Abstract
A simple, fast and environmental friendly vortex assisted-supramolecular solvent based microextraction (VA-SSME) method was developed for the preconcetration of triclosan in wastewater prior to UV spectrophotometric determination. To achieve maximum sensitivity and accuracy for the target analyte, the experimental parameters affecting the VA-SSME procedure were optimized using response surface methodology (RSM). Under optimised conditions, the correlation coefficient (R2) and recoveries were 0.9994 and 100.31-118.5%, respectively. The intra-day (repeatability) and inter-day (reproducibility) precisions expressed in terms of relative standard deviation (RSD) were 2-4% and 5.2%, respectively. The preconcentration factor and limits of detection (LOD) and quantification (LOQ) were found to be 90, 0.28 μg L−1 and 0.92 μg L−1, respectively. The developed VA-SSME/UV method was applied for the determination of triclosan in real samples collected over a period of three months. The analytical results obtained showed that triclosan was frequently detected in influent wastewater samples but was not detected in effluent samples.
1 Introduction
Triclosan (2’-hydroxy-2,4,4’-trichlorodiphenyl ether) is used as a broad-spectrum synthetic antimicrobial agent for medicated agents, toothpaste, hand and body soaps and shampoo [1,2]. Increased cases of bacterial infection have resulted in wide spread utilisation of antimicrobial agents [3]. This has elevated the concentration of these compounds in different environmental media [4]. More alarming is the evidence of transformation of these compounds into more toxic compounds such as 2,8-dichlorodibenzo-p-dioxin and 2,4-dichlorophenol [5,6]. Triclosan is regarded as an androgenic compound, moreover: it has also been found to have endocrine effects in animals and humans [7]. The United States Environmental Protection Agency (USEPA) termed triclosan a high priority pollutant as it decomposes into dioxin derivatives which are potential carcinogens [8]. Due to the potential toxicity to human and animals, the maximum permitted level of triclosan in personal care products is 0.3% (w/w) [6]. Therefore, there is a need to develop rapid, simple, selective and sensitive analytical methods for accurate quantification of trace levels of triclosan in complex environmental samples.
Consequently, several analytical methods have been developed for the assessment of triclosan in different matrices. These include high performance liquid chromatography–ultraviolet spectroscopy (HPLC-UV) [2], liquid chromatography-tandem mass spectroscopy (LC-MS/MS [9,10]), gas chromatography-mass spectroscopy (GC-MS) [11,12]. Although the aforementioned mentioned techniques have their own advantages such selectivity and high sensitivity, they often need time-consuming sample preparation steps, have a high cost of operation and suffer from complex and expensive instrumentation [13]. In addition, these analytical techniques require long analysis times and require highly skilled personnel. In contrast, spectrophotometric techniques such as UV-Vis have attractive advantages such as wide availability of instrumentation, inherent simplicity, low cost, short analysis time, and adequate precision and accuracy [13]. In addition, these merits make the spectrophotometric techniques especially convenient for the routine analysis of different analytes.
Several spectrophotometric methods have been developed for the determination of sulfadiazine (another class of emerging pollutants) in different matrices [14,15,16]. The majority of the developed spectrophotometric techniques for determination of sulfadiazine are based on the formation of a detectable azo dye by the diazotization of sulfadiazine followed by its coupling with different reagents, including 8-hydroxyquinoline [17] and iminodibenzyl [18]. Triclosan has also been determined spectrophotometrically using a similar method [19]. Despite the above-mentioned advantages, the UV–VIS spectrophotometer is seriously limited by its low sensitivity. Hence, separation/ preconcentration methods are required in order to carry out accurate and sensitive determinations [20]. For this reason, different sample preparation methods have been used for preconcentration of different organic pollutants prior to UV-Vis spectrophotometric determination. These sampler pretreatment techniques include dispersive liquid–liquid microextraction (DLLME) [21], solid phase extraction (SPE) [22], solid phase microextraction (SPME) [14] and supramolecular solvent-based microextraction [23], among others.
Supramolecular solvent based microextraction (SSME) uses supramolecular solvents (SUPRAS) as an extractant. SUPRAS are water immiscible liquids synthesized by self-assembly of two amphiphile solutions in a continuous phase in which self-assembly processes occur on the molecular and nano scale [24,25,26]. These supramolecular assemblies of SUPRAS lead to properties useful for extraction of inorganic and organic compounds [27]. Therefore, the principle of the SSME procedure is based on partitioning of an analyte between an alkyl carboxylic acid-based nano-structured solvent and a bulk aqueous sample [28]. SUPRAS have regions of varying polarities, providing different interactions for analytes. Hence, they are attractive solvents to replace organic solvents in analytical extractions [29]. Another feature of SUPRAS is the high concentration of amphiphiles which results in a high number of analyte binding sites. This permits high extraction efficiencies while using low extraction volumes; hence, they are frequently used in microextractions [27].
This study aims to develop a novel simple, rapid and sensitive SUPRAS (made up of decanoic acid and tricaprylymethylammonium chloride)-based microextraction and UV–VIS spectrophotometry for preconcentration and quantification of triclosan in wastewater samples. According to the authors’ knowledge, the use of SUPRAS in order to improve UV-Vis spectrophometric detection capabilities for determination of triclosan or any personal care products has not been previously reported. The experimental parameters affecting the SSME procedure were optimized using response surface methodology (RSM) based on a central composite design (CCD).
2 Experimental
2.1 Reagents and standards
Triclosan (pharmaceutical secondary standard), decanoic acid, tricaprylymethylammonium chloride (Aliquat 336) and methanol were purchased from Sigma-Aldrich (St. Loius, MO, USA). A triclosan stock solution (10 mg L-1) was prepared by diluting an appropriate amount of triclosan in 2 mL of methanol and diluting to 100 mL with ultra-pure water (Direct-Q® 3UV-R purifier system, Millipore, Merck). Stock triclosan was kept refrigerated. Working standard solutions were prepared daily by appropriate dilution of the stock solution with ultra-pure water.
2.2 Instrumentation
A PRO scientific VSM-3 vortex mixer (PRO scientific Inc.,) was used to vortex the samples before using an Eppendorf centrifuge 5702 (Eppendorf, Hamburg, Germany). An OHAUS starter 2100 pH meter (Pine Brook, NJ, USA) was used for pH adjustments of the reagents and to measure the pH of samples. The Shimadzu UV-2450 high performance single monochromator UV-VIS spectrophotometer (Shimadzu Corporation, Tokyo, Japan) with 5 mL quartz cuvettes and a slit width of 0.5 and wavelength of 284.8 nm was used for all sample analyses. Reference studies were carried out using an Agilent 1200 Infinity series HPLC equipped with a photodiode array detector (Agilent Technologies, Waldbronn, Germany). The chromatograms were recorded at 280 nm. An Agilent Zorbax Eclipse Plus C18 column (3.5 µm × 150 mm × 4.6 mm) (Agilent, Newport, CA, USA) was operated at an oven temperature of 25°C. The mobile phase was a mixture of 30% water (mobile phase A) and 70 % acetonitrile (mobile phase C). A flow rate of 1.00 mL min−1 was used throughout the analysis.
2.3 Sampling and sample collection
Influent (after sediment removal) and effluent wastewater samples were collected from Daspoort wastewater treatment plant (WWTP, Pretoria, Gauteng, South Africa). The samples were collected in pre-cleaned 500 mL glass bottles. The samples were then refrigerated at 4 °C. Samples were collected four times a month (meaning once a week) for a period of 3 months. In each week (Mondays at midday (between 11:00 and 13:00)), six samples (three influent and three effluent) were collected and analysed.
2.4 Supramolecular solvent based microextraction
A mixture of 50 µg L−1 decanoic acid in Aliquat-336 was prepared via stirring a mixture of the two for 5 minutes (until a clear homogenous solution formed), forming the supramolecular solvent. Different volumes (100–500 µL)of the solvent were added to centrifuge tube containing a 5 mL sample. This mixture was shaken using a vortex for a few seconds until a cloudy suspension formed. The solution was then centrifuged for a maximum of 10 minutes at 3000 rpm. After centrifuging, the top layer was recovered using a micropipette and was redissolved in 2 mL of methanol and analysed using UV-Vis spectrophotometry at 284.8 nm.
Ethical approval
The conducted research is not related to either human or animals use.
3 Results and discussion
3.1 Optimization
In order to achieve quantitative preconcetration of triclosan with the supramolecular microextraction procedure, the optimization of the most influential parameters, such as sample pH, extraction time (ET) and supramolecular solvent volume (SSV), was carried out using response surface methodology (RSM) based on a central composite design. Table 1 presents the factorial design matrix and the analytical responses (expressed as absorbance at 284.8 nm) obtained in each experiment. A Pareto chart (Figure 1) was generated for the analysis of variance (ANOVA) to explore the significance of the effects on the SSME procedure. The Pareto chart of main effects and their interactions produced is shown in Figure 1. The effect of pH and extraction time according to Figure 1 was not significant in the extraction of triclosan at the 95% confidence level. In contrast, impact of the supramolecular solvent volume (SSV) was significant at the 95% confidence level. The interaction of pH and extraction time was also statistically significant for the extraction of triclosan. The effects of extraction time and pH were only significant when considering quadratic effects, which for the purpose of the study were not considered.
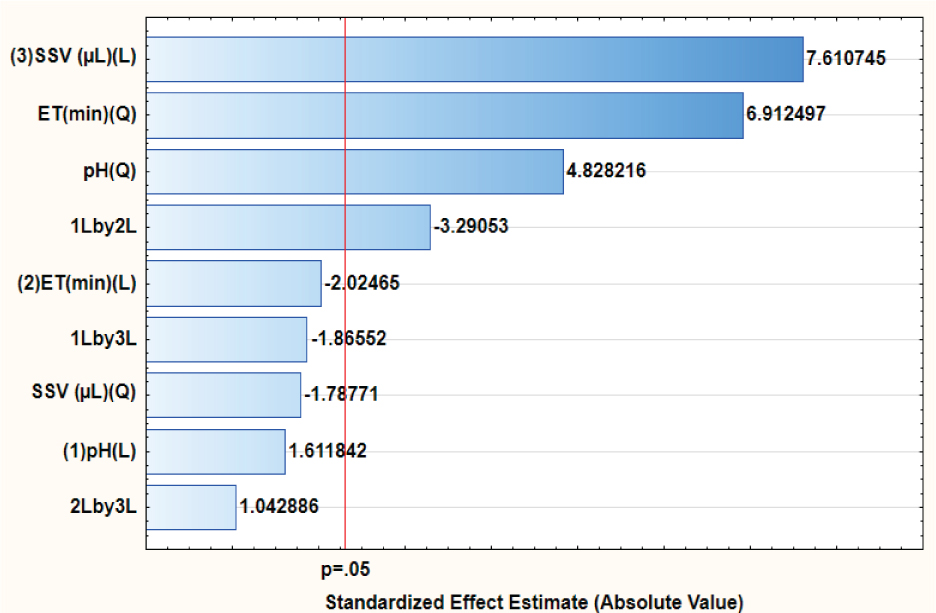
Pareto chart of standardized effects for variables in the preconcentration of triclosan.
Factors and levels used in central composite for supramolecular solvent based microextraction of triclosan.
| Factors | Low level (-1) | Central point (0) | High level (+1) |
|---|---|---|---|
| Sample pH | 3 | 7 | 10 |
| Extraction time (min) | 2 | 6 | 10 |
| SSV (μL) | 100 | 300 | 500 |
Factorial design matrix and the analytical responses (expressed as absorbance at 284.8 nm).
| Exper | pH | ET (min) | SSV (μL) | Abs |
|---|---|---|---|---|
| 1 | 3 | 2 | 300 | 1.334 |
| 2 | 9 | 2 | 300 | 1.464 |
| 3 | 3 | 10 | 300 | 1.704 |
| 4 | 9 | 10 | 300 | 2.344 |
| 5 | 3 | 6 | 100 | 0.049 |
| 6 | 9 | 6 | 100 | 0.45 |
| 7 | 3 | 6 | 500 | 1.75 |
| 8 | 9 | 6 | 500 | 1.448 |
| 9 | 6 | 2 | 100 | 1.016 |
| 10 | 6 | 10 | 100 | 0.53 |
| 11 | 6 | 2 | 500 | 1.498 |
| 12 | 6 | 10 | 500 | 1.405 |
| 13 | 6 | 6 | 300 | 0.666 |
| 14 | 6 | 6 | 300 | 0.6001 |
| 15 | 6 | 6 | 300 | 0.643 |
| 16 | 6 | 6 | 300 | 0.656 |
| 17 | 6 | 6 | 300 | 0.662 |
| 18 | 6 | 6 | 300 | 0.6729 |
Triclosan is a lipophilic compound with a log Kow of 4.8 [30]; this means that it associates with the hydrophobic section of the supramolecular solvent during the dispersion of the solvent in the water sample [31]. In addition to the lipophilicity, the pKa of the analyte played a major role in the SSME [31]. As a result of its pKa (8.1), triclosan is stable over the pH range 4-9. The optimum pH for the extraction of 5 thus falls within the stable range of triclosan. In other words, as a result of its pKa, triclosan existed in its molecular form during the application of the method [32].
ANOVA variables of the response surface quadratic model for the absorbance of triclosan were obtained. The ANOVA results were analysed with quadratic equations for the models to illustrate the dependence of the analytical response with respect to the evaluated main effects [33]. The response surfaces together with quadratic equations (equation not included) were used to calculate the optimum conditions.
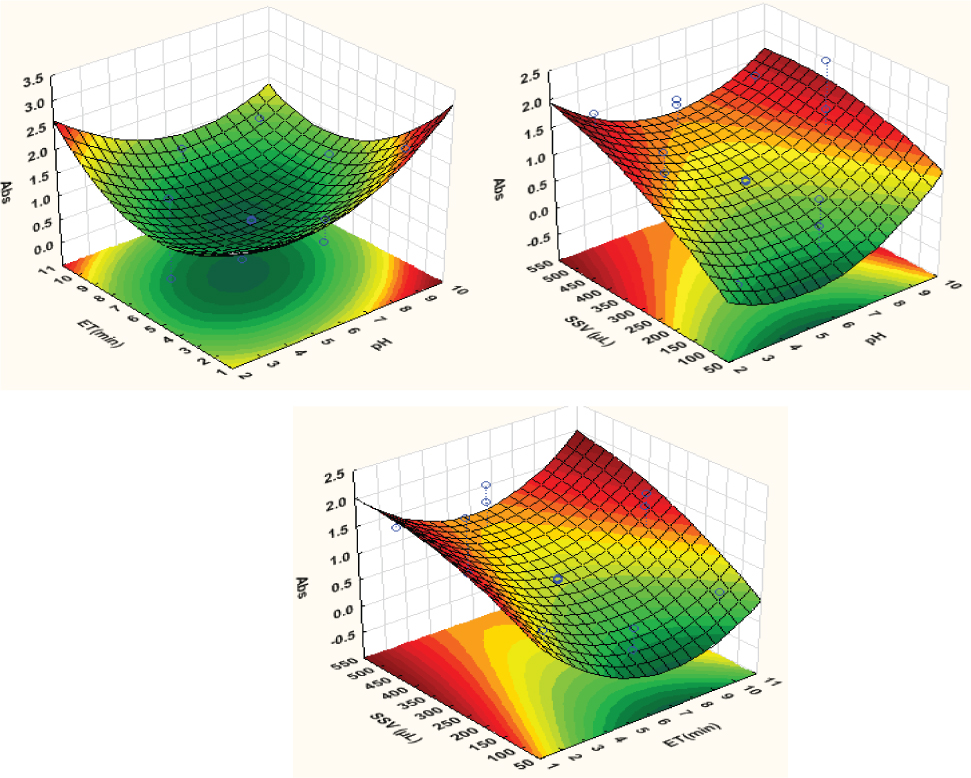
Response surfaces obtained for triclosan after extraction and preconcentration by supramolecular solvent extraction.
The 3D surface response plots were used to investigate the relationship/interaction between the independent variables (sample pH, extraction time and supramolecular solvent volume) on the absorbance of the samples. Based on quadratic expressions [33], the calculated optimum conditions for further processesing and application of the method were a pH of 5, extraction time of 6 minutes and supramolecular solvent volume of 400 µL. The confirmatory experiments were performed to validate the optimum conditions obtained by RSM and the results were not significantly different from the predicted values at 95% confidence level. These conditions were then used for the investigation of the analytical performance and validation and application of the procedure in real samples.
3.2 Analytical figures of merit
Under the determined optimum experimental conditions, the analytical performances of the developed SSME method for preconcentration and spectrophotometric determination of triclosan were investigated. The calibration curves (Figure 3) were obtained after a set of standard solutions (0 to 400 µg L−1) was processed using the described extraction procedure. The concentrations of the analytes in the eluent solutions were quantified with the aid of a UV spectrophotometer. The limits of detection and quantification were calculated using the formulas:
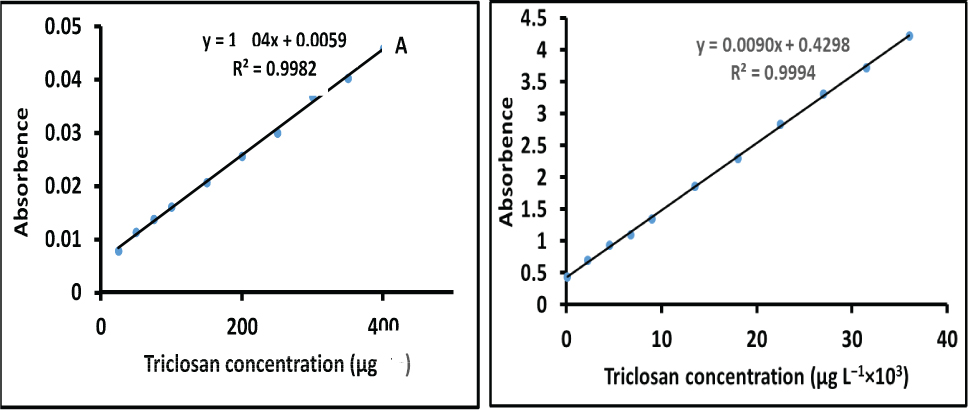
calibration curve for triclosan (A) before and (B) after preconcentration using VA-SSME.
The performance of the developed VA-SSME/UV method for the preconcetration and determination of TCS in wastewater samples was compared with different published analytical procedures (Table 3). It can be seen that the current method has better performance in terms of LOD and LOQ when compared with those reported [34]. In addition, the developed method had a wider DLR compared to several published reports [34,35,37,38,39,40,41] and the % RSD was comparable with all the reported studies except one [37]. This optimal performance can be attributed to the fact that SUPRAS provide a number of possible interaction between the analyte and solvent including high number of binding sites and hydrogen bond interactions which increase the extraction efficiency [27]. However, the LOD and LOQ were higher than those reported by Refs [35,36,37,38,39,40,41].
Comparison of analytical figures of merit for different extraction and detection methods for triclosan and other emerging pollutants.
| Analyte | Matrix | Method | LOD (μgL-1) | LOQ ( μgL-1) | DLR | RSD | References |
|---|---|---|---|---|---|---|---|
| Triclosan | Tapwater | SPME-LC-UV | 0.001 | 0.033 | 0.01-168 | 7 | [37] |
| Triclosan | Surface water | SPME-HPLC-UV | 0.04 | 0.13 | [38] | ||
| Triclosan | Urine | ULLME-HPLC-DAD | 0.11 | 0.36 | 0.5-500 | 0.64-4.6 | [36] |
| Triclosan | River water | SPME-HPLC-UV | 0.00024 | 0.0008 | 0.0005-0.04 | 4 | [39] |
| Triclosan | Cosmetics | IT-USA-SI-LLME-HPLC-UV | 0.00009 | 0.0003 | 0.0004-0.1 | 0.8-5.3 | [40] |
| Triclosan | Water, beverages and urine | DLLME-UV | 4 | 13.3 | 0.02-2 | - | [34] |
| Triclosan | Environmental water | SPE-GC | 0.0001 | 0.0003 | 0.004-5 | 4.7-5.9 | [41] |
| Triclosan | Aqueous samples | IT-USAEME-GC-μECD | 0.004 | 0.013 | 0.02-2 | 2.8-5.4 | [35] |
| Triclosan | Wastewater | SSME-UV | 0.28 | 0.95 | 0.95-400 | 2.4-5.2 | Current work |
SPME= Solid phase microextraction, LC-UV= Liquid chromatography-Ultraviolet detection, HPLC= High pressure liquid chromatography, ULLME= ultrasound-assisted liquid-liquid microextraction, DAD= Diode array detector, IT-USA-LLME= In-tube based ultrasound-assisted salt-induced liquid–liquid microextraction, DLLME= dispersive liquid-liquid microextraction, SPE= Solid phase extraction, GC-Gas chromatography, IT-USAEME = In-tube ultasonication-assisted emulsification microextraction, GC-µECD= Gas chromatography-microelectron-capture detection, SSME= Supramolecular solvent microextraction
3.3 Validation and Application
Due to the absence of reference material, the method was validated by the use of a spiked recovery test. The influent and effluent samples were spiked at two different concentrations as indicated in Table 4. The samples were then preconcentrated in triplicate using the proposed method as described in the experimental section prior to their analysis using a UV spectrophotometer and HPLC-PDA. It can be seen that the results obtained using the current method were comparable with those obtained using HPLC-PDA. In addition, the chromatogram (Figure 4) proved that, under optimum conditions, the developed method was able to extract triclosan from complex matrices.
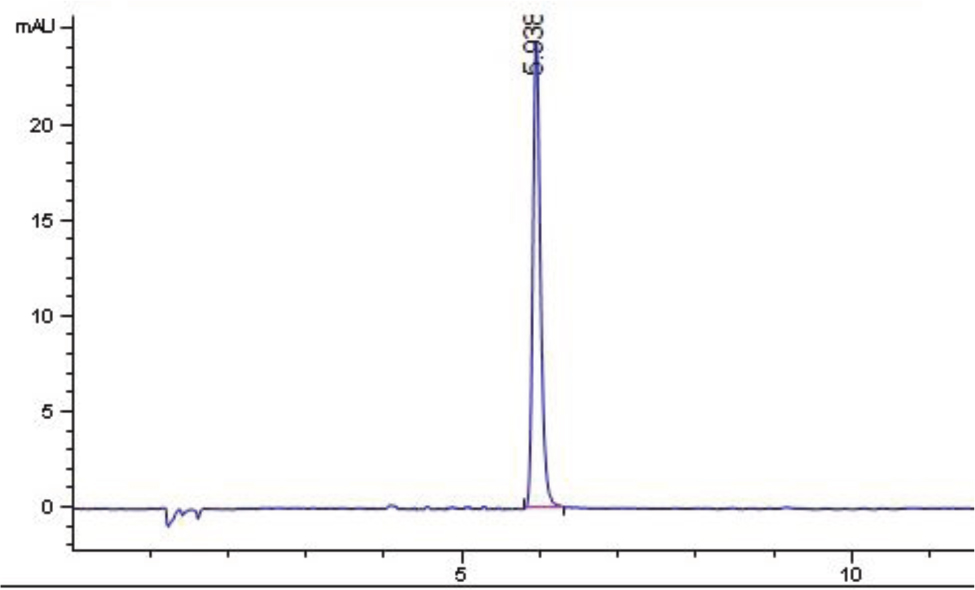
HPLC-PDA chromatogram for triclosan from influent sample spiked with 50 µg L−1 of the analyte after preconcentration using the VA-SSME.
Analysis of wastewater samples (influent and effluent) spiked and unspiked from Daspoort (Pretoria, Gauteng, South Africa) wastewater treatment plant.
| Sample | SSME-UV-Vis | HPLC-PDA | |||
|---|---|---|---|---|---|
| Added (μgL-1) | Found (μgL-1) | %R | Found (μgL-1) | %R | |
| Influent | 0 | 10.01±0.5 | 9.93±1.3 | ||
| 50 | 59.07±0.8 | 98.12 | 59.88±3.7 | 99.90 | |
| 100 | 116.97±1.4 | 107.0 | 109.8±3.3 | 99.87 | |
| Effluent | 0 | nd | nd | ||
| 50 | 50.16±0.9 | 100.31 | 49.89±2.5 | 99.78 | |
| 100 | 101.22±1.5 | 101.22 | 99.97±3.9 | 99.97 |
The described method was applied in the determination of triclosan from wastewater samples collected over a period of 3 months: August, September and October of the same year (Table 5). It should be noted that the above concentrations were determined from influent samples. In the effluent samples, triclosan was not detected. The lack of detectable triclosan in effluent can be explained by the fact that the Daspoort waste water treatment has three types of treatment stages, including chlorinated and ultraviolet treatment before the effluent is released into the nearby river. This secondary treatment can possibly lead to either the degradation of triclosan or transformation into other compounds, as triclosan has been known to be able to be transformed into other compounds [6]. In summary, in the effluent the concentration of triclosan was below the limits of the method. The triclosan concentrations obtained using the developed method was confirmed by a reference method (HPLC-PDA). According to the student t-test, the results were not significantly difference at the 95% confidence level.
Analysis of wastewater samples (influent and effluent) collected from Daspoort (Pretoria, Gauteng, South Africa) wastewater treatment plant over three months (concentration in μg L-1, n = 4).
| Samples | VA-SSME/UV | HPLC-PDA | |
|---|---|---|---|
| August | Influent | 10.82± 0.5 | 11.01±1.6 |
| Effluent | nd | nd | |
| September | Influent | 10.67 ± 0.4 | 10.41±2.3 |
| Effluent | nd | nd | |
| October | Influent | 9.93 ± 0.34 | 10.11±3.1 |
| Effluent | nd | nd |
4 Conclusions
In this study, a rapid and simple SSME/UV-Vis spectrophotometric method for preconcetration and determination of triclosan in wastewater samples was developed. The developed VA-SSME/UV method was solvent minimized, inexpensive, eco-friendly, precise and accurate. The analysis of wastewater samples revealed that the target analyte was present in all influent samples, while it was not detected in effluent samples. These findings suggested that triclosan was transformed to other compounds during the wastewater treatment process. Since even the reference method (HPLC-PDA) did not detect triclosan in the effluent samples, this demonstrated the effectiveness of the tertiary treatment stage.
Acknowledgement
The authors wish to thank the National Research Foundation (NRF, South Africa, grant nos. 99270 & 107975) for financial assistance.
Conflict of interest: Authors state no conflict of interest.
References
[1] Agüera A., Fernández-Alba A.R., Piedra L., Mézcua M., Gómez M.J., Evaluation of triclosan and biphenylol in marine sediments and urban wastewaters by pressurized liquid extraction and solid phase extraction followed by gas chromatography mass spectrometry and liquid chromatography mass spectrometry, Anal. Chim. Acta, 2003, 480(2), 193-205.10.1016/S0003-2670(03)00040-0Suche in Google Scholar
[2] Yang Y., Ma X., Feng F., Dang X., Huang J., Chen H., Magnetic solid-phase extraction of triclosan using core-shell Fe3, Microchim. Acta, 2016, 183(8), 2467-2472.10.1007/s00604-016-1872-xSuche in Google Scholar
[3] Aufiero M., Butler C., Jaser J., An analysis of methods for detecting triclosan and removal of triclosan from water using activated carbon and zeolites, Major Qualifying Project completed in partial fulfillment of the Bachelor of Science Degree at Worcester Polytechnic Institute, Worcester, 2012.Suche in Google Scholar
[4] Tohidi F., Cai Z., GC/MS analysis of triclosan and its degradation by-products in wastewater and sludge samples from different treatments, Environ. Sci. Pollut. Res., 2015, 22(15), 11387-11400.10.1007/s11356-015-4289-xSuche in Google Scholar PubMed
[5] Aranami K., Readman J.W., Photolytic degradation of triclosan in freshwater and seawater, Chemosphere, 2007, 66(6), 1052-1056.10.1016/j.chemosphere.2006.07.010Suche in Google Scholar PubMed
[6] Buth J.M., Steen P.O., Sueper C., Blumentritt D., Vikesland P.J., Arnold W.A., McNeill K., Dioxin photoproducts of triclosan and its chlorinated derivatives in sediment cores, Environ. Sci. Technol., 2010, 44(12), 4545-4551.10.1021/es1001105Suche in Google Scholar PubMed
[7] Anger C.T., Sueper C., Blumentritt D.J., McNeill K., Engstrom D.R., Arnold W.A., Quantification of triclosan, chlorinated triclosan derivatives, and their dioxin photoproducts in lacustrine sediment cores, Environ. Sci. Technol., 2013, 47(4), 1833-1843.10.1021/es3045289Suche in Google Scholar PubMed
[8] Chen M.J., Liu Y.T., Lin C.W., Ponnusamy V.K., Jen J.F., Rapid determination of triclosan in personal care products using new in-tube based ultrasound-assisted salt-induced liquid–liquid microextraction coupled with high performance liquid chromatography-ultraviolet detection, Anal Chim. Acta, 2013, 767, 81-87.10.1016/j.aca.2013.01.014Suche in Google Scholar PubMed
[9] Dejmkova H., Kwiecien J., Cizek K., Cermak J., Vranova E., Mala P., Zima J. Barek J., Voltammetric and LC-MS/MS method for the determination of Triclosan–A comparative study, validation and simultaneous application, Int. J. Electrochem. Sci., 2014, 9, 139-147.Suche in Google Scholar
[10] Chu S., Metcalfe C.D., Simultaneous determination of triclocarban and triclosan in municipal biosolids by liquid chromatography tandem mass spectrometry, J. Chromatogr. A, 2007, 1164(1), 212-218.10.1016/j.chroma.2007.07.024Suche in Google Scholar PubMed
[11] Azzouz A., Rascón A.J., Ballesteros E., Simultaneous determination of parabens, alkylphenols, phenylphenols, bisphenol A and triclosan in human urine, blood and breast milk by continuous solid-phase extraction and gas chromatography–mass spectrometry, J. Pharm. Biomed. Anal., 2016, 119, 16-26.10.1016/j.jpba.2015.11.024Suche in Google Scholar PubMed
[12] Provencher G., Bérubé R., Dumas P., Bienvenu J.F., Gaudreau É., Bélanger P., Ayotte P., Determination of bisphenol A, triclosan and their metabolites in human urine using isotopedilution liquid chromatography–tandem mass spectrometry, J. Chromatogr. A, 2014, 1348, 97-104.10.1016/j.chroma.2014.04.072Suche in Google Scholar PubMed
[13] Kazemi E., Dadfarnia S., Shabani A.M.H., Abbasi A., Vaziri M.R.R., Behjat A., Iron oxide functionalized graphene oxide as an efficient sorbent for dispersive micro-solid phase extraction of sulfadiazine followed by spectrophotometric and mode-mismatched thermal lens spectrometric determination, Talanta, 2016, 147, 561-568.10.1016/j.talanta.2015.10.033Suche in Google Scholar PubMed
[14] Kazemi E., Shabani A.M.H., Dadfarnia S., Application of graphene oxide-silica composite reinforced hollow fibers as a novel device for pseudo-stir bar solid phase microextraction of sulfadiazine in different matrices prior to its spectrophotometric determination, Food Chem., 2017, 221, 783-789.10.1016/j.foodchem.2016.11.103Suche in Google Scholar PubMed
[15] Thomas D., Aggarwal P., Syed A.A., Green spectrophotometric method for the determination of nitrite in environmental samples using sulfadiazine, Int. J. Recent. Sci. Res., 2015, 6(11), 7234-7239.Suche in Google Scholar
[16] Errayess S.A., Lahcen A.A., Idrissi L., Marcoaldi C., Chiavarini S., Amine A., A sensitive method for the determination of Sulfonamides in seawater samples by Solid Phase Extraction and UV–Visible spectrophotometry, Spectrochim. Acta. A. Mol. Biomol. Spectrosc., 2017, 181, 276-285.10.1016/j.saa.2017.03.061Suche in Google Scholar PubMed
[17] Nagaraja P., Naik S., Shrestha A., Shivakumar A., A sensitive spectrophotometric method for the determination of sulfonamides in pharmaceutical preparations, Acta Pharma., 2007, 57(3), 333-342.10.2478/v10007-007-0026-4Suche in Google Scholar PubMed
[18] Nagaraja P., Chamaraja N.A., Shivakumar A., Krishna H., A spectrophotometric method for the assay of peroxidase using para-phenylenediamine dihydrochloride and iminodibenzyl as chromogenic reagents: applications in some plant sources, Chem. Sci. Rev. Lett., 2014,3(12), 1068-1079.Suche in Google Scholar
[19] Lu H., Ma H., Tao G., Spectrophotometric determination of triclosan in personal care products, Spectrochim. Acta. A. Mol. Biomol. Spectrosc., 2009, 73(5), 854-857.10.1016/j.saa.2009.04.007Suche in Google Scholar PubMed
[20] Aydin F., Yilmaz E., Soylak M., A simple and novel deep eutectic solvent based ultrasound-assisted emulsification liquid phase microextraction method for malachite green in farmed and ornamental aquarium fish water samples, Microchem. J., 2017, 132, 280-285.10.1016/j.microc.2017.02.014Suche in Google Scholar
[21] Rastegarzadeh S., Pourreza N., Larki A., Dispersive liquid–liquid microextraction for the microvolume spectrophotometric determination of bismuth in pharmaceutical and human serum samples, Anal. Methods, 2014, 6(10), 3500-3505.10.1039/C4AY00526KSuche in Google Scholar
[22] Ahmadi M., Madrakian T., Afkhami A., Solid phase extraction of amoxicillin using dibenzo-18-crown-6 modified magnetic-multiwalled carbon nanotubes prior to its spectrophotometric determination, Talanta, 2016, 148, 122-128.10.1016/j.talanta.2015.10.051Suche in Google Scholar PubMed
[23] Yigit S., Tuzen M., Soylak M., Dogan M., Supramolecular solvent microextraction of Sudan blue II in environmental samples prior to its spectrophotometric determination, Int, J. Environ. Anal. Chem., 2016, 96(6), 568-575.10.1080/03067319.2016.1172221Suche in Google Scholar
[24] García-Fonseca S., Ballesteros-Gómez A., Rubio S., Pérez-Bendito D., Supramolecular solvent-based microextraction of ochratoxin A in raw wheat prior to liquid chromatography-fluorescence determination, J. Chromatogr. A, 2010, 1217(16), 2376-2382.10.1016/j.chroma.2009.10.085Suche in Google Scholar PubMed
[25] Cardeñosa V., Lunar M.L., Rubio S., Generalized and rapid supramolecular solvent-based sample treatment for the determination of annatto in food, J. Chromatogr. A, 2011, 1218(50), 8996-9002.10.1016/j.chroma.2011.10.041Suche in Google Scholar
[26] Costi E.M., Sicilia M.D., Rubio S., Supramolecular solvents in solid sample microextractions: application to the determination of residues of oxolinic acid and flumequine in fish and shellfish, J. Chromatogr A, 2010, 1217(9), 1447-1454.10.1016/j.chroma.2009.12.073Suche in Google Scholar
[27] Ballesteros-Gómez A., Sicilia M.D., Rubio S., Supramolecular solvents in the extraction of organic compounds. A review, Anal. Chim. Acta, 2010, 677(2), 108-130.10.1016/j.aca.2010.07.027Suche in Google Scholar
[28] Moradi M., Yamini Y., Tayyebi M., Asiabi H., Ultrasound-assisted liquid-phase microextraction based on a nanostructured supramolecular solvent, Anal. Bioanal. Chem., 2013, 405(12), 4235-4243.10.1007/s00216-013-6810-8Suche in Google Scholar
[29] Yazdi A.S., Surfactant-based extraction methods, TrAC Trends Anal. Chem., 2011, 30(6), 918-929.10.1016/j.trac.2011.02.010Suche in Google Scholar
[30] Adolfsson-Erici M., Pettersson M., Parkkonen J., Sturve J., Triclosan, a commonly used bactericide found in human milk and in the aquatic environment in Sweden, Chemosphere, 2002, 46(9), 1485-1489.10.1016/S0045-6535(01)00255-7Suche in Google Scholar
[31] Scheel G.L., Tarley C.R.T., Feasibility of supramolecular solvent-based microextraction for simultaneous preconcentration of herbicides from natural waters with posterior determination by HPLC-DAD, Microchem. J., 2017, 133, 650-657.10.1016/j.microc.2017.03.007Suche in Google Scholar
[32] Montaseri H., Forbes P.B., A review of monitoring methods for triclosan and its occurrence in aquatic environments, TrAC Trends Anal. Chem., 2016, 85, 221-231.10.1016/j.trac.2016.09.010Suche in Google Scholar
[33] Nomngongo P.N., Ngila J.C., Msagati T.A., Moodley B., Chemometric optimization of hollow fiber-liquid phase microextraction for preconcentration of trace elements in diesel and gasoline prior to their ICP-OES determination, Microchem. J., 2014, 114, 141-147.10.1016/j.microc.2013.12.013Suche in Google Scholar
[34] Wang H., Zhang A., Wang W., Zhang M., Liu H., Wang X., Separation and Determination of Triclosan and Bisphenol A in Water, Beverage, and Urine Samples by Dispersive Liquid–Liquid Microextraction Combined with Capillary Zone Electrophoresis–UV Detection, J.AOAC Int., 2013, 96(2), 459-465.10.5740/jaoacint.10-402Suche in Google Scholar PubMed
[35] Shih H.K., Lin C.W., Ponnusamy V.K., Ramkumar A., Jen J.F., Rapid analysis of triclosan in water samples using an in-tube ultrasonication assisted emulsification microextraction coupled with gas chromatography-electron capture detection, Anal. Methods, 2013, 5(9), 2352-2359.10.1039/c3ay40104aSuche in Google Scholar
[36] Wang H., Gao J., Yu N., Qu J., Fang F., Wang H., Wang M., Wang X., Development of a novel naphthoic acid ionic liquid and its application in “no-organic solvent microextraction” for determination of triclosan and methyltriclosan in human fluids and the method optimization by central composite design, Talanta, 2016, 154, 381-391.10.1016/j.talanta.2016.03.092Suche in Google Scholar PubMed
[37] Kim D., Han J., Choi Y., On-line solid-phase microextraction of triclosan, bisphenol A, chlorophenols, and selected pharmaceuticals in environmental water samples by high-performance liquid chromatography–ultraviolet detection, Anal. Bioanal. Chem., 2013, 405(1), 377-387.10.1007/s00216-012-6490-9Suche in Google Scholar PubMed
[38] Liu L., Liu H.X., Li Y., Du X.Z., Electrodeposition of Porous Polyaniline Coating on an Etching Stainless Steel Wire as a Fiber for Solid-Phase Microextraction of Triclosan and Chlorophenols in Water Samples, Appl. Mech. Mater., Trans Tech Publications, 2014, 665, 499-502.10.4028/www.scientific.net/AMM.665.499Suche in Google Scholar
[39] Regiart M., Magallanes J.L., Barrera D., Villarroel-Rocha J., Sapag K., Raba J., Bertolino F.A., An ordered mesoporous carbon modified electrochemical sensor for solid-phase microextraction and determination of triclosan in environmental samples, Sens. Actuators B, 2016, 232, 765-772.10.1016/j.snb.2016.04.031Suche in Google Scholar
[40] Chen M.J., Liu Y.T., Lin C.W., Ponnusamy V.K., Jen J.F., Rapid determination of triclosan in personal care products using new in-tube based ultrasound-assisted salt-induced liquid–liquid microextraction coupled with high performance liquid chromatography-ultraviolet detection, Anal. Chim. Acta, 2013, 767, 81-87.10.1016/j.aca.2013.01.014Suche in Google Scholar PubMed
[41] Azzouz A., Ballesteros E., Trace analysis of endocrine disrupting compounds in environmental water samples by use of solid-phase extraction and gas chromatography with mass spectrometry detection, J. Chromatogr. A, 2014, 1360, 248-257.10.1016/j.chroma.2014.07.059Suche in Google Scholar PubMed
© 2017 Anele Mpupa et al.
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Artikel in diesem Heft
- Regular Articles
- Rare Coumarins Induce Apoptosis, G1 Cell Block and Reduce RNA Content in HL60 Cells
- Regular Articles
- Evaluation of the photocatalytic ability of a sol-gel-derived MgO-ZrO2 oxide material
- Regular Articles
- Extraction Methods for the Isolation of Isoflavonoids from Plant Material
- Regular Articles
- Micro and nanocomposites of polybutadienebased polyurethane liners with mineral fillers and nanoclay: thermal and mechanical properties
- Regular Articles
- Effect of pH on Structural, Magnetic and FMR Properties of Hydrothermally Prepared Nano Ni Ferrite
- Regular Articles
- Statistical approach to study of lithium magnesium metaborate glasses
- Regular Articles
- The effectiveness of biodrying waste treatment in full scale reactor
- Regular Articles
- Chemical comparison of the underground parts of Valeriana officinalis and Valeriana turkestanica from Poland and Kazakhstan
- Regular Articles
- Phytochemical Characterization and Biological Evaluation of the Aqueous and Supercritical Fluid Extracts from Salvia sclareoides Brot
- Regular Articles
- Recent Microextraction Techniques for Determination and Chemical Speciation of Selenium
- Regular Articles
- Compost leachate treatment using polyaluminium chloride and nanofiltration
- Regular Articles
- Facile and Effective Synthesis of Praseodymium Tungstate Nanoparticles through an Optimized Procedure and Investigation of Photocatalytic Activity
- Regular Articles
- Computational Study on Non-linear Optical and Absorption Properties of Benzothiazole based Dyes: Tunable Electron-Withdrawing Strength and Reverse Polarity
- Regular Articles
- Comparative sorption studies of chromate by nano-and-micro sized Fe2O3 particles
- Regular Articles
- Recycling Monoethylene Glycol (MEG) from the Recirculating Waste of an Ethylene Oxide Unit
- Regular Articles
- Antimicrobial activity and thiosulfinates profile of a formulation based on Allium cepa L. extract
- Regular Articles
- The effect of catalyst precursors and conditions of preparing Pt and Pd-Pt catalysts on their activity in the oxidation of hexane
- Regular Articles
- Platinum and vanadate Bioactive Complexes of Glycoside Naringin and Phenolates
- Regular Articles
- Antimicrobial sesquiterpenoids from Laurencia obtusa Lamouroux
- Regular Articles
- Comprehensive spectroscopic (FT-IR, FT-Raman, 1H and 13C NMR) identification and computational studies on 1-acetyl-1H-indole-2,3-dione
- Regular Articles
- A combined experimental and theoretical study on vibrational and electronic properties of (5-methoxy-1H-indol-1-yl)(5-methoxy-1H-indol-2-yl)methanone
- Regular Articles
- Erratum to: Analysis of oligonucleotides by liquid chromatography with alkylamide stationary phase
- Regular Articles
- Non-isothermal Crystallization, Thermal Stability, and Mechanical Performance of Poly(L-lactic acid)/Barium Phenylphosphonate Systems
- Regular Articles
- Vortex assisted-supramolecular solvent based microextraction coupled with spectrophotometric determination of triclosan in environmental water samples
- Regular Articles
- Investigation on Two Compounds of O,O’-dithiophosphate Derivatives as Corrosion Inhibitors for Q235 Steel in Hydrochloric Acid Solution
- Regular Articles
- Evaluation of temporary seasonal variation of heavy metals and their potential ecological risk in Nzhelele River, South Africa
- Regular Articles
- Synthesis, characterization, second and third order non-linear optical properties and luminescence properties of 1,10-phenanthroline-2,9-di(carboxaldehyde phenylhydrazone) and its transition metal complexes
- Regular Articles
- Spectrodensitometric simultaneous determination of esomeprazole and domperidone in human plasma
- Regular Articles
- Computer-aided drug design of capuramycin analogues as anti-tuberculosis antibiotics by 3D-QSAR and molecular docking
- Regular Articles
- Synthesis, characterization, thermal degradation and urease inhibitory studies of the new hydrazide based Schiff base ligand 2-(2-hydroxyphenyl)-3-{[(E)-(2-hydroxyphenyl)methylidene]amino}-2,3-dihydroquinazolin-4(1H)-one
- Regular Articles
- Quaternary salts derived from 3-substituted quinuclidine as potential antioxidative and antimicrobial agents
- Regular Articles
- Bio-concentration of Polycyclic Aromatic Hydrocarbons in the grey Mangrove (Avicennia marina) along eastern coast of the Red Sea
- Regular Articles
- Quantitative Investigation of Roasting-magnetic Separation for Hematite Oolitic-ores: Mechanisms and Industrial Application
- Regular Articles
- Photobleaching characteristics of α-(8-quinolinoxy) zinc phthalocyanine, a new type of amphipathic complex
- Regular Articles
- Methane dry reforming over Ni catalysts supported on Ce–Zr oxides prepared by a route involving supercritical fluids
- Regular Articles
- Thermodynamic Compatibility, Crystallizability, Thermal, Mechanical Properties and Oil Resistance Characteristics of Nanostructure Poly (ethylene-co-methyl acrylate)/Poly(acrylonitrile-co-butadiene) Blends
- Regular Articles
- The crystal structure of compositionally homogeneous mixed ceria-zirconia oxides by high resolution X-ray and neutron diffraction methods
- Topical Issue on Agriculture
- Properties of the filtrate from treatment of pig manure by filtration method
- Topical Issue on Agriculture
- Monitoring content of cadmium, calcium, copper, iron, lead, magnesium and manganese in tea leaves by electrothermal and flame atomizer atomic absorption spectrometry
- Topical Issue on Catalysis
- Application of screen-printed carbon electrode modified with lead in stripping analysis of Cd(II)
- Topical Issue on Research for Natural Bioactive Products
- Burdock (Arctium lappa) Leaf Extracts Increase the In Vitro Antimicrobial Efficacy of Common Antibiotics on Gram-positive and Gram-negative Bacteria
- Topical Issue on Research for Natural Bioactive Products
- A survey of bacterial, fungal and plant metabolites against Aedes aegypti (Diptera: Culicidae), the vector of yellow and dengue fevers and Zika virus
- Topical Issue on Research for Natural Bioactive Products
- ‘Capiture’ plants with interesting biological activities: a case to go
- Topical Issue on Research for Natural Bioactive Products
- Volatile terpenoids as potential drug leads in Alzheimer’s disease
- Topical Issue on Research for Natural Bioactive Products
- Essential Oils as Immunomodulators: Some Examples
- Topical Issue on Research for Natural Bioactive Products
- Phenolic profiling and therapeutic potential of local flora of Azad Kashmir; In vitro enzyme inhibition and antioxidant
- Topical Issue on Research for Natural Bioactive Products
- Chemical profile, antioxidant activity and cytotoxic effect of extract from leaves of Erythrochiton brasiliensis Nees & Mart. from different regions of Europe
Artikel in diesem Heft
- Regular Articles
- Rare Coumarins Induce Apoptosis, G1 Cell Block and Reduce RNA Content in HL60 Cells
- Regular Articles
- Evaluation of the photocatalytic ability of a sol-gel-derived MgO-ZrO2 oxide material
- Regular Articles
- Extraction Methods for the Isolation of Isoflavonoids from Plant Material
- Regular Articles
- Micro and nanocomposites of polybutadienebased polyurethane liners with mineral fillers and nanoclay: thermal and mechanical properties
- Regular Articles
- Effect of pH on Structural, Magnetic and FMR Properties of Hydrothermally Prepared Nano Ni Ferrite
- Regular Articles
- Statistical approach to study of lithium magnesium metaborate glasses
- Regular Articles
- The effectiveness of biodrying waste treatment in full scale reactor
- Regular Articles
- Chemical comparison of the underground parts of Valeriana officinalis and Valeriana turkestanica from Poland and Kazakhstan
- Regular Articles
- Phytochemical Characterization and Biological Evaluation of the Aqueous and Supercritical Fluid Extracts from Salvia sclareoides Brot
- Regular Articles
- Recent Microextraction Techniques for Determination and Chemical Speciation of Selenium
- Regular Articles
- Compost leachate treatment using polyaluminium chloride and nanofiltration
- Regular Articles
- Facile and Effective Synthesis of Praseodymium Tungstate Nanoparticles through an Optimized Procedure and Investigation of Photocatalytic Activity
- Regular Articles
- Computational Study on Non-linear Optical and Absorption Properties of Benzothiazole based Dyes: Tunable Electron-Withdrawing Strength and Reverse Polarity
- Regular Articles
- Comparative sorption studies of chromate by nano-and-micro sized Fe2O3 particles
- Regular Articles
- Recycling Monoethylene Glycol (MEG) from the Recirculating Waste of an Ethylene Oxide Unit
- Regular Articles
- Antimicrobial activity and thiosulfinates profile of a formulation based on Allium cepa L. extract
- Regular Articles
- The effect of catalyst precursors and conditions of preparing Pt and Pd-Pt catalysts on their activity in the oxidation of hexane
- Regular Articles
- Platinum and vanadate Bioactive Complexes of Glycoside Naringin and Phenolates
- Regular Articles
- Antimicrobial sesquiterpenoids from Laurencia obtusa Lamouroux
- Regular Articles
- Comprehensive spectroscopic (FT-IR, FT-Raman, 1H and 13C NMR) identification and computational studies on 1-acetyl-1H-indole-2,3-dione
- Regular Articles
- A combined experimental and theoretical study on vibrational and electronic properties of (5-methoxy-1H-indol-1-yl)(5-methoxy-1H-indol-2-yl)methanone
- Regular Articles
- Erratum to: Analysis of oligonucleotides by liquid chromatography with alkylamide stationary phase
- Regular Articles
- Non-isothermal Crystallization, Thermal Stability, and Mechanical Performance of Poly(L-lactic acid)/Barium Phenylphosphonate Systems
- Regular Articles
- Vortex assisted-supramolecular solvent based microextraction coupled with spectrophotometric determination of triclosan in environmental water samples
- Regular Articles
- Investigation on Two Compounds of O,O’-dithiophosphate Derivatives as Corrosion Inhibitors for Q235 Steel in Hydrochloric Acid Solution
- Regular Articles
- Evaluation of temporary seasonal variation of heavy metals and their potential ecological risk in Nzhelele River, South Africa
- Regular Articles
- Synthesis, characterization, second and third order non-linear optical properties and luminescence properties of 1,10-phenanthroline-2,9-di(carboxaldehyde phenylhydrazone) and its transition metal complexes
- Regular Articles
- Spectrodensitometric simultaneous determination of esomeprazole and domperidone in human plasma
- Regular Articles
- Computer-aided drug design of capuramycin analogues as anti-tuberculosis antibiotics by 3D-QSAR and molecular docking
- Regular Articles
- Synthesis, characterization, thermal degradation and urease inhibitory studies of the new hydrazide based Schiff base ligand 2-(2-hydroxyphenyl)-3-{[(E)-(2-hydroxyphenyl)methylidene]amino}-2,3-dihydroquinazolin-4(1H)-one
- Regular Articles
- Quaternary salts derived from 3-substituted quinuclidine as potential antioxidative and antimicrobial agents
- Regular Articles
- Bio-concentration of Polycyclic Aromatic Hydrocarbons in the grey Mangrove (Avicennia marina) along eastern coast of the Red Sea
- Regular Articles
- Quantitative Investigation of Roasting-magnetic Separation for Hematite Oolitic-ores: Mechanisms and Industrial Application
- Regular Articles
- Photobleaching characteristics of α-(8-quinolinoxy) zinc phthalocyanine, a new type of amphipathic complex
- Regular Articles
- Methane dry reforming over Ni catalysts supported on Ce–Zr oxides prepared by a route involving supercritical fluids
- Regular Articles
- Thermodynamic Compatibility, Crystallizability, Thermal, Mechanical Properties and Oil Resistance Characteristics of Nanostructure Poly (ethylene-co-methyl acrylate)/Poly(acrylonitrile-co-butadiene) Blends
- Regular Articles
- The crystal structure of compositionally homogeneous mixed ceria-zirconia oxides by high resolution X-ray and neutron diffraction methods
- Topical Issue on Agriculture
- Properties of the filtrate from treatment of pig manure by filtration method
- Topical Issue on Agriculture
- Monitoring content of cadmium, calcium, copper, iron, lead, magnesium and manganese in tea leaves by electrothermal and flame atomizer atomic absorption spectrometry
- Topical Issue on Catalysis
- Application of screen-printed carbon electrode modified with lead in stripping analysis of Cd(II)
- Topical Issue on Research for Natural Bioactive Products
- Burdock (Arctium lappa) Leaf Extracts Increase the In Vitro Antimicrobial Efficacy of Common Antibiotics on Gram-positive and Gram-negative Bacteria
- Topical Issue on Research for Natural Bioactive Products
- A survey of bacterial, fungal and plant metabolites against Aedes aegypti (Diptera: Culicidae), the vector of yellow and dengue fevers and Zika virus
- Topical Issue on Research for Natural Bioactive Products
- ‘Capiture’ plants with interesting biological activities: a case to go
- Topical Issue on Research for Natural Bioactive Products
- Volatile terpenoids as potential drug leads in Alzheimer’s disease
- Topical Issue on Research for Natural Bioactive Products
- Essential Oils as Immunomodulators: Some Examples
- Topical Issue on Research for Natural Bioactive Products
- Phenolic profiling and therapeutic potential of local flora of Azad Kashmir; In vitro enzyme inhibition and antioxidant
- Topical Issue on Research for Natural Bioactive Products
- Chemical profile, antioxidant activity and cytotoxic effect of extract from leaves of Erythrochiton brasiliensis Nees & Mart. from different regions of Europe

