Electromechanical characterization of multilayer graphene-reinforced cellulose composite containing 1-ethyl-3-methylimidazolium diethylphosphonate ionic liquid
-
Emine Akar
, Yoldaş Seki
Abstract
In this study, multilayer graphene (Gr)-reinforced cellulose composites were synthesized by using 1-ethyl-3-methylimidazolium diethylphosphonate ionic liquid. The composites were fabricated via dissolving the cellulose in 1-ethyl-3-methylimidazolium diethylphosphonate and Gr loading at different ratios (0.2, 0.4, and 0.6 wt.%). Both sides of the composites were coated with gold leaf to generate electrodes. The effect of Gr loading on chemical functional groups, crystallographic properties, thermal stability, and morphological and mechanical properties of cellulose film was investigated by Fourier transform infrared spectroscopy, X-ray diffraction, thermogravimetric analysis, scanning electron microscopy, and tensile test, respectively. Electromechanical behavior of the cellulose composite films reinforced with Gr (0.2, 0.4, and 0.6 wt.%) was investigated under DC excitation voltages of 1, 3, 5 and 7 V. Gr loading of 0.2 wt.% increased maximum tip displacement by 400% when the actuator is excited with 3 V.
1 Introduction
Electroactive polymers (EAPs) are the polymers that change shape, size, etc., under electrical stimuli [1]. EAPs require low driving voltage (<5 V) [2]. In the last decade, EAPs such as dielectric elastomers [3], conducting polymers [4], [5], ionic polymer metal composites (IPMCs) [6], carbon nanotube actuators [7], and polymer gels [8] have been studied. The application areas of EAPs are biomimetic robots, artificial muscles, sensors, etc. These applications require some properties such as biodegradability and biocompatibility for polymers [1]. Cellulose, one of the biopolymers, meets the requirements mentioned above.
Cellulose, the most abundant resource in the world, provides many advantages to industrial applications such as renewability, biocompatibility, and biodegradablity [1], [9]. These advantages make it valuable in many applications such as in paint, paper, fiber, membrane, and polymer industries [10]. In spite of these valuable properties, handling of cellulose is difficult due to dissolving problems because of hydrogen bonding and crystalline structure [11]. The conventional methods for dissolving cellulose include the cuprammonium and xanthenes processes and require the use of unusual solvents, typically with high ionic strength, and relatively harsh conditions. They are expensive and inadequate for dissolution of cellulose [10], [12], [13], [14], [15], [16]. In addition to these disadvantages, they give serious environmental hazards [15]. Because some of these dissolution solvents are toxic, unstable during cellulose processing, volatile, and difficult to recover, a new “green” method is necessary for dissolution of cellulose [11].
Ionic liquids (ILs) are salts, which are fluid below or around 100°C [17], [18]. Most of the ILs have a wide electrochemical window, a low vapor pressure, a high thermal stability, a wide liquid range, and a high solvation ability for inorganic and organic substances [19], [20], [21]. Cellulose can be dissolved in IL without addition of water, ethanol, methanol, acetone, acetonitrile, etc. [10], [15], [22], [23], [24], [25]. 1-Ethyl-3-methylimidazolium diethylphosphonate ([EMIM]DEP) is a room temperature IL and a suitable solvent for cellulose [26].
Graphene (Gr) is a two-dimensional sheet of sp2-hybridized carbon. It is known that Gr has revealed a kind of electrical and mechanical properties when it was used as reinforcement for polymer matrix. In order to obtain better electrical and mechanical properties in polymer matrix, Gr loading into polymer is useful [27]. It was reported that loading of the Gr improved the actuation behavior of Nafion-based IPMC. It was also revealed that the tensile strength and proton conductivity of Nafion were significantly improved by loading Gr into Nafion membrane [28], [29]. Gr-based actuating systems are situated in a wide range such as sensors, switches, artificial muscles, nano/microelectromechanical devices, etc. [29].
In this study, multilayer Gr-based cellulose membranes were synthesized by using [EMIM]DEP. Gr was used to improve tensile strength and actuating performance. Characterization of the different Gr-loaded samples was evaluated by thermogravimetric analysis (TGA), X-ray diffraction (XRD) analysis, Fourier transform infrared analysis (FTIR), scanning electron microscopy (SEM), and tensile tests measurements. The electroactive characteristics of Gr-loaded films were also investigated at different voltage levels.
2 Materials and methods
2.1 Materials
Cellulose (Cel), [EMIM]DEP, and N,N-dimethyle acetamid (DMAc) were provided from Sigma-Aldrich (Steinheim, Germany). Multilayer Gr, with an average particle diameter of 5–10 μm, was purchased from Grafen Kimya Sanayi A.Ş. (Ankara, Turkey). Gold leaf, which has a thickness of 10 μm, was obtained from L.A. Gold Leaf (Azuza, ABD).
2.2 Fabrication of cellulose-based IPMC actuators
Cel (0.39 g) was dissolved in 5.58 g of [EMIM]DEP at 80°C (in water bath). DMAc (3 ml) as a plasticizer was added to the solution. After the solution was mixed for an hour, the proper amount of Gr was added to the mixture to obtain 0.2, 0.4, and 0.6 wt.% of total mass and was well dispersed by ultrasonic homogenizer. Then, the mixture was cast on a glass plate. The wet film was dried at room temperature for 18 h, and the film, which has a measured thickness of 1.0 mm, was obtained. Both surfaces of the film were coated with gold leaves to fabricate IPMC actuators.
2.3 Characterization methods
FTIR spectra of the cellulose-based samples were analyzed by using Perkin Elmer Spectrum BX-II. The spectra were recorded at a resolution of 4 cm-1 in the range 4000–400 cm-1.
XRD analysis of the cellulose-based samples was done via a Philips X-Pert Diffractometer with Ni-filtered Cu Kα radiation (λ=1.54 Å) at 45 kV and 40 mA. The samples were scanned from 5° to 80° (2θ).
Thermal behavior of the cellulose-based samples was determined by using TGA (Shimadzu, TGA 50). TGA was performed at a heating rate of 10°C/min at a range from 30°C to 600°C under nitrogen atmosphere with a flow rate of 1.0 ml/min.
SEM analysis of Cel-PO4 and Gr-loaded Cel-PO4 films (Cel-PO4-Gr) and the cross-sections of films were conducted by using Quanta FEG 250 SEM (at an accelerating voltage of 5 kV).
The tensile strength and tensile modulus of cellulose-based films and actuators were obtained by a Shimadzu universal testing machine with a 100-N load cell at a cross-head speed of 0.1 mm/min.
2.4 Electroactive properties
Electroactive behaviors of Cel-PO4-based actuators were investigated under DC voltages of 1, 3, 5, and 7 V. As signal source, data acquisition hardware (NI-PXI 7854R) and a buffer circuit were utilized. The maximum tip displacements of actuators were measured by Keyence LK-51 Laser Displacement Sensor.
3 Results and discussion
3.1 FTIR analysis
Figure 1 shows the FTIR spectra of Cel, Cel-PO4, Cel-PO4-Gr0.2, Cel-PO4-Gr0.4, and Cel-PO4-Gr0.6. In the FTIR spectra of cellulose, a broad band between 3600 and 3200 cm-1 indicates the -OH stretching vibrations. The sharp peak at 3342 cm-1 can be related with the -OH stretching vibrations due to the intramolecular hydrogen bonding [30], [31]. The absorption bands between 3000–2800 cm-1 and 1500–1250 cm-1 are attributed to the C-H and CH2stretching vibration, respectively [31], [32], [33]. The absorption peak around 1630 cm-1is caused by the presence of water [34]. The peak at around 1160 cm-1 originated from C-O-C stretching vibration. The peak at 1042 cm-1 is related to C-O stretching vibration [31], [35], [36].

FTIR spectra of samples (A) Cel, (B) Cel-PO4, (C) Cel-PO4-Gr0.2, (D) Cel-PO4-Gr0.4, and (E) Cel-PO4-Gr0.6.
For [EMIM]DEP, the absorption band between 1505 and 1590 cm-1 at around 1570 cm-1 is due to stretching vibration of the phosphate group (PO4)3- [37]. The P-O stretching vibrations are observed between 1200 and 900 cm-1 [38], [39]. The band between 1200 and 1250 cm-1is assigned to asymmetric stretching vibration of P=O [39].
Addition of [EMIM]DEP, Gr, and DMAc led to some changes in the FTIR spectrum of cellulose. The formation of new absorption bands was observed at 1570, 1216, 944, and 794 cm-1. It was observed that the peaks at 1436, 1366, 1164, and 1042 cm-1shifted to the peaks at around 1450, 1390, 1169, and 1056 cm-1. In addition, it can be seen that there are some changes in the spectra of samples after the addition of Gr. The intensity of the absorption peaks at around 3400 and 1366 cm-1 decreased depending on Gr concentration. The strong vibration peak at 1042 cm-1, which is assigned to C-O stretching in cellulose, shifted to 1056, 1047, and 1052 cm-1 after Gr loading of 0.2, 0.4, and 0.6 wt%, respectively.
3.2 XRD analysis
X-ray diffractograms of Cel, Cel-PO4, Cel-PO4-Gr0.2, Cel-PO4-Gr0.4, and Cel-PO4-Gr0.6 are shown in Figure 2. The major crystalline peak of the Cel-PO4 film occurred at 2θ angle of 23.9°. Essentially, the main peak at 2θ angle of 22° for cellulose corresponds to the 002 crystallographic plane of the cellulose I lattice [40]. It is interesting to note that no peak at 2θ angle of 22° was observed for the Cel-PO4 film.

X-ray patterns of (A) cellulose, (B) Cel-PO4, (C) Cel-PO4-Gr0.2, (D) Cel-PO4-Gr0.4, and (E) Cel-PO4-Gr 0.6.
The XRD analysis shows that the main diffraction peaks of Cel-PO4 films, which are loaded with a low amount of Gr, are similar to those of Cel-PO4. According to [41], if the regular stacks of graphite or graphite oxide are destroyed by exfoliation, the diffraction peak becomes weak or even disappears. Because there is no diffraction from the Gr, it is suggested that Gr has been distributed uniformly within the cellulose matrix [42].
3.3 Thermogravimetric analysis
Figure 3 shows the mass loss curves of Cel, Cel-PO4, Cel-PO4-Gr0.2, Cel-PO4-Gr0.4, and Cel-PO4-Gr0.6. The related TGA data of samples are summarized in Table 1. The mass loss of the cellulose occurs in two stages. The first stage resulted from evaporation of trapped moisture in cellulose [43]. The second stage of the mass loss is due to the thermal decomposition of cellulose [44]. The first mass losses of the samples that consist of Cel, [EMIM]DEP, DMAc, and different amounts of Gr are 1.0%, 20.3%, 21.5%, 18.1%, and 18.8% for Cel, Cel-PO4, Cel-PO4-Gr0.2, Cel-PO4-Gr0.4, and Cel-PO4-Gr0.6, respectively. The increase in trapped moisture of the samples resulted from the hydrophilicity of the [EMIM]DEP IL [45].
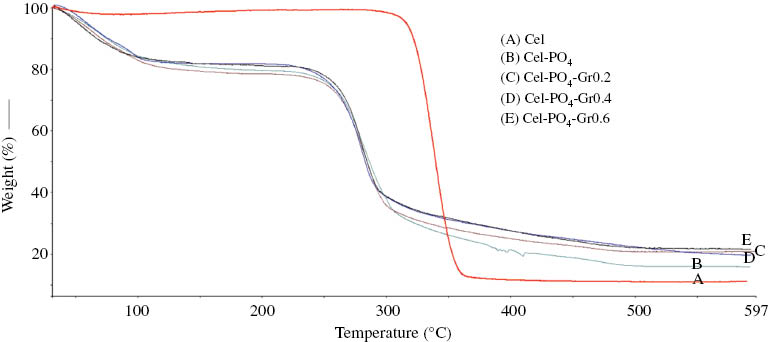
Thermograms of samples: (A) Cel-PO4, (B) Cel-PO4-Gr0.2, (C) Cel-PO4-Gr0.4, and (D) Cel-PO4-Gr0.6.
TGA data for the samples.
| Sample | Mass loss (%) (25°C–110°C) | Mass loss (%) (25°C–600°C) | Mass loss (%) (up to Tmax) | Tinitial(°C) | Tmax (°C) | Tfinal (°C) |
|---|---|---|---|---|---|---|
| Cel | 1.0 | 88.2 | 38 | 312 | 340 | 364 |
| Cel-PO4 | 20.3 | 67.3 | 72 | 247 | 283 | 319 |
| Cel-PO4-Gr0.2 | 21.5 | 57.6 | 65 | 244 | 281 | 320 |
| Cel-PO4-Gr0.4 | 18.1 | 62.3 | 70 | 243 | 276 | 312 |
| Cel-PO4-Gr0.6 | 18.8 | 59.6 | 59 | 241 | 279 | 344 |
Cellulose began to decompose at 312°C, whereas the Cel-PO4, Cel-PO4-Gr0.2, Cel-PO4-Gr0.4, and Cel-PO4-Gr0.6 started to decompose at around 245°C. As can be seen in Table 1, thermal stability of the Cel-PO4 and Gr-loaded samples (Cel-PO4-Gr) (279°C–283°C) was lower than that of original cellulose (340°C) on the basis of maximum decomposition temperatures. The decrease in the thermal stability probably resulted from the partial destruction of the crystalline part and hydrolysis of the cellulose [26]. However, Gr loading into Cel-PO4 has not led to significant variation in maximum decomposition temperature. Besides, Gr loading of 0.6 wt.% decreased the initial decomposition temperature of Cel-PO4 by 6°C. The decrease in thermal stability of Gr-loaded samples can also be associated with the catalyzing effect of the Gr on the thermal decomposition of Cel-PO4[46].
The mass losses in the temperature range 25°C–600°C for Cel, Cel-PO4, Cel-PO4-Gr0.2, Cel-PO4-Gr0.4, and Cel-PO4-Gr0.6 are 88.2%, 67.3%, 57.6%, 62.3%, and 59.6%, respectively. The total mass loss of cellulose is higher than those of Cel-PO4 and Gr-loaded Cel-PO4. The char residue of Cel, Cel-PO4, Cel-PO4-Gr0.2, Cel-PO4-Gr0.4, and Cel-PO4-Gr0.6 are 11.8%, 32.7%, 42.4%, 37.7%, and 40.4%, respectively. The loading of Gr into Cel-PO4sligthly increased the pyrolysis residue [47]. Because the formation of the char layer is important for thermal insulation and flame-retardant properties of samples, it can be expected that Gr loading into Cel-PO4 has led to better thermal insulation and flame-retardant properties [48].
3.4 SEM analysis
SEM micrographs of Cel-PO4 and Gr-loaded Cel-PO4 films are given in Figure 4. Film surfaces (Figure 4A, B, E, and G) appear to be smooth and fairly homogenous without any pores. In order to see the Gr particles, the cross-sections of films (Figure 4C, D, F, and H) were investigated by SEM analysis. A closer examination on the cross-section of films is given in Figure 4D, F, and H for Cel-PO4-Gr0.2, Cel-PO4-Gr0.4, and Cel-PO4-Gr0.6, respectively. Gr particles cannot be seen on the surface and cross-sections of films even at high magnifications.

SEM images of samples: (A) Cel-PO4, (B) Cel-PO4-Gr 0.2, (C) Cel-PO4-Gr0.2 cross-section, (D) Cel-PO4-Gr0.2 cross-section, (E) Cel-PO4-Gr0.4, (F) Cel-PO4-Gr0.4 cross-section, (G) Cel-PO4-0.6Gr, (H) Cel-PO4-0.6Gr cross-section.
3.5 Mechanical properties
Figures 5 and 6 show the tensile strength and modulus with increasing Gr content in Cel-PO4 films. As can be noticed from Figure 5, the filler content affects the tensile strength of Cel-PO4 films. The tensile strength and modulus of Cel-PO4 film were determined to be 12.57 MPa and 0.46 GPa, respectively.

The effect of Gr loading on tensile strength of Cel-PO4, Cel-PO4-Gr0.2, Cel-PO4-Gr0.4, and Cel-PO4-Gr0.6.

The effect of Gr loading on Young’s modulus of Cel-PO4, Cel-PO4-Gr0.2, Cel-PO4-Gr0.4, and Cel-PO4-Gr0.6.
After Gr of 0.2 and 0.4 wt.% were loaded into the Cel-PO4 film, the tensile strength of the Cel-PO4 film increased to 17.61 and 20.15 MPa, respectively. Thus, the tensile modulus of the Cel-PO4film increased by about 50% and 61%, respectively, when Gr of 0.2 and 0.4 wt.% were loaded. These increments may be explained by well dispersion of Gr into the mixture of Cel-PO4. However, when Gr of 0.6 wt.% was added into Cel-PO4, the tensile strength and the modulus of Cel-PO4-Gr0.6 film decreased by about 17% and 12%, respectively, compared with those of Cel-PO4-Gr0.4. The detrimental mechanical properties may be described on the basis of aggregation theory [49].
3.6 Electroactive properties
Electromechanical behavior of the gold-coated films with different Gr loadings (0.2, 0.4, and 0.6 wt.%) was investigated under DC excitation voltages of 1, 3, 5, and 7 V. The tip displacements of the actuators were recorded as their time responses via laser displacement sensor. The observed time response curves are in exponential forms, and any back relaxation did not occur during the experiments. Therefore, the final value of the tip displacements corresponds to the maximum tip displacement values of the actuators. These maximum values, given in Figure 7, are used to evaluate the electromechanical behavior of the actuator.

Maximum tip displacement of gold coated actuators with different Gr loading (1 mm in thickness).
The rows of Figure 7 show the effect of increasing excitation voltage on each actuator performance. It is seen that the maximum tip displacements of unloaded actuator and 0.6 wt.% Gr-loaded actuator increased slightly as excitation voltage increases from 3 to 5 V. On the other hand, the maximum tip displacement of 0.2 and 0.4 wt.% Gr-loaded actuator samples increased when excitation voltage increased from 1 to 3 V and then decreased when excitation voltage increased to 5 V.
The effects of Gr loading on the maximum tip displacements of the actuators are given in Figure 7. It is seen that Gr loadings of 0.2 and 0.4 wt.% increased the actuator performance for excitation voltages of 1 and 3 V. When 5 V experiments are considered, it is seen that the maximum tip displacement of the actuator with 0.2 wt.% Gr loading did not change, and further increase in Gr loading reduced the maximum tip displacement. The highest increase in performance is observed for 0.2 wt.% Gr-loaded actuators. It is seen that 0.2 wt.% Gr loading causes 400% increase in maximum tip displacement when the actuator is excited with 3 V. Lower Gr loading leads to considerable improvement in actuation behavior. Similar results for Nafion-based IPMC actuators have been obtained by Jung et al. [28]. It was emphasized that Gr at extremely low concentrations is advantageous and improves the actuation behavior of Nafion-based IPMC actuators [28]. The actuation performance was decreased clearly by greater Gr loading due to the agglomeration resulting from van der Waals forces and the π–π interactions between the Gr layers. This agglomeration may have restricted the diffusion of ions leading to actuation. Thus, probably, a decrement in maximum tip displacement came into existence.
4 Conclusions
Gr-reinforced cellulose composites were fabricated by dissolving cellulose in [EMIM]DEP, subsequently by Gr loading at different ratios. Dissolving cellulose in [EMIM]DEP and Gr loading affected the cellulose chemical structure; thus, formation of new absorption peaks was observed. Besides, the amount of Gr led to changes in crystallinity of the composites such as shifting for 2θ degree and decrease in the intensity peaks. Thermal stability of the raw cellulose was decreased with dissolving in [EMIM]DEP and Gr loading, whereas pyrolysis residue was increased. The SEM images seemed to be quite smooth. The tensile strength and the Young’s modulus of the composites increased with increasing Gr loading but decreased at 0.6 wt.% Gr content. The tip displacement of unloaded and 0.6 wt.% Gr-loaded actuator (Cel-PO4-Gr0.6) increased slightly as excitation voltage is increased from 3 to 5 V. Besides, 0.2 and 0.4 wt.% Gr-loaded actuators (Cel-PO4-Gr0.2 and Cel-PO4-Gr0.4) exhibited better tip displacement as excitation voltage is increased from 1 to 3 V. Besides, maximum tip displacements decreased when excitation voltage is increased to 5 V. The best tip displacement was obtained by 0.2 wt.% Gr-loaded sample (Cel-PO4-Gr0.2) under excitation voltage of 3 V.
Acknowledgments
Financial support for this study was provided by TUBITAK – The Scientific and Technological Research Council of Turkey (project number: 111M643).
References
[1] Kim SS, Kee C-D. Int. J. Precis. Eng. Manuf. 2014, 15, 315–321.10.1007/s12541-014-0340-ySearch in Google Scholar
[2] Palmre V, Hubbard JJ, Fleming M, Pugal D, Kim S, Kim KJ, Leang KK. Smart Mater. Struct. 2013, 22, 014003.10.1088/0964-1726/22/1/014003Search in Google Scholar
[3] Pelrine R, Kornbluh R, Pei Q, Joseph J. Science 2000, 287, 836–839.10.1126/science.287.5454.836Search in Google Scholar PubMed
[4] Smela E. Adv. Mater. 2003, 15, 481–494.10.1002/adma.200390113Search in Google Scholar
[5] Nguyen CH, Alici G, Wallace GG. Sens. Actuators A 2012, 185, 82–91.10.1016/j.sna.2012.07.018Search in Google Scholar
[6] Lughmani WA, Jho JY, Lee JY, Rhee K. Int. J. Precis. Eng. Manuf. 2009, 10, 131–139.10.1007/s12541-009-0104-2Search in Google Scholar
[7] Baughman RH, Cui C, Zakhidov AA, Iqbal Z, Barisci JN, Spinks GM, Wallace GG, Mazzoldi A, De Rossi D, Rinzler AG, Jaschinski O, Roth S, Kertesz M. Science 1999, 284, 1340–1344.10.1126/science.284.5418.1340Search in Google Scholar PubMed
[8] Osada Y, Okuzaki H, Hori H. Nature 1992, 355, 242–244.10.1038/355242a0Search in Google Scholar
[9] Klemm D, Heublein B, Fink HP, Bohn A. Angew. Chem. Int. Ed. Engl. 2005, 44, 3358–3393.10.1002/anie.200460587Search in Google Scholar PubMed
[10] Swatloski RP, Spear SK, Holbrey JD, Rogers RD. J. Am. Chem. Soc. 2002, 124, 4974–4975.10.1021/ja025790mSearch in Google Scholar PubMed
[11] Cao Y, Wub J, Zhangb J, Lia H, Zhanga Y, He J. Chem. Eng. J. 2009, 147, 13–21.10.1016/j.cej.2008.11.011Search in Google Scholar
[12] Zhu S, Wu Y, Chen Q, Yu Z, Wang C, Jin S, Dinga Y, Wu G. Green Chem. 2006, 8, 325–327.10.1039/b601395cSearch in Google Scholar
[13] Heinze T, Liebert T. Prog. Polym. Sci. 2001, 26, 1689–1762.10.1016/S0079-6700(01)00022-3Search in Google Scholar
[14] Swatloski RP, Rogers RD, Holbrey JD. Dissolution and processing of cellulose using ionic liquids. 2004, Google Patents.Search in Google Scholar
[15] Zhang H, Wu J, Zhang J, He J. Macromolecules 2005, 38, 8272–8277.10.1021/ma0505676Search in Google Scholar
[16] Wu J, Zhang J, Zhang H, He J, Ren Q, Guo M. Biomacromolecules 2004, 5, 266–268.10.1021/bm034398dSearch in Google Scholar
[17] Rogers RD, Seddon KR. Science 2003, 302, 792–793.10.1126/science.1090313Search in Google Scholar
[18] Seddon KR. J. Chem. Technol. Biotechnol. 1997, 68, 351–356.10.1002/(SICI)1097-4660(199704)68:4<351::AID-JCTB613>3.0.CO;2-4Search in Google Scholar
[19] Dupont J, de Souza RF, Suarez PA. Chem. Rev. 2002, 102, 3667–3692.10.1021/cr010338rSearch in Google Scholar
[20] Welton T. Chem. Rev. 1999, 99, 2071–2084.10.1021/cr980032tSearch in Google Scholar
[21] Kosmulski M, Gustafsson J, Rosenholm JB. Thermochim. Acta 2004, 412, 47–53.10.1016/j.tca.2003.08.022Search in Google Scholar
[22] Wang H, Gurau G, Rogers RD. Chem. Soc. Rev. 2012, 41, 1519–1537.10.1039/c2cs15311dSearch in Google Scholar PubMed
[23] Xu A, Wang J, Wang H. Green Chem. 2010, 12, 268–275.10.1039/B916882FSearch in Google Scholar
[24] Vitz J, Erdmenger T, Haensch C, Schubert US. Green Chem. 2009, 11, 417–424.10.1039/b818061jSearch in Google Scholar
[25] Mäki-Arvela P, Anugwom I, Virtanen P, Sjöholm R, Mikkola JP. Ind. Crop. Prod. 2010, 32, 175–201.10.1016/j.indcrop.2010.04.005Search in Google Scholar
[26] Zhao D, Li H, Zhang J, Fu L, Liu M, Fu J, Ren P. Carbohyd. Polym. 2012, 87, 1490–1494.10.1016/j.carbpol.2011.09.045Search in Google Scholar
[27] Feng Y, Zhang X, Shen Y, Yoshino K, Feng W. Carbohyd. Polym. 2012, 87, 644–649.10.1016/j.carbpol.2011.08.039Search in Google Scholar PubMed
[28] Jung J.-H, Jeon J.-H, Sridhar S. Carbon 2011, 49, 1279–1289.10.1016/j.carbon.2010.11.047Search in Google Scholar
[29] Zhao Y, Song L, Zhang Z, Qu L. Energ. Environ. Sci. 2013, 6, 3520–3536.10.1039/c3ee42812eSearch in Google Scholar
[30] Li Q, Renneckar S. Biomacromolecules 2011, 12, 650–659.10.1021/bm101315ySearch in Google Scholar PubMed
[31] Khan A, Khan RA, Salmieri S, Tien CL, Riedl B, Bouchard J, Chauve G, Tan V, Kamal MR, Lacroix M. Carbohyd. Polym. 2012, 90, 1601–1608.10.1016/j.carbpol.2012.07.037Search in Google Scholar PubMed
[32] Nikonenko NA, Buslov DK, Sushko NI, Zhbankov RG. Biopolymers 2000, 57, 257–262.10.1002/1097-0282(2000)57:4<257::AID-BIP7>3.0.CO;2-3Search in Google Scholar
[33] Wang H, Roman M. Biomacromolecules 2011, 12, 1585–1593.10.1021/bm101584cSearch in Google Scholar
[34] Naboka O, Sanz-Velasco A, Lundgren P, Enoksson P, Gatenholm P. J. Colloid Interf. Sci. 2012, 367, 485–493.10.1016/j.jcis.2011.10.030Search in Google Scholar
[35] Nikonenko N, Buslov DK, Sushko NI, Zhbankov RG. J. Mol. Struct. 2005, 752, 20–24.10.1016/j.molstruc.2005.05.015Search in Google Scholar
[36] Kondo T. Cellulose 1997, 4, 281–292.10.1023/A:1018448109214Search in Google Scholar
[37] Trchová M, Šeděnková i, Morávková Z, Stejskal J. Poly. Degrad. Stabi. 2014, 109, 27–32.10.1016/j.polymdegradstab.2014.06.012Search in Google Scholar
[38] Abdu YA, Hull SK, Fayek M, Hawthorne FC. Am. Mineral. 2011, 96, 1433–1442.10.2138/am.2011.3658Search in Google Scholar
[39] Pisarski WA, Żur L, Pisarska J. Opt. lett. 2011, 36, 990–992.10.1364/OL.36.000990Search in Google Scholar
[40] Benyahia A, Merrouche A, Rahmouni ZEA, Rokbi M, Serge W, Kouadri Z. Mech. Ind. 2014, 15, 69–73.10.1051/meca/2013082Search in Google Scholar
[41] Huang LJ, Wang YX, Huang Z, Tang JG, Wang Y, Liu JX, Jiao JQ, Liu JQ, Laurence AB. J Power Sources 2014, 269, 716–722.10.1016/j.jpowsour.2014.07.043Search in Google Scholar
[42] Jin B, Chen G, Zhong X, Liu Y, Zhou K, Sun P, Lu P, Zhang W, Liang J. Ceram. Int. 2014, 40, 10359–10365.10.1016/j.ceramint.2014.03.009Search in Google Scholar
[43] Sullivan A, Ball R. Atmos. Environ. 2012, 47, 133–141.10.1016/j.atmosenv.2011.11.022Search in Google Scholar
[44] Huang F.-Y. Polymers 2012, 4, 1012–1024.10.3390/polym4021012Search in Google Scholar
[45] Nakashima K, Yamaguchi K, Taniguchi N, Arai S, Yamada R, Katahira S, Ishida N, Takahashi H, Ogino C, Kondo A. Green Chem. 2011, 13, 2948–2953.10.1039/c1gc15688hSearch in Google Scholar
[46] Castelaín M, Martínez G, Ellis G, Salavagione HJ. Chem. Commun. 2013, 49, 8967–8969.10.1039/c3cc43729aSearch in Google Scholar PubMed
[47] Wang X, Song L, Yang HY, Lu HD, Hu Y. Ind. Eng. Chem. Res. 2011, 50, 5376–5383.10.1021/ie102566ySearch in Google Scholar
[48] Huang G, Liang H, Wang Y, Wang X, Gao J, Fei Z. Mater. Chem. Phys. 2012, 132, 520–528.10.1016/j.matchemphys.2011.11.064Search in Google Scholar
[49] Dhakate SR, Mathur RB, Sharma S, Borah M, Dhami TL. Energ. Fuels 2009, 23, 934–941.10.1021/ef800744mSearch in Google Scholar
©2017 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Original articles
- Effects of multiwalled carbon nanotube mass fraction on microstructures and electrical resistivity of polycarbonate-based conductive composites
- Preparation and fluid drag reduction properties of superhydrophobic paper-based films comprising carbon nanotubes and fluoropolymers
- Studies on the structural, thermal, and dielectric properties of fabricated Nylon 6,9/CaCu3Ti4O12 nanocomposites
- Metal-matrix composite fabricated with gas tungsten arc melt injection and precoated with NiCrBSi alloy to increase the volume fraction of WC particles
- Construction of polypropylene composite multifilaments filled with sodium perborate trihydrate-treated jute microparticles
- Damage detection of three-dimensional braided composite materials using carbon nanotube thread
- An exact solution for improved metal-composite joints reinforced by FG inter-layers
- Effect of chemical treatment on thermal properties of bagasse fiber-reinforced epoxy composite
- The superior ductility of fine SiCp/Al2014 composites after extrusion
- Optical properties and photocatalytic activity of CdS-TiO2/graphite composite
- Effects of bottom ash and granulated blast furnace slag as fine aggregate on abrasion resistance of concrete
- Surface modification of diamond and its effect on the mechanical properties of diamond/epoxy composites
- Numerical simulation of strain rate effect on the inelastic behavior of metal matrix composites
- Electromechanical characterization of multilayer graphene-reinforced cellulose composite containing 1-ethyl-3-methylimidazolium diethylphosphonate ionic liquid
- The effect of hydroxyapatite reinforcement and preparation methods on the structure and mechanical properties of Mg-HA composites
- The effect of yttrium incorporation on the formation and properties of α-Al2O3 coatings
Articles in the same Issue
- Frontmatter
- Original articles
- Effects of multiwalled carbon nanotube mass fraction on microstructures and electrical resistivity of polycarbonate-based conductive composites
- Preparation and fluid drag reduction properties of superhydrophobic paper-based films comprising carbon nanotubes and fluoropolymers
- Studies on the structural, thermal, and dielectric properties of fabricated Nylon 6,9/CaCu3Ti4O12 nanocomposites
- Metal-matrix composite fabricated with gas tungsten arc melt injection and precoated with NiCrBSi alloy to increase the volume fraction of WC particles
- Construction of polypropylene composite multifilaments filled with sodium perborate trihydrate-treated jute microparticles
- Damage detection of three-dimensional braided composite materials using carbon nanotube thread
- An exact solution for improved metal-composite joints reinforced by FG inter-layers
- Effect of chemical treatment on thermal properties of bagasse fiber-reinforced epoxy composite
- The superior ductility of fine SiCp/Al2014 composites after extrusion
- Optical properties and photocatalytic activity of CdS-TiO2/graphite composite
- Effects of bottom ash and granulated blast furnace slag as fine aggregate on abrasion resistance of concrete
- Surface modification of diamond and its effect on the mechanical properties of diamond/epoxy composites
- Numerical simulation of strain rate effect on the inelastic behavior of metal matrix composites
- Electromechanical characterization of multilayer graphene-reinforced cellulose composite containing 1-ethyl-3-methylimidazolium diethylphosphonate ionic liquid
- The effect of hydroxyapatite reinforcement and preparation methods on the structure and mechanical properties of Mg-HA composites
- The effect of yttrium incorporation on the formation and properties of α-Al2O3 coatings

