Abstract
Polypropylene composite multifilaments filled with surface-treated jute microparticles were successfully spun by melt spinning. To enhance the particle distribution, jute particleos were treated with 5–20% (w/v) aqueous solutions of sodium perborate trihydrate (SP). X-ray photoelectron spectroscopy (XPS) was used to confirm the surface treatment. XPS analysis indicated that the treatments improved the hydrophobicity of the jute by means of increasing the carbon/oxygen ratio of the surface; thus, the maximum increment was achieved after 10% (w/v) SP treatment. After determining the optimum SP concentration, the spinning of polypropylene composite multifilaments containing 0.3–1.4 wt% jute particles was employed. Differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) revealed the nucleating agent effect of the particles during crystallization in the filaments. The addition of fillers did not result in significant changes in the functional groups of polypropylene. The main output of this research is that polypropylene multifilaments incorporating 1.4 wt% jute particles presented the highest moisture absorption and hydrophilic character as determined by TGA, moisture content, and vertical wicking tests. It was concluded that particle content >0.3 wt% showed a tendency to agglomerate in the filament. Consequently, this study provided a new polypropylene filament having moisture absorbability performance, which can create potential applications in the textile industry.
1 Introduction
Polypropylene is a well-known thermoplastic polymer and is one of the most widely used thermoplastic polymers in the textile and plastics industry. Due to some superior properties, such as easy processing, excellent chemical inertness, light weight, and low cost, the production and the consumption of polypropylene fibers are reported to show increasing trends recently [1]. In spite of their beneficial properties, they have also some drawbacks, such as low thermal stability and very low polarity [1], [2], [3], [4]. Due to their highly hydrophobic character and insufficient moisture absorption capability, uncomfortable feeling comes to exist in polypropylene garments. To overcome these shortcomings, some organic or inorganic fillers have been added into molten polypropylene in the extrusion process. These filler particles specify the applications and the performance of the final product to impart functional features to the polymer.
Until now, the effects of different additives on some specific properties of polypropylene fibers were investigated in a number of related scientific researches. Several scientific groups investigated the effects of nanoclay and multiwalled carbon nanotubes on the mechanical performance of polypropylene fibers [1], [5]. In another study, polypropylene fibers incorporating silver nanoparticles are revealed to exhibit excellent antibacterial effects [6], [7]. The effects of TiO2 and SiO2 particles on the UV stability and flame retardancy performance of polypropylene fibers were also studied [4], [8]. Zinc oxide/polypropylene fibers were investigated whether they exhibit antimicrobial activity [9]. The effect of boron phosphate on the mechanical, thermal, and flame retardant performance of polypropylene and polyamide fibers was reported [10]. Some research papers also reported the manufacturing and characterization of polypropylene fibers incorporating wood fibers with maleic anhydride-grafted polypropylene coupling agent [11], [12]. However, there are not many scientific studies focusing on composite polypropylene fibers filled with cellulose-based natural fibers.
Cellulose-based fibers have very polar character due to the presence of the hydroxyl groups. However, the presence of pectin and waxy substances on the surface of the fibers hinder hydroxyl groups from reacting with nonpolar polymers. Additionally, incompatibility with hydrophilic cellulose-based fibers and hydrophobic polymers generally results in poor composite performance [13]. To improve the interfacial interaction between cellulose fibers and relatively nonpolar polymers, the surface characteristic of cellulose fibers should be chemically or physically treated [14].
Sodium perborate, a source of active oxygen, can produce hydrogen peroxide, which is responsible for the oxidation when dissolved in water. Sodium perborate can be a good alternative agent for the surface treatment of cellulose fibers. In our previous study, sodium perborate trihydrate (SP) is found to be the most efficient chemical agent for the treatment of jute fibers by providing the highest interfacial adhesion with polypropylene and the highest surface roughness in comparison with potassium permanganate and potassium dichromate oxidative agents [14].
Nowadays, the demand for new functional fibers filled with natural additives has been increasing due to the growing environmental awareness and substantial interest in ecological concerns. The common structure and attributes between the filler and the polymer interface play an important role in the definition of the physical and mechanical attributes of the produced composite [15]. Literature review provides very limited knowledge on the manufacturing of polypropylene composite filaments added with cellulose-based fillers. In our previous studies, untreated jute particle-doped polypropylene composite fibers were manufactured at 0.5 and 1 wt% contents [16], [17]. Because of the agglomeration of jute particles due to their high polarity, the production of polypropylene composite fibers with higher particle content of jute particles could not be performed. To improve the particle distribution along the polypropylene filaments by decreasing their polarity, jute fibers can be surface treated.
This study focuses on the fabrication and characterization of polypropylene composite multifilaments filled with surface-treated jute microparticles to improve the moisture absorbability performance of polypropylene filaments. For this purpose, surface treatments of jute microparticles with SP (at concentrations of 5%, 10%, 15%, and 20%, w/v) were carried out to provide a more homogeneous particle distribution in the polypropylene filaments. In manufacturing processes, polypropylene composite granules containing surface-treated jute microparticles in three different weight ratios (0.3, 0.8, and 1.4 wt%) were prepared at first, and then the spinning process of the multifilaments was employed. The effects of the treatments on the surface of jute microparticles were examined by X-ray photoelectron spectroscopy (XPS), and the optimum SP concentration was selected for manufacturing the polypropylene composite filaments. The structure, morphology, and thermal stability of the polypropylene filaments were investigated. Additionally, some functional properties such as moisture content, vertical wicking, and thermal conductivity, which play a fairly determinative role on the end use of the fibers, were also studied. It is thought that the findings of this experimental study may open up an alternative pathway for the development of moisture-absorbable composite polypropylene filaments in the textile industry.
2 Materials and methods
2.1 Materials
Jute yarns supplied from Atlantik Halı A.Ş (Turkey) were used as additive materials. The contents of the basic constituents of jute yarns, such as cellulose, hemicellulose, lignin, and the others, were determined as 79.0%, 12.6%, 8.0%, and 0.4%, respectively [13]. SP (NaBO2·H2O2·3H2O) was supplied by Merck Corp (Germany). Isotactic polypropylene, used as matrix polymer in powder form with a melt flow index value of 35 g/10 min, was supplied from Senkroma Global Colors (Turkey).
2.2 Preparation of the jute microparticles
Jute yarns were ground in Retsch Cutting Mill SM 100 (Haan, Germany) grinder using a sieve having holes of 250 μm. Jute yarns in powder form were ground in Fritsch Pulverisette 7 (Oberstein, Germany) grinder at the speed of 850 rpm for 20 min to decrease the size of the jute particles.
2.3 Surface treatment of the jute microparticles
Jute particles were immersed in distilled water for 1.5 h to remove the impurities and dried in an oven at 90°C. Following the pretreatment process, jute microparticles were treated with 5%, 10%, 15%, and 20% (w/v) SP aqueous solutions for 30 min at 75°C. The jute microparticles were rinsed out with distilled water several times to remove the chemical residues and then oven dried at 105°C and kept in a desiccator. The optimum SP concentration was selected for manufacturing polypropylene composite filaments considering the XPS analysis results of surface-treated jute particles.
2.4 Manufacturing of the composite multifilaments
Initially, both treated jute microparticles and polypropylene in powder form were premixed mechanically in which the contents of jute particles are 0, 0.3, 0.8, and 1.4 wt%, dried at 105°C for 4 h, and then melt blended in the extruder (Lab Tech, Thailand) at the speed of 170 rpm. The compounded material passed through six extrusion zones at the temperature of 190°C and finally extruded through the spinneret holes. After the extrusion process, the material was cooled down with cold water and pelletized as granules with the aforementioned concentrations (Figure 1).

Polypropylene granules containing (A) 0 wt%, (B) 0.3 wt%, (C) 0.8 wt%, and (D) 1.4 wt% treated jute microparticles.
Then, the polypropylene composite granules were fed into a laboratory-scale melt spinning machine (Plantex Lab Line, Italy) having a single-screw extruder with two spinning nozzles in trilobal cross-sections. The composite granules passed through six zones with different temperatures in the range of 180°C–215°C from the feeder to the spinneret, respectively. The extrusion speed and the extrusion pressure were kept at 14 dpf and 80 bar, respectively. The spun filaments were drawn with a draw ratio of 3.25. Finally, 100% polypropylene (P0) and polypropylene composite multifilaments containing 0.3 wt% (P03), 0.8 wt% (P08), and 1.4 wt% (P14) treated jute microparticles were fabricated. It should be noted that P0 multifilaments as a control sample were also subjected to the same manufacturing processes.
2.5 XPS analysis of the jute microparticles
XPS analysis was carried out to examine the effects of the treatments on the surface chemistry of the jute microparticles. XPS spectra were obtained using a Thermo Scientific Kα XPS (USA).
2.6 Characterization of the polypropylene multifilaments
2.6.1 Differential scanning calorimetry (DSC)
The melting and the crystallization behavior of the polypropylene multifilaments were investigated using Perkin-Elmer/Pyris 1 DSC (USA) under nitrogen atmosphere. The samples were scanned from 0°C to 200°C with heating and cooling rates of 20°C/min. The percentage of crystallinity was calculated using the melting enthalpy via following formula:
where ΔHm is the melting enthalpy of the polypropylene analyzed in this experimental study and
2.6.2 Fiber tensile test
To examine the tensile properties of the polypropylene filaments, single fiber tensile tests were carried out using Instron tensile testing machine (Bucks, England) according to ASTM D 3822-07. The cross-head speed was kept 60 mm/min, and three different gauge lengths such as 25, 50, and 75 mm were used in this test to check the homogeneity of the polypropylene filaments. Fifty fiber specimens for each polypropylene fiber were tested to check for repeatability.
2.6.3 Thermogravimetric analysis (TGA)
The TGA analyses of the polypropylene multifilaments were conducted by Perkin-Elmer Diamond TG/DTA instrument (USA) with a heating rate of 10°C/min, from 25°C to 600°C, under inert atmosphere of N2 (10 ml/min).
2.6.4 Determination of moisture content
The moisture contents of the polypropylene filaments were tested in accordance with ASTM D 629-08.
2.6.5 Vertical wicking test
Before the vertical wicking test, the knitted fabric samples of the polypropylene filaments were manufactured using Textimat Diamant V bed hand-knitting machine of 11 gauge. The loop densities of all polypropylene supreme knitted fabrics are 5 courses/cm and 5 wales/cm.
The effect of treated jute particles on the vertical wicking of polypropylene was examined by determining the vertical wicking height against gravity along the course direction of the knitted fabric. The related test was performed using a vertical wicking tester in accordance with DIN 53924. A strip of fabric (150×25 mm) was suspended vertically with its lower end (20 mm) immersed in 1% (w/w) K2CrO4 solution to track the movement of the liquid at a regular time interval. The height reached by the liquid in the fabric was measured with respect to the clamped scale [19].
2.6.6 Thermal conductivity
The thermal conductivity performances of the polypropylene multifilaments were tested using C-Therm thermal conductivity analyzer (New Brunswick, Canada). Twenty measurements per each fiber sample were performed to check for repeatability.
2.6.7 Fourier transform infrared (FTIR) analysis
The FTIR spectra of the polypropylene multifilaments were obtained using an FTIR spectrometer (Perkin-Elmer Spectrum BX). Each spectrum was recorded in the range of 400–4000 cm-1 with a resolution of 2 cm-1.
2.6.8 Fluorescence microscopy observation
The fluorescence micrographs of the jute fiber and the polypropylene filaments were taken using an Olympus BX 43 fluorescence microscope for tracking the jute particles in longitudinal views of the polypropylene filaments.
3 Results and discussion
3.1 XPS analysis of the jute microparticles
Table 1 gives the surface chemical composition by means of atomic concentration (%) and carbon/oxygen (C/O) ratios of the untreated jute (J) and treated jute microparticles with different concentrations of SP aqueous solutions (5%, 10%, 15%, and 20% SPJ). After all SP treatments, the C and O contents of the jute particles remain almost similar. It is clearly seen that the C/O ratio of J increased after all chemical treatments. The maximum increment at C/O ratio of J was achieved after 10% (w/v) SP treatment. The C/O ratio can give information about the hydrophobicity of the fiber surface in general. A high C/O ratio modified the surface of the jute from hydrophilic to more hydrophobic [14], [20]. Accordingly, it is determined that 10% (w/v) SP treatment changed the surface of J from hydrophilic to more hydrophobic. It is probable that 10% (w/v) SP treatment creates a hydrophobic environment on the surface of the jute particles. This may be due to the oxidative modification with SP treatment, which reduced the proportion of O-H groups and increased that of O=C groups of the jute particles [14].
Surface chemical compositions (%) of the jute microparticles.
| C | O | C/O | Increase in C/O ratio (%) | |
|---|---|---|---|---|
| J | 66.88 | 33.12 | 2.00 | – |
| 5% SPJ | 67.38 | 30.01 | 2.24 | 12.0 |
| 10% SPJ | 68.90 | 28.29 | 2.43 | 21.5 |
| 15% SPJ | 64.99 | 30.96 | 2.09 | 4.5 |
| 20% SPJ | 63.62 | 29.94 | 2.12 | 6.0 |
The optimum SP concentration was selected for manufacturing polypropylene composite filaments considering the XPS analysis results of surface-treated jute particles.
3.2 DSC analysis of the polypropylene filaments
Table 2 gives the melting and crystallization temperatures, the melting and crystallization enthalpies, the extent of supercooling, and the crystallinity (%) of the polypropylene filaments obtained by DSC analysis. The DSC curves of the polypropylene filaments are shown in Figure 2. It can be mentioned from DSC results that the difference between the melting point and the crystallization temperature of P0 filaments is calculated to be 39.5°C, and this difference is evaluated as the extent of supercooling of the polymer [21], [22]. The addition of 0.3, 0.8, and 1.4 wt% treated jute microparticles reduced the extent of supercooling of P0 filaments by 33%, 26%, and 12%, respectively. To decrease the extent of supercooling, some substances acting as nucleating agents are added to the polymers. The nucleating agents change the crystallization behavior of polypropylene chains and agglomerate in the molten phase. These agents reduce the extent of supercooling of polypropylene by increasing the onset crystallization temperature [23]. Therefore, it can be stated that the treated jute microparticles acted as nucleating agents in the polypropylene, and the best improvement was achieved by the incorporation of 0.3 wt% jute particles. It is also pointed out that the polypropylene filaments exhibited similar crystallinity values. Similarly, Srisawat et al. reported that silica particulate loading acted as nucleating agent by increasing the crystallization temperature of polypropylene fibers [24].
Calorimetric data of the polypropylene filaments.
| Onset melting temperature (°C) | Onset crystallization temperature (°C) | Crystallinity (%) | ΔHmelting (J/g) | ΔHcrystallization (J/g) | Extent of supercooling | |
|---|---|---|---|---|---|---|
| P0 | 157.15 | 117.68 | 40.16 | 83.12 | 94.22 | 39.47 |
| P03 | 154.07 | 127.64 | 39.71 | 87.25 | 95.68 | 26.49 |
| P08 | 154.31 | 124.96 | 40.15 | 82.24 | 95.24 | 29.35 |
| P14 | 157.01 | 122.24 | 41.35 | 85.59 | 97.45 | 34.77 |

(A) Exothermic (crystallization) and (B) endothermic (melting) peaks of the polypropylene filaments.
As presented in Figure 2, the composite filaments have multiple peaks in the melting zone apart from P0 filaments. This may be explained by the difference between melting behaviors of chain folded crystals and the crystals in more extended chain conformation [25], [26]. The formation of the multiple melting peaks is also elucidated by the melting of different sized crystals or disordered crystals [25], [27]. Soitong and Pumchusak revealed that multiwalled carbon nanotube-filled polypropylene composite fibers also presented multiple peaks in the melting zone due to the difference in crystal forms or degree of perfection [28].
3.3 Tensile properties of the polypropylene filaments
To examine the effects of the surface-treated jute microparticles on the tensile properties of the polypropylene filaments at different gauge lengths, single fiber tensile tests were carried out. The tenacity results of P0, P03, P08, and P14 filaments are listed in Table 3. It is obvious that the tenacity results of P0 filament increase with the addition of the treated jute particles. It is also determined that the standard deviation values are noticed to be high for all polypropylene filaments. This may be due to the nonhomogeneous distribution of the treated jute microparticles, which can be seen in the fluorescence images of the polypropylene filaments (Figure 9) or the crystalline regions along the filament.
Tenacity of the polypropylene filaments at different gauge lengths.
| Tenacity (g/denier) | |||
|---|---|---|---|
| 25 mm | 50 mm | 75 mm | |
| P0 | 1.94±0.65 | 1.93±0.43 | 2.26±0.62 |
| P03 | 2.88±0.44 | 2.98±0.31 | 2.83±0.41 |
| P08 | 2.54±1.02 | 2.52±0.71 | 2.98±1.09 |
| P14 | 2.28±0.23 | 2.23±0.64 | 2.26±0.76 |
3.4 TGA analysis of the polypropylene filaments
TGA was conducted to investigate the effect of the surface-treated jute microparticles on the thermal decomposition of the polypropylene filaments. Figures 3 and 4 illustrate the TGA curves of the jute and the polypropylene filaments, respectively. In general, the extrusion in the manufacturing processes of polypropylene granules and polypropylene filaments performs generally at approximately 200°C. On this basis, Figure 3 presents that there is no thermal degradation of jute that existed until 200°C.
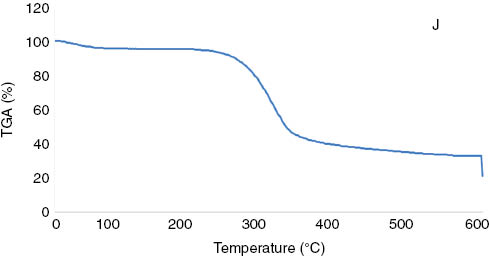
TGA curve of the jute microparticles.

TGA curves of the polypropylene filaments.
As seen from Table 4 and Figure 4, there is no mass loss that existed until 120°C in the TGA curves of P0, P03, and P08 filaments. However, the mass loss is recorded to be 0.7% at this temperature for P14 filament. This can be due to the dehydration of the jute particles in the polypropylene. T05 and T50 are the temperatures at which a sample loses 5% and 50% of its mass, respectively. T05 and T50 for all polypropylene filaments are determined to be approximately 365°C–381°C and 437°C–449°C, respectively. It is noticed from Table 4 that the addition of treated jute microparticles increased the initial thermal degradation temperature of P0 filament. This enhanced thermal stability may be related to the nucleating agent effect of treated jute microparticles in the polypropylene. The thermal degradation of P0, P03, P08, and P14 was completed at 482°C, 480°C, 486°C, and 490°C, respectively. The improved crystallization and the enhanced molecular order with the addition of fillers may hinder the thermal decomposition of polypropylene [29].
TGA results of the polypropylene filaments.
| Sample | Mass loss (%) until 120°C | Tinitial* (°C) | Tfinal* (°C) | 5% loss (T05) (°C) | 50% loss (T50) (°C) | Residue (%) |
|---|---|---|---|---|---|---|
| P0 | 0 | 308 | 482 | 378 | 449 | 0 |
| P03 | 0 | 339 | 480 | 381 | 449 | 0 |
| P08 | 0 | 336 | 486 | 365 | 437 | 2.4 |
| P14 | 0.7 | 316 | 490 | 378 | 447 | 0 |
*Tinitial, initial degradation temperature; Tfinal, final degradation temperature.
3.5 Moisture content of the polypropylene filaments
Figure 5 presents the effect of the surface-treated jute microparticles on the moisture content of the polypropylene filaments. It is pointed out from the representative results that there are slight differences between the moisture content of P0 and P03 filaments. This can be due to the addition of jute particles in low content. However, the addition of 0.8 and 1.4 wt% jute particles increased the moisture content of the polypropylene filament from 0.04% to 0.34% and 0.70%, respectively. According to statistical analysis, the effect of the addition of 0.8 and 1.4 wt% jute particles is statistically significant on the moisture content of the polypropylene filaments. Wang et al. reported that the polypropylene composite fiber-filled microcrystalline cellulose achieved water uptake performance, which will be beneficial in some applications in the textile industry [30].

Moisture content of the polypropylene filaments.
3.6 Vertical wicking of the polypropylene knitted fabrics
The experimental results presented in Figure 6 describe that the incorporation of the surface-treated jute microparticles reduces the vertical wicking of P0 filament. Additionally, the increments in the content of the jute particles negatively influence the vertical wicking of the polypropylene. Das et al. [19] explained this behavior by the absorption and wicking phenomena. Due to high absorbency of jute, the water molecules of the solution form bonds with the absorbing group of jute particles, which results in very less water movement along the fabric. However, higher wicking heights were observed in the case of the polypropylene due to its hydrophobic character, which does not form bond with water molecules and the liquid surface dragged very smoothly. Polypropylene acts as a channel to water and enhances the vertical wicking [19], [31]. Therefore, the wicking results indicate that the polypropylene filaments can achieve hydrophilic character with the incorporation of 1.4 wt% treated jute particles, which is more pronounced.

Vertical wicking of the polypropylene filaments.
3.7 Thermal conductivity of the polypropylene knitted fabrics
Figure 7 summarizes the k-values, the thermal conductivity of the polypropylene filaments. Representative results in Figure 7 reveal that the thermal conductivity of P0 filament decreased by 5%, 32%, and 17% with the addition of 0.3, 0.8, and 1.4 wt% SP-treated jute particles, respectively. This finding is in line with the research by Mounika et al. [32], which focused on the thermal conductivity of bamboo fiber-reinforced polyester composite. It is determined that the thermal insulating of the polypropylene can be improved with incorporating treated jute particles. To examine the details, the interfacial thermal resistance tended to decrease the thermal conductivity of the composite filament at lower content (0.3 and 0.8 wt%) of the jute particles. The enhancement of the heat conduction paths with increasing the particle content counteracted the decreasing effect of the interfacial thermal resistance on the thermal conductivity of the polypropylene composite filament [33].

Thermal conductivity of the polypropylene filaments.
3.8 FTIR analysis of the polypropylene filaments
Figure 8 represents the FTIR spectra of the polypropylene filaments. The absorption bands at 2950, 2918, and 2838 cm-1 are related to asymmetric CH3 and asymmetric and symmetric CH2 stretching vibrations, respectively [34]. The absorption bands at 1456 and 1376 cm-1 are assigned to asymmetric and symmetric CH3 bending vibrations [35]. The band at 1167 cm-1 is attributed to the presence of isotactic bands of polypropylene [36]. It can be noted that the intensity of this peak increased with incorporating 1.4 wt% treated jute microparticles into the polypropylene filament. However, the intensity of the peak observed at 1102 cm-1, which is related to C-C and C-H deformations, decreased in the spectrum of P14 filament. The absorption band at 998 cm-1 is assigned to the characteristic crystalline band of polypropylene [37]. It can be said that the incorporation of the jute microparticles did not modify the functional groups of the polypropylene filament but changed the intensity of isotactic bands, C-C and C-H deformations.

FTIR spectra of the polypropylene filaments.
3.9 Fluorescence microscopy observation of the polypropylene filaments
The longitudinal views of the jute fiber and the polypropylene filaments were investigated using fluorescence microscopy for tracking the jute particles. The micrographs of the jute and the polypropylene filaments are presented in Figure 9. The jute fiber and the jute particles in the polypropylene are seen in different colors due to their autofluorescence characteristics as displayed in Figure 9. It is determined that the sizes of the jute particles increase with increasing particle content in the polypropylene filament. The jute microparticles in higher loading in the polypropylene filament show a tendency to agglomerate due to the interactions between the particles.

Fluorescence micrographs of the jute fiber (×20) and the polypropylene filaments (×5).
4 Conclusion
This research focuses on the preparation and characterization of polypropylene composite multifilaments containing surface-treated jute particles at various contents. Jute particles were treated with SP in different concentrations to reduce the interaction between the jute particles in polypropylene by decreasing their surface hydrophilicity and to facilitate the melt spinning process. Treatment with 10% (w/v) SP provides the highest C/O ratio on the surface of the jute particles, which indicates an improvement in the hydrophobicity of jute particles. It is noteworthy that the melt spinning of 1.4% untreated jute/polypropylene filaments could be performed as a result of the treatment process. The surface-treated jute particles acted as a nucleating agent by decreasing the extent of supercooling and increasing the crystallization temperature, initial thermal degradation temperature, and tenacity of P0 filament. Thus, the highest enhancement in these properties could be achieved by the addition of 0.3 wt% treated jute microparticles. The thermal insulating of polypropylene filaments was enhanced with the addition of modified jute microparticles. The main output of this research is that the polypropylene filament achieved moisture absorbability and hydrophilic character with the addition of 1.4% jute particles as confirmed by TGA analysis, moisture content, and wicking test, respectively. It is concluded that increasing the particle content in the polypropylene filament exhibited a tendency to agglomerate and results in nonhomogeneous distribution. This study is considered to be important for development of manmade textile filaments having moisture absorbency, which are generally produced as nonpolar.
Acknowledgments
This study was supported by the Dokuz Eylül University (project no. 2011.KB.FEN.037 and project name “An investigation on production and properties of natural fibers reinforced composite fibers”) as a scientific project. The authors also gratefully acknowledge the funding by the Scientific and Technological Research Council of Turkey (TÜBİTAK) under grant 111M498.
References
[1] Guo Z, Hagström B. Polym. Eng. Sci. 2013, 53, 2035–2044.10.1002/pen.23463Search in Google Scholar
[2] Karian H. Handbook of Polypropylene and Polypropylene Composites. Revised and expanded. Taylor & Francis, 2003.10.1201/9780203911808Search in Google Scholar
[3] Lewin M. Handbook of Fiber Chemistry. 3rd ed., Taylor & Francis, 2006.10.1201/9781420015270Search in Google Scholar
[4] Erdem N, Erdogan UH, Cireli AA, Onar N. J. Appl. Polym. Sci. 2010, 115, 152–157.10.1002/app.30950Search in Google Scholar
[5] Tambe PB, Bhattacharyya AR, Kamath SS, Kulkarni AR, Sreekumar TV, Srivastav A, Rao KUB, Liu Y, Kumar S. Polym. Eng. Sci. 2012, 52, 1183–1194.10.1002/pen.22186Search in Google Scholar
[6] Yeo SY, Jeong SH. Polym. Int. 2003, 52, 1053–1057.10.1002/pi.1215Search in Google Scholar
[7] Wei Q, Tao D, Deng B, Huang F. J. Ind. Text. 2009, 38, 309–316.10.1177/1528083708092013Search in Google Scholar
[8] Erdem N, Cireli AA, Erdogan UH. J. Appl. Polym. Sci. 2009, 111, 2085–2091.10.1002/app.29052Search in Google Scholar
[9] Dural Erem A, Ozcan G, Skrifvars M. Text. Res. J. 2013.Search in Google Scholar
[10] Doğan M, Bayramlı E. Fiber Polym. 2013, 14, 1595–1601.10.1007/s12221-013-1595-0Search in Google Scholar
[11] Awal A, Ghosh SB, Sain M. J. Mater. Sci. 2009, 44, 2876–2881.10.1007/s10853-009-3380-4Search in Google Scholar
[12] Awal A, Ghosh SB, Sain M. J. Therm. Anal. Calorim. 2010, 99, 695–701.10.1007/s10973-009-0100-xSearch in Google Scholar
[13] Turku I, Karki T. Eur. J. Wood Prod. 2014, 72, 73–79.10.1007/s00107-013-0754-8Search in Google Scholar
[14] Bulut Y, Aksit A. Cellulose 2013, 20, 3155–3164.10.1007/s10570-013-0049-6Search in Google Scholar
[15] Nafchi HR, Abdouss M, Najafi SK, Gargari RM, Mazhar M. Adv. Compos. Mater. 2015, 24, 239–248.10.1080/09243046.2014.891341Search in Google Scholar
[16] Bulut Y, Erdoğan ÜH, Akşit A. An investigation on structural properties of melt spun polypropylene/jute composite fiber. 12th Autex World Textile Conference, Croatia, 2012.Search in Google Scholar
[17] Erdoğan ÜH, Bulut Y, Kartal GE, Kutlu B. Detection of natural additives in composite filaments by using fluorescence microscopy. 1st International Conference on Natural Fibers, Portugal, 2013.Search in Google Scholar
[18] Longo C, Savaris M, Zeni M, Brandalise RN, Grisa AMC. Mater. Res. 2011, 14, 442–448.10.1590/S1516-14392011005000080Search in Google Scholar
[19] Das B, Das A, Kothari V, Fangueiro R, Araujo MD. J. Eng. Fiber Fabr. 2009, 4, 20–28.Search in Google Scholar
[20] Sernek M. Comparative Analysis of Inactivated Wood Surfaces. Faculty of the Virginia Polytechnic Institute, Wood Science and Forest Products, State University, 2002.Search in Google Scholar
[21] Slama I, Mala J. Chem. Pap. 1991, 45, 221–225.Search in Google Scholar
[22] Feng L, Zheng J, Yang H, Guo Y, Li W, Li X. Sol. Energ. Mater. Sol. C 2011, 95, 644–650.10.1016/j.solmat.2010.09.033Search in Google Scholar
[23] Additives: Nucleating and Clarifying Agents, 2013.Search in Google Scholar
[24] Srisawat N, Nithitanakul M, Srikulkit K. J. Compos. Mater. 2012, 46, 99–110.10.1177/0021998311410477Search in Google Scholar
[25] Karacan İ, Benli H. Tekst. Konfeksiyon 2011, 21, 201–209.Search in Google Scholar
[26] Samuels RJ. J. Polym. Sci. Polym. Phys. Ed. 1975, 13, 1417–1446.10.1002/pol.1975.180130713Search in Google Scholar
[27] Tanaka H, Takagi N, Okajima S. J. Polym. Sci. Polym. Chem. Ed. 1974, 12, 2721–2728.10.1002/pol.1974.170121202Search in Google Scholar
[28] Soitong T, Pumchusak J. J. Mater. Sci. 2011, 46, 1697–1704.10.1007/s10853-010-4987-1Search in Google Scholar
[29] Avalos-Belmontes F, Ramos-De Valle LF, Ramirez-Vargas E, Sanchez-Valdes S, Mendez-Nonel J, Zitzumbo-Guzman R. J. Nanomater. 2012, 1–8.10.1155/2012/406214Search in Google Scholar
[30] Wang Y, Jacob K, Netravali A, Pan N, Zhang X, Uçar N. National Textile Center Annual Report, 2010.Search in Google Scholar
[31] Durur G, Öner E. J. Eng. Fiber Fabr. 2013, 8, 1–10.Search in Google Scholar
[32] Mounika M, Ramaniah K, Ratna Prasad AV, Mohana Rao K, Hema Chandra Reddy K. J. Mater. Environ. Sci. 2012, 3, 1109–1116.Search in Google Scholar
[33] Bai G, Jiang W, Chen L. Mater. Trans. 2006, 47, 1247–1249.10.2320/matertrans.47.1247Search in Google Scholar
[34] Gomathi N, Neogi S. Appl. Surf. Sci. 2009, 255, 7590–7600.10.1016/j.apsusc.2009.04.034Search in Google Scholar
[35] Mallakpour SE, Hajipour AR, Zadhoush A, Mahdavian AR. J. Appl. Polym. Sci. 2001, 79, 1317–1323.10.1002/1097-4628(20010214)79:7<1317::AID-APP200>3.0.CO;2-ESearch in Google Scholar
[36] Sinha D. Adv. Appl. Sci. Res. 2012, 3, 1365–1371.Search in Google Scholar
[37] Semba T, Kitagawa K, Endo S, Maeda K, Hamada H. J. Appl. Polym. Sci. 2004, 91, 833–840.10.1002/app.13197Search in Google Scholar
©2017 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Original articles
- Effects of multiwalled carbon nanotube mass fraction on microstructures and electrical resistivity of polycarbonate-based conductive composites
- Preparation and fluid drag reduction properties of superhydrophobic paper-based films comprising carbon nanotubes and fluoropolymers
- Studies on the structural, thermal, and dielectric properties of fabricated Nylon 6,9/CaCu3Ti4O12 nanocomposites
- Metal-matrix composite fabricated with gas tungsten arc melt injection and precoated with NiCrBSi alloy to increase the volume fraction of WC particles
- Construction of polypropylene composite multifilaments filled with sodium perborate trihydrate-treated jute microparticles
- Damage detection of three-dimensional braided composite materials using carbon nanotube thread
- An exact solution for improved metal-composite joints reinforced by FG inter-layers
- Effect of chemical treatment on thermal properties of bagasse fiber-reinforced epoxy composite
- The superior ductility of fine SiCp/Al2014 composites after extrusion
- Optical properties and photocatalytic activity of CdS-TiO2/graphite composite
- Effects of bottom ash and granulated blast furnace slag as fine aggregate on abrasion resistance of concrete
- Surface modification of diamond and its effect on the mechanical properties of diamond/epoxy composites
- Numerical simulation of strain rate effect on the inelastic behavior of metal matrix composites
- Electromechanical characterization of multilayer graphene-reinforced cellulose composite containing 1-ethyl-3-methylimidazolium diethylphosphonate ionic liquid
- The effect of hydroxyapatite reinforcement and preparation methods on the structure and mechanical properties of Mg-HA composites
- The effect of yttrium incorporation on the formation and properties of α-Al2O3 coatings
Articles in the same Issue
- Frontmatter
- Original articles
- Effects of multiwalled carbon nanotube mass fraction on microstructures and electrical resistivity of polycarbonate-based conductive composites
- Preparation and fluid drag reduction properties of superhydrophobic paper-based films comprising carbon nanotubes and fluoropolymers
- Studies on the structural, thermal, and dielectric properties of fabricated Nylon 6,9/CaCu3Ti4O12 nanocomposites
- Metal-matrix composite fabricated with gas tungsten arc melt injection and precoated with NiCrBSi alloy to increase the volume fraction of WC particles
- Construction of polypropylene composite multifilaments filled with sodium perborate trihydrate-treated jute microparticles
- Damage detection of three-dimensional braided composite materials using carbon nanotube thread
- An exact solution for improved metal-composite joints reinforced by FG inter-layers
- Effect of chemical treatment on thermal properties of bagasse fiber-reinforced epoxy composite
- The superior ductility of fine SiCp/Al2014 composites after extrusion
- Optical properties and photocatalytic activity of CdS-TiO2/graphite composite
- Effects of bottom ash and granulated blast furnace slag as fine aggregate on abrasion resistance of concrete
- Surface modification of diamond and its effect on the mechanical properties of diamond/epoxy composites
- Numerical simulation of strain rate effect on the inelastic behavior of metal matrix composites
- Electromechanical characterization of multilayer graphene-reinforced cellulose composite containing 1-ethyl-3-methylimidazolium diethylphosphonate ionic liquid
- The effect of hydroxyapatite reinforcement and preparation methods on the structure and mechanical properties of Mg-HA composites
- The effect of yttrium incorporation on the formation and properties of α-Al2O3 coatings

