Effect of oligosaccharides and aerobic training on hyperglycemia, growth and intestinal microbial diversity of diabetic rats
-
Mariya Atanasova Choneva
, Milen Veselinov Hristozov
, Ivica Dimov
, Krasimir Ognyanov Boyanov
, Iliyan Valeriev Dimitrov
, Mariana Atanasova Murdjeva
, Petar Ivanov Hrischev
, Veselin Atanasov Vasilev
, Katerina Nikolova Georgieva
and Anelia Veselinova Bivolarska
Abstract
Objectives
Type 1 diabetes mellitus is a metabolic disease characterized by dysbiosis. Modulation of the gut microbiota by oligosaccharides and aerobic training are proposed mechanisms that ameliorate the disease through affecting host-microbiota interactions.
Methods
Seventy-two male Wistar rats were randomly divided into 8 groups – 5 with streptozotocin-induced diabetes and 3 healthy controls. The effect of two oligosaccharides – xylo- and galactooligosaccharides, and of aerobic training on the blood glucose concentration, growth and diversity of the gut microbiota was evaluated in the current study.
Results
The galactooligosaccharides positively affected the glycemic status of the experimental animals as the diabetic and healthy rats had lower blood glucose concentration after 6 weeks of treatment (diabetic rats: week 4 vs. week 8, p=0.047; healthy rats: week 2,4,6,10 vs. week 8, p=0.001, p=0.000, p=0.025 and p=0.001, respectively). A positive effect of the galactooligosaccharides on body weight was observed when administered to diabetic rats in comparison to the diabetic control (p=0.020). Similar results were observed for the aerobically trained diabetic rats (p=0.004). The identification of bacterial species showed preserved microbiota diversity and indicated Bifidobacterium indicum, Lactobacillus feritoshensis and E. coli as the most abundant species among the analyzed genera.
Conclusions
Prebiotic treatment beneficially affected the hyperglycemia and growth of type 1 diabetic rats. The most significant effect of the aerobic training was the improvement of the morphological parameters. Oligosaccharide administration and exercise did not affect the diversity of the bacterial species.
Introduction
Type 1 diabetes mellitus (T1DM) is an autoimmune metabolic disease of multifactorial origin, resulting from the interaction between genetic and environmental factors [1]. Although the disorder’s pathogenesis has not been fully clarified, growing evidence indicates that disturbances in the intestinal microbiota composition and abundance, a condition known as dysbiosis, are a major contributor to the development of T1DM [2].
The intestinal microbiome comprises many microbial communities, important for the host’s health, that maintain mucosal homeostasis and regulate epithelial development and immune responses [3, 4]. Several environmental factors such as diet, extensive use of antibiotics and altered living conditions are known to affect the microbiota, decreasing its diversity, which is associated with the development of various diseases, including T1DM [5]. Modulation of the gut microbiota by non-digestible fiber is a proposed method for diabetes alleviation [6].
In 1995, Gibson and Roberfroid defined prebiotics as ‘a non-digestible food ingredient that beneficially affects the host by selectively stimulating the growth and/or the activity of one or a limited number of bacteria in the colon and thus improves health’ [7]. Xylooligosaccharides (XOS) and galactooligosaccharides (GOS) are among the most used prebiotics [8]. GOS are suggested to modulate the gut microbiota not only through enhancing beneficial bacteria, but also by inhibiting the adhesion of pathogenic microbes to the intestinal epithelial cells. GOS supplementation is also found to significantly increase the Lactobacillus spp. count in the feces of alloxan-induced diabetic rats [9]. XOS consumption is found to increase the levels of Bifidobacterium spp. in the gastrointestinal tract of rats [8].
Several studies have reported that exercise can increase the number of beneficial gut bacteria and their responsiveness to homeostatic and physiological variations [4]. Exercise is known to play a pivotal role in the regulation of metabolism and energy expenditure, thus affecting host-microbiota interactions [10]. Physical training is associated with qualitative and quantitative changes in the intestinal microbiota such as increased levels of Lactobacilli and Bifidobacteria [11] as well as butyrate-producing bacteria [12].
The present study was designed to evaluate the effect of two oligosaccharides – XOS and GOS, and of aerobic exercise on hyperglycemia and growth in streptozotocin (STZ)-induced diabetic rats. Furthermore, the diversity of the intestinal microbiota on species level was identified for the first time.
Materials and methods
Animals and ethical approval
The experimental protocols of the present study were approved by the Bulgarian Agency for Food Safety (BAFS resolution №150/09.04.2019) and comply with the ethical standards of the Medical University of Plovdiv (resolution of the University Ethic Committee №2/13.06.2019).
Seventy-two male Wistar rats (8 weeks old, 195 ± 30 g), procured from the vivarium of the Medical University of Plovdiv, were randomly distributed into 8 experimental groups (n=9): 1) diabetic control group on a standard diet (DSD); 2) diabetic prebiotic group, treated with XOS (D-XOS); 3) diabetic prebiotic group, treated with GOS (D-GOS); 4) diabetic trained group on a standard diet (DTSD); 5) diabetic trained group, treated with XOS (DT-XOS); 6) healthy control group on a standard diet (HSD); 7) healthy control group, treated with XOS (H-XOS); 8) healthy control group, treated with GOS (H-GOS).
The animals were kept under standard conditions (4–5 rats/cage, 12-h light/dark cycle, 22 ± 2°C, 55 ± 10 % humidity, free food and water access) for 10 weeks. The stages of the experiment are presented in Supplemental Figure 1. After a few days of acclimatization, the rats of the diabetic groups were injected intraperitoneally with 60 mg kg−1 of STZ for T1DM to be induced [13]. The animals from the three healthy groups were injected with an identical volume of saline.
A week post STZ administration, the rats from the respective prebiotic groups started to receive an XOS and GOS supplement in a dose of 100 mg/kg body weight, which accounted for 5 % of the basal diet. The animals were treated every day for 8 weeks. According to Wang et al., supplementation with such oligosaccharide concentrations beneficially affects the blood glucose levels, lipid and antioxidant profiles of rats on a high-fat diet [14]. The prebiotics were given per os following dilution in distilled water. The distribution of the carbohydrates in the prebiotics was as follows: XOS (Xylooligosaccharide powder, Lenzing AG, Lenzing, Austria) – 13 % with a degree of polymerization (DP) – 2; 19 % with a DP – 3; 11 % with a DP – 4 and 60 % with a DP of 5 and more; GOS (TOS-P, Yakult, Japan) – 2 % with a DP – 2; 48 % with a DP – 3; 38 % with a DP – 4 and 12 % with a DP – 5.
Two weeks post-STZ administration, the rats from groups 4 and 5 were subjected to a physical training program, that continued until the end of the experimental period. At the end of the 10th week, the rats were fasted overnight and sacrificed following treatment with ketamine/xylazine anaesthetic administered in an overdose (87.5/12.5 mg/kg body weight).
Induction of diabetes
STZ was prepared in a citrate buffer (pH 4.5) according to the method of Furman [15]. The buffer contained 5.78 g citric acid ͯ1H2O (M=210.14 g/mol), and 0.71 g Na2HPO4 (M=141.96 g/mol), separately dissolved in 50 mL distilled water. To reach a pH of 4.5, 10 mL of the citric acid solution and 45–50 mL of the Na2HPO4 solution were mixed. One g dry STZ substance was then dissolved in 33.3 mL citrate buffer recalculated in accordance with the dose of 60 mg/kg body weight [13]. Rats with a blood glucose level of above 12 mmol/L were considered diabetic.
Aerobic training
The experimental animals from the respective groups underwent aerobic training from week three until the end of the experiment. The exercise training program was performed five days a week on a treadmill (EXER-3R-Treadmill, Columbus Instruments, Columbus OH, USA) with 16 m/min band speed and 5° slope. On the first day, the rats were only familiarized with the exercise procedure with a training duration of 20 min. After that, the duration was increased with 5 min every other day until it reached 40 min at the end of the fifth week. This load was kept until the end of the experiment. The intensity of the treadmill running was below the maximal steady rate [16] and thus aerobic.
Fecal sample collection and analyses
Fresh fecal samples were collected twice throughout the experiment (at the start and end) and placed in 1.5 mL cryotubes containing glycerol as a preserving agent. The cryotubes were stored in a deep freezer at −80 °C until the conduction of the analysis.
Cultural methods
The fecal specimen was cultivated in ordinary culture medium – Nutrient Agar, and in selective culture media – Rogosa SL Agar, MacConkey Agar and Bifidobacterium Selective Agar.
To determine the total viable count (TVC) in the examined samples we used a surface cultivation method of the persisting microflora in Nutrient Agar (HiMedia, India) medium with appropriate dilution of the samples and cultivation at 37°C for 72 h (until the appearance of single colonies). The following equation for determination of the TVC in colony-forming units (CFU)/mL was used:
∑с – sum of the counted colonies of all the plates;
v – inoculum volume, mL;
n1 – number of plates from the first dilution;
n2 – number of plates from the second dilution;
d – dilution factor of the first dilution.
Identification methods
We aimed to identify the most typical representatives of the gut microbiota – Bifidobacteria, Lactobacilli and Enterobacteria.
For the biochemical identification of Bifidobacteria, we used HiBifido Identification Kit (HiMedia, India), which includes a combination of 12 tests for differentiation of Bifidobacterium spp.
Each test contains a sterile medium for catalase and 11 different utilization tests for carbohydrates: arabinose, cellulose, fructose, lactose, maltose, mannose, melibiose, raffinose, sucrose, xylose and salicin. The test is based on the principle of pH change resulting from the substrate utilization. The microorganism is isolated in Soybean Casein Digest Agar nutrient medium. According to McFarland, one to three single colonies are then taken and homogeneously suspended in 2–3 mL sterile saline, pre-diluted by 0.5. They are incubated at 35–37 °C for 24–48 h and are later determined by the change in the colour of the medium.
For the biochemical identification of Lactobacilli, we used HiLacto Identification Kit (HiMedia, India), which includes a combination of 12 tests for differentiation of Lactobacillus spp.
Each test contains a sterile medium for catalase, esculin and 10 different utilization tests for carbohydrates: xylose, cellobiose, arabinose, maltose, galactose, mannose, melibiose, raffinose, sucrose, and trehalose. The test is based on the principle of pH change resulting from the substrate utilization. The microorganism is isolated in Soybean Casein Digest Agar nutrient medium. One-three single colonies are then taken and homogeneously suspended in 2–3 mL sterile saline, pre-diluted by 0.5 according to McFarland. They are incubated at 35–37 °C for 24–48 h and are later determined by the change in the colour of the medium.
For the biochemical identification of Enterobacteria we used MLT ENTEROtest 24 N (HiMedia, India), which includes a combination of 24 biocehmical tests for quick identification of species of the Enterobacteriaceae family.
The results were interpreted 24 h after the inoculation.
Glucose measurement
The blood glucose concentration of the rats was measured at the beginning of the experimental period, after STZ treatment (week 2) and then every other week until the end of the experiment. The measurement was conducted with a glucometer, and a drop of blood collected from the tail vein.
Morphological measurements
The measured morphological parameters were the weight (g) and naso-anal length (cm) of the rats. The Lee index is a parameter of adiposity but is also used to assess the effect of nutrition on growth [17]. The Lee index (g/mm) was calculated as the cubic root of the weight(g) divided by the naso-anal length (mm) multiplied by 10,000 [18].
Statistical analysis
Statistical analyses were performed with the SPSS program, version 17.0 (SPSS Inc., Chicago, IL, USA). The data are presented as mean ± SEM. Results were regarded as statistically significant at p≤0.05 and tendencies at p<0.1. The differences between groups were analyzed with one-way ANOVA followed by LSD’s post hoc test. The Paired samples t-test was used to compare the parameters measured two or more times during the experiment (blood glucose, morphological parameters).
Results
Blood glucose concentration
The obtained results for the concentration of blood glucose are presented in Figure 1. No significant differences were observed between the groups at the beginning of the experiment. The Paired samples t-test showed within-group differences in the D-GOS (week 4 vs. week 8, p=0.047), H-XOS (week 2, 4, 6 and 10 vs. week 8, p=0.000, p=0.000, p=0.030 and p=0.000, respectively) and H-GOS (week 2, 4, 6 and 10 vs. week 8, p=0.001, p=0.000, p=0.025 and p=0.001, respectively) groups. Despite the lack of a significant difference within the D-XOS group, the data shows that the values for the blood glucose concentrationin week 8 are lower than the ones in the preceding weeks. According to the obtained results, the two oligosaccharides managed to ameliorate the hyperglycemia and the effect was best manifested after 6 weeks of treatment (week 8).
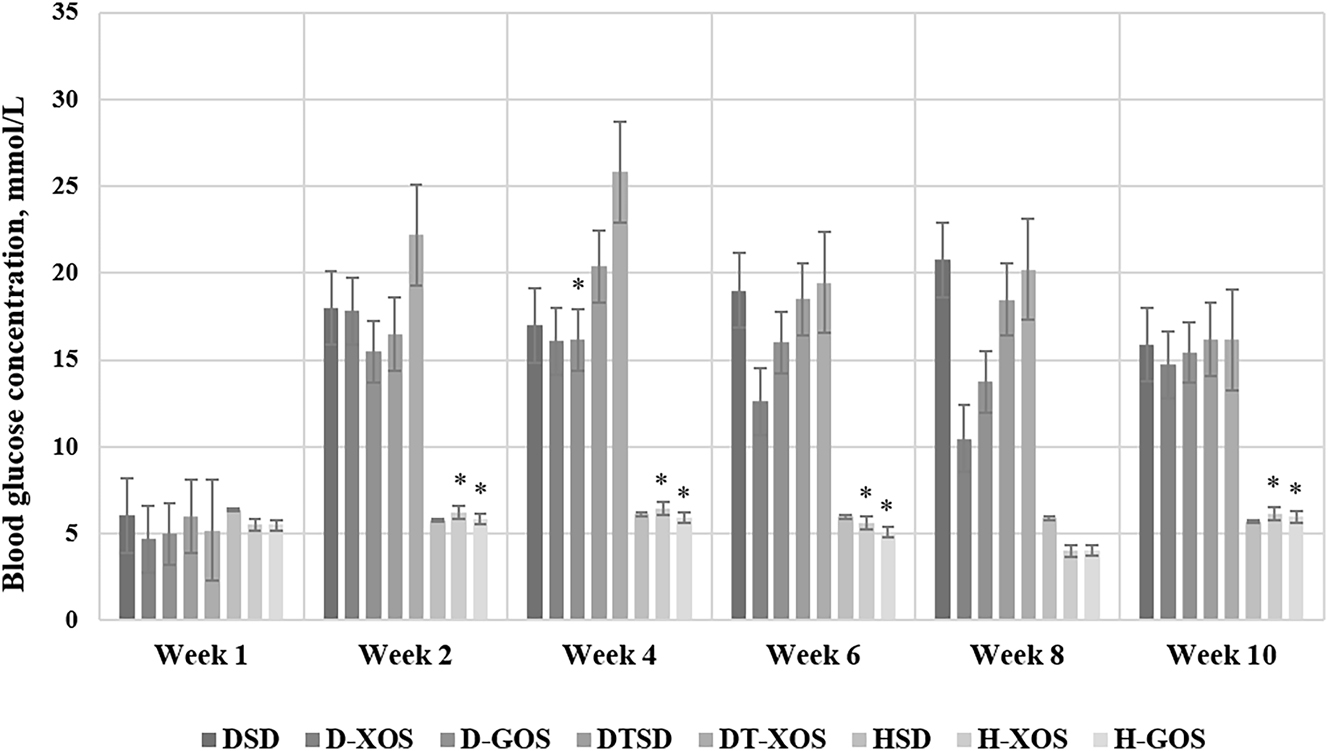
Effect of the oligosaccharides and/or aerobic training on the blood glucose concentration. Data are presented as mean ± SEM. *Significant differences vs. week 8 within a group, p≤0.05 (paired samples t-test); week 0 – pre-STZ-induced diabetes, before treatment with oligosaccharides and aerobic training. DSD, diabetic control group on a standard diet; D-XOS, diabetic prebiotic group, treated with xylooligosaccharides; D-GOS, diabetic prebiotic group, treated with galactooligosaccharides; DTSD, diabetic trained group on a standard diet; DT-XOS, diabetic trained group, treated with xylooligosaccharides; HSD, healthy control group on a standard diet; H-XOS, healthy control group, treated with xylooligosaccharides; H-GOS, healthy control group, treated with galactooligosaccharides.
Morphological parameters
Data regarding the weight of the experimental animals, measured at the beginning and the end of the experiment are presented in Figure 2. There were no significant differences in the glucose concentration measured at week 1.
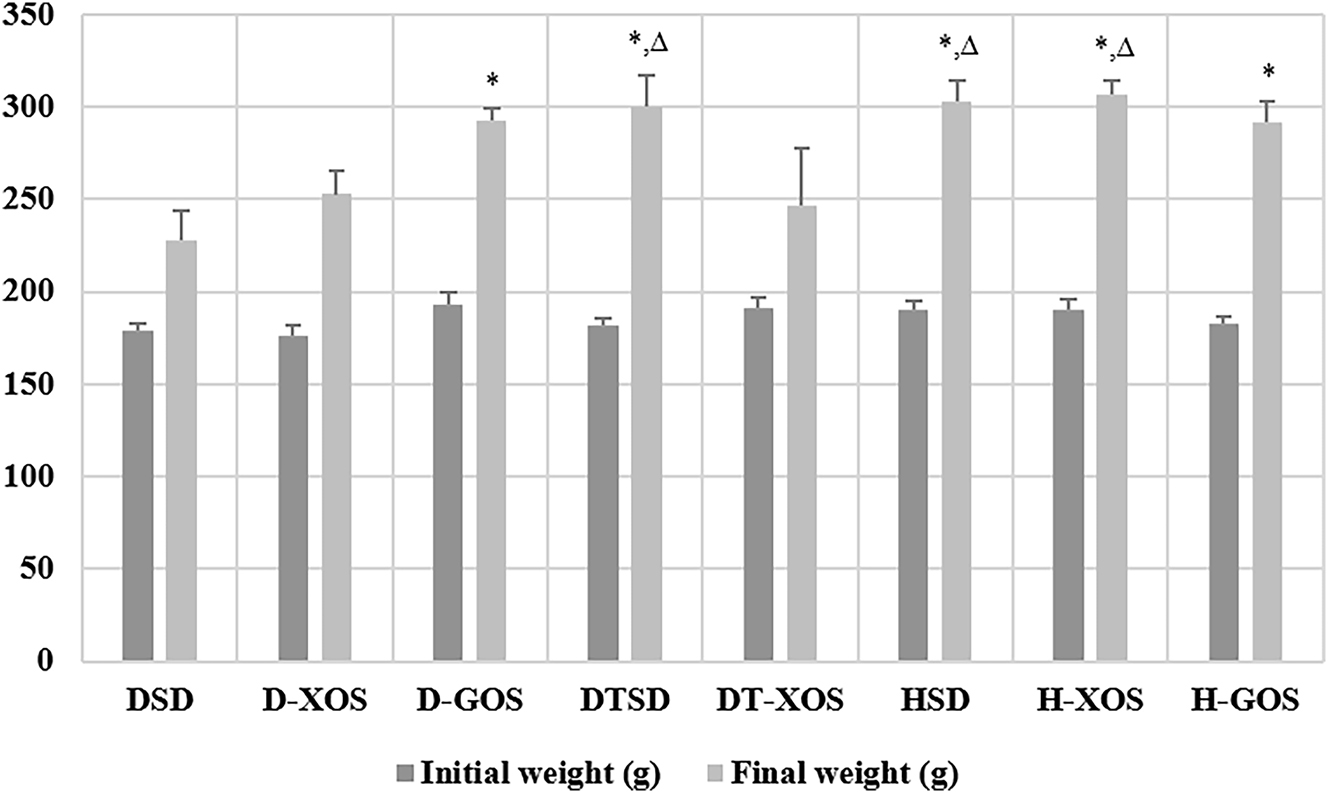
Effect of the oligosaccharides and/or aerobic training on weight. Data are presented as mean ± SEM. *Significant differences vs. DSD, p<0.05; ∆ – significant differences vs. DT-XOS, p<0.05 (one-way ANOVA, LSD). DSD, diabetic control group on a standard diet; D-XOS, diabetic prebiotic group, treated with xylooligosaccharides; D-GOS, diabetic prebiotic group, treated with galactooligosaccharides; DTSD, diabetic trained group on a standard diet; DT-XOS, diabetic trained group, treated with xylooligosaccharides; HSD, healthy control group on a standard diet; H-XOS, healthy control group, treated with xylooligosaccharides; H-GOS, healthy control group, treated with galactooligosaccharides.
The results show that STZ treatment negatively affected the weight of the animals. This effect was less manifested in the D-GOS group as it showed a significant increase in weight at the end of the experiment compared to the DSD group (p=0.020). Similar results were observed for the DTSD vs. DSD group, which implies a positive effect of the aerobic training (p=0.004). The healthy controls had significantly higher weight compared to the DSD group (p=0.001 vs. HSD, p=0.008 vs. H-XOS and p=0.002 vs. H-GOS). The trained diabetic rats fed an XOS supplement (DT-XOS) also showed retarded growth at the end of the experiment with asignificantly lower weight than that of the DTSD (p=0.024), H-XOS (p=0.010) and HSD rats (p=0.015).
The results for the naso-anal length of the rats are presented in Figure 3. No significant differences in the parameter were observed at the beginning of the experiment.
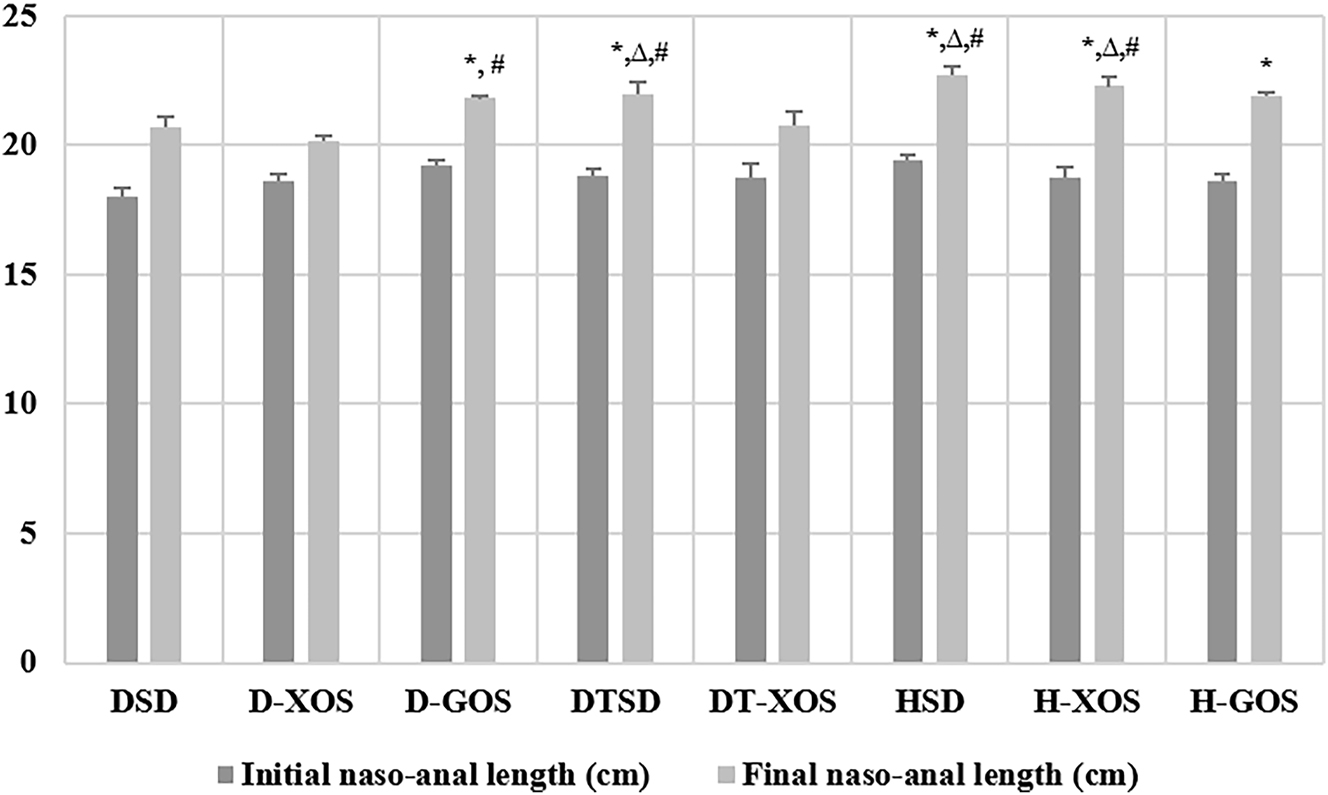
Effect of the oligosaccharides and/or aerobic training on the naso-anal length. Data are presented as mean ± SEM. *Significant differences or tendenciesvs. DSD, p<0.05; ∆ – significant differences vs. DT-XOS, p<0.05; # – significant differences vs. D-XOS (one-way ANOVA, LSD). DSD, diabetic control group on a standard diet; D-XOS, diabetic prebiotic group, treated with xylooligosaccharides; D-GOS, diabetic prebiotic group, treated with galactooligosaccharides; DTSD, diabetic trained group on a standard diet; DT-XOS, diabetic trained group, treated with xylooligosaccharides; HSD, healthy control group on a standard diet; H-XOS, healthy control group, treated with xylooligosaccharides; H-GOS, healthy control group, treated with galactooligosaccharides.
Significant differences in the naso-anal length at the end of the experiment were observed in the DSD group vs. the DTSD, HSD, H-XOS and H-GOS groups (p=0.028, p=0.005, p=0.030 and p=0.000, respectively). There was a tendency for statistical significance between the DSD and D-GOS group (p=0.082). A significantly lower final naso-anal length was found in the DT-XOS group compared to DTSD, HSD and H-XOS (p=0.027, p=0.004, p=0.000, respectively). The statistical analysis showed significant differences between the D-XOS group and the D-GOS, DTSD, H-XOS and HSD groups (p=0.037, p=0.016, p=0.004 and p=0.001, respectively).
Data regarding the Lee index are presented in Figure 4. There were no significant differences in the initial Lee index of the animals.
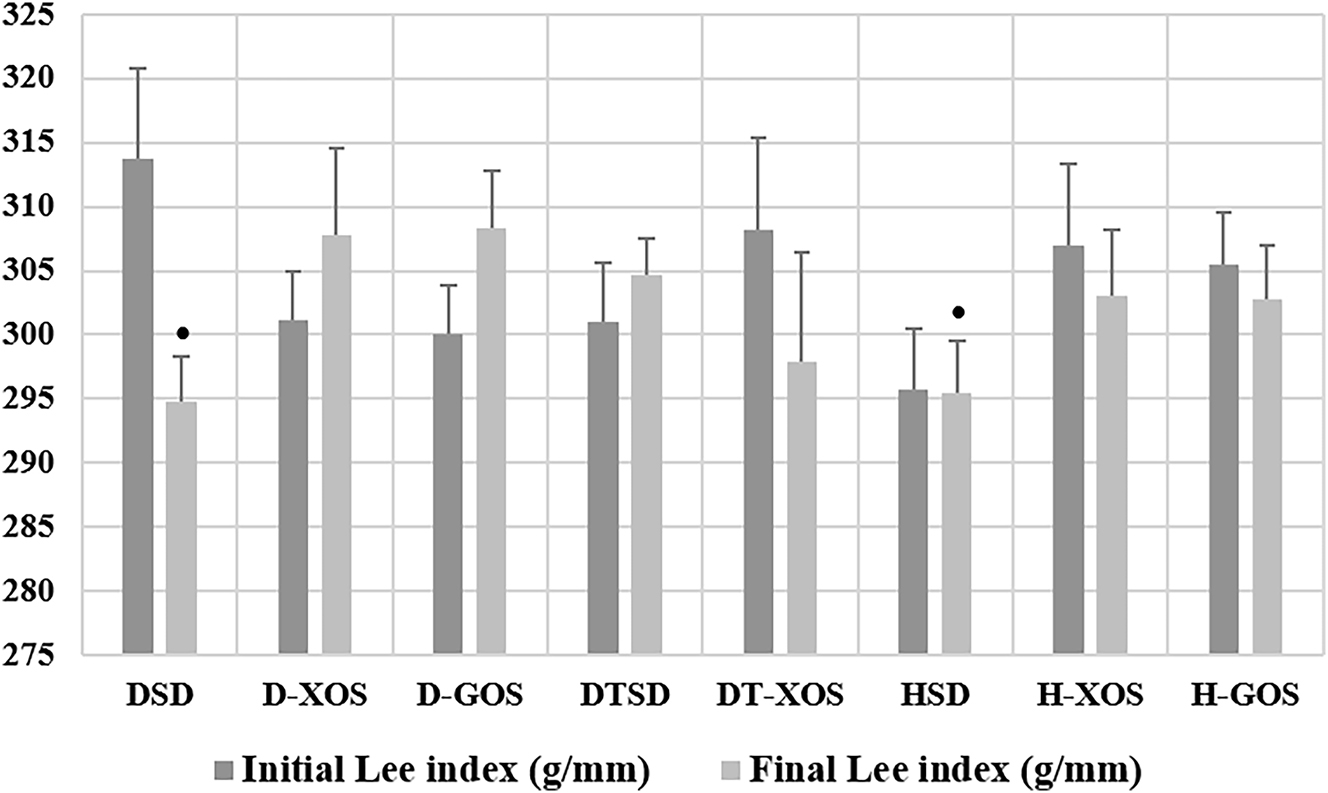
Effect of the oligosaccharides and/or aerobic training on the Lee index. Data are presented as mean ± SEM. *Tendency for a significant difference vs. D-GOS (one-way ANOVA, LSD). DSD, diabetic control group on a standard diet; D-XOS, diabetic prebiotic group, treated with xylooligosaccharides; D-GOS, diabetic prebiotic group, treated with galactooligosaccharides; DTSD, diabetic trained group on a standard diet; DT-XOS, diabetic trained group, treated with xylooligosaccharides; HSD, healthy control group on a standard diet; H-XOS, healthy control group, treated with xylooligosaccharides; H-GOS, healthy control group, treated with galactooligosaccharides.
The Paired sample’s t-test showed a significant decrease of the Lee index in the DSD group (p=0.039). In addition, there was a tendency for statistical significance between the final Lee index of the diabetic rats supplemented with GOS (D-GOS) and diabetic control (DSD) (p=0.071). A similar tendency was observed between the D-GOS and HSD groups (p=0.094).
Intestinal microbiota
The effect of XOS and GOS and aerobic training on the intestinal microbial abundance at the beginning and the end of the experimental period is presented in Table 1.
Effect of the oligosaccharides and/or the aerobic training on the composition of the intestinal microbiota. The most abundant strains amongst the Bifidobacterium spp. and Lactobacillus spp. genera as well as the Enterobacteriaceae family were B. indicum, L. feritoshensis and E. coli, respectively. The abundance of the E. coli strain in the D-XOS (not detected to positive) and the H-XOS (positive to not detected) groups was different throughout the two separate stages of the experiment. The same was observed for the P. rettgeri strain in the D-GOS group (positive to not detected).
| Genus/Famiy | Species | Period | Group | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DSD | D-XOS | D-GOS | DTSD | DT-XOS | HSD | H-XOS | H-GOS | |||
| Bifidobacterium spp. | B. indicum | Beginning | P | P | P | P | P | P | ND | P |
| End | P | P | P | P | P | P | ND | P | ||
| B. minimum | Beginning | ND | ND | ND | ND | ND | ND | ND | P | |
| End | ND | ND | ND | ND | ND | ND | ND | P | ||
| B. tsurumiense | Beginning | P | ND | ND | P | ND | P | P | ND | |
| End | P | ND | ND | P | ND | P | P | ND | ||
| Lactobacillus spp. | L. colehominis | Beginning | ND | P | ND | P | P | P | ND | P |
| End | ND | P | ND | P | P | P | ND | P | ||
| L. durianis | Beginning | ND | ND | ND | ND | ND | P | ND | ND | |
| End | ND | ND | ND | ND | ND | P | ND | ND | ||
| L. feritoshensis | Beginning | P | P | P | P | ND | P | P | P | |
| End | P | P | P | P | ND | P | P | P | ||
| L. fructivorans | Beginning | ND | ND | P | ND | ND | ND | ND | ND | |
| End | ND | ND | P | ND | ND | ND | ND | ND | ||
| L. vaginalis | Beginning | ND | ND | ND | P | ND | ND | ND | ND | |
| End | ND | ND | ND | P | ND | ND | ND | ND | ||
| Enterobacteriaceae | E. coli | Beginning | P | ND | P | P | P | P | P | P |
| End | P | P | P | P | P | P | ND | P | ||
| K. oxytoca | Beginning | ND | P | ND | ND | P | P | P | ND | |
| End | ND | P | ND | ND | P | P | P | ND | ||
| P. rettgeri | Beginning | P | ND | P | ND | ND | ND | ND | ND | |
| End | P | ND | ND | ND | ND | ND | ND | ND | ||
-
P, positive; ND, not detected; DSD, diabetic control group on a standard diet; D-XOS, diabetic prebiotic group, treated with xylooligosaccharides; D-GOS, diabetic prebiotic group, treated with galactooligosaccharides; DTSD, diabetic trained group on a standard diet; DT-XOS, diabetic trained group, treated with xylooligosaccharides; HSD, healthy control group on a standard diet; H-XOS, healthy control group, treated with xylooligosaccharides; H-GOS, healthy control group, treated with galactooligosaccharides.
Data obtained regarding the TVC of the rats at the beginning and the end of the experiment are presented in Figure 5. A statistically significant increase in the bacterial count was observed over time in the DSD group (p=0.045). The two oligosaccharides led to an insignificant decrease of the microorganisms in the two diabetic treated groups (D-XOS and D-GOS). The two aerobically trained groups also showed an elevated TVC towards the end of the experiment with a tendency for a statistically significant increase in the DT-XOS group (p=0.093). The results regarding the healthy controls were diverse. A significant increase of the microbial count was observed for the HSD group (p=0.049), while in the H-GOS group it was insignificant. A decrease of the number of bacteria was observed in the H-XOS group.
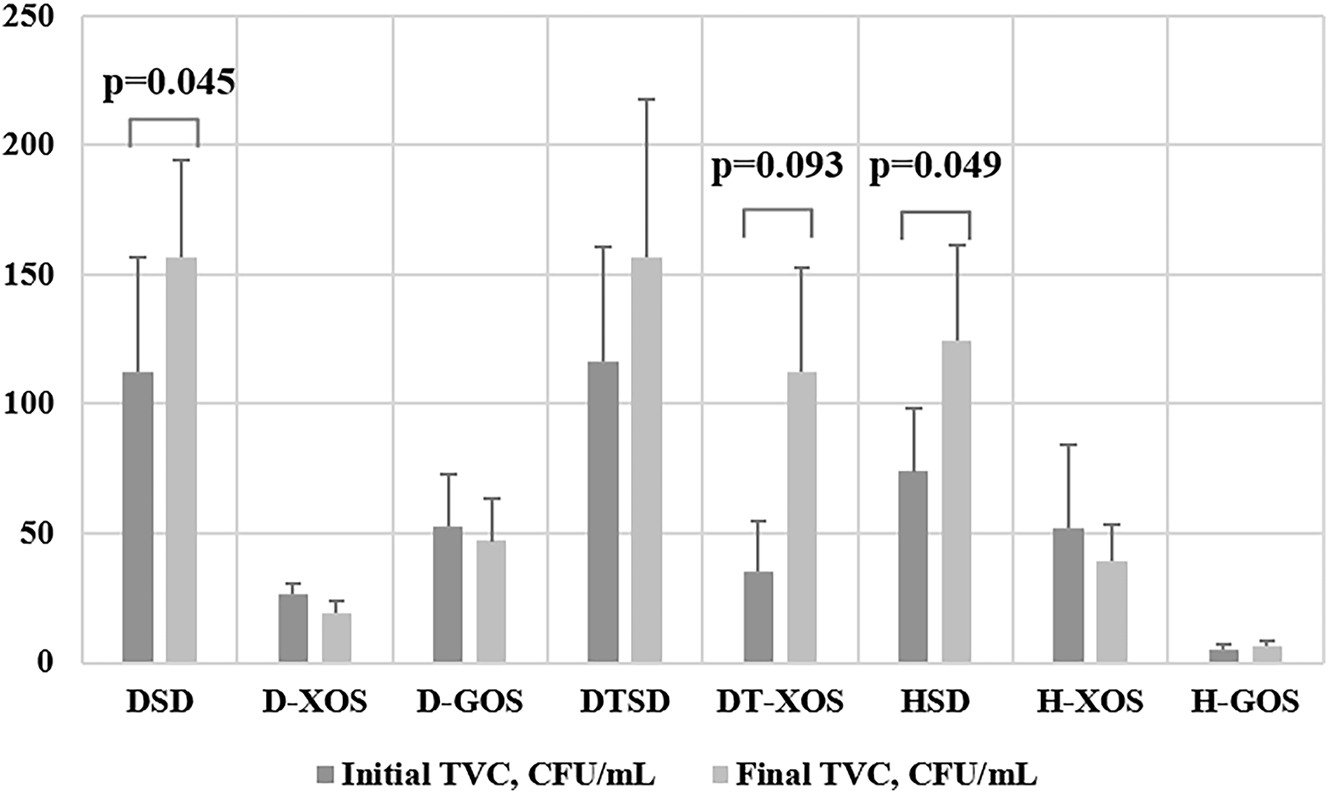
Effect of prebiotics and/or aerobic training on the TVC (paired samples t-test; results are presented as mean ± sem×104). DSD, diabetic control group on a standard diet; D-XOS, diabetic prebiotic group, treated with xylooligosaccharides; D-GOS, diabetic prebiotic group, treated with galactooligosaccharides; DTSD, diabetic trained group on a standard diet; DT-XOS, diabetic trained group, treated with xylooligosaccharides; HSD, healthy control group on a standard diet; H-XOS, healthy control group, treated with xylooligosaccharides; H-GOS, healthy control group, treated with galactooligosaccharides.
Discussion
In the present study we report that GOS and XOS and/or aerobic exercise beneficially affect the glucose homeostasis and growth but show no effect on intestinal microbiota composition of type 1 diabetic rats.
Treatment with GOS and XOS exerted a beneficial effect on the glycemic status of STZ-treated diabetic rats after a period of 6 weeks. These results follow the ones reported by Sangwan et al. and Gobinath et al. [6, 9]. A proposed mechanism for oligosaccharides’ blood glucose lowering effect includes reduced gastric emptying time, accompanied by shorter transit time through the small intestine [19].
In the present study, aerobic training did not significantly improve the hyperglycemic state of the diabetic rats. These results are in accordance with experimental data published by several authors [20, 21]. The lack of a positive effect of aerobic exercise on the blood glucose concentration may be a result of an insufficient duration of the training period. According to Li et al., the duration of physical activity is a key factor that determines its beneficial effects [22].
The obtained results regarding the growth of the animals show that GOS administration stimulates weight gain and increases the naso-anal length, thus beneficially affects the growth retardation of the diabetic rats. Prebiotics are shown to suppress the loss of glucose in the urine, typical of type 1 diabetes [23]. The amelioration of glucosuria and loss of calories is a possible mechanism for the observed improvement in the growth of diabetic animals. Similar results were also reported by Byung-Sung [24] and Gobinath et al. [6].
Aerobic training confers a positive effect on the growth of diabetic animals by significantly increasing their weight and naso-anal length compared to the diabetic controls. These results support the experimental data reported by Heyman et al. [25]. The beneficial effect of aerobic exercise on the weight of the diabetic rats could be explained with the improvement of glucose homeostasis, resulting from an increased insulin sensitivity, increased binding of insulin to its receptor, stimulation of glucose transporter-4 translocation to the muscle cell surface and thus, an improved glucose uptake [26]. The activation of the skeletal muscles also increases nitric oxide production in the endothelium, which stimulates blood glucose uptake [27].
The role of the intestinal microbiota in maintaining the normal physiology and homeostasis of the gut is essential [4]. In our experiment we studied the effect of prebiotic treatment and aerobic training on the composition of the intestinal microbiota on the species level focusing on the Bifidobacterium spp. and Lactobacillus spp. genera as well as the Enterobacteriaceae family. There are many reports on the effect of different prebiotics on the bacterial diversity on the phylum, family and genera level, but none on the level of the species. In the current study we report for the first time that prebiotic treatment and aerobic training alone or in combination do not lead to changes in the diversity of the studied bacterial species. The maintenance of bacterial balance is a proven effect of prebiotics [8]. The preservation of gut homeostasis is important for the development and function of the innate immune system and the regulation of the host’s immune responses [28].
Several scientific studies have reported that T1DM is associated with abnormally increased Bacteroidetes:Firmicutes ratio [28, 29]. Costa et al. reported that STZ treatment leads to alterations in the gut microbiota composition, including increased count of several bacterial genera, all associated with increased T1DM incidence [30]. Patterson et al. have observed an increase in the fecal count of several bacterial genera like Parasuterella, Bifidobacterium and Bacteroides five weeks post-STZ treatment. Furthermore, the dysbiosis resulting from T1DM changes the intestinal environment, allowing representatives of the Actinobacteria and Proteobacteria phyla to thrive [31]. In our study we found that diabetes leads to an increased TVC in sedentary rats on a standard diet. We assume that this increase could be associated with the above mentioned genera and species.
The results obtained in the present study demonstrate that aerobic training, alone and in combination with xylooligosaccharides, leads to an elevated microbial count in diabetic rats. It has been reported by several authors that exercise positively affects the gut microbiota by increasing the number of beneficial bacteria [12, 32]. Furthermore, controlled physical training is associated with an increased number of lactic acid-producing bacteria and ones that convert the produced lactate to butyrate – a compound that conveys beneficial effects regarding intestinal epithelium protection and mucin synthesis [4].
Conclusions
We conclude that prebiotic treatment beneficially affects the hyperglycemic status and growth of type 1 diabetic rats. The aerobic training was most effective in the improvement of the morphological parameters. The bacterial species identification showed no significant changes in the diversity of the microbiota under the influence of the studied factors. It indicated Bifidobacterium indicum, Lactobacillus feritoshensis and E. coli as the most abundant species amongst the analyzed genera.
Limitations
The main limitation of the experiment was the small sample size, which however is common in animal studies and is in accordance with the ethical guidelines. Although the TVC in the fecal samples of the experimental animals was determined, the study lacks evaluation on the count of the studied species, and we consider this to be another limitation of this scientific work. More thorough analysis of the gut microbiota will be needed for the exact effect of XOS, GOS and aerobic training on its abundance and count to be evaluated.
Funding source: Medical University of Plovdiv
Award Identifier / Grant number: Project â„– HO-07/2019
-
Research ethics: The research related to animals’ use has complied with all the relevant national regulations and institutional polices for the care and use of animals [Bulgarian Agency for Food Safety (BAFS) resolution №150/09.04.2019; Ethical standards of the Medical University of Plovdiv - resolution of the University Ethic Committee №2/13.06.2019].
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: Authors state no conflict of interest.
-
Research funding: This work was supported by the Medical University of Plovdiv [Project № HO-07/2019].
References
1. Ussar, S, Fujisaka, S, Kahn, CR. Interactions between host genetics and gut microbiome in diabetes and metabolic syndrome. Mol Metabol 2016;5:795–803. https://doi.org/10.1016/j.molmet.2016.07.004.Search in Google Scholar PubMed PubMed Central
2. Musso, G, Gambino, R, Cassader, M. Interactions between gut microbiota and host metabolism predisposing to obesity and diabetes. Annu Rev Med 2011;62:361–80. https://doi.org/10.1146/annurev-med-012510-175505.Search in Google Scholar PubMed
3. Holscher, HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microb 2017;8:172–84. https://doi.org/10.1080/19490976.2017.1290756.Search in Google Scholar PubMed PubMed Central
4. Monda, V, Villano, I, Messina, A, Valenzano, A, Esposito, T, Moscatelli, F, et al.. Exercise modifies the gut microbiota with positive health effects. Oxid Med Cell Longev 2017;2017:1–8. https://doi.org/10.1155/2017/3831972.Search in Google Scholar PubMed PubMed Central
5. Knip, M, Honkanen, J. Modulation of type 1 diabetes risk by the intestinal microbiome. Curr Diabetes Rep 2017;17:105. https://doi.org/10.1007/s11892-017-0933-9.Search in Google Scholar PubMed
6. Gobinath, D, Madhu, AN, Prashant, G, Srinivasan, K, Prapulla, SG. Beneficial effect of xylo-oligosaccharides and fructo-oligosaccharides in streptozotocin-induced diabetic rats. Br J Nutr 2010;104:40–7. https://doi.org/10.1017/s0007114510000243.Search in Google Scholar
7. Gibson, GR, Roberfroid, MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr 1995;125:1401–12. https://doi.org/10.1093/jn/125.6.1401.Search in Google Scholar PubMed
8. Lin, SH, Chou, LM, Chien, YW, Chang, JS, Lin, CI. Prebiotic effects of xylooligosaccharides on the improvement of microbiota balance in human subjects. Gastroenterol Res Pract 2016;2016:1–6. https://doi.org/10.1155/2016/5789232.Search in Google Scholar PubMed PubMed Central
9. Sangwan, V, Tomar, SK, Ali, B, Singh, RR, Singh, AK. Hypoglycaemic effect of galactooligosaccharides in alloxan-induced diabetic rats. J Dairy Res 2015;82:70–7. https://doi.org/10.1017/s0022029914000582.Search in Google Scholar
10. Petriz, BA, Castro, AP, Almeida, JA, Gomes, CP, Fernandes, GR, Kruger, RH, et al.. Exercise induction of gut microbiota modifications in obese, non-obese and hypertensive rats. BMC Genom 2014;15:511. https://doi.org/10.1186/1471-2164-15-511.Search in Google Scholar PubMed PubMed Central
11. Codella, R, Luzi, L, Terruzzi, I. Exercise has the guts: how physical activity may positively modulate gut microbiota in chronic and immune-based diseases. Dig Liver Dis 2018;50:331–41. https://doi.org/10.1016/j.dld.2017.11.016.Search in Google Scholar PubMed
12. Campbell, SC, Wisniewski, PJ, Noji, M, McGuinness, LR, Häggblom, MM, Lightfoot, SA, et al.. The effect of diet and exercise on intestinal integrity and microbial diversity in mice. PLoS One 2016;11:e0150502. https://doi.org/10.1371/journal.pone.0150502.Search in Google Scholar PubMed PubMed Central
13. Akbarzadeh, A, Norouzian, D, Mehrabi, MR, Jamshidi, S, Farhangi, A, Verdi, AA, et al.. Induction of diabetes by Streptozotocin in rats. Indian J Clin Biochem 2007;22:60–4. https://doi.org/10.1007/bf02913315.Search in Google Scholar PubMed PubMed Central
14. Wang, J, Cao, Y, Wang, C, Sun, B. Wheat bran xylooligosaccharides improve blood lipid metabolism and antioxidant status in rats fed a high-fat diet. Carbohydr Polym 2011;86:1192–7. https://doi.org/10.1016/j.carbpol.2011.06.014.Search in Google Scholar
15. Furman, BL. Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol 2015;70:5.47.1–20. https://doi.org/10.1002/0471141755.ph0547s70.Search in Google Scholar PubMed
16. Manchado-Gobatto, FB, Gobatto, CA, Contarteze, RVL, Papoti, M, De Mello, MAR. Maximal lactate steady state in running rats. J Exerc Physiol Online 2005;8:29–35.Search in Google Scholar
17. Pérez-Cano, FJ, Castell, M, Castellote, C. The suckling rat as a model for immunonutrition studies in early life. Clin Dev Immunol 2012;2012:1–16. https://doi.org/10.1155/2012/537310.Search in Google Scholar PubMed PubMed Central
18. Bernardis, LL, Bellinger, LL, Spinner, LI, Brooks, S. Feeding studies in weanling rats with dorsomedial hypothalamic lesions: effect of high fat and high carbohydrate diet and nutrient completeness on food choice and intake. J Nutr 1978;108:753–8. https://doi.org/10.1093/jn/108.5.753.Search in Google Scholar PubMed
19. Swennen, K, Courtin, CM, Delcour, JA. Non-digestible oligosaccharides with prebiotic properties. Crit Rev Food Sci Nutr 2006;46:459–71. https://doi.org/10.1080/10408390500215746.Search in Google Scholar PubMed
20. Huang, HH, Farmer, K, Windscheffel, J, Yost, K, Power, M, Wright, DE, et al.. Exercise increases insulin content and basal secretion in pancreatic islets in type 1 diabetic mice. Exp Diabetes Res 2011;2011:1–10. https://doi.org/10.1155/2011/481427.Search in Google Scholar PubMed PubMed Central
21. Yoon, H, Thakur, V, Isham, D, Fayad, M, Chattopadhyay, M. Moderate exercise training attenuates inflammatory mediators in DRG of Type 1 diabetic rats. Exp Neurol 2015;267:107–14. https://doi.org/10.1016/j.expneurol.2015.03.006.Search in Google Scholar PubMed
22. Li, J, Zhang, W, Guo, Q, Liu, X, Zhang, Q, Dong, R, et al.. Duration of exercise as a key determinant of improvement in insulin sensitivity in type 2 diabetes patients. Tohoku J Exp Med 2012;227:289–96. https://doi.org/10.1620/tjem.227.289.Search in Google Scholar PubMed
23. Imaizumi, K, Nakatsu, Y, Sato, M, Sedarnawati, Y, Sugano, M. Effects of xylooligosaccharides on blood glucose, serum and liver lipids and cecum short-chain fatty acids in diabetic rats. Agric Biol Chem 1991;55:199–205. https://doi.org/10.1080/00021369.1991.10870553.Search in Google Scholar
24. Byung-Sung, P. Effect of oral administration of Jerusalem artichoke inulin on reducing blood lipid and glucose in STZ-induced diabetic rats. J Anim Vet Adv 2011;10:2501–7. https://doi.org/10.3923/javaa.2011.2501.2507.Search in Google Scholar
25. Heyman, E, Toutain, C, Delamarche, P, Berthon, P, Briard, D, Youssef, H, et al.. Exercise training and cardiovascular risk factors in type 1 diabetic adolescent girls. Pediatr Exerc Sci 2007;19:408–19. https://doi.org/10.1123/pes.19.4.408.Search in Google Scholar PubMed
26. Crespilho, DM, de Almeida Leme, JA, de Mello, MA, Luciano, E. Effects of physical training on the immune system in diabetic rats. Int J Diabetes Dev Ctries 2010;30:33–7. https://doi.org/10.4103/0973-3930.60010.Search in Google Scholar PubMed PubMed Central
27. Cimbiz, A, Ozay, Y, Yurekdeler, N, Caycı, K, Colak, T, Caner, T, et al.. The effect of long-term exercise training on the blood glucose level and weight in alloxan administered mice. Sci Res Essays 2011;6:66–70.Search in Google Scholar
28. Mishra, SP, Wang, S, Nagpal, R, Miller, B, Singh, R, Taraphder, S, et al.. Probiotics and prebiotics for the amelioration of type 1 diabetes: present and future perspectives. Microorganisms 2019;7:67. https://doi.org/10.3390/microorganisms7030067.Search in Google Scholar PubMed PubMed Central
29. Han, H, Li, Y, Fang, J, Liu, G, Yin, J, Li, T, et al.. Gut microbiota and type 1 diabetes. Int J Mol Sci 2018;19:995. https://doi.org/10.3390/ijms19040995.Search in Google Scholar PubMed PubMed Central
30. Costa, FR, Françozo, MC, de Oliveira, GG, Ignacio, A, Castoldi, A, Zamboni, DS, et al.. Gut microbiota translocation to the pancreatic lymph nodes triggers NOD2 activation and contributes to T1D onset. J Exp Med 2016;213:1223–39. https://doi.org/10.1084/jem.20150744.Search in Google Scholar PubMed PubMed Central
31. Patterson, E, Marques, TM, O’Sullivan, O, Fitzgerald, P, Fitzgerald, GF, Cotter, PD, et al.. Streptozotocin-induced type-1-diabetes disease onset in Sprague-Dawley rats is associated with an altered intestinal microbiota composition and decreased diversity. Microbiology 2015;161:182–93. https://doi.org/10.1099/mic.0.082610-0.Search in Google Scholar PubMed
32. Quiroga, R, Nistal, E, Estébanez, B, Porras, D, Juárez-Fernández, M, Martínez-Flórez, S, et al.. Exercise training modulates the gut microbiota profile and impairs inflammatory signaling pathways in obese children. Exp Mol Med 2020;52:1048–61. https://doi.org/10.1038/s12276-020-0459-0.Search in Google Scholar PubMed PubMed Central
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/tjb-2023-0070).
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Review
- Metabolomics: a review of liquid chromatography mass spectrometry-based methods and clinical applications
- Opinion Papers
- Green transformation in the health sector and medical laboratories, adaptation to climate change in Türkiye
- Forward steps in green medical laboratory practices for a sustainable future
- Research Articles
- Comparison of the immunoassay method with the commercial and in-house LC-MS/MS methods for substance abuse in urine
- Peroxisome proliferator-activated receptor gamma and osteoprotegerin levels as an indicator and diagnostic predictor of endothelial dysfunction
- Gingival status and prophylactic oral hygiene measures modulate salivary amino acids’ profile in children with plaque-induced gingivitis
- FIB4 score is increased in severe preeclampsia
- The effects of a single dialysis session on serum hepcidin levels
- Gestational diabetes mellitus is associated with a low serum level of mitochondrial-derived peptide-MOTS-C
- Ideal timing of labor in terms of oxidative stress – which term period is best?
- Synergistic role of thymoquinone and 5-fluorouracil in U-251MG glioblastoma cell line
- Effect of oligosaccharides and aerobic training on hyperglycemia, growth and intestinal microbial diversity of diabetic rats
- Association of AdipoQ (G>T) gene polymorphism with obesity and hypertension in North Indian postmenopausal women of Punjab
- The attraction of paraoxonase-1 associated with the MAPK pathway on colon carcinoma cells
- Resveratrol modulates miRNA machinery proteins in different types of colon cancer cells
- The relationship between ASIC3 gene polymorphism and fibromyalgia syndrome
- Expression levels of genes involved in lipogenesis and cholesterol synthesis in adenomyosis
- Frequency of thrombophilia-associated mutations and polymorphisms in pregnant women with a history of thrombosis or pregnancy complications
Articles in the same Issue
- Frontmatter
- Review
- Metabolomics: a review of liquid chromatography mass spectrometry-based methods and clinical applications
- Opinion Papers
- Green transformation in the health sector and medical laboratories, adaptation to climate change in Türkiye
- Forward steps in green medical laboratory practices for a sustainable future
- Research Articles
- Comparison of the immunoassay method with the commercial and in-house LC-MS/MS methods for substance abuse in urine
- Peroxisome proliferator-activated receptor gamma and osteoprotegerin levels as an indicator and diagnostic predictor of endothelial dysfunction
- Gingival status and prophylactic oral hygiene measures modulate salivary amino acids’ profile in children with plaque-induced gingivitis
- FIB4 score is increased in severe preeclampsia
- The effects of a single dialysis session on serum hepcidin levels
- Gestational diabetes mellitus is associated with a low serum level of mitochondrial-derived peptide-MOTS-C
- Ideal timing of labor in terms of oxidative stress – which term period is best?
- Synergistic role of thymoquinone and 5-fluorouracil in U-251MG glioblastoma cell line
- Effect of oligosaccharides and aerobic training on hyperglycemia, growth and intestinal microbial diversity of diabetic rats
- Association of AdipoQ (G>T) gene polymorphism with obesity and hypertension in North Indian postmenopausal women of Punjab
- The attraction of paraoxonase-1 associated with the MAPK pathway on colon carcinoma cells
- Resveratrol modulates miRNA machinery proteins in different types of colon cancer cells
- The relationship between ASIC3 gene polymorphism and fibromyalgia syndrome
- Expression levels of genes involved in lipogenesis and cholesterol synthesis in adenomyosis
- Frequency of thrombophilia-associated mutations and polymorphisms in pregnant women with a history of thrombosis or pregnancy complications

