Abstract
Carbon/silica composite (C/SiO2) aerogels were synthesized by using tetraethyl orthosilicate, 3-aminopropyltriethoxysilane, trimethylchlorosilane, resorcinol as the silicon and carbon sources, and ethanol as solvent, via the sol–gel process followed by supercritical ethanol drying and thermal treatment. The influence of thermal treatment process on the structure of C/SiO2 composite aerogels was analyzed mainly and measured by Fourier transform infrared spectrometer, X-ray diffraction and nitrogen adsorption–desorption analysis. The results showed that the optimum thermal treatment temperature of C/SiO2 composite aerogels was 1,300°C. With the increase in the thermal treatment time, the linear shrinkage of the composite aerogels increased gradually, and the pore volume, average pore diameter and specific surface area of the composite aerogels increased first and then decreased.
1 Introduction
Aerogels are unique porous materials with a distinctive microstructure consisting of pores and particles in the nanometer size range. Recently, increased attention has been given to monolithic aerogels due to the combination of a compact integral structure and porous microstructure in a material that exhibits low density, good mechanical behavior, large internal void space and high specific surface area [1,2,3,4]. Among these, carbon/silicon carbide composite (C/SiC) aerogels have excellent properties, including chemical and thermal stability, high conductivity, high surface area and high porosity, and can potentially be used as adsorbents, thermal insulators or electrode materials [5,6,7].
The basic prepared method of C/SiO2 aerogel is the sol–gel method. There were three categories ways of C/SiO2 which had the aerogel-structure: copolymerization, immersion, polymer precursor pyrolysis [8,9,10]. Leventis et al. [11] prepared C/SiO2 aerogels first by resorcinol-formaldehyde/SiO2 (RF/SiO2) composite aerogel at the carbonization temperature of 700–900°C. Kong et al. [12] used resorcinol and formaldehyde solution as carbon source, 3-aminopropyltriethoxysilane (APTES) as silicon source and ethanol as solvent. Mesoporous α-SiC was prepared through the series of processes such as sol–gel, supercritical drying and heat treatment. At present, the application requirements of catalysis, high temperature insulation, adsorption, hydrogen storage and photoelectricity have been put forward as higher requirements for the high temperature resistance of aerogel materials.
In this study, we propose a novel method of synthesizing C/SiO2 aerogels. First, RF/SiO2 aerogels were prepared by sol–gel process and supercritical CO2 drying. C/SiO2 aerogels were obtained by heat treatment from RF/SiO2 aerogels. Different heat treatment time influence the pore structure of C/SiO2 composite aerogels, and further influence the chemical properties of the obtained samples. A series of characterization methods were used to certify the properties of C/SiO2 aerogels. According to the results, C/SiO2 aerogels with different heat treatment time exhibit different pore properties [13,14,15]. Fourier transform infrared spectrometer (FTIR) analysis and X-ray diffraction (XRD) patterns all exhibit the structure changes of the C/SiO2 aerogels with different heat treatment time, and the results were analyzed in detail according to refs [16–20]. The network structure and high temperature resistance of C/SiO2 aerogel materials will be studied systematically, which will provide a useful reference for the application of C/SiO2 aerogels in many fields.
2 Experiment
This section describes the chemicals as well as the equipment used in the experiment.
2.1 C/SiO2 aerogel preparation
All chemicals were analytical grade and used as received without further purification. Certain amount of tetraethyl orthosilicate, APTES, trimethylchlorosilane, resorcinol, anhydrous alcohol and deionized water (molar ratio = 25:15:5:12:40:1) were mixed and stirred in a pot at room temperature for 30 min, then the compound was placed in an air oven at 50°C. The wet gel was aged in an oven at 50°C for 48 h (the solvent was replaced with absolute ethanol every 8 h). Then, the gel was dried by supercritical drying of carbon dioxide (HELIX 1.1 system, Applied Separations Inc., Allentown, PA) at 45°C and 8.0 MPa for 5 h, then RF/SiO2 composite aerogel was obtained. The RF/SiO2 composite aerogel was heat-treated and carbonized at a temperature of 1,200–1,400°C with a heating rate of 2°C/min and Ar flow rate of 100 cm3/min.
2.2 Characterization method
The microstructure and energy spectrum of the specimens were surveyed by LEO-1530VP scanning electron microscopy (SEM). Surface areas, average pore diameter, pore volume and pore distribution were measured by nitrogen adsorption–desorption porosimeter using a Micromeritics ASAP2020 surface area and pore distribution analyzer after the samples were degassed in a vacuum at 200°C for 6 h. The molecular structure of aerogel composites was analyzed by Nicolet 670 Nexus FTIR. The phase composition of the sample was evaluated by XRD using a Cu-Kα radiation.
3 Results and discussion
This section reflects the SEM, N2 adsorption–desorption, FTIR and XRD results, and analyses the properties of the samples in detail.
3.1 Analysis of samples and morphology before and after heat treatment of C/SiO2 aerogel
Figure 1 shows the photographs of C/SiC aerogels before and after heat treatment. C/SiC aerogel exhibits significant mass loss and shrinkage, shows complete shape with no cracks on the surface after supercritical drying and thermal treatment. Figure 2 shows the SEM images of C/SiC aerogels before and after heat treatment. C/SiO2 aerogels exhibit the disordered, porous structures of a typical colloidal gel. After carbothermal reduction, the particles of C/SiC aerogels are distinguishable and non-spherical. It presents disordered porous nanostructures with “pearl chain” morphology with cross-linked nanoparticles compactly packed.
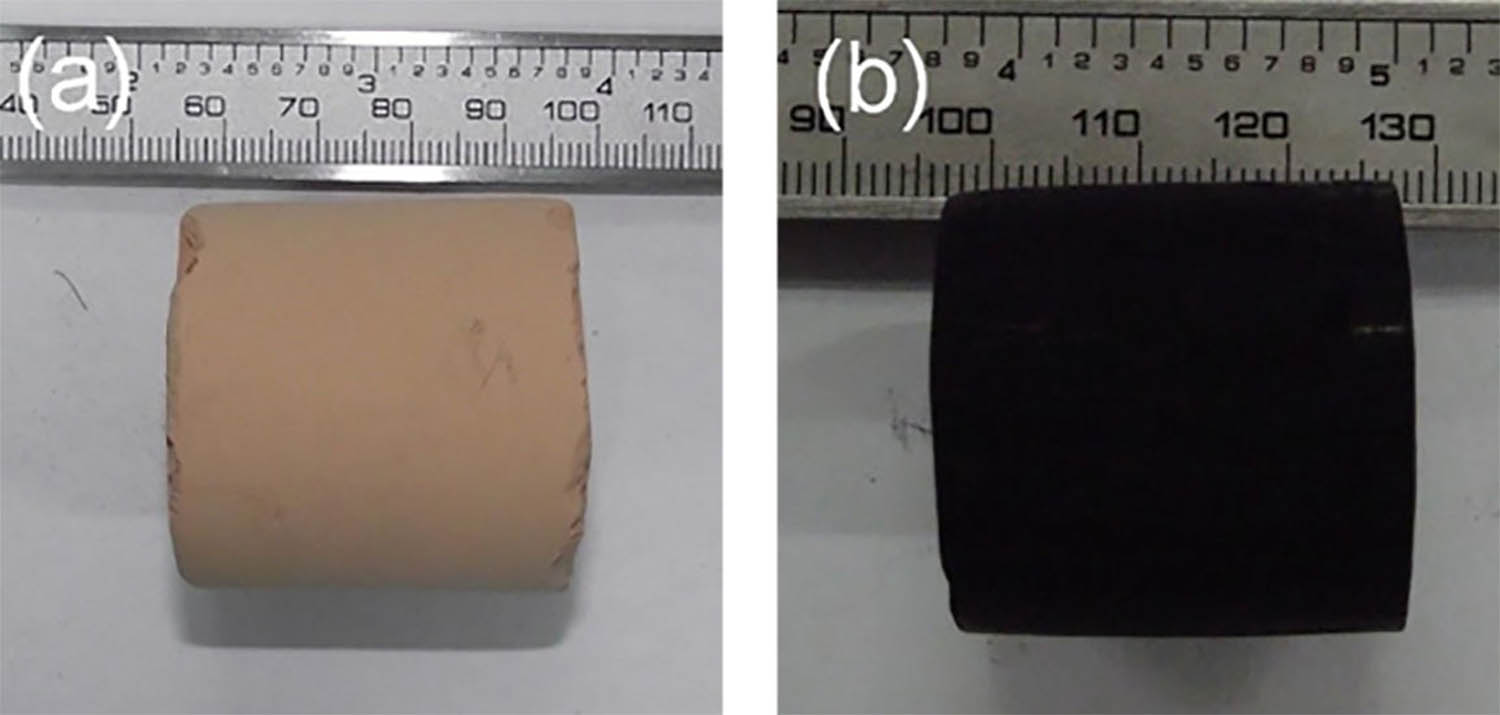
Sample of C/SiO2 composite aerogel before (a) and after (b) heat treatment.

SEM of C/SiO2 composite aerogel before (a) and after (b) heat treatment.
3.2 Effect of heat treatment temperature on pore structure of C/SiO2 composite aerogels
The heat treatment temperature is one of the main factors that affect the pore structure of C/SiO2 composite aerogels. The linear shrinkage and loss of the composite aerogels increase with the increase in the carbonization temperature. The density, specific surface area, pore volume and average pore size of the aerogels are increased to a certain extent compared with those before treatment. The pore structure of C/SiC aerogels is evaluated using nitrogen adsorption–desorption analysis. The pore structure characterization for C/SiC aerogels are shown in Figure 3. They are all Type IV curves with type H1 hysteresis loop in the IUPAC classification, suggesting that they are mesoporous under three different heat treatments [13].

N2 adsorption desorption isotherms (a) and pore size distribution curves (b) of C/SiO2 aerogels with different heat treatment temperatures.
The adsorption capacity of heat-treated sample at 1,200°C increases sharply when the relative pressure is close to 1, which indicates there are a large number of macropores in the samples. The heat-treated samples at 1,300°C still have a high adsorption capacity when the relative pressure is close to 0, which indicates that there are more micropores in the material. Pore size distribution curves of C/SiO2 aerogels under three heat treatment temperatures show many macropores appearing in the heat-treated samples at 1,200°C, especially in the range of 20–110 nm, but the number of holes below 10 nm decreased significantly. However, the relatively small holes (<10 nm) in the heat-treated samples at 1,300–1,400°C increased significantly, the number of macro holes decreased significantly, and the pore size distribution tended to be smaller [14]. All pertinent physical and pore structure characterization data for aerogels are summarized in Table 1.
Pore structure data of C/SiO2 aerogel at different temperatures
| Temperature (°C) | S BET (m2/g) | S mic (m2/g) | V p (cm3/g) | V mic (cm3/g) | Porosity (%) |
|---|---|---|---|---|---|
| RF/SiO2 | 384 | 77 | 1.44 | 0.032 | 93.0 |
| 1,200 | 220 | 59 | 1.84 | 0.012 | 93.0 |
| 1,300 | 653 | 242 | 2.36 | 0.073 | 93.2 |
| 1,400 | 376 | 174 | 1.93 | 0.060 | 93.1 |
3.3 Effect of heat treatment time on pore structure of C/SiO2 composite aerogels
The heat treatment time has an important effect on the pore structure of C/SiO2 composite aerogels [15]. The linear shrinkage and ignition loss of the composite aerogels all increased with the increase in heat treatment time. The density, specific surface area, pore volume and average pore diameter of the aerogels increased first and then decreased.
Figure 4 and Table 2 show the N2 adsorption–desorption isotherms of C/SiO2 aerogels with three different heat treatment times. It can be seen from the Figure that the adsorption–desorption isotherms of the three heat treatment time samples are all of type IV, typical mesoporous structure, and the type of hysteresis loop is H1, indicating that the pore morphology in the sample network structure is cylindrical or conical. When the relative pressure of the sample with treatment time of 2 h is close to 1, the adsorption capacity increases sharply. It indicates that there are many macropores existing. When the relative pressure is close to 0, the sample after 5 h of heat treatment still has a high adsorption capacity. It indicates that there are more micropores in the material. Figure 4 shows the pore size distribution curves of C/SiO2 aerogels under three carbonization time. It can be seen from the Figure that there are many macropores in the samples after 2 h heat treatment, especially in the range of 20–100 nm. However, the number of holes below 10 nm decreased significantly.

N2 adsorption desorption isotherms (a) and pore size distribution curves (b) of C/SiO2 aerogels with different heat treatment time.
Pore structure data of C/SiO2 aerogel with different thermal treatment time at 1,300°C
| Time (h) | S BET (m2/g) | S mic (m2/g) | V p (cm3/g) | V mic (cm3/g) | Porosity (%) |
|---|---|---|---|---|---|
| 2 | 275 | 111 | 1.60 | 0.051 | 92.6 |
| 5 | 653 | 142 | 2.41 | 0.073 | 93.2 |
| 10 | 325 | 120 | 1.84 | 0.055 | 92.9 |
3.4 FTIR analysis
Figure 5 is the FTIR diagram of the C/SiO2 composite aerogel prepared before and after heat treatment. The absorption peak at 1,620 cm−1 represents the absorption spectrum of water molecules on the surface of the sample. The stretching vibration absorption peaks at 1,385 and 1,210 cm−1 correspond to the C–H bonds in –CH3 and –CH2, respectively [16]. The stretching vibration absorption peak at 1,110 cm−1 corresponds to the Si–O–Si bond and the Si–C bond of the tested sample. The stretching vibration absorption peak near 920 cm−1 is Si–OH [17].

FTIR of C/SiO2 composite aerogel before and after heat treatment.
3.5 XRD analysis
Figure 6 is the XRD spectrum of C/SiO2 composite aerogel before and after heat treatment. Figure 6 shows that the composite aerogel behaved as a typical amorphous material before heat treatment. With the increase in the heat treatment temperature, the composite aerogel after 1,300°C heat treatment has pure carbon grain phase appearing obviously, though the amorphous form still exists [18,19]. After 1,400°C heat treatment, the composite aerogel has obvious SiC crystalline phase appearing clearly and carbon with crystalline form has gradually disappeared, besides the amorphous form. From the change in XRD spectra of composite aerogels, it can be referred that as the oxidation temperature continues to rise, the C/SiO2 functional surface of the C/SiO2 surface before heat treatment gradually disappears. The main reason is ablative or partial functional groups react with the Si–O bond, and finally form a more obvious Si–C or Si–O–C bond with diffraction peaks [20].
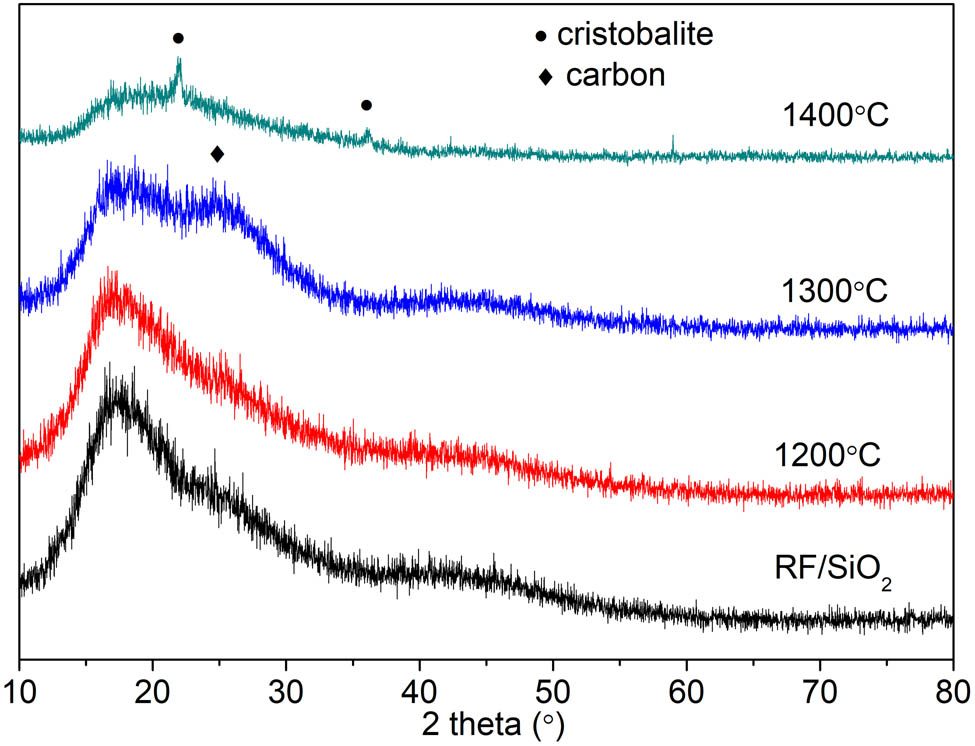
XRD of C/SiO2 composite aerogel before and after heat treatment.
4 Conclusion
Based on sol–gel method, C/SiO2 composite aerogels were prepared by high temperature heat treatment under the protection of Ar atmosphere, using four tetraethyl orthosilicate, Resorcinol, APTES and three methyl siloxanes, combined with supercritical ethanol drying. It was found that the heat treatment time had an important effect on the pore structure of C/SiO2 composite aerogels during the study of heat treatment process of C/SiO2 composite aerogels.
With the increase in heat treatment time, the linear shrinkage of composite aerogels increases gradually, and the pore volume, average pore diameter and specific surface area of composite aerogels increase first and then decrease. The optimum heat treatment time of C/SiO2 composite aerogels was 1,300°C, also the effective heat resistance temperature of C/SiO2 composite aerogels.
In this study, carbon materials with various forms were combined with SiO2. After carbonization, C/SiO2 composites with different aerogel structure characteristics could be formed. Reasonable carbonization time and higher heat treatment temperature could improve the network structure of C/SiO2 aerogels significantly. This composite material with aerogel structure presents new characteristics, such as light weight, high thermal stability, high adsorption rate, high temperature radiation resistance and high electrocatalytic activity. With the rich combination of silicon and carbon, the C/SiO2 composites with aerogel structure has new structural characteristics and characteristics, which opens new directions for catalysis, high temperature insulation, adsorption, hydrogen storage and photoelectricity. The study of C/SiO2 aerogel has good application value.
Acknowledgement
This study originated from the Industry Program of Jiangyin Polytechnic College (XJ2022LG006).
-
Conflict of interest: The authors declare that there are no conflict of interest.
References
[1] Smirnova I, Gurikov P. Aerogel production: Current status, research directions, and future opportunities. J Supercrit Fluids. 2018;134:228–33.10.1016/j.supflu.2017.12.037Search in Google Scholar
[2] Ming-Wei LI, Xiao-Dong HE, Min-Han F, Wan-Jun YU, Fei HE. An overview on silica aerogels synthesized by siloxane co-precursors. J Inorg Mater. 2015;30(12):1243–53.10.15541/jim20150223Search in Google Scholar
[3] Zhao S, Malfait WJ, Guerrero-Alburquerque N, Koebel MM, Nyström G. Biopolymer aerogels and foams: Chemistry, properties, and applications. Angew Chem Int Ed. 2018;57(26):7580–608.10.1002/anie.201709014Search in Google Scholar
[4] Hüsing N, Schubert U. Aerogels—airy materials: chemistry, structure, and properties. Angew Chem Int Ed. 1998;37(1–2):22–45.10.1002/(SICI)1521-3773(19980202)37:1/2<22::AID-ANIE22>3.0.CO;2-ISearch in Google Scholar
[5] Ye X, Chen Z, Ai S, Zhang J, Hou B, Zhou Q, et al. Mechanical and thermal properties of reticulated SiC aerogel composite prepared by template method. J Compos Mater. 2019;53(28–30):4117–24.10.1177/0021998319851190Search in Google Scholar
[6] Maleki H. Recent advances in aerogels for environmental remediation applications: A review. Chem Eng J. 2016;300:98–118.10.1016/j.cej.2016.04.098Search in Google Scholar
[7] Xu Y, Xu N, Zhang W, Zhu J. A multi-layer integrated thermal protection system with C/SiC composite and Ti alloy lattice sandwich. Compos Struct. 2019;230:111507.10.1016/j.compstruct.2019.111507Search in Google Scholar
[8] Bruzzoniti MC, Appendini M, Rivoira L, Onida B, Del Bubba M, Jana P, et al. Polymer‐derived ceramic aerogels as sorbent materials for the removal of organic dyes from aqueous solutions. J Am Ceram Soc. 2018;101(2):821–30.10.1111/jace.15241Search in Google Scholar
[9] Kong Y, Shen X, Cui S, Fan M. Preparation of monolith SiC aerogel with high surface area and large pore volume and the structural evolution during the preparation. Ceram Int. 2014;40(6):8265–71.10.1016/j.ceramint.2014.01.025Search in Google Scholar
[10] Wu X, Shao G, Shen X, Cui S, Chen X. Evolution of the novel C/SiO2/SiC ternary aerogel with high specific surface area and improved oxidation resistance. Chem Eng J. 2017;330:1022–34.10.1016/j.cej.2017.08.052Search in Google Scholar
[11] Leventis N, Sadekar A, Chandrasekaran N, Sotiriou-Leventis C. Click synthesis of monolithic silicon carbide aerogels from polyacrylonitrile-coated 3D silica networks. Chem Mater. 2010;22(9):2790–803.10.1021/cm903662aSearch in Google Scholar
[12] Kong Y, Zhong Y, Shen X, Cui S, Fan M. Effect of silica sources on nanostructures of resorcinol–formaldehyde/silica and carbon/silicon carbide composite aerogels. Microporous Mesoporous Mater. 2014;197:77–82.10.1016/j.micromeso.2014.05.032Search in Google Scholar
[13] He F, Li Y, Luo J, Fang MH, He XD. Development of SiO2/C and SiC/C composites featuring aerogel structures. J Inorg Mater. 2017;32(5):449–58.10.15541/jim20160380Search in Google Scholar
[14] Kong Y, Zhang J, Zhao Z, Jiang X, Shen X. Monolithic silicon nitride-based aerogels with large specific surface area and low thermal conductivity. Ceram Int. 2019;45(13):16331–7.10.1016/j.ceramint.2019.05.160Search in Google Scholar
[15] An Z, Zhang R, Fang D. Synthesis of monolithic SiC aerogels with high mechanical strength and low thermal conductivity. Ceram Int. 2019;45(9):11368–74.10.1016/j.ceramint.2019.02.216Search in Google Scholar
[16] Ye X, Chen Z, Ai S, Hou B, Zhang J, Liang X, et al. Novel three-dimensional SiC/melamine-derived carbon foam-reinforced SiO2 aerogel composite with low dielectric loss and high impedance matching ratio. ACS Sustain Chem Eng. 2019;7(2):2774–83.10.1021/acssuschemeng.8b05966Search in Google Scholar
[17] Cheng Y, Tan M, Hu P, Zhang X, Sun B, Yan L, et al. Strong and thermostable SiC nanowires/graphene aerogel with enhanced hydrophobicity and electromagnetic wave absorption property. Appl Surf Sci. 2018;448:138–44.10.1016/j.apsusc.2018.04.132Search in Google Scholar
[18] Wu Z, Cheng X, Zhang L, Li J, Yang C. Sol–gel synthesis of preceramic polyphenylsilsesquioxane aerogels and their application toward monolithic porous SiOC ceramics. Ceram Int. 2018;44(12):14947–51.10.1016/j.ceramint.2018.05.115Search in Google Scholar
[19] Zhang X-F, Chen Z, Feng Y, Qiu J, Yao J. Low-temperature transformation of C/SiO2 nanocomposites to β-SiC with high surface area. ACS Sustain Chem Eng. 2018;6(1):1068–73.10.1021/acssuschemeng.7b03375Search in Google Scholar
[20] Su L, Wang H, Niu M, Fan X, Ma M, Shi Z, et al. Ultralight, recoverable, and high-temperature-resistant SiC nanowire aerogel. ACS Nano. 2018;12(4):3103–11.10.1021/acsnano.7b08577Search in Google Scholar PubMed
© 2022 Na Xu and Xiao-dong Shen, published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- Experimental investigations of a novel pressure microfoam preparation device for dust removal
- Influence of hydrothermal aging on the mechanical performance of foam core sandwich panels subjected to low-velocity impact
- Experimental study on surface wrapping strengthening of EPS particles and its concrete performance
- Modification of mechanical properties of Shanghai clayey soil with expanded polystyrene
- A new EPS beads strengthening technology and its influences on axial compressive properties of concrete
- A novel superabsorbent material based on soybean straw: Synthesis and characterization
- Use of line laser scanning thermography for the defect detection and evaluation of composite material
- Research on back analysis of meso-parameters of hydraulic cemented sand and gravel based on Box-Behnken design response surface
- Hot deformation behavior and microstructure of a 0.5 wt% graphene nanoplatelet reinforced aluminum composite
- Analysis of electromagnetic characteristics of the proposed composite four-rail electromagnetic launcher
- Preparation and characterization of a graphene hybridizing polyurethane damping composite
- Effects of layup parameters and interference value on the performance of CFRP–metal interference fit joints
- Vibration and noise reduction of pipelines using shape memory alloy
- Finite element analysis of behavior and ultimate strength of composite column
- Dynamic response of functionally graded plate under harmonic load with variable gradient parameters
- Deformation behavior of rubber composite based on FEA and experimental verification
- Effects of Z-pin on moisture absorption property and damage mode under flexural load for carbon fiber composite
- Design and testing of a smart rubber stave for marine water-lubricated bearings
- Study of carbon nano-modifier of fly ash in cement concrete mixtures of civil engineering
- Analysis of multiple impact tests’ damage to three-dimensional four-directional braided composites
- Theoretical analysis of aluminum honeycomb sandwich panel supported by reinforced concrete wall under low-speed impact load
- Effects of local fiber discontinuity on the fatigue strength parameter at the fiber inclusion corner in fiber-reinforced composites
- Experimental investigation on compressive properties of three-dimensional five-directional braided composites in hygrothermal environment
- Failure process of steel–polypropylene hybrid fiber-reinforced concrete based on numerical simulations
- A simple method for measuring the monofilament diameter of continuous filament yarn with high bending stiffness via synthetic laser imaging
- Span length effect on flexural properties of composite laminate reinforced with a plain weave carbon fiber fabric in a polymer matrix
- Mechanical properties improving and microstructure characterization of inorganic artificial stone binder
- Effect of thermal treatment process on the structure of C/SiO2 composite aerogels
- Mechanical and corrosion resistance analysis of laser cladding layer
- Wear and corrosion mechanisms of Ni–WC coatings modified with different Y2O3 by laser cladding on AISI 4145H steel
- Damage and failure analysis of composite stiffened panels under low-velocity impact and compression after impact with damp-heat aging
- In-situ CT characterization of 2D woven SiCf/SiC composite loading under compression
- Effect of the manufacturing process on the equivalency qualification of glass fiber reinforced polymer
- Study of concrete properties based on crushed stone sand mixture and fiber of fly ash of thermal power plants
- Establishment of wear mechanism distribution diagram of ZTAp-reinforced iron matrix composites
- Calculation method of elastic modulus for carbon fiber-reinforced plastics considering inhomogeneous interphase
- An experimental study on the failure and enhancement mechanism of bolt-strengthening GFRP T-joint subjected to tensile loading
- The viability of cell that encapsulated in calcium alginate hydrogel beads
- Discussion of ceramic bar reinforced TWIP steel composite structure
- A theoretical framework underlying an accelerated testing method and its application to composites under constant strain rates and fatigue loading
- Theoretical analysis of interfacial design and thermal conductivity in graphite flakes/Al composites with various interfacial coatings
- Multiscale heat conduction and fractal oxidation behaviors of needle-punched carbon/carbon composites
- Numerical simulation of composite grid sandwich structure under low-velocity impact
- Wear properties of Al/TiO2 composites fabricated via combined compo-casting and APB process
- Review Articles
- Application of melanin as biological functional material in composite film field
- Review on research progress of cemented sand and gravel dam
- Communication
- Fabrications and microstructure analysis of cobalt-based coatings by an easy-coating and sintering process
- Letter to the Editor
- Investigation on mechanical and conductive behaviors of nano-graphite-based concrete
Articles in the same Issue
- Regular Articles
- Experimental investigations of a novel pressure microfoam preparation device for dust removal
- Influence of hydrothermal aging on the mechanical performance of foam core sandwich panels subjected to low-velocity impact
- Experimental study on surface wrapping strengthening of EPS particles and its concrete performance
- Modification of mechanical properties of Shanghai clayey soil with expanded polystyrene
- A new EPS beads strengthening technology and its influences on axial compressive properties of concrete
- A novel superabsorbent material based on soybean straw: Synthesis and characterization
- Use of line laser scanning thermography for the defect detection and evaluation of composite material
- Research on back analysis of meso-parameters of hydraulic cemented sand and gravel based on Box-Behnken design response surface
- Hot deformation behavior and microstructure of a 0.5 wt% graphene nanoplatelet reinforced aluminum composite
- Analysis of electromagnetic characteristics of the proposed composite four-rail electromagnetic launcher
- Preparation and characterization of a graphene hybridizing polyurethane damping composite
- Effects of layup parameters and interference value on the performance of CFRP–metal interference fit joints
- Vibration and noise reduction of pipelines using shape memory alloy
- Finite element analysis of behavior and ultimate strength of composite column
- Dynamic response of functionally graded plate under harmonic load with variable gradient parameters
- Deformation behavior of rubber composite based on FEA and experimental verification
- Effects of Z-pin on moisture absorption property and damage mode under flexural load for carbon fiber composite
- Design and testing of a smart rubber stave for marine water-lubricated bearings
- Study of carbon nano-modifier of fly ash in cement concrete mixtures of civil engineering
- Analysis of multiple impact tests’ damage to three-dimensional four-directional braided composites
- Theoretical analysis of aluminum honeycomb sandwich panel supported by reinforced concrete wall under low-speed impact load
- Effects of local fiber discontinuity on the fatigue strength parameter at the fiber inclusion corner in fiber-reinforced composites
- Experimental investigation on compressive properties of three-dimensional five-directional braided composites in hygrothermal environment
- Failure process of steel–polypropylene hybrid fiber-reinforced concrete based on numerical simulations
- A simple method for measuring the monofilament diameter of continuous filament yarn with high bending stiffness via synthetic laser imaging
- Span length effect on flexural properties of composite laminate reinforced with a plain weave carbon fiber fabric in a polymer matrix
- Mechanical properties improving and microstructure characterization of inorganic artificial stone binder
- Effect of thermal treatment process on the structure of C/SiO2 composite aerogels
- Mechanical and corrosion resistance analysis of laser cladding layer
- Wear and corrosion mechanisms of Ni–WC coatings modified with different Y2O3 by laser cladding on AISI 4145H steel
- Damage and failure analysis of composite stiffened panels under low-velocity impact and compression after impact with damp-heat aging
- In-situ CT characterization of 2D woven SiCf/SiC composite loading under compression
- Effect of the manufacturing process on the equivalency qualification of glass fiber reinforced polymer
- Study of concrete properties based on crushed stone sand mixture and fiber of fly ash of thermal power plants
- Establishment of wear mechanism distribution diagram of ZTAp-reinforced iron matrix composites
- Calculation method of elastic modulus for carbon fiber-reinforced plastics considering inhomogeneous interphase
- An experimental study on the failure and enhancement mechanism of bolt-strengthening GFRP T-joint subjected to tensile loading
- The viability of cell that encapsulated in calcium alginate hydrogel beads
- Discussion of ceramic bar reinforced TWIP steel composite structure
- A theoretical framework underlying an accelerated testing method and its application to composites under constant strain rates and fatigue loading
- Theoretical analysis of interfacial design and thermal conductivity in graphite flakes/Al composites with various interfacial coatings
- Multiscale heat conduction and fractal oxidation behaviors of needle-punched carbon/carbon composites
- Numerical simulation of composite grid sandwich structure under low-velocity impact
- Wear properties of Al/TiO2 composites fabricated via combined compo-casting and APB process
- Review Articles
- Application of melanin as biological functional material in composite film field
- Review on research progress of cemented sand and gravel dam
- Communication
- Fabrications and microstructure analysis of cobalt-based coatings by an easy-coating and sintering process
- Letter to the Editor
- Investigation on mechanical and conductive behaviors of nano-graphite-based concrete

