Abstract
For the first time silica gel was observed to activate peroxides in oxidative coupling reactions. Here we report silica gel mediated oxidative C–O coupling of β-dicarbonyl compounds with cyclic diacyl peroxides affording α-acyloxy derivatives with 100% atom efficiency. The highest yields of coupling products were achieved in solvent free conditions. C–O coupling products were prepared in yields up to 86%.
Introduction
Cross-dehydrogenative coupling is an intensively developing field of organic synthesis. This type of chemical bond construction is highly atom-efficient owing to the absence of pre-functionalization of substrates with the help of leaving groups (–Hal, –OTf, –BR2, –SnR3, –SiR3, –ZnHal, –MgHal et al.) which are used in nucleophilic substitution of metal complex catalysis [1], [2], [3], [4]. Oxidative C–C coupling reactions were studied most thoroughly. Among other less common coupling types, such as C–N, C–P, and C–O, the latter is the least studied and the most difficult in realizing one [5], [6] since C-partner can be easily transformed into carbonyl compounds by the action of oxidant [7], [8], [9].
Previously we developed an efficient lanthanide-catalyzed oxidative C–O coupling in which one of the reagents, diacyl peroxide, acts both as an O-component for coupling and as the oxidizing agent for C-components – β-dicarbonyl compounds [10], [11]. The advantageous feature of the disclosed process is the unusual chemical behavior of the peroxide: the oxygen atom of the peroxide links the two partners together to afford the coupling product instead of oxygen-atom transfer.
Cyclic diacyl peroxides known since the 1950s [12], [13], [14], [15], [16], [17] have found application in organic synthesis only recently. Nowadays cyclic diacyl peroxides are used for the stereoselective dihydroxylation of alkenes [18], [19], [20], [21], [22], [23], [24], [25], [26], arene oxidation [27], [28], [29], [30], [31] and arynes generation [32].
The oxyfunctionalization of β-dicarbonyl compounds previously was limited to hydroxylation [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], peroxidation [54], [55], [56], [57], [58], coupling of N–O fragment [59], [60], [61] and phenols [62]. A few studies where substituted α-acyloxydicarbonyl products were synthesized by using hypervalent iodine compounds [63], [64], [65], Bu4NI/t-BuOOH [66], [67], manganese(III) acetate [68], [69], lead(IV) acetate [70], and iron(III) salts [71] were conducted. To achieve benzoyloxylation with the use of benzoyl peroxide, β-dicarbonyl substrates require preliminary activation through their transformation into enamines [72], [73], [74], [75], [76], copper complexes [77] or enolates [78], [79], [80], [81], [82].
Currently, significant challenge consists of avoiding the use of organic solvents in order to reduce environmental damage, and the cost of the final products [83], [84], [85]. Herein we report an effective application of silica gel as heterogeneous catalyst for oxidative C–O coupling of β-dicarbonyl compounds with diacyl peroxides in solvent free conditions (Scheme 1).
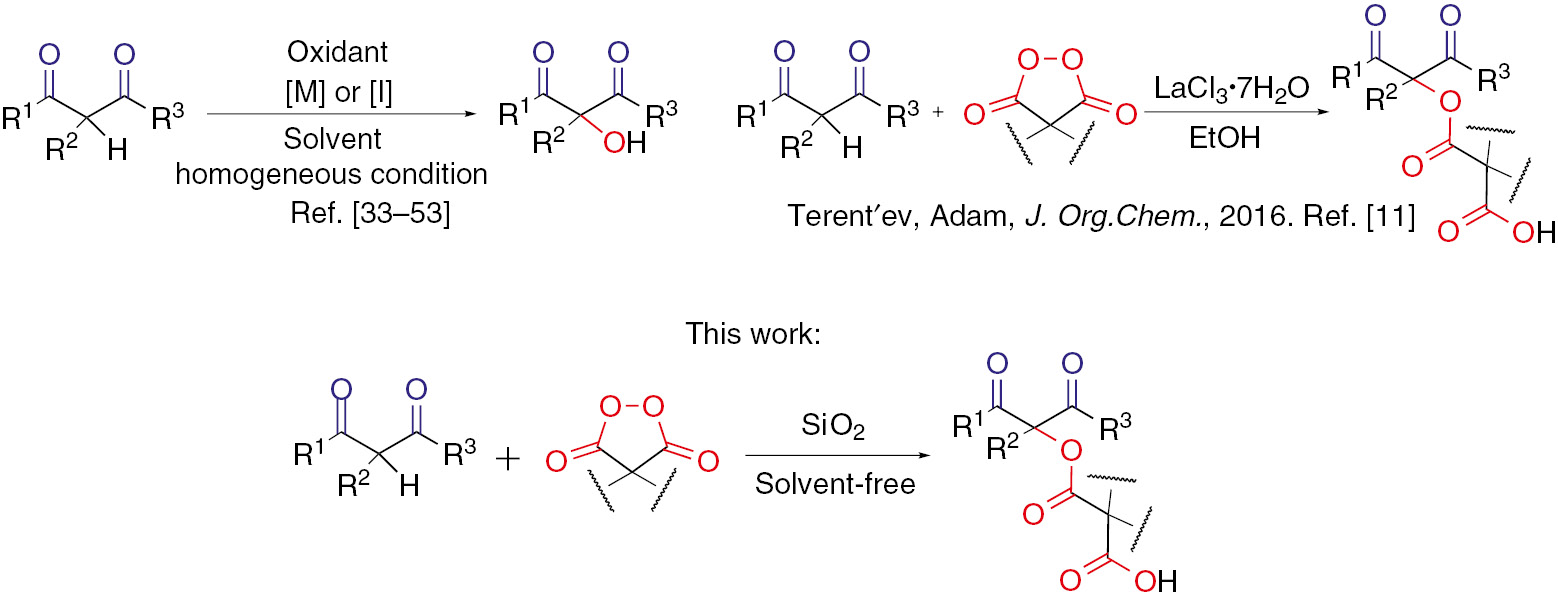
Oxyfunctionaliazation of 1,3-dicarbonyl compounds.
This investigation cover three important fields: heterogeneous catalysis with the use of silica gel, peroxides-involved oxidative coupling processes and selective functionalization of dicarbonyl compounds.
Silica gel and relative materials find enormous application in chemical, medical and many other industries [86], [87], [88]. Diversity of usages of silica gel is determined by its availability, possibility for properties control (porosity, particle size and shape), a highly selective adsorption capacity, feasibility of creation of developed porous structure and large specific surface area, the thermal stability and simple regeneration without dramatic changes in properties [89].
Silica gel is one of the most widespread catalyst support for various reagents [90], [91], [92], [93], and catalytic systems: metals [94], their oxides [95], salts (including Lewis acids) [96], [97], complexes [98], Bronsted acids [99], [100], [101], [102], heteropoly acids [103], covalently bonded with SiO2 organocatalysts [104], [105]. Silica gel is used as a catalyst for the oxidation of alcohols, dibenzothiophene and its 4,6-dimethyl derivative [106], [107], halogenation of ketones [108], nitration of arenes [109], nucleophilic substitution [110] and cleavage of cyclic products from Diels–Alder reaction of furans with methyl 3-bromopropiolate [111]. Condensation of aldehydes with activated organic halides or methyl acrylate [112], epoxides with nitrogen heterocycles [113], Diels–Alder reaction of furans with methyl 3-bromopropiolate [114], three-component synthesis of 2,3-disubstituted 4-thiazolidinones [115], cyclization of o-phenylenediamines with nitroolefins [116], and aromatic aldehydes [117], and also generation of episulfonium ions [118] were performed with the use of silica gel. Multi-component cyclizations [119], [120], [121], S→O acetyl migration to synthesize thiols [122], rearrangement of allylic acetates into their most stable regioisomers [123], and synthesis of 1,2- or 1,3-dithioethers via the reactions of allyl bromide with thiol [124] were carried out with SiO2 as a catalyst. In the majority of studies the catalytic activity of SiO2 is explained by the influence of acidic Si–OH groups, but complete understanding of the catalytic properties of silica gel and supported catalyst systems based on it, still represents an actual fundamental problem. Wide application, low cost, high stability and low toxicity of silica gel make it perspective material for the development of heterogeneous catalytic systems in accordance with current requirements of green chemistry.
Experimental section
Caution: Although we have encountered no difficulties in working with peroxides, precautions such as the use of safety shield, fume hood should be taken, the use of redox-active transition-metal salts, heating and vigorous shaking should be avoided!
General materials and methods
NMR spectra were recorded on a commercial instrument (300.13 MHz for 1Н, 75.48 MHz for 13С) in СDCl3. High resolution mass spectra (HRMS) were measured using electrospray ionization (ESI) [125]. The measurements were done in a positive ion mode (interface capillary voltage 4500 V); the mass ratio was from m/z 50 to 3000 Da; external/internal calibration was done with Electrospray Calibrant Solution. A syringe injection was used for solutions in MeCN (flow rate 3 μL/min). Nitrogen was applied as a dry gas; interface temperature was set at 180°C.
The TLC analysis was carried out on standard silica gel chromatography plates. The melting points were determined on a Kofler hot-stage apparatus. Chromatography was performed on silica gel (0.060–0.200 mm, 60 Å, CAS 7631-86-9, Acros).
2-Acetylcyclopentanone (1d), 2-acetylcyclohexanone (1e), ethyl 2-methylacetoacetate (1g), ethyl 2-oxocyclopentanecarboxylate (1k), ethyl 2-oxocyclohexanecarboxylate (1l), α-acetylbutyrolactone (1m), 2,2-diethyl malonic acid were purchased from commercial sources and was used as is. All solvents were distilled before use using standard procedures. Ethyl 4-acetyl-5-oxohexanoate (1a) [126], 3-benzylpentane-2,4-dione (1b) [127], 3-(4-chlorobenzyl)pentane-2,4-dione (1c) [128], 3-acetylheptane-2,6-dione (1f) [129], ethyl 2-acetylhexanoate (1h) [130], ethyl 2-acetyl-4-cyanobutanoate (1i) [131], ethyl 2-benzyl-3-oxobutanoate (1j) [132] and diethylmalonyl peroxide (2a) [20] were synthesized according to the literature.
The six types of commercial available silica gel were used. 60–200 μm: 60 Å, appearance (color) – white, appearance (form) – powder, pH – 6.5 to 7.5 (10% w/w suspension), specific surface area 470–530 m2/g, pore diameter 58–68 Å, pore volume 0.70–0.85 mL/g, volatile matter 3–6% (160°C), particle size 5.0% max. (under size), 5.0% max. (over size). 40–60 μm: 60 Å, appearance (color) – white, appearance (form) – powder, pH 6.5–7 (10% w/w suspension), specific surface area 460–510 m2/g, pore volume 0.75–0.8 mL/g, volatile matter ≤6.0% (160°C), particle size 10.0% max. (under size), 10.0% max. (over size) was purchased from Acros. 40–63 μm: type A: 60 Å, appearance (color) – white, appearance (form) – powder, pH 6.5–7.5 (10% w/w suspension), specific surface area 480–540 m2/g, pore volume 0.74–0.84 mL/g, volatile matter ≤9.0% (150°C), particle size 30–40 μm (d10), 55–65 μm (d50), 95–110 μm (d90) was purchased from Merck. 40–63 μm: type B: 60 Å, appearance (color) – white, appearance (form) – powder, specific surface area approx. 500 m2/g, pore volume approx. 0.75 mL/g, particle size 230–400 μm was purchased from Macherey-Nagel. 5–40 μm: 60 Å, appearance (color) – white, appearance (form) – powder. Silica fumed S 5505, 14 nm, Sigma-Aldrich. Titanium(IV) oxide (anatase, nanopowder, <25 nm particle size) was purchased from Sigma. Aluminium oxide (neutral, 50–200 μm 60 Å), aluminium oxide (weakly acidic 50–200 μm 60 Å), aluminium oxide (basic, 50–200 μm 60 Å). ZSM-5 (SiO2/Al2O3 mole ratio 80; surface area 425 m2/g; Na2O weight 0.05%).
Experimental for Table 1: SiO2-mediated oxidative coupling of ethyl 2-benzyl-3-oxobutanoate 1j with diethylmalonyl peroxide 2a
Ethyl 2-benzyl-3-oxobutanoate 1j (100.0 mg, 0.45 mmol) was added to SiO2 (27.2–81.7 mg, 0.45–1.36 mmol, 1–3 mol SiO2/1 mol 1j; excluding entries 1, 10) (particles size: 60–200 μm, entries 2–4, 10, 11, 13–19; 40–60 μm, entry 5; 40–63 μm, entries 6, 7; 5–40 μm, entry 8; nano, entry 9). After that diethylmalonyl peroxide 2a (107.6 mg, 0.68 mmol, 1.5 mol 2a/1 mol 1j) was added dropwise under stirring for 5 min. The mixture was stirred for 2–24 h at 20–190°С. [a] In entries 16, 17 solution of 1j and 2a in 2 mL of СH2Cl2 was added to SiO2. [b] In entry 18 solution of 1j and 2a in 3 mL of toluene was added to SiO2. [c] In entry 19 solution of 1j and 2a in 3 mL of MeOH was added to SiO2 (3 mL). The reaction mixture was suspended in CH2Cl2 (10 mL), catalyst was filtered, washed with CH2Cl2 (3×3 mL). Combined CH2Cl2 was concentrated under vacuum of water jet pump. Target coupling product was isolated using column chromatography of organic residue on SiO2 using petroleum ether (40–70)/ethyl acetate eluent with gradient volume ratio from 20/1 to 2/1.
Experimental for Table 2: Oxidative coupling of ethyl 2-benzyl-3-oxobutanoate 1j with diethylmalonyl peroxide 2a with the use of TiO2, Al2O3, and ZSM-5
Ethyl 2-benzyl-3-oxobutanoate 1j (100.0 mg, 0.45 mmol) was added to TiO2 (anatase) (72.5 mg, 0.91 mmol, 2 mol TiO2/1 mol 1j) or Al2O3 (neutral) (92.6 mg, 0.91 mmol, 2 mol Al2O3/1 mol 1j) or ZSM-5 (SiO2/Al2O3=80) (100.0 mg). After that diethylmalonyl peroxide 2a (107.6 mg, 0.68 mmol, 1.5 mol 2a/1 mol 1j) was added dropwise under stirring for 5 min. The mixture was stirred for 24 h at 20–25°С. [a] In entry 3 weakly acidic Al2O3 (50–200 μm) (92.6 mg, 0.91 mmol, 2 mol Al2O3/1 mol 1j) was used. [b] In entry 4 basic Al2O3 (50–200 μm) (92.6 mg, 0.91 mmol, 2 mol Al2O3/1 mol 1j) was used. The reaction mixture was suspended in CH2Cl2 (10 mL), catalyst was filtered, washed with CH2Cl2 (3×3 mL). Combined CH2Cl2 was concentrated under vacuum of water jet pump. Target coupling product was isolated using column chromatography of organic residue on SiO2 using petroleum ether (40–70)/ethyl acetate eluent with gradient volume ratio from 20/1 to 2/1.
Experimental for Table 3: The C–O coupling products 3aa–ma, 3jb, 3jc and 3jd synthesized from β-diketones 1a–e, β,δ-triketone 1f, β-oxoesters 1g–l and lactone 1m in reactions with malonyl peroxides 2a–d
SiO2 (60–200 μm) (267.0–475.6 mg, 4.45–7.93 mmol, 2 mol SiO2/1 mol 1a–m) was added into 5 mL flask. After that β-diketone 1a–f (500.0 mg, 2.23–3.96 mmol), β-oxoester 1g–l (500.0 mg, 2.27–3.47 mmol) or lactone 1m (500.0 mg, 3.90 mmol) was added. Malonyl peroxide 2a–d (527.4–939.4 mg, 3.34–5.95 mmol, 1.5 mol 2a–d/1 mol 1a–m) was added dropwise under stirring for 5 min. The mixture was stirred for 24 h at 20–25°С. [a] Diethylmalonyl peroxide 2a was added dropwise under stirring for 30 min at 0°С in the case of 3da, 3ea, 3ka, 3la, and then the mixture was stirred for 24 h at 20–25°С. [b] In the case of 3ja silica gel (60–200 μm) was reused by means of filtration after each experiment. [c] In the case of 3jb the mixture was stirred for 2 h at 90°С. [d] In the case of 3jc the mixture was stirred for 24 h at 60°С. [e] In the case of 3jd the mixture was stirred for 24 h at 40°С. The reaction mixture was suspended in CH2Cl2 (10 mL), catalyst was filtered, washed with CH2Cl2 (3×3 mL). Combined CH2Cl2 was concentrated under vacuum of water jet pump. Target coupling products were isolated using column chromatography of organic residue on SiO2 using petroleum ether (40–70)/ethyl acetate eluent with gradient volume ratio from 20/1 to 2/1.
2-((1,1-diacetyl-4-ethoxy-4-oxobutoxy)carbonyl)-2-ethylbutanoic acid (3aa)
Colorless oil. Rf=0.37 (PE/EtOAc 5:1+2% AcOH); 1Н NMR (300.13 MHz, CDCl3, 25°С): δ=0.94 (t, J=7.3 Hz, 6H), 1.23 (t, J=7.3 Hz, 3H), 2.03 (q, J=7.3 Hz, 4H), 2.20–2.29 (m, 8H), 2.63 (t, J=8.1 Hz, 2H), 4.10 (q, J=7.3 Hz, 2H), 7.55 ppm (broad s., 1H); 13C NMR (75.48 MHz, CDCl3, 25°С): δ=8.1, 13.9, 24.9, 26.4, 27.9, 28.2, 58.4, 60.8, 93.3, 169.9, 172.0, 175.9, 201.0 ppm.
2-((3-benzyl-2,4-dioxopentan-3-yloxy)carbonyl)-2-ethylbutanoic acid (3ba)
White solid, M.p. 83–86°С; Rf=0.63 (PE/EtOAc 5:1+2% AcOH); 1Н NMR (300.13 MHz, CDCl3, 25°С): δ=0.87 (t, 6H, J=7.3 Hz), 1.96 (q, 4H, J=7.3 Hz), 2.13 (s, 6H), 3.56 (s, 2H), 7.05–7.06 (m, 2H), 7.21–7.25 (m, 3H), 10.07 ppm (broad s., 1H); 13C NMR (75.48 MHz, CDCl3, 25°С): δ=8.2, 25.2, 27.3, 39.8, 58.6, 94.5, 127.4, 128.4, 130.0, 133.4, 170.6, 176.5, 201.8 ppm.
2-((1-acetyl-1-(4-chlorobenzyl)-2-oxopropoxy)carbonyl)-2-ethylbutanoic acid (3сa)
White solid. M.p. 113–115°С; Rf=0.57 (PE/EtOAc 10:1+2% AcOH); 1Н NMR (300.13 MHz, CDCl3, 25°С): δ=0.87 (t, J=7.3 Hz, 6H), 1.97 (q, J=7.3 Hz, 4H), 2.14 (s, 6H), 3.55 (s, 2H), 7.00 (d, J=8.2 Hz, 2H), 7.20 (d, J=8.2 Hz, 2H), 8.96 ppm (broad s., 1H); 13C NMR (75.48 MHz, CDCl3, 25°С): δ=8.5, 25.1, 27.3, 38.9, 59.4, 94.0, 128.7, 131.5, 132.1, 133.5, 172.0, 175.2, 202.2 ppm.
2-((1-acetyl-2-oxocyclopentyloxy)carbonyl)-2-ethylbutanoic acid (3da)
Colorless oil. Rf=0.48 (PE/EtOAc 5:1+2% AcOH); 1Н NMR (300.13 MHz, CDCl3, 25°С): δ=0.86–0.93 (m, 6H), 1.92–2.18 (m, 7H), 2.25–2.40 (m, 4H), 2.51–2.68 (m, 2H), 9.87 ppm (broad s., 1H); 13CNMR (75.48 MHz, CDCl3, 25°С): δ=8.2, 8.3, 18.4, 25.4, 26.1, 31.1, 36.0, 58.2, 90.7, 170.2, 176.2, 202.7, 208.7 ppm.
2-((1-acetyl-2-oxocyclohexyloxy)carbonyl)-2-ethylbutanoic acid (3ea)
Colorless oil. Rf=0.43 (PE/EtOAc 5:1+2% AcOH); 1Н NMR (300.13 MHz, CDCl3, 25°С): δ=0.95 (t, J=7.3 Hz, 6H), 1.79–1.83 (m, 3H), 1.95–2.07 (m, 5H), 2.15–2.32 (m, 5H), 2.64–2.67 (m, 2H), 10.12 ppm (broad s., 1H); 13C NMR (75.48 MHz, CDCl3, 25°С): δ=8.3, 8.4, 21.3, 25.5, 25.7, 26.0, 34.2, 40.8, 58.6, 90.7, 170.5, 176.1, 202.2, 202.3 ppm.
2-((3-acetyl-2,6-dioxoheptan-3-yloxy)carbonyl)-2-ethylbutanoic acid (3fa)
White solid. M.p. 76–79°С; Rf=0.21 (PE/EtOAc 10:1+2% AcOH); 1Н NMR (300.13 MHz, CDCl3, 25°С): δ=0.93 (t, J=7.3 Hz, 6H), 2.01 (q, J=7.3 Hz, 4H), 2.09 (s, 3H), 2.24 (s, 6H), 2.38 (t, J=7.3 Hz, 2H), 2.52 (t, J=7.3 Hz, 2H), 10.13 ppm (broad s., 1H);13C NMR (75.48 MHz, CDCl3, 25°С): δ=8.2, 25.1, 26.5, 26.6, 29.7, 37.1, 58.5, 93.4, 170.0, 176.1, 201.2, 206.7 ppm.
2-((1-(ethoxycarbonyl)-1-methyl-2-oxopropoxy)carbonyl)-2-ethylbutanoic acid (3ga)
Colorless oil. Rf=0.45 (PE/EtOAc 5:1+2% AcOH); 1Н NMR (300.13 MHz, CDCl3, 25°С): δ=0.88 (t, J=7.3 Hz, 6H), 1.25 (t, J=7.3 Hz, 3H), 1.70 (s, 3H), 1.96 (q, J=7.3 Hz, 4H), 2.31 (s, 3H), 4.21 (q, J=7.3 Hz, 2H), 6.92 ppm (broad s., 1H); 13C NMR (75.48 MHz, CDCl3, 25°С): δ=8.2, 8.3, 13.7, 19.1, 25.5, 25.6, 58.6, 62.4, 86.1, 166.9, 170.2, 176.1, 201.2 ppm.
2-((3-(ethoxycarbonyl)-2-oxoheptan-3-yloxy)carbonyl)-2-ethylbutanoic acid (3ha)
Colorless oil. Rf=0.47 (PE/EtOAc 10:1+2% AcOH); 1Н NMR (300.13 MHz, CDCl3, 25°С): δ=0.83–0.92 (m, 9H), 1.21–1.29 (m, 7H), 1.98–2.00 (m, 4H), 2.12–2.17 (m, 2H), 2.32 (s, 3H), 4.20 (q, J=7.3 Hz, 2H), 8.67 ppm (broad s., 1H); 13C NMR (75.48 MHz, CDCl3, 25°С): δ=8.4, 13.7, 13.8, 22.5, 25.2, 26.1, 27.1, 33.5, 58.7, 62.3, 88.9, 166.8, 171.0, 175.9, 200.8 ppm.
2-((1-cyano-3-(ethoxycarbonyl)-4-oxopentan-3-yloxy)carbonyl)-2-ethylbutanoic acid (3ia)
Colorless oil. Rf=0.31 (PE/EtOAc 5:1+2% AcOH); 1Н NMR (300.13 MHz, CDCl3, 25°С): δ=0.88–0.97 (m, 6H), 1.24 (t, J= 7.3 Hz, 3H), 1.94–2.09 (m, 4H), 2.28–2.42 (m, 5H), 2.53–2.75 (m, 2H), 4.18–4.26 (m, 2H), 9.77 ppm (broad s., 1H); 13C NMR (75.48 MHz, CDCl3, 25°С): δ=8.1, 8.2, 11.7, 13.7, 25.2, 26.7, 28.3, 58.6, 63.0, 86.9, 118.2, 165.3, 169.8, 176.0, 200.0 ppm.
2-((1-benzyl-1-(ethoxycarbonyl)-2-oxopropoxy)carbonyl)-2-ethylbutanoic acid (3ja)
White solid. M.p. 89–93°С; Rf=0.40 (PE/EtOAc 5:1+2% AcOH); 1Н NMR (300.13 MHz, CDCl3, 25°С): δ=0.83–0.91 (m, 6H), 1.16 (t, J=7.3 Hz, 3H), 1.94–2.02 (m, 4H), 2.24 (s, 3H), 3.43 (d, J=14.7 Hz, 1H, CH2), 3.52 (d, J=14.7 Hz, 1H, CH2), 4.15 (q, J=7.3 Hz, 2H), 7.10–7.13 (m, 2H), 7.23–7.26 (m, 3H), 9.33 ppm (broad s., 1H); 13C NMR (75.48 MHz, CDCl3, 25°С): δ=8.4, 13.7, 25.4, 25.6, 27.7, 40.0, 59.0, 62.3, 88.2, 127.4, 128.3, 130.2, 133.5, 166.7, 171.8, 175.0, 201.6 ppm.
1-((1-benzyl-1-(ethoxycarbonyl)-2-oxopropoxy)carbonyl)cyclopropanecarboxylic acid (3jb)
Colorless oil. Rf=0.20 (PE/EtOAc 2:1+2% AcOH); 1Н NMR (300.13 MHz, CDCl3, 25°С): δ=1.18 (t, J=7.3 Hz, 3H), 1.49–1.56 (m, 1H), 1.77–1.89 (m, 3H), 2.26 (s, 3H), 3.44 (s, 2H), 4.18 (q, J=7.3 Hz, 2H), 6.99–7.02 (m, 2H), 7.25–7.27 ppm (m, 3H); 13C NMR (75.48 MHz, CDCl3, 25°С): δ=13.8, 22.5, 25.4, 27.3, 39.7, 62.8, 89.2, 127.9, 128.6, 129.7, 132.9, 165.8, 169.8, 174.4, 199.4 ppm.
1-((1-benzyl-1-(ethoxycarbonyl)-2-oxopropoxy)carbonyl)cyclobutanecarboxylic acid (3jc)
Colorless oil. Rf=0.31 (PE/EtOAc 5:1+2% AcOH); 1Н NMR (300.13 MHz, CDCl3, 25°С): δ=1.18 (t, J=7.3 Hz, 3H), 1.94–2.05 (m, 3H), 2.17 (s, 3H), 2.52–2.62 (m, 4H), 3.44 (s, 2H), 4.18 (q, J=7.3 Hz, 2H), 6.99–7.02 (m, 2H), 7.25–7.27 ppm (m, 3H); 13C NMR (75.48 MHz, CDCl3, 25°С): δ=13.8, 22.5, 25.4, 27.3, 39.7, 62.8, 89.2, 127.9, 128.6, 129.7, 132.9, 165.8, 169.8, 174.4, 199.4 ppm; HRMS (ESI) m/z [M+Na]+: Calcd for [C19H22NaO7]+: 385.1258. Found: 385.1244; Elemental analysis: Calcd (%) for C19H22O7 (362.37): С 62.97, Н 6.12; found: С 62.58, Н 6.39.
1-((1-benzyl-1-(ethoxycarbonyl)-2-oxopropoxy)carbonyl)cyclopentanecarboxylic acid (3jd)
White solid. M.p. 68–69 °C; Rf=0.51 (PE/EtOAc 2:1+2% AcOH); 1Н NMR (300.13 MHz, CDCl3, 25°С): δ= 1.17 (t, 3H, J=7.3 Hz), 1.69 (m, 4H), 2.15–2.27 (m, 7H), 3.47 (d, 1H, J=13.9 Hz, CH2), 3.54 (d, 1H, J=13.9 Hz, CH2), 4.11–4.18 (m, 2H), 7.07–7.10 (m, 2H), 7.21–7.25 (m, 3H), 10.44 ppm (broad s., 1H); 13C NMR (75.48 MHz, CDCl3, 25°С): 13.7, 25.5, 27.4, 34.4, 39.5, 60.3, 62.3, 88.8, 127.4, 128.3, 130.2, 133.6, 166.2, 170.6, 177.6, 201.5 ppm.
2-(((1-(ethoxycarbonyl)-2-oxocyclopentyl)oxy)carbonyl)-2-ethylbutanoic acid (3ka)
Colorless oil. Rf=0.57 (PE/EtOAc 5:1+2% AcOH); 1Н NMR (300.13 MHz, CDCl3, 25°С): δ=0.91 (t, J=7.3 Hz, 6H), 1.26 (t, J=7.3 Hz, 3H), 1.93–2.15 (m, 6H), 2.22–2.29 (m, 1H), 2.48–2.56 (m, 2H), 2.74–2.80 (m, 1H), 4.22 (q, J=7.3 Hz, 2H), 8.16 ppm (broad s., 1H); 13C NMR (75.48 MHz, CDCl3, 25°С): δ=8.4, 13.9, 18.4, 26.1, 33.0, 35.8, 58.5, 62.3, 84.4, 166.6, 170.9, 176.1,207.2 ppm.
2-((1-(ethoxycarbonyl)-2-oxocyclohexyloxy)carbonyl)-2-ethylbutanoic acid (3la)
Colorless oil. Rf=0.80 (PE/EtOAc 5:1+2% AcOH); 1Н NMR (300.13 MHz, CDCl3, 25°С): δ=0.87 (t, J=7.3 Hz, 6H), 1.20 (t, J=7.3 Hz, 3H), 1.65–1.98 (m, 8H), 2.11–2.16 (m, 1H), 2.31–2.37 (m, 1H), 2.54–2.62 (m, 1H), 2.71–2.80 (m, 1H), 4.18 (q, J=7.3 Hz, 2H), 8.85 ppm (broad s., 1H); 13C NMR (75.48 MHz, CDCl3, 25°С): δ=8.2, 13.7, 20.5, 25.6, 27.1, 35.8, 39.9, 58.5, 62.0, 85.9, 167.2, 170.5, 175.6, 201.3 ppm.
2-((3-acetyl-2-oxotetrahydrofuran-3-yloxy)carbonyl)-2-ethylbutanoic acid (3ma)
Colorless oil. Rf=0.22 (PE/EtOAc 10:1+2% AcOH); 1Н NMR (300.13 MHz, CDCl3, 25°С): δ=0.86–0.93 (m, 6H), 1.93–2.04 (m, 4H), 2.33–2.46 (m, 4H), 2.96–3.04 (m, 1H), 4.34–4.54 (m, 2H), 10.34 ppm (broad s., 1H); 13C NMR (75.48 MHz, CDCl3, 25°С): δ=8.1, 8.2, 25.1, 25.3, 30.0, 58.4, 66.3, 85.0, 169.3, 170.1, 176.2, 200.4 ppm.
Results and discussion
SiO2-mediated oxidative C–O coupling of dicarbonyl compounds 1a–m: α-substituted β-diketones 1a–e, β,δ-triketone 1f, β-oxoesters 1g–l and lactone 1m with malonyl peroxides 2a–d was carried out in solvent free heterogeneous conditions with preparation of 3aa–ma, 3jb, 3jc, 3jd as major products and 4aa–ma, 4jb, 4jc, 4jd as by products (Scheme 2).
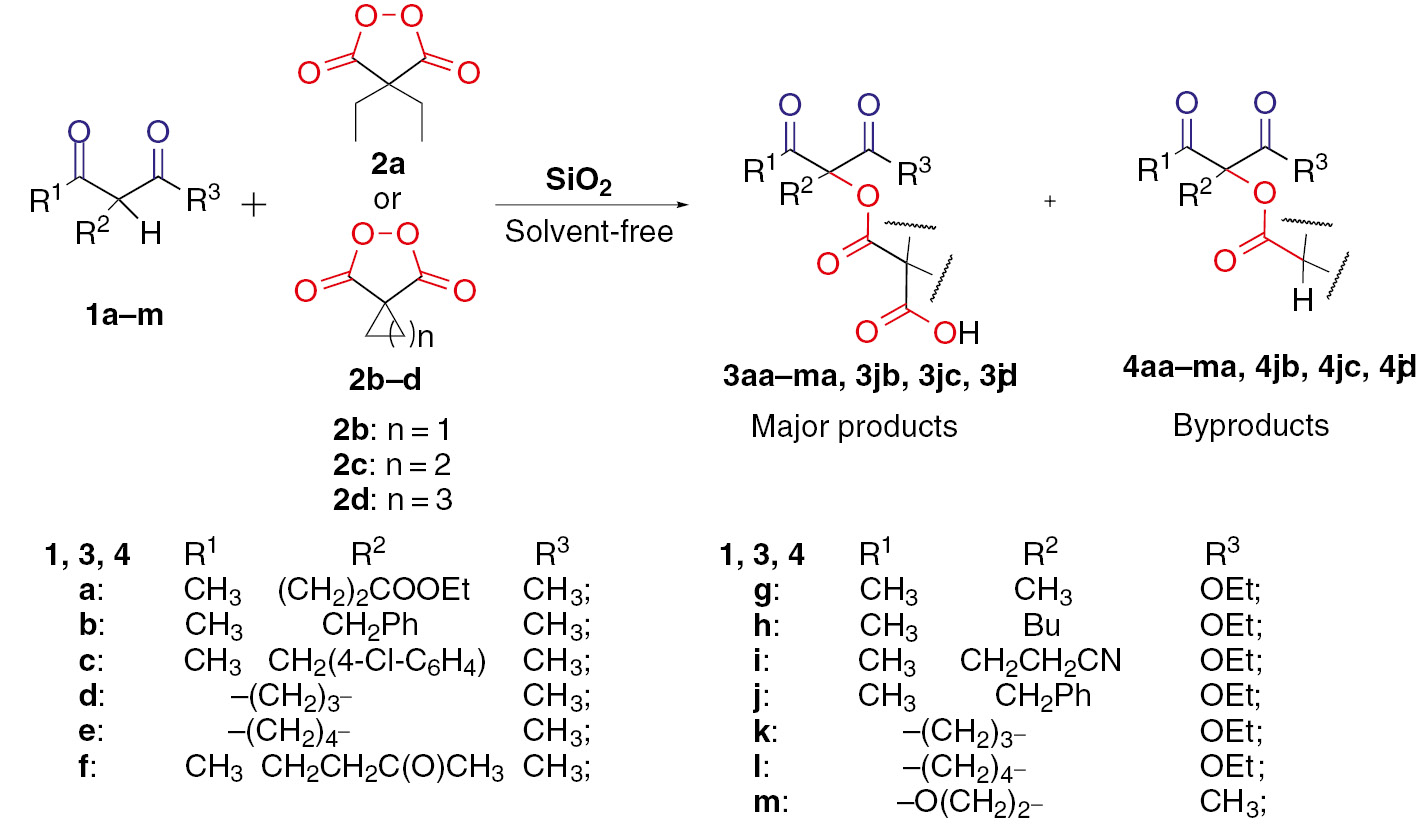
SiO2-mediated oxidative C–O coupling of dicarbonyl compounds 1a–m with malonyl peroxides 2a–d with subsequent decarboxylation.
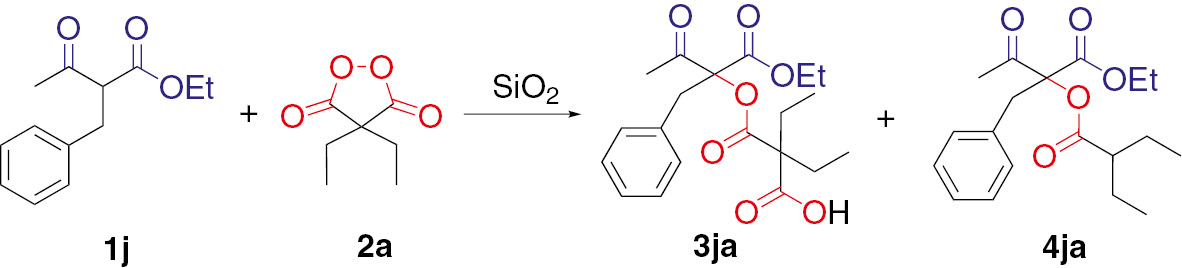
The oxidative C–O coupling of ethyl 2-benzyl-3-oxobutanoate 1j with diethylmalonyl peroxide 2a was chosen as a model reaction for study of catalytic activity of silica gel. The effect of SiO2 type, its amount, reaction time and temperature on the yield of products 3ja and 4ja was explored (Table 1).
SiO2-mediated oxidative coupling of ethyl 2-benzyl-3-oxobutanoate 1j with diethylmalonyl peroxide 2a.
| Entry | Type of SiO2 (particle size, μm) (mol SiO2/1 mol 1j) | t,°С | τ, h | Conversion of 1j, % | Yield of 3ja, % | Yield of 4ja, % |
|---|---|---|---|---|---|---|
| 1 | Without | r.t. | 24 | <10% | Trace | – |
| 2 | SiO2 (60–200) (3) | r.t. | 24 | 81 | 60 | Trace |
| 3 | SiO2 (60–200) (2) | r.t. | 24 | 89 | 83 | Trace |
| 4 | SiO2 (60–200) (1) | r.t. | 24 | 54 | 43 | Trace |
| 5 | SiO2 (40–60) (3) | r.t. | 24 | 85 | 66 | Trace |
| 6 | SiO2 (40–63, A) (2) | r.t. | 24 | 84 | 71 | Trace |
| 7 | SiO2 (40–63, B) (2) | r.t. | 24 | 75 | 62 | Trace |
| 8 | SiO2 (5–40) (2) | r.t. | 24 | 69 | 38 | Trace |
| 9 | SiO2 (nano) (2) | r.t. | 12 | 60 | 47 | Trace |
| 10 | SiO2 (60–200) (2) | 40 | 3 | 67 | 43 | Trace |
| 11 | SiO2 (60–200) (2) | 40 | 24 | 92 | 74 | Trace |
| 12 | Without | 40 | 24 | 49 | 37 | – |
| 13 | SiO2 (60–200) (2) | 70 | 2 | <95 | 68 | 19 |
| 14 | SiO2 (60–200) (2) | 70 | 9 | <95 | 66 | 24 |
| 15 | SiO2 (60–200) (2) | 100 | 2 | <95 | 58 | 29 |
| 16a | SiO2 (60–200) (3) | r.t. | 24 | 41 | 31 | Trace |
| 17a | SiO2 (60–200) (3) | 40 | 24 | 84 | 52 | Trace |
| 18b | SiO2 (60–200) (3) | 40 | 24 | 83 | 61 | Trace |
| 19c | SiO2 (60–200) (3) | 40 | 24 | 87 | 41 | Trace |
-
General procedure: Ethyl 2-benzyl-3-oxobutanoate 1j (100.0 mg, 0.45 mmol) was added to SiO2 (27.2–81.7 mg, 0.45–1.36 mmol, 1–3 mol SiO2/1 mol 1j) (particles size: 60–200 μm; 40–60 μm; 40–63 μm; 5–40 μm). After that diethylmalonyl peroxide 2a (107.6 mg, 0.68 mmol, 1.5 mol 2a/1 mol 1j) was added dropwise under stirring for 5 min. The mixture was stirred for 2–24 h at 20–190°С. a1j and 2a were added to SiO2 in СH2Cl2 (2 mL). b1j and 2a were added to SiO2 in toluene (3 mL). c1j and 2a were added to SiO2 in MeOH (3 mL).
In the absence of SiO2 at room temperature, the reaction did not occur (Table 1, entry 1). In contrast, excellent catalytic activity was achieved with silica gels, affording the coupling product 3ja in 38–83% yields (entries 2–9). The optimal conditions for C–O coupling were found (entry 3) by variation of SiO2 type, its amount and reaction time (entries 2–9). Molar ration of 2 mol of SiO2 (60–200 μm) per mol of 1j is optimal for effective catalysis of C–O coupling reaction and permits easy mixing of the reaction components in solvent-free conditions affording target product 3ja in 83% yield (entry 3). Applying of silica gel with smaller particles size (entries 5–9) reduces the yield of C–O coupling product 3ja to 38–71%. The temperature has a crucial influence on yields and ratio of products 3ja and 4ja: when the reaction mixture is heated from 20–25°С to 40°С (entries 10–11) the yield of 3ja slightly decreases. Without silica gel at 40°С the coupling reaction proceeds with low yield (entry 12). Conducting the reaction at 70°C allows to achieve full conversion of ethyl 2-benzyl-3-oxobutanoate 1j (entries 13, 14), however in this case together with desired C–O coupling product 3ja the byproduct 4ja is prepared in the result of 3ja decarboxylation. Further increasing of the temperature up to 100°С (entry 15) causes the significant conversion of 3ja into corresponding decarboxylation product 4ja with 29% yield. When solvents are used [methylene chloride (entries 16, 17), toluene (entry 18), MeOH (entry 19)] the yield of 3ja does not exceed 61%.
Unlike SiO2, other related sorbents and supports – TiO2, Al2O3 and ZSM-5 catalyze oxidative C–O coupling far worse. It is demonstrated by the reaction of ethyl 2-benzyl-3-oxobutanoate 1j with diethylmalonyl peroxide 2a (Table 2). In all entries, low conversion of 1j is observed, the product 3ja is formed only with yields 9–32%, decarboxylation product 4ja is absent.
Oxidative coupling of ethyl 2-benzyl-3-oxobutanoate 1j with diethylmalonyl peroxide 2a with the use of TiO2, Al2O3, and ZSM-5.
| Entry | Catalyst (mol per mol of 1j) | Conversion of 1j, % | Yield 3ja, % |
|---|---|---|---|
| 1 | TiO2 (2) | 41 | 12 |
| 2 | Al2O3 (2) | 59 | 17 |
| 3a | Al2O3 (2) | 60 | 32 |
| 4b | Al2O3 (2) | 45 | 31 |
| 5 | ZSM-5 | 34 | 9 |
-
General procedure: Ethyl 2-benzyl-3-oxobutanoate 1j (100.0 mg, 0.45 mmol) was added to TiO2 (anatase) (72.5 mg, 0.91 mmol, 2 mol TiO2/1 mol 1j) or Al2O3 (neutral) (92.6 mg, 0.91 mmol, 2 mol Al2O3/1 mol 1j) or ZSM-5 (SiO2/Al2O3=80) (100.0 mg). After that diethylmalonyl peroxide 2a (107.6 mg, 0.68 mmol, 1.5 mol 2a/1 mol 1j) was added dropwise under stirring for 5 min. The mixture was stirred for 24 h at 20–25°С. aAl2O3 weakly acidic (50–200 μm) (92.6 mg, 0.91 mmol, 2 mol Al2O3/1 mol 1j) was used. bAl2O3 basic (50–200 μm) (92.6 mg, 0.91 mmol, 2 mol Al2O3/1 mol 1j) was used.
The investigation of different types of silica gel used in this study by field emission scanning electron microscopy showed that each type of silica gel mainly contained particles of fixed size. Silica gel (60–200 μm) contains particles with size about 100 μm (Figs. 1 and 2). The morphology of the silica gel surface is irregular.
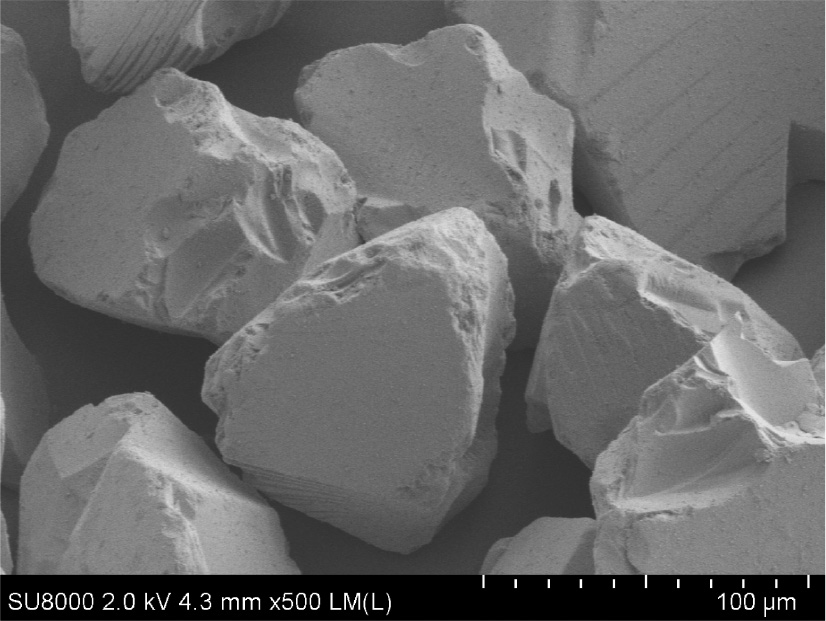
SEM images of silica gel 60–200 μm.

SEM images of silica gel 60–200 μm.
The energy dispersive X-ray microanalysis (EDX) showed that tested silica gel samples did not contain impurities of heavy metals (Fig. 3).

Energy-dispersive X-ray (EDX) spectra of silica gel 60–200 μm.
β-Dicarbonyl compounds with various nucleophilic reactivity were investigated in the reaction with malonyl peroxides 2a–d, in order to test the scope of substrate structure in silica gel mediated oxidative C–O coupling. The results for β-diketones 1a–e, β,δ-triketone 1f, β-oxoesters 1g–l and lactone 1m are summarized in Table 3. The coupling reactions were carried out under the optimized conditions presented in Table 1, entry 3.
The C–O coupling products 3aa–ma, 3jb, 3jc and 3jd synthesized from β-diketones 1a–e, β,δ-triketone 1f, β-oxoesters 1g–l and lactone 1m in reactions with malonyl peroxides 2a–d.
| Structure and yield of C–O coupling products, % |
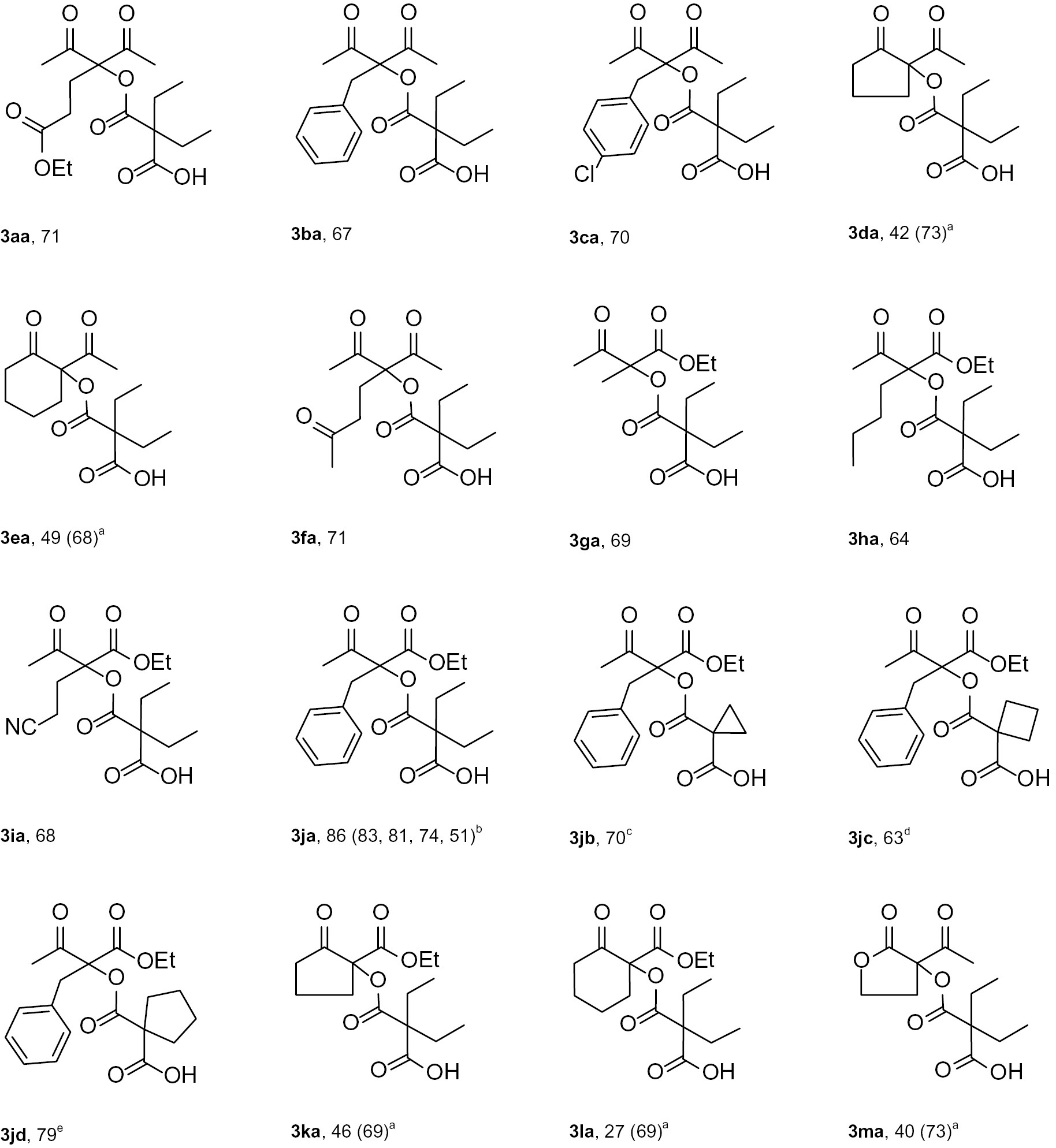 |
-
General procedure: SiO2 (60–200 μm) (267.0–475.6 mg, 4.45–7.93 mmol, 2 mol SiO2/1 mol 1a–m) was added into 5 mL flask. After that β-diketone 1a–f (500.0 mg, 2.23–3.96 mmol), β-oxoester 1g–l (500.0 mg, 2.27–3.47 mmol) or lactone 1m (500.0 mg, 3.90 mmol) was added. Malonyl peroxide 2a–d (527.4–939.4 mg, 3.34–5.95 mmol, 1.5 mol 2a–d/1 mol 1a–m) was added dropwise under stirring for 5 min. The mixture was stirred for 24 h at 20–25°С. aDiethylmalonyl peroxide 2a was added dropwise under stirring for 30 min at 0°С. The mixture was stirred for 24 h at 20–25°С. bIn the case of 3ja silica gel (60–200 μm) was reused by means of filtration after each experiment. cThe mixture was stirred for 2 h at 90°С. dThe mixture was stirred for 24 h at 60°С. eThe mixture was stirred for 24 h at 40°С.
The coupling products 3aa–ca, 3fa–ja from diketones 1a–c, 1f and even from the less reactive oxoesters 1g–j were obtained in good yields (64–71%) demonstrating the usefulness of silica gel (Table 3). An important advantage of the developed coupling method is the possibility of employing various malonyl peroxides 2a–d: corresponding C–O coupling products 3ja, 3jb, 3jc and 3jd were obtained with yields from 63 to 86% (Table 3). Reusability of SiO2 catalyst (60–200 μm) was tested in four cycles on the synthesis of coupling product 3ja (Table 3). In all cycles the loss of SiO2 mass after regeneration was low, 3–5% after each cycle. Diethyl 2-benzylmalonate and acetophenone did not react with malonyl peroxides 2a–d under the optimized conditions.
Based on obtained experimental data and investigations of previous researchers [121], [122], we can conclude that the active sites of the silica gel catalyst are its hydroxyl groups. We propose a reasonable mechanism for oxidative coupling (Scheme 3). β-Dicarbonyl compound likely reacts through its enol form generated with the help of acidic hydroxyl group of SiO2 (step A). The peroxide bond is activated for nucleophilic attack by coordination of the acidic hydroxyl group of SiO2 to the carbonyl oxygen of the diacyl peroxide (step B). The enol form of β-dicarbonyl compound attacks the peroxide partner as a nucleophile (step C). The final step is desorption of the product from the silica gel surface (step D).
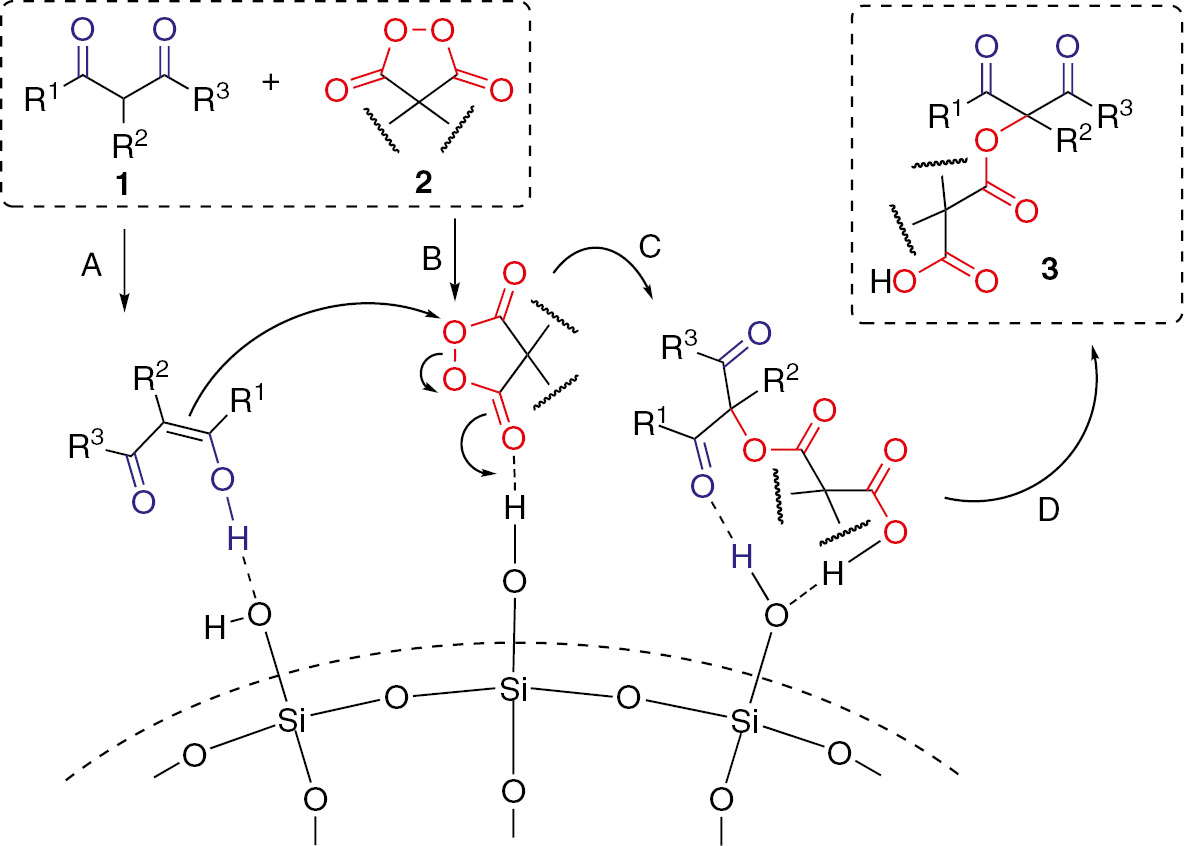
Proposed mechanism of SiO2-mediated oxidative coupling of β-dicarbonyl compound with diacyl peroxide.
Conclusions
It was found that silica gel possesses high catalytic activity in oxidative C–O coupling reaction of α-substituted β-diketones, β-oxoesters and acetyl-lactone with malonyl peroxides. This method avoids the use of toxic, expensive, hazardous chemicals, and provides an easy and rapid access to potentially valuable precursors for the synthesis of natural products and pharmaceuticals. The method offers many advantages over the reported strategies such as easy isolation, good yield of the products and the absence of organic solvent.
Article note
A collection of invited papers based on presentations at the 6th international IUPAC Conference on Green Chemistry (ICGC-6), Venice (Italy), 4–8 September 2016.
Acknowledgements
This work is supported by the Russian Foundation for Basic Research (Grant 16-29-10678). We thank the staff of the Section of Structural Studies of the N. D. Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, for scanning electron microscopy studies of the samples.
References
[1] C.-J. Li. Acc. Chem. Res.42, 335 (2009).10.1021/ar800164nSuche in Google Scholar PubMed
[2] C. S. Yeung, V. M. Dong. Chem. Rev.111, 1215 (2011).10.1021/cr100280dSuche in Google Scholar PubMed
[3] S. A. Girard, T. Knauber, C.-J. Li. Angew. Chem. Int. Ed.52, 2 (2013).Suche in Google Scholar
[4] I. B. Krylov, V. A. Vil’, A. O. Terent’ev. Beilstein J. Org. Chem.11, 92 (2015).10.3762/bjoc.11.13Suche in Google Scholar PubMed PubMed Central
[5] C. Zhang, C. Tanga, N. Jiao. Chem. Soc. Rev.41, 3464 (2012).10.1039/c2cs15323hSuche in Google Scholar PubMed
[6] R. Samanta, K. Matcha, A. P. Antonchick. Eur. J. Org. Chem.26, 5769 (2013).10.1002/ejoc.201300286Suche in Google Scholar
[7] L. Melone, C. Punta. Beilstein J. Org. Chem.9, 1296 (2013).10.3762/bjoc.9.146Suche in Google Scholar PubMed PubMed Central
[8] B. Han, Q. Liu, Z. Liu, R. Mu, W. Zhang, Z.-L. Liu, W. Yu. Synlett15, 2333 (2005).Suche in Google Scholar
[9] G. Yang, Q. Zhang, H. Miao, X. Tong, J. Xu. Org. Lett.7, 263 (2005).10.1021/ol047749pSuche in Google Scholar PubMed
[10] A. O. Terent’ev, V. A. Vil’, G.I. Nikishin, W. Adam. Synlett26, 802 (2015).10.1055/s-0034-1379982Suche in Google Scholar
[11] A. O. Terent’ev, V. A. Vil’, E. S. Gorlov, G. I. Nikishin, K. K. Pivnitsky, W. Adam. J. Org. Chem.81, 810 (2016).10.1021/acs.joc.5b02233Suche in Google Scholar PubMed
[12] W. Adam, J. W. Diehl. J. Chem. Soc. Chem. Commun.13, 797 (1972).10.1039/c39720000797Suche in Google Scholar
[13] C. L. Perrin, T. Arrhenius. J. Am. Chem. Soc.100, 5249 (1978).10.1021/ja00484a078Suche in Google Scholar
[14] F. D. Greene. J. Am. Chem. Soc.78, 2246 (1956).10.1021/ja01591a060Suche in Google Scholar
[15] F. D. Greene, W. W. Rees. J. Am. Chem. Soc.80, 3432 (1958).10.1021/ja01546a058Suche in Google Scholar
[16] W. Adam, R. Rucktäschel. J. Org. Chem.43, 3886 (1978).10.1021/jo00414a021Suche in Google Scholar
[17] M. J. Darmon, G. B. Schuster. J. Org. Chem.47, 4658 (1982).10.1021/jo00145a011Suche in Google Scholar
[18] M. Schwarz, O. Reiser. Angew. Chem. Int. Ed.50, 10495 (2011).10.1002/anie.201104009Suche in Google Scholar PubMed
[19] C. Yuan, A. Axelrod, M. Varela, L. Danysh, D. Siegel. Tetrahedron Lett.52, 2540 (2011).10.1016/j.tetlet.2011.03.005Suche in Google Scholar
[20] J. C. Griffith, K. M. Jones, S. Picon, M. J. Rawling, B. M. Kariuki, M. Campbell, N. C. O Tomkinson. J. Am. Chem. Soc.132, 14409 (2010).10.1021/ja1066674Suche in Google Scholar PubMed
[21] S. Picon, M. Rawling, M. Campbell, N. C. O. Tomkinson. Org. Lett.14, 6250 (2012).10.1021/ol3030154Suche in Google Scholar PubMed
[22] K. M. Jones, N. C. O. Tomkinson. J. Org. Chem.77, 921 (2012).10.1021/jo202084wSuche in Google Scholar PubMed
[23] M. J. Rawling, J. H. Rowley, M. Campbell, A. R. Kennedy, J. A. Parkinson, N. C. O. Tomkinson. Chem. Sci.5, 1777 (2014).10.1039/C3SC53256ASuche in Google Scholar
[24] M. J. Rawling, N. C. O. Tomkinson. Org. Biomol. Chem.11, 1434 (2013).10.1039/c3ob27387cSuche in Google Scholar PubMed
[25] C. Alamillo-Ferrer, S. C. Davidson, M. J. Rawling, N. H. Theodoulou, M. Campbell, P. G. Humphreys, A. R. Kennedy, N. C. O. Tomkinson. Org. Lett.17, 5132 (2015).10.1021/acs.orglett.5b02674Suche in Google Scholar PubMed
[26] C. Alamillo-Ferrer, M. Karabourniotis-Sotti, A. R. Kennedy, M. Campbell, N. C. O. Tomkinson. Org. Lett.18, 3102 (2016).10.1021/acs.orglett.6b01253Suche in Google Scholar PubMed
[27] C. Yuan, Y. Liang, T. Hernandez, A. Berriochoa, K. N. Houk, D. Siegel. Nature499, 192 (2013).10.1038/nature12284Suche in Google Scholar PubMed
[28] C. Yuan, A. M. Eliasen, A. M. Camelio, D. Siegel. Nature Protocols9, 2624 (2014).10.1038/nprot.2014.175Suche in Google Scholar PubMed
[29] A. Dragan, T. M. Kubczyk, J. H. Rowley, S. Sproules, N. C. O. Tomkinson. Org. Lett.17, 2618 (2015).10.1021/acs.orglett.5b00953Suche in Google Scholar PubMed
[30] A. M. Camelio, Y. Liang, A. M. Eliasen, T. C. Johnson, C. Yuan, A. W. Schuppe, K. N. Houk, D. Siegel. J. Org. Chem.80, 8084 (2015).10.1021/acs.joc.5b01079Suche in Google Scholar PubMed
[31] A. M. Eliasen, M. Christy, K. R. Claussen, R. Besandre, R. P. Thedford, D. Siegel. Org. Lett.17, 4420 (2015).10.1021/acs.orglett.5b02008Suche in Google Scholar PubMed
[32] D. Chang, D. Zhu, L. Shi. J. Org. Chem.80, 5928 (2015).10.1021/acs.joc.5b00517Suche in Google Scholar PubMed
[33] W. Adam, A. K. Smerz. Tetrahedron52, 5799 (1996).10.1016/0040-4020(96)00231-1Suche in Google Scholar
[34] A. M. R. Smith, D. Billen, K. K. Hii. Chem. Commun.26, 3925 (2009).10.1039/b907151bSuche in Google Scholar PubMed
[35] A. M. R. Smith, H. S. Rzepa, A. J. P. White, D. Billen, K. K. Hii. J. Org. Chem.75, 3085 (2010).10.1021/jo1002906Suche in Google Scholar PubMed
[36] J. Christoffers, T. Werner, W. Frey, A. Baro. Eur. J. Org. Chem.24, 4879 (2003).10.1002/ejoc.200300439Suche in Google Scholar
[37] J. Christoffers. J. Org. Chem.64, 7668 (1999).10.1021/jo9909094Suche in Google Scholar
[38] A. M. Richardson, C.-H. Chen, B. B. Snider. J. Org. Chem.72, 8099 (2007).10.1021/jo701512wSuche in Google Scholar PubMed
[39] J. Christoffers, T. Kauf, T. Werner, M. Rossle. Eur. J. Org. Chem.11, 2601 (2006).10.1002/ejoc.200600061Suche in Google Scholar
[40] H. Asahara, N. Nishiwaki. J. Org. Chem.79, 11735 (2014).10.1021/jo501985uSuche in Google Scholar PubMed
[41] Y.-F. Liang, N. Jiao. Angew. Chem. Int. Ed.53, 548 (2014).10.1002/anie.201308698Suche in Google Scholar PubMed
[42] L. Zou, B. Wang, H. Mu, H. Zhang, Y. Song, J. Qu. Org. Lett.15, 3106 (2013).10.1021/ol401306hSuche in Google Scholar PubMed
[43] H. Yao, M. Lian, Z. Li, Y. Wang, Q. Meng. J. Org. Chem.77, 9601 (2012).10.1021/jo3016242Suche in Google Scholar PubMed
[44] C. De Fusco, S. Meninno, C. Tedesco, A. Lattanzi. Org. Biomol. Chem. 11, 896 (2013).10.1039/c2ob27283kSuche in Google Scholar PubMed
[45] H. Miyamura, S. Kobayashi. Chem. Lett. 41, 976 (2012).10.1246/cl.2012.976Suche in Google Scholar
[46] M. R. Acocella, O. G. Mancheno, M. Bella, K. A. Jørgensen. J. Org. Chem.69, 8165 (2004).10.1021/jo048655wSuche in Google Scholar PubMed
[47] G. J. Chuang, W. Wang, E. Lee, T. Ritter. J. Am. Chem. Soc.133, 1760 (2011).10.1021/ja108396kSuche in Google Scholar PubMed PubMed Central
[48] B. Gong, Q. Meng, T. Su, M. Lian, Q. Wang, Z. Gao. Synlett16, 2659 (2009).10.1055/s-0029-1217758Suche in Google Scholar
[49] X. Gu, Y. Zhang, Z.-J. Xu, C.-M. Che. Chem. Commun.50, 7870 (2014).10.1039/c4cc01631aSuche in Google Scholar PubMed
[50] J. Li, G. Chen, Z. Wang, R. Zhang, X. Zhang, K. Ding. Chem. Sci.2, 1141 (2011).10.1039/c0sc00607fSuche in Google Scholar
[51] M. Lu, D. Zhu, Y. Lu, X. Zeng, B. Tan, Z. Xu, G. Zhong. J. Am. Chem. Soc.131, 4562 (2009).10.1021/ja8088907Suche in Google Scholar PubMed
[52] Z. Li, T. Li, J. Li, L. He, X. Jia, J. Yang. Synlett26, 2863 (2015).10.1055/s-0035-1560647Suche in Google Scholar
[53] Y. Wang, Z. Li, T. Xiong, J. Zhao, Q. Meng. Synlett25, 2155 (2014).10.1055/s-0034-1378548Suche in Google Scholar
[54] A. O. Terent’ev, D. A. Borisov, I. A. Yaremenko, V. V. Chernyshev, G. I. Nikishin. J. Org. Chem.75, 5065 (2010).10.1021/jo100793jSuche in Google Scholar PubMed
[55] A. O. Terent’ev, D. A. Borisov, V. V. Semenov, V. V. Chernyshev, V. M. Dembitsky, G. I. Nikishin. Synthesis13, 2091 (2011).10.1055/s-0030-1260027Suche in Google Scholar
[56] M. T. Rahman, H. Nishino. Org. Lett.5, 2887 (2003).10.1021/ol034939bSuche in Google Scholar PubMed
[57] M. A. Haque, H. Nishino. Synth. Comm.42, 608 (2012).10.1287/inte.1120.0664Suche in Google Scholar
[58] A. O. Terent’ev, V. A. Vil’, O. V. Bityukov, G. I. Nikishin. Russ. Chem. Bull.63, 2461 (2014).10.1007/s11172-014-0763-8Suche in Google Scholar
[59] A. O. Terent’ev, I. B. Krylov, V. P. Timofeev, Z. A. Starikova, V. M. Merkulova, A. I. Ilovaisky, G. I. Nikishin. Adv. Synth. Catal.355, 2375 (2013).10.1002/adsc.201300341Suche in Google Scholar
[60] I. B. Krylov, A. O. Terent’ev, V. P. Timofeev, B. N. Shelimov, R. A. Novikov, V. M. Merkulova, G. I. Nikishin. Adv. Synth. Catal.356, 2266 (2014).10.1002/adsc.201400143Suche in Google Scholar
[61] Y.-Y. Liu, X.-H. Yang, J. Yang, R.-J. Song, J.-H. Li. Chem. Commun.50, 6906 (2014).10.1039/c4cc02084gSuche in Google Scholar PubMed
[62] Regev, H. Shalit, D. Pappo. Synthesis47, 1716 (2015).10.1055/s-0034-1380360Suche in Google Scholar
[63] J. Yu, J. Tian, C. Zhanga. Adv. Synth. Catal.352, 531 (2010).Suche in Google Scholar
[64] R. M. Moriarty, R. K. Vaid, T. E. Hopkins, B. K. Vaid, O. Prakash. Tetrahedron Lett.31, 201 (1990).10.1016/S0040-4039(00)94370-3Suche in Google Scholar
[65] W.-B. Liu, C. Chen, Q. Zhang, Z.-B. Zhu. Beilstein J. Org. Chem.7, 1436 (2011).10.3762/bjoc.7.167Suche in Google Scholar PubMed PubMed Central
[66] M. Uyanik, D. Suzuki, T. Yasui, K. Ishihara. Angew. Chem. Int. Ed.50, 5331 (2011).10.1002/anie.201101522Suche in Google Scholar PubMed
[67] X. Li, C. Zhou, X. Xu. Arkivoc9, 150 (2012).10.3998/ark.5550190.0013.913Suche in Google Scholar
[68] A. Citterio. J. Org. Chem.54, 2703 (1989).10.1021/jo00272a046Suche in Google Scholar
[69] Z. Cong, T. Miki, O. Urakawa, H. Nishino. J. Org. Chem.74, 3978 (2009).10.1021/jo9002773Suche in Google Scholar PubMed
[70] M. G. Moloney, E. Nettleton, K. Smithies. Tetrahedron Lett.43, 907 (2002).10.1016/S0040-4039(01)02288-2Suche in Google Scholar
[71] A. Citterio, A. Cerati, R. Sebastiano. Tetrahedron Lett.30, 1289 (1989).10.1016/S0040-4039(00)72739-0Suche in Google Scholar
[72] J. Lee, S. Oya, J. K. Snyder. Tetrahedron Lett.32, 5899 (1991).10.1016/S0040-4039(00)79421-4Suche in Google Scholar
[73] O. Lifchits, N. Demoulin, B. List. Angew. Chem. Int. Ed.50, 9680 (2011).10.1002/anie.201104244Suche in Google Scholar PubMed
[74] D. Wang, C. Xu, L. Zhang, S. Luo. Org. Lett.17, 576 (2015).10.1021/ol503592nSuche in Google Scholar PubMed
[75] M. S. Jadhav, P. Righi, E. Marcantoni, G. Bencivenni. J. Org. Chem.77, 2667 (2012).10.1021/jo2024976Suche in Google Scholar PubMed
[76] T. Kanemitsu, M. Sato, M. Yoshida, E. Ozasa, M. Miyazaki, Y. Odanaka, K. Nagata, T. Itoh. Org. Lett.18, 5484 (2016).10.1021/acs.orglett.6b02682Suche in Google Scholar PubMed
[77] M. E. Lloris, N. Gálvez, J. Marquet, M. Moreno-Mañas. Tetrahedron47, 8031 (1991).10.1016/S0040-4020(01)81955-4Suche in Google Scholar
[78] R. Salomon, M. Salomon, M. Zagorski, J. Reuter, D. Coughlin. J. Am. Chem. Soc.104, 1008 (1982).10.1021/ja00368a015Suche in Google Scholar
[79] M. Chmielewski, O. Achmatowicz, A. Zamojski. Bull. Pol. Acad. Sci. Chem.32, 19 (1984).Suche in Google Scholar
[80] C. Gandolfi, L. Cotini, M. Mantovanini, G. Caselli, G. Clavenna, C. Omini. US 5656656A1 (1997).Suche in Google Scholar
[81] I. Uchida, H. Hatanaka, K. Nitta, S. Hashimoto, M. Okuhara, H. Murai, M. Hashimoto. US 5061730A (1991).Suche in Google Scholar
[82] Z. Zhang, W. Zheng, J. C. Antilla. Angew. Chem. Int. Ed.50, 1135 (2011).10.1002/anie.201006595Suche in Google Scholar PubMed PubMed Central
[83] M. B. Gawande, V. D. B. Bonifácio, R. Luque, P. S. Branco, R. S. Varma. ChemSusChem7, 24 (2014).10.1002/cssc.201300485Suche in Google Scholar PubMed
[84] M. S. Singh, S. Chowdhury. RSC Adv.2, 4547 (2012).10.1039/c2ra01056aSuche in Google Scholar
[85] C. Descorme, P. Gallezot, C. Geantet, C. George. ChemCatChem4, 1897 (2012).10.1002/cctc.201200483Suche in Google Scholar
[86] M. Pagliaro, Silica-Based Materials for Advanced Chemical Applications, RSC Publishing, Cambridge (2009).10.1039/9781847557162Suche in Google Scholar
[87] R. Ciriminna, M. Sciortino, G. Alonzo, A. de Schrijver, M. Pagliaro. Chem. Rev.111, 765 (2011).10.1021/cr100161xSuche in Google Scholar PubMed
[88] R. Ciriminna, A. Fidalgo, V. Pandarus, F. Béland, L. M. Ilharco, M. Pagliaro. Chem. Rev.113, 6592 (2013).10.1021/cr300399cSuche in Google Scholar PubMed
[89] Silica Gel. Van Nostrand’s Scientific Encyclopedia (2007).Suche in Google Scholar
[90] U. D. Neue. Silica Gel and Its Derivatization for Liquid Chromatography. Encyclopedia of Analytical Chemistry (2009).10.1002/9780470027318.a5915.pub2Suche in Google Scholar
[91] B. C. Ranu, A. Sarkar, A. Majee. J. Org. Chem.62, 1841 (1997).10.1021/jo961736aSuche in Google Scholar
[92] M. E. González-Núñez, R. Mello, A. Olmos, G. Asensio. J. Org. Chem.71, 6432 (2006).10.1021/jo060753pSuche in Google Scholar PubMed
[93] J.-G. Li, Y.-Q. Peng. J. Chin. Chem. Soc.57, 305 (2010).10.1002/jccs.201000045Suche in Google Scholar
[94] X. Cui, F. Shi, Y. Deng. Chem. Commun.48, 7586 (2012).10.1039/c2cc31438jSuche in Google Scholar PubMed
[95] G. Bartoli, M. Bartolacci, M. Bosco, G. Foglia, A. Giuliani, E. Marcantoni, L. Sambri, E. Torregiani. J. Org. Chem.68, 4594 (2003).10.1021/jo034303ySuche in Google Scholar PubMed
[96] H. R. Shaterian, A. Hosseinian, M. Ghashang. Phosphorus, Sulfur Silicon Relat. Elem.183, 3136 (2008).10.1080/10426500802066096Suche in Google Scholar
[97] W.-H. Cheung, W.-Y. Yu, W.-P. Yip, N.-Y. Zhu, C.-M. Che. J. Org. Chem.67, 7716 (2002).10.1021/jo0204404Suche in Google Scholar PubMed
[98] P. N. Liu, F. Xia, Q. W. Wang, Y. J. Ren, J. Q. Chen. Green Chem. 12, 1049 (2010).10.1039/b926142gSuche in Google Scholar
[99] A. Agarwal, S. Rani, Y. D. Vankar. J. Org. Chem. 69, 6137 (2004).10.1021/jo049415jSuche in Google Scholar PubMed
[100] Y. Shimoda, H. Yamamoto. Tetrahedron Lett.56, 3090 (2015).10.1016/j.tetlet.2014.12.046Suche in Google Scholar
[101] A. K. Chakraborti, R. Gulhane. Chem. Commun.15, 1896 (2003).10.1039/B304178FSuche in Google Scholar
[102] S. Shikata, S. Nakata, T. Okuhara, M. Misono. J. Catal.166, 263 (1997).10.1006/jcat.1997.1502Suche in Google Scholar
[103] E. L. Margelefsky, R. K. Zeidan, M. E. Davis. Chem. Soc. Rev.37, 1118 (2008).10.1039/b710334bSuche in Google Scholar PubMed
[104] V. Pandarus, G. Gingras, F. Béland, R. Ciriminna, M. Pagliaro. Catal. Sci. Technol.1, 1600 (2011).10.1039/c1cy00232eSuche in Google Scholar
[105] L. Wang, L. Green, Z. Li, J. McCabe Dunn, X. Bu, C. J. Welch, C. Li, T. Wang, Q. Tu, E. Bekos, D. Richardson, J. Eckert, J. Cui. Org. Process Res. Dev.15, 1371 (2011).10.1021/op2001657Suche in Google Scholar
[106] J. M. French, C. A. Caras, S. T. Diver. Org. Lett.15, 5416 (2013).10.1021/ol402339eSuche in Google Scholar PubMed
[107] M. Li, M. Li, C. Feng, Q. Zeng. Appl. Surf. Sci.314, 1063 (2014).10.1016/j.apsusc.2014.06.038Suche in Google Scholar
[108] J. M. Bobbitt. J. Org. Chem.63, 9367 (1998).Suche in Google Scholar
[109] K. J. Stanger, R. J. Angelici. Energy Fuels20, 1757 (2006).10.1021/ef050327rSuche in Google Scholar
[110] M. Reddy, V. V. R. Kumar, N. C. G. Reddy, S. M. Rao. Chin. Chem. Lett.25, 179 (2014).10.1016/j.cclet.2013.09.014Suche in Google Scholar
[111] S. Onitsuka, Y. Z. Jin, A. C. Shaikh, H. Furuno, J. Inanaga. Molecules.17, 11469 (2012).10.3390/molecules171011469Suche in Google Scholar PubMed PubMed Central
[112] H. Kotsuki, K. Hayashida, T. Shimanouchi, H. Nishizawa. J. Org. Chem. 61, 984 (1996).10.1021/jo951106tSuche in Google Scholar
[113] H. Shinohara, M. Sonoda, N. Hayagane, S. Kita, S. Tanimori, A. Ogawa. Tetrahedron Lett.55, 5302 (2014).10.1016/j.tetlet.2014.07.084Suche in Google Scholar
[114] M. P. Thakare, P. Kumar, N. Kumar, S. K. Pandey. Tetrahedron Lett.55, 2463 (2014).10.1016/j.tetlet.2014.03.007Suche in Google Scholar
[115] C. Li, F. Zhang, Z. Yang, C. Qi. Tetrahedron Lett. 55, 5430 (2014).10.1016/j.tetlet.2014.08.022Suche in Google Scholar
[116] A. K. Chaturvedi, A. S. Negi, P. Khare. RSC Adv.3, 4500 (2013).10.1039/c3ra22435jSuche in Google Scholar
[117] L. Caggiano, D. J. Fox, S. Warren. Chem. Commun.21, 2528 (2002).10.1039/b207542nSuche in Google Scholar
[118] A. Sagar, V. N. Babu, D. S. Sharada. RSC Adv. 5, 29066 (2015).10.1039/C5RA02491ASuche in Google Scholar
[119] H. Wu, W. Lin, Y. Wan, H. Xin, D. Shi, Y. Shi, R. Yuan, R. Bo, W. Yin. J. Comb. Chem.12, 31 (2010).Suche in Google Scholar
[120] S. Agarwal, U. Aware, A. Patil, V. Rohera, M. Ghate, M. Jain, P. Patel. Bull. Korean Chem. Soc.33, 377 (2012).10.5012/bkcs.2012.33.2.377Suche in Google Scholar
[121] Y. Jin, J. Li, L. Peng, C. Gao. Chem. Commun.51, 15390 (2015).10.1039/C5CC05396JSuche in Google Scholar PubMed
[122] A. Serra-Muns, A. Guérinot, S. Reymond, J. Cossy. Chem. Commun.46, 4178 (2010).10.1039/c0cc00035cSuche in Google Scholar PubMed
[123] S. Kundu, B. Roy, B. Basu. Beilstein J. Org. Chem.10, 26 (2014).10.3762/bjoc.10.5Suche in Google Scholar PubMed PubMed Central
[124] A. Alexakis, J. E. Bäckvall, N. Krause, O. Pàmies, M. Dié- guez. Chem. Rev.108, 2796 (2008).10.1021/cr0683515Suche in Google Scholar PubMed
[125] A. M. Tsedilin, A. N. Fakhrutdinov, D. B. Eremin, S. S. Zalesskiy, A. O. Chizhov, N. G. Kolotyrkina, V. P. Ananikov. Mend. Comm.25, 454 (2015).10.1016/j.mencom.2015.11.019Suche in Google Scholar
[126] R. W. Kluiber, F. Oberender, C. Rossi. J. Org. Chem.25, 1069 (1960).10.1021/jo01076a627Suche in Google Scholar
[127] J. Olguin, S. Brooker. New J. Chem.35, 1242 (2011).10.1016/S1499-2671(11)52117-3Suche in Google Scholar
[128] J. Marquet, M. Moreno-Manas, P. Pacheco, M. Prat, A. R. Katritzky, B. Brycki. Tetrahedron46, 5333 (1990).10.1016/S0040-4020(01)87840-6Suche in Google Scholar
[129] A. O. Terent’ev, V. A. Vil’, I. A. Yaremenko, O. V. Bityukov, D. O. Levitsky, V. V. Chernyshev, G. I. Nikishin, F. Fleury. New J. Chem.38, 1493 (2014).10.1039/C3NJ01454ASuche in Google Scholar
[130] R. Deng, Y. Wang, Y. Jiang. Synth. Comm.24, 111 (1994).10.1080/00397919408012633Suche in Google Scholar
[131] N. F. Albertson. J. Am. Chem. Soc.72, 2594 (1950).10.1021/ja01162a068Suche in Google Scholar
[132] J. E. Beddow, S. G. Davies, K. B. Ling, P. M. Roberts, A. J. Russell, A. D. Smith, J. E. Thomson. Org. Biomol. Chem.5, 2812 (2007).10.1039/b707689dSuche in Google Scholar PubMed
Supplemental Material
The online version of this article (DOI: https://doi.org/10.1515/pac-2017-0312) offers supplementary material, available to authorized users.
©2018 IUPAC & De Gruyter. This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. For more information, please visit: http://creativecommons.org/licenses/by-nc-nd/4.0/
Artikel in diesem Heft
- Frontmatter
- In this issue
- Conference papers
- Papers from the 6th International IUPAC Conference on Green Chemistry (ICGC-6)
- Microwave assisted synthesis of glycerol carbonate from glycerol and urea
- Silica gel mediated oxidative C–O coupling of β-dicarbonyl compounds with malonyl peroxides in solvent-free conditions
- Definition of green synthetic tools based on safer reaction media, heterogeneous catalysis, and flow technology
- Heavy metal removal from waste waters by phosphonate metal organic frameworks
- A clean and simple method for deprotection of phosphines from borane complexes
- Development and treatment procedure of arsenic-contaminated water using a new and green chitosan sorbent: kinetic, isotherm, thermodynamic and dynamic studies
- Bio-adsorbent derived from papaya peel waste and magnetic nanoparticles fabricated for lead determination
- 5-Membered cyclic ethers via phenonium ion mediated cyclization through carbonate chemistry
- Synergy in food, energy and advanced materials production from biomass
- Step economy strategy for the synthesis of amphoteric aminoaldehydes, key intermediates for reduced hydantoins
- Separation technology meets green chemistry: development of magnetically recoverable catalyst supports containing silica, ceria, and titania
- Green chemistry and sustainable development: approaches to chemical footprint analysis
- Greener solvents for solid-phase organic synthesis
- Photocatalytic hydrogenolysis of allylic alcohols for rapid access to platform chemicals and fine chemicals
- IUPAC Recommendations
- Definition of the mole (IUPAC Recommendation 2017)
- Terminology of separation methods (IUPAC Recommendations 2017)
Artikel in diesem Heft
- Frontmatter
- In this issue
- Conference papers
- Papers from the 6th International IUPAC Conference on Green Chemistry (ICGC-6)
- Microwave assisted synthesis of glycerol carbonate from glycerol and urea
- Silica gel mediated oxidative C–O coupling of β-dicarbonyl compounds with malonyl peroxides in solvent-free conditions
- Definition of green synthetic tools based on safer reaction media, heterogeneous catalysis, and flow technology
- Heavy metal removal from waste waters by phosphonate metal organic frameworks
- A clean and simple method for deprotection of phosphines from borane complexes
- Development and treatment procedure of arsenic-contaminated water using a new and green chitosan sorbent: kinetic, isotherm, thermodynamic and dynamic studies
- Bio-adsorbent derived from papaya peel waste and magnetic nanoparticles fabricated for lead determination
- 5-Membered cyclic ethers via phenonium ion mediated cyclization through carbonate chemistry
- Synergy in food, energy and advanced materials production from biomass
- Step economy strategy for the synthesis of amphoteric aminoaldehydes, key intermediates for reduced hydantoins
- Separation technology meets green chemistry: development of magnetically recoverable catalyst supports containing silica, ceria, and titania
- Green chemistry and sustainable development: approaches to chemical footprint analysis
- Greener solvents for solid-phase organic synthesis
- Photocatalytic hydrogenolysis of allylic alcohols for rapid access to platform chemicals and fine chemicals
- IUPAC Recommendations
- Definition of the mole (IUPAC Recommendation 2017)
- Terminology of separation methods (IUPAC Recommendations 2017)


