboxylic acid, hydrochloride (1c·HCl)
Yield 98%; white crystals; mp 144–146°C; 1H NMR (D2O) δ 8.30 (s, 1H), 5.04 (dd, J = 11.6, 3.5 Hz, 1H), 3.60 (d, J = 12.6 Hz, 1H), 3.24 (td, J = 12.5, 2.8 Hz, 1H), 2.24–1.98 (m, 4H), 1.89–1.67 (m, 2H); 13C NMR (DMSO-d6) δ 162.0, 152.1, 151.6, 129.0, 50.3, 44.3, 27.0, 21.5, 21.2; ESI- MS: m/z 197 (MH+). Anal. Calcd for C9H13ClN2O3: C, 46.46; H, 5.63; Cl, 15.24; N, 12.04. Found: C, 46.27; H, 6.01; Cl, 15.54; N 11.73.
5-(Piperidin-3-yl)oxazole-4-carboxylic acid, hydrochloride (1d·HCl)
Yield 95%; white crystals; mp 131–133°C; 1H NMR (D2O): δ 8.16 (s, 1H), 4.01–3.90 (m, 1H), 3.57 (dd, J = 12.7, 3.0 Hz, 1H), 3.46 (d, J = 12.7 Hz, 1H), 3.28 (t, J = 12.1 Hz, 1H), 3.09 (t, J = 11.9 Hz, 1H), 2.16–1.79 (m, 4H); 13C NMR (D2O): δ 166.1, 159.7, 153.4, 128.7, 47.4, 45.9, 33.5, 27.9, 23.6; ESI-MS: m/z 197 (MH+). Anal. Calcd for C9H13ClN2O3: C, 46.46; H, 5.63; Cl, 15.24; N, 12.04. Found: C, 46.38; H, 5.95; Cl, 15.36; N, 11.71.
5-(Piperidin-4-yl)oxazole-4-carboxylic acid, hydrochloride (1e·HCl)
Yield 98%; white crystals; mp 245–247°C; 1H NMR (D2O) δ 8.15 (s, 1H), 3.92–3.80 (m, 1H), 3.56 (d, J = 13.2 Hz, 2H), 3.20 (td, J = 12.2, 2.3 Hz, 2H), 2.24–2.02 (m, 4H); 13C NMR (D2O): δ 166.4, 162.3, 153.0, 127.7, 45.5, 33.0, 28.0; ESI-MS: m/z 197 (MH+), 153 (MH+–CO2). Anal. Calcd for C9H13ClN2O3: C, 46.46; H, 5.63; Cl, 15.24; N, 12.04. Found: C, 46.40; H, 5.49; Cl, 14.91; N, 12.16.
References
[1] Koehn, F. E. Drug discovery from natural products. Nature Rev. Drug Discov.2009, 8, 678.10.1038/nrd2950Search in Google Scholar
[2] Koehn. F. E.; Carter, G. T. The evolving role of natural products in drug discovery. Nature Rev. Drug Discov.2005, 4, 206–220.10.1038/nrd1657Search in Google Scholar
[3] Mishra, B. B.; Tiwari, V. K. Natural products: an evolving role in future drug discovery. Eur. J. Med. Chem.2011, 46, 4769–4807.10.1016/j.ejmech.2011.07.057Search in Google Scholar
[4] Li, J. W.-H.; Vederas, J. C. Drug discovery and natural products: end of an era or an endless frontier? Science2009, 325, 161–165.10.1126/science.1168243Search in Google Scholar
[5] Rizzo, S.; Waldmann, H. Development of a natural-product-derived chemical toolbox for modulation of protein function. Chem. Rev.2014, 114, 4621–4639.10.1021/cr400442vSearch in Google Scholar
[6] Roy, R. S.; Gehring, A. M.; Milne, J. C.; Belshaw, P. J.; Walsh, C. T. Thiazole and oxazole peptides: biosynthesis and molecular machinery. Nat. Prod. Rep.1999, 16, 249–263.10.1039/a806930aSearch in Google Scholar
[7] Yeh, V. S. C. Recent advances in the total syntheses of oxazole-containing natural products. Tetrahedron2004, 60, 11995–12042.10.1016/j.tet.2004.10.001Search in Google Scholar
[8] Melby, J. O.; Nard, N. J.; Mitchell, D. A. Thiazole/oxazole-modified microcins: complex natural products from ribosomal templates. Curr. Opin. Chem. Biol.2011, 15, 369–378.10.1016/j.cbpa.2011.02.027Search in Google Scholar
[9] Davyt, D.; Serra, G. Thiazole and oxazole alkaloids: isolation and synthesis. Mar. Drugs2010, 8, 2755–2780.10.3390/md8112755Search in Google Scholar PubMed PubMed Central
[10] Hall, S. E.; Han, W.-C.; Harris, D. H.; Goldenberg, H.; Michel, I. M.; Monshizadegan, H.; Webb, M. L. Synthesis of pyrrolidine oxazoles as thromboxane A 2/endoperoxide receptor antagonists. Bioorg. Med. Chem. Lett.1993, 3, 1263–1266.10.1016/S0960-894X(00)80328-5Search in Google Scholar
[11] Macdonald, S. J.; Dowle, M. D.; Harrison, L. A.; Clarke, G. D.; Inglis, G. G.; Johnson, M. R.; Shah, P.; Smith, R. A.; Amour, A.; Fleetwood, G.; et al. Discovery of further pyrrolidine trans-lactams as inhibitors of human neutrophil elastase (HNE) with potential as development candidates and the crystal structure of HNE complexed with an inhibitor (GW475151). J. Med. Chem.200245, 3878–3890.10.1021/jm020881fSearch in Google Scholar
[12] Swanson, D. M.; Shah, C. R.; Lord, B.; Morton, K.; Dvorak, L. K.; Mazur, C.; Apodaca, R.; Xiao, W.; Boggs, J. D.; Feinstein, M.; et al. Heterocyclic replacement of the central phenyl core of diamine-based histamine H3 receptor antagonists. Eur. J. Med. Chem.2009, 44, 4413–4425.10.1016/j.ejmech.2009.06.007Search in Google Scholar
[13] Pellegrino, S.; Contini, A.; Gelmi, M. L.; Lo Presti, L.; Soave, R.; Erba, E. Asymmetric modular synthesis of a semirigid dipeptide mimetic by cascade cycloaddition/ring rearrangement and borohydride reduction. J. Org. Chem.2014, 79, 3094–3102.10.1021/jo500237jSearch in Google Scholar
[14] de Meijere, A.; Ernst, K.; Zuck, B.; Brandl, M.; Kozhushkov, S. I.; Tamm, M.; Yufit, D. S.; Howard J. A. K.; Labahn, T. Convenient syntheses of novel α- and β-amino acids with spiropentyl groups. Eur. J. Org. Chem.1999, 1999, 3105–3115.10.1002/(SICI)1099-0690(199911)1999:11<3105::AID-EJOC3105>3.0.CO;2-1Search in Google Scholar
[15] Chernykh, A. V.; Radchenko, D. S.; Grygorenko, O. O.; Volochnyuk, D. M.; Shishkina, S. V.; Shishkin, O. V.; Komarov, I. V. Conformationally restricted glutamic acid analogues: stereoisomers of 1-aminospiro[3.3]heptane-1,6-dicarboxylic acid. RSC Adv.2014, 4, 10894–10902.10.1039/c3ra47725hSearch in Google Scholar
[16] Radchenko, D. S.; Grygorenko, O. O.; Komarov, I. V. Synthesis of conformationally restricted glutamic acid analogs based on the spiro[3.3]heptane scaffold. Tetrahedron: Asymmetry2008, 19, 2924–2930.10.1016/j.tetasy.2008.12.016Search in Google Scholar
[17] Pellicciari, R.; Marinozzi, M.; Natalini, B.; Costantino, G.; Luneia, R.; Giorgi, G.; Moroni, F.; Thomsen,C. Synthesis and pharmacological characterization of all sixteen stereoisomers of 2-(2′-carboxy-3′-phenylcyclopropyl)glycine. Focus on (2S,1′S,2′S,3′R)-2-(2′-carboxy-3′-phenylcyclopropyl)glycine, a novel and selective group II metabotropic glutamate receptors antagonist. J. Med. Chem.1996, 39, 2259–2269.10.1021/jm960059+Search in Google Scholar
[18] Marinozzi, M.; Serpi, M.; Amori, L.; Gavilan Diaz, M.; Costantino, G.; Meyer, U.; Flor, P. J.; Gasparini, F.; Heckendorn, R.; Kuhn, R.; et al. Synthesis and preliminary pharmacological evaluation of the four stereoisomers of (2S)-2-(2′-phosphono-3′-phenylcyclopropyl)glycine, the first class of 3′-substituted trans C1′-2′-2-(2′-phosphonocyclopropyl)glycines. Bioorg. Med. Chem.2007, 15, 3161–3170.10.1016/j.bmc.2007.02.040Search in Google Scholar
[19] Chernykh, A. V.; Radchenko, D. S.; Grygorenko, O. O.; Daniliuc, C. G.; Volochnyuk, D. M.; Komarov, I. V. Synthesis and structural analysis of angular monoprotected diamines based on spiro[3.3]heptane scaffold. J. Org. Chem.2015, 80, 3974–3981.10.1021/acs.joc.5b00323Search in Google Scholar
[20] Kasai, S.; Mcgee, K. F. Jr. Derivatives of N-acyl-N′-phenylpiperazine useful (inter alia) for the prophylaxis or treatment of diabetes. U. S. Pat. 2012071489, 2012.Search in Google Scholar
[21] Kanojia, R. M.; Jordan, A. D.; Reitz, A. B.; Macielag, M. J.; Zhao, B. Neurotrophic pyrrolidines and piperidines, and related compositions and methods. U. S. Pat. 6809107, 2004.Search in Google Scholar
[22] Kamal, A.; Sathish, M.; Srinivasulu, V.; Chetna, J.; Shekar, K. C.; Nekkanti, S.; Tangella, Y.; Shankaraiah, N. Asymmetric Michael addition of ketones to nitroolefins: pyrrolidinyl-oxazole-carboxamides as new efficient organocatalysts. Org. Biomol. Chem.2014, 12, 8008–8018.10.1039/C4OB01223BSearch in Google Scholar
[23] Senwar, K. R.; Sharma, P.; Nekkanti, S.; Sathish, M.; Kamal, A.; Sridhard, B.; Shankaraiah, N. A one-pot ‘click’ reaction from spiro-epoxides catalyzed by Cu(I)-pyrrolidinyl-oxazole-carboxamide. New J. Chem.,2015, 39, 3973–3981.10.1039/C4NJ02131BSearch in Google Scholar
[24] Instant JChem was used for prediction of the physico-chemical properties of the compounds, Instant JChem 15.2.16.0, 2015, ChemAxon (http://www.chemaxon.com).Search in Google Scholar
[25] Nadin, A.; Hattotuwagama, C.; Churcher, I. Lead-oriented synthesis: a new opportunity for synthetic chemistry. Angew. Chem. Int. Ed.2012, 51, 1114–1122.10.1002/anie.201105840Search in Google Scholar
[26] Blankson, G.; Rzuczek, S. G.; Bishop, C.; Pilch, D. S.; Liu, A.; Liu, L.; LaVoie, E. J.; Rice, J. E. Macrocyclic pyridyl polyoxazoles: structure-activity studies of the aminoalkyl side-chain on G-quadruplex stabilization and cytotoxic activity. molecules2013, 18, 11938–11963.10.3390/molecules181011938Search in Google Scholar
[27] Théveau, L.; Verrier, C.; Lassalas, P.; Martin, T.; Dupas, G.; Querolle, O.; Van Hijfte, L.; Marsais, F.; Hoarau, C. Mechanism Selection for regiocontrol in base-assisted, palladium-catalysed direct C–H coupling with halides: first approach for oxazole- and thiazole-4-carboxylates. Chem. Eur. J.2011, 17, 14450–14463.10.1002/chem.201101615Search in Google Scholar
[28] Tormyshev, V. M.; Mikhalina, T. V.; Rogozhnikova, O. Yu.; Troitskaya, T. I.; Trukhin, D. V. A combinatorially convenient version of synthesis of 5-substituted oxazole-4-carboxylic acid ethyl esters. Russ. J. Org. Chem.2006, 42, 1031–1035.10.1134/S1070428006070177Search in Google Scholar
[29] Baumann, M.; Baxendale, I. R.; Ley, S. V.; Smith, C. D.; Tranmer, G. K. Fully automated continuous flow synthesis of 4,5-disubstituted oxazoles. Org. Lett.2006, 8, 5231–5234.10.1021/ol061975cSearch in Google Scholar
[30] Yang, K.; Zhang, C.; Wang, P.; Zhang, Y.; Ge, H. Nickel-catalyzed decarboxylative acylation of heteroarenes by sp2 C–H functionalization. Chem. Eur. J.2014, 20, 7241–7244.10.1002/chem.201402516Search in Google Scholar
[31] Meng, L.; Kamada, Y.; Muto, K.; Yamaguchi, J.; Itami, K. C–H alkenylation of azoles with enols and esters by nickel catalysis. Angew. Chem. Int. Ed.2013, 52, 10048–10051.10.1002/anie.201304492Search in Google Scholar
[32] Amaike, K.; Muto, K.; Yamaguchi, J.; Itami, K. Decarbonylative C–H coupling of azoles and aryl esters: unprecedented nickel catalysis and application to the synthesis of muscoride A. J. Am. Chem. Soc.2012, 134, 13573–13576.10.1021/ja306062cSearch in Google Scholar
[33] Yamazaki, Y.; Tanaka, K.; Nicholson, B.; Deyanat-Yazdi, G.; Potts, B.; Yoshida, T.; Oda, A.; Kitagawa, T.; Orikasa, S.; Kiso, Y.; et al. Synthesis and structure–activity relationship study of antimicrotubule agents phenylahistin derivatives with a didehydropiperazine-2,5-dione structure. J. Med. Chem.2012, 55; 1056–1071.10.1021/jm2009088Search in Google Scholar PubMed
[34] Muto, K.; Yamaguchi, J.; Itami, K. Nickel-catalyzed C–H/C–O coupling of azoles with phenol derivatives. J. Am. Chem. Soc.2012, 134, 169–172.10.1021/ja210249hSearch in Google Scholar
[35] Makino, K.; Goto, T.; Ohtaka, J.; Hamada, Y. Highly stereoselective synthesis of (2R,3R)-2-amino-3-cyclohexyl-3-hydroxypropionic acid using asymmetric hydrogenation. Heterocycles2009, 77, 629–634.10.3987/COM-08-S(F)46Search in Google Scholar
[36] Makino, K.; Goto, T.; Hiroki, Y.; Hamada, Y. Direct anti-selective asymmetric hydrogenation of α-amino-β-keto esters through dynamic kinetic resolution using Ru-axially chiral phosphine catalysts – stereoselective synthesis of anti-β-hydroxy-α-amino acids. Tetrahedron: Asymmetry2008, 19, 2816–2828.10.1016/j.tetasy.2008.12.024Search in Google Scholar
[37] Brescia, M. R.; Rokosz, L. L.; Cole, A. G.; Stauffer, T. M.; Lehrach, J. M.; Auld, D. S.; Henderson, I.; Webb, M. L. Discovery and preliminary evaluation of 5-(4-phenylbenzyl)oxazole-4-carboxamides as prostacyclin receptor antagonists. Bioorg. Med. Chem. Lett.2007, 17, 1211–1215.10.1016/j.bmcl.2006.12.025Search in Google Scholar
[38] Hamada, Y.; Shioiri, T. New methods and reagents in organic synthesis. 22. Diphenyl phosphorazidate as a reagent for C-acylation of methyl isocyanoacetate with carboxylic acids. Tetrahedron Lett.1982, 23, 235–236.10.1016/S0040-4039(00)86794-5Search in Google Scholar
[39] Ramsbeck, D.; Buchholz, M.; Koch, B.; Boehme, L.; Hoffmann, T.; Demuth, H.-U.; Heiser, U. Structure–activity relationships of benzimidazole-based glutaminyl cyclase inhibitors featuring a heteroaryl scaffold. J. Med. Chem.2013, 56, 6613–6625.10.1021/jm4001709Search in Google Scholar
©2015 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Preliminary Communications
- Triphenylphosphine catalyzed domino reaction of dialkyl acetylenedicarboxylate with 3-aryl- 2-benzoylcyclopropane-1,1-dicarbonitrile
- Synthesis of colletotrichumine A
- Research Articles
- Synthesis of the spiroacetal fragments of spirofungins A and B, antibiotics isolated from Streptomyces violaceusniger Tü 4113
- Efficient synthesis and fungicidal activities of strobilurin analogues containing benzofuro [3,2-d]-1,2,4-triazolo[1,5-a]pyrimidinone side chains
- Synthesis of 2-amino-6,7,8,9-tetrahydro-6-phenethyl-3H-pyrimido[4,5-e][1,4]diazepin-4(5H)-one: a model for a potential pyrimido[4,5-e][1,4]diazepine-based folate anti-tumor agent
- Cascade assembling of pyrazolin-5-ones and benzylidenemalononitriles: the facile and efficient approach to medicinally relevant spirocyclopropylpyrazolone scaffold
- Synthesis, characterization and bioactivity of novel 5,6-dihydropyrrolo[3,4-c]pyrazol-4- (1H)one derivatives
- Molecular modeling and synthesis of new 1,5-diphenylpyrazoles as breast cancer cell growth inhibitors
- An efficient synthesis of 11-aryl-10-oxo-7,8,10,11-tetrahydro-1H-[1,2,3]triazolo [4′,5′:3,4]benzo[1,2-b][1,6]naphthyridine derivatives under catalyst-free conditions
- Mechanochemical synthesis of 2,2-difluoro-4, 6-bis(β-styryl)-1,3,2-dioxaborines and their use in cyanide ion sensing
- Visible-light-mediated radical aryltrichloromethylation of N-arylacrylamides for the synthesis of trichloromethyl-containing oxindoles
- A stereolibrary of conformationally restricted amino acids based on pyrrolidinyl/piperidinyloxazole motifs
- Synthesis of [1,3]thiazolo[3,2-b][1,2,4]triazol-7-ium and [1,2,4]triazolo[5,1-b][1,3]thiazin-4-ium salts via regioselective electrophilic cyclization of 3-[(2-alken-1-yl)sulfanyl]-4H-1,2,4-triazoles
Articles in the same Issue
- Frontmatter
- Preliminary Communications
- Triphenylphosphine catalyzed domino reaction of dialkyl acetylenedicarboxylate with 3-aryl- 2-benzoylcyclopropane-1,1-dicarbonitrile
- Synthesis of colletotrichumine A
- Research Articles
- Synthesis of the spiroacetal fragments of spirofungins A and B, antibiotics isolated from Streptomyces violaceusniger Tü 4113
- Efficient synthesis and fungicidal activities of strobilurin analogues containing benzofuro [3,2-d]-1,2,4-triazolo[1,5-a]pyrimidinone side chains
- Synthesis of 2-amino-6,7,8,9-tetrahydro-6-phenethyl-3H-pyrimido[4,5-e][1,4]diazepin-4(5H)-one: a model for a potential pyrimido[4,5-e][1,4]diazepine-based folate anti-tumor agent
- Cascade assembling of pyrazolin-5-ones and benzylidenemalononitriles: the facile and efficient approach to medicinally relevant spirocyclopropylpyrazolone scaffold
- Synthesis, characterization and bioactivity of novel 5,6-dihydropyrrolo[3,4-c]pyrazol-4- (1H)one derivatives
- Molecular modeling and synthesis of new 1,5-diphenylpyrazoles as breast cancer cell growth inhibitors
- An efficient synthesis of 11-aryl-10-oxo-7,8,10,11-tetrahydro-1H-[1,2,3]triazolo [4′,5′:3,4]benzo[1,2-b][1,6]naphthyridine derivatives under catalyst-free conditions
- Mechanochemical synthesis of 2,2-difluoro-4, 6-bis(β-styryl)-1,3,2-dioxaborines and their use in cyanide ion sensing
- Visible-light-mediated radical aryltrichloromethylation of N-arylacrylamides for the synthesis of trichloromethyl-containing oxindoles
- A stereolibrary of conformationally restricted amino acids based on pyrrolidinyl/piperidinyloxazole motifs
- Synthesis of [1,3]thiazolo[3,2-b][1,2,4]triazol-7-ium and [1,2,4]triazolo[5,1-b][1,3]thiazin-4-ium salts via regioselective electrophilic cyclization of 3-[(2-alken-1-yl)sulfanyl]-4H-1,2,4-triazoles

