Chalcogenative spirocyclization of N-aryl propiolamides with diselenides/disulfides promoted by Selectfluor
-
Jin-Wei Yuan
, Guang-Chao Huang
Abstract
A practical and efficient synthetic route to construct a variety of 3-arylselenenyl/3-arylthio spiro[4.5]trienones was developed using Selectfluor reagent as a mild oxidant. This reaction proceeds via a sequence of electrophilic cation addition, spirocyclization and dearomatization, then offers an approach to introduce Se/S-centered cation into the C–C triple bonds. The utility of this protocol were justified by the excellent compatibility of a wide range of functional groups, good yields and scalability under mild reaction conditions.
1 Introduction
Spirocycles have recently received significant attention as they are found in the core of a variety of privileged scaffolds like natural products, functional materials, and pharmaceuticals (Figure 1) [1], [2], [3], [4], [5], [6]. Among the various spirocycles, more attention has been drawn towards the synthesis of azaspiro[4,5]trienones due to their excellent biological activities and their widespread applications in the synthesis of complex molecular frameworks [7], [8], [9], [10], [11]. Consequently, the development of new methods for the construction of these spirocycles is of high interest. Generally, the spiro[4,5]trienone structures can be constructed via the oxidative spirocyclization of phenol derivatives [12], [13], [14], electrophilic ipso-cyclization [15], [16], [17], [18], transition-metal mediated intramolecular nucleophilic ipso-spirocyclization of alkenes or alkynes [19], [20], [21], [22], [23], [24], and the radical coupling ipso-cyclization [25], [26], [27], [28]. Indeed, enormous efforts have been paid to the synthesis of functionalized azaspiro[4,5]trienones via cascade ipso-spirocyclization of N-arylpropiolamides, involving either electrophilic [29], [30], [31], [32] or radical addition [33], [34], [35]. Through this strategy, some functionalities such as halogen [36], [37], [38], [39], [40], [41], [42], carbonyl [43], [44], [45], alkyl [46], [47], [48], [49], [50], phosphory [51, 52] nitro [53], thiocyanato [54], silyl groups [55, 56], and sulfonyl [57], [58], [59], etc. [60], could be introduced into the azaspiro[4.5]-trienone framework.
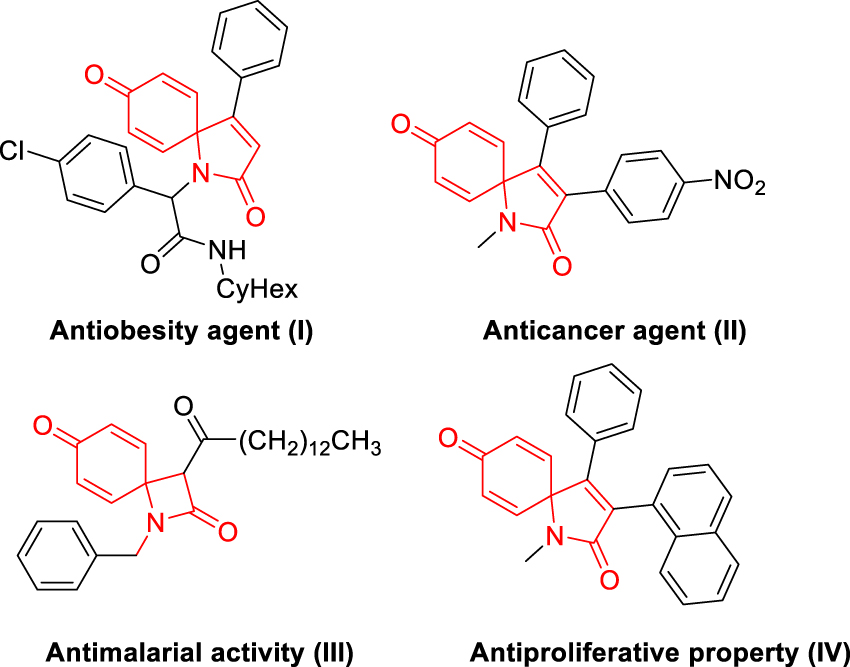
Structure of representative spirocyclic bioactive molecules.
Selenium is a very important element in our body, and selenium-containing compounds are widely distributed in bioactive compounds and natural products [61, 62]. Moreover, they have gained considerable interest due to their well-known biological activities and fluorescent properties [63], [64], [65], and wide applications in pharmaceutical, food chemistry and material science [66], [67], [68]. Therefore, many efforts have been devoted for the synthesis of more valuable organoselenides [69], [70], [71], [72], [73], [74], [75]. The introduction of an organoselenium or organosurfur group to the spiro[4,5]trienones has attracted growing attention during the past decades, due to the wide and interesting biological properties associated with these unique functional groups. In 2015, Wei’s group reported a silver-catalyzed direct oxidative spirocyclization of N-arylpropiolamides with thiophenols to synthesize various 3-thioazaspiro[4,5]trienones at 80 °C for 10–24 h [76]. A copper-catalyzed strategy for the synthesis of 3-arylthio/3-arylselenenyl azaspiro[4,5]trienones via the oxidative ipso-cyclization of N-arylpropiolamides with diselenides/disulfides in the presence of O2 at 100 °C for 24 h was disclosed by Li’s group (Scheme 1a) [77]. Photoredox catalysis enabled by visible light has emerged as a fascinating and powerful synthetic protocol to promote a wide range of synthetically useful organic transformations under mild conditions [78], [79], [80], [81], [82], [83]. In 2017, Wang’s group developed a visible light induced method for the construction of 3-sulfenyl azaspiro[4,5]trienones through metal-free difunctionalization of N-arylpropiolamides with disulfides, using Na2-eosin Y as a photocatalyst under air for 6–24 h [84]. In 2018, Baidya’s group reported a visible light-induced synthetic approach for selenylative spirocyclization of N-arylpropiolamides with molecular oxygen as oxidant for 24–36 h [85]. A visible light-promoted spirocyclization reaction of N-arylpropiolamides with thiophenols via recyclable Pd/ZrO2 nanoparticle as a catalyst was also disclosed by Guo’s group (Scheme 1b) [86]. In recent years, as a reliable alternative for redox reagents, electrochemistry has been recognized as an environmental friendly and economy tool to promote chemical reactions [87], [88], [89], [90], [91], [92]. An electrochemical synthetic route for 3-selenylative azaspiro[4,5]trienones through radical initiated dearomative spirocyclization of N-arylpropiolamides was developed by Guo’s group (Scheme 1c) [93]. Moreover, a selenium-promoted electrophilic cyclization of arylpropiolamides allowing the synthesis of 3-selenyl spiro[4,5]trienones using arylselenyl bromide as a selenyl source was described by Zeni’s group [94]. Baidya’s group devised a selenium radical triggered ipso-cyclization of N-arylpropiolamides to access 3-selenospiro[4,5]trienones using K2S2O8 as oxidant under a N2 atmosphere at 80 °C for 24 h [95]. Regardless of their merits, the current strategies suffer from disadvantages such as transition-metal as a catalyst, the inevitability of a strong oxidant, high reaction temperature, limited substrate scope, harsh or complex reaction conditions. Therefore, more general and mild approaches for the construction of diversely chalcogenative spiro[4,5]trienones are still of great interest.
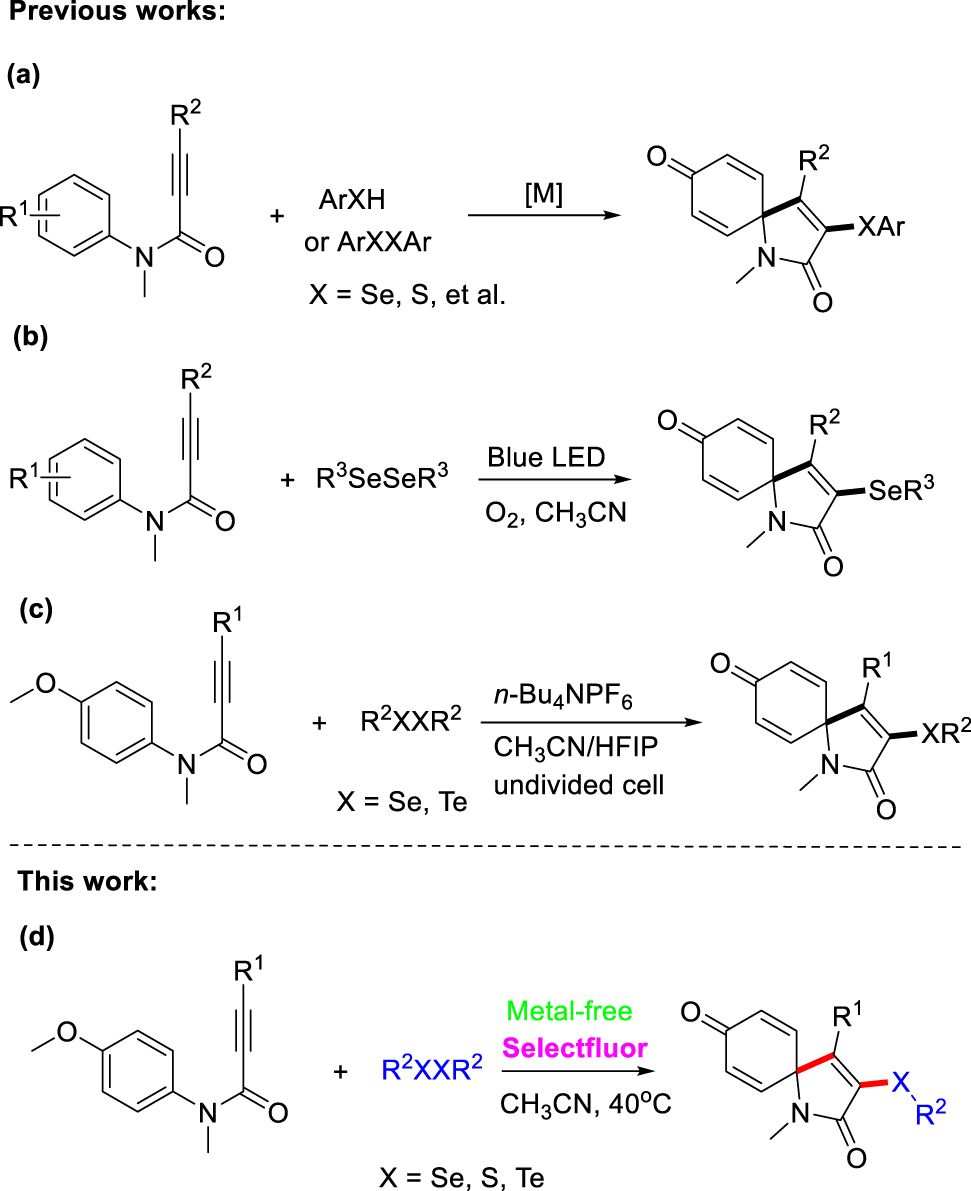
Chalcogenative spirocyclization of N-aryl propiolamides.
Selectfluor is commercially available, exceptionally stable, and useful for a powerful fluorination regent and oxidant [96], [97], [98], [99], [100], [101], [102]. Synthesis of several functionalized heterocyclic compounds has been realized successfully using Selectfluor as a mild oxidant [103], [104], [105], [106], [107], [108], [109], [110], [111], [112]. Recently, our group has also developed several practical synthetic route to realize some functionalized heterocycles construction employing Selectfluor reagent as an electrophilic reagent or oxidant [113, 114]. Expanding the application of Selectflour for the cross-coupling reaction is still challenging works. To the best of our knowledge, using stable and readily available diselenides/disulfides as selenium/sulfur reagent, transition-metal free Selectfluor-promoted ipso-spirocyclization of N-arylpropiolamides to access 3-arylselenenyl/3-arylthio azaspiro[4,5]trienones remains yet elusive. As a part of our continuous interest in forming C–X bond promoted by Selectfluor reagent, herein, we reported a direct chalcogenation of N-arylpropiolamides to achieve dearomative spirocyclization through Selectfluor-promoted oxidative coupling reaction under metal-free conditions (Scheme 1d). This protocol features good to excellent yields, without the need for toxic metals, ligands, and bases, and could serve as an efficient approach for the construct 3-arylselenenyl/3-arylthio azaspiro[4,5]trienones under mild conditions.
2 Results and discussion
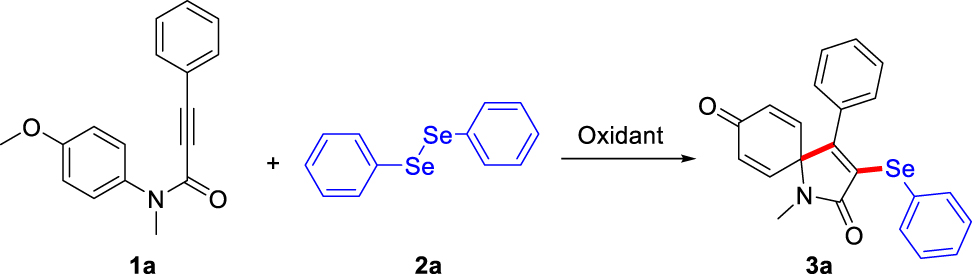
Initially, the reaction of N-(4-methoxyphenyl)-N-methyl-3-phenylpropiolamide 1a with commercially available diphenyldiselenide 2a was screened as model reaction to identify suitable reaction conditions (Table 1). To our delight, when the mixture of 1a (0.2 mmol) and 2a (1.0 equiv.) in CH3CN was exposed to I2 (1.0 equiv.) oxidant at 90 °C for 6.0 h, the selenium functionalized ipso-spirocyclization product 3a was obtained in 43% isolated yield (entry 1, Table 1). The structure of 3a was unambiguously confirmed by IR, NMR, and HR MS. Encouraged by this preliminary result, other oxidants including PhI(OAc)2, PhI(OCOCF3)2, Selectfluor, and O2 were screened, the result showed that PhI(OAc)2 and O2 are noneffective, however PhI(OCOCF3)2 and Selectfluor gave in 65% and 80% yields, respectively (entries 2–5, Table 1). Selectfluor was used as the best oxidant for this transformation. The effect of the Selectfluor loading was also screened, and 1.0 equiv. was found to be the best choice to produce 80% yield (entries 4, 6–8, Table 1). The solvents screening indicated that replacing CH3CN with other solvents, including DMF, DMSO, Toluene, THF, CH3OH, DCE, dioxane, H2O, and co-solvent CH3CN-H2O (1:1, v:v) only led to inferior results (entries 4, 9–17, Table 1). It was noted that CH3CN, the reported efficient solvent for the electrophilic ipso-halocyclization reaction [37], was also highly reactive for the current reaction. Raising the temperature from 20 °C to 40 °C, the yield increased from 76% to 80%. But upon increasing it further to 90 °C, the yield could not increase obviously (entries 4, 18–20, Table 1). Then, the molar ratio of 1a and 2a was investigated. Increasing the amount of 2a from 0.3 to 1.0 equiv., the yield of 3a was improved sharply, and the best yield was 80%. However, when 1.5 equiv. 2a was used, no obvious change in yield was observed even with prolonged reaction time (ESI, entries 1–4, Supplementary Material Table S1). The effect of reaction time was also tested. 1.5 h was proved be optimal time, and could provide the yield of 80% (entries 19, 21–24, Table 1). When the reaction proceeded in the absence of Selectfluor reagent, no desired product 3a was detected (entry 25, Table 1), thus indicating that Selectfluor is crucial for the reaction to occur. Thus, the optimized conditions were identified as follows: the molar ratio of 1a and 2a is 1:1, 1.0 equiv. Selectfluor as the oxidant in CH3CN at 40 °C for 1.5 h.
Optimization of reaction conditions.a
|
|||||
|---|---|---|---|---|---|
| Entry | Oxidant (equiv.) | Solvent | Temp (°C) | Time (h) | Yieldb (%) |
| 1 | I2 (1.0) | CH3CN | 90 | 6.0 | 43 |
| 2 | PhI(OAc)2 (1.0) | CH3CN | 90 | 6.0 | 0 |
| 3 | PhI(OCOCF3)2 (1.0) | CH3CN | 90 | 6.0 | 65 |
| 4 | Selectfluor (1.0) | CH3CN | 90 | 6.0 | 80 |
| 5c | O2 (1.0) | CH3CN | 90 | 6.0 | 0 |
| 6 | Selectfluor (0.2) | CH3CN | 90 | 6.0 | 45 |
| 7 | Selectfluor (0.5) | CH3CN | 90 | 6.0 | 68 |
| 8 | Selectfluor (1.5) | CH3CN | 90 | 6.0 | 80 |
| 9 | Selectfluor (1.0) | DMF | 90 | 6.0 | 55 |
| 10 | Selectfluor (1.0) | DMSO | 90 | 6.0 | 0 |
| 11 | Selectfluor (1.0) | Toluene | 90 | 6.0 | 0 |
| 12 | Selectfluor (1.0) | THF | 90 | 6.0 | 0 |
| 13 | Selectfluor (1.0) | CH3OH | 90 | 6.0 | 38 |
| 14 | Selectfluor (1.0) | DCE | 90 | 6.0 | 10 |
| 15 | Selectfluor (1.0) | Dioxane | 90 | 6.0 | 0 |
| 16 | Selectfluor (1.0) | H2O | 90 | 6.0 | 0 |
| 17 | Selectfluor (1.0) | CH3CN:H2O = 1:1 | 90 | 6.0 | Trace |
| 18 | Selectfluor (1.0) | CH3CN | 20 | 6.0 | 76 |
| 19 | Selectfluor (1.0) | CH3CN | 40 | 6.0 | 80 |
| 20 | Selectfluor (1.0) | CH3CN | 60 | 6.0 | 79 |
| 21 | Selectfluor (1.0) | CH3CN | 40 | 0.5 | 58 |
| 22 | Selectfluor (1.0) | CH3CN | 40 | 1.0 | 72 |
| 23 | Selectfluor (1.0) | CH3CN | 40 | 1.5 | 80 |
| 24 | Selectfluor (1.0) | CH3CN | 40 | 2.0 | 80 |
| 25c | – | CH3CN | 40 | 1.5 | 0 |
-
aReaction conditions: N-(4-methoxyphenyl)-N-methyl-3-phenylpropiolamide 1a (0.2 mmol, 53.0 mg), diphenyl diselenide 2a (0.2 mmol, 62.8 mg), Selectfluor agent in solvent (2.0 mL). bIsolated yield. cNo Selectflour was added.
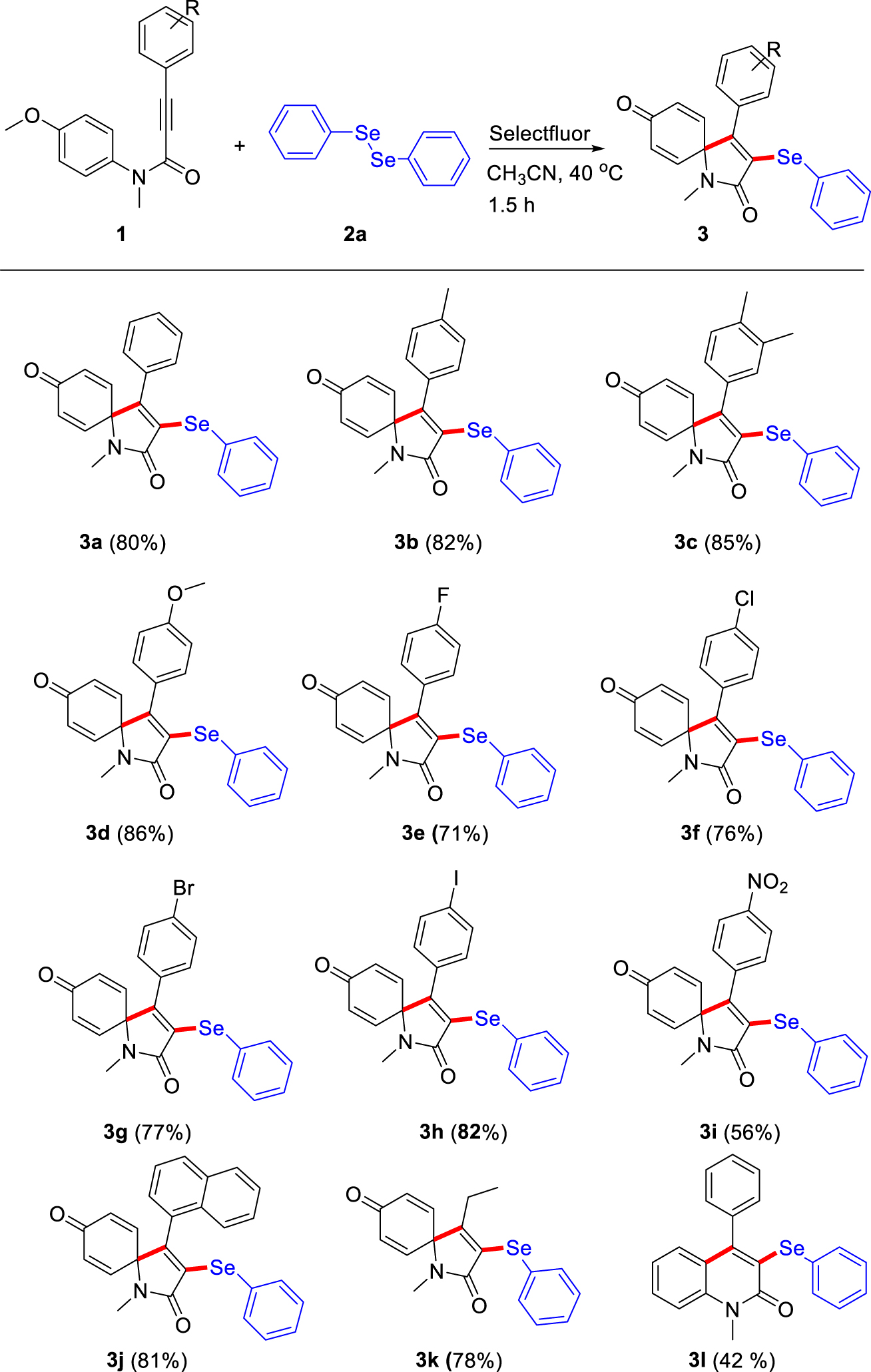
With the optimal reaction conditions in hand, the substrate scope and generality of dearomative spirocyclization of N-aryl propiolamides 1 with diphenyl diselenide 2a was investigated (Table 2). To our delight, the reaction of N-aryl propiolamides 1 bearing both electron-donating or electron-withdrawing substituents attached on the alkyne showed great compatibility to prepare the corresponding products in good to excellent yields (3a-3j, 56–86%). Generally, N-aryl propiolamides with electron-donating groups (–Me, –OMe) gave higher yields in comparison with those with electron-withdrawing groups (–F, –Cl, –Br, –I, –NO2) due to the good stability of the intermediates formed by N-aryl propiolamides with electron-donating groups. Notedly, when alkynamide with a strong electron-withdrawing –NO2 group 1i was employed, the oxidative ipso-cyclization reaction could tolerate the reaction conditions to provide 3i in 56% yield. Gratifyingly, when aliphatic alkynamide, N-(4-methoxyphenyl)-N-methylpent-2-ynamide, was employed, the reaction could also proceed smoothly to obtain the desired 3k in excellent yields (78%). When naphthalene-1-yl alkyne, N-(4-methoxyphenyl)-N-methyl-3-(naphthalen-1-yl)propiolamide 1j, was employed, the reaction could provide excellent yield (3j, 81%), which indicated that the steric factor did not obstruct the process of this reaction. Unfortunately, alkynamide with a free N-H group has no reactivity for the ipso-cyclization reaction. Interestingly, N-methyl-N,3-diphenylpropiolamide 1l was well tolerated to provide the cyclization product 3l in 42% yield, instead of ipso-cyclization product.
Scope of N-aryl propiolamides for the spirocyclization.a,b
|
-
aReaction conditions: N-aryl propiolamides 1 (0.2 mmol), diphenyl diselenide 2a (0.2 mmol, 62.8 mg), Selectfluor agent (0.2 mmol, 70.8 mg) in CH3CN (2.0 mL) at 40 °C for 1.5 h. bIsolated yield.
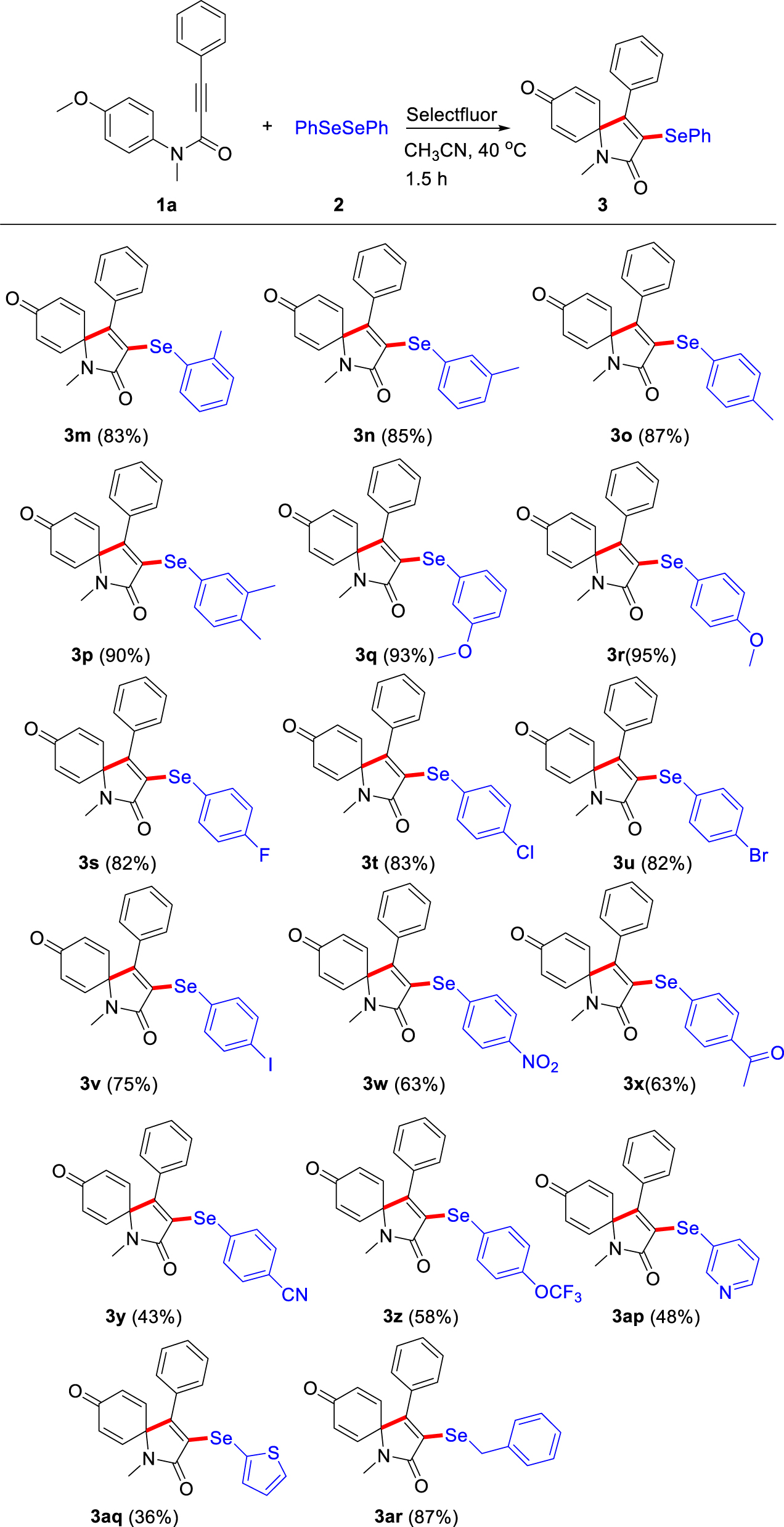
Subsequently, we turned our attention to explore the substrate range of diselenides under the standard conditions. As shown in Table 3, diaryldiselenides 2 with the electron-donating and electron-withdrawing groups all work well with N-aryl alkynamide 1a, providing the corresponding 3-arylselenenyl azaspiro[4,5]trienone derivatives in considerable yields (3m-3aq, 36–95%). The diaryldiselenides with electron-donating groups (–Me, –OMe) could provide higher yields than those with electron-withdrawing (–F, –Cl, –Br, –I, –NO2, –COMe, –CN, –OCF3) substituents. It is possible that the arylselenyl intermediates have good stability. Gratifyingly, when diaryldiselenides with electron-withdrawing groups (–NO2, –COMe, –CN, –OCF3) were employed, the ipso-cyclization reaction could proceed smoothly to obtain the corresponding spirocyclization products (3w-3z) in moderate to good yields (43–63%). Gratifyingly, heteroarene diselenides, 1,2-di(pyridin-3-yl)diselane and 1,2-di(thiophen-2-yl)diselane, could also provide the desired products 3ap and 3aq in moderate yields (48%, and 36%), respectively. Notably, we were pleased that the synthetic routes involving dialkyl disulfide, 1,2-dibenzyldiselane, proceeded smoothly to generate the desired product 3ar in excellent yield (87%).
Scope of diselenides on the spirocyclization of N-aryl propiolamides.a,b
|
-
aReaction conditions: N-(4-methoxyphenyl)-N-methyl-3-phenylpropiolamide 1a (0.2 mmol, 53.0 mg), diaryldiselenide 2 (0.2 mmol), Selectfluor agent (0.2 mmol, 70.8 mg) in CH3CN (2.0 mL) at 40 °C for 1.5 h. bIsolated yield.
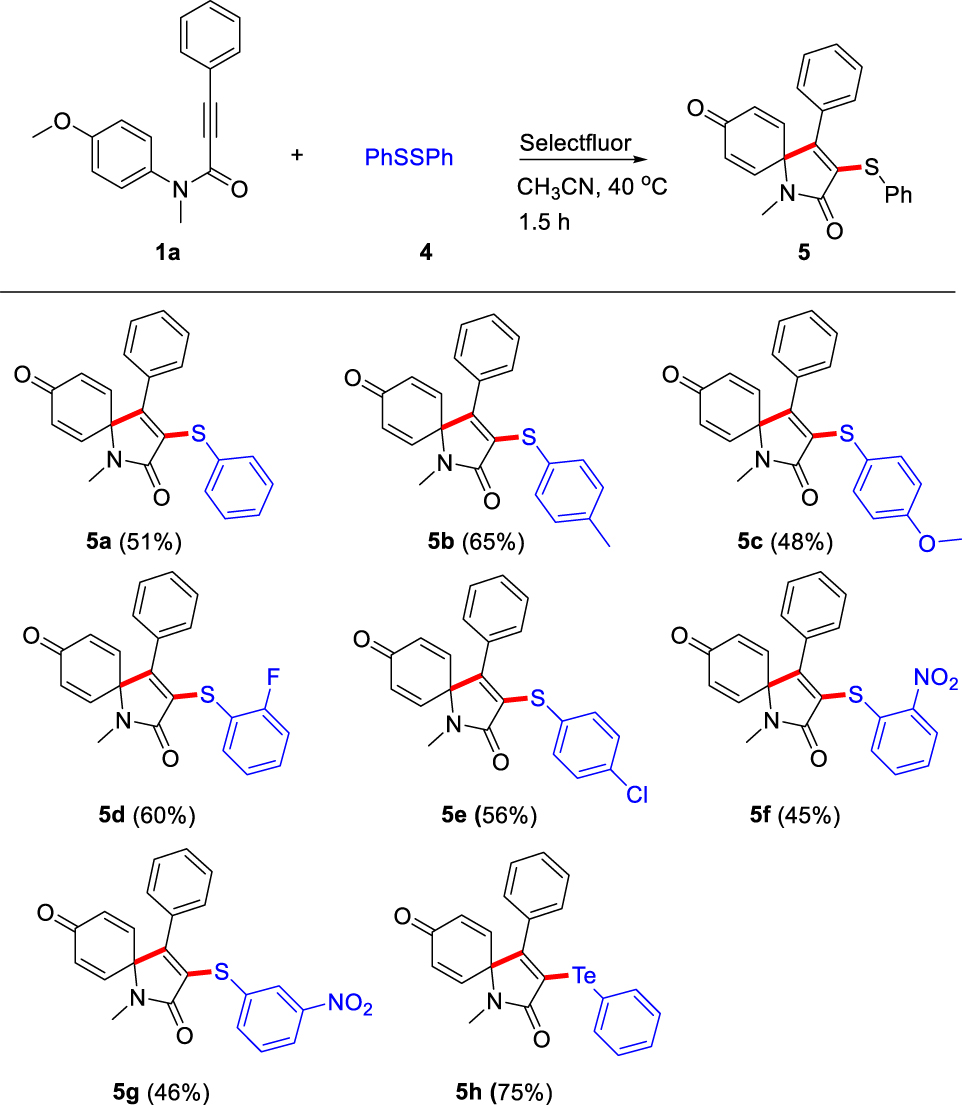
Next, the Selectfluor-promoted synthesis of 3-arylsulfenylated azaspiro[4,5]trienone derivatives was explored (Table 4). We were glad to find that our protocol was applied well in the synthesis of 3-arylsulfenylated azaspiro[4,5]trienones when ArS-SAr was used instead in albeit slightly lower yields (45–65%) comparison to their selenylated analogs. In general, substrates with either an electron-donating groups (–Me, –OMe), or electron-withdrawing groups (–F, –Cl, –NO2) all reacted smoothly to give the corresponding spirocyclization products 5a-5g in good yields (45–65%). Unexpectedly, the use of diaryldisulfide with an electron-donating group (–OMe) resulted in relatively low yield (48%) of the corresponding product 5c compared to 5b with electron-donating group (–Me), potentially due to the high reactivity of this electron rich intermediate, thus producing a complex mixture. To our delight, diaryldisulfides with strong electron-withdrawing group (–NO2) could also provide the desired products (5f and 5g) in moderate yields (45%, and 46%). Gratifyingly, 1,2-diphenylditellane also actively participated in this transformation to dispense the desired 3-phenyltellanyl azaspiro[4,5]trienone derivative 5h in 75% isolated yield.
Scope of disulfides on the spirocyclization of N-aryl propiolamides.a,b
|
-
aReaction conditions: N-(4-methoxyphenyl)-N-methyl-3-phenylpropiolamide 1a (0.2 mmol, 53.0 mg), diaryldisulfides 4 (0.2 mmol), Selectfluor agent (0.2 mmol, 70.8 mg) in CH3CN (2.0 mL) at 40 °C for 1.5 h. bIsolated yield.
Additionally, to verify the utility and robustness of this protocol, gram-scale reactions were conducted using substrate 1a (4.0 mmol, 1.06 g) with diphenyldiselenide 2a under the standard conditions. As expected, the reaction proceeded well to produce the corresponding ipso-cyclization product 3a in 76% yield (Scheme 2a), which provides promising application in preparative synthesis. Then, an intermolecular competition experiment was carried out to explore the selectivity of this spirocyclization reaction of N-(4-methoxyphenyl)-N-methyl-3-phenylpropiolamide 1a with diphenyldiselenide 2a and diphenyl disulfide 4a. The result showed that 3-phenylselenyl azaspiro[4,5]trienone was provided in 80% yield, and 3-phenylsulfenyl azaspiro[4,5]trienone was only obtained in 3% yield observed by HPLC, which indicated that diaryldiselenides 2 had much higher reactivity than that of diaryldisulfides 4 (Scheme 2b), possibly as a result of instability of arylsulfenyl intermediates.
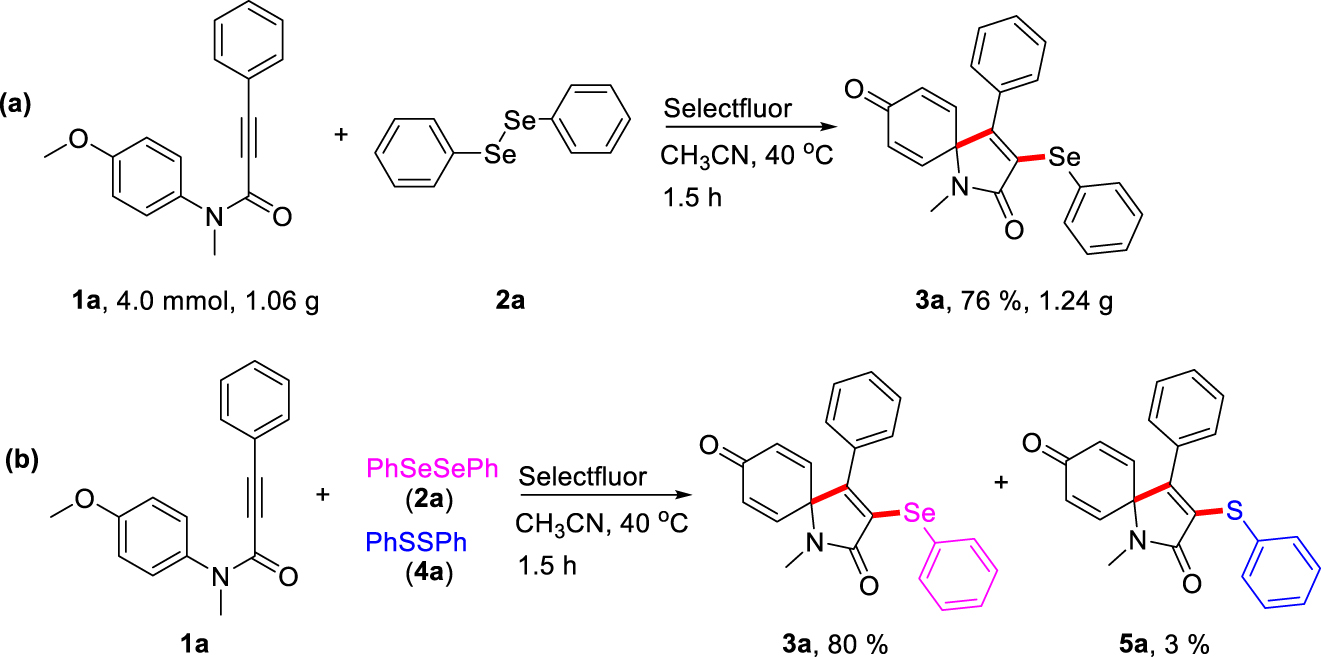
Investigation and application of this protocol.
(a) Gram-scale synthesis experiment. (b) Intermolecular competition experiments.
In order to explore the possible reaction pathway, some control experiments were carried out as shown in Scheme 3. While 2,2,6,6-tetramethyl-1-piperidinyloxyl (TEMPO, 2.0 equiv.) as radical-trapping reagent was subjected to the spirocyclization reaction under standard reaction conditions, the yield of 3a didn’t change obviously. 2,6-Di-tert-butyl-4-methylphenol (BHT, 3.0 equiv.) was added to the reaction system, the same result was observed. These results reveal that this transformation may proceed via an ion pathway, instead of a radical pathway.
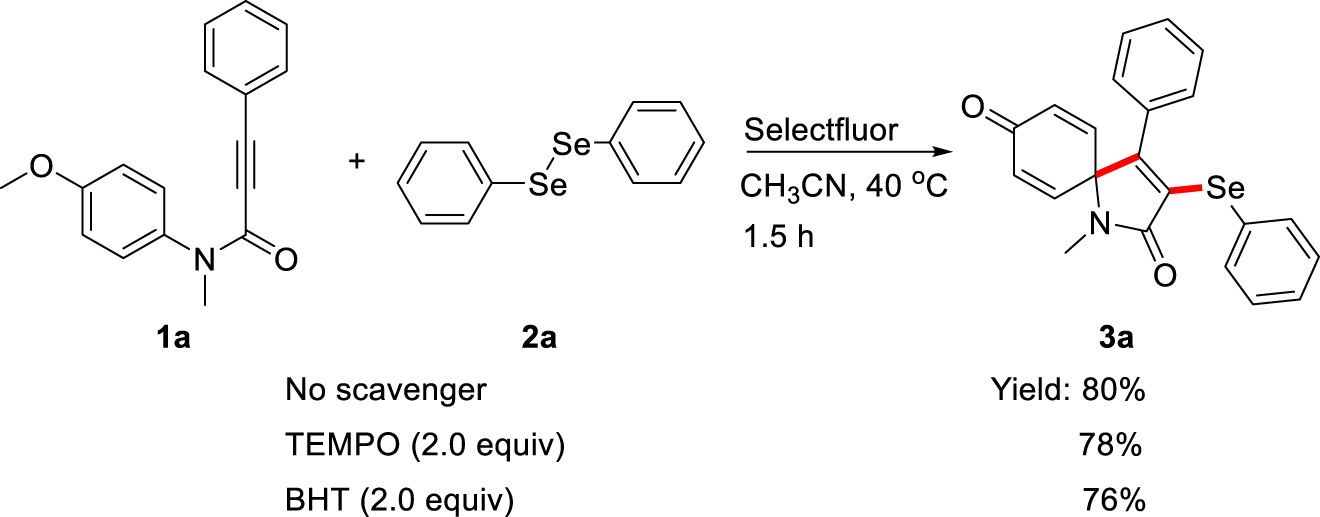
Control experiments.
On the basis of these experimental results and previous reports [71, 115, 116], a plausible catalytic cycle was proposed and depicted in Scheme 4. Initially, the reaction involves the oxidation of diphenyl diselenide by Selectfluor reagent to form the electrophilic species I and II [101, 103]. Then, these species attack C–C triple bond of N-(4-methoxyphenyl)-N-methyl-3-phenylpropiolamide 1a to produce selenonium salt III. Subsequently, the intramolecular electrophilic attack of the carbon atom of the C-N moiety on the Csp2 center proceeds to the carbon cation intermediate IV. The intermediate IV is combined with hydroxyl anion from the ionization of H2O to form the molecule V [39, 45, 58, 59]. Finally, the expected product 3a is formed by losing a CH3OH molecule.
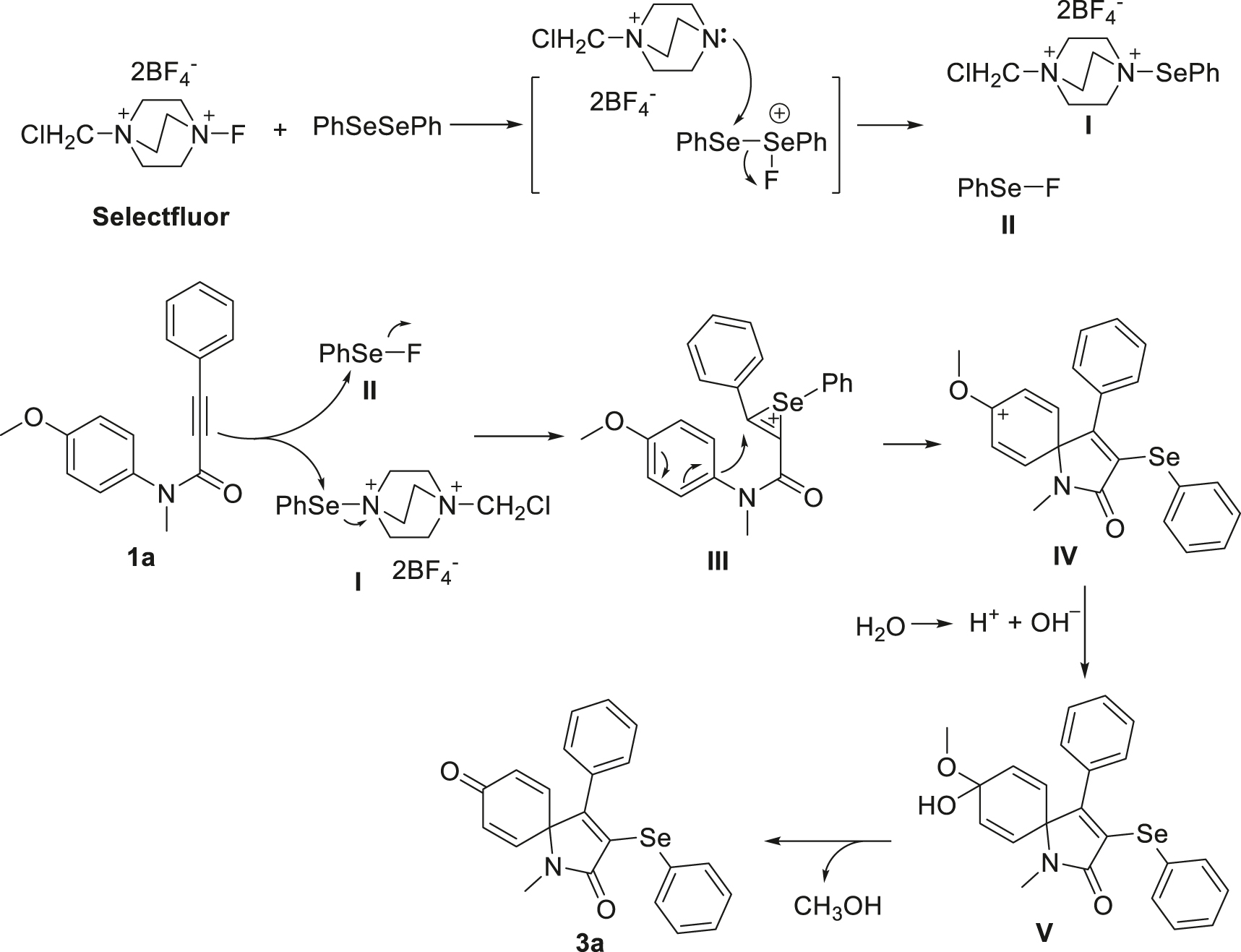
Proposed reaction mechanism.
3 Conclusions
In conclusion, we have developed an intriguing Selectfluor-promoted, metal-free, mild and efficient synthetic approach to access 3-arylselenyl and 3-arylsulfenyl azaspiro[4,5]trienones from N-arylpropiolamides and easily available diarylselenides/diaryldisulfides. One Striking feature of the current method is that it does not require the use of any metal for the reaction to occur. Furthermore, this approach exhibits a broad substrate scope, simple procedure, mild reaction condition and high-yielding product. Further studies of the applications of Selectfluor/diarylselenides species is currently in progress.
4 Experimental section
4.1 General
All chemicals were commercially available and used as received without further purification. Column chromatography was performed using 300–400 mesh silica. Nuclear magnetic resonance spectra were recorded on Bruker Avance 400 MHz spectrometer. 1H NMR spectra were recorded in ppm from tetramethylsilane. Data were reported as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, m = multiplet and br = broad), coupling constant in Hz and integration. 13C NMR spectra were recorded in parts per million from tetramethylsilane. 19F NMR spectra were recorded in ppm with fluorobenzene as external standard. High resolution mass spectra (HR MS) were obtained on Thermo Scientific LTQ Orbitrap XL instrument using the ESI technique. IR spectra were recorded on WQF-510 Fourier transform infrared spectrophotometer. Melting points were determined on an XT4A microscopic apparatus and are uncorrected.
4.2 Synthesis of 3-arylselenenyl azaspiro[4,5]trienones (3)
N-Aryl propiolamides 1 (0.2 mmol), diaryldiselenide 2 (0.2 mmol), Selectfluor agent (0.2 mmol, 70.8 mg), and acetonitrile (2.0 mL) were added to a 10 mL reaction tube. The mixture was stirred at 40 °C for 1.5 h. After completion of the reaction, the solvent was distilled under vacuum. Then, the resulting mixture was dissolved with ethyl acetate (15 mL), washed with saturated sodium chloride solution (10 mL × 2). The organic phase was dried over anhydrous Na2SO4 and concentrated under vacuum. The residue was purified by silica gel column chromatography to give 3-arylselenenyl azaspiro[4,5]trienones 3 using ethyl acetate/dichloromethane as eluant.
4.2.1 1-Methyl-4-phenyl-3-(phenylselanyl)-1-azaspiro[4.5]deca-3,6,9-triene-2,8-dione (3a)
Pale yellow crystal; yield: 65.1 mg (80%); m.p. 142–143 °C (lit.94 135.1–135.9 °C). − IR (KBr): ν = 1702, 1688, 1668, 1438, 1359, 868 cm−1. − 1H NMR (400 MHz, CDCl3): δ = 7.36 (dd, JH-H = 8.0, 1.1 Hz, 2H), 7.24 (t, JH-H = 7.3 Hz, 1H), 7.19–7.14 (m, 3H), 7.11–7.08 (m, 4H), 6.51 (d, JH-H = 10.2 Hz, 2H), 6.42 (d, JH-H = 10.2 Hz, 2H), 2.89 (s, 3H). − 13C NMR (100 MHz, CDCl3): δ = 183.9 (C=O), 168.8 (C=O), 154.1, 145.0 (CH), 133.9 (CH), 133.1 (CH), 131.1, 130.1, 129.4 (CH), 129.0 (CH), 128.2 (CH), 128.0 (CH), 127.9 (CH), 127.1, 69.0, 26.4 (CH3). − HR MS ((+)ESI): m/z = 430.0312 (calcd. 430.0317 for C22H17NO2SeNa [M + Na]+).
4.2.2 1-Methyl-3-(phenylselanyl)-4-(p-tolyl)-1-azaspiro[4.5]deca-3,6,9-triene-2,8-dione (3b) 93
Colorless acicular crystal; yield: 69.0 mg (82%); m.p. 166–167 °C. − IR (KBr): ν = 1704, 1688, 1669, 1411, 1362, 826, 739 cm−1. − 1H NMR (400 MHz, CDCl3): δ = 7.39–7.37 (m, 2H), 7.19–7.15 (m, 1H), 7.13–7.09 (m, 2H), 7.01 (q, JH-H = 6.5 Hz, 4H), 6.50 (d, JH-H = 10.2 Hz, 2H), 6.43 (d, JH-H = 10.2 Hz, 2H), 2.88 (s, 3H), 2.27 (s, 3H). − 13C NMR (100 MHz, CDCl3): δ = 184.0 (C=O), 168.8 (C=O), 154.8, 145.2 (CH), 139.7, 133.6 (CH), 133.0 (CH), 129.3, 129.0 (CH), 128.9 (CH), 128.3, 127.9 (CH), 127.7 (CH), 127.5, 68.9, 26.3 (CH3), 21.3 (CH3). − HR MS ((+)ESI): m/z = 422.0646 (calcd. 422.0654 for C23H20NO2Se [M + H]+).
4.3 Synthesis of 3-arylthio azaspiro[4,5]trienones (5)
N-(4-Methoxyphenyl)-N-methyl-3-phenylpropiolamide 1a (0.2 mmol, 53.0 mg), diaryldisulfides 4 (0.2 mmol), Selectfluor agent (0.2 mmol, 70.8 mg), and acetonitrile (2.0 mL) were added to a 10 mL reaction tube. The mixture was stirred at 40 °C for 1.5 h. After completion of the reaction, the solvent was distilled under vacuum. Then, the resulting mixture was dissolved with ethyl acetate (15 mL), washed with saturated sodium chloride solution (10 mL × 2). The organic phase was dried over anhydrous Na2SO4 and concentrated under vacuum. The residue was purified by silica gel column chromatography to give 3-arylthio azaspiro[4,5]trienones 5 using ethyl acetate/dichloromethane as eluant.
4.3.1 1-Methyl-4-phenyl-3-(phenylthio)-1-azaspiro[4.5]deca-3,6,9-triene-2,8-dione (5a) 84
Colorless crystal; yield: 36.6 mg (51%); m.p. 169–171 °C. − IR (KBr): ν = 2919, 1697, 1667, 1625, 1603, 1381, 1368, 872 cm−1. − 1H NMR (400 MHz, CDCl3): δ = 7.28–7.26 (m, 3H), 7.22 (d, JH-H = 4.5 Hz, 4H), 7.19–7.14 (m, 3H), 6.54 (d, JH-H = 10.2 Hz, 2H), 6.46 (d, JH-H = 10.2 Hz, 2H), 2.88 (s, 3H). − 13C NMR (100 MHz, CDCl3): δ = 183.9, 167.6, 152.9, 145.1 (CH), 133.1 (CH), 132.1, 131.7, 130.8 (CH), 130.6, 129.6 (CH), 128.9 (CH), 128.3 (CH), 128.1 (CH), 127.4 (CH), 67.7, 26.3 (CH3). − HR MS ((+)ESI): m/z = 360.1053 (calcd. 360.1053 for C22H18NO2S [M + H]+).
4.3.2 1-Methyl-4-phenyl-3-(p-tolylthio)-1-azaspiro[4.5]deca-3,6,9-triene-2,8-dione (5b)
Colorless acicular crystal; yield: 48.5 mg (65%); m.p. 170–172 °C (lit.84 159.3–160.2 °C). − IR (KBr): ν = 2920, 1708, 1691, 1668, 1366, 870 cm−1. − 1H NMR (400 MHz, CDCl3): δ = 7.29–7.19 (m, 7H), 6.98 (d, JH-H = 7.9 Hz, 1H), 6.51 (d, JH-H = 10.3 Hz, 2H), 6.45 (d, JH-H = 10.3 Hz, 2H), 2.87 (s, 3H), 2.26 (s, 3H). − 13C NMR (100 MHz, CDCl3): δ = 183.9 (C=O), 167.7 (C=O), 151.6, 145.2 (CH), 137.8, 133.1 (CH), 132.8, 131.7 (CH), 130.7, 129.7 (CH), 129.5 (CH), 128.3 (CH), 128.2 (CH), 127.6, 67.6, 26.2 (CH3), 21.1 (CH3). − 19F NMR (376 MHz, CDCl3): δ = −107.5. − HR MS ((+)ESI): m/z = 374.1202 (calcd. 374.1209 for C23H20NO2S [M + H]+).
5 Supporting information
The experimental section, copies of 1H NMR, and 13C NMR spectra and characterization of data (3c-3ar, 5c-5h) can be found in supporting information.
Funding source: Natural Science Foundation in Henan Province Department of Education
Award Identifier / Grant number: 21A150016
Funding source: Innovative Funds Plan of Henan University of Technology
Award Identifier / Grant number: 2020ZKCJ29
Funding source: Fundamental Research Funds for the Henan Provincial Colleges and Universities in Henan University of Technology
Award Identifier / Grant number: 2017RCJH08
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by the Natural Science Foundation in Henan Province Department of Education (No. 21A150016), the Innovative Funds Plan of Henan University of Technology (No. 2020ZKCJ29), and the Fundamental Research Funds for the Henan Provincial Colleges and Universities in Henan University of Technology (No. 2017RCJH08).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Cai, Y. S., Guo, Y. W., Krohn, K. Nat. Prod. Rep. 2010, 27, 1840–1870; https://doi.org/10.1039/c0np00031k.Suche in Google Scholar PubMed
2. Gravel, E., Poupon, E. Nat. Prod. Rep. 2010, 27, 32–56; https://doi.org/10.1039/b911866g.Suche in Google Scholar PubMed
3. Antunes, E. M., Copp, B. R., Davier-Coleman, M. T., Samaai, T. Nat. Prod. Rep. 2005, 22, 62–72; https://doi.org/10.1039/b407299p.Suche in Google Scholar PubMed
4. Yoneda, K., Yamagata, E., Nakanishi, T., Nagashima, T., Kawasaki, I., Yoshida, T., Mori, H., Miura, I. Photochemistry 1984, 23, 2068–2069; https://doi.org/10.1016/s0031-9422(00)84976-6.Suche in Google Scholar
5. Yugandhar, D., Nayak, V. L., Archana, S., Shekar, K. C., Srivastava, A. K. Eur. J. Med. Chem. 2015, 101, 348–357; https://doi.org/10.1016/j.ejmech.2015.06.050.Suche in Google Scholar PubMed
6. Winkler, M., Maynadier, M., Wein, S., Lespinasse, M., Boumis, G., Miele, A. E., Vial, H., Wong, Y. Org. Biomol. Chem. 2015, 13, 2064–2077; https://doi.org/10.1039/c4ob02459a.Suche in Google Scholar PubMed
7. Leon, R., Jawalekar, A., Redert, T., Gaunt, M. J. Chem. Sci. 2011, 2, 1487–1490; https://doi.org/10.1039/c1sc00218j.Suche in Google Scholar
8. Jia, M. Q., You, S. L. Chem. Commun. 2012, 48, 6363–6636; https://doi.org/10.1039/c2cc32783j.Suche in Google Scholar PubMed
9. Roche, S. T., Porco, J. A. Angew. Chem. Int. Ed. 2011, 50, 4068–4409; https://doi.org/10.1002/anie.201006017.Suche in Google Scholar PubMed PubMed Central
10. Zhuo, C. X., Zhang, W., You, S. L. Angew. Chem. Int. Ed. 2012, 51, 12662–12686; https://doi.org/10.1002/anie.201204822.Suche in Google Scholar PubMed
11. Reddy, C. R., Prajapti, S. K., Warudikar, K., Ranjan, R., Rao, B. B. Org. Biomol. Chem. 2017, 15, 3130–3051; https://doi.org/10.1039/c7ob00405b.Suche in Google Scholar PubMed
12. Dohi, T., Maruyama, A., Yoshimura, M., Morimoto, K., Tohma, H., Kita, Y. Angew. Chem. Int. Ed. 2005, 44, 6193–9196; https://doi.org/10.1002/anie.200501688.Suche in Google Scholar PubMed
13. Magdziak, D., Meek, S. J., Pettus, T. R. R. Chem. Rev. 2004, 104, 1383–1429; https://doi.org/10.1021/cr0306900.Suche in Google Scholar PubMed
14. Pouységu, L., Deffieux, D., Quideau, S. Tetrahedron 2010, 66, 2235–2261.10.1016/j.tet.2009.12.046Suche in Google Scholar
15. Yugandhar, D., Kuriakose, S., Nanubolu, J. B., Srivastava, A. K. Org. Lett. 2016, 18, 1040–1043; https://doi.org/10.1021/acs.orglett.6b00164.Suche in Google Scholar PubMed
16. Yu, Q. F., Zhang, Y. H., Yin, W., Tang, B. X., Tang, R. Y., Zhang, P., Li, J. H. J. Org. Chem. 2008, 73, 3568–3661; https://doi.org/10.1021/jo800328a.Suche in Google Scholar PubMed
17. Yin, Q., You, S. L. Org. Lett. 2012, 14, 3526–3529; https://doi.org/10.1021/ol301531z.Suche in Google Scholar PubMed
18. Wang, L. J., Zhu, H. T., Qiu, Y. F., Liu, X. Y., Liang, Y. M. Org. Biomol. Chem. 2014, 12, 643–650; https://doi.org/10.1039/c3ob42020e.Suche in Google Scholar PubMed
19. Tnay, Y. L., Chen, C., Chua, Y. Y., Zhang, L., Chiba, S. Org. Lett. 2012, 14, 3550–3553; https://doi.org/10.1021/ol301583y.Suche in Google Scholar PubMed
20. Wu, Q. F., Liu, W. B., Zhuo, C. X., Rong, Z. Q., Ye, K. Y., You, S. L. Angew. Chem. Int. Ed. 2011, 50, 4455–4458; https://doi.org/10.1002/anie.201100206.Suche in Google Scholar PubMed
21. Rousseaux, S., García-ortanet, J., Del Aguila Sanchez, M. A., Buchwald, S. L. J. Am. Chem. Soc. 2011, 133, 9282–9285; https://doi.org/10.1021/ja203644q.Suche in Google Scholar PubMed PubMed Central
22. Pigge, F. C., Coniglio, J. J., Dalvi, R. J. Am. Chem. Soc. 2006, 128, 3498–3499; https://doi.org/10.1021/ja058342y.Suche in Google Scholar PubMed
23. Matsuura, B. S., Condie, A. G., Buff, R. C., Karahalis, G. J., Stephenson, C. R. J. Org. Lett. 2011, 13, 6320–6632; https://doi.org/10.1021/ol202881q.Suche in Google Scholar PubMed PubMed Central
24. Bansode, A. H., Shaikh, S. R., Gonnade, R. G., Patil, N. T. Chem. Commun. 2017, 53, 9081–9084; https://doi.org/10.1039/c7cc04010e.Suche in Google Scholar PubMed
25. Zheng, D., Yu, J., Wu, J. Angew. Chem. Int. Ed. 2016, 55, 11925–11929; https://doi.org/10.1002/anie.201607292.Suche in Google Scholar PubMed
26. Yang, X. L., Long, Y., Chen, F., Han, B. Org. Chem. Front. 2016, 3, 184–189; https://doi.org/10.1039/c5qo00352k.Suche in Google Scholar
27. Ouyang, X. H., Song, R. J., Liu, B., Li, J. H. Chem. Commun. 2016, 52, 2573–2576; https://doi.org/10.1039/c5cc08952b.Suche in Google Scholar PubMed
28. Ni, S., Cao, J., Mei, H., Han, J., Li, S., Pan, Y. Green Chem. 2016, 18, 3935–3939; https://doi.org/10.1039/c6gc01027j.Suche in Google Scholar
29. Schumacher, R. F., Rosario, A. R., Souza, A. C. G., Menezes, P. H., Zeni, G. Org. Lett. 2010, 12, 1952–1955; https://doi.org/10.1021/ol1003753.Suche in Google Scholar PubMed
30. Godoi, B., Schumacher, R. F., Zeni, G. Chem. Rev. 2011, 111, 2937–2980; https://doi.org/10.1021/cr100214d.Suche in Google Scholar PubMed
31. Zhang, X., Larock, R. C. J. Am. Chem. Soc. 2005, 127, 12230–12231; https://doi.org/10.1021/ja053079m.Suche in Google Scholar PubMed
32. Tang, B. X., Tang, D. J., Tang, S., Yu, O. F., Zhang, Y. H., Liang, Y., Zhong, P., Li, J. H. Org. Lett. 2008, 10, 1063–1066; https://doi.org/10.1021/ol703050z.Suche in Google Scholar PubMed
33. Wen, J., Wei, W., Xue, S., Yang, D., Lou, Y., Gao, C., Wang, H. J. Org. Chem. 2015, 80, 4966–4972; https://doi.org/10.1021/acs.joc.5b00361.Suche in Google Scholar PubMed
34. Hua, H. L., He, Y. T., Qiu, Y. F., Li, Y. X., song, B., Gao, P., Song, X. R., Guo, D. H., Liu, X. Y., Liang, Y. M. Chem. Eur. J. 2015, 21, 1468–1473; https://doi.org/10.1002/chem.201405672.Suche in Google Scholar PubMed
35. Yang, W. C., Zhang, M. M., Feng, J. G. Adv. Synth. Catal. 2020, 362, 4446–4461; https://doi.org/10.1002/adsc.202000636.Suche in Google Scholar
36. Tang, B. X., Zhang, Y. H., Song, R. J., Tang, D. J., Deng, G. B., Wang, Z. Q., Xie, Y. X., Xia, Y. Z., Li, J. H. J. Org. Chem. 2012, 77, 2837–2849; https://doi.org/10.1021/jo300037n.Suche in Google Scholar PubMed
37. Yu, Q. F., Zhang, Y. H., Yin, Q., Tang, B. X., Tang, R. Y., Zhang, P., Li, J. H. J. Org. Chem. 2008, 73, 3658–3661; https://doi.org/10.1021/jo800328a.Suche in Google Scholar PubMed
38. Liu, T., Li, Y., Jiang, L., Wang, J., Jin, K., Zhang, R., Duan, C. Org. Biomol. Chem. 2020, 18, 1933–1939; https://doi.org/10.1039/d0ob00057d.Suche in Google Scholar PubMed
39. Nair, A. M., Shinde, A. H., Kumar, S., Volla, C. M. R. Chem. Commun. 2020, 56, 12367–12370; https://doi.org/10.1039/d0cc04800c.Suche in Google Scholar PubMed
40. Tang, B. X., Yin, Q., Tang, R. Y., Li, J. H. J. Org. Chem. 2008, 73, 9008–9011; https://doi.org/10.1021/jo8018297.Suche in Google Scholar PubMed
41. Yu, K., Kong, X., Yang, J., Li, G., Xu, B., Chen, Q. J. Org. Chem. 2021, 86, 917–928; https://doi.org/10.1021/acs.joc.0c02429.Suche in Google Scholar PubMed
42. Chen, P., Xie, J., Chen, Z., Xiong, B. Q., Liu, Y., Yang, C. A., Tang, K. W. Adv. Synth. Catal. 2021, 363, 4440–4446; https://doi.org/10.1002/adsc.202100852.Suche in Google Scholar
43. Ouyang, X. H., Song, R. J., Li, Y., Liu, B., Li, J. H. J. Org. Chem. 2014, 79, 4582–4589; https://doi.org/10.1021/jo5005982.Suche in Google Scholar PubMed
44. Liu, Y., Wang, Q. L., Zhou, C. S., Xiong, B. Q., Zhang, P. L., Yang, C., Tang, K. W. J. Org. Chem. 2018, 83, 2210–2218; https://doi.org/10.1021/acs.joc.7b03104.Suche in Google Scholar PubMed
45. Reddy, C. R., Kolgave, D. H., Subbarao, M., Aila, M., Prajapti, S. K. Org. Lett. 2020, 22, 5342–5346; https://doi.org/10.1021/acs.orglett.0c01588.Suche in Google Scholar PubMed
46. Wei, W. T., Song, R. J., Ouyang, X. H., Li, Y., Li, H. B., Li, J. H. Org. Chem. Front. 2014, 1, 484–489; https://doi.org/10.1039/c4qo00006d.Suche in Google Scholar
47. Wang, C. S., Roisnel, T., Dixneuf, P. H., Soulé, J. F. Adv. Synth. Catal. 2019, 361, 445–450; https://doi.org/10.1002/adsc.201801203.Suche in Google Scholar
48. Manna, S., Ashwathappa, P. K. S., Prabhu, K. R. Chem. Commun. 2020, 56, 13165–13168; https://doi.org/10.1039/d0cc01217c.Suche in Google Scholar PubMed
49. Li, M., Song, R. J., Li, J. H. Chin. J. Chem. 2017, 35, 299–302; https://doi.org/10.1002/cjoc.201600749.Suche in Google Scholar
50. Reddy, C. R., Yarlagadda, S., Ramesh, B., Reddy, M. R., Sridhar, B., Reddy, B. V. S. Eur. J. Org. Chem. 2017, 2017, 2332–2337; https://doi.org/10.1002/ejoc.201700058.Suche in Google Scholar
51. Wang, L. J., Wang, A. Q., Xia, Y., Wu, X. X., Liu, X. Y., Liang, Y. M. Chem. Commun. 2014, 50, 13998–14001; https://doi.org/10.1039/c4cc06923d.Suche in Google Scholar PubMed
52. Zeng, F. L., Chen, X. L., Sun, K., Zhu, H. L., Yuan, X. Y., Liu, Y., Qu, L. B., Zhao, Y. F., Yu, B. Org. Chem. Front. 2021, 8, 760–766; https://doi.org/10.1039/d0qo01410a.Suche in Google Scholar
53. Yang, X. H., Ouyang, X. H., Wei, W. T., Song, R. J., Li, J. H. Adv. Synth. Catal. 2015, 357, 1161–1166; https://doi.org/10.1002/adsc.201400895.Suche in Google Scholar
54. Chen, Y., Chen, Y. J., Guan, Z., He, Y. H. Tetrahedron 2019, 75, 130763; https://doi.org/10.1016/j.tet.2019.130763.Suche in Google Scholar
55. Wu, L. J., Tan, F. L., Li, M., Song, R. J., Li, J. H. Org. Chem. Front. 2017, 4, 350–353; https://doi.org/10.1039/c6qo00691d.Suche in Google Scholar
56. Gao, P., Zhang, W., Zhang, Z. Org. Lett. 2016, 18, 5820–5823; https://doi.org/10.1021/acs.orglett.6b02781.Suche in Google Scholar PubMed
57. Nair, A. M., Halder, I., Khan, S., Volla, C. M. R. Adv. Synth. Catal. 2020, 362, 224–229; https://doi.org/10.1002/adsc.201901321.Suche in Google Scholar
58. Liu, Y., Wang, Q. L., Xiong, B. Q., Zhang, P. L., Yang, C. A., Gong, Y. X., Liao, J., Zhou, Q. Synlett 2018, 29, 2396–2403; https://doi.org/10.1055/s-0037-1609948.Suche in Google Scholar
59. Liu, Y., Wang, Q. L., Chen, Z., Zhou, Q., Xiong, B. Q., Zhang, P. L., Tang, K. W. Chem. Commun. 2019, 55, 12212–12215; https://doi.org/10.1039/c9cc05949k.Suche in Google Scholar PubMed
60. Jin, D. P., Gao, P., Chen, D. Q., Chen, S., Wang, J., Liu, X. Y., Liang, Y. M. Org. Lett. 2016, 18, 3486–3489; https://doi.org/10.1021/acs.orglett.6b01702.Suche in Google Scholar PubMed
61. Brutchey, R. L. Acc. Chem. Res. 2015, 48, 2918–2926; https://doi.org/10.1021/acs.accounts.5b00362.Suche in Google Scholar PubMed
62. Modha, S. G., Mehtab, V. P., der Eycken, E. V. V. Chem. Soc. Rev. 2013, 42, 5042–5055; https://doi.org/10.1039/c3cs60041f.Suche in Google Scholar PubMed
63. Fourmigue, M., Dhaka, A. Coord. Chem. Rev. 2020, 403, 213084–213100; https://doi.org/10.1016/j.ccr.2019.213084.Suche in Google Scholar
64. Chen, Z., Lai, H., Hou, L., Chen, T. Chem. Commun. 2020, 56, 179–196; https://doi.org/10.1039/c9cc07683b.Suche in Google Scholar PubMed
65. Frieben, E. E., Amin, S., Sharma, A. K. J. Med. Chem. 2019, 62, 5261–5275; https://doi.org/10.1021/acs.jmedchem.8b01698.Suche in Google Scholar PubMed
66. Csonka, A., Kincses, A., Nove, M., Vadas, Z., Spengler, G., Csonka, A., Sanmartin, C., Sanmartin, C., Dominguez-Alvarez, E. Anticancer Res. 2019, 39, 3777–3783; https://doi.org/10.21873/anticanres.13526.Suche in Google Scholar PubMed
67. Vahter, J., Viht, K., Uri, A., Manoharan, G. B., Enkvist, E. Bioorg. Med. Chem. 2018, 26, 5062–5068; https://doi.org/10.1016/j.bmc.2018.09.003.Suche in Google Scholar PubMed
68. Tian, M., Yang, Y., Avila, F. W., Fish, T., Yuan, H., Hui, M., Thannhauser, T. W., Li, L., Tian, M., Pan, S. J. Agric. Food Chem. 2018, 66, 8036–8044; https://doi.org/10.1021/acs.jafc.8b03396.Suche in Google Scholar PubMed
69. Wang, X. Y., Zhong, Y. F., Mo, Z. Y., Wu, S. H., Xu, Y. L., Tang, H. T., Pan, Y. M. Adv. Synth. Catal. 2021, 363, 208–214; https://doi.org/10.1002/adsc.202001192.Suche in Google Scholar
70. Song, Z., Ding, C., Wang, S., Dai, Q., Sheng, Y., Zheng, Z., Liang, G. Chem. Commun. 2020, 56, 1847–1850; https://doi.org/10.1039/c9cc09001k.Suche in Google Scholar PubMed
71. Ai, Z., Xiao, J., Li, Y., Guo, B., Du, Y., Zhao, K. Org. Chem. Front. 2020, 7, 3935–3940; https://doi.org/10.1039/d0qo01175d.Suche in Google Scholar
72. Ghosh, P., Chhetri, G., Perl, E., Das, S. Adv. Synth. Catal. 2021, 363, 2148–2156; https://doi.org/10.1002/adsc.202001426.Suche in Google Scholar
73. Sun, K., Wang, S., Feng, R., Zhang, Y., Wang, X., Zhang, Z., Zhang, B. Org. Lett. 2019, 21, 2052–2055; https://doi.org/10.1021/acs.orglett.9b00240.Suche in Google Scholar PubMed
74. Kosso, A. R. O., Kabri, Y., Broggi, J., Redon, S., Vanelle, P. J. Org. Chem. 2020, 85, 3071–3081; https://doi.org/10.1021/acs.joc.9b02963.Suche in Google Scholar PubMed
75. Brahmachari, G., Bhowmick, A., Karmakar, I. J. Org. Chem. 2021, 86, 9658–9669; https://doi.org/10.1021/acs.joc.1c00919.Suche in Google Scholar PubMed
76. Cui, H., Wei, W., Yang, D., Zhang, J., Xu, Z., Wen, J., Wang, H. RSC Adv. 2015, 5, 84657–84661; https://doi.org/10.1039/c5ra16548b.Suche in Google Scholar
77. Qian, P. C., Liu, Y., Song, R. J., Xiang, J. N., Li, J. H. Synlett 2015, 26, 1213–1216.10.1055/s-0034-1380573Suche in Google Scholar
78. Skubi, K. L., Blum, T. R., Yoon, T. P. Chem. Rev. 2016, 116, 10035–10074; https://doi.org/10.1021/acs.chemrev.6b00018.Suche in Google Scholar PubMed PubMed Central
79. Romero, N. A., Nicewicz, D. A. Chem. Rev. 2016, 116, 10075–10166; https://doi.org/10.1021/acs.chemrev.6b00057.Suche in Google Scholar PubMed
80. Xuan, J., Xiao, W. J. Angew. Chem. Int. Ed. 2012, 51, 6828–6838; https://doi.org/10.1002/anie.201200223.Suche in Google Scholar PubMed
81. Nicewicz, D. A., MacMillan, D. W. C. Science 2008, 322, 77–80; https://doi.org/10.1126/science.1161976.Suche in Google Scholar PubMed PubMed Central
82. Yoon, T. P., Ischay, M. A. Du J. Nat. Chem. 2010, 2, 527–532; https://doi.org/10.1038/nchem.687.Suche in Google Scholar PubMed
83. Shi, L., Xia, W. Chem. Soc. Rev. 2012, 41, 7687–7697; https://doi.org/10.1039/c2cs35203f.Suche in Google Scholar PubMed
84. Wei, W., Cui, H., Yang, D., Yue, H., He, C., Zhang, Y., Wang, H. Green Chem. 2017, 19, 5608–5613; https://doi.org/10.1039/c7gc02330h.Suche in Google Scholar
85. Sahoo, H., Mandal, A., Dana, S., Baidya, M. Adv. Synth. Catal. 2018, 360, 1099–1103; https://doi.org/10.1002/adsc.201701410.Suche in Google Scholar
86. Zhang, N., Zuo, H., Xu, C., Pan, J., Sun, J., Guo, C. Chin. Chem. Lett. 2020, 31, 337–340; https://doi.org/10.1016/j.cclet.2019.06.008.Suche in Google Scholar
87. Wang, H., Gao, X., Lv, Z., Abdelilah, T., Lei, A. Chem. Rev. 2019, 119, 6769–6787; https://doi.org/10.1021/acs.chemrev.9b00045.Suche in Google Scholar PubMed
88. Moeller, K. D. Chem. Rev. 2018, 118, 4817–4833; https://doi.org/10.1021/acs.chemrev.7b00656.Suche in Google Scholar PubMed
89. Yan, M., Kawamata, Y., Baran, P. S. Chem. Rev. 2017, 117, 13230–13319; https://doi.org/10.1021/acs.chemrev.7b00397.Suche in Google Scholar PubMed PubMed Central
90. Waldvogel, S. R., Lips, S., Selt, M., Riehl, B., Kampf, C. J. Chem. Rev. 2018, 118, 6706–6765; https://doi.org/10.1021/acs.chemrev.8b00233.Suche in Google Scholar PubMed
91. Zhao, Y., Xia, W. Chem. Soc. Rev. 2018, 47, 2591–2608; https://doi.org/10.1039/c7cs00572e.Suche in Google Scholar PubMed
92. Yan, M., Kawamata, Y., Baran, P. S. Angew. Chem. Int. Ed. 2018, 57, 4149–4155; https://doi.org/10.1002/anie.201707584.Suche in Google Scholar PubMed PubMed Central
93. Hua, J., Fang, Z., Bian, M., Ma, T., Yang, M., Xu, J., Liu, C. K., He, W., Zhu, N., Yang, Z., Guo, K. ChemSusChem 2020, 13, 2053–2059; https://doi.org/10.1002/cssc.202000098.Suche in Google Scholar PubMed
94. Recchi, A. M. S., Rosa, P. H. P., Back, D. F., Zeni, G. Org. Biomol. Chem. 2020, 18, 3544–3551; https://doi.org/10.1039/d0ob00609b.Suche in Google Scholar PubMed
95. Sahoo, H., Grandhi, G. S., Ramakrishna, I., Baidya, M. Org. Biomol. Chem. 2019, 17, 10163–10166; https://doi.org/10.1039/c9ob02177a.Suche in Google Scholar PubMed
96. Nyffeler, P. T., Durón, S. G., Burkart, M. D., Vincent, S. P., Wong, C. H. Angew. Chem. Int. Ed. 2005, 44, 192–212; https://doi.org/10.1002/anie.200400648.Suche in Google Scholar PubMed
97. Liu, P., Gao, Y., Gu, W., Shen, Z., Sun, P. J. Org. Chem. 2015, 80, 11559–11565; https://doi.org/10.1021/acs.joc.5b01961.Suche in Google Scholar PubMed
98. Li, J. L., Lin, E., Han, X. L., Li, Q., Wang, H. Org. Lett. 2019, 21, 4255–4258; https://doi.org/10.1021/acs.orglett.9b01428.Suche in Google Scholar PubMed
99. Xu, P., Guo, S., Wang, L., Tang, P. Angew. Chem., Int. Ed. 2014, 53, 5955–5958; https://doi.org/10.1002/anie.201400225.Suche in Google Scholar PubMed
100. Xie, L. Y., Qu, J., Peng, S., Liu, K. J., Wang, Z., Ding, M. H., Wang, Y., Cao, Z., He, W. M. Green Chem. 2018, 20, 760–764; https://doi.org/10.1039/c7gc03106h.Suche in Google Scholar
101. Ban, Y. L., You, L., Feng, K. W., Ma, F. C., Jin, X. L., Liu, Q. J. Org. Chem. 2021, 86, 5274–5283; https://doi.org/10.1021/acs.joc.1c00167.Suche in Google Scholar PubMed
102. Yang, L., Ma, Y., Song, F., You, J. Chem. Commun. 2014, 50, 3024–3026; https://doi.org/10.1039/c3cc49851d.Suche in Google Scholar PubMed
103. Niu, L., Liu, J., Liang, X. A., Wang, S., Lei, A. Nat. Commun. 2019, 10, 1–7; https://doi.org/10.1038/s41467-019-08413-9.Suche in Google Scholar PubMed PubMed Central
104. Xavier, M. C. D. F., Sandagorda, E. M. A., Neto, J. S. S., Schumacher, R. F., Silva, M. S. RSC Adv. 2020, 10, 13975–13983; https://doi.org/10.1039/d0ra01907k.Suche in Google Scholar PubMed PubMed Central
105. Xie, L. Y., Peng, S., Liu, F., Yi, J. Y., Wang, M., Tang, Z., Xu, X., He, W. M. Adv. Synth. Catal. 2018, 360, 4259–4264; https://doi.org/10.1002/adsc.201800918.Suche in Google Scholar
106. Kong, Y., Sun, X., Weng, J. Chin. J. Org. Chem. 2020, 40, 2641–2657; https://doi.org/10.6023/cjoc202004005.Suche in Google Scholar
107. Wang, X., Wang, Q., Xue, Y., Sun, K., Wu, L., Zhang, B. Chem. Commun. 2020, 56, 4436–4439; https://doi.org/10.1039/d0cc01079k.Suche in Google Scholar PubMed
108. Galloway, J. D., Mai, D. N., Baxter, R. D. Org. Lett. 2017, 19, 5772–5775; https://doi.org/10.1021/acs.orglett.7b02706.Suche in Google Scholar PubMed
109. Liang, X. A., Niu, L., Wang, S., Liu, J., Lei, A. Org. Lett. 2019, 21, 2441–2444; https://doi.org/10.1021/acs.orglett.9b00744.Suche in Google Scholar PubMed
110. Kong, Y., Yu, W., Liu, X., Weng, J. Chin. Chem. Lett. 2020, 31, 3245–3249; https://doi.org/10.1016/j.cclet.2020.05.022.Suche in Google Scholar
111. Fei, H., Xu, Z., Wu, H., Zhu, L., Jalani, H. B., Li, G., Fu, Y., Lu, H. Org. Lett. 2020, 22, 2651–2656; https://doi.org/10.1021/acs.orglett.0c00620.Suche in Google Scholar PubMed
112. Zhao, H., Jin, J. Org. Lett. 2019, 21, 6179–6184; https://doi.org/10.1021/acs.orglett.9b01635.Suche in Google Scholar PubMed
113. Mai, W. P., Yuan, J. W., Zhu, J. L., Li, Q. Q., Yang, L. R., Xiao, Y. M., Mao, P., Qu, L. B. ChemistrySelect 2019, 4, 11066–11070; https://doi.org/10.1002/slct.201903478.Suche in Google Scholar
114. Yuan, J., Zeng, F., Mai, W., Yang, L., Xiao, Y., Mao, P., Wei, D. Org. Biomol. Chem. 2019, 17, 5038–5046; https://doi.org/10.1039/c9ob00509a.Suche in Google Scholar PubMed
115. Zhang, J. R., Liu, H. Y., Fan, T., Chen, Y. Y., Xu, Y. L. Adv. Synth. Catal. 2021, 363, 497–504; https://doi.org/10.1002/adsc.202000983.Suche in Google Scholar
116. Belladona, A. L., Cervo, R., Alves, D., Barcellos, T., Cargnelutti, R., Schumacher, R. F. Tetrahedron Lett. 2020, 61, 152035; https://doi.org/10.1016/j.tetlet.2020.152035.Suche in Google Scholar
Supplementary Material
The online version of this article offers supplementary material (https://doi.org/10.1515/znb-2021-0154).
© 2021 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- In this issue
- Research Articles
- A new phenanthrene derivative from Entada abyssinica with antimicrobial and antioxidant properties
- Antileishmanial, antibacterial and cytotoxicity activity of the extracts, fractions, and compounds from the fruits and stem bark extracts of Pentadesma butyracea Sabine
- Two 2D Co(II)/Mn(II) coordination polymers based on the quinoline-2,3-dicarboxylate ligand: synthesis, crystal structure, and fluorescence properties
- Synthesis, hydrogen bond interactions and crystal structure elucidation of some stable 2H-imidazolium salts
- Molecular and crystal structure of a copper(II) complex of sildenafil
- A convenient approach for the electrochemical bromination and iodination of pyrazoles
- Furanone-functionalized benzothiazole derivatives: synthesis, in vitro cytotoxicity, ADME, and molecular docking studies
- Si⋯O proximity in imidosilanes – absence of orbital interactions
- A new luminescent metal-organic framework with 2,6-di(1H-imidazol-1-yl)naphthalene and biphenyl-3,4′,5-tricarboxylic acid
- Chalcogenative spirocyclization of N-aryl propiolamides with diselenides/disulfides promoted by Selectfluor
- [Msim]CuCl3: as an efficient catalyst for the preparation of 5-amino-1H-pyrazole-4-carbonitriles by anomeric based oxidation
- Synthesis and structure of an asymmetrical sila[1]magnesocenophane
Artikel in diesem Heft
- Frontmatter
- In this issue
- Research Articles
- A new phenanthrene derivative from Entada abyssinica with antimicrobial and antioxidant properties
- Antileishmanial, antibacterial and cytotoxicity activity of the extracts, fractions, and compounds from the fruits and stem bark extracts of Pentadesma butyracea Sabine
- Two 2D Co(II)/Mn(II) coordination polymers based on the quinoline-2,3-dicarboxylate ligand: synthesis, crystal structure, and fluorescence properties
- Synthesis, hydrogen bond interactions and crystal structure elucidation of some stable 2H-imidazolium salts
- Molecular and crystal structure of a copper(II) complex of sildenafil
- A convenient approach for the electrochemical bromination and iodination of pyrazoles
- Furanone-functionalized benzothiazole derivatives: synthesis, in vitro cytotoxicity, ADME, and molecular docking studies
- Si⋯O proximity in imidosilanes – absence of orbital interactions
- A new luminescent metal-organic framework with 2,6-di(1H-imidazol-1-yl)naphthalene and biphenyl-3,4′,5-tricarboxylic acid
- Chalcogenative spirocyclization of N-aryl propiolamides with diselenides/disulfides promoted by Selectfluor
- [Msim]CuCl3: as an efficient catalyst for the preparation of 5-amino-1H-pyrazole-4-carbonitriles by anomeric based oxidation
- Synthesis and structure of an asymmetrical sila[1]magnesocenophane

