Acute exercise of painful muscles does not reduce the hypoalgesic response in young healthy women – a randomized crossover study
-
Anders Mohrsen
Abstract
Objectives
Exercise-induced hypoalgesia (EIH) is characterized by an increase in pain threshold following acute exercise. EIH is reduced in some individuals with chronic musculoskeletal pain, although the mechanisms are unknown. It has been hypothesized that this may relate to whether exercises are performed in painful or non-painful body regions. The primary aim of this randomized experimental crossover study was to investigate whether the presence of pain per se in the exercising muscles reduced the local EIH response. The secondary aim was to investigate if EIH responses were also reduced in non-exercising remote muscles.
Methods
Pain-free women (n=34) participated in three separate sessions. In session 1, the maximal voluntary contraction (MVC) for a single legged isometric knee extension exercise was determined. In sessions 2 and 3, pressure pain thresholds (PPT) were assessed at the thigh and shoulder muscles before and after a 3-min exercise at 30 % of MVC. Exercises were performed with or without thigh muscle pain, which was induced by either a painful injection (hypertonic saline, 5.8 %) or a non-painful injection (isotonic saline, 0.9 %) into the thigh muscle. Muscle pain intensity was assessed with an 11-point numerical rating scale (NRS) at baseline, after injections, during and after exercises.
Results
PPTs increased at thigh and shoulder muscles after exercise with painful (14.0–24.9 %) and non-painful (14.3–19.5 %) injections and no significant between-injection EIH differences were observed (p>0.30). Muscle pain intensity was significantly higher following the painful injection compared to the non-painful injection (p<0.001).
Conclusions
Exercising painful muscles did not reduce the local or remote hypoalgesic responses, suggesting that the pain-relieving effects of isometric exercises are not reduced by exercising painful body regions.
Ethical committee number
S-20210184.
Trial registration number
NCT05299268.
Introduction
Musculoskeletal pain is a global problem on the rise, affecting up to 47 % of the general population [1, 2]. Unfortunately, women are disproportionately affected with both a higher prevalence of musculoskeletal pain conditions and experiencing more widespread pain [3, 4] although the underlying reason is poorly understood. Exercise is recommended as an essential part of a multimodal approach to manage musculoskeletal pain [5], [6], [7], [8], and there is evidence to support a positive effect of exercise on pain for 1 in 2 individuals with musculoskeletal pain conditions [9], [10], [11].
In those without pain there is evidence to suggest that the pain experience and pain sensitivity may differ in response to experimental pain between the sexes [12] but while there are contrasting findings in the literature, there is no clear evidence of sex influencing EIH responses [7, 8]. In pain-free populations an immediate hypoalgesic effect to noxious stimuli, including mechanical pressure, is a common finding following exercise and is termed exercise induced hypoalgesia (EIH) [7, 8, 13, 14]. The characteristic EIH response last up to 30 min, with a local and remote increase in pain thresholds following exercise when compared to a pre-exercise assessment [7, 8, 14]. The underlying mechanisms of EIH is not fully understood, but the response could reflect the sum of several local and systemic pain inhibitory mechanisms [7, 8, 13, 15].
Interestingly, EIH is often reduced in musculoskeletal painful conditions, although the magnitude of the EIH response vary between studies [7]. The mechanisms behind the lack of hypoalgesia are unknown but could be related to whether the exercise is performed with the painful body region or not [7, 13]. Several studies across different painful conditions have observed reduced EIH responses immediately after exercises performed with the painful body region [16], [17], [18]. Recently, Hansen and colleagues [19] investigated the effect of experimental muscle pain in the exercising muscle on the EIH response compared to exercise in a non-painful muscle in pain free individuals. The local and remote EIH responses were comparable between exercises at painful and non-painful muscles; however, the interpretation of the results was limited by the fact the participants exercised both the painful and non-painful thigh simultaneously using a wall-squat. Exercising a non-painful body region could have counteracted a potential reduced local EIH response when exercising the painful region. Whether exercises limited to the painful region result in reduced EIH is still unclear. Thus, the primary aim of this randomized crossover study was to investigate whether a local exercise in a painful body region would produce a smaller EIH response compared to the same local exercise in a non-painful body region. Secondary aims were to explore whether the EIH responses in the non-exercising thigh and shoulder were reduced after exercise in a painful body region.
Methods
This study is reported in accordance with the CONSORT NPT recommendations [20]. The study was approved by the ethical committee (S-20210184) and pre-registered at ClinicalTrials.gov (NCT05299268). Participants were enrolled after providing verbal and written informed consent and data was collected in a laboratory setting at Aalborg University between March 8th and April 12th, 2022.
Participants
Pain-free women between 18 and 50 years who were proficient in Danish were recruited from a university setting, through social media and word of mouth.
A verbal screening of exclusion criteria was conducted: Pain during the past 3 months; any pain on test days; any rheumatic, neurological-and/or psychological disorders; surgery in the lower extremities within the last 12 months; pregnancy; addictive behaviour to any kind of euphoric or analgesic substances; alcohol intake on test days. Furthermore, participants were excluded if they experienced any significant side effect during the study such as feeling ill after injections or exercise.
The study was powered to detect a moderate difference in the local EIH re sponse (i.e., effect size of 0.50) between sessions with painful and non-painful muscle exercises. Using G*Power (version 3.1.9.2., Dusseldorf, Germany), it was estimated that 34 participants were required in this cross-over study to be able to detect this difference with a power of 80 % and a two-sided α of 0.05.
Protocol
The participants attended three 40-min sessions separated by approximately one week (Figure 1) to avoid crossover effects such as delayed onset muscle soreness [21].
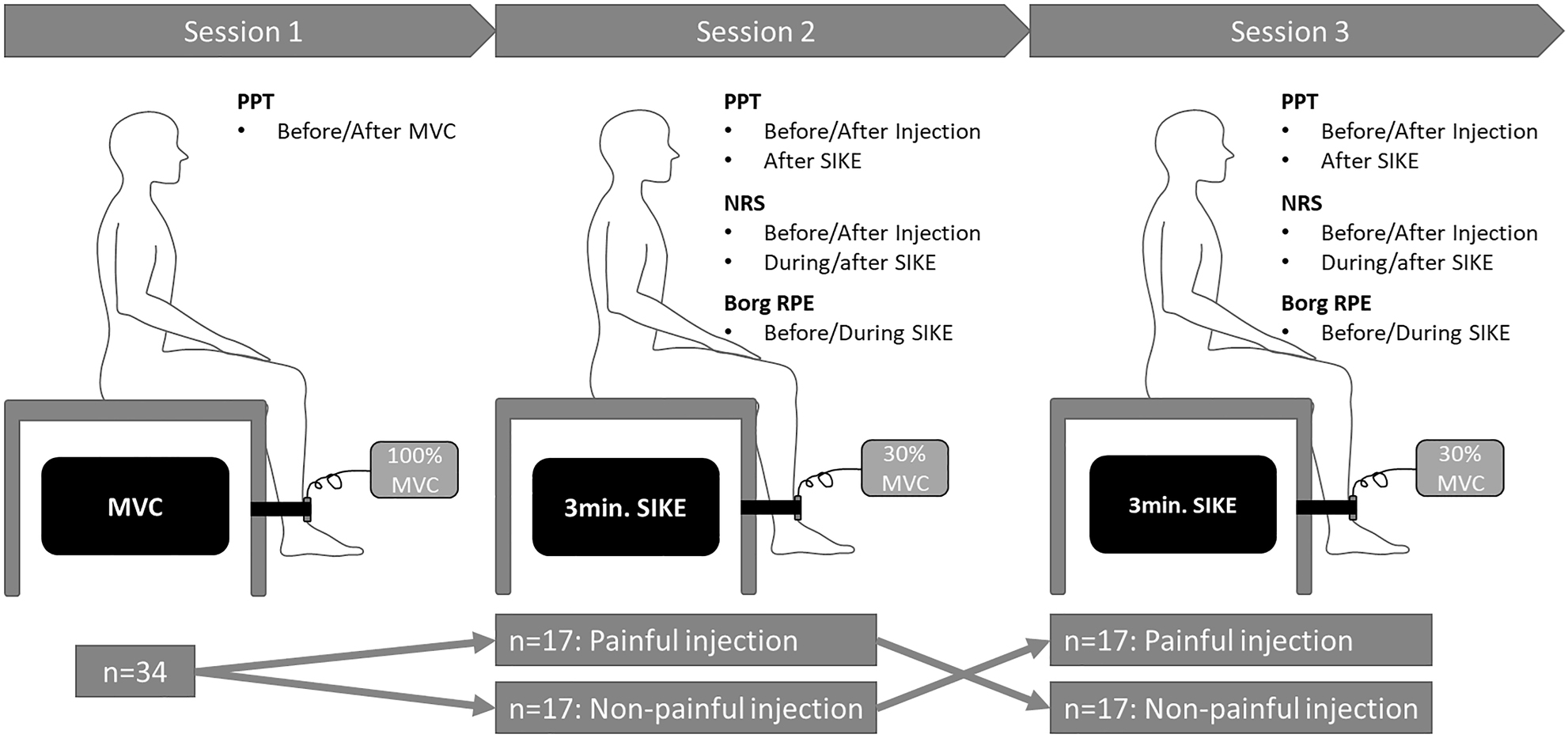
Overview of experimental procedures. Pressure pain thresholds (PPT) were assessed repeatedly during all sessions over the bilateral vastus medialis muscles and the non-dominant upper trapezius muscle. Session 1: The maximal voluntary contraction (MVC) in seated isometric knee extension (SIKE) was assessed. Session 2 and 3: SIKE exercise (30 % of MVC) for 3 min or until exhaustion. Muscle pain intensity was assessed by 0–10 numerical rating scale (NRS) at baseline, after the injections and after 1, 2, and 3 min. of the SIKE and immediately after SIKE. The rate of perceived exertion (Borg RPE: 6–20) was assessed along NRS at baseline and after 1, 2 and 3 min of SIKE. Sessions were separated by approximately one week.
Session 1: First, demographic data (age and gender) and leg dominance were recorded. Next, as participants were naïve to the study protocol, they were familiarized with the procedure of the session, including assessment of pressure pain threshold (PPT) above the lateral epicondyle of the elbow (common extensor tendon). Following familiarization, PPTs were assessed at both thigh muscles and the non-dominant shoulder before and after assessment of maximal voluntary contraction (MVC). MVC was measured for the dominant leg using a seated isometric knee extension (SIKE) exercise and 100 % MVC was defined as the mean of three maximal contractions of 3–5 s, separated by a 1-min rest period. MVCs were conducted with the participants seated on a table with their hands in their lap, the thigh fully supported and with the lower leg hanging over the side and the knees flexed to approximately 90° [22]. The dominant leg was fixated to the table leg using a belt placed above the ankle joint. Between the belt and the participant’s lower leg a force dynamometer (Commander Muscle Tester, Powertrack II; JTECH Medical, Salt Lake City, Utah, USA) was placed. In the end of session 1, participants were randomized into one of two groups (balanced): (a) Non-painful/Painful group receiving injection with isotonic saline (control) in session 2 and injection with hypertonic saline in session 3, or (b) Painful/Non-painful group receiving injection with hypertonic saline in session 2 and injection with isotonic saline (control) in session 3. The randomization was done by having the participants choose an opaque envelope containing the session order. Only the researcher (the same in all sessions, AM) responsible for the injections were aware of the allocation while the other researchers and participants were blinded. Similarly, the researchers responsible for the statistical analysis (HL, SZR) were blinded to the group allocation.
Session 2 and 3: PPTs were assessed at both thighs and the non-dominant shoulder followed by an injection (either painful or non-painful) in the dominant thigh. Immediately after the injection, PPTs were assessed and followed by a 3-min SIKE exercise at 30 % of MVC (visible in real time on the dynamometer’s display) in the same position as MVC was recorded. Verbal motivation was provided for the participants to complete the exercise. Ratings of perceived exertion (RPE) was assessed using the Borg scale (Borg RPE: 6=rest, 20=maximal exertion) [23] at baseline, and at min 1, 2 and 3 during SIKE. After the SIKE, PPTs were reassessed.
Induction of experimental thigh muscle pain
Experimental thigh muscle pain was induced by an intramuscular injection of 1 mL sterile hypertonic saline (5.8 %). The injection was administered in the participant’s dominant medial vastus muscle, 20 cm proximally on a line from the centre of the basis of patella towards the umbilicus [19] using a 1 mL syringe with a disposable needle (27G × 1 1/2″, 0.4 mm × 40 mm). Isotonic saline (1 mL, 0.9 %) was used as a non-painful control injection [19]. Injections (painful or non-painful depending on the randomization order) were administered by the same researcher (AM), who was not blinded to group allocation. Saline has previously been used in several studies as a safe way to induce an intense and short-lasting experimental pain [24]. Prior to the injections, the location and depth of the medial vastus muscle on the dominant leg was confirmed using an ultrasound scanner (Nextgen Logiq e R8, General Electric Company, Boston, Massachusetts, USA) mounted with a 12 MHz linear G4-12T-RS probe [25, 26].
Muscle pain intensity in the dominant thigh was determined using a 11-point numerical rating scale (NRS; 0=no pain, 10=worst imaginable pain) which has previously shown to be valid and reliable [27, 28]. Muscle pain intensity scores were obtained at baseline, immediately after the injection, at min 1, 2 and 3 during exercise and 30 s after exercise.
Outcomes
The primary outcome was the absolute change in pressure pain threshold (PPT) at the dominant thigh (i.e., the local EIH response). Secondary outcomes were the absolute change in PPTs of the non-dominant thigh and non-dominant shoulder (i.e., the remote EIH responses) as well as muscle pain intensity scores in the injected thigh. Other pre-specified outcome measures were change in PPTs before and after determining MVC in session 1.
Pressure pain threshold
Pressure pain threshold (PPT) was assessed using a handheld digital pressure algometer (Somedic Production AB, Hörby, Sweden) wired with a stop button and mounted with a 1 cm2 probe covered by a thin disposable plastic sleeve. For the assessment, participants were seated upright on a chair with a back rest and the probe was placed perpendicular to the skin over the assessment site and the pressure was increased at 30 kPa/s. PPT was defined as the first time the pressure went from being a pressure to first becoming painful [29], at which point the participants pushed the wired button and PPT was recorded. PPT was assessed over three pre-defined locations based [19]: (1) The dominant medial vastus muscle, 15 cm proximal to the basis of patella in a line aiming at the umbilicus (5 cm distally from the injection site); (2) The non-dominant medial vastus muscle as described for the dominant leg; (3) The non-dominant upper trapezius muscle, 10 cm from the acromion on a horizontal line towards the spinous process of the 7th cervical vertebra. Three rounds of PPT recordings were conducted over approximately 3–5 min depending on the individual threshold, separating assessment of the same site by at least 20 s, and the average of each location was used for analysis [19, 30, 31]. All PPT assessments were conducted by the same assessor (HL) who was blinded to the group allocation.
Statistical analysis
Data distribution was explored using Shapiro-Wilks test after which the appropriate statistical approach was chosen. For the primary aim, a Wilcoxon Signed-Rank test was used to compare between session difference in PPT at the dominant thigh following exercise + painful/non-painful injections. Similarly, the secondary aim was explored using a Wilcoxon Signed-Rank test to compare between session difference in PPTs at the non-dominant thigh and shoulder following exercise + painful/non-painful injections. Effect sizes were expressed as Eta squared (η2). Difference in muscle pain intensity scores between the two sessions (painful/non-painful injections) were compared at each time point (baseline, after injection, after 1-, 2-and 3 min of exercise, and 30 s after exercise) using a Wilcoxon Signed-Rank tests with a Bonferroni corrected α (0.05/6). Similarly, differences in RPE during exercise between the two sessions (painful/non-painful injections) were compared at each time point (baseline and after 1-, 2-and 3 min of exercise) using a Bonferroni corrected paired sample t-tests (α 0.05/4). Finally, an exploratory analysis using Wilcoxon Signed-Rank tests was conducted to investigate changes in PPTs after the MVC assessment in session 1 by comparing PPTs from immediately before and after the MVC. All analysis were conducted using Stata/MP 17 (StataCorp, College Station, Texas, USA). The results in text and tables are presented as either median and interquartile range (25th – 75th percentile) or mean ± standard deviation (SD) and 95 % confidence intervals (CI) depending where relevant.
Results
Forty-eight women showed interest in the study and 38 pain-free women were enrolled. Four dropped out or were excluded after inclusion (Figure 2). Two participants felt unwell for approximately 5 min following one of the injections and wished to withdraw from the study. One withdrew due to personal reasons while another sustained a painful injury prior to the final test-session. In total, 34 participants completed all three sessions (age: 26.5 [24], [25], [26], [27], [28], [29], [30] years, BMI: 23.4 ± 2.9 kg/m2, 32 right-legged). The full 3 min of the SIKE exercise was completed by 33 participants in both sessions 2 and 3 while one participant stopped due to exhaustion after 2:03 min in session 2.
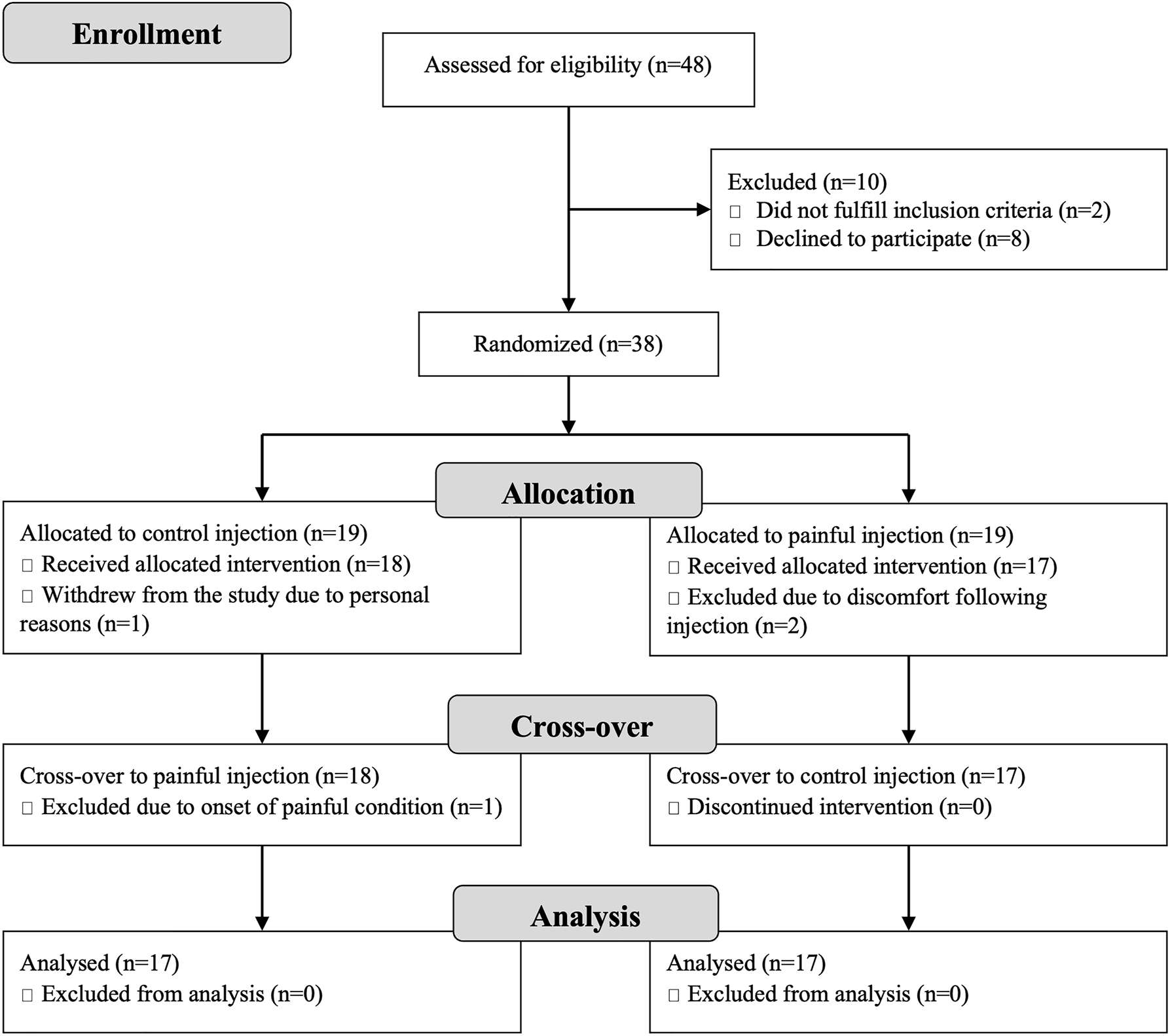
Flow Diagram showing numbers assessed and allocated to group in this cross-over study along with number of dropouts and exclusions as well as the reason for each of these.
For the primary outcome, PPT at the dominant thigh increased after exercises with painful (24.9 %) and non-painful injections (19.5 %) with no significant between-injection difference (Figure 3; Table 1). Similarly, for the secondary outcomes, PPTs increased at the non-dominant thigh and shoulder following exercise with painful (thigh: 14.0 %, shoulder: 19.0 %) and non-painful (thigh: 14.6 %, shoulder: 14.3 %) injections with no between-injection differences (Figure 3). Following the painful injection, muscle pain intensity scores were significantly higher compared to the non-painful injection while no significant differences were observed for ratings of perceived exercise exertion between sessions (Table 2).
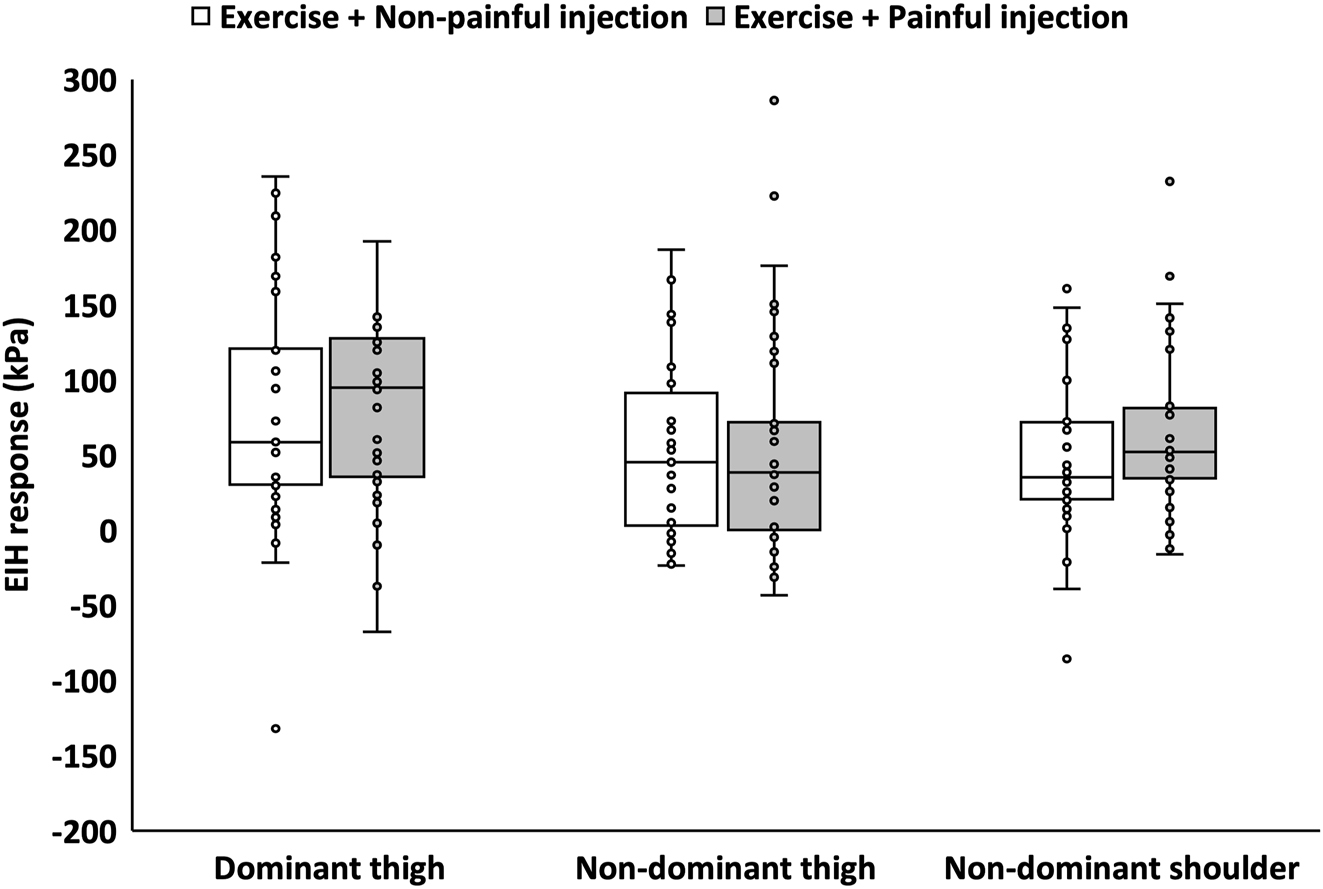
Exercise induced hypoalgesia (EIH) responses for all participants (n=34) at all assessment sites (dominant thigh, non-dominant thigh, and shoulder) for sessions with painful and non-painful injections and exercise. EIH (absolute change in pressure pain thresholds (PPTs)) are presented as median and interquartile range (25th – 75th percentile) as well as individual data-points.
Pressure pain thresholds (PPT) for all participants (n=34) and assessment sites (dominant thigh, non-dominant thigh and shoulder) for sessions with painful and non-painful injections and exercise. PPTs (kPa) are presented as median and interquartile range [25th – 75th percentile] in addition to mean ± SD (95 % CI) for baseline, after injection and after the seated isometric knee extension (SIKE) exercise.
| Pressure pain thresholds during sessions with painful and non-painful injections and exercise | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Session with non-painful control injection | Session with painful injection | |||||||||
| Baseline | After injection | After SIKE | EIH | Baseline | After injection | After SIKE | EIH | p-Value | Effect size (η2) | |
| Dominant thigh | 352 kPa [254–483] | 362 kPa [254–500] | 434 kPa [312–634] | 59 kPa [30–121] | 352 kPa [255–457] | 351 kPa [272–503] | 461 kPa [314–578] | 95 kPa [35–128] | 0.651 | 0.003 |
| 394 kPa ± 190 (328–461) | 411 kPa ± 195 (343–479) | 471 kPa ± 220 (339–548) | 77 kPa ± 79 (49–104) | 394 kPa ± 188 (328–459) | 418 kPa ± 217 (342–493) | 492 kPa ± 261 (400–583) | 98 kPa ± 102 (63–134) | |||
| Non-dominant thigh | 356 kPa [266–527] | 376 kPa [281–522] | 431 kPa [289–606] | 45 kPa [2–98] | 328 kPa [246–483] | 452 kPa [333–572] | 385 kPa [290–509] | 39 kPa [0–72] | 0.447 | 0.009 |
| 398 kPa ± 166 (339–456) | 427 kPa ± 186 (362–492) | 456 kPa ± 197 (386–524) | 58 kPa ± 73 (33–83) | 385 kPa ± 172 (324–444) | 484 kPa ± 205 (412–555) | 439 kPa ± 207 (366–511) | 54 kPa ± 75 (28–81) | |||
| Non-dominant shoulder | 271 kPa [242–349] | 299 kPa [255–457] | 317 kPa [253–471] | 35 kPa [20–72] | 279 kPa [225–345] | 344 kPa [271–439] | 329 kPa [280–471] | 52 kPa [34–83] | 0.317 | 0.015 |
| 336 kPa ± 173 (276–396) | 362 kPa ± 169 (303–421) | 385 kPa ± 189 (318–450) | 48 kPa ± 55 (29–68) | 327 kPa ± 163 (271–385) | 401 kPa ± 189 (335–467) | 390 kPa ± 195 (322–458) | 62 kPa ± 54 (43–81) | |||
-
The difference from baseline to after SIKE (exercise induced hypoalgesia (EIH) response, gray shading) is presented along with the Wilcoxon Signed-Rank test (p-value) and effect sizes (Eta squared; η2) where relevant.
Muscle pain intensity and ratings of perceived exertion in the dominant thigh for sessions with painful and non-painful injections and exercise. Data is presented as median and interquartile range [25th – 75th percentile] and mean ± SD (95 % CI).
| Muscle pain intensity and rating of perceived exercise exertion during sessions with painful and non-painful injections and exercise | ||||||
|---|---|---|---|---|---|---|
| Baseline | After injection | SIKE min 1 | SIKE min 2 | SIKE min 3 | 30 s after SIKE | |
| Non-painful injection session Pain intensity (NRS: 0–10) | 0 [0–0] | 0 [0–0] | 0 [0–2] | 1 [0–3] | 2 [0–4] | 0 [0 - 0] |
| 0 ± 0 (0–0) | 0.3 ± 0.7 (0–0.5) | 0.9 ± 1.2 (0.4–1.3) | 1.7 ± 2 (1–2.4) | 2.3 ± 2.3 (1.5–3.1) | 0.2 ± 0.5 (0–0.4) | |
| Perceived exertion (RPE: 6–20) | 6 [6–6] | – | 11 [9–12] | 13 [11–14] | 15 [13–18] | – |
| 6 ± 0 (6–6) | – | 10.6 ± 2.0 (9.8–11.3) | 13 ± 2.9 (12–14) | 15.3 ± 2.5 (14.4–16.1) | – | |
| Painful injection session | 0 [0–0] | 3.5 [2–4]a | 3 [2–4]a | 3 [2–5]a | 4 [1–6]a | 1 [0–2]a |
| Pain intensity (NRS: 0–10) | 0 ± 0 (0–0) | 3.2 ± 2.2 (2.5–4) | 2.9 ± 1.8 (2.3–3.5) | 3.3 ± 2.0 (2.6–4.0) | 3.8 ± 2.6 (2.8–4.7) | 1.2 ± 1.5 (0.7–1.7) |
| Perceived exertion (RPE: 6–20) | 6 [6–6] | – | 11 [10–12] | 13.5 [12–15] | 15.5 [13–17] | – |
| 6 ± 0 (6–6) | – | 10.9 ± 2.2 (10.1–11.7) | 13.1 ± 2.6 (12.2–14) | 14.9 ± 3.0 (13.9–15.9) | – | |
-
Where relevant, aindicates significant difference compared to the session with non-painful injection (Wilcoxon Signed Rank test: p<0.001).
PPTs were not significantly different after MVC testing compared with before (Table 3). Mean, SD and 95 % CI for changes in PPTs after MVC and exercises are also represented in the respective tables for ease of interpretation.
Pressure pain thresholds (PPT) for all participants (n=34) during the MVC test session. PPT data (kPa) is presented as median and interquartile range [25th – 75th percentile] in addition to mean ± SD (95 % CI) for baseline and after the MVC test for the seated isometric knee extension (SIKE) exercise along with the difference (exercise induced hypoalgesia (EIH) response, gray shading).
| Pressure pain thresholds during MVC test session | |||||
|---|---|---|---|---|---|
| Baseline | After MVC | EIH | p-Value | Effect size (η2) | |
| Dominant thigh | 391 kPa [264–542] | 386 kPa [318–481] | 18 kPa [−40 to 54] | 0.352 | 0.013 |
| 418 kPa ± 177 (356–480) | 437 kPa ± 214 (363–512) | 19 kPa ± 94 (−14 to 52) | |||
| Non-dominant thigh | 367 kPa [278–492] | 377 kPa [270–561] | 16 kPa [−15 to 83] | 0.059 | 0.052 |
| 409 kPa ± 174 (349–470) | 432 kPa ± 191 (365–498) | 23 kPa ± 73 (−3 to 48) | |||
| Non-dominant shoulder | 327 kPa [226–409] | 325 kPa [238–436] | 23 kPa [−19 to 57] | 0.101 | 0.04 |
| 356 kPa ± 201 (285–426) | 374 kPa ± 205 (302–445) | 18 kPa ± 64 (−5 to 40) | |||
-
Where relevant, baseline and after MVC values were compared with the Wilcoxon Signed-Rank test (p-value) and the effect sizes are presented as Eta squared (η2).
Discussion
This randomized experimental crossover study investigated whether the presence of pain per se in the exercising muscles reduced the local and remote EIH responses. Unexpectedly, no significant differences in EIH responses were found between the painful and non-painful injection sessions, despite there being a significantly higher reported thigh muscle pain intensity during the painful condition.
The hypothesis of the current study was that the local EIH response would be impaired when exercising a painful muscle compared to a non-painful muscle. Had the hypothesis been confirmed, it would have been in line with what is seen in painful clinical conditions such as knee osteoarthritis, shoulder myalgia and neck pain where exercising the painful region resulted in an impaired EIH response [16], [17], [18]. However, in the current study, experimental muscle pain did not reduce the EIH response at any site, which is in accordance with the findings of Hansen and colleagues [19]. Nevertheless, in the previous study [16] the lack of difference between conditions could potentially be explained by choice of exercise, isometric wall squat, where participants were exercising the painful and non-painful leg simultaneously which was not the case in the current study. One explanation for the current findings could be related to the experimental pain model. While the hypertonic saline injection caused mild to moderately intense pain [27, 32] in the current study, this is short lasting and although it may produce comparable characteristics to some clinical painful conditions [24], it has been criticized for being unable to replicate clinical features of pain and hyperalgesia as observed in chronic musculoskeletal pain [33]. It may be that the duration of pain following a hypertonic saline injection is not long enough to inhibit the EIH response as seen in persistent clinical pain. Future studies may consider using a different experimental model, such as the injection of nerve growth factor (NGF) which can cause pain and hyperalgesia lasting for days [34, 35]. In fact, a recent study using NGF to cause a low intensity, long-lasting experimental neck muscle pain did see a differentiated EIH response over time when compared to a pain-free group [35]. However, it is unclear if a longer lasting, more intense pain, would impact the EIH response in an otherwise healthy population, similar to what has been observed in clinical populations.
Higher pain intensity following hypertonic saline injection compared to isotonic saline were a feature of both the current study and the one by Hansen and colleagues [19]. With this in mind, it could be speculated whether the noxious stimulus from the injected hypertonic saline may have caused a pain inhibits pain response and the potential impact of pain on EIH may have been counteracted by this. A pain inhibits pain response would be in line with results of a previous study where increased PPT recordings was observed in the neck region following bilateral injection of hypertonic saline in the upper trapezius muscle [36]. In the literature a link between the pain inhibits pain phenomenon and EIH has been proposed and may potentially work through similar inhibitory pathways although this is not yet fully understood [8, 37] and studies have shown that a larger pain inhibits pain response may predict a greater EIH response [38]. That the pain inhibits pain phenomenon and EIH may work through the same pathways is further supported by a previous study [39] which showed that if pain inhibits pain is first elicited this may reduce a subsequent EIH response. The opposite, a decreased pain inhibits pain response following exercise have also been observed [19, 40, 41] and taken together, these results indicate that the endogenous pain modulatory response may be exhaustible. With this in mind, if both pain inhibits pain and EIH is indeed working though the same mechanism [19], the current results could also reflect a ceiling effect for remote areas where, once activated through pain inhibits pain following a painful injection, no further activation through exercise is possible. However, when interpreting the results from studies such as the current one, using a healthy population, it is important to consider that these findings may merely reflect how a healthy system is impacted by pain and this may not reflect what would be expected from clinical populations where EIH responses are commonly reported to be impaired [7, 8].
A final point to consider is thigh muscle pain caused by the exercise itself. Ellingson et al. [42] found a greater EIH response using painful exercises compared to non-painful exercises in pain-free individuals. In the current study, thigh muscle pain, although less, was also caused by the exercise in the session with the non-painful injection suggesting that pain during exercise results in higher EIH, but that more or less pain does not further affect the EIH response.
Strengths and limitations
This study is strengthened by pre-registration, blinded allocation and analysis, and its randomized crossover design, ensuring that participants act as their own controls and thereby accounts for any potential inter-individual response that could potentially have influenced the results. In the current study, no recording of the duration or spatial distribution of injection pain was conducted. However, based on a previous study [43] using a similar experimental pain model and injection location, the experienced pain would be expected to have covered the local PPT site over the dominant thigh and lasting for more than 10 min. In addition, a limitation is the lack of a non-exercise control group. It is therefore not possible to conclude that the change in PPTs, although similar to previous studies [19, 35], were due to the exercise. Another potential limitation is the setup used for MVC testing which was similar to the SIKE exercise. Here, participants were sitting with their hands in their lap, which may have impaired their ability to generate maximal force. In addition, this study was powered to detect a moderate between-injection effect and thus not powered to detect a smaller difference in the EIH response. Finally, this study only included women and the results may therefore not be representative of what may be found in a male population.
Conclusions
This study showed that exercising painful muscles did not reduce the local or remote hypoalgesic responses in young healthy women, suggesting that the pain-relieving effects of acute isometric exercises are not reduced by exercising painful body regions. This adds insight regarding the impact of acute pain and adds to the discussion whether exercising painful or non-painful regions are optimal for a hypoalgesic response.
-
Research funding: Authors state no funding involved.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: Authors state no conflict of interest.
-
Informed consent: Informed consent has been obtained from all individuals included in this study.
-
Ethical approval: Research involving human subjects complied with all relevant national regulations, institutional policies and is in accordance with the tenets of the Helsinki Declaration (as amended in 2013) and has been approved by the Research Ethical Committee (S-20210184).
References
1. El-Tallawy, SN, Nalamasu, R, Salem, GI, LeQuang, JAK, Pergolizzi, JV, Christo, PJ. Management of musculoskeletal pain: an update with emphasis on chronic musculoskeletal pain. Pain Ther 2021;10:181–209. https://doi.org/10.1007/s40122-021-00235-2.Search in Google Scholar PubMed PubMed Central
2. Cieza, A, Causey, K, Kamenov, K, Hanson, SW, Chatterji, S, Vos, T. Global estimates of the need for rehabilitation based on the global burden of disease study 2019: a systematic analysis for the global burden of disease study 2019. Lancet 2021;396:2006–17. https://doi.org/10.1016/s0140-6736(20)32340-0.Search in Google Scholar
3. Fillingim, RB, King, CD, Ribeiro-Dasilva, MC, Rahim-Williams, B, Riley, JL3rd. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain 2009;10:447–85. https://doi.org/10.1016/j.jpain.2008.12.001.Search in Google Scholar PubMed PubMed Central
4. Wijnhoven, HA, de Vet, HC, Picavet, HS. Prevalence of musculoskeletal disorders is systematically higher in women than in men. Clin J Pain 2006;22:717–24. https://doi.org/10.1097/01.ajp.0000210912.95664.53.Search in Google Scholar PubMed
5. Pedersen, BK, Saltin, B. Exercise as medicine – evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports 2015;25:1–72. https://doi.org/10.1111/sms.12581.Search in Google Scholar PubMed
6. Law, LF, Sluka, KA. How does physical activity modulate pain? Pain 2017;158:369–70. https://doi.org/10.1097/j.pain.0000000000000792.Search in Google Scholar PubMed PubMed Central
7. Vaegter, HB, Jones, MD. Exercise-induced hypoalgesia after acute and regular exercise: experimental and clinical manifestations and possible mechanisms in individuals with and without pain. Pain Rep 2020;5:e823. https://doi.org/10.1097/pr9.0000000000000823.Search in Google Scholar PubMed PubMed Central
8. Rice, D, Nijs, J, Kosek, E, Wideman, T, Hasenbring, MI, Koltyn, K, et al.. Exercise-induced hypoalgesia in pain-free and chronic pain populations: state of the art and future directions. J Pain 2019;20:1249–66. https://doi.org/10.1016/j.jpain.2019.03.005.Search in Google Scholar PubMed
9. Roos, EM, Gronne, DT, Skou, ST, Zywiel, MG, McGlasson, R, Barton, CJ, et al.. Immediate outcomes following the GLA:D(R) program in Denmark, Canada and Australia. A longitudinal analysis including 28,370 patients with symptomatic knee or hip osteoarthritis. Osteoarthritis Cartilage 2021;29:502–6. https://doi.org/10.1016/j.joca.2020.12.024.Search in Google Scholar PubMed
10. Hayden, JA, Ellis, J, Ogilvie, R, Malmivaara, A, van Tulder, MW. Exercise therapy for chronic low back pain. Cochrane Database Syst Rev 2021;9:CD009790. https://doi.org/10.1002/14651858.cd009790.pub2.Search in Google Scholar PubMed PubMed Central
11. Fransen, M, McConnell, S, Harmer, AR, Van der Esch, M, Simic, M, Bennell, KL. Exercise for osteoarthritis of the knee: a Cochrane systematic review. Br J Sports Med 2015;49:1554–7. https://doi.org/10.1136/bjsports-2015-095424.Search in Google Scholar PubMed
12. Loram, L, Horwitz, E, Bentley, A. Gender and site of injection do not influence intensity of hypertonic saline-induced muscle pain in healthy volunteers. Man Ther 2009;14:526–30. https://doi.org/10.1016/j.math.2008.09.002.Search in Google Scholar PubMed
13. Naugle, KM, Fillingim, RB, Riley, JL3rd. A meta-analytic review of the hypoalgesic effects of exercise. J Pain 2012;13:1139–50. https://doi.org/10.1016/j.jpain.2012.09.006.Search in Google Scholar PubMed PubMed Central
14. Koltyn, KF. Exercise-induced hypoalgesia and intensity of exercise. Sports Med 2002;32:477–87. https://doi.org/10.2165/00007256-200232080-00001.Search in Google Scholar PubMed
15. Da Silva Santos, R, Galdino, G. Endogenous systems involved in exercise-induced analgesia. J Physiol Pharmacol 2018;69:3–13. https://doi.org/10.26402/jpp.2018.1.01.Search in Google Scholar PubMed
16. Lannersten, L, Kosek, E. Dysfunction of endogenous pain inhibition during exercise with painful muscles in patients with shoulder myalgia and fibromyalgia. Pain 2010;151:77–86. https://doi.org/10.1016/j.pain.2010.06.021.Search in Google Scholar PubMed
17. Burrows, NJ, Booth, J, Sturnieks, DL, Barry, BK. Acute resistance exercise and pressure pain sensitivity in knee osteoarthritis: a randomised crossover trial. Osteoarthritis Cartilage 2014;22:407–14. https://doi.org/10.1016/j.joca.2013.12.023.Search in Google Scholar PubMed
18. Christensen, SW, Hirata, RP, Graven-Nielsen, T. Altered pain sensitivity and axioscapular muscle activity in neck pain patients compared with healthy controls. Eur J Pain 2017;21:1763–71. https://doi.org/10.1002/ejp.1088.Search in Google Scholar PubMed
19. Hansen, S, Petersen, KK, Sloth, E, Manum, LA, McDonald, AK, Andersen, PG, et al.. Hypoalgesia after exercises with painful vs. non-painful muscles in healthy subjects – a randomized cross-over study. Scand J Pain 2022;22:614–21. https://doi.org/10.1515/sjpain-2021-0161.Search in Google Scholar PubMed
20. Boutron, I, Altman, DG, Moher, D, Schulz, KF, Ravaud, P. CONSORT statement for randomized trials of nonpharmacologic treatments: a 2017 update and a CONSORT extension for nonpharmacologic trial abstracts. Ann Intern Med 2017;167:40–7. https://doi.org/10.7326/m17-0046.Search in Google Scholar
21. Lau, WY, Blazevich, AJ, Newton, MJ, Wu, SS, Nosaka, K. Changes in electrical pain threshold of fascia and muscle after initial and secondary bouts of elbow flexor eccentric exercise. Eur J Appl Physiol 2015;115:959–68. https://doi.org/10.1007/s00421-014-3077-5.Search in Google Scholar PubMed
22. Vaegter, HB, Handberg, G, Graven-Nielsen, T. Similarities between exercise-induced hypoalgesia and conditioned pain modulation in humans. Pain 2014;155:158–67. https://doi.org/10.1016/j.pain.2013.09.023.Search in Google Scholar PubMed
23. Borg, GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;14:377–81. https://doi.org/10.1249/00005768-198205000-00012.Search in Google Scholar
24. Graven-Nielsen, T. Fundamentals of muscle pain, referred pain, and deep tissue hyperalgesia. Scand J Rheumatol Suppl 2006;122:1–43. https://doi.org/10.1080/03009740600865980.Search in Google Scholar PubMed
25. Christensen, SW, Hirata, RP, Graven-Nielsen, T. Bilateral experimental neck pain reorganize axioscapular muscle coordination and pain sensitivity. Eur J Pain 2017;21:681–91. https://doi.org/10.1002/ejp.972.Search in Google Scholar PubMed
26. Christensen, SWM, Peolsson, A, Agger, SM, Svindt, M, Graven-Nielsen, T, Hirata, RP. Head repositioning accuracy is influenced by experimental neck pain in those most accurate but not when adding a cognitive task. Scand J Pain 2019;20:191–203. https://doi.org/10.1515/sjpain-2019-0093.Search in Google Scholar PubMed
27. Karcioglu, O, Topacoglu, H, Dikme, O, Dikme, O. A systematic review of the pain scales in adults: which to use? Am J Emerg Med 2018;36:707–14. https://doi.org/10.1016/j.ajem.2018.01.008.Search in Google Scholar PubMed
28. Modarresi, S, Lukacs, MJ, Ghodrati, M, Salim, S, MacDermid, JC, Walton, DM, et al.. A systematic review and synthesis of psychometric properties of the numeric pain rating scale and the visual analog scale for use in people with neck pain. Clin J Pain 2021;38:132–48. https://doi.org/10.1097/ajp.0000000000000999.Search in Google Scholar
29. Jensen, K, Andersen, HO, Olesen, J, Lindblom, U. Pressure-pain threshold in human temporal region. Evaluation of a new pressure algometer. Pain 1986;25:313–23. https://doi.org/10.1016/0304-3959(86)90235-6.Search in Google Scholar PubMed
30. Rolke, R, Magerl, W, Campbell, KA, Schalber, C, Caspari, S, Birklein, F, et al.. Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur J Pain 2006;10:77–88. https://doi.org/10.1016/j.ejpain.2005.02.003.Search in Google Scholar PubMed
31. Pfau, DB, Krumova, EK, Treede, RD, Baron, R, Toelle, T, Birklein, F, et al.. Quantitative sensory testing in the German research network on neuropathic pain (DFNS): reference data for the trunk and application in patients with chronic postherpetic neuralgia. Pain 2014;155:1002–15. https://doi.org/10.1016/j.pain.2014.02.004.Search in Google Scholar PubMed
32. Boonstra, AM, Stewart, RE, Koke, AJ, Oosterwijk, RF, Swaan, JL, Schreurs, KM, et al.. Cut-off points for mild, moderate, and severe pain on the numeric rating scale for pain in patients with chronic musculoskeletal pain: variability and influence of sex and catastrophizing. Front Psychol 2016;7:1466. https://doi.org/10.3389/fpsyg.2016.01466.Search in Google Scholar PubMed PubMed Central
33. Ford, B, Halaki, M, Diong, J, Ginn, KA. Acute experimentally-induced pain replicates the distribution but not the quality or behaviour of clinical appendicular musculoskeletal pain. A systematic review. Scand J Pain 2021;21:217–37. https://doi.org/10.1515/sjpain-2020-0076.Search in Google Scholar PubMed
34. Bergin, MJ, Hirata, R, Mista, C, Christensen, SW, Tucker, K, Vicenzino, B, et al.. Movement evoked pain and mechanical hyperalgesia after intramuscular injection of nerve growth factor: a model of sustained elbow pain. Pain Med 2015;16:2180–91. https://doi.org/10.1111/pme.12824.Search in Google Scholar PubMed
35. Christensen, SWM, Elgueta-Cancino, E, Simonsen, MB, Silva, PB, Sorensen, LB, Graven-Nielsen, T, et al.. Effect of prolonged experimental neck pain on exercise-induced hypoalgesia. Pain 2022;163:2411–20. https://doi.org/10.1097/j.pain.0000000000002641.Search in Google Scholar PubMed
36. Ge, HY, Madeleine, P, Cairns, BE, Arendt-Nielsen, L. Hypoalgesia in the referred pain areas after bilateral injections of hypertonic saline into the trapezius muscles of men and women: a potential experimental model of gender-specific differences. Clin J Pain 2006;22:37–44. https://doi.org/10.1097/01.ajp.0000149799.01123.38.Search in Google Scholar PubMed
37. Smith, SA, Micklewright, D, Winter, SL, Mauger, AR. Muscle pain induced by hypertonic saline in the knee extensors decreases single-limb isometric time to task failure. Eur J Appl Physiol 2020;120:2047–58. https://doi.org/10.1007/s00421-020-04425-2.Search in Google Scholar PubMed PubMed Central
38. Lemley, KJ, Hunter, SK, Bement, MK. Conditioned pain modulation predicts exercise-induced hypoalgesia in healthy adults. Med Sci Sports Exerc 2015;47:176–84. https://doi.org/10.1249/mss.0000000000000381.Search in Google Scholar PubMed
39. Sorensen, LB, Gazerani, P, Sluka, KA, Graven-Nielsen, T. Repeated injections of low-dose nerve growth factor (NGF) in healthy humans maintain muscle pain and facilitate ischemic contraction-evoked pain. Pain Med 2020;21:3488–98. https://doi.org/10.1093/pm/pnaa315.Search in Google Scholar PubMed
40. Shiozawa, S, Hirata, RP, Graven-Nielsen, T. Reorganised anticipatory postural adjustments due to experimental lower extremity muscle pain. Hum Mov Sci 2013;32:1239–52. https://doi.org/10.1016/j.humov.2013.01.009.Search in Google Scholar PubMed
41. Henriksen, M, Alkjaer, T, Lund, H, Simonsen, EB, Graven-Nielsen, T, Danneskiold-Samsoe, B, et al.. Experimental quadriceps muscle pain impairs knee joint control during walking. J Appl Physiol 2007;103:132–9. https://doi.org/10.1152/japplphysiol.01105.2006.Search in Google Scholar PubMed
42. Ellingson, LD, Koltyn, KF, Kim, JS, Cook, DB. Does exercise induce hypoalgesia through conditioned pain modulation? Psychophysiology 2014;51:267–76. https://doi.org/10.1111/psyp.12168.Search in Google Scholar PubMed
43. Gallina, A, Salomoni, SE, Hall, LM, Tucker, K, Garland, SJ, Hodges, PW. Location-specific responses to nociceptive input support the purposeful nature of motor adaptation to pain. Pain 2018;159:2192–200. https://doi.org/10.1097/j.pain.0000000000001317.Search in Google Scholar PubMed
© 2023 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Systematic Review
- Comparison of the effectiveness of eHealth self-management interventions for pain between oncological and musculoskeletal populations: a systematic review with narrative synthesis
- Topical Review
- Shifting the perspective: how positive thinking can help diminish the negative effects of pain
- Clinical Pain Researches
- Pain acceptance and psychological inflexibility predict pain interference outcomes for persons with chronic pain receiving pain psychology
- A feasibility trial of online Acceptance and Commitment Therapy for women with provoked vestibulodynia
- Relations between PTSD symptom clusters and pain in three trauma-exposed samples with pain
- Short- and long-term test–retest reliability of the English version of the 7-item DN4 questionnaire – a screening tool for neuropathic pain
- Chronic post-thoracotomy pain after lung cancer surgery: a prospective study of preoperative risk factors
- Pain sensitivity after Roux-en-Y gastric bypass – associations with chronic abdominal pain and psychosocial aspects
- Barriers in chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) management: perspectives from health practitioners
- Observational studies
- Spontaneous self-affirmation: an adaptive coping strategy for people with chronic pain
- COVID-19 and processes of adjustment in people with persistent pain: the role of psychological flexibility
- Presence and grade of undertreatment of pain in children with cerebral palsy
- Sex-related differences in migraine clinical features by frequency of occurrence: a cross-sectional study
- Recurrent headache, stomachache, and backpain among adolescents: association with exposure to bullying and parents’ socioeconomic status
- Original Experimentals
- Temporal stability and responsiveness of a conditioned pain modulation test
- Anticipatory postural adjustments mediate the changes in fear-related behaviors in individuals with chronic low back pain
- The role of spontaneous vs. experimentally induced attentional strategies for the pain response to a single bout of exercise in healthy individuals
- Acute exercise of painful muscles does not reduce the hypoalgesic response in young healthy women – a randomized crossover study
- Short Communications
- Nation-wide decrease in the prevalence of pediatric chronic pain during the COVID-19 pandemic
- A multidisciplinary transitional pain service to improve pain outcomes following trauma surgery: a preliminary report
Articles in the same Issue
- Frontmatter
- Systematic Review
- Comparison of the effectiveness of eHealth self-management interventions for pain between oncological and musculoskeletal populations: a systematic review with narrative synthesis
- Topical Review
- Shifting the perspective: how positive thinking can help diminish the negative effects of pain
- Clinical Pain Researches
- Pain acceptance and psychological inflexibility predict pain interference outcomes for persons with chronic pain receiving pain psychology
- A feasibility trial of online Acceptance and Commitment Therapy for women with provoked vestibulodynia
- Relations between PTSD symptom clusters and pain in three trauma-exposed samples with pain
- Short- and long-term test–retest reliability of the English version of the 7-item DN4 questionnaire – a screening tool for neuropathic pain
- Chronic post-thoracotomy pain after lung cancer surgery: a prospective study of preoperative risk factors
- Pain sensitivity after Roux-en-Y gastric bypass – associations with chronic abdominal pain and psychosocial aspects
- Barriers in chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) management: perspectives from health practitioners
- Observational studies
- Spontaneous self-affirmation: an adaptive coping strategy for people with chronic pain
- COVID-19 and processes of adjustment in people with persistent pain: the role of psychological flexibility
- Presence and grade of undertreatment of pain in children with cerebral palsy
- Sex-related differences in migraine clinical features by frequency of occurrence: a cross-sectional study
- Recurrent headache, stomachache, and backpain among adolescents: association with exposure to bullying and parents’ socioeconomic status
- Original Experimentals
- Temporal stability and responsiveness of a conditioned pain modulation test
- Anticipatory postural adjustments mediate the changes in fear-related behaviors in individuals with chronic low back pain
- The role of spontaneous vs. experimentally induced attentional strategies for the pain response to a single bout of exercise in healthy individuals
- Acute exercise of painful muscles does not reduce the hypoalgesic response in young healthy women – a randomized crossover study
- Short Communications
- Nation-wide decrease in the prevalence of pediatric chronic pain during the COVID-19 pandemic
- A multidisciplinary transitional pain service to improve pain outcomes following trauma surgery: a preliminary report

