Abstract
This work contributes to the extant literature by providing experimental results showing an improvement in the interfacial adhesion between diamond and epoxy resin by modifying the surface properties of diamond using KH550 silane coupling agent in (i) aqueous alcohol solvent (KH550:H2O:C2H5OH=1:1:8) and (ii) toluene solvent (KH550:C7H8=1:9), and the weight ratio of diamond to the above-mixed solution was 1:4. This in turn has been demonstrated to provide improved mechanical properties of the corresponding diamond/resin composite material synthesized from this study. The results obtained from this study were analyzed by using Fourier-transform infrared spectroscopy, X-ray photoelectron spectra, field emission scanning electron microscopy. and scanning electron microscopy. Besides these examinations, bend strength and hardness tests were also conducted, which lend further credence to this study. Finally, toluene solvent was observed to provide better adhesion at the interface of diamond and epoxy resin, and consequently, this resulted in improved bend strength of the corresponding diamond/resin composite by 14.3%.
1 Introduction
The ubiquitous use of diamond as a cutting tool or as a grinding wheel due to its exceptional properties such as high hardness and wear resistance is well documented in the literature [1], [2], [3], [4]. It has lately been realized that bonding of diamond with epoxy resin results in the formation of a newer composite material that has shown promising results when such a diamond/resin composite is used as a grinding wheel [5], [6], [7]. However, bonding of diamond with any material is a challenging problem, as diamond is chemically inert to most of the chemical agents [8], [9]. It is therefore very difficult in practice to wet the diamond particles and bond them together using epoxy resin. Consequently, the binding forces between the diamond and epoxy composite are governed by the mechanical bonding rather than chemical bonding or metallurgical bonding [10]. Such a material succumbs to wear when subjected to large grinding loads during grinding applications [11]. This limitation has motivated several researchers to use wet plating methods and vacuum plating methods to form a metal layer (e.g. nickel or cooper) on diamond surface in order to prolong the useful life of the grinding wheel [12], [13], [14]. While a modicum of success has been attained in the past, a severe problem concerning metal plating is that the ductility of thick metal plating reduces the self-sharpening ability of diamond causing a reduced self-dressing ability of diamond grinding wheels prepared by such methods. To tackle this problem, Wang et al. [15] developed corundum-coated diamond. In their work, diamond grits were coated with corundum powder by binding a vitreous layer. However, because of the brittleness of the inorganic coating, the self-sharpening ability of diamond and the self-dressing ability of diamond wheel were not observed to change a lot. Thus, the common limitation of the abovementioned methods is that they do not change the mechanical anchor effect between diamond and resin.
Studies also exist in the literature where silane coupling agents were employed to improve the compatibility and adhesion between inorganic particles and organic matrix [16], [17], [18], [19]. One of the most noticeable works related to diamond/resin composite is that of Semba et al. [20], who used radio-frequency magnetron sputtering to prepare SiO2 deposited diamond and then used silane coupling agent to modify the diamond surface. The subsequent grinding tests showed that the gripping strength of SiO2 sputtered diamond grains exceeded the strength of nickel-plated grains and silane-coated SiO2 sputtered diamond could increase the chemical binding effect of diamond to melamine resin matrix. McHale [21] paid much attention to the method of modifying the metal-coated super-abrasive with silane coupling agent, but no experimental data revealed the effect of treatment. To simplify the treatment process of diamond, Yin et al. [22] replaced the wetting agent with silane coupling agent during the diamond/resin composite preparation process and found that the grinding ratio of wheel could be increased by 25%. Chen et al. [23] used silane coupling agent to modify the aqueous alkali pre-treated diamond in aqueous alcohol solvent and also investigated the effect on phenolic resin and polyimide resin matrix. The grinding results showed that silane treatment could improve the bonding state between the diamond and resin and the grinding ratio of polyimide resin wheel increased a lot by 109.9%. Although results of these researches showed that silane-treated diamond could increase the grinding ratio of different resin wheels, no research was conducted on the structure and mechanical properties of epoxy resin bonded wheel. Also, none of them have illustrated or provided a coherent description of the modification mechanism of silane-treated diamond. Furthermore, when modification is conducted in aqueous alcohol solvent, the unimolecular products of silane coupling agent are not stable and may self-polymerize [24], [25]. In order to address these problems, this work compares both alcohol solvent and toluene solvent as media during treatment of diamond with KH550. Based on the experimental observations, a mechanism of modification of diamond surface is also proposed, which may help to refine the existing industrial processes.
2 Materials and methods
2.1 Materials
Diamond with average size of 5–10 μm employed in this study was supplied by Jinrui Diamond Co., Ltd (Changsha, China). Epoxy resin (E-54) and the curing agent methyl tetrahydrophthalic anhydride (MTHPA) were purchased from Wuxi Hongxing Chemical Industry Plant (Wuxi, China). The reaction accelerator N,N-dimethyl benzyl amine was obtained from Sinopharm Chemical Reagent Co., Ltd (Beijing, China). Anhydrous ethanol, toluene and acetone, used as diluting agent, were purchased from Tianjin Kemiou Chemical Reagent Co., Ltd (Tianjin, China). 3-Aminopropyltriethoxy silane (KH-550), supplied by Nanjing Shuguang Chemical Co., Ltd (Nanjing, China), was used as coupling agent.
2.2 Sample preparation
Before proceeding with the actual experiments, samples of diamond were washed in aqueous alkali (0.11 g/ml NaOH) at 90°C for 4 h and then rinsed in distilled water before complete drying.
2.2.1 Modification in aqueous alcohol solvent
Ten percent silane coupling agent aqueous alcohol solution was prepared by ultrasonic dispersion for 10 min followed by pre-hydrolysis for 2 h. The weight ratio of KH550, distilled water and anhydrous ethanol was 1:1:8. Diamond was added into the abovementioned solution in the weight ratio of 1:4. The system was stirred and heated up to 80°C using heating magnetic stirrer. After stirring for about 4 h, the suspension was filtrated and the modified diamond was obtained after a repeated wash using anhydrous ethanol. These diamond particles were dried for 10 h at 100°C in a vacuum oven, and this helped to obtain modified diamond treated in aqueous alcohol solvent.
2.2.2 Modification in toluene solvent
KH550 was added to the toluene in a weight ratio of 1:9, which was dispersed ultrasonically for 10 min. Diamond particles were added into the KH550 toluene solution in the same weight ratio of diamond to solution of 1:4. After allowing the reaction to occur for about 4 h at a temperature of 100°C, the suspension was filtrated and the diamond was washed repeatedly with acetone. Finally, the diamond particles were dried for 10 h at 100°C in a vacuum oven. This process helped to obtain modified diamond treated in toulene solvent.
2.3 Preparation of diamond/epoxy composite
Epoxy resin, MTHPA and diamond particles were mixed in the weight ratio of 31:28:41. Slurry (100 g) was prepared by the addition of N,N-dimethyl benzyl amineto (0.4 ml). After stirring, the slurry was injected into the mold and cured at 130°C for 4 h. Finally, the samples were cut into small pieces of dimensions 40 mm×5 mm×5 mm using a cutting disc.
2.4 Characterization of samples
The functional group, surface topography and the chemical analysis of the diamond surface were done by using Fourier-transform infrared spectroscopy (FTIR, WQF-410, Beijing Second Optical Instrument Factory, Beijing, China), field emission scanning electron microscopy (JSM-6700F, Japan Electron Optics Laboratory Co., Ltd., Tokyo, Japan) and X-ray photoelectron spectroscopy (EscaLab 250 Xi, Thermo Fisher Scientific, Warrington, UK). The mechanical properties and the interface properties of diamond/epoxy resin were examined by motorized bending tester (DKZ-5000, Wuxi Built Instrument Machinery Co., Ltd, Wuxi, China), Rockwell apparatus (HR150DT, Shanghai Material Test Factory, Shanghai, China) and environmental scanning electron microscope (FEI QUANTA-200, FEI Co., Eindhoven, The Netherlands).
3 Results and discussion
3.1 Diamond particles
3.1.1 The surface groups of diamond particle
Figure 1 shows the FTIR spectra of diamond particles. The peaks at 3430 cm-1and 1645 cm-1 in Figure 1A are that of stretching vibration and deformation vibration of -OH, and the peak of 1394 cm-1could be attributed to C-H vibration [26]. In Figure 1B and 1C, the peaks at 3430 cm-1 and 1645 cm-1 are narrowed and weakened. These are likely the absorption bands of rest of the -OH of diamond [27] and of -NH2 of KH550. The peaks at 871 cm-1 of Figure 1B and 885 cm-1of Figure 1C belong to -Si-OH [17]. New peaks of 1468 cm-1(-CH2-)and 1040 cm-1(C-O-Si/Si-O-Si) [17], [28] indicate that KH550 may be chemically grafted into the diamond surface both in aqueous alcohol and in toluene solvent. However, in comparison to Figure 1B, the peaks at 1468 cm-1 and 1040 cm-1 in Figure 1C are more intense, signifying the presence of more amount of KH550 on the diamond surface when treated in toluene solvent. Based on this, the following modification mechanism is proposed to happen during the process.
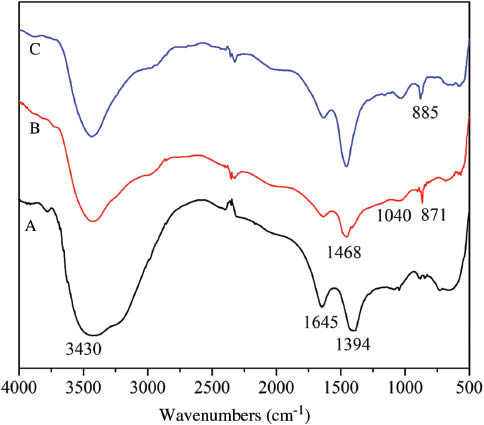
FTIR spectra of (A) pristine diamond, (B) KH550 modified diamond in aqueous alcohol, (C) KH550 modified diamond in toluene.
3.1.2 Modification mechanism
In this paper, the chemical kinetics of the modification mechanism in aqueous alcohol solvent is hypothesized, as per the details shown in Figure 2. As shown in Figure 2, because of the presence of aqueous solvent, KH550 molecules will hydrolyze. However, if the experimental conditions are not controlled, the unimolecular hydrolysis products will easily self-polymerize into bigger molecules instead of reacting with -OH on the diamond surface. Also, the reaction of bigger molecules with the hydroxyl of the diamond surface is unfavorable, which couples further with the fact that the bigger molecules will hinder the reaction between unimolecular silanol and diamond. Thus, the number of molecules of KH550 reacting with hydroxyl on the diamond surface decreases, thereby making the amount of KH550 molecules less on the surface of diamond.

The action mechanism of KH550 on the diamond surface in aqueous alcohol solvent.
Figure 3 shows schematically the plausible reaction mechanism of KH550 on the diamond surface in toluene solvent. In toluene solvent, the alkoxy groups of KH550 directly react with the hydroxyl on the diamond surface. During subsequent filtration process, the -Si-O-C2H5groups may hydrolyze to -Si-OH because of the steam in air and polymerize with each other subsequently. This would be expected to provide better adhesion, which has been detailed in the next sections.
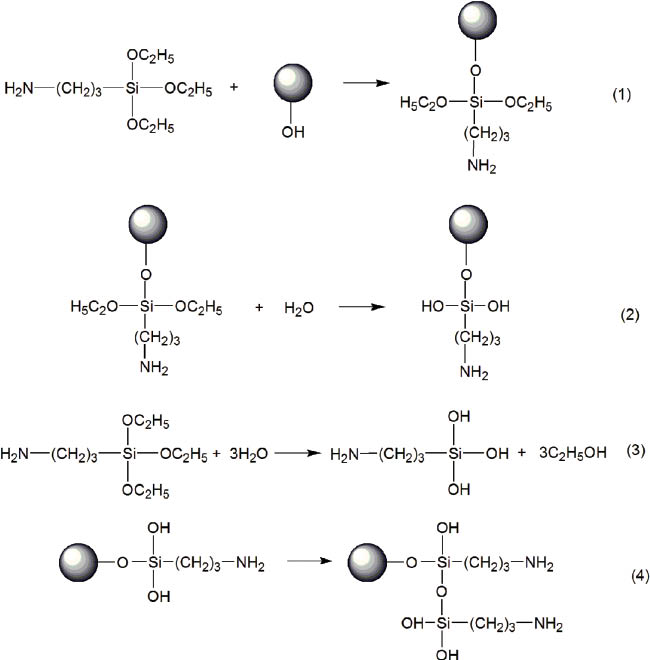
The modification mechanism of diamond with KH550 in toluene solvent.
3.1.3 The surface topography of diamond particles
Surface characterization of diamond under three different experimental conditions was performed using scanning electron microscopy (SEM) examination, which is shown in Figure 4. From Figure 4B and 4C, the presence of KH550 molecules on diamond surface is evident. However, the amount of KH550 on the surface of diamond modified in aqueous alcohol solvent is comparatively lower. Figure 4C shows that a silane layer was formed on diamond surface when treated with toluene solvent, which could offer better adhesion between diamond and resin.

FE-SEM photographs of (A) pristine diamond, (B) KH550 modified diamond in aqueous alcohol, (C) KH550 modified diamond in toluene.
3.1.4 Chemical analysis of diamond particles using XPS
X-ray photoelectron spectra (XPS) analysis was considered reliable to unravel the understanding of the characteristics of bonding between diamond and KH550. Figure 5 and Table 1 show the XPS spectra of the pristine diamond and a comparison with the spectra of diamond obtained after modification by KH550. The contents of N (9.48%) and Si (13.87%) on diamond surface modified in toluene solvent were observed to be more than N (2.21%) and Si (3.11%) on the diamond treated in aqueous alcohol solvent. This is in agreement with the results of FTIR spectra and SEM investigation. Figure 6 displays the XPS spectra of Si 2p on the surface of the modified diamond. The binding energy of C1s (284.8 eV) is used as reference. The peaks at 101.53 eV, 102.3 eV and 103.15 eV are the binding energy of C-Si-O-Si, C-Si-O-H and C-Si-O-C, respectively [29]. The presence of C-O-Si-C indicates that the chemical reaction between KH550 and the hydroxyl group of diamond would proceed both in aqueous alcohol and toluene solvent. It can also be seen that much of the Si element on the surface of diamond modified in aqueous alcohol solvent is in the form of C-Si-O-Si. It means that most of the unimolecular hydrolysis products of silane coupling agent polymerized before reacting with the hydroxyl group of diamond. Moreover, through the integration of area enclosed by the peak of C-Si-O-C and the baseline, the area in Figure 6A and 6B is 1794 and 2406, respectively. It indicates that more C-Si-O-C was formed during the reaction between KH550 and -OH on diamond surface in toluene solvent.

XPS spectra of (A) pristine diamond, (B) KH550 modified diamond in aqueous alcohol, (C) KH550 modified diamond in toluene.
The element content analysis of diamond surface obtained by using XPS analysis.
| Element | Original diamond (%) | Diamond modified in aqueous alcohol (%) | Diamond modified in toluene (%) |
|---|---|---|---|
| C1s | 90.68 | 81.04 | 55.54 |
| O1s | 8.71 | 13.64 | 21.11 |
| N1s | 0.21 | 2.21 | 9.48 |
| Si2p | 0.40 | 3.11 | 13.87 |

Si 2p XPS spectrum of diamond (A) KH550 modified diamond in aqueous alcohol, (B) KH550 modified diamond in toluene.
3.2 Diamond/epoxy composite
3.2.1 Mechanical properties of composite
After proceeding with the chemical investigation, mechanical analysis was performed to draw a comparison in the mechanical properties of three types of diamonds. Figure 7 shows the influence of the chemical treatment on the mechanical properties of diamond/epoxy composites under three test conditions. It can be seen that the bend strength of diamond/epoxy composite increases from 97.3 MPa to 103.6 MPa and 111.2 MPa, respectively, after diamond particles were treated with KH550 in aqueous alcohol and toluene solvent. This is because of the fact that during the curing process, the amine groups on the surface of modified diamond produce chemical cross-linking with the epoxy groups of resin, which increases the interfacial bonding strength. Therefore, the bend strength of the diamond/epoxy composite improves by 6.5% and 14.3% in respective conditions. Furthermore, as shown above, adhesion of more number of KH550 molecules in toluene solvent also contributes to higher bending strength. However, the hardness (Rockwell HB) of different samples (52.58, 53.13 and 55.17) showed a very small variation. It is anticipated that the acting force of the indenter was mainly applied on resin, which might be the reason that the hardness obtained here may not provide a good assessment.

The bend strength and hardness of diamond/epoxy composites (i) 1#: sample with pristine diamond; (ii) 2#: sample with KH550 modified diamond in aqueous alcohol; and (iii) 3#: sample with KH550 modified diamond in toluene.
3.2.2 Interfacial conditions
Figure 8 is a SEM micrograph of the fracture surface of diamond/resin composite. Figure 8A shows the presence of several cracks (white arrows) at the interface of pristine diamond and resin. The surface of pristine diamond is hydrophilic, while the resin is hydrophobic. Therefore, the poor compatibility results in the appearance of cracks. However, post-treatment with KH550, the surface property of diamond was observed to change, which results in improved wettability between diamond and resin and eventually a better interfacial adhesion. After the diamond specimen was modified in aqueous alcohol solvent, a relatively smaller amount of KH550 molecule was chemically grafted into the surface of diamond (Figure 8B). Finally, when the modification was conducted in toluene solvent, more KH550 gets chemically grafted to the surface of diamond. This indicated that more -NH2 groups will react with epoxy groups through chemical cross-linking, which accounts for better interfacial state between diamond and epoxy resin, and no cracks were observed as a result (Figure 8C).

SEM micrograph of fracture surface of diamond/resin composite (A) sample with pristine diamond, (B) sample with KH550 modified diamond in aqueous alcohol, (C) sample with KH550 modified diamond in toluene; D represents diamond particles. The white arrows in (A) and (B) indicate racks.
4 Conclusion
This paper details the results of an experimental study wherein an attempt was made to modify the diamond surface with KH550 silane coupling agent in the presence of (i) aqueous alcohol solvent (KH550:H2O:C2H5OH=1:1:8) and (ii) toluene solvent (KH550:C7H8=1:9), and the weight ratio of diamond to the above-mixed solution was 1:4. Key observations were that although both processes improve the mechanical properties of diamond/epoxy composite, the latter, in particular, provides superior performance. It has been shown that when a diamond specimen is treated in aqueous alcohol solvent, the hydrolysis products of coupling agent can polymerize to big molecules resulting in a decelerated reaction rate between -Si-OH and -OH on the diamond surface. On the other hand, the process of treating diamond in toluene solvent results in the availability of more KH550 molecules on the surface of diamond, which improves the chemical kinetics (atom by atom activity) at the interface between diamond and epoxy resin. Thus, interfacial adhesion of diamond/epoxy composite is greatly improved, and the bend strength of the resulting composite material was found to increase by 14.3%. This study in its current form thereby provides a roadmap to design grinding wheels and cutting tools exhibiting improved strength and mechanical properties than currently possible.
Acknowledgments
This project is supported by the National Natural Science Foundation of China (Grant No. 51375157).
References
[1] Yu H, Li S, Hu E. Diam. Relat. Mater. 1994, 3, 222–226.10.1016/0925-9635(94)90083-3Search in Google Scholar
[2] Goel S, Luo X, Reuben RL, Rashid WB. Mater. Lett. 2012, 68, 507–509.10.1016/j.matlet.2011.11.028Search in Google Scholar
[3] Liu Y-K, Tso P-L. Int. J. Adv. Manuf. Technol. 2003, 22, 396–400.10.1007/s00170-003-1545-xSearch in Google Scholar
[4] Li W, Wang Y, Fan S, Xu J. Mater. Lett. 2007, 61, 54–58.10.1016/j.matlet.2006.04.004Search in Google Scholar
[5] Luo S, Liu Y. J. Mater. Process Technol. 1999, 96, 215–224.10.1016/S0924-0136(99)00355-6Search in Google Scholar
[6] de Oliveira OC, Bobrovnitchii GS, de Oliveira LJ, da Rocha Paranhos RP, Aigueira RB, Filgueira M. Mater. Character. 2009, 60, 869–874.10.1016/j.matchar.2009.02.005Search in Google Scholar
[7] Wang S-X, Geng L, Liu X-J, Geng B, Niu S-C. J. Mater. Process Technol. 2009, 209, 1871–1876.10.1016/j.jmatprotec.2008.04.045Search in Google Scholar
[8] Tsubota T, Tanii S, Ida S, Nagata M, Matsumoto Y. Phys. Chem. Chem. Phys. 2003, 5, 1474–1480.10.1039/b210248jSearch in Google Scholar
[9] Tsubota T, Ida S, Hirabayashi O, Nagaoka S, Nagata M, Matsumoto Y. Phys. Chem. Chem. Phys. 2002, 4, 3881–3886.10.1039/b202345hSearch in Google Scholar
[10] Webster J, Tricard M. CIRP Ann. A Manuf. Technol. 2004, 53, 597–617.10.1016/S0007-8506(07)60031-6Search in Google Scholar
[11] Luo S, Liao Y, Chou C, Chen J. J. Mater. Process Technol. 1997, 69, 289–296.10.1016/S0924-0136(97)00032-0Search in Google Scholar
[12] Abdullah A, Pak A, Farahi M, Barzegari M. J. Mater. Process Technol. 2007, 183, 165–168.10.1016/j.jmatprotec.2006.09.038Search in Google Scholar
[13] Ishizuka H, Suzuki K. US Patent 4278448 A. 1981 Jul. 14.Search in Google Scholar
[14] Pipkin NJ. US Patent 4399167 A. 1983 Aug. 16.Search in Google Scholar
[15] Wang YH, Zhao YC, Wang MZ, Zang JB. Key Eng. Mater. 2003, 250, 94–98.10.4028/www.scientific.net/KEM.250.94Search in Google Scholar
[16] Hoikkanen M, Honkanen M, Vippola M, Lepistö T, Vuorinen J. Prog. Org. Coat. 2011, 72, 716–723.10.1016/j.porgcoat.2011.08.002Search in Google Scholar
[17] Lin J, Siddiqui JA, Ottenbrite RM. Polym. Advan. Technol. 2001, 12, 285–292.10.1002/pat.64Search in Google Scholar
[18] Monticelli F, Toledano M, Osorio R, Ferrari M. Dent. Mater. 2006, 22, 1024–1028.10.1016/j.dental.2005.11.024Search in Google Scholar
[19] Castellano M, Conzatti L, Turturro A, Costa G, Busca G. J. Phys. Chem. B 2007, 111, 4495–4502.10.1021/jp0702144Search in Google Scholar
[20] Semba T, Fujiyama H, Sato H. CIRP Ann A Manuf. Technol. 1998, 47, 271–274.10.1016/S0007-8506(07)62832-7Search in Google Scholar
[21] McHale J. US Patent 20030005646 A1. 2003 Jan. 9.Search in Google Scholar
[22] Yin XM, Sun BS. J. Zhengzhou Polytech. Ins. 2002, 4, 19–20.Search in Google Scholar
[23] Chen L, Zeng LM, Ye XC, Zhang C, Song YX. Diamond Abras. Eng. 2010, 30, 54–57.Search in Google Scholar
[24] Zhou B. Wuhan University of Technology, Master thesis, 2006.Search in Google Scholar
[25] Arkles B, Steinmetz J, Zazyczny J, Mehta P. J. Adhes. Sci. Technol. 1992, 6, 193–206.10.1163/156856192X00133Search in Google Scholar
[26] Mironov E, Koretz A, Petrov E. Diamond Relat. Mater. 2002, 11, 872–876.10.1016/S0925-9635(01)00723-3Search in Google Scholar
[27] Li X, Song R, Jiang Y, Wang C, Jiang D. Appl. Surf. Sci. 2013, 276, 761–768.10.1016/j.apsusc.2013.03.167Search in Google Scholar
[28] Singh B, Gupta M, Verma A, Tyagi OS. Polym. Int. 2000, 49, 1444–1451.10.1002/1097-0126(200011)49:11<1444::AID-PI526>3.0.CO;2-9Search in Google Scholar
[29] Yin R, Zhang L, Dong X, Ji X, Zhang X, Cao W, Shi X. Acta Metall. Sinica-Chinese Edition- 2007, 43, 404.Search in Google Scholar
©2017 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Original articles
- Effects of multiwalled carbon nanotube mass fraction on microstructures and electrical resistivity of polycarbonate-based conductive composites
- Preparation and fluid drag reduction properties of superhydrophobic paper-based films comprising carbon nanotubes and fluoropolymers
- Studies on the structural, thermal, and dielectric properties of fabricated Nylon 6,9/CaCu3Ti4O12 nanocomposites
- Metal-matrix composite fabricated with gas tungsten arc melt injection and precoated with NiCrBSi alloy to increase the volume fraction of WC particles
- Construction of polypropylene composite multifilaments filled with sodium perborate trihydrate-treated jute microparticles
- Damage detection of three-dimensional braided composite materials using carbon nanotube thread
- An exact solution for improved metal-composite joints reinforced by FG inter-layers
- Effect of chemical treatment on thermal properties of bagasse fiber-reinforced epoxy composite
- The superior ductility of fine SiCp/Al2014 composites after extrusion
- Optical properties and photocatalytic activity of CdS-TiO2/graphite composite
- Effects of bottom ash and granulated blast furnace slag as fine aggregate on abrasion resistance of concrete
- Surface modification of diamond and its effect on the mechanical properties of diamond/epoxy composites
- Numerical simulation of strain rate effect on the inelastic behavior of metal matrix composites
- Electromechanical characterization of multilayer graphene-reinforced cellulose composite containing 1-ethyl-3-methylimidazolium diethylphosphonate ionic liquid
- The effect of hydroxyapatite reinforcement and preparation methods on the structure and mechanical properties of Mg-HA composites
- The effect of yttrium incorporation on the formation and properties of α-Al2O3 coatings
Articles in the same Issue
- Frontmatter
- Original articles
- Effects of multiwalled carbon nanotube mass fraction on microstructures and electrical resistivity of polycarbonate-based conductive composites
- Preparation and fluid drag reduction properties of superhydrophobic paper-based films comprising carbon nanotubes and fluoropolymers
- Studies on the structural, thermal, and dielectric properties of fabricated Nylon 6,9/CaCu3Ti4O12 nanocomposites
- Metal-matrix composite fabricated with gas tungsten arc melt injection and precoated with NiCrBSi alloy to increase the volume fraction of WC particles
- Construction of polypropylene composite multifilaments filled with sodium perborate trihydrate-treated jute microparticles
- Damage detection of three-dimensional braided composite materials using carbon nanotube thread
- An exact solution for improved metal-composite joints reinforced by FG inter-layers
- Effect of chemical treatment on thermal properties of bagasse fiber-reinforced epoxy composite
- The superior ductility of fine SiCp/Al2014 composites after extrusion
- Optical properties and photocatalytic activity of CdS-TiO2/graphite composite
- Effects of bottom ash and granulated blast furnace slag as fine aggregate on abrasion resistance of concrete
- Surface modification of diamond and its effect on the mechanical properties of diamond/epoxy composites
- Numerical simulation of strain rate effect on the inelastic behavior of metal matrix composites
- Electromechanical characterization of multilayer graphene-reinforced cellulose composite containing 1-ethyl-3-methylimidazolium diethylphosphonate ionic liquid
- The effect of hydroxyapatite reinforcement and preparation methods on the structure and mechanical properties of Mg-HA composites
- The effect of yttrium incorporation on the formation and properties of α-Al2O3 coatings

