Abstract
Soybean products are popular because of its taste, digestibility, and health benefits. However, soybean lacks vitamin, mainly the low water-soluble vitamin B12. This study investigated the effects of fermentation conditions on the synthesis of vitamin B12, production of metabolites, and growth of Lactobacillus reuteri and Propionibacterium shermainii in fermented soy-milk. A Lotka Volterra model was successfully employed to describe the competition relationship between the two microorganisms under various fermentation conditions. A quadratic function between the ratio of interaction coefficients and vitamin B12 content was found. Higher vitamin B12 in soy-milk can be produced when the ratio of interaction coefficients approach to one. Compared with other fermented soybean products, fermented soy-milk contains more acetate, ethanol, and propionic acid. This study successfully demonstrated a mathematical model to enhance soy-milk vitamin B12 production.
Özet
Soya ürünleri, tadı, sindirilebilirliği ve sağlık yararları nedeniyle popülerdir. Bununla birlikte, soya fasulyesi, esas olarak düşük suda çözünür B12 vitamini olan vitaminlerden yoksundur. Bu çalışmada fermantasyon koşullarının fermente soya sütlerinde B12 vitamini sentezi, metabolit üretimi ve Lactobacillus reuteri ve Propionibacterium shermainii gelişimi üzerine etkileri araştırıldı. Bir Lotka Volterra modeli, çeşitli fermantasyon koşulları altında iki mikroorganizma arasındaki rekabet ilişkisini tanımlamak için başarıyla kullanılmıştır. Etkileşim katsayıları ve B12 vitamini içeriği arasındaki ikinci dereceden bir fonksiyon bulunmuştur. Etkileşim katsayıları oranı bire yaklaştığında soya sütünde daha yüksek B12 vitamini üretilebilir. Diğer fermente soya fasülyesi ürünleri ile karşılaştırıldığında, mayalanmış soya sütü daha fazla asetat, etanol ve propiyonik asit içerir. Bu çalışma, soya sütü B12 üretimini arttırmak için bir matematik modelini başarıyla göstermiştir.
Introduction
Soybean products have been demonstrated to be a good substitution of meat protein due to its similar taste, high digestibility and health benefits [1, 2]. Traditional tofu, as a typical oriental soybean product, is made through a serial process of soaking, grinding, heating, fiber removal, coagulation, and pressing [3]. Furu and stinky tofu are fermented tofu products with brine by mold and bacteria [4]. Compared to soy-milk, these fermented products have no or less characteristic beany flavor and the bitter or astringent taste [5]. They are normally inexpensive and highly nutritious, therefore, studies had demonstrated them to be substitutions of meat; when cooked together with vegetables or in soups as a high quality protein supplementation, they are also suitable for the seniors and vegetarians [3]. However, sufu and stinky tofu have high concentration of salt and ammonia [3, 6], which is not acceptable for many customers. A high sodium daily-intake might cause a long-term risk of cardiovascular issues [7]. Soy-milk, on the other hand, does not have high salt and ammonia, but it contains a low vitamin content, especially the water-soluble vitamin B12 [8]. Vitamin B12 (cobalamin) works as a cofactor involved in a variety of enzymatic reactions [9]. Vitamin B12 deficiency might lead to the disturbance in cell division, neuropathy, nervous system disease, and pernicious anemia [10]. To prevent such fatal deficiency diseases, 2.4 μg of vitamin B12 daily intake is suggested [11]. Vitamin B12 is exclusively synthesized by certain bacteria and archaea, and is accumulated in predators’ bodies in the food chain [12].
A co-fermentation with Propionibacterium shermanii and Lactobacillus reuteri was employed to solve the problem of low cobalamin concentrations in fermented soy-milk. Propionibacterium shermanii with an ability of high cobalamin production prefers to consume lactate as the main energy and carbon source [13, 14]. This can reduce the stress of lactate on L. reuteri and retard the decrease of pH. Moreover, Propionibacterium spp. has a 100-fold stronger activity of hydrolyzing triglycerides of fat, compared with lactic acid bacteria [15]. Hence glycerol produced by P. shermanii induces vitamin B12 dependent enzyme in L. reuteri. The synthesis of DMBI 5,6-dimethylbenzimidazole, an important precursor of cobalamin, can only be formed in the presence of oxygen by Propionibacterium freudenreichii and P. shermanii [16]. Because of this, after several days of fermentation under anaerobic conditions with P. freudenreichii or P. shermanii, the fermentation should be switched to aerobic conditions. However, Santos et al. [17] found that L. reuteri has the ability to form DMBI without oxygen. In previous work, vitamin B12 production from co-fermentation was obviously higher than those from L. reuteri or P. shermanii single fermentations [18], indicating a big influence of fermentation type on the interaction of bacteria and synthesis of vitamin B12. A Lotka Volterra model of competition, historically proposed in ecology as a mechanistic model, was introduced into this work to interpret the interactions between two microorganisms under different conditions. Coefficients were used to explain the underling mechanism. This model has been introduced into several experiments such as the growth of LAB, coliforms, pseudomonads, Brochothrix, Salmonella, and yeasts on sliced pork shoulder; growth of Aeromonas hydrophila on fish; and interactions of yeast to yeast and yeast to bacterium during the ripening of cheeses [19]. As to our knowledge, this model can be applied to provide guidance and evaluate results of co-fermentation.
Materials and methods
Microorganisms and culture conditions
Lactobacillus reuteri ZJ03 and P. shermanii ZJ01 were taken from the culture collection of Key Laboratory for Food Safety of Zhejiang Province. The stock of cells was maintained in glycerol 50% (v/v) at −70°C. The bacteria were propagated in vitamin B12 test broth (Luqiao, Beijing, China) in standing cultures overnight at 37°C.
One milliliter of fermented soy-milk was mixed with 9 mL of saline solution. The sample was diluted to corresponding concentrations and spread onto solid agars such as MRS (pH of 5.0) and NaLa agar [20]. MRS agar was incubated at 37°C for 72 h and NaLa agar was incubated at 30°C for 7 days. Lactobacillus reuteri ZJ03 was counted from MRS agar of the white shiny smooth colonies. Propionibacterium shermanii ZJ01 was identified and counted from NaLa agar by the morphology of 1.0–2.5 mm, dull brown, lighter margin colonies. A subtraction method, as a control, could also be used to calculate counts of Propionibacteria by subtracting the number of L. reuteri from the total count in NaLa agar.
Fermentation
A basic medium in a glass flask was prepared using 100 mL soy-milk, 5 g glucose, 1 mL of 108 CFU/mL of Lactobacillus reuteri inoculum and 1 mL of 108 CFU of P. shermanii inoculum. The medium were adjusted pH to 6.5 and conducted at 30°C, if it was not mentioned separately.
To study the effect of pH on fermentations, initial pH values were adjusted to 6.0, 6.5, 7.0, 7.5, and 8.0 for this set of experiments. To study the effect of temperature on fermentations, experiments were conducted under 28, 30, 35, and 37°C. To study the combination effects of aerobic/anaerobic fermentation, experiments were conducted respectively under 6-day anaerobic and 1-day aerobic, 5-day anaerobic and 2-day aerobic, and 4-day anaerobic and 3-day aerobic fermentation conditions, respectively. All other trials, unless specified, were carried out at 30°C under 5-day anaerobic followed by 2-day aerobic fermentation conditions.
Determination of vitamin B12 and metabolites
Vitamin B12 was analyzed using a microbiological assay modified by Denter [21] and HPLC method [22]. Vitamin B12 was extracted from 1 mL of soy-milk with 10 mL sodium acetate buffer (pH 6.0) in present of KCN and heated in a water bath for 30 min at 70°C. Then, the pH of the solution was adjusted to 7.0 and mixed with 10 mL hexane (Extra pure N-hexane, Merck, Darmstadt, Germany) then centrifuged for 15 min at 4010 g (Varifuge 3.0, Heraeus centrifuge, Heraeus Instruments, Hanau, Germany). The aqueous phase was collected and passed through a solid phase extraction column (SPE) (CEC181M6 United Chemical Technologies, Bristol, PA, USA), which had been washed by 3 mL methanol (Merck, Darmstadt, Germany) and 3 mL double distilled water (DDW) [from Reversed osmosis Mill-Q water (18 Ω) (Millipore, Billerica, MA, USA)], with the aid of a pump (AL 15, Knf Neuberger, Hamburg, Germany) to control the speed of drops at one drop per second. After 3 times washing by ultra purified water, 3 mL methanol was used as the eluate. After the solvent was evaporated to dry, the residue was dissolved by 1 mL ultra purified water. The solvent was filtered through a membrane filter (0.2 μm) (Macherey-Nagel, Düren, Germany) and the filtrate was analyzed by HPLC (Waters E2695, USA) using a RP-18 column (250*4 mm I.D., 5 μm, Merck, Darmstadt, Germany).
All chromatographic separations were carried out at room temperature. A flow of 0.5 mL/min, methanol with 0.1% formic acid (A) (Merck, Darmstadt, Germany) and ultra purified water with 0.1% formic acid (B), which were degassed by an ultrasonic water bath (Sonorex TK 52, ultrasonic waterbath, Bandelin electronics, Berlin, Germany), were used as mobile phases and the gradient elution was programmed as follows; 0–2 min 20% A; 2–3 min 20–25% A; 3–11 min 25–35% A; 11–19 min 35–20% A; 20–22 min 100–100% A; 22–26 min 100–20% A; 26–36 min 20% A. The injection volume was 100 μL and the column eluate was monitored by Diode Array Detector (Waters, USA) at 361 nm wavelength.
Metabolites were detected using HPLC (Waters E2695, USA) with an organic acid column (850 BP-OA H+, 300*7.8 mm, Benson Polymeric, Sparks, USA) as a solid phase. All chromatographic separations were carried out at 60°C. A flow of 0.6 mL/min with 26 mM sulfuric acid was used as the mobile phase. The injection volume was 10 μL and the column eluate was monitored by Lachrom RI Dectector. One milliliter fermentation liquid was centrifuged for 10 min at 17,000 g (Biofuge pico centrifuge, Heraeus Instruments, Hanau, Germany). Ten microliter supernatant was injected into HPLC for analysis.
Lotka Volterra model development/implementation
An assumption was made that both microorganisms grew naturally without any inhibition from themselves. L and P stand for CFU of L. reuteri and P. shermanii at time t. Q1 and Q2 respectively represent the physiological state of both microorganisms. μmaxL and μmaxP separately show the maximum species growth rate. Lmax and Pmax stand for the maximum CFU during fermentation. The coefficients of a and b means the interspecific competition parameters of P. shermanii on L. reuteri, vice versa.
According to the assumption made, Qi/(1+Qi) was set as 1. The integration of equation was made from ti−1 to ti. The differential equations (Eq. 1, Eq. 2) can not be solved manually. Hence, least squares method was introduced to estimate the coefficients of a and b using Matlab (Version 5.3.0.10183, Mathworks Inc., Natick, MA, USA). The transpose of A is AT.
Results and discussions
Temperature effects on co-fermentation
Temperature displayed a mild influence on interactions between these bacteria. With the increase of temperature, the inhibition effects of both bacteria increased (Table 1). In this study (Figure 1), 30°C is an optimal temperature for co-fermentation to produce vitamin B12. An unexpected drop in cell numbers for L. reuteri and P. shermanii was observed from 28 to 37°C (Figure 2). The decrease of final concentrations of ethanol (Table 2) and increase of final concentrations of propionic acid (Table 2) from 28 to 37°C can be recognized as a reason for low cobalamin production and low cell densities of L. reuteri, confirmed by increasing values of interaction coefficient a (Table 1). Our data suggested that dramatic changes of metabolism were observed at 37°C. It is may due to the suitable temperature for L. reuteri to produce more lactate. Meanwhile, P. shermanii prefers to utilize lactate as primary carbon resources, leading to increasing of propionic acid. There is no report about the relationship between temperature and cobalamin production before. But some researchers [14, 23, 24, 25] reported a correlation between temperature and 3-HPA production or propionic acid, in which a cobalamin dependent enzyme was involved. Doleyres et al. [23] reported no significant difference in 3-HPA production at temperatures between 15 and 37°C. Another contradictory result suggested that 3-HPA production at 37°C was significantly higher than at other temperatures in any kind of media [25]. The optimal growth temperature for Propionibacterium spp. is however, almost 30°C [24].
Interaction coefficients (a and b) under different fermentation conditions.
| Conditions | Interaction coefficients | |
|---|---|---|
| a | b | |
| Aerobic for 1 day | −43.59±2.89 | −30.66±4.93 |
| Aerobic for 2 days | −0.16±0.08 | −0.83±0.12 |
| Aerobic for 3 days | 0.01±0.01 | 0.02±0.01 |
| pH 6.0 | 0.45±0.11 | 0.98±0.23 |
| pH 6.5 | 13.54±1.08 | 16.64±2.30 |
| pH 7.0 | −10.19±2.14 | −10.31±2.66 |
| pH 7.5 | −12.09±0.99 | −17.39±0.63 |
| pH 8.0 | −9.26±3.00 | −8.52±1.96 |
| 28°C | −0.11±0.01 | −0.09±0.05 |
| 30°C | −0.16±0.07 | −0.21±0.01 |
| 35°C | 2.74±0.09 | 2.74±0.53 |
| 37°C | 2.89±0.13 | 4.35±0.23 |
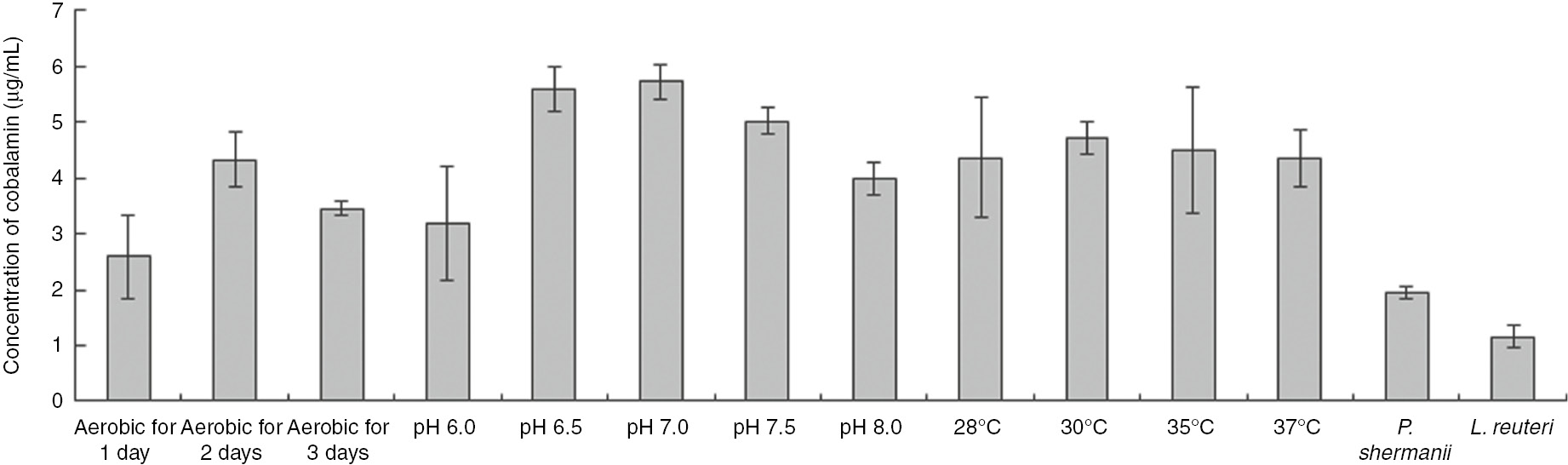
Vitamin B12 production under various conditions. Each experiment was repeated for 5 times.

Microorganisms content on 7th day under various conditions. Each experiment was repeated for 5 times.
Final metabolites production under different conditions.
| g/L | Lactate | Acetate | Propionic acid | Ethanol |
|---|---|---|---|---|
| Aerobic for 1 day | 10.76±0.11 | 5.22±0.02 | 1.27±0.06 | 1.78±0.05 |
| Aerobic for 2 days | 9.49±0.03 | 5.12±0.04 | 1.24±0.02 | 1.38±0.06 |
| Aerobic for 3 days | 7.83±0.12 | 6.57±0.05 | 1.23±0.03 | 0.76±0.09 |
| 28°C | 21.29±0.02 | 7.79±0.01 | 1.51±0.06 | 5.23±0.12 |
| 30°C | 22.03±0.11 | 8.07±0.10 | 1.54±0.12 | 4.44±0.15 |
| 35°C | 19.31±0.14 | 6.40±0.09 | 1.43±0.05 | 3.68±0.07 |
| 37°C | 34.06±0.17 | 2.52±0.09 | 2.03±0.09 | 0.79±0.19 |
| pH 6.0 | 18.95±0.34 | 7.72±0.29 | 1.27±0.73 | 5.18±0.99 |
| pH 6.5 | 19.32±0.23 | 5.38±0.33 | 1.72±0.98 | 2.85±0.21 |
| pH 7.0 | 28.55±0.136 | 4.36±0.56 | 4.35±0.24 | 7.59±0.73 |
| pH 7.5 | 18.76±0.95 | 7.53±0.58 | 1.31±0.39 | 16.05±0.99 |
| pH 8.0 | 22.67±0.41 | 10.67±0.39 | 1.41±0.87 | 6.25±0.074 |
Each detection was repeated for 5 times.
Initial pH effects on co-fermentation
With the increase of initial pH values, both bacteria had a synergistic effect on each other. But these phenomena did not enhance the production of vitamin B12. pH is essentially important in influencing metabolites and cobalamin production. Both microorganisms have their own optimal pH and the corresponding suitable ranges. The optimal pH for 3-HPA production is 6.0 [24], whereas the best pH for propionic acid is between 7.0 and 7.2 [14]. The question of the optimal initial pH for cobalamin production in co-fermentation was solved by our work (Figure 1). At pH 6.5–7.0 the highest values of cobalamin were reached. Interestingly, as is shown in Table 1, the ratio of a/b at pH 6.5 and pH 7.0 were 13.54/16.64 and −10.19/−10.31, respectively. Except for final concentrations of L. reuteri at pH 6.5, no huge difference was observed between both microorganisms content among various pH conditions (Figure 2). At pH 6.5 they had an inhibitory effect, but at pH 7.0 they changed to a synergistic effect. Moreover, a very strong influence on production of ethanol, propionic acid, and acetate was observed (Table 2). A possible interpretation of high cobalamin production is that a higher production of propionic acid needs more cobalamin under acidic condition (pH 6.5–7.0), as reported by Hsu and Yang [26]. Another explanation could be the low activity of 3-HPA generation above pH 7.0 [24], whereas more ethanol was generated to balance the redox reaction in L. reuteri.
Oxygen supplementation effects on co-fermentation
Oxygen, as mentioned above, is involved in the DMBI generation in P. freudenreichii. In the presence of oxygen, growth is slower due to the inhibition of propionic acid, acetate, and succinate formation, but pyruvate is accumulated [27]. However, propionic acid, an inhibitory factor of both microorganisms, can be decomposed in the presence of oxygen. Some researchers [28, 29] have conducted an oxygen cycle to improve cobalamin production by mediating catabolism of glucose to propionic acid and acetate in the presence and absence of oxygen [14]. Oxygen also affects L. reuteri to synthesize more heme against toxic forms of oxygen [30]. In this study (Figure 1), 2-day aerobic fermentation showed a higher productivity of cobalamin than 1-day or 3-day aerobic fermentation. But an obvious increase inhibitory effect of bacteria can be observed from an increase of interaction coefficients during the increase of aerobic fermentation duration. The CFU of L. reuteri increased during the increase of oxygen supplied days (Figure 2). Propionibacterium shermanii demonstrated the opposite trend (Figure 2). Some researchers [29, 31] found that low dissolved oxygen was advantageous for cell growth, propionate decomposition and acetate production by P. shermanii decrement, which was also confirmed in this study (Table 2). The dissolved oxygen obviously led to a reduction of final ethanol concentrations (Table 2).
Interaction and co-fermentation
A Lotka Volterra model, known as an ecological predator-prey model, was applied to describe the competition between microorganisms. The interaction coefficients, which describe the antagonistic activities, were obtained by fitting the Lotka Volterra model with least square methods. The coefficients of a and b can be interpreted as the interspecific competition parameters of P. shermanii on L. reuteri, and vice versa. The average values of interaction coefficients of a (−4.66) and b (−3.60) from this study represented a less negative effect from P. shermanii on L. reuteri and a negative effect from L. reuteri on P. shermanii. However, some researchers [24] reported that the maximal cell numbers of L. acidophilus and P. shermanii were higher than in single culture fermentation.
With the exception of pH 6.5, all fermentations with high productions of cobalamin did not show strong antagonistic effects between the two microorganisms (Table 1). Experiments of oxygen supply for 1 day acquired huge negative values of interaction coefficients. It can be explained that oxygen to some extent became the main inhibitor for the growth of both microorganisms. Interaction coefficients were changing from positive values to negative values during fermentations with an increase of initial pH from 6.0 to 8.0. This means a high initial pH value is beneficial for growth of both bacteria. No significant difference of interactions was found in fermentation under different temperatures.
A synergistic effect in the co-fermentation of L. acidophilus and P. shermanii was described by Liu and Moon [24]. They reported no lactate accumulation in the medium. Acetic acid production rates per generation were lower in mixed cultures and growth rate was faster than before. Results from this study agreed with previous results [24] and partly confirmed them as well. The robust growth of mixed cultures was also indicated in this study. Fast increase of propionic acid, ethanol production, and OD values were observed (data not shown), along with an accumulation of lactate observed, particularly in fermentation at 37°C (Table 2). Propionibacterium sp. can reduce the lactate stress on L. reuteri and retard the decrease of pH. Moreover, at the same time, L. reuteri can decompose proteins from soymilk relying on full proteolytic system to meet the nitrogen requirement of itself and P. freudenreichii with a low ability of protease. Another hypothetical assumption is about the synthesis of Dmbi. 5,6-dimethylbenzimidazole, an important precursor of cobalamin, can only be formed in the presence of oxygen by P. freudenreichii and P. shermanii [16]. Because of this, after several days of fermentation under anaerobic conditions with P. freudenreichii or P. shermanii, the fermentation should be switched to aerobic conditions. However, Santos et al. [17] found that the gene of cobT of L. reuteri is 59% similar with Salmonella typhimurium, which could mean that L. reuteri has the ability to form Dmbi without oxygen. Furthermore, some analogs can improve production of cobalamin by protecting an inhibitory riboswitch [32].
Some researchers demonstrated that spent media cultured with LAB strains led to a low cell concentration of P. shermanii, which produced more cobalamin than before [33]. Nevertheless, mixed cultures in this study produced 1.6–2.4 fold more cobalamin than single fermentation (data not shown). For further work, a doubtless conclusion can be made that a co-fermentation with P. shermanii and L. reuteri can lead to a high production of cobalamin in soy-milk. Correlations between the ratio of a/b and vitamin B12 production were also found. According to Figure 3, an obvious conclusion can be made that more vitamin B12 production (Figure 1) can be produced when values of a/b approaches to 1. This means that both bacteria contribute to vitamin B12 production. The model is is therefore demonstrated its feasibility in describing the response of vitamin B12 production and predicting a response value within the appropriate ranges.
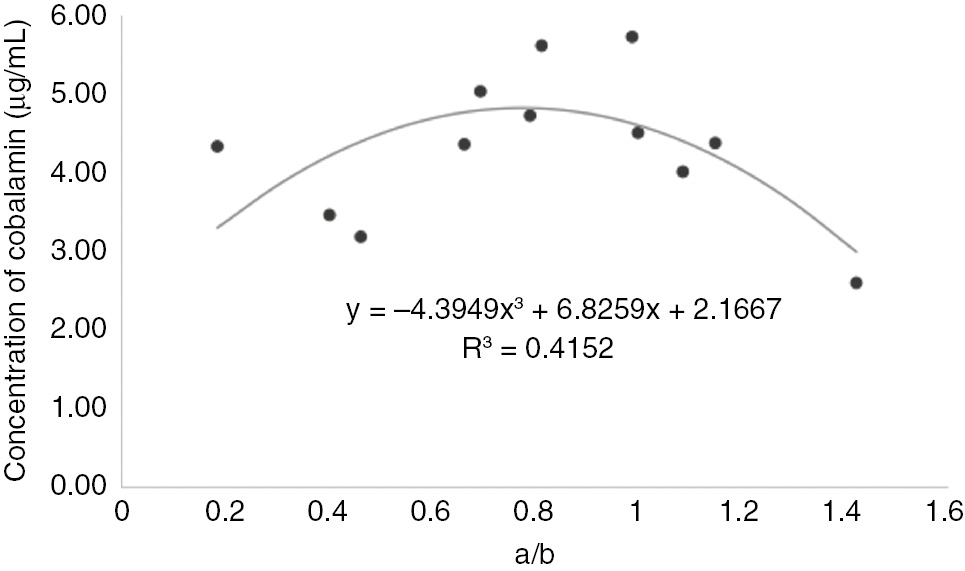
Concentration of cobalamin production under various a/b.
Conclusions
A Lotka Volterra model was successfully employed to describe the competition relationship between L. reuteri and P. fruedenreichii microorganisms under various fermentation conditions of anaerobic/aerobic, initial pH, and temperature. With the increase of aerobic fermentation days and the rising of fermentation temperature, the inhibition effects between microorganisms were rising. During the increase of initial pH values, the synergistic effects were developing. A quadratic function was set up to predict vitamin B12 by values of a/b. This model has achieved to explain interactions of two bacteria and provide a useful approach to further increase yielding of vitamin B12, even other relative fermented products.
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 31501452
Award Identifier / Grant number: 31401570
Funding statement: This work was supported by National Natural Science Foundation of China (No. 31501452 and 31401570), the Scientific Research Foundation of Education Department of Zhejiang Province (No. Y201432277), Foundation of Food Science and Engineering, the most important Discipline of Zhejiang Province (No. JYTsp20141082), and Project of international communication for construction of the first ranked discipline of Zhejiang Gongshang Univerisity (No. 2017SICR106).
Conflict of interest statement: Authors have no conflict of interest.
References
1. Liu KS. Soybeans: chemistry, technology and utilization. New York: Aspen Publishers, 1997.10.1007/978-1-4615-1763-4Search in Google Scholar
2. Watanabe F, Yabuta Y, Tanioka Y. Biologically active vitamin B12 compounds in foods for preventing deficiency among vegetarians and elderly subjects. J Agric Food Chem 2014;61:6769–75.10.1021/jf401545zSearch in Google Scholar PubMed
3. Liu KS. Soybean as functional foods and ingredients. Beijing: Chinese Light Industry Press, 2009.Search in Google Scholar
4. Friberg S, Hui YH. Handbook of food and beverage fermentation technology. Boca Raton: CRC Press, 2005.Search in Google Scholar
5. Rekha CR, Vijayalakshmi G. Influence of processing parameters on the quality of soycurd (tofu). J Food Sci Technol 2013;50: 176–180.10.1007/s13197-011-0245-zSearch in Google Scholar PubMed PubMed Central
6. Han BZ, Rombouts FM, Nout MJ. Amino acid profiles of sufu, a Chinese fermented soybean food. J Food Comp Anal 2004;17:689–98.10.1016/j.jfca.2003.09.012Search in Google Scholar
7. Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP). BMJ 2007;334:885–8.10.1136/bmj.39147.604896.55Search in Google Scholar PubMed PubMed Central
8. Mo H, Kariluoto S, Piironen V, Zhu Y, Sanders MG, Vincken JP, et al. Effect of soybean processing on content and bioaccessibility of folate, vitamin B12 and isoflavones in tofu and tempe. Food Chem 2013;141:2418–25.10.1016/j.foodchem.2013.05.017Search in Google Scholar PubMed
9. Schneider Z, Stroinski A. Comprehensive B12: chemistry, biochemistry, nutrition, ecology, medicine. Berlin: Verlag Walter de Gruyter, 1987.10.1515/9783110844795Search in Google Scholar
10. Allen LH. Bioavailability of vitamin B12. Int J Vitam Nutr Res 2010;80:330–5.10.1024/0300-9831/a000041Search in Google Scholar PubMed
11. Rucker BR, Suttie JW, McCormick BD, Machilin LJ. Handbook of vitamins. New York: Marcel Dekker, Inc., 2001.Search in Google Scholar
12. Martens JH, Barg H, Warren MJ, Jahn D. Microbial production of vitamin B12. Appl Microbiol Biotechnol 2002;58:275–85.10.1007/s00253-001-0902-7Search in Google Scholar PubMed
13. Lee IH, Fredrickson AG, Tsuchiya HM. Diauxic growth of Propionibacterium shermanii. Appl Microbiol Biotechnol 1974;28:831–5.10.1128/am.28.5.831-835.1974Search in Google Scholar PubMed PubMed Central
14. Piveteau P. Metabolism of lactate and sugars by dairy propionibacteria: a review. Lait 1999;79:23–41.10.1051/lait:199912Search in Google Scholar
15. Dupuis C, Corre C, Boyaval P. Lipase and esterase activities of Propionibacterium freudenreichii subsp. freudenreichii. Appl Environ Microbiol 1993;59:4004–9.10.1128/aem.59.12.4004-4009.1993Search in Google Scholar
16. Hoellriegl V, Lamm L, Rowold J, Hoerig J, Renz P. Biosynthesis of vitamin B12. Arch Microb 1982;132:155–8.10.1007/BF00508722Search in Google Scholar
17. Santos F, Wegkamp A, de Vos M. High-level folate production in fermented foods by the B12 producer lactobacillus reuteri JCM1112. Appl Environ Microbiol 2008;74:3291–4.10.1128/AEM.02719-07Search in Google Scholar
18. Hugenschmidt S, Schwenninger SM, Lacroix C. Concurrent high production of natural folate and vitamin B12 using a co-culture process with Lactobacillus plantarum SM39 and Propionibacterium freudenreichii DF13. Process Biochem 2011;46:1063–70.10.1016/j.procbio.2011.01.021Search in Google Scholar
19. Cornu M, Billoir E, Bergis H, Beaufort A, Zuliani V. Modeling microbial competition in food: application to the behavior of Listeria monocytogenes and lactic acid flora in pork meat products. Food Microbiol 2011;28:639–47.10.1016/j.fm.2010.08.007Search in Google Scholar
20. Tharmaraj N, Shah NP. Selective enumeration of Lactobacillus delbrueckii ssp. bulgaricus, Streptococcus thermophilus, Lactobacillus acidophilus, bifidobacteria, Lactobacillus casei, Lactobacillus rhamnosus, and propionibacteria. J Dairy Sci 2003;86:2288–96.10.3168/jds.S0022-0302(03)73821-1Search in Google Scholar
21. Denter J, Bisping B. Formation of B-vitamins by bacteria during the soaking process of soybeans for tempe fermentation. Int J Food Microbiol 1994;22:23–31.10.1016/0168-1605(94)90004-3Search in Google Scholar
22. Gauch R, Leuenberger U, Mueller U. Bestimmung der wasserloeslichen Vitamine B1, B2, B6 und B12 in Milch durch HPLC. Z Lebensm Unters For 1992;195:312–5.10.1007/BF01187905Search in Google Scholar PubMed
23. Doleyres Y, Beck P, Vollenweider S. Production of 3-hydroxypropionaldehyde using a two-step process with Lactobacillus reuteri. Appl Microb and Biotech 2005;68:467–4.10.1007/s00253-005-1895-4Search in Google Scholar PubMed
24. Liu JA, Moon NJ. Commensalistic Interaction Between Lactobacillus acidophilus and Propionibacterium shermanii. Appl Environ Microb 1982;44:715–22.10.1128/aem.44.3.715-722.1982Search in Google Scholar
25. Luthi-Peng Q, Scharer S, Puhan Z. Production and stability of 3-hydroxypropionaldehyde in Lactobacillus reuteri. Appl Microbiol Biotechnol 2002;60:73–80.10.1007/s00253-002-1099-0Search in Google Scholar
26. Hsu ST, Yang ST. Propionic acid fermentation of lactose by Propionibacterium acidipropionici: effects of pH. Biotechnol Bioengin 1991;38:571–8.10.1002/bit.260380603Search in Google Scholar
27. Schwartz AC, Mertens B, Voss KW, Hahn H. Inhibition of acetate and propionate formation upon aeration of resting cells of the anaerobic Propionibacterium shermanii: evidence on the PASTEUR reaction. Z Allg Mikrobiol 1976;16:123–31.10.1002/jobm.19760160206Search in Google Scholar
28. Miyano K, Ye K, Shimizu K. Improvement of vitamin B12 fermentation by reducing the inhibitory metabolites by cell recycle system and a mixed culture. Biochem Engin J 2000;6:207–14.10.1016/S1369-703X(00)00089-9Search in Google Scholar
29. Ye K, Shijo M, Jin S. Efficient production of vitamin B12 from propionic acid bacteria under periodic variation of dissolved oxygen concentration. J Ferm Bioengin 1996;82:484–91.10.1016/S0922-338X(97)86988-7Search in Google Scholar
30. Wolf G, Strahl A, Meisel J, Hammes WP. Heme-dependent catalase activity of lactobacilli. Inter J Food Microbiol 1991;12:133–40.10.1016/0168-1605(91)90062-TSearch in Google Scholar
31. Ye K, Shijo M, Miyano K. Metabolic pathway of Propionibacterium growing with oxygen: enzyme, 13C NMR analysis, and its application for vitamin B12 production with periodic fermentation. Biotechnol Prog 1999;15:201–7.10.1021/bp990012sSearch in Google Scholar PubMed
32. Thirupathaiah Y, Swarupa RC, Sudhakara RM, Venkateswar Rao L. Effect of chemical and microbial vitamin B12 analogues on production of vitamin B12. World J Microbiol Biotechnol 2012;28:2267–71.10.1007/s11274-012-1011-8Search in Google Scholar PubMed
33. Gardner N, Champagne CP. Production of Propionibacterium shermanii biomass and vitamin B12 on spent media. J Appl Microbiol 2005;99:1236–45.10.1111/j.1365-2672.2005.02696.xSearch in Google Scholar PubMed
©2018 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Research Articles
- Effects of calcium hydroxide and N-acetylcysteine on MMP-2, MMP-9, TIMP-1 and TIMP-2 in LPS-stimulated macrophage cell lines
- Synthesis of fused 1,4-dihydropyridines as potential calcium channel blockers
- Optimization of fermentation conditions for efficient ethanol production by Mucor hiemalis
- Covalent immobilization of an alkaline protease from Bacillus licheniformis
- Major biological activities and protein profiles of skin secretions of Lissotriton vulgaris and Triturus ivanbureschi
- Optimized production, purification and molecular characterization of fungal laccase through Alternaria alternata
- Adsorption of methyl violet from aqueous solution using brown algae Padina sanctae-crucis
- Protective effect of dexpanthenol (vitamin B5) in a rat model of LPS-induced endotoxic shock
- Purification and biochemical characterization of a β-cyanoalanine synthase expressed in germinating seeds of Sorghum bicolor (L.) moench
- Molecular cloning and in silico characterization of two alpha-like neurotoxins and one metalloproteinase from the maxilllipeds of the centipede Scolopendra subspinipes mutilans
- Improvement of delta-endotoxin production from local Bacillus thuringiensis Se13 using Taguchi’s orthogonal array methodology
- Enhancing vitamin B12 content in co-fermented soy-milk via a Lotka Volterra model
- Species and number of bacterium may alternate IL-1β levels in the odontogenic cyst fluid
- Rheo-chemical characterization of exopolysaccharides produced by plant growth promoting rhizobacteria
- Benzo(a)pyrene degradation pathway in Bacillus subtilis BMT4i (MTCC 9447)
- Indices
- Reviewers 2018
- Yazar Dizini/Author Index
Articles in the same Issue
- Frontmatter
- Research Articles
- Effects of calcium hydroxide and N-acetylcysteine on MMP-2, MMP-9, TIMP-1 and TIMP-2 in LPS-stimulated macrophage cell lines
- Synthesis of fused 1,4-dihydropyridines as potential calcium channel blockers
- Optimization of fermentation conditions for efficient ethanol production by Mucor hiemalis
- Covalent immobilization of an alkaline protease from Bacillus licheniformis
- Major biological activities and protein profiles of skin secretions of Lissotriton vulgaris and Triturus ivanbureschi
- Optimized production, purification and molecular characterization of fungal laccase through Alternaria alternata
- Adsorption of methyl violet from aqueous solution using brown algae Padina sanctae-crucis
- Protective effect of dexpanthenol (vitamin B5) in a rat model of LPS-induced endotoxic shock
- Purification and biochemical characterization of a β-cyanoalanine synthase expressed in germinating seeds of Sorghum bicolor (L.) moench
- Molecular cloning and in silico characterization of two alpha-like neurotoxins and one metalloproteinase from the maxilllipeds of the centipede Scolopendra subspinipes mutilans
- Improvement of delta-endotoxin production from local Bacillus thuringiensis Se13 using Taguchi’s orthogonal array methodology
- Enhancing vitamin B12 content in co-fermented soy-milk via a Lotka Volterra model
- Species and number of bacterium may alternate IL-1β levels in the odontogenic cyst fluid
- Rheo-chemical characterization of exopolysaccharides produced by plant growth promoting rhizobacteria
- Benzo(a)pyrene degradation pathway in Bacillus subtilis BMT4i (MTCC 9447)
- Indices
- Reviewers 2018
- Yazar Dizini/Author Index

