Purification and biochemical characterization of a β-cyanoalanine synthase expressed in germinating seeds of Sorghum bicolor (L.) moench
-
Ruth Ololade Amiola
Abstract
Background
β-Cyanoalanine synthase plays essential roles in germinating seeds, such as in cyanide homeostasis.
Methods
β-Cyanoalanine synthase was isolated from sorghum seeds, purified using chromatographic techniques and its biochemical and catalytic properties were determined.
Results
The purified enzyme had a yield of 61.74% and specific activity of 577.50 nmol H2S/min/mg of protein. The apparent and subunit molecular weight for purified β-cyanoalanine synthase were 58.26±2.41 kDa and 63.4 kDa, respectively. The kinetic parameters with sodium cyanide as substrate were 0.67±0.08 mM, 17.60±0.50 nmol H2S/mL/min, 2.97×10−1 s−1 and 4.43×102 M−1 s−1 for KM, Vmax, kcat and kcat/KM, respectively. With L-cysteine as substrate, the kinetic parameters were 2.64±0.37 mM, 63.41±4.04 nmol H2S/mL/min, 10.71×10−1 s−1 and 4.06×102 M−1 s−1 for KM, Vmax, kcat and kcat/KM, respectively. The optimum temperature and pH for activity were 35°C and 8.5, respectively. The enzyme retained more than half of its activity at 40°C. Inhibitors such as HgCl2, EDTA, glycine and iodoacetamide reduced enzyme activity.
Conclusion
The biochemical properties of β-cyanoalanine synthase in germinating sorghum seeds highlights its roles in maintaining cyanide homeostasis.
Özet
Giriş
β-siyanoalanin sentaz, çimlenen tohumlarda, siyanür homeostazı gibi önemli roller oynamaktadır.
Yöntemler
β-siyanoalanin sentaz, sorgum tohumlarından izole edildi, kromatografik teknikler kullanılarak saflaştırıldı ve biyokimyasal ve katalitik özellikleri belirlendi.
Bulgular
Saflaştırılmış enzim,% 61.74’lük bir verim ve 577.50 nmol H2S/dak/mg protein spesifik aktivitesine sahipti. Saflaştırılmış β-siyanoalanin sentaz için görünür ve alt birim molekül ağırlığı sırasıyla 58.26±2.41 kDa ve 63.4 kDa idi. Substrat olarak sodyum siyanür ile kinetik parametreler, KM, Vmax, kcat ve kcat/KM için sırasıyla 0.67±0.08 mM, 17.60±0.50 nmol H2S/mL/dak, 2.97×10−1 s−1 ve 4.43×102 M−1 s−1 olarak belirlendir. Substrat olarak L-sistein ile kinetik parametreler KM, Vmax, kcat ve kcat/KM için sırasıyla 2.64±0.37 mM, 63.41±4.04 nmol H2S/mL/dak, 10.71×10−1 s−1 ve 4.06×102 M−1 s−1 idi. Aktivite için optimum sıcaklık 35°C ve optimum pH 8.5 idi. Enzim, aktivitesinin yarısından fazlasını 40°C’de korudu. HgCl2, EDTA, glisin ve iyodoasetamid gibi inhibitörler enzim aktivitesini düşürdü.
Sonuç
Çimlenmekte olan sorgum tohumlarındaki β-siyanoalanin sentazın biyokimyasal özellikleri, siyanür homeostazının korunmasındaki rolünü vurgulamaktadır.
Introduction
Cyanogenesis, defined as the biological process in which living organisms (plants) release hydrogen cyanide, is a well-defined concept known for several centuries. The idea behind cyanogenesis had been established in most higher plants, ferns, etc. [1], [2], [3]. Cyanogenesis is not exclusive to cyanogenic plants, plant species that produce cyanogenic glycosides in storage forms of nitrogen and defensive compounds [4], [5] or plant species accumulating cyanolipids but all higher plants probably form low levels of hydrogen cyanide (HCN) as a co-product of ethylene biosynthesis [6]. Plants use the toxicity of cyanide for protection. Hydrolysis of cyanogenic glycosides in response to attack by herbivores or other tissue damage [7], [8] or during decomposition of plant material in soil [9], [10] leads to the release of cyanide. This makes the degradation of cyanogenic compounds a major source by which cyanide is produced in higher plants.
Cyanide in plants is mostly from biosynthesis of ethylene. The mechanism behind this process occurs via the conversion of 1-amino-cyclopropane-1-carboxylic acid (ACC) to ethylene and the concomitant release of cyanoformic acid. The rapid decarboxylation of cyanoformic acid results ultimately in the release of cyanide [10], [11]. The process leading to the biosynthesis of ethylene occurs throughout the development and growth of the plant. Another contributing factor for the increased cyanide accumulation through ethylene synthesis occurs via stress (biotic and abiotic) in which the plants are exposed to [12], [13], which then leads to increased cyanide production [14], [15]. This theory provides possible explanation as to why there is the presence (albeit in relatively small amount) of this cyanide detoxifying enzymes in hitherto non-cyanogenic plants.
The toxicity of cyanide is well known and the mechanism of action involves the formation of iron/magnesium complex in enzymes hence disrupting vital biologic processes such as respiration, CO2 fixation and nitrate reduction [16], [17]. It must therefore be rapidly metabolized in biological systems to reduce or prevent its adverse effects. Two fundamental strategies exist for removing cyanide from biological systems: degradation (breakdown to simpler inorganic molecules) or assimilation (incorporation of cyanide into primary metabolites). Degradation is the primary strategy for cyanide detoxification in eubacteria and this includes: hydrolytic, reductive, or oxidative pathways forming simple nitrogenous compounds such as formamide and ammonium [17], [18].
Two pathways possibly exist for which assimilation of cyanide can occur. Sulfur tranferases mediate the first pathway where these enzymes (3-mercaptopyruvate transferase and rhodanese) are involved in the transfer to a recipient a thiol (-SH) group. The recipient in terms of cyanide detoxification is cyanide. The product of this process is a thiocyanate [19], [20], [21], [22], [23], [24]. Cyanide assimilation also occurs via a second pathway where there is the incorporation of cyanide into nitrogen metabolism through synthesis of aspartate and asparagine. β-Cyanoalanine pathway is the most prevalent example of this type of cyanide assimilation [25], [26] in which the first step is catalyzed by β-cyanoalanine synthase (β-CAS), an enzyme that mediates a reaction which substitutes the sulfhydryl moiety of cysteine (or another alanyl donor like serine) with cyanide, forming the non-protein amino acid, β-cyanoalanine (or the nitrile cyanoalanine) with the concomitant release of hydrogen sulfide [27], [28], [29].
The second step is catalyzed by a bi-functional enzyme, nitrilase 4 (NIT4) [E. C. 3.5.5.1], which is capable of carrying out both nitrilase and nitrile hydratase activities simultaneously, using β-cyanoalanine as the substrate. The nitrile hydratase activity leads to the conversion of cyanoalanine to asparagine while the nitrilase activity forms aspartate and ammonium [30], [31].
Cyanogenesis has been studied extensively in higher plants. As a matter of fact, it was in sorghum that the pathway necessary for cyanogenic glycoside production was initially identified [32], [33]. Sorghum [Sorghum bicolor (L.) Moench] is an upright, quick growing grass which is a member of the Poaceae family. The grass blades are flat, stems are rigid, and there are no creeping rhizomes. The grain is predominately red or reddish brown. Sorghum is valued for its grain, stalks and leaves, which makes it one of the world’s major cereal crops and an important fodder crop [34]. Sorghum is a well-known cyanogenic plant which releases cyanide upon hydrolysis of its stored cyanogenic glycosides [35]. Another instance where cyanide production increases is during germination, where there is increased production of ethylene. The increased production of ethylene further increases cyanide production [15]. The presence and need for the cyanide detoxifying enzyme, β-cyanoalanine synthase can therefore not be overemphasized.
Hence this study investigated the biochemical and catalytic properties of a purified cyanide detoxifying enzyme, β-cyanoalanine synthase, in germinating sorghum seeds. This will provide a broader perspective in the use of β-cyanoalanine synthase in possible cyanide bioremediation.
Materials and methods
Materials
Reagents used were of analytical grade. L-cysteine, sodium cyanide, Tris-base, hydrochloric acid, ferric chloride, N,N-dimethyl-p-phenylenediamine sulfate was obtained from Fisher Scientific, Loughborough, UK. Glycerol, Borate, boric acid, citric acid, trisodium citrate, were obtained from BDH Chemicals Limited, Poole, England. Sodium hydroxide was obtained from Merck Millipore International, Darmstadht, Germany. Glycine, ethylenediamine tetraacetic acid (EDTA), bovine serum albumin (BSA) were obtained from Sigma-Aldrich Chemical Company Limited, St. Louis, MO, USA. DEAE-cellulose and Sephacryl S-200 were obtained from Pharmacia Fine Chemicals, Uppsala, Sweden. Sorghum grains were obtained from “Oja tutun” market in Ile-Ife and identified as S. bicolor (L.) Moench at the IFE Herbarium, Department of Botany, Obafemi Awolowo University, Ile-Ife.
Steeping and germination
About 50 g of carefully sorted S. bicolor seeds (red cultivar grains) was thoroughly washed with distilled water and then soaked in the different growth media: distilled water, 10 mM Tris-HCl buffer (pH 7.0, pH 7.5, pH 8.0, pH 8.5 and pH 9.0) for approximately 24 h at room temperature (25°C) to break seed dormancy and speed up the germination process [25], [36] with the soaking water/buffer being changed at 12-h interval so as to prevent fermentation. After 24 h of steeping, the seeds were evenly spread on damp papers and covered to ensure darkness. The germination process was carried out in the dark at room temperature and sprinkled with the respective growth media: distilled water, 10 mM Tris-HCl buffer (pH 7.0, pH 7.5, pH 8.0, pH 8.5 and pH 9.0) twice per day. The seeds were allowed to germinate for 3, 4 and 5 days (1 day of steeping inclusive).
Enzyme extraction
After the third day, a portion of the sprouted seeds, germinated with different media was collected. β-Cyanoalanine synthase was extracted by homogenizing the germinating seeds in 2.5 volumes of 50 mM Tris-HCI buffer, pH 8.5. The homogenate was stirred and passed through a sieve cloth to remove the chaff. The resulting solution was centrifuged at 10,000×g for 20 min at 4°C in a cold centrifuge. The supernatant recovered was used as the crude extract. The same procedure was repeated for the sprouted seeds collected on the fourth and fifth days for seeds germinated with the different growth media which include distilled water, 10 mM Tris-HCl buffer (pH 7.0, pH 7.5, pH 8.0, pH 8.5 and pH 9.0). β-Cyanoalanine synthase activity was carried out on each crude extract obtained from different growth media and each day of germination; the crude extract with the highest activity was used for purification and characterization.
Enzyme and protein assay
β-Cyanoalanine synthase assay was according to the method of Ogunlabi and Agboola [37] which is a modification of the methods of both Hendrickson and Conn [28] and Yip and Yang [38]. This is based on the rate of formation of hydrogen sulfide (H2S), one of the end-products; sulfide liberated from cysteine was assayed by spectrophotometry after conversion to methylene blue.
Assay was performed in a 10-mL serum bottle with the rubber cork. The assay mixture contained 1 mL of the substrate solution (25 mM L-cysteine and 25 mM NaCN in 100 mM Tris-HCl buffer, pH 8.5) and 1 mL of appropriately diluted enzyme solution. The mixture was incubated at 30°C for 10 min and the reaction terminated by the addition of 0.5 mL of 20 mM N,N-dimethyl-p-phenylenediamine (in 7.2 N HCl) and 0.5 mL of 30 mM FeCl3 in (1.2 N HCl) through the rubber cork using a calibrated syringe and needle. The mixture was shaken vigorously and placed in the dark for 20 min. The solution was clarified by centrifugation and the absorbance was read at 650 nm. The amount of sulfide produced was calculated using the correlation by Hendrickson and Conn [28] in which A650 of 1.0 is equivalent to 0.5 μmol of sulfide produced under the assay condition. One unit of enzyme activity was defined as the amount of enzyme yielding 1.0 nmol of H2S/min under the assay condition.
The protein concentration was determined by the method described by Bradford using BSA as standard [39].
β-Cyanoalanine synthase purification
The crude β-cyanoalanine synthase was concentrated by dialysis against 50% glycerol in 50 mM Tris-HCl buffer, pH 8.5 and was left in the refrigerator overnight. The concentrated enzyme was used for ion-exchange chromatography on DEAE-cellulose.
Ion-exchange chromatography on DEAE-cellulose
DEAE-cellulose was prepared according to the Whatman instruction manual and packed into a 1.5×20 cm column. A sample of the concentrated enzyme was layered on the column. The column was then washed with the buffer to remove the unbound protein, followed by a stepwise elution with 0.5–1.0 M NaCl in 50 mM Tris-HCl buffer, pH 8.5. Fractions of 5 mL were collected at a flow rate of 40 mL/h. Protein was monitored spectrophotometrically at 280 nm. The fractions were also assayed for β-cyanoalanine synthase activity. The active fractions were pooled and dialysed against 50% glycerol in 50 mM Tris-HCl buffer, pH 8.5.
Gel filtration chromatography on Sephacryl S-200
Sephacryl S-200 was packed into a 1.5×40 cm column. The post DEAE-cellulose sample dialysed against 50% glycerol in 50 mM Tris-HCl buffer, pH 8.5 was layered on the column. The column was eluted with 200 mL of 50 mM Tris-HCl buffer, pH 8.5. Fractions of 5 mL were collected from the column at a flow rate of 20 mL/h. Protein was monitored spectrophotometrically at 280 nm. The fractions were also assayed for β-cyanoalanine synthase activity. The active fractions were pooled and stored by dialysis against 50% glycerol in 50 mM Tris-HCl buffer, pH 8.5.
Characterisation of the purified β-cyanoalanine synthase
Determination of kinetic parameters
The kinetic parameters (KM and Vmax) of β-cyanoalanine synthase were determined by varying the concentrations of L-cysteine between 0.5 mM and 5 mM at fixed concentration of 10 mM sodium cyanide (NaCN). Also, the concentration of NaCN was varied between 0.5 mM and 5 mM at fixed concentration of 10 mM L-cysteine. Plots of the reciprocal of initial reaction rate (1/V) versus reciprocal of the varied substrates 1/[S] at each fixed concentrations of the other substrate were made according to Lineweaver and Burk [40] using Graph Pad Prism 5.
Determination of molecular weight of S. bicolor β-CAS
Determination of native molecular weight of S. bicolor β-CAS on Sephacryl S-200
The native molecular weight of the enzyme was estimated by gel filtration on a Sephacryl S-200 column (1.5×40 cm) using the following protein markers: lysozyme (14,000 Da) α-chymotrypsinogen (25,000 Da), peroxidase (44,000 Da) and bovine serum albumin (66,000). The marker proteins at 2 mg/mL were run separately and the column was eluted with 100 mM Tris-HCl buffer, pH 8.5 at a flow rate of 20 mL/h. Fractions of 5 mL were collected and monitored spectrophotometrically by taking absorbance at 280 nm and the elution volume of each protein was estimated. The pure enzyme (5 mL) was then passed through the same column. A plot of logarithm of the molecular weight of the standard proteins against Kav (partition coefficient) was made. The molecular weight of S. bicolor β-CAS was interpolated from the curve.
Determination of molecular weight of S. bicolor β-cyanoalanine synthase by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
SDS-PAGE was carried out according to the method of Weber and Osborn [41] using Tris-glycine buffer system to determine purity as well as subunit molecular weight. The enzyme preparation was denatured by boiling for 5 min in boiling water and was loaded on different wells of the gel (4% stacking, 12% resolving) slab. The standard protein was also applied to a different well on the same gel along with the sample.
Effect of temperature on enzyme activity
The enzyme was assayed at temperatures between 10°C and 60°C to investigate the effect of temperature on the activity of the purified enzyme and also to determine the optimum temperature. The reaction mixture containing 1 mL of the substrate solution (25 mM L-cysteine and 25 mM NaCN in 100 mM Tris-HCl buffer, pH 8.5) was incubated at the indicated temperature and initiated by the addition of an aliquot of the enzyme. The residual enzyme activity was plotted against the different temperatures.
Thermal stability
The thermal stability of the enzyme was studied by incubating 1 mL of the enzyme at temperatures around the optimum temperature (35°C, 40°C, 45°C and 50°C) for 1 h. 0.1 mL was withdrawn at 15 min interval and assayed for residual activity. The residual activity at each temperature was expressed as a percentage of the activity at zero time which was taken to be 100%. The percentage residual activity was plotted against incubation time.
Effect of pH on enzyme activity
The activity of purified β-cyanoalanine synthase in different buffers at different pH values ranging between pH 5.0 and pH 11.0 was investigated. All buffers were 100 mM in concentration. The buffers used were sodium citrate buffer (pH 5.0–7.5), Tris–HCl (pH 7.5–9.0), Borate buffer (pH 8.5–10.0) and Glycine-NaOH buffer (pH range 10.0–11.0). The reaction mixture contained the substrate (25 mM L-cysteine and 25 mM NaCN) in respective buffers and 1 mL of appropriately diluted enzyme.
Effect of salts on enzyme activity
Effect of salts on β-cyanoalanine synthase activity was studied using the following salts in final concentrations of 1 mM, 5 mM and 10 mM: NaCl, KCl, NH4Cl, MgCl2, MnCl2 and CaCl2. The salt was incorporated into the substrate solution, containing 25 mM L-cysteine and 25 mM NaCN in 100 mM Tris-HCl buffer, pH 8.5. The reaction was initiated by the addition of 1 mL appropriately diluted enzyme. Reaction mixtures without salts were taken as control with 100% activity.
Effect of inhibitor on enzyme activity
The effect of some representative inhibitory compounds was examined. These include glycine, EDTA, iodoacetamide, HgCl and β-mercaptoethanol in final concentrations of 1.0 mM, 5 mM and 10 mM, incorporated into the substrate solution of 25 mM L-cysteine and 25 mM NaCN in 100 mM Tris-HCl buffer, pH 8.5. One milliliter of appropriately diluted enzyme was added to initiate the reaction. Reaction mixture without inhibitor was taken as control with 100% activity.
Results
Germination optimization for β-cyanoalanine synthase activity
Sorghum grains grown with 10 mM Tris-HCl buffer, pH 8.5 for 3 days has the highest β-cyanoalanine synthase activity (Figure 1). The crude extract obtained from this was used for purification and characterization of the enzyme.

Germination optimization for β-cyanoalanine synthase activity.
Purification of S. bicolor β-cyanoalanine synthase by ion-exchange chromatography on DEAE-cellulose and gel filtration chromatography on Sephacryl S-200
A summary of a typical purification procedure of β-cyanoalanine synthase from S. bicolor is presented in Table 1. The elution profile of the enzyme on ion exchange chromatography on DEAE-cellulose is shown in Figure 2. A single peak of activity was obtained which was pooled and dialysed against 50% glycerol and then layered on gel filtration chromatography on Sephacryl S-200. The elution profile on Sephacryl S-200 is shown in Figure 3. A single peak of activity was obtained with a yield of 61.74% and a purification fold of 6.64.
Summary of purification procedure for S. bicolor β-cyanoalanine synthase.
| Volume (mL) | Total activity (U) | Total protein (mg) | Specific activity (U/mg) | Yield (%) | Purification fold | |
|---|---|---|---|---|---|---|
| Crude | 41 | 3423.50 | 39.36 | 86.98 | 100.00 | 1.00 |
| Ion-exchange on DEAE-cellulose | 40 | 2560.00 | 24.00 | 106.67 | 74.80 | 1.23 |
| Dialysis against 50% glycerol | 18 | 2151.00 | 19.80 | 108.64 | 62.83 | 1.25 |
| Gel filtration on Sephacryl S-200 | 61 | 2113.65 | 3.66 | 577.50 | 61.74 | 6.64 |

Ion-exchange chromatography of S. bicolor β-cyanoalanine synthase on DEAE-cellulose.

Gel filtration chromatography of S. bicolor β-cyanoalanine synthase on Sephacryl S-200.
Molecular weight determination of S. bicolor β-cyanoalanine synthase
Native molecular weight of S. bicolor β-cyanoalanine synthase by gel filtration chromatography on Sephacryl S-200
The native molecular weight obtained from the plot of the logarithms of molecular weight of standard proteins against the partition coefficient was 58.3 kDa. The plot of the Kav values against the logarithm of the molecular weight is shown in Figure 4.
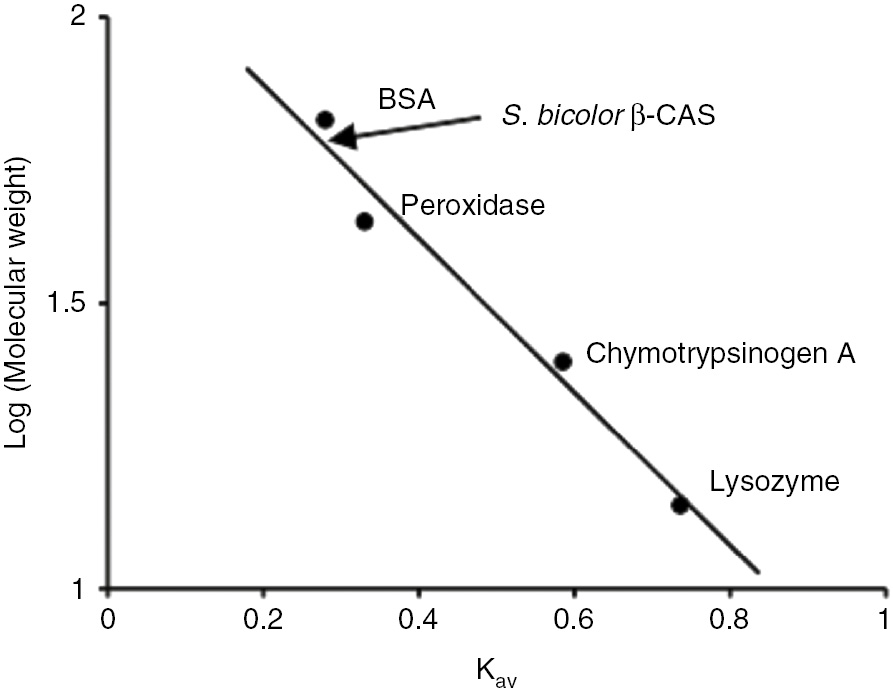
Plot of logarithm of molecular weight against partition coefficient.
Marker proteins used include lysozyme (14 kDa), chymotrypsinogen A (25 kDa), peroxidase (44 kDa) and BSA (66 kDa). The position of S. bicolor β-cyanoalanine synthase is indicated by the arrow.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) of S. bicolor β-cyanoalanine synthase
A distinct band was obtained whose molecular weight is estimated to be 63.4 kDa (Figure 5A). The plot of the logarithms of molecular weight of standard proteins against the relative mobility Rf is shown in Figure 5B.

A distinct band whose molecular weight is estimated to be 63.4 kDa and the plot of the logarithms of molecular weight of standard proteins against the relative mobility.
(A) Electrophoregram of SDS-polyacrylamide gel electrophoresis of S. bicolor β-cyanoalanine synthase. Proteins were stained with Coomassie brilliant Blue R-250. Lane 1 is the molecular weight ladder while lane 2 is S. bicolor β-cyanoalanine synthase. (B) Plot of logarithm of molecular weight of protein standards against the relative mobility (Rf). The relative mobility of protein standards and S. bicolor β-CAS on the SDS-PAGE gel were determined. The molecular weight of S. bicolor β-CAS was interpolated from the plot of the logarithm of molecular weight of protein standards against the relative mobility. The protein mixture for the protein standard include myosin (212 kDa), β-galactosidase (118 kDa), serum albumin (66 kDa), carbonic anhydrase (29 kDa), trypsin inhibitor (20 kDa) and lysozyme (14 kDa).
Kinetic parameters for S. bicolor β-cyanoalanine synthase
Lineweaver-Burk plots for varying concentration of sodium cyanide at fixed concentration of L-cysteine gave KM and Vmax of 0.67±0.08 mM and 17.60±0.50 nmol H2S/mL/min, respectively (Figure 6) while that of varying concentration of L-cysteine at fixed concentration of sodium cyanide are respectively 2.64±0.37 mM and 63.41±4.07 nmol H2S/mL/min (Figure 7). The values of the kinetic parameters of the Lineweaver-Burk plots are presented in Table 2.

Lineweaver-burk plot for S. bicolor β-cyanoalanine synthase by varying concentration of NaCN between 0.5 mM and 5 mM at fixed concentration of 10 mM L-cysteine.
The value shown represents the average from triplicate experiment. Error bar represent the standard deviation.

Lineweaver-burk plot for S. bicolor β-cyanoalanine synthase by varying concentration of L-cysteine between 0.5 mM and 5 mM at fixed concentration of 10 mM NaCN.
The value shown represents the average from triplicate experiment. Error bar represent the standard deviation.
Summary for the kinetic parameters for S. bicolor β-cyanoalanine synthase.
| Substrate | KM (mM) | Vmax (nmol H2S/mL/min) | kcat (s−1)×10−1 | kcat/KM (M−1 s−1)×102 |
|---|---|---|---|---|
| NaCN | 0.67±0.08 | 17.60±0.50 | 2.97 | 4.43 |
| L-cysteine | 2.64±0.37 | 63.41±4.04 | 10.71 | 4.06 |
Effect of temperature on the activity of S. bicolor β-cyanoalanine synthase
The effect of temperature on the activity of S. bicolor β-CAS is shown in Figure 8A. The enzyme showed optimum activity at 35°C. The activation energy, Ea obtained from the Arrhenius plot are 131.75 J/mol/K and –103.54 J/mol/K (Figure 8B).

The effect of temperature on the activity of S. bicolor β-cyanoalanine synthase and the Arrhenius plot for the effect.
(A) The activity-temperature profile of β-CAS from S. bicolor was obtained by varying the temperature between 10°C and 60°C. The values shown represent the average from triplicate experiments. Error bars represent the standard deviation. (B) From the Arrhenius equation, the logarithm of activity was plotted against the inverse of temperature in Kelvins. The activation energy was obtained from the slope of the graph which is −Ea/(2.303R), where R=8.314 J mol−1 K−1. The activation energy of the reaction was then calculated from the slope of the linear portion with negative slope. The positive slope indicates the onset of protein denaturation.
Heat stability of S. bicolor β-cyanoalanine synthase
β-Cyanoalanine synthase from S. bicolor was stable at 30–40°C as it retained about 50% or more of its activity after incubating for 1 h at this temperature (Figure 9). At increased temperatures ranging from 45 to 50°C, the enzyme lost about 90% of its activity after incubating for 1 h.
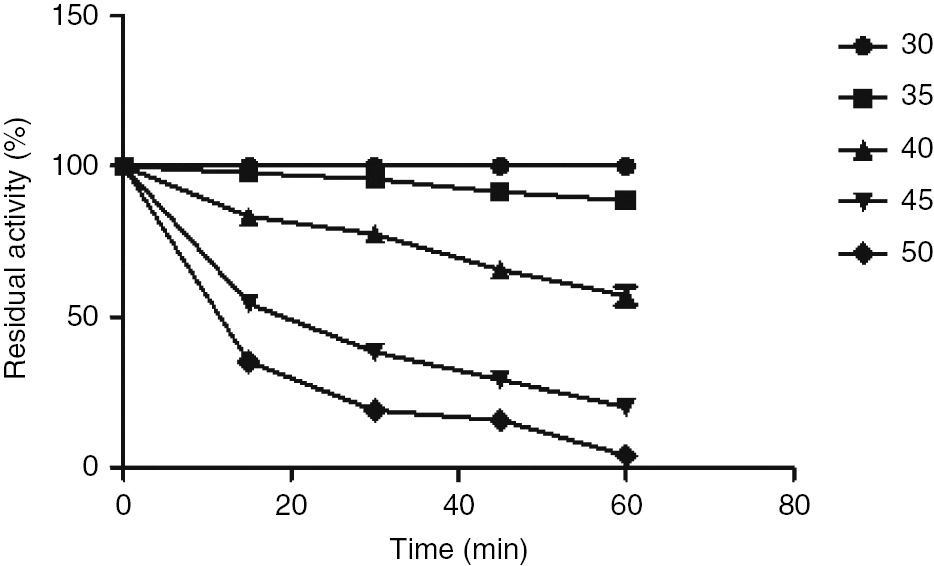
Heat stability of S. bicolor β-cyanoalanine synthase.
Aliquots of β-CAS were incubated at different temperatures (35°C–50°C) for 1 h. An aliquot of the enzyme solution was taken at every 15-min interval and assayed for β-CAS activity and the residual activity was determined under the standard reaction conditions. The activity at zero time was taken as 100%. The residual activity was plotted against the time of incubation.
Effect of pH on the activity of S. bicolor β-cyanoalanine synthase
The effect of pH on the activity of S. bicolor β-CAS is shown in Figure 10. The enzyme showed no activity at pH 5.0–6.0. There was a stable increase in activity between pH 6.5–8.5 followed by a gradual decrease. The enzyme showed optimum activity at pH 8.5.

Effect of pH on the activity of S. bicolor β-cyanoalanine synthase.
Optimum pH was obtained using 100 mM citrate buffer (pH 5.0–7.5), 100 mM Tris-HCl buffer (pH 7.5–9.0), 100 mM borate buffer (pH 8.5–10.0) and 100 mM glycine-NaOH buffer (pH 10.0–11.0).
Effect of salts on the activity of S. bicolorββ-cyanoalanine synthase
Salts of monovalent ions (Na+, K+ and NH4+) had little effect, with 1 mM–10 mM concentrations causing about 15%–20% stimulation. The divalent ions (Mg2+, Ca2+ and Mn2+) also had little effect, with 10 mM concentration causing about 10% stimulation of activity (Table 3).
Effect of salts on the activity of S. bicolor β-cyanoalanine synthase.
| Salts | Relative activity (%) | ||
|---|---|---|---|
| 1.0 mM | 5.0 mM | 10 mM | |
| MnCl2 | 100.57±2.91 | 102.0±1.2 | 109.7±2.4 |
| MgCl2 | 102.9±2.4 | 107.1±3.6 | 111.9±3.1 |
| CaCl2 | 103.7±1.2 | 106.3±2.4 | 110.6±1.2 |
| KCl | 115.7±1.2 | 122.6±3.6 | 123.4±2.4 |
| NH4Cl | 117.4±3.6 | 120.0±2.4 | 118.3±2.4 |
| NaCl | 120.0±2.4 | 124.3±3.6 | 117.4±1.2 |
Assays were carried out in final concentrations of 1.0–10.0 mM of chloride salts of mangenese, magnesium, calcium, potassium, ammonium and sodium. The relative activity was determined by measuring β-CAS activity in the control that contained no chloride salt and taken as 100%. The values shown represent the average from triplicate experiments.
Effect of inhibitors on S. bicolor β-cyanoalanine synthase
Activity of S. bicolor β-cyanoalanine synthase was greatly affected by mercuric chloride, EDTA, and Iodoacetamide with about 80% loss of activity even at 1 mM concentration of EDTA (Table 4).
Effect of inhibitors on the activity of S. bicolor β-cyanoalanine synthase.
| Inhibitor | Relative activity (%) | ||
|---|---|---|---|
| 1.0 mM | 5.0 mM | 10 mM | |
| Mercuric chloride | 88.14±3.7 | 5.65±2.1 | 0 |
| EDTA | 7.34±2.1 | 0 | 0 |
| Glycine | 96.35±1.7 | 84.86±0.9 | 66.43±2.9 |
| Iodoacetamide | 62.84±1.5 | 22.14±0.8 | 0 |
Assays were carried out in final concentrations of 1.0–10.0 mM of inhibitors. The relative activity was determined by measuring β-CAS activity in the control that contained no inhibitor and taken as 100%. The values shown represent the average from triplicate experiments.
Discussion
β-Cyanoalanine synthase (β-CAS) is an enzyme that catalyses the conversion of cyanide and cysteine to β-cyanoalanine [25]. It is the key enzyme for cyanide detoxification in plants [37], [42]. It has been detected in all plants examined though levels of activity vary considerably between species and between different tissues of the same plant. In plant physiology, β-CAS is regarded as the main cyanide detoxifying enzyme necessary for the removal of cyanide that is produced in the life cycle of the plant [28], [42], [43], [44], [45]. Ethylene biosynthesis is the ubiquitous source of cyanide in plants. Conversion of 1-amino-cyclopropane-1-carboxylic acid (ACC) to ethylene releases cyanoformic acid, which spontaneously decarboxylates to release CN− [10], [11]. Ethylene synthesis occurs throughout plant growth and development, but increases significantly when plants are subjected to either biotic or abiotic stress [12], [13], which then leads to increased cyanide production [14], [15].
This research work reported the existence of β-cyanoalanine synthase from germinating seeds of S. bicolor((L.) Moench). After extensive literature search, there has been no known report exclusively on the purification and characterization of this enzyme in S. bicolor. The highest activity for β-cyanoalanine synthase in germinating seeds of sorghum was observed on the third day of germination with Tris-HCl buffer, pH 8.5 (Figure 1). Plant species, such as cocklebur, sorghum, barley and almond, store cyanogenic compounds as a source of nitrogen; there is increased evolution and release of endogenous cyanide immediately prior to and during germination, to help break seed dormancy and promote germination [46], [47], [48]. As a result, β-CAS activity increases considerably during imbibition following an ethylene burst [49].
In the purification method adopted in this study, β-cyanoalanine synthase was purified about seven-fold from germinating seeds of S. bicolor. The purification fold is lower compared to β-CAS purified from previous studies. For example, Hendrickson and Conn [28] purified β-cyanoalanine synthase 140-fold from mitochondrial acetone powder of blue lupine seedlings using a combination of ammonium sulfate precipitation, acetone precipitation, fractionation on Sephadex G-100 column chromatography and preparative gel electrophoresis. β-CAS was purified 6200-fold from fresh spinach leaves by a procedure including the preparation of acetonized mitochondria, ammonium sulfate fractionation, ion-exchange chromatography on DEAE-Sephadex A-50, gel filtration on Sephadex G-100 or Ultrogel AcA 44, hydrophobic chromatography on AH-Sepharose 4B and preparative polyacrylamide gel electrophoresis [28]. β-CAS was purified 17-fold from the cytosolic fraction of the gut of grasshopper Zonocerus variegatus (L.) by ion-exchange chromatography on DEAE-cellulose and gel filtration on Sephadex G-100 columns [37]. β-CAS from S. bicolor has a specific activity of 577.50 nmol H2S/min/mg and a yield of 61.74%. Ikegami et al. [50] reported a specific activity of 33.8 mmol/mL/mg and a yield of 9.8% from 16.5 g of acetone powder extract of blue lupine shoots while [42] obtained a yield of 24.3% and specific activity of 43.5 mmol/mL/mg from 2.5 g of protein of blue lupine seedlings. Yields of 12%, 15% and 12% and specific activities of 13.8, 6.3 and 16.5 mmol/mL/mg, respectively were obtained by [51] starting with 83.2, 69.8 and 85.3 mg of protein of the leaf, rind and tuber of cassava, respectively. The specific activity of β-CAS purified from the cytosolic fraction of the gut of grasshopper, Z. variegatus was 37.5 nmol H2S/min/mg and had a yield of 25.6% [37]. The specific activity obtained for β-CAS from S. bicolor may be as a result of difference in the nature of starting material, cytosolic fractions as against the mitochondrial fractions reported from other plant sources.
The native molecular weight obtained for S. bicolor β-CAS was 58.3 kDa (Figure 4) and the sub-unit molecular weight was estimated to be 63.4 kDa (Figure 5A) which shows it is a monomeric enzyme. In plants, two classes of β-CAS have been identified, based on differences in amino acid composition and protein structure [50] with molecular weight which varies from 50 kDa to 62 kDa [28], [50], [51]. In blue lupine, β-CAS is a monomeric enzyme, with a molecular weight of about 52 kDa, and contains one mole pyridoxal phosphate per mole of protein, which is essential for the catalytic activity [42]. In spinach (Spinacia oleracea) and Lathyrus latifolius, the enzyme contains two identical subunits of 28 kDa–30 kDa, each containing one molecule of pyridoxal phosphate, similar to the β-CAS of the cyanide-producing eubacterium Chromatium violaceum [52], [53].
The kinetic parameters for S. bicolor β-CAS compared very well with reported values of β-CAS from other plant sources, showing that S. bicolor β-CAS has a high affinity for cyanide than for L-cysteine and is able to rapidly detoxify cyanide that is produced during germination [54], [55]. Lineweaver-Burk plots gave apparent KM values of 2.64 mM and 0.67 mM for L-cysteine and cyanide, respectively (Figures 6 and 7). The KM value for L-cysteine was almost the same value as that determined for blue lupine, 2.5 mM [28], spinach (S. oleracea), 2.3 mM [50] and particulate fractions of potato tubers, 2.82 mM. It was however lower than that determined for Vicia angustifolia, which has a KM value of 3.6 mM for L-cysteine [50]. The KM value for sodium cyanide was lower than that determined for (S. oleracea) which has a KM value of 0.73 mM but higher than that determined for blue lupine, 0.55 mM [28] V. angustifolia, 0.5 mM [50] and potato tubers, which has a KM value of 0.235 mM for sodium cyanide. The maximum velocity, Vmax, obtained for S. bicolor β-CAS was 63.41 nmol H2S/mL/min and 17.60 nmol H2S/mL/min for L-cysteine and sodium cyanide, respectively [37] reported Vmax values of 2.17 nmol H2S/mL/min and 20.0 nmol H2S/mL/min for L-cysteine and NaCN, respectively for β-CAS from Z. variegatus. KM is equivalent to the substrate concentration at which the reaction rate is half maximal and is often used as an indicator of the affinity of an enzyme for its substrate [57]; a high KM indicates weak binding, that is, low affinity of the enzyme for the substrate while a low KM indicates strong binding that is high affinity of the enzyme for the substrate [57]. The maximal rate, Vmax, reveals the turnover number of an enzyme, which is the number of substrate molecules converted into product by an enzyme molecule in a unit time when the enzyme is fully saturated with substrate [57]. The turnover number, kcat, for S. bicolor β-CAS was 10.71×10−1 s−1 for L-cysteine and 2.97×10−1 s−1 for sodium cyanide. The specificity constant, kcat/KM is the rate constant for the interaction of substrate and enzyme and can be used as a measure of catalytic efficiency, to know how efficient an enzyme converts a substrate into product. The kcat/KM obtained for S. bicolor β-CAS was 4.06×102 M−1 s−1 and 4.43×102 M−1 s−1 for L-cysteine and sodium cyanide, respectively. The kcat/KM ratios of the enzymes superoxide dismutase, acetylcholinesterase, and triose phosphate isomerase are between 108 and 109 M−1 s−1, which represent the upper limit for kcat/KM. Enzymes such as these that have kcat/KM ratios at the upper limits are said to have achieved catalytic perfection [56], [57].
The optimum temperature obtained for S. bicolor β-CAS was 35°C (Figure 8A). The optimum temperature for β-CAS from the grasshopper Z. variegatus was 30°C with activity reducing to near zero at 45°C. An optimum temperature of 30°C has been reported for both immobilized and dissolved β-CAS [57]. Also, β-CAS from cassava had maximum activity at 30°C when studied over a temperature range of 20–45°C [51]. It can be concluded that this enzyme is most active at around 30–35°C and loses activity as temperature increases above 35°C. The energy required to form the transition state from the substrate, that is, a minimum amount of energy reactants must first acquire to transform into products, is called the activation energy, Ea. Enzymes therefore function to lower the activation energy; in other words, enzymes facilitate the formation of the transition state. The lower activation energy means that more molecules have the required energy to reach the transition state, and hence, a faster reaction [57], [58] The Arrhenius plot of the effect of temperature on the reaction rates for S. bicolor β-CAS consists of two linear segments with a break occurring at 35°C. The apparent activation energy values obtained from these slopes were 131.75 J/mol/K and –103.54 J/mol/K (Figure 8B).
Thermal stability studies on the purified enzyme showed that the enzyme was relatively stable at 30–40°C (Figure 9) as it retained about 50% or more of its activity after incubating for 1 h at this temperature. At increased temperatures ranging from 45 to 50°C, the enzyme lost nearly 90% of its activity after incubating for 1 h. This is similar to the β-CAS from cassava where studies over a temperature range of 20–45°C showed that the enzyme activity decreased significantly above 40°C [51]. A sharp decline in enzyme activity for S. bicolor β-CAS at 35°C could imply that this enzyme is sensitive to thermal inactivation, a process which apparently results from thermally induced transitions of the native structure which leads to the exposure of hydrophobic surfaces and irreversible protein association. Consequently, an enzyme loses its compact three-dimensional structure at extremely high temperature leading to loss of activity.
The optimum pH values reported for β-CAS from different sources fall within the alkaline pH of 8.0–10.0. Hendrickson and Conn [28] reported an optimum pH of about 9.5 for β-CAS from 10-day-old etiolated blue lupine seedlings. The optimum pH obtained for β-CAS from the cyanide-producing bacterium, Chromobacterium violaceum was found to be pH 9.15, with diethanolamine-HCl as the preferred buffer [59]. Similarly, β-CAS purified from the leaves of spinach (S. oleracea) exhibited a single pH optimum at around pH 9.0–9.5 with Tris-HCl buffer [50] and pH optimum for β-CAS from immature seeds of V. angustifolia was found to be pH 9.4–9.5, also with Tris-HCl buffer [50]. β-CAS from potato tubers was reported to have an optimum pH of 8.0–9.0 [61]. An alkaline pH of 9.0 for optimum activity was reported for β-CAS from grasshopper (Z. variegatus) [37]. The optimum pH obtained for S. bicolor β-CAS was 8.5, which also falls within the alkaline pH range.
Hendrickson and Conn [28] investigated the effect of salts on the activity of β-CAS purified from the mitochondria of 10-day-old etiolated blue lupine seedlings and no effect was observed when NH4+, Na+, K+, acetate, or chloride ions were incubated with the enzyme. However, salts of NH4+ and metal ions such as Na+, K+ was reported to relatively stimulate the activity of β-CAS purified from the cyanide producing bacteria, C. violaceum with 1 mM K+ causing 15%–20% stimulation of activity; divalent ions such as Ca2+, Mg2+ and Zn2+ also had little effect, with 10 mM Ca2+ causing 10% stimulation of activity [59]. Similar observation was made for β-CAS from germinating seeds of S. bicolor with salts such as NaCl, KCl, and NH4Cl causing about 15%–20% stimulation of activity at 1 mM–10 mM concentrations. MgCl2, CaCl2 and MnCl2 which are divalent metal salts also stimulated the enzyme activity, with 10 mM concentration causing about 10% stimulation. The influence of these metals on enzyme activity is probably due to the fact that they enhance the protein folding thereby enhancing catalysis. Also, there is also the possibility that β-CAS is a metal activated protein as the inclusion of these metals allowed for enhanced biocatalysis.
Activity of S. bicolor β-CAS was greatly affected by iodoacetamide and mercuric chloride which are known site-specific inhibitors (Table 4). Iodoacetamide can inactivate an enzyme by reacting with a critical cysteine residue [57]. The reaction catalyzed by β-CAS begins with binding of cysteine to the active site; pyridoxal-5-phosphate (PLP) co-factor in the β-CAS structure identifies the active site. In the first half reaction, the α-amine of the cysteine reacts with the Cys-49 of the PLP-Lys-95 Schiff base to release the Lys residue. Formation of PLP-Cys allows Lys-95 to act as a general base in the α, β-elimination of sulfide resulting in the formation of the α-aminoacrylate intermediate [60], [61], [62]. The chelator EDTA caused more than 90% of inhibition at 1 mM concentration. Total inhibition of activity was observed for β-CAS from C. violaceum at 380 μM. The inhibition was 85% relieved by 2 mM Mg2+ suggesting that a divalent metal was required for activity [59].
Conclusion
Pathways for the detoxification of cyanide have been studied in many plants. The results obtained from this study shows that the purified β-cyanoalanine synthase from the germinating sorghum seeds is present to maintain cyanide homeostasis produced during germination. The purified β-cyanoalanine synthase in this study has biochemical and catalytic properties similar to that from other sources and these characteristics makes it unique in the cyanogenesis during germination of the sorghum. The possible role of β-cyanoalanine synthase in cyanide removal via bioremediation will be further exploited as well as investigating the possible roles other cyanide removing enzymes (rhodanese and mercaptopyruvate transferase) play in this seed.
Conflict of interest statements: The authors declare no conflict of interest.
References
1. Conn EE. Cyanogenic glycosides. New York: Academic Press, 1981:479–500.10.1016/B978-0-12-675407-0.50022-1Search in Google Scholar
2. Møller BL. Functional diversifications of cyanogenic glucosides. Curr Opin Plant Biol 2010;13:338–47.10.1016/j.pbi.2010.01.009Search in Google Scholar
3. Lechtenberg M. Cyanogenesis in higher plants and animals. Chichester: John Wiley and Sons, 2011.10.1002/9780470015902.a0001921.pub2Search in Google Scholar
4. Selmar D, Lieberei R, Biehl B. Mobilization and utilization of cyanogenic glycosides. The linustatin pathway. Plant Physiol 1988;86:711–6.10.1104/pp.86.3.711Search in Google Scholar
5. Vetter J. Plant cyanogenic glycosides. Toxicon 1988;38:11–36.10.1016/S0041-0101(99)00128-2Search in Google Scholar
6. Kende H. Enzymes of ethylene biosynthesis. Plant Physiol 1989;91:1–4.10.1104/pp.91.1.1Search in Google Scholar PubMed PubMed Central
7. Seigler DS. Cyanide and cyanogenic glycosides. In: Herbivores: their interactions with secondary plant metabolites. San Diego, CA: Academic Press, 1991:35–77.10.1016/B978-0-12-597183-6.50007-3Search in Google Scholar
8. Kadow D, Voß K, Selmar D, Lieberei R. The cyanogenic syndrome in rubber tree Hevea brasiliensis: tissue-damage-dependent activation of linamarase and hydroxynitrile lyase accelerates hydrogen cyanide release. Ann Bot 2012;109:1253–62.10.1093/aob/mcs057Search in Google Scholar PubMed PubMed Central
9. Widmer TL, Abawi GS. Relationship between levels of cyanide in sudangrass hybrids incorporated into soil and suppression of Meloidogyne hapla. J Nematol 2002;34:16–22.Search in Google Scholar
10. Peiser GD, Tsu-Tsuen W, Hoffman NE, Shang Y, Liu HW, Walsh CT. Formation of cyanide from carbon 1 of 1-aminocyclopropane-1-carboxylic acid during its conversion to ethylene. Proc Natl Acad Sci USA 1984;81:3059–63.10.1073/pnas.81.10.3059Search in Google Scholar PubMed PubMed Central
11. Manning K. Ethylene production and β-cyanoalanine synthase activity in carnation flowers. Planta 1986;168:61–6.10.1007/BF00407010Search in Google Scholar PubMed
12. Morgan PW, Drew MC. Ethylene and plant responses to stress. Physiol Plant 1997;100:620–30.10.1111/j.1399-3054.1997.tb03068.xSearch in Google Scholar
13. Seo S, Mitsuhara I, Feng J, Iwai T, Hasegawa M, Ohashi Y. Cyanide, a co-product of plant hormone ethylene biosynthesis, contributes to the resistance of rice to blast fungus. Plant Physiol 2011;155:502–14.10.1104/pp.110.162412Search in Google Scholar
14. Woodrow IE, Slocum DJ, Gleadow RM. Influence of water stress on cyanogenic capacity in Eucalyptus cladocalyx. Funct Plant Biol 2002;29:103–10.10.1071/PP01116Search in Google Scholar
15. Liang WS. Drought stress increases both cyanogenesis and β-cyanoalanine synthase activity in tobacco. Plant Science 2003;165:1109–15.10.1016/S0168-9452(03)00306-6Search in Google Scholar
16. Donato DB, Nichols O, Possingham H, Moore M, Ricci PF, Noller BN. A critical review of the effects of gold cyanide-bearing tailings solutions on wildlife. Environ Int 2007;33:974–84.10.1016/j.envint.2007.04.007Search in Google Scholar
17. Samiotakis M, Ebbs SD. Possible evidence for transport of an iron cyanide complex by plants. Environ Pollut 2004;127:169–73.10.1016/j.envpol.2003.08.002Search in Google Scholar
18. Gupta N, Balomajumder C, Agarwal VK. Enzymatic mechanism and biochemistry for cyanide degradation: a review. J Hazard Mater 2010;176:1–13.10.1016/j.jhazmat.2009.11.038Search in Google Scholar
19. Sorbo BJ. Rhodanese. Methods Enzymol 1955;2:334–7.10.1016/S0076-6879(55)02207-6Search in Google Scholar
20. Hatzfeld Y, Saito K. Evidence for the existence of rhodanese (thiosulfate:cyanide sulfurtransferase) in plants: preliminary characterization of two rhodanese cDNAs from Arabidopsis thaliana. FEBS Lett 2000;470:147–50.10.1016/S0014-5793(00)01311-9Search in Google Scholar
21. Papenbrock J, Schmidt A. Characterization of a sulfurtransferase from Arabidopsis thaliana. Eur J Biochem 2000;267:145–54.10.1046/j.1432-1327.2000.00980.xSearch in Google Scholar PubMed
22. Ezzi-Mufaddal I, Lynch JM. Cyanide catabolizing enzymes in Trichoderma spp. Enzyme Microb Technol 2002;31:1042–7.10.1016/S0141-0229(02)00238-7Search in Google Scholar
23. Meyer T, Burow M, Bauer M, Papenbrock J. Arabidopsis sulfurtransferases: investigation of their function during senescence and in cyanide detoxification. Planta 2003;217:1–10.10.1007/s00425-002-0964-5Search in Google Scholar
24. Saidu Y. Physicochemical features of rhodanese: a review. Afr J Biotechnol 2004;3:370–4.10.5897/AJB2004.000-2071Search in Google Scholar
25. Miller JM, Conn EE. Metabolism of hydrogen cyanide by higher plants. Plant Physiol 1980;65:1199–202.10.1104/pp.65.6.1199Search in Google Scholar
26. Yu XZ, Lu PC, Yu Z. On the role of β-cyanoalanine synthase (CAS) in metabolism of free cyanide and ferri-cyanide by rice seedlings. Ecotoxicology 2012;21:548–56.10.1007/s10646-011-0815-xSearch in Google Scholar
27. Blumenthal SG, Hendrickson HR, Abrol YP, Conn EE. Cyanide metabolism in higher plants III. The biosynthesis of β-cyanoalanine. J Biol Chem 1968;243:5302–7.10.1016/S0021-9258(18)91950-2Search in Google Scholar
28. Hendrickson HR, Conn EE. Cyanide metabolism in higher plants. IV. Purification and properties of the β-cyanoalanine synthase of blue lupine. J Biol Chem 1969;244:2632–40.10.1016/S0021-9258(18)83446-9Search in Google Scholar
29. Warrilow AG, Hawkesford MJ. Separation, subcellular location and influence of sulphur nutrition on isoforms of cysteine synthase in spinach. J Exp Bot 1998;49:1625–36.10.1093/jxb/49.327.1625Search in Google Scholar
30. Piotrowski M, Schonfelder S, Weiler EW. The Arabidopsis thaliana isogene NIT4 and its orthologs in tobacco encode β-cyano-L-alanine hydratase/nitrilase. J Biol Chem 2001;276:2616–21.10.1074/jbc.M007890200Search in Google Scholar PubMed
31. Piotrowski M, Volmer JJ. Cyanide metabolism in higher plants: cyanoalanine hydratase is a NIT4 homolog. Plant Mol Biol 2006;61:111–22.10.1007/s11103-005-6217-9Search in Google Scholar PubMed
32. Møller BL, Conn EE. The biosynthesis of cyanogenic glucosides in higher plants: N-hydroxytyrosine as an intermediate in the biosynthesis of dhurrin by Sorghum bicolor (Linn) Moench. J Biol Chem 1979;254:8575–83.10.1016/S0021-9258(19)86931-4Search in Google Scholar
33. Halkier BA, Scheller HV, Møller BL. Cyanogenic glucosides: the biosynthetic pathway and the enzyme system involved. Chichester, UK: John Wiley and Sons, 1988:49–66.10.1002/9780470513712.ch5Search in Google Scholar
34. Barkworth M. Sorghum moench. In: Flora of North America Vol 25 magnoliophyta: commelinidae (in part): Poaceae, Part 2. New York: Oxford Univ. Press, 2003:626–30.Search in Google Scholar
35. Busk PK, Møller BL. Dhurrin synthesis in sorghum is regulated at the transcriptional level and induced by nitrogen fertilization in older plants. Plant Physiol 2002;129:1222–31.10.1104/pp.000687Search in Google Scholar
36. Claver IP, Zhang H, Li Q, Zhou H, Zhu, K. Optimized conditions of steeping and germination and their effect on Sorghum [Sorghum bicolor (L.) Moench] composition. PJN 2010;9:686–95.10.3923/pjn.2010.686.695Search in Google Scholar
37. Ogunlabi OO, Agboola FK. A soluble β-cyanoalanine synthase from the gut of the variegated grasshopper Zonocerus variegatus (L.). Insect Biochem Mol Biol 2007;37:72–9.10.1016/j.ibmb.2006.10.003Search in Google Scholar
38. Yip WK, Yang SF. Cyanide metabolism in relation to ethylene production in plant tissues. Plant Physiol 1988;88:473–6.10.1104/pp.88.2.473Search in Google Scholar
39. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54.10.1016/0003-2697(76)90527-3Search in Google Scholar
40. Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc 1934;56:658–66.10.1021/ja01318a036Search in Google Scholar
41. Weber K, Osborn M. Proteins and Sodium dodecyl sulfate: molecular weight determination on polyacrylamide gels and related procedures. New York: Academic Press, 1975:179–223.10.1016/B978-0-12-516301-9.50007-3Search in Google Scholar
42. Meyers DM, Ahmad S. Link between L-3-cyanoalanine activity and differential cyanide sensitivity of insects. Biochim Biophys Acta 1991;1075:195–7.10.1016/0304-4165(91)90252-CSearch in Google Scholar
43. Akopyan TN, Braunstein AE, Goryachenkova EV. β-Cyanoalanine synthase: purification and characterization. Proc Natl Acad Sci USA 1965;72:1617–21.10.1073/pnas.72.4.1617Search in Google Scholar PubMed PubMed Central
44. Wurtele ES, Nikolau BJ, Conn, EE. Subcellular and developmental distribution of β-cyanoalanine synthase in barley leaves. Plant Physiol 1985;78:285–90.10.1104/pp.78.2.285Search in Google Scholar PubMed PubMed Central
45. Manning K. Detoxification of cyanide by plants and hormone action – In Cyanide compounds in biology, Ciba Foundation Symposium 140, Evered D, Garnety SF, editors. Chichester: Wiley, 1988:92–110.10.1002/9780470513712.ch7Search in Google Scholar
46. Poulton JE. Cyanogenesis in plants 1. Plant Physiol 1990;94:401–5.10.1104/pp.94.2.401Search in Google Scholar
47. Bogatek R, Lewak S. Cyanide controls enzymes involved in lipid and sugar catabolism in dormant apple embryos during culture. Physiol Plant 1991;83:422–6.10.1111/j.1399-3054.1991.tb00115.xSearch in Google Scholar
48. Esashi Y, Maruyama A, Sasaki S, Tani A, Yoshiyama M. Involvement of cyanogens in the promotion of germination of cocklebur seeds in response to various nitrogenous compounds, inhibitors of respiratory and ethylene. Plant Cell Physiol 1996;37:545–9.10.1093/oxfordjournals.pcp.a028978Search in Google Scholar
49. Oracz K, El-Maarouf-Bouteau H, Bogatek R, Corbineau F, Bailly C. Release of sunflower seed dormancy by cyanide: cross-talk with ethylene signalling pathway. J Exp Bot 2008;59:2241–51.10.1093/jxb/ern089Search in Google Scholar
50. Ikegami F, Takayama K, Tajima C, Murakoshi I. Purification and properties of [β]-cyano-l-alanine synthase from Spinacia oleracea. Phytochemistry 1988;27:2011–16.10.1016/0031-9422(88)80087-6Search in Google Scholar
51. Sudhakaran PR, Elias M, Nambisan B. Purification and characterization of β-cyanoalanine synthase from cassava tissues. Phytochemistry 1997;46:469–72.10.1016/S0031-9422(97)00305-1Search in Google Scholar
52. Ikegami F, Takayama K, Murakoshi I. Purification and properties of [β]-cyano-l-alanine synthase from Lathyrus latifolius. Phytochemistry 1988;27:3385–9.10.1016/0031-9422(88)80736-2Search in Google Scholar
53. Macadam AM, Knowles CJ. Purification and properties of β-cyanoalanine synthase from the cyanide-producing bacterium, Chromobacterium violaceum. Biochim Biophys Acta 1984;786:123–32.10.1016/0167-4838(84)90081-5Search in Google Scholar
54. Esashi Y, Isuzugawa K, Matsuyama S, Ashino H, Hasegawa R. Endogenous evolution of HCN during pre-germination periods in many seed species. Physiol Plant 1991;83:27–33.10.1111/j.1399-3054.1991.tb01277.xSearch in Google Scholar
55. Bethke PC, Libourel IG, Reinohl V, Jones RL. Sodium nitroprusside, cyanide, nitrite, and nitrate break Arabidopsis seed dormancy in a nitric oxide-dependent manner. Planta 2006;223:805–12.10.1007/s00425-005-0116-9Search in Google Scholar
56. Ikegami F, Takayama K, Kurihara T, Horiuchi S, Tajima C, Shirai R, et al. Purification and properties of [β]-cyano-l-alanine synthase from Vicia angustifolia. Phytochemistry 1989;28:2285–91.10.1016/S0031-9422(00)97969-XSearch in Google Scholar
57. Nelson DL, Cox MM. Lehninger principles of biochemistry, 4th ed. New York: W. H. Freeman, 2004.Search in Google Scholar
58. Berg JM, Tymoczko JL, Stryer L. Biochemistry, 5th ed. New York: W. H. Freeman, 2002.Search in Google Scholar
59. Svenson A, Andersson B. The application of cyanide metabolizing enzymes to environmental control: preparation and characterization of Rhodanase and β-cyanoalanine synthase, immobilized on solid supports. Anal Biochem 1977;83:739–45.10.1016/0003-2697(77)90079-3Search in Google Scholar
60. Maruyama A, Ishizawa K, Takagi T. Purification and characterization of β-cyanoalanine synthase and cysteine synthases from potato tubers: are β-cyanoalanine synthase and mitochondrial cysteine synthase same enzyme? Plant Cell Physiol 2000;41:200–8.10.1093/pcp/41.2.200Search in Google Scholar PubMed
61. Maruyama A, Yoshiyama M, Adachi Y, Tani A, Hasegawa R, Esashi Y. Promotion of cocklebur seed germination by allyl, sulfur and cyanogenic compounds. Plant Cell Physiol 1996;37:1054–8.10.1093/oxfordjournals.pcp.a029053Search in Google Scholar
62. Yi H, Juergens M, Jez JM. Structure of soybean β-cyanoalanine synthase and the molecular basis for cyanide detoxification in plants. Plant Cell 2012;24:2696–706.10.1105/tpc.112.098954Search in Google Scholar PubMed PubMed Central
©2018 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Research Articles
- Effects of calcium hydroxide and N-acetylcysteine on MMP-2, MMP-9, TIMP-1 and TIMP-2 in LPS-stimulated macrophage cell lines
- Synthesis of fused 1,4-dihydropyridines as potential calcium channel blockers
- Optimization of fermentation conditions for efficient ethanol production by Mucor hiemalis
- Covalent immobilization of an alkaline protease from Bacillus licheniformis
- Major biological activities and protein profiles of skin secretions of Lissotriton vulgaris and Triturus ivanbureschi
- Optimized production, purification and molecular characterization of fungal laccase through Alternaria alternata
- Adsorption of methyl violet from aqueous solution using brown algae Padina sanctae-crucis
- Protective effect of dexpanthenol (vitamin B5) in a rat model of LPS-induced endotoxic shock
- Purification and biochemical characterization of a β-cyanoalanine synthase expressed in germinating seeds of Sorghum bicolor (L.) moench
- Molecular cloning and in silico characterization of two alpha-like neurotoxins and one metalloproteinase from the maxilllipeds of the centipede Scolopendra subspinipes mutilans
- Improvement of delta-endotoxin production from local Bacillus thuringiensis Se13 using Taguchi’s orthogonal array methodology
- Enhancing vitamin B12 content in co-fermented soy-milk via a Lotka Volterra model
- Species and number of bacterium may alternate IL-1β levels in the odontogenic cyst fluid
- Rheo-chemical characterization of exopolysaccharides produced by plant growth promoting rhizobacteria
- Benzo(a)pyrene degradation pathway in Bacillus subtilis BMT4i (MTCC 9447)
- Indices
- Reviewers 2018
- Yazar Dizini/Author Index
Articles in the same Issue
- Frontmatter
- Research Articles
- Effects of calcium hydroxide and N-acetylcysteine on MMP-2, MMP-9, TIMP-1 and TIMP-2 in LPS-stimulated macrophage cell lines
- Synthesis of fused 1,4-dihydropyridines as potential calcium channel blockers
- Optimization of fermentation conditions for efficient ethanol production by Mucor hiemalis
- Covalent immobilization of an alkaline protease from Bacillus licheniformis
- Major biological activities and protein profiles of skin secretions of Lissotriton vulgaris and Triturus ivanbureschi
- Optimized production, purification and molecular characterization of fungal laccase through Alternaria alternata
- Adsorption of methyl violet from aqueous solution using brown algae Padina sanctae-crucis
- Protective effect of dexpanthenol (vitamin B5) in a rat model of LPS-induced endotoxic shock
- Purification and biochemical characterization of a β-cyanoalanine synthase expressed in germinating seeds of Sorghum bicolor (L.) moench
- Molecular cloning and in silico characterization of two alpha-like neurotoxins and one metalloproteinase from the maxilllipeds of the centipede Scolopendra subspinipes mutilans
- Improvement of delta-endotoxin production from local Bacillus thuringiensis Se13 using Taguchi’s orthogonal array methodology
- Enhancing vitamin B12 content in co-fermented soy-milk via a Lotka Volterra model
- Species and number of bacterium may alternate IL-1β levels in the odontogenic cyst fluid
- Rheo-chemical characterization of exopolysaccharides produced by plant growth promoting rhizobacteria
- Benzo(a)pyrene degradation pathway in Bacillus subtilis BMT4i (MTCC 9447)
- Indices
- Reviewers 2018
- Yazar Dizini/Author Index

