Abstract
The transformation of glycerol into value-added chemicals is an interesting topic in Green Chemistry. The glycerolysis of urea into glycerol carbonate is an important route of glycerol transformation. In this work, the synthesis of glycerol carbonate from glycerol and urea was performed under microwave irradiation and solvent free conditions. The catalytic properties of ZnSO4, ZnCl2, CuSO4 and MgSO4 were tested and ZnSO4 showed the best performance. The effects of catalyst amount, temperature, reaction time, microwave power, molar ratio of urea to glycerol on the reaction were studied using ZnSO4 as the catalyst. It was demonstrated that microwave irradiation could promote the reaction effectively, and the yield of desired product glycerol carbonate could reach 93.7%.
Introduction
Glycerol is regarded as one of the most important bio-platform molecules. As a by-product from biodiesel production, glycerol is largely available as a result of the increase in the production of biodiesel. Effective and efficient conversion of glycerol to value-added products is an interesting topic. Glycerol is polyhydric, allowing some value-added products to be produced from it by different reactions, such as esterification [1], dehydration [2], oxidation [3], and hydrogenolysis [4].
Glycerol carbonate can be used as an intermediate in chemical reactions, especially as a new versatile building block for producing many chemicals [5, 6]. Glycerol carbonate can be synthesized by using different reactants, such as glycerol and glycidol [7]. In recent years, synthesis of glycerol carbonate from glycerol has received much attention. Glycerol can react with carbonyl chloride, carbon monoxide [8], carbon dioxide [9], dimethyl carbonate [10, 11], diethyl carbonate [12], cyclic carbonate [13] and urea to produce glycerol carbonate. The glycerolysis of urea will release ammonia as by-product (as shown in Scheme 1), making it very easy to separate product mixtures. Hydrotalcite-like solid catalysts [14], Zn/Al mixed oxide [15], metal monoglycerolates [16], lanthanum oxide [17], zinc chloride and zinc sulfate [18] have been used as catalysts for the synthesis of glycerol carbonate from glycerol and urea, but the reaction usually need long reaction time and/or excess of one reagent.

Microwave assisted synthesis of glycerol carbonate from glycerol and urea.
Microwave irradiation have been used in various processes, such as chemical reaction [19, 20], material synthesis [21], nanotechnology [22] and biochemical processes [23, 24]. It has been reported that microwave irradiation could raise the yield and reduced the reaction time in the synthesis of carbonates from CO2 and epoxide [25], NaHCO3 and olefins [26], CO and diols [27], and polycarbonates from open-ring polymerization of trimethylene carbonate [28]. In this work, we studied the effect of microwave irradiation on glycerol carbonate synthesis from glycerol and urea, hoping to get similar results. It was demonstrated that microwave irradiation could enhance the efficiency of the reaction effectively.
Materials and methods
Materials
Urea (purity≥99%) was provided by Sinopharm Chemical Reagent Co. Ltd. ZnSO4·7H2O (purity≥99%) was purchased from J&k Scientific Ltd. Glycerol 1,2-carbonate (glycerol carbonate, purity≥90%) was obtained from Tokyo Chemical Industry. Glycerol (purity≥99%), La2O3 (purity≥99%), CuSO4 (purity≥99%) and ZnCl2(purity≥98%) were supplied by Acros Organics. n-Butanol (purity≥99%), ethanol (purity≥99%), NaOH (purity≥99%) and MgSO4 (purity≥99%)were provided by Beijing Chemical Company.
Catalyst preparation
The ZnSO4·7H2O crystal was dried in a baking oven at 100°C for 16 h to eliminate most water. Then the solid was calcined in a chamber electric furnace at 300°C for 1 h and ZnSO4 was obtained.
Synthesis of glycerol carbonate
The reaction was carried out in a XH-MC-1 microwave oven with a maximum output power of 900 W and microwave frequency of 2450 MHz (Beijing Xiang Hu Science and Technology Development Co., Ltd.). The temperature of the microwave oven was measured by a built-in sensor.
In a typical microwave-assisted experiment, urea, glycerol and catalyst was added to a round-bottom flask with a built-in thermocouple that monitored the temperature of the reaction mixture. Then the flask was set in the microwave oven. The reaction was carried out at 15 KPa (controlled by a vacuum pump) and desired temperature, microwave power, fixed stirring rate for certain time. After reaction, the product mixture was cooled to room temperature and was diluted by ethanol to reduce its viscosity and solid catalyst was separated by centrifuge. The liquid phase was analyzed by gas chromatography (Agilent 6820 Gas Chromatography) with FID detector using n-butanol as internal standard.
In the experiment without using microwave, the round-bottom flask was set in an oil bath. Other operation procedures were the same as the microwave-assisted experiment.
Results and discussion
The influence of the concentration of catalyst, temperature, reaction time, microwave power and molar ratio on the synthesis of glycerol carbonate from glycerol and urea were studied in this work. Several commonly used catalysts [18, 29] for urea alcoholysis, including ZnCl2, ZnSO4, CuSO4, MgSO4 were tested, and the results are shown in Table 1. All the catalysts were effective for the reaction, and ZnSO4 showed the best performance. In the following research, ZnSO4 was chosen as catalyst for further optimization of the reaction conditions.
The effect of different catalysts on the conversion of glycerol and the yield of glycerol carbonate.a
| Catalysts | Conversion (%) | Yield (%) |
|---|---|---|
| ZnCl2 | 60.7 | 60.3 |
| MgSO4 | 58.1 | 57.2 |
| CuSO4 | 55.8 | 53.1 |
| ZnSO4 | 81.8 | 80.6 |
-
aConcentration of catalyst 2.8 mol% to glycerol, temperature 150°C, reaction time 40 min, 600 W microwave irradiation, molar ratio of urea to glycerol=1:1, pressure 15 KPa.
The effect of ZnSO4 amount on the conversion and yield of glycerol carbonate under microwave irradiation is presented in Fig. 1. The reaction could take place under catalyst free conditions, but the catalyst could accelerate reaction rate effectively. The yield of glycerol carbonate reached 90.0% when 2.8 mol% ZnSO4 was added, and the change of the yield was not obvious with further increasing the amount of the catalyst.
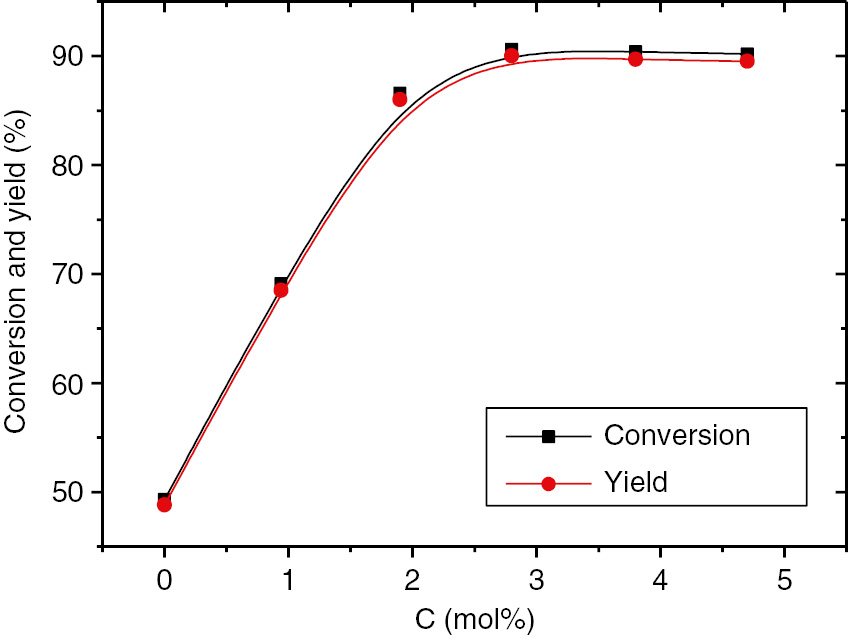
Effect of the concentration of catalyst (ZnSO4) on the conversion of glycerol and the yield of glycerol carbonate. Reaction conditions: temperature 150°C, reaction time 100 min, 600 W microwave irradiation, molar ratio of urea to glycerol =1:1, pressure 15 KPa.
The effect of temperature on the conversion of glycerol and yield of glycerol carbonate under microwave irradiation is shown in Fig. 2. Under microwave irradiation, glycerol and urea could react at lower temperature, 50.9% of glycerol was transformed into glycerol carbonate at 125°C in 100 min. The yield of glycerol carbonate increased with temperature, and reached 90.0% at 150°C. With further increase of temperature, the yield of glycerol carbonate reduced, this may come from too fast decomposition of urea and some lost before reaction result from instability around 160°C.

Effect of temperature on the conversion of glycerol and the yield of glycerol carbonate. Reaction conditions: concentration of catalyst 2.8 mol% to glycerol, reaction time 100 min, 600 W microwave irradiation, molar ratio of urea to glycerol=1:1, pressure 15 KPa.
The dependence of conversion of glycerol and yield of glycerol carbonate on reaction time is illustrated in Fig. 3. The yield increased rapidly with time during the initial period, and reached 80.6% in 40 min under microwave (M) irradiation. Then the yield increased slowly with further extension of reaction time to 90.0% at 100 min. For comparison, the reaction was also carried out at the same temperature without (C) microwave and the results are also shown in Fig. 3. The yield of glycerol carbonate was 39.8% at 40 min and 61.0% at 100 min. The results indicate that microwave irradiation could enhance the yield of glycerol carbonate effectively when other reaction conditions were the same.

Effect of reaction time on the conversion of glycerol and the yield of glycerol carbonate with (M) and without (C) microwave irradiation (600 W). Reaction conditions: concentration of catalyst 2.8 mol% to glycerol, temperature 150°C, molar ratio of urea to glycerol=1:1, pressure 15 KPa.
The effect of microwave power on the conversion of glycerol and yield of glycerol carbonate is illustrated in Fig. 4. It can be seen from the figure that the conversion and yield increased with increasing power in lower power range. However, the conversion and yield became independent of the power when the power exceeded 600 W.

Effect of microwave power on the conversion of glycerol and the yield of glycerol carbonate. Reaction conditions: concentration of catalyst 2.8 mol% to glycerol, temperature 150°C, reaction time 100 min, molar ratio of urea to glycerol =1:1, pressure 15 KPa.
We also conducted the reaction at different urea to glycerol molar ratios (Ru), and the results are given in Fig. 5. It can be known from the figure that as the increase of the molar ratio of urea to glycerol, the conversion of glycerol increased synchronously, nearly all the glycerol could be converted (conversion 97.6%), while the yield of glycerol carbonate grew to 93.7% at 1.3:1 Ru and then started to decline slightly.

Effect of urea to glycerol molar ratio (Ru) on the reaction. Reaction conditions: concentration of catalyst 2.8 mol% to glycerol, temperature 150°C, reaction time 100 min, 600 W microwave irradiation, pressure 15 KPa.
Conclusions
The reaction of glycerol and urea to produce glycerol carbonate has been studied with and without microwave irradiation. It is shown that microwave can enhance the reaction effectively when ZnSO4 is used as the catalyst. The conversion of glycerol and yield of the desired product depend on reaction conditions, such as amount of catalyst, reaction time, temperature, power of microwave, molar ratio of urea to glycerol. At optimized condition, the yield of the product can be as high as 93.7%. We believe that this route has promising potential of application in the synthesis of glycerol carbonate from glycerol and urea.
Article note
A collection of invited papers based on presentations at the 6th international IUPAC Conference on Green Chemistry (ICGC-6), Venice (Italy), 4–8 September 2016.
Acknowledgements
The authors thank the National Natural Science Foundation of China (Grant No. 21673255, 21533011), and the Chinese Academy of Sciences (QYZDY-SSW-SLH013).
References
[1] H. R. Prakruthi, B. S. Jai Prakash, Y. S. Bhat. J. Mol. Catal. A: Chem.408, 214 (2015).10.1016/j.molcata.2015.07.036Search in Google Scholar
[2] R. Bai, H. Zhang, F. Mei, S. Wang, T. Li, Y. Gu, G. Li. Green Chem.18, 6144 (2016).10.1039/C6GC90102FSearch in Google Scholar
[3] C. Zhang, T. Wang, X. Liu, Y. Ding. J. Mol. Catal. A: Chem.424, 91 (2016).10.1016/j.molcata.2016.08.018Search in Google Scholar
[4] M. Lee, Y. K. Hwang, J.-S. Chang, H.-J. Chae, D. W. Hwang. Catal. Commun.84, 5 (2016).10.1016/j.catcom.2016.05.022Search in Google Scholar
[5] M. O. Sonnati, S. Amigoni, E. P. Taffin de Givenchy, T. Darmanin, O. Choulet, F. Guittard. Green Chem.15, 283 (2013).10.1039/C2GC36525ASearch in Google Scholar
[6] S. Holmiere, R. Valentin, P. Marechal, Z. Mouloungui. J. Colloid Interface Sci.487, 418 (2017).10.1016/j.jcis.2016.10.072Search in Google Scholar PubMed
[7] B. R. Buckley, A. P. Patel, K. G. Wijayantha. Chem. Commun. (Cambridge, U. K.)47, 11888 (2011).10.1039/c1cc15467bSearch in Google Scholar PubMed
[8] F. Doro, P. Winnertz, W. Leitner, A. Prokofieva, T. E. Müller. Green Chem.13, 292 (2011).10.1039/c0gc00817fSearch in Google Scholar
[9] J. Liu, Y. Li, J. Zhang, D. He. Appl. Catal., A513, 9 (2016).Search in Google Scholar
[10] J. R. Ochoa-Gómez, O. Gómez-Jiménez-Aberasturi, B. Maestro-Madurga, A. Pesquera-Rodríguez, C. Ramírez-López, L. Lorenzo-Ibarreta, J. Torrecilla-Soria, M. C. Villarán-Velasco. Appl. Catal., A366, 315 (2009).10.1016/j.apcata.2009.07.020Search in Google Scholar
[11] J. Granados-Reyes, P. Salagre, Y. Cesteros. Appl. Clay Sci.132, 216 (2016).10.1016/j.clay.2016.06.008Search in Google Scholar
[12] A. Takagaki, K. Iwatani, S. Nishimura, K. Ebitani. Green Chem.12, 578 (2010).10.1039/b925404hSearch in Google Scholar
[13] M. J. Climent, A. Corma, P. De Frutos, S. Iborra, M. Noy, A. Velty, P. Concepción. J. Catal.269, 140 (2010).10.1016/j.jcat.2009.11.001Search in Google Scholar
[14] Y. Sun, X. Tong, Z. Wu, J. Liu, Y. Yan, S. Xue. Energy Technol.2, 263 (2014).10.1002/ente.201300135Search in Google Scholar
[15] Y. B. Ryu, J. H. Baek, Y. Kim, M. S. Lee. J. Nanosci. Nanotechnol.15, 321 (2015).10.1166/jnn.2015.8343Search in Google Scholar PubMed
[16] T. W. Turney, A. Patti, W. Gates, U. Shaheen, S. Kulasegaram. Green Chem.15, 1925 (2013).10.1039/c3gc37028cSearch in Google Scholar
[17] L. Wang, Y. Ma, Y. Wang, S. Liu, Y. Deng. Catal. Commun.12, 1458 (2011).10.1016/j.catcom.2011.05.027Search in Google Scholar
[18] S.-i. Fujita, Y. Yamanishi, M. Arai. J. Catal.297, 137 (2013).10.1016/j.jcat.2012.10.001Search in Google Scholar
[19] J. D. Moseley, C. O. Kappe. Green Chem.13, 794 (2011).10.1039/c0gc00823kSearch in Google Scholar
[20] N. Kaur. Synth. Commun.45, 1599 (2015).10.1080/00397911.2013.828755Search in Google Scholar
[21] N. A. Khan, S. H. Jhung. Coord. Chem. Rev.285, 11 (2015).10.1016/j.ccr.2014.10.008Search in Google Scholar
[22] M. G. Ma, J. F. Zhu, Y. J. Zhu, R. C. Sun. Chem. -Asian J.9, 2378 (2014).10.1002/asia.201402288Search in Google Scholar PubMed
[23] D. D. Young, J. Nichols, R. M. Kelly, A. Deiters. J. Am. Chem. Soc.130, 10048 (2008).10.1021/ja802404gSearch in Google Scholar PubMed
[24] W. H. Xiao, X. M. Zhang, X. Wang, W. J. Niu, L. J. Han. Bioresources10, 4038 (2015).10.15376/biores.10.3.4038-4047Search in Google Scholar
[25] J. Tharun, G. Mathai, A. C. Kathalikkattil, R. Roshan, J.-Y. Kwak, D.-W. Park. Green Chem.15, 1673 (2013).10.1039/c3gc40729bSearch in Google Scholar
[26] X. Yang, J. Wu, X. Mao, T. F. Jamison, T. A. Hatton. Chem. Commun.50, 3245 (2014).10.1039/c4cc00252kSearch in Google Scholar PubMed
[27] D. M. Pearson, N. R. Conley, R. M. Waymouth. Adv. Synth. Catal.353, 3007 (2011).10.1002/adsc.201100240Search in Google Scholar
[28] L. Liao, C. Zhang, S. Gong. Eur. Polym. J.43, 4289 (2007).10.1016/j.eurpolymj.2007.07.009Search in Google Scholar
[29] Z. Zhang, C. Wu, J. Ma, J. Song, H. Fan, J. Liu, Q. Zhu, B. Han. Green Chem.17, 1633 (2015).10.1039/C4GC02199ASearch in Google Scholar
©2018 IUPAC & De Gruyter. This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. For more information, please visit: http://creativecommons.org/licenses/by-nc-nd/4.0/
Articles in the same Issue
- Frontmatter
- In this issue
- Conference papers
- Papers from the 6th International IUPAC Conference on Green Chemistry (ICGC-6)
- Microwave assisted synthesis of glycerol carbonate from glycerol and urea
- Silica gel mediated oxidative C–O coupling of β-dicarbonyl compounds with malonyl peroxides in solvent-free conditions
- Definition of green synthetic tools based on safer reaction media, heterogeneous catalysis, and flow technology
- Heavy metal removal from waste waters by phosphonate metal organic frameworks
- A clean and simple method for deprotection of phosphines from borane complexes
- Development and treatment procedure of arsenic-contaminated water using a new and green chitosan sorbent: kinetic, isotherm, thermodynamic and dynamic studies
- Bio-adsorbent derived from papaya peel waste and magnetic nanoparticles fabricated for lead determination
- 5-Membered cyclic ethers via phenonium ion mediated cyclization through carbonate chemistry
- Synergy in food, energy and advanced materials production from biomass
- Step economy strategy for the synthesis of amphoteric aminoaldehydes, key intermediates for reduced hydantoins
- Separation technology meets green chemistry: development of magnetically recoverable catalyst supports containing silica, ceria, and titania
- Green chemistry and sustainable development: approaches to chemical footprint analysis
- Greener solvents for solid-phase organic synthesis
- Photocatalytic hydrogenolysis of allylic alcohols for rapid access to platform chemicals and fine chemicals
- IUPAC Recommendations
- Definition of the mole (IUPAC Recommendation 2017)
- Terminology of separation methods (IUPAC Recommendations 2017)
Articles in the same Issue
- Frontmatter
- In this issue
- Conference papers
- Papers from the 6th International IUPAC Conference on Green Chemistry (ICGC-6)
- Microwave assisted synthesis of glycerol carbonate from glycerol and urea
- Silica gel mediated oxidative C–O coupling of β-dicarbonyl compounds with malonyl peroxides in solvent-free conditions
- Definition of green synthetic tools based on safer reaction media, heterogeneous catalysis, and flow technology
- Heavy metal removal from waste waters by phosphonate metal organic frameworks
- A clean and simple method for deprotection of phosphines from borane complexes
- Development and treatment procedure of arsenic-contaminated water using a new and green chitosan sorbent: kinetic, isotherm, thermodynamic and dynamic studies
- Bio-adsorbent derived from papaya peel waste and magnetic nanoparticles fabricated for lead determination
- 5-Membered cyclic ethers via phenonium ion mediated cyclization through carbonate chemistry
- Synergy in food, energy and advanced materials production from biomass
- Step economy strategy for the synthesis of amphoteric aminoaldehydes, key intermediates for reduced hydantoins
- Separation technology meets green chemistry: development of magnetically recoverable catalyst supports containing silica, ceria, and titania
- Green chemistry and sustainable development: approaches to chemical footprint analysis
- Greener solvents for solid-phase organic synthesis
- Photocatalytic hydrogenolysis of allylic alcohols for rapid access to platform chemicals and fine chemicals
- IUPAC Recommendations
- Definition of the mole (IUPAC Recommendation 2017)
- Terminology of separation methods (IUPAC Recommendations 2017)

