Gingival status and prophylactic oral hygiene measures modulate salivary amino acids’ profile in children with plaque-induced gingivitis
-
Bistra Tzaneva Galunska
, Ayshe Seyhan Salim
, Miglena Nikolaeva Nikolova
, Sirma Todorova Angelova
, Yoana Dimitrova Kiselova-Kaneva
, Stefan Vasilev Peev
und Diana Georgieva Ivanova
Abstract
Objectives
Plaque-induced gingivitis is one of the most widely distributed periodontal disorder during childhood. The control of the pathogenic potential of the plaque is associated with oral hygiene status at individual, group, and population levels. We hypothesize that regular application of complex oral hygiene-prophylaxis could beneficially modulate salivary amino acids profile in children with different stage of plaque-induced gingivitis. Therefore, we aimed to study the salivary amino acids’ profile in relation to certain clinical indicators and environmental variables for plaque-induced gingivitis in children.
Methods
Fifty children (29 girls, 21 boys; mean age 8.18 ± 2.32 years) without anamnestic data for common diseases, no medication, and no data for allergy were selected. Plaque and gingival indexes were determined for assessment oral hygiene and plaque accumulation. Unstimulated whole saliva was collected, centrifuged and supernatants stored at −80 °C. Amino acid analysis was performed by liquid chromatography using analytical grade AccQ·Tag-Ultra-derivatization kit.
Results
Gingivitis was indicated in most of the examined children over 6 years. More than half (63.6 %) of them revealed moderate stage of the disease and a tendency to satisfactory good oral hygiene and degree of gingival inflammation. Salivary glycine, proline, arginine, serine, lysine, aspartate, glutamate, threonine, methionine, and isoleucine were higher in gingivitis children, while cysteine, tyrosine and phenylalanine decrease. In gingivitis children without regular oral hygiene-prophylaxis, some structural amino acids like glycine and proline were increased, while amino acids with protective antioxidant potential like cysteine were diminished.
Conclusions
Plaque-induced gingivitis is associated with increased salivary levels of certain amino acids. These may serve as distinguishing markers among children with gingivitis.
Introduction
Saliva, as a fluid component of the oral cavity, plays a definite role in the maintenance of the dynamic equilibrium of homeostasis, related to the functionality of hard teeth structures, oral mucosa and periodontal tissues. Proteins, glycoproteins and enzymes are among the basic ingredients of saliva with an explicit antimicrobial activity [1].
Sterile saliva obtained from the glands contains not all known amino acids, but only a few of them [2]. Saliva is characterized as a transudate of blood plasma. As a result of the intensive exchange between these body fluids, the characteristics of the diet affect the free amino acid composition of saliva. Several studies demonstrate that in the case of oral inflammatory diseases, the predominant portion of salivary free amino acids originates from proteolytic degradation of salivary proteins by proteases with microbial or endogenous origin [3], [4], [5], [6].
Plaque-induced gingivitis is determined as a reversible inflammation of gingival soft tissues as a consequence of human organisms’ reactivity towards dental-plaque microorganisms and their metabolic products [7]. It is one of the most widely distributed periodontal disorder in different periods of childhood. The etiology of that oral-health disorder is mainly associated with dental plaque [8]. The control of the pathogenic potential of the plaque is associated with the oral hygiene status at individual, group, and population levels [9]. The adequate, age-related individual and professional oral hygiene cares reduce the accumulation of dental plaque and diminish its pathogenic capacity [10]. That effect is enhanced by the application of different forms and means of exogenous fluoride in accordance with the age specifics [11]. The ultimate goal is to reduce the risk of inflammation of gingival tissues.
We hypothesize that regular application of complex oral hygiene care including tooth brushing, topical fluoride prophylaxis, endogenous fluoride prophylaxis, and usage of additional oral hygiene products could beneficially modulate salivary amino acids profile in children with different stage of plaque-induced gingivitis. Therefore, we aimed to study the salivary amino acids’ profile in relation to certain clinical indicators and environmental variables for plaque-induced gingivitis in children.
Materials and methods
Participants
The study included 50 children from both genders (29 girls and 21 boys), aged between 4 and 14 years (mean age 8.18 ± 2.32 years) who visited the University Dental Medicine Center. Clinically healthy children without anamnestic data for common or chronic diseases, no medicine intake, including homeopathic remedies, no anamnestic data for known allergic reactions, no use of toothpaste with arginine or homeopathic ingredients, and no vegan or vegetarians were selected for the study. All participants were tested for periodontitis by registration of periodontal pocket depth. Participants with confirmed periodontitis were excluded from the study (Supplementary Figure 1).
The calculated sample size for the group with plaque-induced gingivitis was 45 and for the control group was 7 (95 % confidence level; α=0.05; SD with a margin of error 0.8, for both groups) [12].
Age and presence of common diseases, allergic reactions, and current use of medications or supplements, including homeopathic therapy were assessed through a structured interview. Participants were divided into two groups based on the registered Gingival Index GI Löe-Silness: control group without gingival inflammation (GI=0; n=8); group with clinically manifested plaque-induced gingivitis (0<GI≤3; n=42).
Gingival status examination
Dental examination to determine gingival and plaque indexes was performed by only one examiner. Conventional diagnostic methods were used for determination both plaque index Silness-Löe (PLI) and gingival index (Löe and Silness) (GI). In brief, Plaque index Silness-Löe was applied for the evaluation of dental plaque accumulation based on its registration on Ramfjörd teeth: 16, 22, 24, 36, 42, 44. In primary and mixed dentition are estimated the respective primary teeth or the mesially located ones. The index was recorded by scratching with an atraumatic periodontal probe on the vestibular, oral, mesial, and distal surfaces along the contact with the marginal gingiva. The degree of dental plaque accumulation was assessed as 0, 1, 2, 3, where 0 corresponds to lack of dental plaque; 1 accounts to a very small amount of dental plaque; 2 is related to a moderate amount of dental plaque; 3 is equivalent to a considerable, naked eye-visible accumulation of dental plaque. The results were scaled as very good to good oral hygiene (0.1<PLI≤1.1); good to satisfactory oral hygiene (1.2≤PLI≤2.0); satisfactory to poor oral hygiene, parallel to the increase of PLI level of (2.1≤PLI≤3.0). The gingival index [13] is applied for the evaluation of the gingival state and indicates alterations in the gingival tissue. GI records the condition of the marginal and interproximal sites separately in the range between 0 and 3, where GI=0 designates normal gingiva; 0<GI≤1.0 corresponds to mild inflammation; 1.1≤GI≤2.0 accounts to moderate inflammation; 2.1≤GI≤3.0 signifies severe inflammation [14]. Caries index is not a subject of our study.
Saliva sample collection
Unstimulated whole saliva sample collection was performed between 9.00 and 11.30 a.m. All participants were instructed to properly brush their teeth just before the sample collection with toothpaste without fluoride and arginine content. Saliva samples were collected in 2 mL sterile polypropylene DNase-and RNase-free collection tubes before the dental examination. The collected saliva samples were centrifuged at 1000×g for 10 min and supernatants were frozen immediately and stored at −80 °C until further analysis.
Oral hygiene prophylaxis
The oral hygiene prophylaxis was evaluated through a questionnaire survey related to the application of topical and/or endogenic fluoride prophylaxis, additional oral hygiene products and measures (interdental brushes, interdental flosses, mouth washes, fluoride-containing tooth pastes) and frequency of tooth brushing (once or twice daily).
Amino acid analysis
Chemicals and reagents
Analytical grade AccQ·Tag Ultra derivatization kit for aminoacids and amino acid mix standard H were obtained from Waters (Millford, MA, USA). Deionized water was prepared with water purification system Milli-Q® IQ 7005 (Merck, Germany).
Preparing standards and samples
The calibration was done by external standard method using amino acid standard mixture containing a 2.5 mM of each of the dissolved in 0.1 N HCl amino acids, except cystine (1.25 mM).
For calibration, a series of working standard solutions with concentrations 25, 50, 100, 200, and 250 nmol/mL corresponding to the concentrations of free amino acids in saliva were prepared.
The saliva samples were thawed and centrifuged at 1000×g for 10 min for protein removal. The supernatants were separated and filtered through 0.2 μm PTFA filter and the filtrates were derivatized as described.
The derivatization procedure was performed using analytical grade AccQ·Tag Ultra derivatization kit for aminoacids (Waters, Millford, MA, USA) following the manufacturer’s protocol.
Chromatographic analysis
The chromatographic analysis was performed on Acquity UPLC PDA-QDa chromatographic system (Waters, Millford, MA, USA). Data were acquired, calibrated and quantified by Waters® Empower 3 Software Chromatography Data (CDS).
The chromatographic separation was performed on AccQ·Tag Ultra RP C18 Column (2.1 × 100 mm, 1.7 μm) using mobile phase A (5 % AccQ·Tag Ultra Eluent A) and mobile phase B (100 % AccQ·Tag Ultra solvent B) in a nonlinear gradient mode: 0.1 % B (0–0.54 min); 9.1–21.2 % B (5.74–7.74 min); 59.6 % B (8.0 min); 90 % B (8.05 min); 0.1 % B (8.73–9.5 min). The column temperature was 55 °C; flow rate 0.7 mL/min, injection volume 1 μL; 2D Channel absorbance mode at 260 nm.
Statistical analyses
Data were presented as mean ± SD, or number (n) and percentage (%), as appropriate. Data analysis was performed on GraphPad Prism v. 8.3., USA and SPSS v. 23, USA. Standard statistical methods such as descriptive statistics, unpaired Student’s t-test for normally distributed parameters and one-way ANOVA were used. Statistical significance was considered at p-value<0.05.
Ethics
The study was approved by the Local Ethics Committee (Protocol №82/28.03.2019). A parent or legal guardian of each participant signed a written informed consent.
Results
General characteristics of the studied population
Most of the examined children were over 6 years of age and represented the presence of gingivitis. More than half (63.6 %) of the gingivitis subjects revealed a moderate stage of the disease (1.1≤GI≤2.0). More than half of the studied children practiced good to satisfactory oral hygiene (1.2≤PLI≤2.0). The most widely practiced teeth prophylactics were topical fluoride application and teeth brushing. Milk and meat proteins dominated in their weekly nutrition regimen (Supplementary Table 1A, B).
Age-related salivary free amino acid variations
Among the participants with plaque-induced gingivitis, four children were up to the age of 6, with primary dentition. Thirty-three participants were between 6 and 14 years of age, respectively with mixed and permanent dentition. In the control group, two children were younger than 6 years of age (with primary dentition) and four children were older than 6 years (mixed and permanent dentition).
The mean value of the plaque index (PLI) among the children over 6 years of age was 1.24-fold significantly higher than the mean PLI value among the subjects under 6 years of age (p-Value=0.018). Similar results were found for the gingival index (GI). It was 1.7-fold significantly higher in the children older than 6 years compared to the subjects under 6 years (p-value=0.046).
Significantly higher mean values for most of the salivary amino acids among the children over the age of 6 years compared to those between 0 and 6 years were established. Six of the elevated amino acids were essential ones (Table 1).
Age dependent variations in salivary amino acid concentrations and gingival indexes.
| Parameter | Age, years | Mean ± SD, mmol/L (n) | p-Value | Parameter | Age, years | Mean ± SD, mmol/L (n) | p-Value |
|---|---|---|---|---|---|---|---|
| HISa | 0–6 | 0.041 ± 0.002 (4) | 0.87 | LYSa | 0–6 | 0.079 ± 0.010 (6) | 0.001 |
| >6 | 0.041 ± 0.004 (18) | >6 | 0.113 ± 0.047 (38) | ||||
| SER | 0–6 | 0.108 ± 0.037 (6) | 0.22 | TYR | 0–6 | 0.132 ± 0.037 (6) | 0.01 |
| >6 | 0.131 ± 0.060 (36) | >6 | 0.198 ± 0.120 (38) | ||||
| ARG | 0–6 | 0.181 ± 0.069 (6) | 0.92 | METa | 0–6 | 0.031 ± 0.001 (6) | 0.001 |
| >6 | 0.184 ± 0.111 (38) | >6 | 0.036 ± 0.007 (38) | ||||
| GLY | 0–6 | 0.198 ± 0.037 (6) | 0.001 | VALa | 0–6 | 0.043 ± 0.009 (6) | 0.003 |
| >6 | 0.317 ± 0.174 (38) | >6 | 0.066 ± 0.038 (38) | ||||
| ASP | 0–6 | 0.020 ± 0.010 (6) | 0.22 | ILEa | 0–6 | 0.041 ± 0.006 (6) | 0.01 |
| >6 | 0.026 ± 0.012 (36) | >6 | 0.052 ± 0.021 (38) | ||||
| GLU | 0–6 | 0.072 ± 0.005 (6) | 0.005 | LEUa | 0–6 | 0.051 ± 0.011 (6) | 0.01 |
| >6 | 0.110 ± 0.077 (38) | >6 | 0.071 ± 0.039 (38) | ||||
| THRa | 0–6 | 0.039 ± 0.008 (6) | 0.03 | PHEa | 0–6 | 0.063 ± 0.018 (6) | 0.51 |
| >6 | 0.050 ± 0.021 (38) | >6 | 0.069 ± 0.029 (38) | ||||
| ALA | 0–6 | 0.069 ± 0.021 (6) | 0.04 | PLI | 0–6 | 1.203 ± 0.2669 (9) | 0.02 |
| >6 | 0.095 ± 0.044 (36) | >6 | 1.496 ± 0.445 (41) | ||||
| PRO | 0–6 | 0.207 ± 0.058 (6) | 0.03 | GI | 0–6 | 0.624 ± 0.754 (9) | 0.05 |
| >6 | 0.294 ± 0.168 (38) | >6 | 1.233 ± 0.641 (41) | ||||
| CYS | 0–6 | 0.018 ± 0.025 (6) | 0.54 | ||||
| >6 | 0.012 ± 0.007 (38) |
-
aEssential amino acids; His, histidine; Ser, serine; Arg, arginine; Gly, glycine; Asp, aspartate; Glu, glutamate; Thr, threonine; Ala, alanine; Pro, proline; Cys, cysteine; Lys, lysine; Tyr, tyrosine; Met, methionine; Val, valine; Ile, isoleucine; Leu, leucine; Phe, phenylalanine; PLI, plaque index; GI, gingival index; statistical significance was indicated at p-value<0.05; n, number of studied participants.
Salivary free amino acid levels according to gingival status
Although nonsignificant, the mean values of glycine, proline, arginine, serine, lysine, aspartate, glutamate, threonine, methionine, and isoleucine concentrations in saliva were higher in the group with plaque-induced gingivitis, while cysteine, tyrosine, and phenylalanine were decreased. Salivary cysteine revealed the lowest mean values for both studied groups (plaque-induced gingivitis and controls), while salivary glycine and proline were with the highest mean values. Glycine and proline were elevated in the gingivitis group vs. controls (by 10 and 8 %, respectively), while cysteine was decreased by 42 % compared to the controls (Figure 1).

Amino acid concentrations in saliva of children with plaque-induced gingivitis and healthy controls. Data are given as mean ± SD; AA, amino acids; His, histidine; Ser, serine; Arg, arginine; Gly, glycine; Asp, aspartate; Glu, glutamate; Thr, threonine; Ala, alanine; Pro, proline; Cys, cysteine; Lys, lysine; Tyr, tyrosine; Met, methionine; Val, valine; Ile, isoleucine; Leu, leucine; Phe, phenylalanine.
Regarding the stage of gingivitis, the participants were stratified into three groups – mild (0<GI ≤1.0), moderate (1.1≤GI≤2.0), and severe (2.1≤GI≤3.0) gingivitis. We did not find any significant changes in salivary amino acid levels between the groups. Most of the tested amino acids were increased in the group with severe gingivitis. More pronounced elevation was found in serine, threonine, alanine, cysteine, lysine, tyrosine, valine, and isoleucine levels. Two amino acids, arginine and proline, decreased with the severity of gingivitis (Figure 2).

Variations in salivary amino acid concentrations according to the severity of gingivitis as evaluated by GI. Data are given as mean ± SD; AA, amino acid; His, histidine; Ser, serine; Arg, arginine; Gly, glycine; Asp, aspartate; Glu, glutamate; Thr, threonine; Ala, alanine; Pro, proline; Cys, cysteine; Lys, lysine; Tyr, tyrosine; Met, methionine; Val, valine; Ile, isoleucine; Leu, leucine; Phe, phenylalanine; GI, gingival index. Gingivitis stage: mild (0<GI≤1.0), moderate (1.1≤GI≤2.0), and severe (2.1≤GI≤3.0).
Salivary free amino acid levels according to oral hygiene prophylaxis
The Plaque index (PLI) Silness-Löe was used as an indicator for the degree of oral hygiene and dental plaque accumulation, where higher PLI corresponded to poorer oral hygiene. The largest increase was found in salivary glutamate (37.1 %, p-value=0.20) followed by aspartate (30.8 %, p-Value=0.15), alanine (31.4 %, p-value=0.22), serine (20.2 %, p-Value=0.28), and lysine (19.7 %, p-value =0.23), while salivary arginine decreased at the highest extent (by 16.9 %, p-Value=0.38) with worsening of oral hygiene. In individuals with poor oral hygiene, GI increases significantly more than fivefold (Figure 3).

Variations in salivary amino acid concentrations according to the oral hygiene as evaluated by PLI. Data are given as mean ± SD; AA, amino acid; His, histidine; Ser, serine; Arg, arginine; Gly, glycine; Asp, aspartate; Glu, glutamate; Thr, threonine; Ala, alanine; Pro, proline; Cys, cysteine; Lys, lysine; Tyr, tyrosine; Met, methionine; Val, valine; Ile, isoleucine; Leu, leucine; Phe, phenylalanine; PLI, plaque index Silness-Löe. Oral hygiene stage: very good to good (PLI 0.1–1.1), good to satisfactory (PLI 1.2–2.0), satisfactory to poor (PLI 2.1–3.0).
Frequency of teeth brushing
The effect of various hygiene measures such as frequency of teeth brushing, and topical and/or endogenous fluoride prophylaxis on salivary amino acids concentrations were examined.
Although the frequency of teeth brushing did not significantly affect the levels of salivary amino acids, we found higher levels of salivary cysteine (1.21-fold), methionine (1.04-fold), and histidine (1.03-fold) among the participants with diagnosed plaque-induced gingivitis and regular tooth brushing twice per day compared to the subjects with gingivitis and rare or once per day tooth brushing. In addition, salivary cysteine, tyrosine, and phenylalanine were higher among the controls who brushed their teeth twice per day in comparison to the children with plaque-induced gingivitis who brush their teeth twice per day, 1.64–fold vs. 1.20-fold, and 1.16-fold, respectively. The teeth brushing beneficially affected PLI and GI. PLI and GI among the children who brushed their teeth rarely or once per day were greater compared to the subjects with regular tooth brushing twice per day, 1.09-fold vs. 1.13-fold, respectively (Figure 4).

Free salivary amino acid concentration according to frequency of teeth brushing. Data are given as mean ± SD; AA, amino acid; His, histidine; Ser, serine; Arg, arginine; Gly, glycine; Asp, aspartate; Glu, glutamate; Thr, threonine; Ala, alanine; Pro, proline; Cys, cysteine; Lys, lysine; Tyr, tyrosine; Met, methionine; Val, valine; Ile, isoleucine; Leu, leucine; Phe, phenylalanine. Frequency of teeth brushing: rare, once daily or less; frequent, twice daily or more.
Topical fluoride prophylaxis
The regular application of topical fluoride prophylaxis is associated with significant elevation of valine (p-Value=0.039) and threonine levels (p-value=0.042), and a decrease in salivary arginine (p-value=0.030). Non-significant but pronounced elevation was found for alanine, tyrosine, methionine, isoleucine, leucine, and phenylalanine while serine, glutamate, and proline levels decreased (Figure 5).
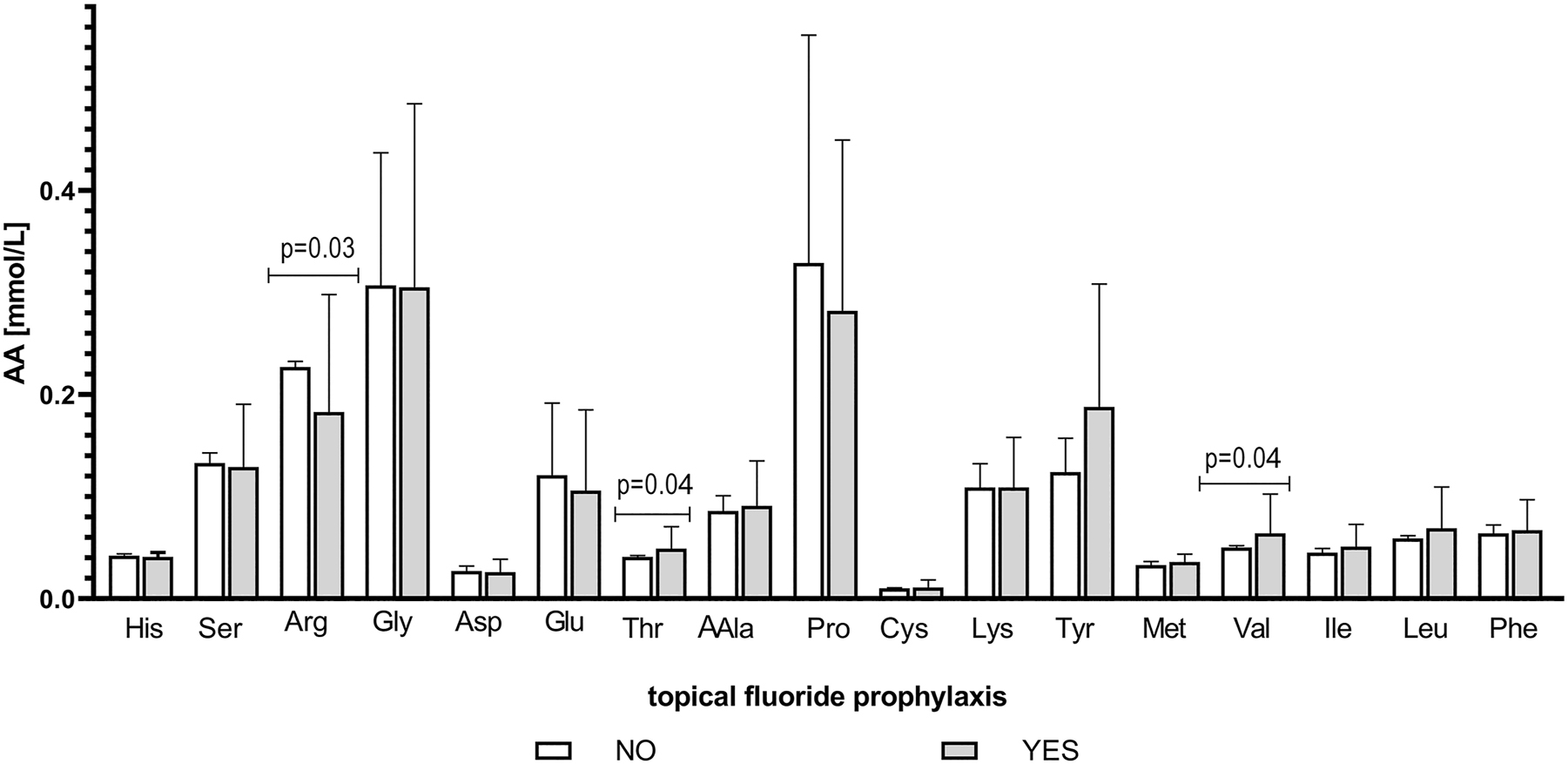
Changes in the concentration of free salivary amino acids in children with plaque-induced gingivitis according to topical fluoride prophylaxis. Data are given as mean ± SD; AA, amino acid; His, histidine; Ser, serine; Arg, arginine; Gly, glycine; Asp, aspartate; Glu, glutamate; Thr, threonine; Ala, alanine; Pro, proline; Cys, cysteine; Lys, lysine; Tyr, tyrosine; Met, methionine; Val, valine; Ile, isoleucine; Leu, leucine; Phe, phenylalanine. NO, not applying topical fluoride prophylaxis; YES, applying topical fluoride prophylaxis; p-value<0.05 was considered as significant.
Endogenic fluoride prophylaxis
The application of endogenic fluoride prophylaxis resulted in the elevation of all tested salivary amino acids except for cysteine (0.009 ± 0.003 vs. 0.012 ± 0.009 mmol/L) and methionine (0.034 ± 0.006 vs. 0.036 ± 0.008 mmol/L), which levels tend to decrease (p-value>0.05). Only the increase in arginine was statistically significant (0.268 ± 0.149 vs. 0.150 ± 0.062 mmol/L, p-value=0.021). In addition, significantly decreased were PLI (1.22 ± 0.14 vs. 1.69 ± 0.34) and GI (0.75 ± 0.36 vs. 1.54 ± 0.51) in children pursuing endogenic fluoride prophylaxis (p-value<0.001).
Additional oral hygiene prophylaxis products
A tendency for an increase of all salivary amino acids except cysteine was established among children with plaque-induced gingivitis not applying additional oral hygiene products and measures, while only alanine and tyrosine were elevated in the control group. Salivary cysteine revealed opposite changes in children with plaque-induced gingivitis – it decreased in those who did not apply additional oral hygiene products (0.008 ± 0.002 vs. 0.013 ± 0.008, p-value=0.016). The same tendency was observed in the controls, salivary cysteine was 2.53-fold higher among children who applied additional oral hygiene products in comparison to the subjects who did not use additional products. PLI and GI tended also to decrease in children pursuing additional oral hygiene measures.
Discussion
Nowadays the oral disorder of plaque-induced gingivitis is interpreted not only as an inflammatory disease of gingival tissues, but also as a behavioral pattern–associated disease, clinically manifested on individual and community levels. The oral disorder of plaque-induced gingivitis affects approximately 73 % of children between 6 and 11 years of age in developed countries. In 50 %–99 % of adolescents, it is accompanied by negligence in oral health care [15]. This oral health disorder is typical, especially for periods of mixed dentition, and is associated with the intensive processes of the eruption of permanent teeth provoking the condition of pre-eruptive and eruptive gingivitis [16].
The subjects with mixed and permanent dentition (over the age of 6 years) are characterized by a higher level of dental plaque accumulation associated with a higher degree of clinically manifested plaque-induced gingivitis. Parallel to the increase in age there is a process of aggravation from a mild to a moderate degree of plaque-induced gingivitis. On the other hand, the increase in child’s age, and especially the period of early school age to adolescence, is associated with an inclination of the individuals to ignore the significance of tooth brushing, additional means of oral hygiene, including products of topical fluoride prophylaxis and age-related requirements and recommendations as a whole. That results in an unsatisfactory oral hygiene state, abnormal accumulation of dental plaque, and as a consequence, initiation, and progression of plaque-induced gingival inflammation [17, 18]. In the current study, we established a tendency for an increase in salivary free amino acids among children over the age of 6 years compared to those between 0 and 6 years of age. Other studies also revealed significantly enhanced levels of salivary glycine and lysine with aging and age-related quantitative and qualitative changes in saliva amino acids composition [4, 19, 20].
The inflammation of the gingiva, especially under conditions of moderate and severe degrees of plague-induced gingivitis, is accompanied by a reversible damage of its connective tissue [21]. Collagens represent the predominant ratio of approximately 60 % of the total amount of proteins in normal healthy gingiva. It is established that the destruction of collagen can be accompanied by the release of hydroxyproline, proline, and glycine representing approximately 57 % of the amino acids ingredients in collagen. The main source of salivary free amino acids in gingivitis is the proteolytic degradation of salivary proteins by proteases with bacterial or endogenous origin or the degradation of collagen. The most abundant proteins in human saliva are the proline-rich proteins (PRPs) [22, 23]. Approximately 70 % of the amino acids in PRPs are glutamine, glycine, and proline [24, 25]. The degradation of PRPs is a source of salivary proline, aspartate, glutamate, lysine, arginine, glycine, and histidine. However, there are some tendency to increase the salivary glycine, proline, arginine, serine, glutamate, lysine, threonine, and aspartate in the gingivitis group. It might be supposed, that the elevation in these amino acids is associated with plaque-induced gingival inflammation. On the other hand, the decrease of salivary cysteine (by 1.7-fold), in children with plaque-induced gingivitis as compared to the controls with healthy gingiva may be linked to the increased rate of inflammation-related reactive oxygen species generation that provokes the depletion of free amino acids with greater anti-oxidative capacity, such as cysteine [26].
The concept of timely control of dental plaque is associated with multifaceted prophylactic care and procedures, including tooth brushing, application of age-appropriate additional oral hygiene products, and topical fluoride prophylaxis. These are essential for the inhibition of dental plaque accumulation, combined with bacteriostatic activity [27, 28]. The usage of additional oral hygiene products is important with regard to prophylactics of pre-eruptive and eruptive gingivitis under conditions of mixed dentition [29]. The protective potential of salivary cysteine is demonstrated in our study by the result that the salivary cysteine was significantly 1.57-fold elevated in children with healthy gingiva who used additional oral hygiene products in comparison to those who did not (p-Value=0.05). Furthermore, the mean value of cysteine among the controls who brushed their teeth twice per day was 1.64–fold higher in comparison to the children with plaque-induced gingivitis who brushed their teeth rarely. The result confirms the protective role of cysteine in non-stimulated mixed saliva under conditions of plaque-induced gingival inflammation. Similar to the levels of cysteine related to frequency of tooth brushing and application of additional oral hygiene measures, here is ascertained its reactivity under conditions of improper oral hygiene.
On the other hand, the levels of salivary proline, a structural amino acid of healthy gingiva, were decreased in children with regularly practiced oral hygiene prophylaxis, such as teeth bushing, topical fluoride prophylaxis, and/or additional hygiene measures. Based on the established levels of salivary amino acids, our findings confirm the great importance of proper adequate oral hygiene measures for the control of the initiation and progression of plaque-induced gingivitis. [30].
The salivary amino acids (glycine, proline, arginine, lysine, serine, aspartate, glutamate, and threonine) which are increased among the children with plaque-induced gingivitis as compared to controls showed the same tendency of an increase in case of irregular tooth brushing and no usage of additional oral hygiene products. These results correspond to the enhanced metabolic activity and proteolytic capacity of the pathogenic dental plaque in plaque-induced gingivitis.
A great number of studies are focused on the role of fluoride prophylaxis for the maintenance of proper oral dental health [28, 29]. Our results about the variation of some salivary amino acids in children with plaque-induced gingivitis correspond to the established bacteriostatic and plaque-inhibiting properties of regularly applied topical fluoride products. Namely, the mean values of salivary amino acids histidine, serine, arginine, glycine, aspartate, glutamate, and proline are reduced under conditions of regular topical fluoride prophylaxis in comparison to the mean levels of the same amino acids among the participants who do not apply topical fluoride products. A similar trend for a decline of almost all tested amino acids was established in participants applying additional hygiene measures (see above). The release of free amino acids in saliva by bacterial proteases is decreased as a result of inhibited bacterial growth by applied topical fluoride products and additional hygiene measures.
Age-dependent differences in salivary amino acid levels and changes in arginine, valine, and threonine after topical fluoride application in children with plaque-induced gingivitis are among the strengths of the current study.
Limitations
The small sample size does not allow for more definitive conclusions regarding the changes in the studied salivary amino acids depending on age, gingival status, oral hygiene, and diet. In addition, the studied group is heterogenic in terms of the type of dentition (mixed and permanent). Nevertheless, being the first study exploring the amino acid content in children with gingivitis in relation to oral prophylactics measures and nutrition the study could serve as a landmark for further investigations in the field.
Conclusions
We have demonstrated that the free salivary amino acid composition was most considerably influenced by the gingival health status and the application of prophylactic oral hygiene measures in children with plaque-induced gingivitis. Future studies are needed to identify the potential of selected salivary amino acids as prospective non-invasive diagnostic biomarkers of plaque-induced gingivitis in children.
Funding source: Fund “Science”, Medical University “Prof. Dr. Paraskev Stoyanov, Varna, Bulgaria
Award Identifier / Grant number: 18036, FN-12/11.02.2019
-
Research ethics: The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and approved by the Local Ethics Committee (Protocol №82/28.03.2019).
-
Informed consent: Informed consent was obtained from parents or legal guardians of all children included in this study.
-
Author contributions: The authors have accepted responsibility for the entire content of this manuscript and approved its submission. Conceptualization – YK; Design – SA; Supervision – DI; Data collection &/or processing – AS, SA, MN; Analysis and/or interpretation – MN, AS, BG; Literature search – AS, MN, SA; Writing – BG, AS; Critical review – DI, YK, SP.
-
Competing interests: The authors state no conflict of interest.
-
Research funding: This research was funded by Fund “Science” MU-Varna, Bulgaria, Project Number 18036, FN-12/11.02.2019.
-
Data availability: The raw data can be obtained on request fromthecorrespondingauthor.
References
1. Lee, Y, Zimmerman, J, Siqueira, W, Xiao, Y, Basiri, T, Hatibovic-Kofman, S, et al.. Proteomic evaluation of acquired enamel pellicle during in vivo formation. PLoS One 2013;8:e67919. https://doi.org/10.1371/journal.pone.0067919.Suche in Google Scholar PubMed PubMed Central
2. Gonçalves Lda, R, Soares, M, Alves, G, Garcia, CHS, Camisasca, DR, Domont, G, et al.. Analysis of the salivary proteome in gingivitis patients. J Periodontal Res 2011;46:599–606. https://doi.org/10.1111/j.1600-0765.2011.01378.x.Suche in Google Scholar PubMed
3. Fonteles, C, Guerra, M, Ribeiro, T, Mendonca, D, de Carvalho, C, Monteiro, A, et al.. Association of free amino acids with caries experienceand mutans streptococci levels in whole saliva of childrenwith early childhood caries. Arch Oral Biol 2009;54:80–5. https://doi.org/10.1016/j.archoralbio.2008.07.011.Suche in Google Scholar PubMed
4. Tanaka, S, Machino, M, Sakagami, H, Yokote, Y. Changes in salivary amino acid composition during aging. Vivo 2010;24:853–6.Suche in Google Scholar
5. Balci, N, Kurgan, Ş, Çekici, A, Çakır, T, Serdar, MA. Free amino acid composition of saliva in patients with healthy periodontium and periodontitis. Clin Oral Invest 2021;25:4175–83. https://doi.org/10.1007/s00784-021-03977-7.Suche in Google Scholar PubMed
6. Chen, J, Du, Y, Zhang, P. Salivary metabolic profiling in patients with periodontitis. Sichuan Da Xue Xue Bao Yi Xue Ban 2022;53:842–50. https://doi.org/10.12182/20220960207.Suche in Google Scholar PubMed PubMed Central
7. Cole, M, Evans, M, Bowden, G, Johnson, J, Pearce, C, Sheridan, MJ, et al.. Pioneer oral streptococci produce immunoglobulin A1 protease. Infect Immun 1994;62:2165–8. https://doi.org/10.1128/iai.62.6.2165-2168.1994.Suche in Google Scholar PubMed PubMed Central
8. Jenkinson, H, Lamont, R, LeBlanc, D. Oral microbial ecology. In: Lamont, RJ, Lantz, MS, editors. Oral microbiology and immunology. Washington, DC: ASM Press; 2006.Suche in Google Scholar
9. Ketabi, M, Tazhibi, M, Mohebrasool, S. The prevalance and risk factors of gingivitis among the children referred to Isfahan Islamic Azad University (Khorasgan Branch) dental school, in Iran. J Dent Res 2006;3:1–4.Suche in Google Scholar
10. Mitova, N, Rashkova, M, Popova, H, Kozarov, AS. Subgingival microbiota during formation of permanent dentition. Folia Med 2018;60:617–23. https://doi.org/10.2478/folmed-2018-0066.Suche in Google Scholar PubMed
11. Peycheva, S, Apostolova, E, Murdjeva, M, Gardjeva, PA, Shishmanova-Doseva, MS. Oral microbial Flora in Bulgarian adolescents with moderate plaque-induced gingivitis. Folia Med 2019;61:522–8. https://doi.org/10.3897/folmed.61.e47734.Suche in Google Scholar PubMed
12. El-Patal, M, Khalil, M, Shipl, W, Barakat, I, Youssef, E, Attar, S, et al.. Detection of soluble urokinase type plasminogen activator receptors in children with gingivitis and normal subjects. BMC Oral Health 2022;22:436. https://doi.org/10.1186/s12903-022-02478-7.Suche in Google Scholar PubMed PubMed Central
13. Löe, H, Silness, J. Periodontal disease in pregnancy. Acta Odontol Scand 1963;21:533–51. https://doi.org/10.3109/00016356309011240.Suche in Google Scholar PubMed
14. Rebelo, M, Queiroz, A. Gingival diseases – their Aetiology, prevention and treatment. In: Panagakos, F, Davies, R, editors. Gingival indices: state of art. IntechOpen; 2011:1–16 pp.Suche in Google Scholar
15. Trombelli, L, Farina, R, Tatakis, D. Plaque-induced gingivitis: case definition and diagnostic considerations. J Clin Periodontol 2018;45:44–67. https://doi.org/10.1111/jcpe.12939.Suche in Google Scholar PubMed
16. Fan, W, Liu, C, Zhang, Y, Huang, S, Li, J. Epidemiology and associated factors of gingivitis in adolescents in Guangdong Province, Southern China: a cross-sectional study. BMC Oral Health 2021;21:311. https://doi.org/10.1186/s12903-021-01666-1.Suche in Google Scholar PubMed PubMed Central
17. Popa, Ș, Păunică, S, Didilescu, AC, Bodnar, D, Suciu, I, Totan, A, et al.. Dental biofilm-induced gingivitis in children and adolescents. A literature review. Rom Biotechnol Lett 2021;26:2664–70. https://doi.org/10.25083/rbl/26.3/2664-2670.Suche in Google Scholar
18. Pari, A, Reddy, PI, Parthasarthy, H, Katamreddy, V. Gingival diseases in childhood – a review. J Clin Diagn Res 2014;8:01–4. https://doi.org/10.7860/JCDR/2014/9004.4957.Suche in Google Scholar PubMed PubMed Central
19. Maciejczyk, M, Nesterowicz, M, Zalewska, A. Oxidation, Glycation, and Carbamylation of salivary Biomolecules in healthy children, Adults, and the elderly: can saliva Be used in the assessment of aging? J Inflamm Res 2022;28:2051–73. https://doi.org/10.2147/jir.s356029.Suche in Google Scholar PubMed PubMed Central
20. Nahed, A. Saliva can be an indicator for aging. A review. Curr Sci Intl. 2022;11:84–98.Suche in Google Scholar
21. Amado, F, Vitorino, R, Duarte, J, Lobo, MJC. Analysis of the human saliva proteome. Expet Rev Proteonomics 2005;2:521–39. https://doi.org/10.1586/14789450.2.4.521.Suche in Google Scholar PubMed
22. ThamaraiSelvi, V, Brundha, M. Salivaomics – a review. EJMCM 2020;7:2914–293.Suche in Google Scholar
23. Séguier, S, Gogly, B, Brousse, N, Godeau, G. Is collagen breakdown during periodontitis linked to inflammatory cells and expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human gingival tissue? J Periodontol 2001;72:1398–406. https://doi.org/10.1902/jop.2001.72.10.1398.Suche in Google Scholar PubMed
24. Hajishengallis, G, Russell, MW. Innate humoral defense factors. Mucosal Immunol 2015;1:251–70.10.1016/B978-0-12-415847-4.00015-XSuche in Google Scholar
25. Levine, M. Susceptibility to dental caries and the salivary proline-rich proteins. Int J Dent 2011;2011:953412. https://doi.org/10.1155/2011/953412.Suche in Google Scholar PubMed PubMed Central
26. Xu, N, Chen, G, Liu, H. Antioxidative categorization of twenty amino acids based on experimental evaluation. Molecules 2017;22:2066.10.3390/molecules22122066Suche in Google Scholar PubMed PubMed Central
27. Davidovich, E, Grender, A, Zini, A. Factors associated with dental plaque, gingivitis, and caries in a pediatric population: a records-based cross-sectional study. Int J Environ Res Public Health 2020;17:8595–606. https://doi.org/10.3390/ijerph17228595.Suche in Google Scholar PubMed PubMed Central
28. American Academy of Pediatric Dentistry. Fluoride therapy. The reference manual of pediatric dentistry. Chicago, Ill: American Academy of Pediatric Dentistry; 2021:302–5 pp.Suche in Google Scholar
29. Munteanu, A, Holban, A, Farcas, iu C, Imre, M, Farcașiu, AT, Farcașiu, C. Review of professionally applied fluorides for preventing dental caries in children and adolescents. Appl Sci 2022;12:1054. https://doi.org/10.3390/app12031054.Suche in Google Scholar
30. Al-Kamel, A, Al-Hajj, W, Halboub, E, Abdulrab, S, Al-Tahami, K, Al-Hebshi, N. N-acetyl cysteine versus chlorhexidine mouthwashes in prevention and treatment of experimental gingivitis: a randomized, triple-blind, placebo-controlled clinical trial. Clin Oral Invest 2019;23:3833–42. https://doi.org/10.1007/s00784-019-02813-3.Suche in Google Scholar PubMed
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/tjb-2023-0107).
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Review
- Metabolomics: a review of liquid chromatography mass spectrometry-based methods and clinical applications
- Opinion Papers
- Green transformation in the health sector and medical laboratories, adaptation to climate change in Türkiye
- Forward steps in green medical laboratory practices for a sustainable future
- Research Articles
- Comparison of the immunoassay method with the commercial and in-house LC-MS/MS methods for substance abuse in urine
- Peroxisome proliferator-activated receptor gamma and osteoprotegerin levels as an indicator and diagnostic predictor of endothelial dysfunction
- Gingival status and prophylactic oral hygiene measures modulate salivary amino acids’ profile in children with plaque-induced gingivitis
- FIB4 score is increased in severe preeclampsia
- The effects of a single dialysis session on serum hepcidin levels
- Gestational diabetes mellitus is associated with a low serum level of mitochondrial-derived peptide-MOTS-C
- Ideal timing of labor in terms of oxidative stress – which term period is best?
- Synergistic role of thymoquinone and 5-fluorouracil in U-251MG glioblastoma cell line
- Effect of oligosaccharides and aerobic training on hyperglycemia, growth and intestinal microbial diversity of diabetic rats
- Association of AdipoQ (G>T) gene polymorphism with obesity and hypertension in North Indian postmenopausal women of Punjab
- The attraction of paraoxonase-1 associated with the MAPK pathway on colon carcinoma cells
- Resveratrol modulates miRNA machinery proteins in different types of colon cancer cells
- The relationship between ASIC3 gene polymorphism and fibromyalgia syndrome
- Expression levels of genes involved in lipogenesis and cholesterol synthesis in adenomyosis
- Frequency of thrombophilia-associated mutations and polymorphisms in pregnant women with a history of thrombosis or pregnancy complications
Artikel in diesem Heft
- Frontmatter
- Review
- Metabolomics: a review of liquid chromatography mass spectrometry-based methods and clinical applications
- Opinion Papers
- Green transformation in the health sector and medical laboratories, adaptation to climate change in Türkiye
- Forward steps in green medical laboratory practices for a sustainable future
- Research Articles
- Comparison of the immunoassay method with the commercial and in-house LC-MS/MS methods for substance abuse in urine
- Peroxisome proliferator-activated receptor gamma and osteoprotegerin levels as an indicator and diagnostic predictor of endothelial dysfunction
- Gingival status and prophylactic oral hygiene measures modulate salivary amino acids’ profile in children with plaque-induced gingivitis
- FIB4 score is increased in severe preeclampsia
- The effects of a single dialysis session on serum hepcidin levels
- Gestational diabetes mellitus is associated with a low serum level of mitochondrial-derived peptide-MOTS-C
- Ideal timing of labor in terms of oxidative stress – which term period is best?
- Synergistic role of thymoquinone and 5-fluorouracil in U-251MG glioblastoma cell line
- Effect of oligosaccharides and aerobic training on hyperglycemia, growth and intestinal microbial diversity of diabetic rats
- Association of AdipoQ (G>T) gene polymorphism with obesity and hypertension in North Indian postmenopausal women of Punjab
- The attraction of paraoxonase-1 associated with the MAPK pathway on colon carcinoma cells
- Resveratrol modulates miRNA machinery proteins in different types of colon cancer cells
- The relationship between ASIC3 gene polymorphism and fibromyalgia syndrome
- Expression levels of genes involved in lipogenesis and cholesterol synthesis in adenomyosis
- Frequency of thrombophilia-associated mutations and polymorphisms in pregnant women with a history of thrombosis or pregnancy complications

