Abstract
Objective
The aim of this study was to synthesize ten 1,4-dihydropyridine (DHP) derivatives in which substituted cyclohexane rings were fused to the DHP ring and to determine how different ester groups and the benzoyl substituent introduced in 4-phenyl ring affected their calcium channel blocking activity.
Methods
A microwave-assisted one-pot method was applied for the synthesis of compound 1–5 according to a modified Hantzsch reaction. The benzoyl moiety was introduced in the 4-phenyl ring of these dihydropyridines by refluxing with benzoyl chloride in acetone in the presence of anhydrous potassium carbonate. Synthesized products were characterized by elemental analysis, IR, 1H-NMR and 13C-NMR spectroscopy. The inhibitory actions of compounds 1–10 on calcium channel blocking activity were tested on isolated rat aorta preparations.
Results
The obtained pharmacological results showed that although all compounds are potent relaxing agents on isolated rat aorta smooth muscle, introduction of a benzoyloxy substitiuent on the phenyl ring (compound 6–10) decreased the relaxant effect of these compunds.
Conclusion
The reported 1,4-DHP derivatives have calcium channel blocking activity on rat aorta smooth muscle.
Özet
Amaç
Bu çalışmanın amacı, sübstitüe siklohekzan halkasının 1,4-dihidropiridin (DHP) halkasına kaynaştırıldığı on DHP türevi sentezlemek ve farklı ester grupları ile 4-fenil halkasına eklenen benzoil sübstitüentinin kalsiyum kanal bloke edici aktiviteyi ne kadar etkilediğini belirlemektir.
Yöntem: 1-5
no’lu bileşiklerin sentezi için mikrodalga yardımıyla tek basamak olarak modifiye Hantzsch reaksiyonu uygulanmıştır. Benzoil grubu, bu dihidropiridinlerin 4-fenil halkasına, susuz potasyum karbonat varlığında aseton içinde benzoil klorür ile kaynatılarak sübstitüe edilmiştir. Sentezlenmiş bileşikler, eleman analizi, IR, 1H-NMR ve 13C-NMR spektroskopi ile karakterize edilmiştir. 1-10 no’lu bileşiklerin kalsiyum kanalı inhibe edici etkileri izole sıçan aort preparatları üzerinde test edilmiştir.
Sonuç
Elde edilen farmakolojik sonuçlar, tüm bileşiklerin izole sıçan aort düz kasında güçlü bir gevşetici ajan olmasına rağmen, fenil halkasına (bileşik 6-10) bir benzoiloksi sübstitüentinin katılmasının, bu bileşiklerin gevşetici etkisini azalttığını göstermiştir.
Tartışma
Bildirilen 1,4-DHP türevleri, sıçan aortu düz kası üzerinde kalsiyum kanal bloke edici aktiviteye sahiptir.
Introduction
Calcium channels play a critical role both in the normal biological functions and also in various pathological processes that occur in neuronal, muscle and neurosecretory cells [1], [2]. Although several types of calcium channels have been identified; L-type channels are typically confined to cell bodies and are responsible for regulating contractility in muscle cells [3], [4].
Calcium channel blockers are the class of drugs that inhibit selectively the calcium movement through voltage sensitive calcium channels [5], [6]. 1,4-dihydropyridines (DHPs) are an important class of L-type calcium channel blockers that are used to treat cardiovascular diseases such as hypertension and angina [7], [8]. Their principal target in the cardiovascular system is thought to be the Cav1.2 L-type calcium channel isoform [9].
The versatility of the 1,4-DHP scaffold, with its wide range of therapeutic benefits and high potency, has made 1,4-DHPs one of the most studied class of drugs since their introduction into clinical medicine. Important chemical modifications have been carried out on the structure of nifedipine, the prototype of DHPs (Figure 1), in order to elucidate the structure-activity relationships and enhance calcium modulating effects [6], [10].
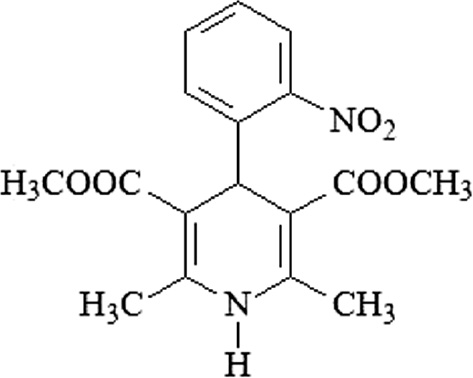
Structure of nifedipine.
Fused DHPs such as hexahydroquinolines, indenopyridines and acridines, which could be obtained by introducing the DHP ring into condensed ring systems, were active derivatives exhibiting calcium antagonistic effects [11], [12], [13]. It has been previously showed that L-type channel inhibition is sensitive to the substitution at the six-position of the hexahydroquinoline ring [14].
The nature and position of C-4-aryl ring substituents optimize activity. Although rather simple as well as more complex modifications such as introducing xanthone, indole and benzofuroxan into 4-position of the 1,4-DHP nucleus were carried out; previous studies have shown that the preferred substituent at the C-4 position of DHPs is a phenyl ring because of animal toxicity observed with heteroaromatic rings [9], [15], [16], [17]. The analysis among 4-phenyl-1,4-DHP analogs revealed that biological activity depends on the hydrophilic, electronic and steric properties of the substituents on the phenyl ring [18].
In addition, ester functionalities at C-3 and C-5 position are of utmost importance to modulate activity and tissue selectivity [19]. It has been reported that asymmetrical substituents in C-3 and C-5 alter the activity [20]. X-ray structural investigations, theoretical calculations of fused 1,4-DHPs indicated that at least one ester must be in the cis arrangement to the double bond of DHP to allow for hydrogen bonding to the receptor [9].
Among the performed modifications at C-3 and C-5, the introduction of bulky and lipophilic substituents as one of the esterifying groups led to novel, potent calcium antagonists including nicardipine, barnidipine and benidipine [21], [22], [23].
The aim of this study is to evaluate the relaxant effect of ten DHP derivatives in which substituted cyclohexane rings are fused to the DHP ring and determine how different ester groups and the addition of the second ester moiety affect the calcium channel block.
Materials and methods
Experimental chemistry
All chemicals used in this study were purchased from Aldrich and Fluka (Steinheim, Germany). Some reactions were carried out in Discover Microwave Apparatus (CEM). Thin layer chromatography (TLC) was run on Merck aluminium sheets, Silica gel 60 F254 (Darmstadt, Germany), mobile phase ethyl acetate-hexane: (1:1) and ultraviolet (UV) absorbing spots were detected by short-wavelength (254 nm) UV light (Camag UV Cabinet, Wiesloch, Germany). Melting points were determined on a Thomas Hoover Capillary Melting Point Apparatus (Philadelphia, PA, USA) and were uncorrected. Infrared spectra were recorded on a Perkin Elmer FT-IR Spectrum BX (USA). 1H-NMR and 13C-NMR spectra were obtained in dimethylsulphoxide (DMSO) solutions on a Varian Mercury 400, 400 MHz High Performance Digital FT-NMR Spectrometer (Palo Alto, CA, USA). Chemical shifts are reported in parts per million (ppm) relative to tetramethylsilane. Mass spectra were obtained on an Agilent 5973 Network Mass Selective Detector by electron ionization (Philadelphia, PA, USA). Elemental analyses were performed on a Leco CHNS-932 Elemental Analyzer (Philadelphia, PA, USA).
Synthesis
Synthesis of compound 1–10 has been described previously [24] but briefly: Compound 1–5 were achieved by the reaction of 4,4-dimethyl-1,3-cyclohexanedione, 5-nitrosalicylaldehyde, appropriate alkyl acetoacetate and ammonium acetate under microwave irradiation. In order to determine the effect of the second ester group; the benzoyl moiety was introduced in the 4-phenyl ring of these dihydropyridines by refluxing with benzoyl chloride in acetone in the presence of anhydrous potassium carbonate (compound 6–10).
General procedure for the preparation of alkyl 4-(2-hydroxy-5-nitrophenyl)-2,6,6-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylates (Compound 1–5)
One-pot four component mixture of 0.002 mol 4,4-dimethyl-1,3-cyclohexanedione, 0.002 mol 5-nitrosalicylaldehyde, 0.002 mol appropriate alkyl acetoacetate and 0.01 mol ammonium acetate was filled into 35 mL-microwave pressure vial and heated under microwave irradiation (power 50 W, maximum temperature 120°C) for 10 min in 5 mL methanol. After the reaction was completed, monitored by TLC, the reaction mixture was poured into ice-water, the obtained precipitate was filtered and crystallized from methanol-water.
General procedure for the preparation of alkyl 4-(2-(benzoyloxy)-5-nitrophenyl)-2,6,6-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylates (Compound 6–10)
0.001 mol synthesized 1,4-dihydropyridine derivative (Compound 1–5), 0.0015 mol benzoyl chloride and 2 g anhydrous potassium carbonate were refluxed in 15 mL acetone for 4 h. The resulting slurry was filtered out and the solvent was removed using a rotary evaporator. The obtained sticky residue was crystallized from ethanol-water to achieve the target compound.
The synthetic route used to prepare the compounds has been outlined in Figure 2.
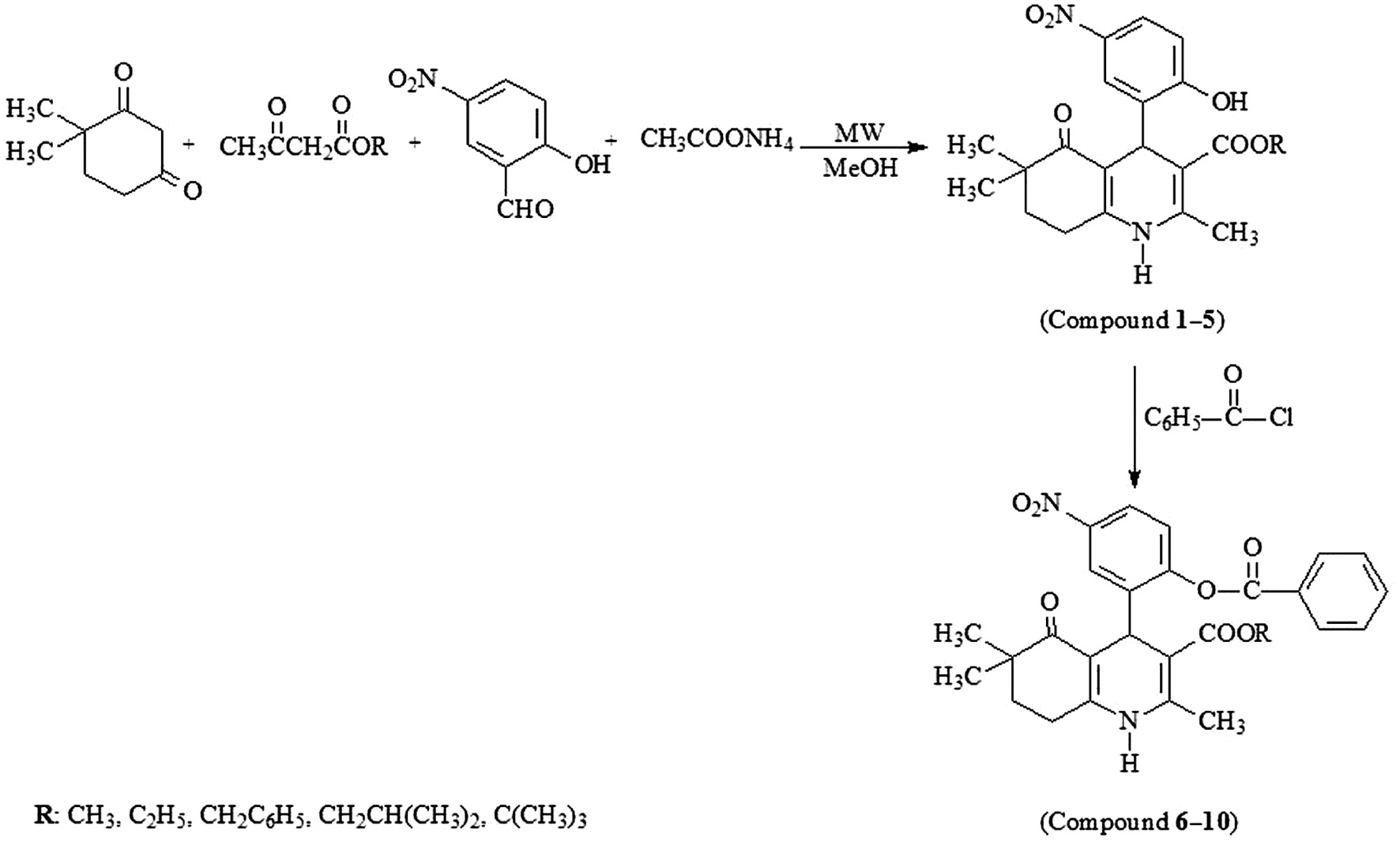
Synthesis of the compound 1–10.
Methyl 4-(2-hydroxy-5-nitrophenyl)-2,6,6-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (Compound 1)
Yield: 85%. m.p. 200–202°C. 1H-NMR (δ, DMSO-d6): 0.91 (3H; s; 6-CH3), 1.01 (3H; s; 6-CH3), 1.58–1.76 (2H; m; H-7), 2.30 (3H; s; 2-CH3), 2.49–2.55 (2H; m; H-8), 3.32 (1H; s; OH), 3.48 (3H; s; COOCH3), 4.99 (1H; s; 4-H), 6.69 (1H; d; J: 8.4 Hz; Ar-H3), 6.80 (1H; d; J: 2.8 Hz; Ar-H6), 6.98 (1H; dd; J: 2.4/8.4 Hz; Ar-H4), 9.42 (1H; s; NH). 13C-NMR (δ, DMSO-d6): 18.1, 22.9, 24.1, 24.9, 31.4, 38.8, 40.0, 50.3, 104.8, 110.7, 122.4, 125.0, 127.6, 129.7, 136.2, 142.2, 144.0, 149.3, 167.4, 199.5. MS (m/z): 386 [M]+. Anal. Calcd. for C20H22N2O6: C, 62.17; H, 5.74; N, 7.25. Found: C, 62.14; H, 5.76; N, 7.28.
Ethyl 4-(2-hydroxy-5-nitrophenyl)-2,6,6-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (Compound 2)
Yield: 82%. m.p. 208–210°C. 1H-NMR (δ, DMSO-d6): 0.89 (3H; s; 6-CH3), 1.00 (3H; s; 6-CH3), 1.11 (3H; t; J: 7.2 Hz; COOCH2CH3), 1.55–1.76 (2H; m; H-7), 2.30 (3H; s; 2-CH3), 2.48–2.54 (2H; m; H-8), 3.93 (1H; dq; COOCH2A-CH3), 4.09 (1H; dq; COOCH2B-CH3), 4.97 (1H; s; 4-H), 6.84 (1H; d; J: 9.2 Hz; Ar-H3), 7.81 (1H; d; J: 2.8 Hz; Ar-H6), 6.98 (1H; dd; J: 2.8/9.2 Hz; Ar-H4), 9.36 (1H; s; NH), 10.91 (1H; s; OH). 13C-NMR (δ, DMSO-d6): 13.8, 18.1, 23.0, 24.1, 24.9, 31.4, 34.0, 40.0, 58.8, 105.1, 110.8, 124.8, 125.0, 125.8, 126.1, 130.3, 143.9, 147.0, 149.2, 167.0, 199.5. MS (m/z): 399 [M−1]+. Anal. Calcd. for C21H24N2O6: C, 62.99; H, 6.04; N, 7.00. Found: C, 62.95; H, 6.01; N, 6.98.
Benzyl 4-(2-hydroxy-5-nitrophenyl)-2,6,6-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (Compound 3)
Yield: 80%. m.p. 185–187°C. 1H-NMR (δ, DMSO-d6): 0.90 (3H; s; 6-CH3), 1.00 (3H; s; 6-CH3), 1.58–1.77 (2H; m; H-7), 2.33 (3H; s; 2-CH3), 2.49–2.5 (2H; m; H-8), 3.33 (1H; s; OH), 4.90, 5.02 (2H; AB system; JAB=12.8 Hz, COOCH2C6H5), 5.00 (1H; s; 4-H), 6.84–7.91 (8H; m; Ar-H) 9.47 (1H; s; NH), 9.82 (1H; s; OH). 13C-NMR (δ, DMSO-d6): 18.3, 23.0, 24.1, 24.9, 31.4, 33.9, 40.0, 64.5, 104.7, 110.9, 124.9, 125.0, 125.6, 125.7, 126.1, 126.2, 127.5, 127.6, 128.1, 130.3, 132.7, 136.4, 146.7, 149.2, 166.7, 199.5. MS (m/z): 462 [M]+. Anal. Calcd. for C26H26N2O6: C, 67.52; H, 5.67; N, 6.06. Found: C, 67.56; H, 5.65; N, 6.10.
Isobutyl 4-(2-hydroxy-5-nitrophenyl)-2,6,6-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (Compound 4)
Yield: 78%. m.p. 170–171°C. 1H-NMR (δ, DMSO-d6): 0.71 (3H; d; J: 6.8 Hz;COOCH2CHCH3), 0.73 (3H; d; J: 6.8 Hz; COOCH2 CHCH3), 0.92 (3H; s; 6-CH3), 1.02 (3H; s; 6-CH3), 1.46–1.53 (1H; m; CH(CH3)2), 1.59–1.78 (2H; m; H-7), 2.34 (3H; s; 2-CH3), 2.48–2.56 (2H; m; H-8), 3.61 (1H; dd; J: 10.8/6.4 Hz; CH2ACH(CH3)2), 3.73 (1H; dd; J: 10.8/6.4 Hz; CH2BCH(CH3)2), 4.96 (1H; s; 4-H), 6.85 (1H; d; J: 8.8 Hz; Ar-H3), 7.80 (1H; d; J: 2.4 Hz; Ar-H6), 7.89 (1H; dd; J: 8.8/2.4 Hz; Ar-H4), 9.48 (1H; s; NH), 11.02 (1H; s; OH). 13C-NMR (δ, DMSO-d6): 18.4, 18.9, 19.2, 22.9, 24.1, 25.1, 27.2, 34.0, 36.2, 40.0, 69.2, 102.6, 109.1, 125.1, 126.6, 127.2, 127.5, 131.5, 135.2, 145.5, 149.6, 166.9, 199.5. MS (m/z): 428 [M]+. Anal. Calcd. for C23H28N2O6: C, 64.47; H, 6.59; N, 6.54. Found: C, 64.40; H, 6.61; N, 6.58.
Tert-butyl 4-(2-hydroxy-5-nitrophenyl)-2,6,6-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (Compound 5)
Yield: 82%. m.p. 198–200°C. 1H-NMR (δ, DMSO-d6): 0.86 (3H; s; 6-CH3), 1.01 (3H; s; 6-CH3), 1.21 (9H; s; COOC(CH3)3), 1.52–1.74 (2H; m; H-7), 2.22–2.43 (2H; m; H-8), 2.35 (3H; s; 2-CH3), 2.83 (1H; s; OH), 4.34 (1H; s; 4-H), 6.93 (1H; d; J: 9.2 Hz; Ar-H3), 7.91 (1H; dd; J: 2.4/9.2 Hz; Ar-H4), 7.98 (1H; d; J: 2.4 Hz; Ar-H6), 8.31 (1H; s; NH). 13C-NMR (δ, DMSO-d6): 18.0, 23.0, 24.1, 25.0, 27.7, 27.9, 28.1, 32.0, 33.9, 40.0, 78.8, 107.0, 110.2, 124.8, 125.8, 126.2, 127.5, 130.3, 132.7, 146.4, 149.5, 166.7, 199.4. MS (m/z): 428 [M]+. Anal. Calcd. for C23H28N2O6: C, 64.47; H, 6.59; N, 6.54. Found: C, 64.42; H, 6.55; N, 6.50.
Methyl 4-(2-(benzoyloxy)-5-nitrophenyl)-2,6,6-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (Compound 6)
Yield: 65%. m.p. 230–232°C. 1H-NMR (δ, DMSO-d6): 0.83 (3H; s; 6-CH3), 0.94 (3H; s; 6-CH3), 1.55–1.75 (2H; m; H-7), 2.20 (3H; s; 2-CH3), 2.49–2.56 (2H; m; H-8), 3.35 (3H; s; COOCH3), 5.22 (1H; s; 4-H), 7.43–8.25 (8H, m, Ar-H), 9.01 (1H; s; NH). 13C-NMR (δ, DMSO-d6): 18.3, 22.8, 24.1, 25.1, 34.0, 35.9, 40.0, 50.6, 102.5, 109.0, 124.8, 125.1, 125.7, 126.5, 127.2, 127.4, 127.6, 131.5, 132.8, 133.6, 138.9, 145.0, 145.4, 149.8, 167.4, 199.5, 200.5. MS (m/z): 489 [M−1]+. Anal. Calcd. for C27H26N2O7: C, 66.11; H, 5.34; N, 5.71. Found: C, 66.06; H, 5.33; N, 5.73.
Ethyl 4-(2-(benzoyloxy)-5-nitrophenyl)-2,6,6-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (Compound 7)
Yield: 68%. m.p. 236–238°C. 1H-NMR (δ, DMSO-d6): 0.83 (3H; s; 6-CH3), 0.92 (3H; s; 6-CH3), 0.99 (3H; t; J: 7.2 Hz; COOCH2CH3), 1.58–1.70 (2H; m; H-7), 2.05–2.37 (2H; m; H-8), 2.30 (3H; s; 2-CH3), 3.86 (1H; dq; COOCH2A-CH3), 3.89 (1H; dq; COOCH2B-CH3), 5.17 (1H; s; 4-H), 7.42–8.19 (8H, m, Ar-H), 8.86 (1H; s; NH). 13C-NMR (δ, DMSO-d6): 14.1, 18.3, 22.9, 24.1, 25.0, 34.1, 36.2, 40.0, 59.0, 102.9, 108.9, 125.10, 125.15, 125.7, 126.6, 127.2, 127.3, 127.6, 131.5, 132.7, 136.9, 138.2, 145.0, 145.1, 149.8, 166.9, 199.5, 200.4. MS (m/z): 504 [M]+. Anal. Calcd. for C28H28N2O7: C, 66.66; H, 5.59; N, 5.55. Found: C, 66.70; H, 5.61; N, 5.55.
Benzyl 4-(2-(benzoyloxy)-5-nitrophenyl)-2,6,6-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (Compound 8)
Yield: 57%. m.p. 182–183°C. 1H-NMR (δ, DMSO-d6): 0.81 (3H; s; 6-CH3), 0.91 (3H; s; 6-CH3), 1.60–1.71 (2H; m; H-7), 2.07–2.38 (2H; m; H-8), 2.16 (3H; s; 2-CH3), 4.92, 4.95 (2H; AB system; JAB=12.4 Hz, COOCH2C6H5), 5.16 (1H; s; 4-H), 7.05–8.13 (13H; m; Ar-H), 8.89 (1H; s; NH). 13C-NMR (δ, DMSO-d6): 18.8, 23.4, 24.6, 25.5, 34.5, 36.7, 40.6, 65.2, 102.9, 109.6, 125.6, 125.7, 126.1, 127.1, 127.7, 127.9, 128.11, 128.15, 128.2, 128.6, 132.1, 133.2, 135.1, 136.9, 137.1, 142.2, 143.1, 145.5, 146.4, 150.2, 167.1, 200.0, 200.9. MS (m/z): 565 [M−1]+. Anal. Calcd. for C33H30N2O7: C, 69.95; H, 5.34; N, 4.94. Found: C, 69.94; H, 5.33; N, 4.96.
Isobutyl 4-(2-(benzoyloxy)-5-nitrophenyl)-2,6,6-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (Compound 9)
Yield: 68%. m.p. 225–227°C. 1H-NMR (δ, DMSO-d6): 0.65 (3H; d; J: 6.8 Hz;COOCH2CHCH3), 0.74 (3H; d; J: 6.8 Hz; COOCH2CHCH3), 0.81 (3H; s; 6-CH3), 0.90 (3H; s; 6-CH3), 1.58–1.67 (2H; m; H-7), 1.68-1.73 (1H; m; CH(CH3)2), 2.02–2.38 (2H; m; H-8), 2.13 (3H; s; 2-CH3), 3.62 (1H; dd; J: 10.8/6.4 Hz; CH2ACH(CH3)2), 3.68 (1H; dd; J: 10.8/6.4 Hz; CH2BCH(CH3)2), 5.14 (1H; s; 4-H), 7.38–8.17 (8H; m; Ar-H), 8.81 (1H; s; NH). 13C-NMR (δ, DMSO-d6): 18.3, 18.5, 19.1, 23.0, 24.2, 25.1, 26.9, 34.5, 38.2, 41.1, 69.5, 101.9, 110.2, 125.5, 126.8, 127.2, 127.9, 128.5, 131.5, 133.5, 135.2, 136.4, 138.2, 139.3, 140.8, 145.5, 149.6, 166.9, 199.5, 200.4. MS (m/z): 532 [M]+. Anal. Calcd. for C30H32N2O7: C, 67.66; H, 6.06; N, 5.26. Found: C, 67.68; H, 6.07; N, 5.29.
Tert-butyl 4-(2-(benzoyloxy)-5-nitrophenyl)-2,6,6-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (Compound 10)
Yield: 70%. m.p. 235–237°C. 1H-NMR (δ, DMSO-d6): 0.83 (3H; s; 6-CH3), 0.90 (3H; s; 6-CH3), 1.26 (9H; s; COOC(CH3)3), 1.57–1.65 (2H; m; H-7), 1.86–2.31 (2H; m; H-8), 2.07 (3H; s; 2-CH3), 5.05 (1H; s; 4-H), 7.39–8.21 (8H; m; Ar-H), 8.61 (1H; s; NH). 13C-NMR (δ, DMSO-d6): 18.2, 23.5, 24.1, 25.1, 27.4, 27.8, 28.0, 34.1, 36.7, 40.0, 78.7, 102.1, 108.7, 125.0, 125.2, 125.7, 126.7, 127.2, 127.5, 127.9, 128.2, 131.5, 132.7, 135.5, 136.1, 143.8, 145.3, 166.4, 199.3, 200.4. MS (m/z): 532 [M]+. Anal. Calcd. for C30H32N2O7: C, 67.66; H, 6.06; N, 5.26. Found: C, 66.70; H, 6.03; N, 5.22. Spectral data of selected compounds are provided as supplementary material.
Pharmacology
The inhibitory actions of compounds 1–10 on calcium channel activity were tested on isolated rat aorta preparations according to the previous study [25]. Male Wistar albino rats weighing 200–250 g were used. Following the diethyl ether anesthesia, animals were sacrified by exsanguination and their thoraces were opened and the thoracic part of the aorta was gently removed. Isolated aorta was cleaned of the fat and connective tissues and then 3–5 mm wide rings were obtained. All these preperation procedures were conducted in Krebs-Henseleit solution gassed with carbogen (95% O2/5% CO2). Aorta rings were mounted in isolated organ baths containing 50 mL Ca2+-free Krebs-Henseleit solution (mmol: NaCl 118, KCl 4.7, MgSO4 1.2, NaHCO3 25, KH2PO4 1.2, glucose 11.5) and kept at 37°C and gassed with carbogen. A resting tension of ~1 g was applied and the muscle contractions were recorded using force-displacement transducer and digitized data acquisition system (PowerLab/8sp, Adinstruments, Australia). All aorta preperations were allowed to equilibrate in the Ca2+-free Krebs-Henseleit solution for about 45 min while washing out the tissues every ~15 min and subsequently high K+ (80 mM) Krebs-Henseleit solution without Ca2+ was applied. The rings were then contracted with 2.5 mM Ca2+. Following the maximal contractile response with Ca2+, data required for the concentration-response curves were obtained by cumulative administration of drugs under investigation. In order to achieve maximal relaxation at the end of cumulative drug administrations, all rings were treated with 10−4 M papaverine. For each drug, six trials were conducted, the obtained data was fit into a curve and EC50, pD2 and Emax values were calculated using GraphPad Prism 5 software (GraphPad, UK). The potencies of the compounds were compared to that of nifedipine. To exclude relaxations that can be induced by the mechanisms other than the Ca2+ channels, cyclooxygenase (COX), adrenergic and nitregic systems were all blocked by indomethacin (COX inhibitor, 10−5 M), guanethidine (an adrenergic nerve blocker, 10−6 M) and L-NAME (Nω-Nitro-L-arginine methyl ester hydrochloride, the nitric oxide synthase inhibitor, 10−4 M), respectively. All test compounds and nifedipine were dissolved in DMSO. The final concentration of DMSO was 0.1% and found to have no effect on aorta activity.
This study was approved by the Ethics Committee of Hacettepe University, Faculty of Medicine, Ankara, Turkey (Approval Number: 2013/47-04). All procedures involving animals and their care were conducted in conformity with international laws and policies.
Statistical analysis
The data were expressed as mean±standard error of themean (SEM). Statistical analysis was carried out using the GraphPad Prism 5. The differences were considered to be significant when p<0.05.
Results and discussion
Chemistry
A microwave-assisted one-pot method was applied for the synthesis of compound 1–5. These compounds were achieved by the reaction of 4,4-dimethyl-1,3-cyclohexanedione, 5-nitrosalicylaldehyde, alkyl acetoacetate and ammonium acetate under microwave irradiation in methanol, according to a modified Hantzsch reaction. The benzoyl moiety was introduced in the 4-phenyl ring of these dihydropyridines by refluxing with benzoyl chloride in acetone in the presence of anhydrous potassium carbonate (compound 6–10) [24]. Structures and chemical characteristics of the synthesized compounds are given in Table 1.
Structural data of the synthesized compounds.
| Compound | R | Melting point (°C) | Empirical formula | Molecular weight |
|---|---|---|---|---|
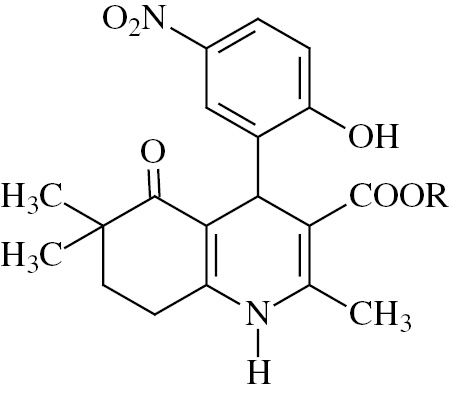 | ||||
| 1 | CH3 | 200–202 | C20H22N2O6 | 386 |
| 2 | C2H5 | 208–210 | C21H24N2O6 | 400 |
| 3 | CH2C6H5 | 185–187 | C26H26N2O6 | 462 |
| 4 | CH2CH(CH3)2 | 170–171 | C23H28N2O6 | 428 |
| 5 | C(CH3)3 | 198–200 | C23H28N2O6 | 428 |
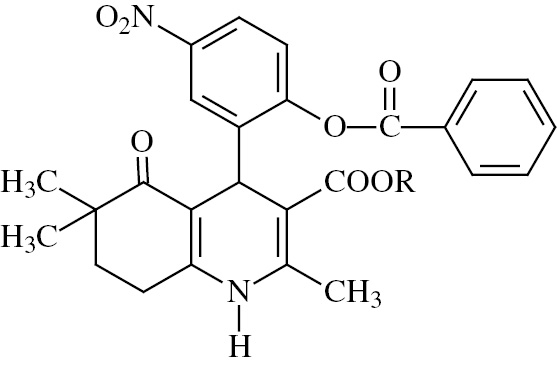 | ||||
| 6 | CH3 | 230–232 | C27H26N2O7 | 490 |
| 7 | C2H5 | 236–238 | C28H28N2O7 | 504 |
| 8 | CH2C6H5 | 182–183 | C33H30N2O7 | 566 |
| 9 | CH2CH(CH3)2 | 225–227 | C30H32N2O7 | 532 |
| 10 | C(CH3)3 | 235–237 | C30H32N2O7 | 532 |
The appearance of the products was monitored by TLC and the reaction time was determined for compound 1–5 as 10 min, which is quite a short time compared to conventional heating [26].
We reported the conventional synthesis of some compounds, which have similar structures to compound 1–5 in previous papers, so it is obvious that this microwave-assisted method reduces the solvent use and reaction time [11], [27], [28].
The structures of the compounds were confirmed by spectral methods 1H-NMR, 13C-NMR and mass spectra and elemental analysis.
In the 1H-NMR spectra, the protons of the methyl substituents at the six-position of the hexahydroquinoline ring were observed at 0.81–1.02 ppm separately and as singlets. The methylene groups of the same ring were at 1.52–2.56 ppm. The N-H protons of the DHP ring were seen at 8.31–9.82 ppm and the signal of O-H proton at the two-position of the phenyl ring disappeared after the introduction of the benzoyl moiety as the second ester group.
In the 13C-NMR spectra the number of the signals fitted exactly the number of carbon atoms.
The mass spectra of the compounds were recorded via the electron ionization technique. The molecular ion peak (M+) or the M−1 peak due to the aromatization of the dihydropyridine ring were seen in the spectra of all compounds. Cleavage of the ester groups and substituted phenyl rings from the parent molecule are the next most observed fragmentations.
Elemental analysis results were within±0.4% of the theoretical values for all compounds.
Pharmacology
The inhibitory actions of compounds 1–10 on calcium channel activity were tested on isolated rat aorta preparations. The maximum relaxant effects (Emax) and the negative logarithm of the concentration for the half-maximal inhibitory response values (pD2) of the compounds and nifedipine on isolated strips of rat aorta smooth muscle are given in Table 2. The pharmacological analysis of Ca2+ block action of all compounds yielded concentration-dependent responses in the rat aorta rings precontracted with Ca2+ (2.5 mM). While Emax values (a measure of efficacy) of compound 2, 3 and 4 were higher, the pD2 values (a measure of potency) of all compounds were found to be lower than that of nifedipine.
Emax and pD2 values on precontracted tissues with Ca2+ (2.5 mM) and high K+ of the compounds and nifedipine on rat aorta rings.
| Compound | Emax | pD2 |
|---|---|---|
| 1 | 95.92±1.87 | 6.20±0.14a |
| 2 | 97.65±1.89 | 5.94±0.13a |
| 3 | 97.64±0.75 | 7.24±0.23 |
| 4 | 97.38±2.30 | 6.94±0.37 |
| 5 | 95.74±1.58 | 7.16±0.49 |
| 6 | 94.50±1.92 | 6.27±0.48a |
| 7 | 76.93±8.31a | 5.57±0.83a |
| 8 | 77.45±6.15a | 5.44±0.39a |
| 9 | 68.09±4.50a | 4.91±0.28a |
| 10 | 67.26±0.87a | 6.25±0.08a |
| Nifedipine | 96.81±0.93 | 7.74±0.04 |
ap<0.001, compounds 1–10 were compared with nifedipine responses (n=6 for each compounds and nifedipine).
Pretreatment of the strips with indomethacin, guanethidine and L-NAME did not significantly alter the relaxant responses to the compounds indicating that cyclooxygenase, adrenergic and nitric oxide (NO) pathways do not play a role in relaxations evoked by these substances.
Given that the main difference between compound 1–5 and compound 6–10 is the second ester group (benzoyloxy substituent on the phenyl ring); this suggests that adding a bulky ring to the phenyl ring dramatically increases the size of the molecules (as shown in Figure 3) and may have a negative effect on the ability of these compounds to block calcium channel.
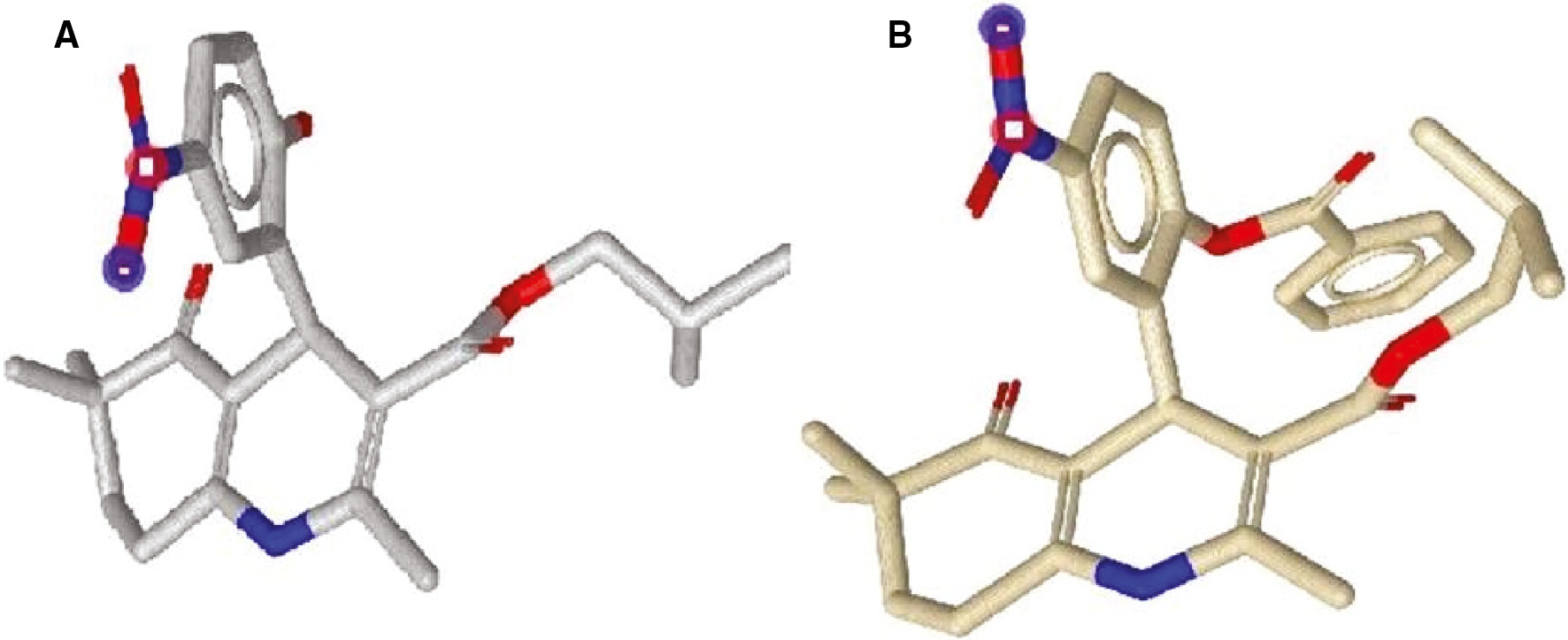
Geometrically optimized and energy minimized conformations of compound 4 (A) and compound 9 (B).
Although electron-withdrawing groups at the ortho- or meta-position of the 4-phenyl ring are important for L-type calcium channel blocking activity [9], the present study demonstrated that hydroxyl group at two-position of the phenyl ring also played a key role in the ability of these compounds to block calcium channels.
Two methyl groups at six-position of the hexahydroquinoline ring are present in all compounds thus they could not be the critical components for the relaxant effect. In compound 1–5 series, increasing the side chain of the ester group from methyl to ethyl and isobutyl, or introducing a ring structure (compound 3) at this position mediated a slight increase in relaxant activity.
A pharmacophore model was generated for compound 2, which was found to be more efficient than nifedipine and proved to block L-type calcium channel effectively [24]. Color-coded pharmacophore features are represented as follows: hydrophobic feature (yellow sphere), electron donor group (red vector), hydrogen bonding domain feature (green vector) and aromatic ring (blue circle) [29]. As demonstrated in Figure 4; the generated ligand-based pharmacophore model features have been found consistent with the reported structure-activity relationships for 1,4-DHP derivatives [6], [20], [30].
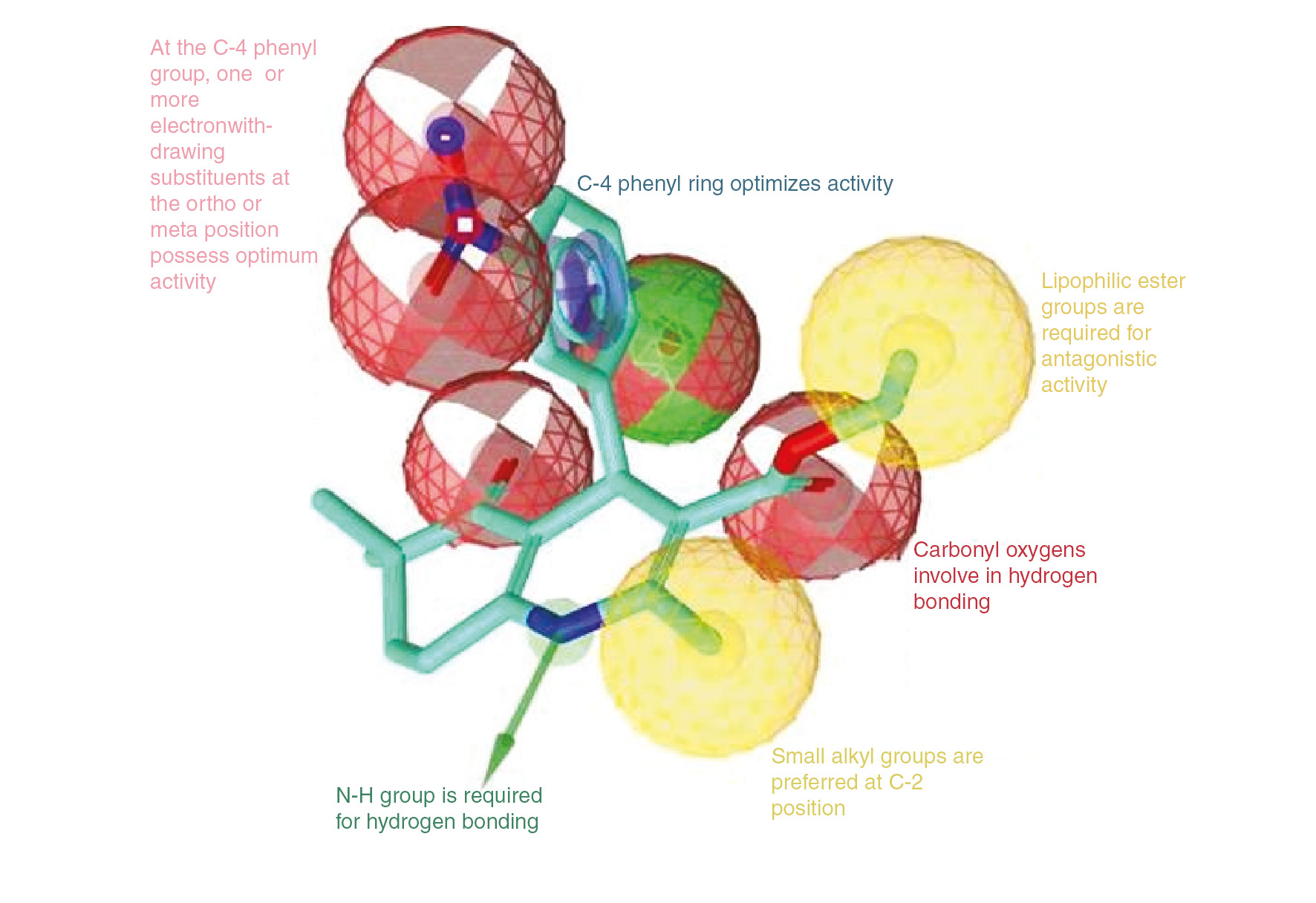
Structure-activity relationships for 1,4-DHPs binding to L-type calcium channels.
In summary, a series of condensed 1,4-DHPs as calcium channel blockers was reported in the study. The obtained pharmacological results showed that although all compounds are potent relaxing agents on isolated rat aorta smooth muscle, introduction of a benzoyloxy substitiuent on the phenyl ring (compound 6–10) decreased the relaxant effect of these compunds.
Acknowledgements
Alper B. Iskit has been supported by the Turkish Academy of Sciences, in the framework of the Young Scientist Award Program (EA-TUBA-GEBIP/2001-2-11).
Conflict of interest statement: The authors have no conflict of interest.
References
1. Dolphin AC. A short history of voltage-gated calcium channels. Br J Pharmacol 2006;147:S56–62.10.1038/sj.bjp.0706442Suche in Google Scholar
2. Camerino DC, Desaphy JF, Tricarico D, Pierno S, Liantonio A. Therapeutic approaches to ion channel diseases. Adv Genet 2008;64:81–145.10.1016/S0065-2660(08)00804-3Suche in Google Scholar
3. Carafoli E. Special issue: calcium signaling and disease – Preface. Biochem Bioph Res Co 2004;322:1097.10.1016/j.bbrc.2004.08.049Suche in Google Scholar
4. Zamponi GW. Antagonist binding sites of voltage-dependent calcium channels. Drug Develop Res 1997;42:131–43.10.1002/(SICI)1098-2299(199711/12)42:3/4<131::AID-DDR4>3.0.CO;2-RSuche in Google Scholar
5. Schleifer KJ. Stereoselective characterization of the 1,4-dihydropyridide binding site at L-type calcium channels in the resting state and the opened inactivated state. J Med Chem 1999;42:2204–11.10.1021/jm981114cSuche in Google Scholar
6. Edraki N, Mehdipour AR, Khoshneviszadeh M, Miri R. Dihydropyridines: evaluation of their current and future pharmacological applications. Drug Discov Today 2009;14:1058–66.10.1016/j.drudis.2009.08.004Suche in Google Scholar
7. Triggle DJ. 1,4-dihydropyridine calcium channel ligands: Selectivity of action. The roles of pharmacokinetics, state-dependent interactions, channel isoforms, and other factors. Drug Develop Res 2003;58:5–17.10.1002/ddr.10124Suche in Google Scholar
8. Safak C, Simsek R. Fused 1,4-dihydropyridines as potential calcium modulatory compounds. Mini-Rev Med Chem 2006;6:747–55.10.2174/138955706777698606Suche in Google Scholar
9. Goldmann S, Stoltefuss J. 1,4-Dihydropyridines – effects of chirality and conformation on the calcium-antagonist and calcium agonist activities. Angew Chem Int Edit 1991;30:1559–78.10.1002/anie.199115591Suche in Google Scholar
10. Gordeev MF, Patel DV, England BP, Jonnalagadda S, Combs JD, Gordon EM. Combinatorial synthesis and screening of a chemical library of 1,4-dihydropyridine calcium channel blockers. Bioorgan Med Chem 1998;6:883–9.10.1016/S0968-0896(98)00048-0Suche in Google Scholar
11. Safak C, Gunduz MG, Ilhan SO, Simsek R, Isli F, Yildirim S, et al. Synthesis and myorelaxant activity of fused 1,4-dihydropyridines on isolated rabbit gastric fundus. Drug Develop Res 2012;73:332–42.10.1002/ddr.21024Suche in Google Scholar
12. Rose U. 5-Oxo-1,4-Dihydroindenopyridines – calcium modulators with partial calcium agonistic activity. J Heterocyclic Chem 1990;27:237–42.10.1002/jhet.5570270223Suche in Google Scholar
13. Tu SJ, Miao CB, Fang F, Feng YJ, Li TJ, Zhuang QY, et al. New potential calcium channel modulators: design and synthesis of compounds containing two pyridine, pyrimidine, pyridone, quinoline and acridine units under microwave irradiation. Bioorg Med Chem Lett 2004;14:1533–6.10.1016/j.bmcl.2003.12.092Suche in Google Scholar
14. Lipkind GM, Fozzard HA. Molecular modeling of interactions of dihydropyridines and phenylalkylamines with the inner pore of the L-type Ca2+ channel. Mol Pharmacol 2003;63:499–511.10.1124/mol.63.3.499Suche in Google Scholar
15. El-Khouly A, Gunduz MG, Cengelli C, Simsek R, Erol K, Safak C, et al. Microwave-assisted synthesis and spasmolytic activity of 4-indolylhexahydroquinoline derivatives. Drug research 2013;63:579–85.10.1055/s-0033-1348261Suche in Google Scholar
16. Bisi A, Budriesi R, Rampa A, Fabbri G, Chiarini A, Valenti P. Synthesis and pharmacological profile of some chloroxanthone-1,4-dihydropyridine derivatives. Arzneimittel-Forsch 1996;46:848–51.10.1002/chin.199702123Suche in Google Scholar
17. Ermondi G, Visentin S, Boschi D, Fruttero R, Gasco A. Structural investigation of Ca2+ antagonists benzofurazanyl and benzofuroxanyl-1,4-dihydropyridines. J Mol Struct 2000;523:149–62.10.1016/S0022-2860(99)00386-5Suche in Google Scholar
18. Coburn RA, Wierzba M, Suto MJ, Solo AJ, Triggle AM, Triggle DJ. 1,4-Dihydropyridine antagonist activities at the calcium-channel – a quantitative structure activity relationship approach. J Med Chem 1988;31:2103–7.10.1021/jm00119a009Suche in Google Scholar
19. Miri R, Javidnia K, Sarkarzadeh H, Hemmateenejad B. Synthesis, study of 3D structures, and pharmacological activities of lipophilic nitroimidazolyl-1,4-dihydropyridines as calcium channel antagonist. Bioorgan Med Chem 2006;14:4842–9.10.1016/j.bmc.2006.03.016Suche in Google Scholar
20. Ioan P, Carosati E, Micucci M, Cruciani G, Broccatelli F, Zhorov BS, et al. 1,4-Dihydropyridine scaffold in medicinal chemistry, the story so far and perspectives (Part 1): action in ion channels and GPCRs. Curr Med Chem 2011;18:4901–22.10.2174/092986711797535173Suche in Google Scholar
21. Leonardi A, Motta G, Pennini R, Testa R, Sironi G, Catto A, et al. Asymmetric N-(3,3-diphenylpropyl)amimoalkyl esters of 4-aryl-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylic acids with antihypertensive activity. Eur J Med Chem 1998;33:399–420.10.1016/S0223-5234(98)80015-9Suche in Google Scholar
22. Tamazawa K, Arima H, Kojima T, Isomura Y, Okada M, Fujita S, et al. Stereoselectivity of a potent calcium-antagonist, 1-benzyl-3-pyrrolidinyl methyl 2,6-dimethyl-4-(Meta-Nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate. J Med Chem 1986;29:2504–11.10.1021/jm00162a013Suche in Google Scholar
23. Gkogkos K, Pavlidis G, Karakozoglou A, Kopras A, Memi E, Tsoutsouli V. Barnidipine: assessment of safety and efficacy: 1 year experience. J Hypertens 2006;24:S32.Suche in Google Scholar
24. Bladen C, Gunduz MG, Simsek R, Safak C, Zamponi GW. Synthesis and evaluation of 1,4-dihydropyridine derivatives with calcium channel blocking activity. Pflugers Archiv 2014;466:1355–63.10.1007/s00424-013-1376-zSuche in Google Scholar
25. Ozer EK, Gunduz MG, El-Khouly A, Sara MY, Simsek R, Iskit AB, et al. Microwave-assisted synthesis of condensed 1,4-dihydropyridines as potential calcium channel modulators. Turk J Chem 2015;39:886–96.10.3906/kim-1412-72Suche in Google Scholar
26. Lidstrom P, Tierney J, Wathey B, Westman J. Microwave assisted organic synthesis – a review. Tetrahedron 2001;57:9225–83.10.1016/S0040-4020(01)00906-1Suche in Google Scholar
27. Gunduz MG, Safak C, Kaygisiz B, Kosar BC, Simsek R, Erol K, et al. Synthesis of cyclopentapyridine and thienopyridine derivatives as potential calcium channel modulators. Arzneimittelforsch 2012;62:167–75.10.1055/s-0031-1299744Suche in Google Scholar PubMed
28. Gunduz MG, Ozturk GS, Vural IM, Simsek R, Sarioglu Y, Safak C. Evaluation of myorelaxant activity of 7-substituted hexahydroquinoline derivatives in isolated rabbit gastric fundus. Eur J Med Chem 2008;43:562–8.10.1002/chin.200830146Suche in Google Scholar
29. Wolber G, Langer T. LigandScout: 3-d pharmacophores derived from protein-bound Ligands and their use as virtual screening filters. J Chem Inf Model 2005;45:160–9.10.1021/ci049885eSuche in Google Scholar PubMed
30. Carosati E, Ioan P, Micucci M, Broccatelli F, Cruciani G, Zhorov BS, et al. 1,4-Dihydropyridine scaffold in medicinal chemistry, the story so far and perspectives (Part 2): action in other targets and antitargets. Curr Med Chem 2012;19:4306–23.10.2174/092986712802884204Suche in Google Scholar PubMed
Supplementary Material
The online version of this article (DOI: https://doi.org/10.1515/tjb-2016-0247) offers supplementary material, available to authorized users.
©2018 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Research Articles
- Effects of calcium hydroxide and N-acetylcysteine on MMP-2, MMP-9, TIMP-1 and TIMP-2 in LPS-stimulated macrophage cell lines
- Synthesis of fused 1,4-dihydropyridines as potential calcium channel blockers
- Optimization of fermentation conditions for efficient ethanol production by Mucor hiemalis
- Covalent immobilization of an alkaline protease from Bacillus licheniformis
- Major biological activities and protein profiles of skin secretions of Lissotriton vulgaris and Triturus ivanbureschi
- Optimized production, purification and molecular characterization of fungal laccase through Alternaria alternata
- Adsorption of methyl violet from aqueous solution using brown algae Padina sanctae-crucis
- Protective effect of dexpanthenol (vitamin B5) in a rat model of LPS-induced endotoxic shock
- Purification and biochemical characterization of a β-cyanoalanine synthase expressed in germinating seeds of Sorghum bicolor (L.) moench
- Molecular cloning and in silico characterization of two alpha-like neurotoxins and one metalloproteinase from the maxilllipeds of the centipede Scolopendra subspinipes mutilans
- Improvement of delta-endotoxin production from local Bacillus thuringiensis Se13 using Taguchi’s orthogonal array methodology
- Enhancing vitamin B12 content in co-fermented soy-milk via a Lotka Volterra model
- Species and number of bacterium may alternate IL-1β levels in the odontogenic cyst fluid
- Rheo-chemical characterization of exopolysaccharides produced by plant growth promoting rhizobacteria
- Benzo(a)pyrene degradation pathway in Bacillus subtilis BMT4i (MTCC 9447)
- Indices
- Reviewers 2018
- Yazar Dizini/Author Index
Artikel in diesem Heft
- Frontmatter
- Research Articles
- Effects of calcium hydroxide and N-acetylcysteine on MMP-2, MMP-9, TIMP-1 and TIMP-2 in LPS-stimulated macrophage cell lines
- Synthesis of fused 1,4-dihydropyridines as potential calcium channel blockers
- Optimization of fermentation conditions for efficient ethanol production by Mucor hiemalis
- Covalent immobilization of an alkaline protease from Bacillus licheniformis
- Major biological activities and protein profiles of skin secretions of Lissotriton vulgaris and Triturus ivanbureschi
- Optimized production, purification and molecular characterization of fungal laccase through Alternaria alternata
- Adsorption of methyl violet from aqueous solution using brown algae Padina sanctae-crucis
- Protective effect of dexpanthenol (vitamin B5) in a rat model of LPS-induced endotoxic shock
- Purification and biochemical characterization of a β-cyanoalanine synthase expressed in germinating seeds of Sorghum bicolor (L.) moench
- Molecular cloning and in silico characterization of two alpha-like neurotoxins and one metalloproteinase from the maxilllipeds of the centipede Scolopendra subspinipes mutilans
- Improvement of delta-endotoxin production from local Bacillus thuringiensis Se13 using Taguchi’s orthogonal array methodology
- Enhancing vitamin B12 content in co-fermented soy-milk via a Lotka Volterra model
- Species and number of bacterium may alternate IL-1β levels in the odontogenic cyst fluid
- Rheo-chemical characterization of exopolysaccharides produced by plant growth promoting rhizobacteria
- Benzo(a)pyrene degradation pathway in Bacillus subtilis BMT4i (MTCC 9447)
- Indices
- Reviewers 2018
- Yazar Dizini/Author Index

