Effects of calcium hydroxide and N-acetylcysteine on MMP-2, MMP-9, TIMP-1 and TIMP-2 in LPS-stimulated macrophage cell lines
-
Eda Ezgi Aslantaş
Abstract
Aim
This study was evaluated the effects of N-acetylcysteine (NAC) and calcium hydroxide (Ca(OH)2) on the expression levels of matrix metalloproteinase -2, -9 (MMP-2, -9) and tissue inhibitor metalloproteinase -1, -2 (TIMP-1, -2) in lipopolysaccharide (LPS)-stimulated human macrophages.
Methods
Human monocyte precursor cells (THP-1) were differentiated into macrophage-adherent cells and were stimulated with LPS for 24 h. Then individually incubated with NAC or Ca(OH)2 for 24, 48 and 72 h. Following incubation, protein expression and mRNA levels of MMP-2, -9 and TIMP-1, -2 were evaluated using enzyme-linked immunosorbent assay (ELISA) and quantitative real-time polymerase chain reaction (qRT-PCR). Data were statistically analysed using two-way ANOVA, to followed by Bonferroni test at α=0.05.
Results
NAC significantly decreased mRNA expression and protein levels of MMP-9, while Ca(OH)2 decreased mRNA expression alone at 24 h. NAC and Ca(OH)2 decreased mRNA expression of MMP-2 at 24 h, while NAC increased this expression at 48 h. Although NAC and Ca(OH)2 decreased the mRNA expression of TIMP-1, -2 at 24 h, only NAC increased mRNA expression of TIMP-1 at 48 h.
Conclusion
At the early stages of inflammation, NAC and Ca(OH)2 have anti-inflammatory effects on macrophages.
Özet
Amaç
Bu çalışmanın amacı lipopolisakkaritle (LPS) stimule edilmiş insan makrofaj hücrelerinde N-asetilsistein (NAC) ve kalsiyum hidroksit’in (Ca(OH)2), matriks metalloproteinaz-2 ve -9 (MMP-2, -9) ve doku inhibitor metalloproteinaz-1 ve -2 (TIMP-1, -2) salınımı üzerine etkilerini belirlemektir.
Yöntemler
İnsan monosit precursor hücreleri (THP-1) makrofaj adherent hücrelere dönüştürülmüştür. Makrofaj hücreleri 24 saat LPS ile stimule edilmiş ve NAC veya Ca(OH)2 ile 24, 48 ve 72 saat inkübe edilmişlerdir. İnkübasyonu takiben, MMP-2, -9 ve TIMP-1,-2 mRNA seviyeleri ve protein salınımı enzim ilintili immun test (ELISA) ve kantitatif eş zamanlı polimeraz zincir reaksiyonu (qRT-PCR) ile değerlendirilmiştir. Veriler istatistiksel olarak Bonferronni testini takiben (α=0.05) iki yönlü varyans analizi (ANOVA) ile analiz edilmiştir.
Bulgular
Ca(OH)2 24. saatte sadece MMP-9 mRNA salınımını azaltırken, NAC, hem MMP-9 protein hem de mRNA salınımını belirgin bir şekilde azaltmıştır ancak her iki materyal de 48.saatte MMP-9 protein ve mRNA salınımını arttırmıştır. Dahası NAC ve Ca(OH)2 MMP-2 mRNA salınımını 24. saatte azaltırken, NAC bu salınımı 48. saatte arttırmıştır. NAC and Ca(OH)2 TIMP-1 ve -2 mRNA salınımını 24. saatte azaltırken, sadece NAC, TIMP-1 mRNA salınımını 48. saatte arttırmıştır.
Sonuç
Sonuçlarımız, inflamasyonun erken safhasında NAC and Ca(OH)2’ in makrofajlar üzerinde anti-inflamatuar etkilerinin olduğunu göstermiştir.
Introduction
Bacterial infection within the root canal induces tissue breakdown around the apical area as a result of local immune response by degradation of several extracellular matrix (ECM) components such as collagen, fibronectin and laminin [1], [2], [3]. ECM components mediates tissue remodeling as well as cell growth and differentiation and are destroyed by matrix-degrading enzymes called matrix metalloproteinases (MMPs). MMPs represent a family of Zn++ and Ca++ dependent endopeptidases, including collagenases, gelatinases and stromelysins [4], [5], [6]. MMP-2 and MMP-9, also called gelatinases, play crucial roles in degradation of collagen and other ECM components [3], [4]. During tissue remodeling, MMP activity is primarily regulated by tissue inhibitor metalloproteinases (TIMPs), which may inhibit active forms of all MMPs. Four different TIMPs have been identified. The primary inhibitors of MMP-9 and MMP-2 are TIMP-1 and TIMP-2 [5], [6].
Studies have reported that gelatinases play a vital role in pulp and periradicular tissue breakdown that occurs during the initial phase of lesion development [7], [8], [9], [10], [11], [12]. During root canal treatment of teeth with periradicular lesions, calcium hydroxide (Ca(OH)2) is primarily recommended as an intracanal medicament in order to eliminate or reduce the bacterial contamination present in the root canal, and induce healing of periradicular tissues [13], [14], [15]. It was reported that Ca(OH)2 significantly decreased mRNA expression of MMP-1 and increased that of TIMP-1, in addition to presentation of more organized ECM, when used as a root canal dressing [14]. However, Ca(OH)2 application has some disadvantages such as decreasing of dentin strength [16] and disruption of the adhesion between endodontic sealer and dentin [17]. Moreover, it was found to be cytotoxic and less effective against certain endodontic pathogens such as Enterococcus faecalis (E. faecalis) and Candida albicans [18], [19], [20].
In LPS-activated cells, inflammation and oxidative stress occur at the same time; antioxidants may inhibit these processes. N-acetylcysteine (NAC) is a thiol-containing compound that can act both as a precursor of reduced glutathione (GSH) and as a direct scavenger of reactive oxygen species (ROS). NAC is an important cellular antioxidant [21], [22]. In previous studies, NAC suppressed LPS-induced inflammatory responses in gingival fibroblasts [23] and macrophage cell lines [24] as well as prevention of alveolar bone loss in the rat model [25]. In addition, studies have reported to decrease the gelatinolytic activity in macrophage cell lines [26], [27]. NAC also has antibacterial effects against certain microorganisms and was found to be effective in both planktonic and biofilm forms of E. faecalis which is the major microorganism of failed root canal treatment [28].
In our previous publication, we have reported the pro- and anti-inflammatory effects of NAC and Ca(OH)2 on lipopolysaccharide (LPS)-stimulated human macrophage cell lines and showed that NAC has antiinflammatory effect [24].
Therefore, we aimed to evaluate the effects of NAC, compared with Ca(OH)2, using similar conditions with our previous study, on MMP-2, MMP-9 and their tissue inhibitors, TIMP-1 and TIMP-2, mRNA expression and protein levels in LPS-stimulated human macrophage cell lines.
Materials and methods
Preparation of compounds
After sterilization of Ca(OH)2 (Sultan Healthcare, Hackensack, USA) and NAC (Sigma Chemical Company, St. Louis, MO, USA) powder under ultraviolet (UV), stock solutions of NAC and Ca(OH)2 were prepared by dissolving these in RPMI-1640 medium (Roswell Park Memorial Institute) and stored at +4°C. The optimum concentrations of NAC and Ca(OH)2 were determined according to our previous study by using flow cytometry analysis with propidium iodide (PI) regarding cell cytotoxicity that represents the viability of at least 50% cells [24].
Cell culture and treatment
In this study, human promonocytic cell lines (THP-1) (ATCC, Rockville, MD, USA) were cultured at 37°C in a humidified incubator with 5% CO in RPMI-1640 medium with 10% foetal bovine serum (FBS), 1% penicillin, streptomycin and glutamine and plated in six-well culture plates at a density of 1×106 cells/mL. Subsequently, THP-1 monocyte cell lines were first incubated with phorbol myristate acetate (PMA) at a concentration of 400 nM for 72 h, followed by fresh medium without PMA for 96 h, Differentiated macrophage cells adhered to the flask, whereas undifferentiated monocytic cells in suspension were removed by washing with PBS (pH=7.4) [24].
For treatment, in order to completely prevent cells from apoptotic DNA fragmentation, the cells were incubated with culture media containing 10 mM NAC (NAC group) and 40 μg/mL Ca(OH)2 (Ca[OH]2 group) for 24 h. The control group did not receive any treatment. Following washing with PBS (1X) three times, the cells were incubated with 10 ng/mL Escherichia coli (E. coli) (Sigma Chemical Company, St. Louis, MO, USA) LPS, for 24, 48 or 72 h.
Enzyme-linked immunosorbent assay (ELISA)
Following LPS stimulation, the protein levels of MMP-9, TIMP-1, MMP-2 and TIMP-2 in the supernatant of macrophage cell lines were analysed using an ELISA reader (SpectraMax M2, Molecular Device, USA) with an ELISA kit (R&D Systems, USA) according to the manufacturer’s instructions at 450 nm and normalized with the standard solution. All experiments were performed in triplicate for two independent experiment sets.
Quantitative real-time polymerase chain reaction (qRT-PCR)
mRNA expression levels of MMP-9, TIMP-1, MMP-2 and TIMP-2 was determined using quantitative real-time polymerase chain reaction (qRT-PCR) assay. The RNeasy Mini kit (Qiagen, Hilden, Germany) was used for total RNA extraction according to the manufacturer’s instructions. RNA yield and quality were detected on the basis of spectrophotometric measurements at wavelengths of 260 and 280 nm with NanoDrop ND 1000 (Thermo Scientific, Wilmington, USA). Reverse transcription was performed using a Transcriptor High Fidelity cDNA synthesis kit (Roche Diagnostics GmbH, Germany) with 1 ug total RNA according to manufacturer’s instructions. After cDNA synthesis was completed, samples were stored at −20°C. qRT-PCR analyses were performed in triplicates using the LightCycler 480 Probes Master kit on Light Cycler 480 II (Roche Applied Science). Amplification conditions of PCR cycles were 95°C for 10 s, 60°C for 30 s and 72°C for 1 s. The relative amount of the target gene was normalized relative to the control gene.
The following primer sequences were used in the RT-PCR reactions: hMMP-9 F:5′CCTCTGGAGGTTCGACGTG3′, hMMP-9 R: 5′CCTGGCAGAAATAGGCTTTC3′; hTIMP-1 F: 5′CTGTTGTTGCTGTGGCTGAT3′ R: 5′AACTTGGCCCTGATGACG3′; hMMP-2 F:5′TATTTGATGGCATCGCTCAG3′, 5′CCAAATGAACCGGTCCTTG3′; 5′GAGCCTGAACCACAGGTACCA3′; hMMP-2 R: hTIMP-2 F: hTIMP-2 R: 5′CCATCCAGAGGCACTCGT3′ and Hprt-1 Real-Time Ready single assay (Roche Diagnostics GmbH, Germany).
Statistical analysis
The results of the experimental groups were statistically analysed using two-way ANOVA followed by Bonferroni test for pair-wise comparisons with a significance level of p<0.05.
Results
Two-way ANOVA revealed significant differences among the experimental groups (p<0.001).
MMP-9 assays
Compared with the control group, NAC significantly decreased both mRNA expression and protein levels at 24 h, whereas Ca(OH)2 only mRNA expression alone at 24 h (p<0.05). However, at 48 h, NAC and Ca(OH)2 increased mRNA expression as well as protein levels (p<0.05: Figure 1A and B).
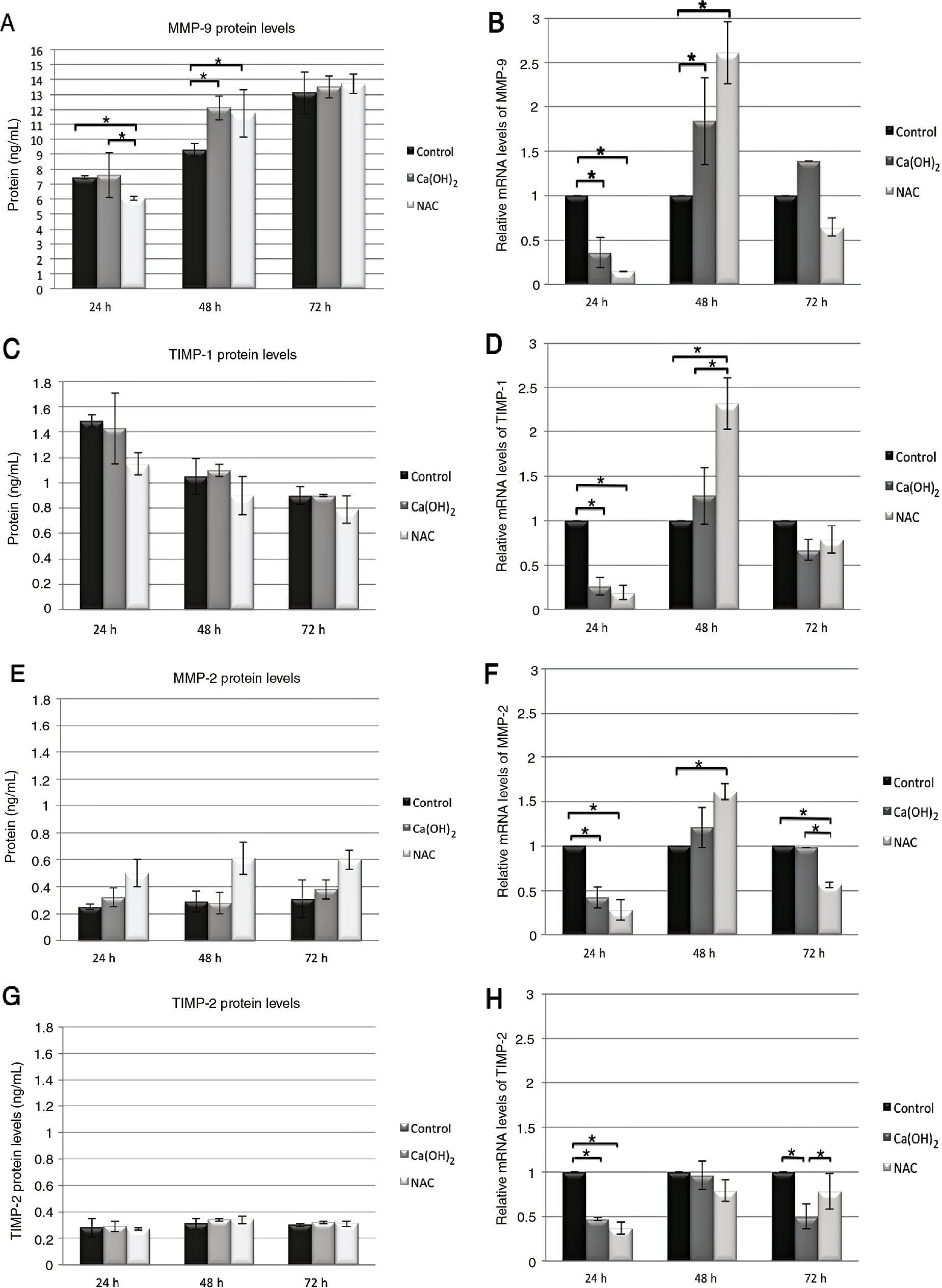
The time (24, 48 and 72 h) and medication NAC (10 mM) and Ca(OH)2 (40 μg/mL) dependent protein and the mRNA expression levels of MMP-9, TIMP-1, MMP-2 and TIMP-2 on THP-1 macrophage cell line were shown in graphics. Values and error bars of average protein and mRNA expression levels represent the means and SD of experimental groups. The asterisks show the statically difference between groups by the Bonferroni test for pair-wise comparison at α=0.05. 1×106 cell were used in the experiment sets.
TIMP-1 assays
NAC and Ca(OH)2 significantly decreased mRNA expression at 24 h compared with the control group (p<0.05). In contrast, NAC increased the mRNA expression at 48 h compared with the Ca(OH)2 and control groups (p<0.05; Figure 1D).
MMP-2 assays
Compared with the control group, NAC and Ca(OH)2 significantly decreased mRNA expression at 24 h (p<0.05). On the other hand, only NAC decreased mRNA expression compared with the control and Ca(OH)2 groups at 72 h (p<0.05). However, NAC increased the mRNA expression at 48 h compared with the control group (p<0.05: Figure 1F).
TIMP-2 assays
NAC and Ca(OH)2 significantly decreased mRNA expression at 24 h compared with the control group (p<0.05). Moreover, Ca(OH)2 decreased mRNA expression compared with the control and NAC groups at 72 h (p<0.05; Figure 1H).
Discussion
In the present study, effects of NAC on gelatinolytic activity were evaluated in macrophage cell lines by comparing to Ca(OH)2. NAC significantly decreased mRNA expression and protein levels of MMP-9. Moreover, during the same time intervals, NAC decreased only mRNA expression of MMP-2. However, NAC has been reported to decrease protein levels of MMP-2 in gingival fibroblasts [23] and endothelial cells [27]. The findings of the present study suggest that MMP-2 could not be translated to its protein owing to other facts affecting the signaling pathway, which might be depending on using different cell types and experimental procedures. According to a previous study, MMP-2 is primarily produced in vitro by fibroblasts and endothelial cells while MMP-9 by macrophages and other inflammatory cells [6]. Consistent with this information, in the present study, MMP-9 and TIMP-1 expressions were high but MMP-2 expression levels are low in THP-1 macrophages.
In our previous publication, we have reported the effects of NAC and Ca(OH)2 on TNF-α and TGF-β in LPS-stimulated macrophages cell lines. The results showed that NAC and Ca(OH)2 significantly inhibited TNF-α expression at both the protein and mRNA expression level at the 4th h. It seems that both materials have a strong anti-inflammatory effect at the early phase of inflammation. These materials only increased TGF-β1, one of the cytokines involved in the wound repair process in periradicular lesions, mRNA level at the 24th h [24].
Cytokines induce MMP release for matrix degradation and turnover [3], [6], TNF-α induces MMP-9 and MMP-2 expression in a variety of cell types including macrophages [29], [30]. Under the lights of our previous publication and literature results; we hypothesized that NAC could affect the gelatinolytic activity, which plays an important role in degradation of collagen and other ECM components during inflammation after cytokine release [3], [4], [15].
NAC is a dithiol that induces intracellular GSH synthesis and directly neutralizes ROS by donating a hydrogen atom from its thiol group [6], [21], [22], [23], [27] It was reported that NAC affect the MMP binding to the Zn atom to block catalysis [3], [4], [6]. Previous studies have indicated the possible role of NAC in the signaling pathway. In fact, ROS production leads to activation of ERK1/2 and subsequent activation of nuclear factor-kB (NF-kB). The promoter of MMP-9, acting as regulatory elements of transcription factors, has an NF-kB binding site inside, and NAC inhibits NF-kB activation directly or the promoter of MMP-9, which, in turn, blocks MMP-9 activity. On the other hand, NAC probably has a direct inhibitory effect on the gelatinolytic capacity of MMP-2 [27], [30].
In this experiment, NAC (10 mM) and Ca(OH)2 (40 μg/mL) concentrations were selected according to our previous research that yielded 50% viable cells and compatible with literature results [24], [26], [27]. It was reported that lower NAC concentrations had greater remodeling effect, whereas higher concentrations was expected to have better antibacterial effects [21]. NAC has been shown to effectively reduce biofilm formation in various gram-positive and gram-negative bacteria, especially for E. faecalis which is the major microorganism of persistant endodontic infections [28]. NAC was most bactericidal at pH 11 when combined with 2% chlorhexidine; [31]. Since pH adjustment might alter the real activity of NAC molecule, in present study, NAC was prepared at pH 7.4 to evaluate cellular MMP expressions for prolonged time periods.
In literature, the gelatinolytic activity of NAC has been evaluated mostly for 24 h [26], [27]; the results of these studies were consistent with the 24-h results of our study. On the other hand, in our study, NAC was not effective in inhibiting both MMP-2 and -9 expressions at 48 and 72 h. Considering the lack of certain data in literature on the activity duration of NAC molecule in cell culture studies, additional studies are needed to evaluate effects of different time points.
In the present study, Ca(OH)2 only suppressed mRNA expression of MMP-2 and MMP-9 at 24 h. In light of these findings, the effectiveness of Ca(OH)2 does not seem to prolong after 24 h. A previous study reported that Ca(OH)2 induced MMP-2 expression and decreased cell viability in fibroblast cell culture after 24 h [19]. On the other hand, in teeth subjected to root canal treatment using Ca(OH)2 as the root canal dressing, a lower inflammatory index was observed, accompanied by an increased proportion of fibroblasts and a decreased MMP-2, MMP-8 and MMP-9 expression compared with single-visit root canal treatment in vivo [15]. Moreover, treatment of Prevotella nigrescens (P. nigrescens) LPS with Ca(OH)2 resulted in down regulation of MMP-1 depending on different time intervals, whereas E. coli LPS treatment did not alter this gene expression levels in osteoblastic cell lines [14]. These differences in results might be attributed to differences in the experimental models.
In this study, 10 ng/mL E. coli LPS was used as bacterial stimulator. E. coli is the most common commercially found gram-negative bacteria, and its chemical structure and stimulatory effects have been extensively investigated [14]. It was reported that LPS induces strong inflammation by expression of proinflammatory cytokines and MMPs [23], [24]. Therefore, E. coli LPS was used to create effective inflammation in a representative model although it is not an actual periapical pathogen. However, mixed bacterial population in root canal infection may possibly be more complex than that observed in in vitro models. Additional studies are needed to evaluate the possible effects of various bacterial components on the destructive enzymes.
The results of our study showed that Ca(OH)2 and NAC decreased the mRNA expression of TIMP-1 and TIMP-2 at 24 h, whereas NAC increased both TIMP-1 and TIMP-2 expressions at 48 h. It was reported that TIMPs have different cellular activities that seem to be independent of their primary inhibitory action on MMPs, which has not been detected yet [5]. On the other hand, in our study, increase of mRNA expression and protein levels of TIMP-1 were compatible with MMP-9 following NAC treatment. When MMP-9 expression increases, TIMP-1 expression may also increase to effectively inhibit the MMP-9 expression. However, a study revealed that Ca(OH)2 effectively stimulated TIMP-1 mRNA expression with P. nigrescens and E. coli LPS stimulation depending on treatment duration and LPS concentration [14]. Such differences observed in different studies might be explained by the use of different cell lines and Ca(OH)2 concentrations.
In conclusion, NAC, similar to Ca(OH)2, decreased the gelatinolytic activity at 24 h. On the other hand, NAC seems to be more effective on TIMPs than Ca(OH)2 and might be considered as an alternate candidate therapeutical agent to Ca(OH)2. Additional studies are needed to evaluate the effectiveness of NAC molecules at higher pH with different endodontic pathogens.
Acknowledgments
This study was supported by The Scientific and Technological Research Council of Turkey (TÜBİTAK) (Project number: 112S004).
Conflict of interest statement: The authors have no conflict of interest.
References
1. Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol 1965;20: 340–9.10.1016/0030-4220(65)90166-0Search in Google Scholar
2. Stashenko P, Teles R, D’Souza R. Periapical inflammatory responses and their modulation. Crit Rev Oral Biol Med 1998;9:498–521.10.1177/10454411980090040701Search in Google Scholar PubMed
3. Sorsa T, Tjaderhane L, Salo T. Matrix metalloproteinases (MMPs) in oral diseases. Oral Dis 2004;10:311–8.10.1111/j.1601-0825.2004.01038.xSearch in Google Scholar PubMed
4. Woessner JF, Jr. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J 1991;5:2145–54.10.1096/fasebj.5.8.1850705Search in Google Scholar
5. Lambert E, Dasse E, Haye B, Petitfrere E. TIMPs as multifacial proteins. Crit Rev Oncol Hematol 2004;49:187–98.10.1016/j.critrevonc.2003.09.008Search in Google Scholar PubMed
6. Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, et al. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med 1993;4:197–250.10.1177/10454411930040020401Search in Google Scholar PubMed
7. Shin SJ, Lee JI, Baek SH, Lim SS. Tissue levels of matrix metalloproteinases in pulps and periapical lesions. J Endod 2002;28:313–5.10.1097/00004770-200204000-00013Search in Google Scholar PubMed
8. Itoh T, Nakamura H, Kishi J, Hayakawa T. The activation of matrix metalloproteinases by a whole-cell extract from Prevotella nigrescens. J Endod 2009;35:55–9.10.1016/j.joen.2008.09.012Search in Google Scholar PubMed
9. Buzoglu HD, Unal H, Ulger C, Mert S, Kucukyildirim S, Er N. The zymographic evaluation of gelatinase (MMP-2 and -9) levels in acute and chronic periapical abscesses. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;108:e121–6.10.1016/j.tripleo.2009.07.014Search in Google Scholar PubMed
10. Corotti MV, Zambuzzi WF, Paiva KB, Menezes R, Pinto LC, Lara VS, et al. Immunolocalization of matrix metalloproteinases-2 and -9 during apical periodontitis development. Arch Oral Biol 2009;54:764–71.10.1016/j.archoralbio.2009.04.013Search in Google Scholar PubMed
11. Letra A, Ghaneh G, Zhao M, Ray H, Jr., Francisconi CF, Garlet GP, et al. MMP-7 and TIMP-1, new targets in predicting poor wound healing in apical periodontitis. J Endod 2013;39:1141–6.10.1016/j.joen.2013.06.015Search in Google Scholar PubMed
12. Pereira Faustino IS, Azevedo RS, Takahama A, Jr. Metalloproteinases 2 and 9 immunoexpression in periapical lesions from primary endodontic infection: possible relationship with the histopathological diagnosis and the presence of pain. J Endod 2016;42:547–51.10.1016/j.joen.2015.12.020Search in Google Scholar PubMed
13. Sjogren U, Figdor D, Spangberg L, Sundqvist G. The antimicrobial effect of calcium hydroxide as a short-term intracanal dressing. Int Endod J 1991;24:119–25.10.1111/j.1365-2591.1991.tb00117.xSearch in Google Scholar PubMed
14. Yang WK, Kim MR, Lee Y, Son HH, Lee W. Effect of calcium hydroxide-treated Prevotella nigrescens on the gene expression of matrix metalloproteinase and its inhibitor in MG63 cells. J Endod 2006;32:1142–5.10.1016/j.joen.2006.05.002Search in Google Scholar PubMed
15. Paula-Silva FW, da Silva LA, Kapila YL. Matrix metalloproteinase expression in teeth with apical periodontitis is differentially modulated by the modality of root canal treatment. J Endod 2010;36:231–7.10.1016/j.joen.2009.10.030Search in Google Scholar PubMed PubMed Central
16. Sahebi S, Moazami F, Abbott P. The effects of short-term calcium hydroxide application on the strength of dentine. Dent Traumatol 2010;26:43–6.10.1111/j.1600-9657.2009.00834.xSearch in Google Scholar PubMed
17. Barbizam JV, Trope M, Teixeira EC, Tanomaru-Filho M, Teixeira FB. Effect of calcium hydroxide intracanal dressing on the bond strength of a resin-based endodontic sealer. Braz Dent J 2008;19:224–7.10.1590/S0103-64402008000300009Search in Google Scholar
18. Mohammadi Z, Dummer PM. Properties and applications of calcium hydroxide in endodontics and dental traumatology. Int Endod J 2011;44:697–730.10.1111/j.1365-2591.2011.01886.xSearch in Google Scholar PubMed
19. Silva EJ, Accorsi-Mendonca T, Almeida JF, Ferraz CC, Gomes BP, Zaia AA. Evaluation of cytotoxicity and up-regulation of gelatinases in human fibroblast cells by four root canal sealers. Int Endod J 2012;45:49–56.10.1111/j.1365-2591.2011.01946.xSearch in Google Scholar PubMed
20. Portenier I, Waltimo TM, Haapasalo M. Enterococcus faecalis-the root canal survivor and ‘star’ in post treatment disease. Endodontic Topics 2003;6:341–5.10.1111/j.1601-1546.2003.00040.xSearch in Google Scholar
21. Sadowska AM, Manuel YK, De Backer WA. Antioxidant and anti-inflammatory efficacy of NAC in the treatment of COPD: discordant in vitro and in vivo dose-effects: a review. Pulm Pharmacol Ther 2007;20:9–22.10.1016/j.pupt.2005.12.007Search in Google Scholar PubMed
22. Aksoy Y, Kesik K, Canpınar H. Does N-acetyl cysteine protect against apoptosis in HL-60 cell line. Tur J Biochem 2010;35:333–9.Search in Google Scholar
23. Kim DY, Jun JH, Lee HL, Woo KM, Ryoo HM, Kim GS, et al. N-acetylcysteine prevents LPS-induced pro-inflammatory cytokines and MMP2 production in gingival fibroblasts. Arch Pharm Res 2007;30:1283–92.10.1007/BF02980269Search in Google Scholar PubMed
24. Karapinar SP, Akkaya Ulum YZ, Ozcelik B, Dogan Buzoglu H, Ceyhan D, Balci Peynircioglu B, et al. The effect of N-acetylcysteine and calcium hydroxide on TNF-α and TGF-β1 in lipopolysaccharide-activated macrophages. Arch Oral Biol 2016;68:48–54.10.1016/j.archoralbio.2016.03.017Search in Google Scholar PubMed
25. Toker H, Ozdemir H, Eren K, Ozer H, Sahin G. N-acetylcysteine, a thiol antioxidant, decreases alveolar bone loss in experimental periodontitis in rats. J Periodontol 2009;80:672–8.10.1902/jop.2009.080509Search in Google Scholar PubMed
26. Galis ZS, Asanuma K, Godin D, Meng X. N-acetyl-cysteine decreases the matrix-degrading capacity of macrophage-derived foam cells: new target for antioxidant therapy? Circulation 1998;97:2445–53.10.1161/01.CIR.97.24.2445Search in Google Scholar
27. Bogani P, Canavesi M, Hagen TM, Visioli F, Bellosta S. Thiol supplementation inhibits metalloproteinase activity independent of glutathione status. Biochem Biophys Res Commun 2007;363:651–5.10.1016/j.bbrc.2007.09.018Search in Google Scholar PubMed
28. Quah SY, Wu S, Lui JN, Sum CP, Tan KS. N-acetylcysteine inhibits growth and eradicates biofilm of Enterococcus faecalis. J Endod 2012;38:81–5.10.1016/j.joen.2011.10.004Search in Google Scholar PubMed
29. Centrella M, McCarthy TL, Canalis E. Transforming growth factor-beta and remodeling of bone. J Bone Joint Surg Am 1991;73:1418–28.10.2106/00004623-199173090-00022Search in Google Scholar
30. Han YP, Tuan TL, Wu H, Hughes M, Gardner WL. TNF-alpha stimulates activation of pro-MMP2 in human skin through NF-(kappa)B mediated induction of MT1-MMP. J Cell Sci 2001;114(Pt1):131–9.10.1242/jcs.114.1.131Search in Google Scholar PubMed PubMed Central
31. Palaniswamy U, Lakkam SR, Arya S, Aravelli S. Effectiveness of N-acetyl cysteine, 2% chlorhexidine, and their combination as intracanal medicaments on Enterococcus faecalis biofilm. J Conserv Dent 2016;19:17–20.10.4103/0972-0707.173186Search in Google Scholar PubMed PubMed Central
©2018 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Research Articles
- Effects of calcium hydroxide and N-acetylcysteine on MMP-2, MMP-9, TIMP-1 and TIMP-2 in LPS-stimulated macrophage cell lines
- Synthesis of fused 1,4-dihydropyridines as potential calcium channel blockers
- Optimization of fermentation conditions for efficient ethanol production by Mucor hiemalis
- Covalent immobilization of an alkaline protease from Bacillus licheniformis
- Major biological activities and protein profiles of skin secretions of Lissotriton vulgaris and Triturus ivanbureschi
- Optimized production, purification and molecular characterization of fungal laccase through Alternaria alternata
- Adsorption of methyl violet from aqueous solution using brown algae Padina sanctae-crucis
- Protective effect of dexpanthenol (vitamin B5) in a rat model of LPS-induced endotoxic shock
- Purification and biochemical characterization of a β-cyanoalanine synthase expressed in germinating seeds of Sorghum bicolor (L.) moench
- Molecular cloning and in silico characterization of two alpha-like neurotoxins and one metalloproteinase from the maxilllipeds of the centipede Scolopendra subspinipes mutilans
- Improvement of delta-endotoxin production from local Bacillus thuringiensis Se13 using Taguchi’s orthogonal array methodology
- Enhancing vitamin B12 content in co-fermented soy-milk via a Lotka Volterra model
- Species and number of bacterium may alternate IL-1β levels in the odontogenic cyst fluid
- Rheo-chemical characterization of exopolysaccharides produced by plant growth promoting rhizobacteria
- Benzo(a)pyrene degradation pathway in Bacillus subtilis BMT4i (MTCC 9447)
- Indices
- Reviewers 2018
- Yazar Dizini/Author Index
Articles in the same Issue
- Frontmatter
- Research Articles
- Effects of calcium hydroxide and N-acetylcysteine on MMP-2, MMP-9, TIMP-1 and TIMP-2 in LPS-stimulated macrophage cell lines
- Synthesis of fused 1,4-dihydropyridines as potential calcium channel blockers
- Optimization of fermentation conditions for efficient ethanol production by Mucor hiemalis
- Covalent immobilization of an alkaline protease from Bacillus licheniformis
- Major biological activities and protein profiles of skin secretions of Lissotriton vulgaris and Triturus ivanbureschi
- Optimized production, purification and molecular characterization of fungal laccase through Alternaria alternata
- Adsorption of methyl violet from aqueous solution using brown algae Padina sanctae-crucis
- Protective effect of dexpanthenol (vitamin B5) in a rat model of LPS-induced endotoxic shock
- Purification and biochemical characterization of a β-cyanoalanine synthase expressed in germinating seeds of Sorghum bicolor (L.) moench
- Molecular cloning and in silico characterization of two alpha-like neurotoxins and one metalloproteinase from the maxilllipeds of the centipede Scolopendra subspinipes mutilans
- Improvement of delta-endotoxin production from local Bacillus thuringiensis Se13 using Taguchi’s orthogonal array methodology
- Enhancing vitamin B12 content in co-fermented soy-milk via a Lotka Volterra model
- Species and number of bacterium may alternate IL-1β levels in the odontogenic cyst fluid
- Rheo-chemical characterization of exopolysaccharides produced by plant growth promoting rhizobacteria
- Benzo(a)pyrene degradation pathway in Bacillus subtilis BMT4i (MTCC 9447)
- Indices
- Reviewers 2018
- Yazar Dizini/Author Index

