Direct contribution of angiogenic factors to neurodevelopment: a focus on angiopoietins
-
Robert Luck
Abstract
Over the last two decades, it has become clear that classical molecules that regulate neurodevelopment also play an important role in directly regulating the development of the vascular system and vice versa. The prototypical angiogenic ligand vascular endothelial growth factor (VEGF) is by now also regarded as a molecular regulator of different neurodevelopmental processes, such as neuronal progenitor proliferation, migration and differentiation, dendritic and axonal branching and synaptogenesis. The direct effect of other classical angiogenic factors, such as angiopoietins and its receptor Tie2, on neurodevelopmental processes remains less defined. Recent work from our group indicates that the angiopoietin-Tie2 pathway does not only regulate blood vessel formation and stabilization but also simultaneously affect neuronal dendritogenesis in a cell-autonomous manner. In this mini-review, we will integrate our findings within the current understanding of the neurovascular link and within the previous knowledge of the potential effects of angiopoietins in the neuronal context.
Zusammenfassung
Während der letzten beiden Jahrzehnte hat sich gezeigt, dass Moleküle die bekanntermaßen die neuronale Entwicklung regulieren, auch eine wichtige Rolle bei der Entwicklung des vaskulären Systems spielen. Der prototypische angiogene Wachstumsfaktor Vascular Endothelial Growth Factor (VEGF) wird heute auch als molekularer Regulator verschiedener neuronaler Entwicklungsprozesse angesehen, welche von der Proliferation, Migration und Differenzierung neuronaler Vorläuferzellen bis hin zur Verzweigung von Dendriten und Axonen sowie der Bildung von Synapsen reichen. Der direkte Effekt von anderen klassischen, angiogenen Faktoren, wie beispielsweise Angiopoietine und deren Rezeptor Tie2, auf die neuronale Entwicklung ist sehr viel weniger untersucht. In einer aktuellen Studie unserer Gruppe konnten wir zeigen, dass der Angiopoietin-Tie2 Signalweg nicht nur das Blutgefäßwachstum sondern simultan auch die neuronale Dendritogenese in einer zell-autonomem Weise reguliert. In diesem Mini-Review wollen wir unsere Ergebnisse in die heutigen Erkenntnisse über die Neurovaskuläre-Wechselwirkung sowie die bekannten Effekte von Angiopoietinen im neuronalen Kontext einbinden.
The concept of the neurovascular link
Research of the past decades highlighted the term neurovascular link, as a research concept trying to understand the similarities and parallelisms between the vascular and the nervous system. Powered by advancing technologies and increasing experimental sensitivity, a number of research studies have demonstrated that both the neuronal and the vascular systems have many more similarities than previously anticipated. Signaling pathways that were classically discovered in either the vascular or the neuronal context are today known to be cross-expressed and functionally affect the respective other one (Paredes et al., 2018; Walchli et al., 2015). In particular, from the vascular perspective, the best-studied molecule is the prototypic angiogenic factor vascular endothelial growth factor (VEGF). Extensive research at the interface of neuro and vascular biology has shown that VEGF is able to directly act on neural cells and participate in the regulation of different neural-related processes (Carmeliet and Ruiz de Almodovar, 2013). Work from us and other research groups contributed to the characterization of VEGF as a factor regulating neurogenesis, axon guidance, neuronal migration, motoneuron vascularization and hippocampal dendritic and axon branching (Erskine et al., 2011; Harde et al., 2019; Himmels et al., 2017; Luck et al., 2019; Mackenzie and Ruhrberg, 2012; Ruiz de Almodovar et al., 2010, 2011; Schwarz et al., 2004). Particularly for the latter, with the support from the Schram Foundation, we were able to characterize that the VEGF receptor VEGFR2 is expressed in CA3 hippocampal neurons and that a direct VEGF/VEGFR2 signaling is required for proper CA3 axon branching during development (Luck et al., 2019). While the function of VEGF in the nervous system is starting to be well understood, little is known about whether other essential angiogenic pathways, such as the angiopoietin-Tie pathway, can act as potential factors signaling directly on neural cells, or as neurovascular communication signals.
The angiopoietin-Tie pathway
The Tie (Tyrosine kinase receptors with Immunoglobulin-like and EGF-like domain) receptors, Tie1 and Tie2, were first described in the 1990s to be highly expressed in the vascular system (Dumont et al., 1992; Partanen et al., 1992; Tanaka et al., 1993). Their amino-terminal ectodomain is composed of two Ig-like domains, followed by three epidermal growth factor (EGF)-like repeats, one Ig-like domain and three fibronectin-type III domains (Figure 1A) (Barton et al., 2006; Fiedler et al., 2003; Macdonald et al., 2006). Shortly after the discovery of the receptors, researchers identified specific ligands for Tie2 named angiopoietins, with the main two being Ang1 and Ang2 (Davis et al., 1996; Maisonpierre et al., 1997; Reiss et al., 2007). Angiopoietins are glycosylated, secreted proteins composed of a N-terminal superclustering domain followed by the coiled-coil oligomerization domain and the C-terminal fibrinogen-like domain (Figure 1C) (Davis et al., 2003; Kim et al., 2005). Even though Tie1 and Tie2 share a high sequence homology, it is only Tie2 that can bind directly and signal via angiopoietins (Barton et al., 2006). Tie1 on the other hand is important for modulating the signaling properties of Tie2 by forming a heterodimeric complex (Figure 1B) (Saharinen et al., 2005; Seegar et al., 2010). Tie1 additionally controls Tie2 surface presentation (Savant et al., 2015), and it further modulates Tie2 signaling via regulated cleavage of its extracellular domain (Kim et al., 2016; Korhonen et al., 2016) Until recently, Tie1 was considered an orphan receptor that acts as a coreceptor for Tie2. However, leukocyte cell-derived chemotaxin 2 (LECT2) was recently identified as a functional ligand of Tie1 (Xu et al., 2019). The crucial role of Tie receptors in the vascular system is highlighted in studies performed with knockout mice. The complete knockout of Tie2 leads to severe vascular and venous malformations, and mutant embryos die early (E10.5) due to defects in cardiac development (Chu et al., 2016; Dumont et al., 1994; Katoh et al., 1995). Similarly, in the absence of Tie1 mice have an increased and leaky vascular network and die latest at birth due to respiratory failure (Katoh et al., 1995; Puri et al., 1995; Yuan et al., 2007). In the adult, vascular loss of Tie receptors does not cause lethality but leads to reduced angiogenic sprouting and vascular density in case of Tie1 and to reduced arterial angiogenesis and increased venous sprouting in case of Tie2 (Chu et al., 2016).
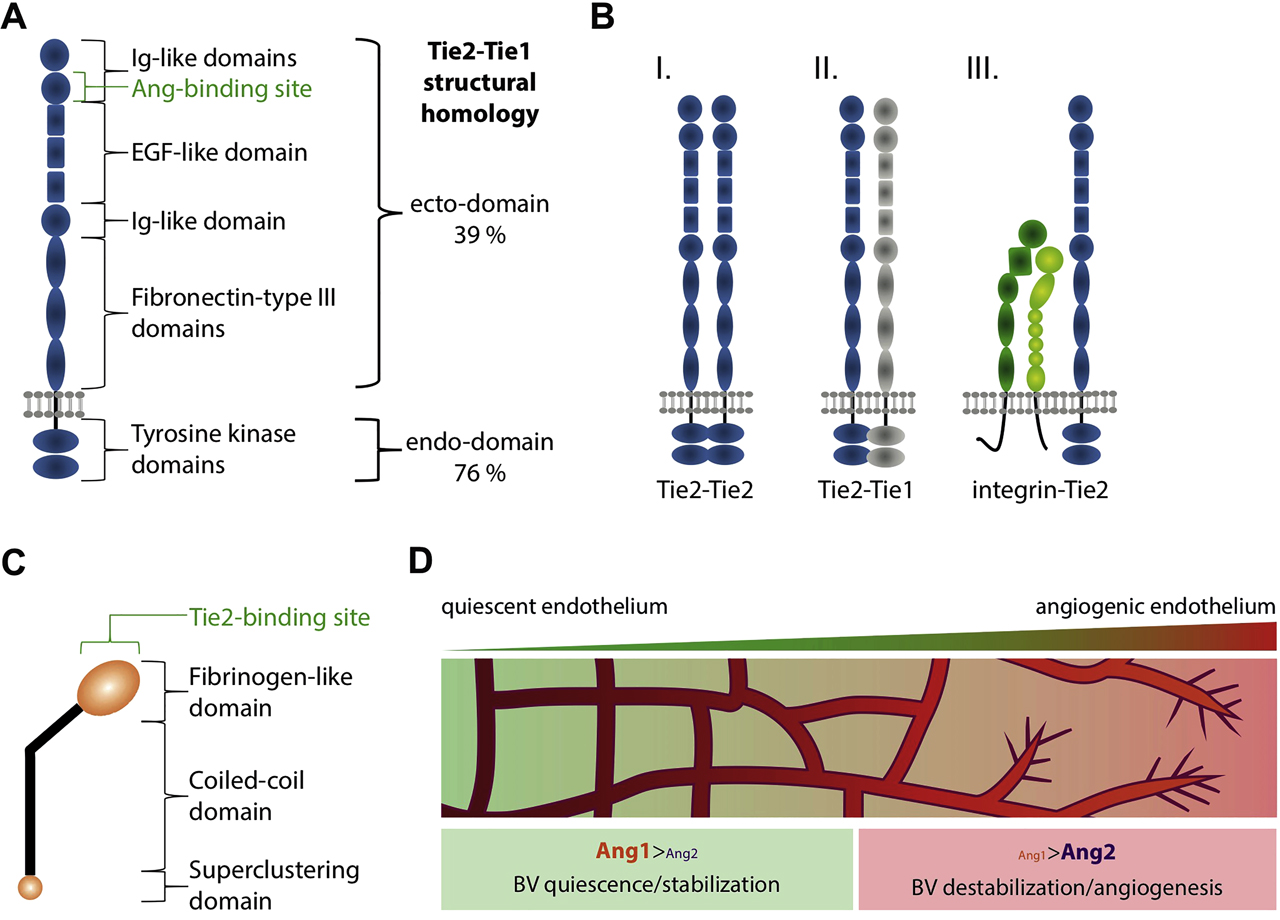
The angiopoietin-Tie2 signaling pathway. (A) The molecular structure of Tie2 (Tyrosine kinase receptors with Immunoglobulin-like and EGF-like domain) is composed of two N-terminal Ig-like domains, followed by three EGF-like repeats, one Ig-like domain and three fibronectin-type III domains. The C-terminal endo-domain shows kinase activity. (B) The Tie2 receptor can form homodimers (I.) or heterodimers with Tie1 (II.) as well as with integrins (III.), what changes its affinity to bind angiopoietin ligands and the downstream signaling. (C) Angiopoietins are glycosylated, secreted proteins containing three main domains, the N-terminal superclustering domain followed by the coiled-coil oligomerization domain and the C-terminal fibrinogen-like domain. (D) The balance of Ang1/Ang2 is indicative for the angiogenic potential in the vascular system. Ang1 induces vascular quiescence, whereas Ang2 promotes angiogenesis through endothelial destabilization. BV: blood vessels.
Angiopoietins in the vascular system
Here we just give a brief overview of the main and classical roles of Ang1 and Ang2 signaling via Tie2 in the vascular system. However, it is important to mention that in the vascular system, angiopoietins can also signal in a Tie2-independent manner via integrins (Bae et al., 2020) For further details, we refer the reader to excellent reviews in the topic (Eklund and Saharinen, 2013; Koh, 2013; Saharinen et al., 2005, 2017). In the angiogenic context, the spatiotemporal expression and role of angiopoietins has been extensively characterized. Vascular endothelial cells comprise the predominant source of Ang2 (Fiedler et al., 2006); however, recent studies have shown that other cell types also express and signal via Ang2, including adipocytes (Bae et al., 2020) and tumor cells (Abdul Pari et al., 2020). On the other hand, Ang1 expression and secretion can be detected from various cells in the proximity of blood vessels, including pericytes and astrocytes (Acker et al., 2001; Davis et al., 1996; Kim et al., 2000). Angiopoietin ligands affect the vascular system in an opposing fashion, with Ang1 and Ang2 showing agonistic and antagonistic functions, respectively (Figure 1D). These opposing roles have been unraveled in studies performed both in vivo and in vitro. More specifically, Ang1 stimulation of endothelial cells in vitro has a proangiogenic effect, leading to a time- and dose-dependent phosphorylation of Tie2 receptors (Bogdanovic et al., 2006). Thus, Tie2 activation through Ang1 reduces vascular leakage and induces endothelial quiescence (Oh et al., 2015). In this line, Ang1-knockout mice show severe vascular malformations and die embryonically, resembling the phenotype observed upon Tie2 loss of function (Suri et al., 1996). Ang2, however, seems to act as a partial agonist. Ang2-knockout animals are generally viable and are characterized by a reduced inflammatory response (Benest et al., 2013). The excess of Ang2 activates endothelial cells and induces angiogenesis, whereby it can counteract the effect of Ang1 (Maisonpierre et al., 1997; Witzenbichler et al., 1998). Therefore, the overexpression of Ang2 causes a phenotype similar to the loss of function of Ang1 and Tie2 (Maisonpierre et al., 1997).
Angiopoietins in the nervous system
Ang/Tie2 in neurogenesis and neuronal survival
In vitro studies show that Ang1 stimulation leads to proliferation and differentiation in neural progenitor cells (NPCs) and neuronal survival in sensory and cortical neurons (Bai et al., 2009; Lim et al., 2015; Rosa et al., 2010; Valable et al., 2003). In adulthood, NPCs of the subventricular zone (SVZ) neurogenic niche express Ang1, which was shown to act as a proneurogenic factor and regulate stem cell dynamics by promoting stem cell proliferation and differentiation in a Tie2-dependent manner (Rosa et al., 2010). Ang2 is expressed in the developing cortex, and specific knockdown of Ang2 in the cortex during embryonic development leads to impaired radial migration of cortical neurons (Marteau et al., 2011). In a pathological setting, Ang2 becomes more highly expressed in endothelial cells (Beck et al., 2000) and in NPCs after stroke and leads to endothelial cell proliferation (Beck et al., 2000) and neuronal differentiation in a Tie2-dependent manner (Androutsellis-Theotokis et al., 2009; Liu et al., 2009).
In summary, there is accumulating evidence suggesting that the angiopoietin-Tie2 pathway contributes to physiological neurogenesis as well as regulates disease progression in the adult central nervous system (CNS) (Figure 2). Future experiments will be needed to better understand its function and its effect in pathology.
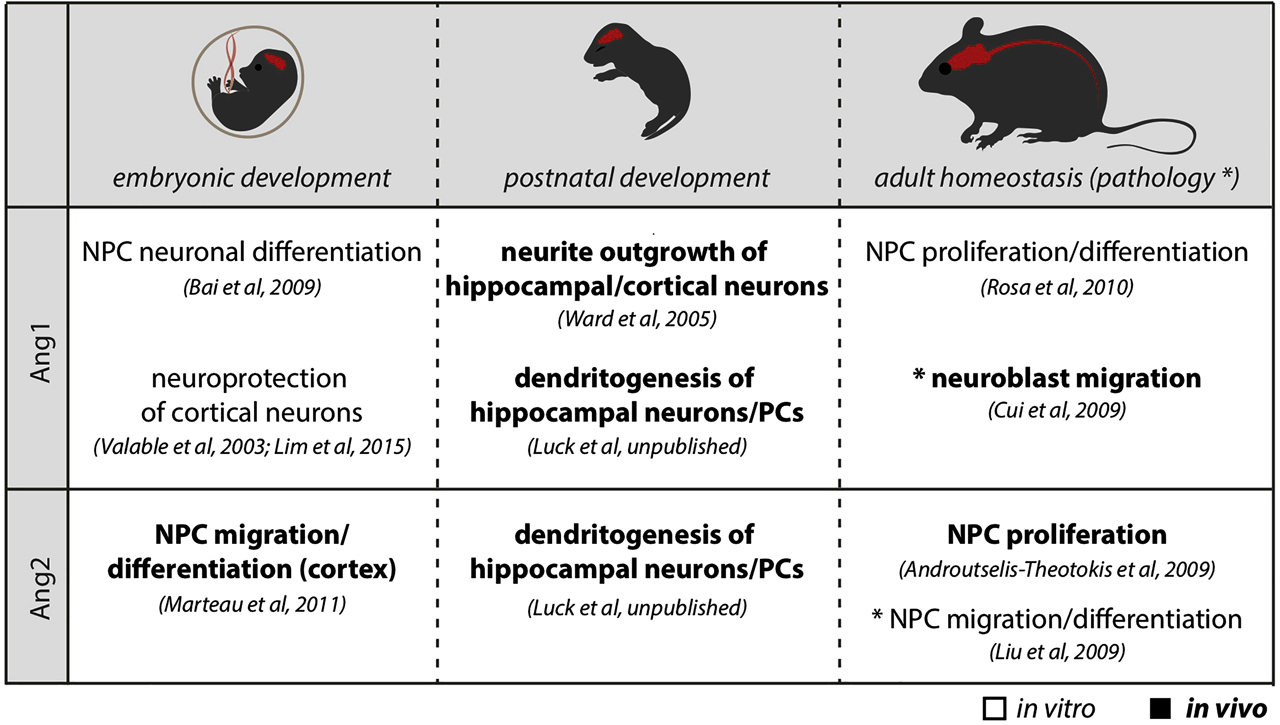
The role of angiopoietins in the CNS. The table summarizes the effect of Ang1 and Ang2 on neural cells of the CNS during development and adulthood. CNS is shown in red. NPCs: neural progenitor cells; PCs: Purkinje cells; CNS: central nervous system.
Ang/Tie2 in neuronal maturation
The contribution of Ang–Tie2 signaling to later neurodevelopmental processes, such as dendritogenesis and synaptogenesis, has so far received little attention. In this line, it was shown that Ang1 stimulation promoted neurite outgrowth of dorsal root ganglia in vitro in a Tie2-dependent (Kosacka et al., 2005) or Tie2-independent manner (Chen et al., 2009). Consistently, Ang1 overexpression in forebrain neurons during development resulted in increased dendritic length as well as qualitative changes in dendritic morphology, suggesting a possible regulatory function of angiopoietins during later stages of neuronal maturation (Ward et al., 2005). However, whether physiological levels of Ang1 would also regulate dendritogenesis and whether a direct signaling via Tie2 in neurons might be responsible for these observations are still open questions (Figure 2).
With the support of the Schram Foundation, our work contributed to answer these open questions as we identified a role for angiopoietin-Tie2 signaling in dendritogenesis of hippocampal neurons and Purkinje cells (PCs) during development. During development, astroglia cells in the hippocampus and cerebellum express Ang1, whereas Tie2 expression is found in CA1 pyramidal neurons and in PCs (Luck et al., unpublished). In the hippocampus, neural cell-specific deletion of Ang1 resulted in aberrant dendritic development with reduced branch complexity of CA1 neurons, which is in line with the above-mentioned studies where Ang1 was administrated or overexpressed (Kosacka et al., 2005; Ward et al., 2005). Interestingly, the role of Ang1 during dendritogenesis does not seem to be restricted to hippocampal neurons as dendritic branching in PCs of the cerebellum was also affected in neural-specific Ang1-knockout mice. PC dendrites are unique, as they show a particular high degree of arborization that is characterized by a strong planar orientation and dendritic self-avoidance. Neural loss of Ang1 caused a reduced dendritic complexity of PCs with aberrant dendritic planarity and dendritic self-avoidance, suggesting a more general role of Ang1 on dendritic development (Figure 2). Consistently, specific deletion of Tie2 in PCs in vivo caused a reduced PC-dendritic development. Simultaneously, Ang2 is expressed in endothelial cells and Ang2-knockout mice present a similar PC branching phenotype (Luck et al., unpublished). This study showed that angiopoietins do not just regulate early angiogenesis and progenitor development but also contribute to later processes of differentiation and cellular maintenance.
Concluding remarks
Research of the last decade has highlighted that to understand CNS development and functionality, one should study not only neuronal function but also other cellular components of the CNS, such as glia cells and blood vessels. Indeed, there are many examples where neurovascular communication is essential for proper CNS formation and function. Thus, understanding the intercellular communication and the molecular pathways of such interactions, among them VEGF and angiopoietins signaling pathways in neural cells, will bring further insights into the complex regulation of CNS formation and function.
Funding source: Schram Foundation
About the authors

Dr. Robert Luck: postdoctoral researcher

Dr. Andromachi Karakatsani: postdoctoral researcher

Prof. Dr. Carmen Ruiz de Almodovar: professor for vascular dysfunction
Acknowledgments
The authors thank the Schram Foundation for supporting this work.
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: None declared.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
Abdul Pari, A.A., Singhal, M., Hubers, C., Mogler, C., Schieb, B., Gampp, A., Gengenbacher, N., Reynolds, L.E., Terhardt, D., Geraud, C., et al. (2020). Tumor cell-derived angiopoietin-2 promotes metastasis in melanoma. Cancer Res. 80, 2586–2598. https://doi.org/10.1158/0008-5472.can-19-2660.Suche in Google Scholar
Acker, T., Beck, H., and Plate, K.H. (2001). Cell type specific expression of vascular endothelial growth factor and angiopoietin-1 and -2 suggests an important role of astrocytes in cerebellar vascularization. Mech. Dev. 108, 45–57. https://doi.org/10.1016/s0925-4773(01)00471-3.Suche in Google Scholar
Androutsellis-Theotokis, A., Rueger, M.A., Park, D.M., Mkhikian, H., Korb, E., Poser, S.W., Walbridge, S., Munasinghe, J., Koretsky, A.P., Lonser, R.R., et al. (2009). Targeting neural precursors in the adult brain rescues injured dopamine neurons. Proc. Natl. Acad. Sci. USA 106, 13570–13575. https://doi.org/10.1073/pnas.0905125106.Suche in Google Scholar
Bae, H., Hong, K.Y., Lee, C.K., Jang, C., Lee, S.J., Choe, K., Offermanns, S., He, Y., Lee, H.J., and Koh, G.Y. (2020). Angiopoietin-2-integrin alpha5beta1 signaling enhances vascular fatty acid transport and prevents ectopic lipid-induced insulin resistance. Nat. Commun. 11, 2980. https://doi.org/10.1038/s41467-020-16795-4.Suche in Google Scholar
Bai, Y., Cui, M., Meng, Z., Shen, L., He, Q., Zhang, X., Chen, F., and Xiao, J. (2009). Ectopic expression of angiopoietin-1 promotes neuronal differentiation in neural progenitor cells through the Akt pathway. Biochem. Biophys. Res. Commun. 378, 296–301. https://doi.org/10.1016/j.bbrc.2008.11.052.Suche in Google Scholar
Barton, W.A., Tzvetkova-Robev, D., Miranda, E.P., Kolev, M.V., Rajashankar, K.R., Himanen, J.P., and Nikolov, D.B. (2006). Crystal structures of the Tie2 receptor ectodomain and the angiopoietin-2-Tie2 complex. Nat. Struct. Mol. Biol. 13, 524–532. https://doi.org/10.1038/nsmb1101.Suche in Google Scholar
Beck, H., Acker, T., Wiessner, C., Allegrini, P.R., and Plate, K.H. (2000). Expression of angiopoietin-1, angiopoietin-2, and tie receptors after middle cerebral artery occlusion in the rat. Am. J. Pathol. 157, 1473–1483. https://doi.org/10.1016/s0002-9440(10)64786-4.Suche in Google Scholar
Benest, A.V., Kruse, K., Savant, S., Thomas, M., Laib, A.M., Loos, E.K., Fiedler, U., and Augustin, H.G. (2013). Angiopoietin-2 is critical for cytokine-induced vascular leakage. PloS One 8, e70459. https://doi.org/10.1371/journal.pone.0070459.Suche in Google Scholar PubMed PubMed Central
Bogdanovic, E., Nguyen, V.P., and Dumont, D.J. (2006). Activation of Tie2 by angiopoietin-1 and angiopoietin-2 results in their release and receptor internalization. J. Cell Sci. 119, 3551–3560. https://doi.org/10.1242/jcs.03077.Suche in Google Scholar PubMed
Carmeliet, P. and Ruiz de Almodovar, C. (2013). VEGF ligands and receptors: implications in neurodevelopment and neurodegeneration. Cell. Mol. Life Sci. 70, 1763–1778. https://doi.org/10.1007/s00018-013-1283-7.Suche in Google Scholar PubMed
Chen, X., Fu, W., Tung, C.E., and Ward, N.L. (2009). Angiopoietin-1 induces neurite outgrowth of PC12 cells in a Tie2-independent, beta1-integrin-dependent manner. Neurosci. Res. 64, 348–354. https://doi.org/10.1016/j.neures.2009.04.007.Suche in Google Scholar
Chu, M., Li, T., Shen, B., Cao, X., Zhong, H., Zhang, L., Zhou, F., Ma, W., Jiang, H., Xie, P., et al. (2016). Angiopoietin receptor Tie2 is required for vein specification and maintenance via regulating COUP-TFII. Elife 5. https://doi.org/10.7554/elife.21032.Suche in Google Scholar
D’Amico, G., Korhonen, E.A., Anisimov, A., Zarkada, G., Holopainen, T., Hagerling, R., Kiefer, F., Eklund, L., Sormunen, R., Elamaa, H., et al. (2014). Tie1 deletion inhibits tumor growth and improves angiopoietin antagonist therapy. J. Clin. Invest. 124, 824–834. https://doi.org/10.1172/JCI68897.Suche in Google Scholar
Davis, S., Aldrich, T.H., Jones, P.F., Acheson, A., Compton, D.L., Jain, V., Ryan, T.E., Bruno, J., Radziejewski, C., Maisonpierre, P.C., et al. (1996). Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell 87, 1161–1169. https://doi.org/10.1016/s0092-8674(00)81812-7.Suche in Google Scholar
Davis, S., Papadopoulos, N., Aldrich, T.H., Maisonpierre, P.C., Huang, T., Kovac, L., Xu, A., Leidich, R., Radziejewska, E., Rafique, A., et al. (2003). Angiopoietins have distinct modular domains essential for receptor binding, dimerization and superclustering. Nat. Struct. Biol. 10, 38–44. https://doi.org/10.1038/nsb880.Suche in Google Scholar PubMed
Dumont, D.J., Gradwohl, G., Fong, G.H., Puri, M.C., Gertsenstein, M., Auerbach, A., and Breitman, M.L. (1994). Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 8, 1897–1909. https://doi.org/10.1101/gad.8.16.1897.Suche in Google Scholar PubMed
Dumont, D.J., Yamaguchi, T.P., Conlon, R.A., Rossant, J., and Breitman, M.L. (1992). tek, a novel tyrosine kinase gene located on mouse chromosome 4, is expressed in endothelial cells and their presumptive precursors. Oncogene 7, 1471–1480.Suche in Google Scholar
Eklund, L. and Saharinen, P. (2013). Angiopoietin signaling in the vasculature. Exp. Cell Res. 319, 1271–1280. https://doi.org/10.1016/j.yexcr.2013.03.011.Suche in Google Scholar PubMed
Erskine, L., Reijntjes, S., Pratt, T., Denti, L., Schwarz, Q., Vieira, J.M., Alakakone, B., Shewan, D., and Ruhrberg, C. (2011). VEGF signaling through neuropilin 1 guides commissural axon crossing at the optic chiasm. Neuron 70, 951–965. https://doi.org/10.1016/j.neuron.2011.02.052.Suche in Google Scholar PubMed PubMed Central
Fiedler, U., Krissl, T., Koidl, S., Weiss, C., Koblizek, T., Deutsch, U., Martiny-Baron, G., Marme, D., and Augustin, H.G. (2003). Angiopoietin-1 and angiopoietin-2 share the same binding domains in the Tie-2 receptor involving the first Ig-like loop and the epidermal growth factor-like repeats. J. Biol. Chem. 278, 1721–1727. https://doi.org/10.1074/jbc.m208550200.Suche in Google Scholar PubMed
Fiedler, U., Reiss, Y., Scharpfenecker, M., Grunow, V., Koidl, S., Thurston, G., Gale, N.W., Witzenrath, M., Rosseau, S., Suttorp, N., et al. (2006). Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat. Med. 12, 235–239. https://doi.org/10.1038/nm1351.Suche in Google Scholar PubMed
Harde, E., Nicholson, L., Furones Cuadrado, B., Bissen, D., Wigge, S., Urban, S., Segarra, M., Ruiz de Almodovar, C., and Acker-Palmer, A. (2019). EphrinB2 regulates VEGFR2 during dendritogenesis and hippocampal circuitry development. Elife 8. https://doi.org/10.7554/elife.49819.Suche in Google Scholar PubMed PubMed Central
Himmels, P., Paredes, I., Adler, H., Karakatsani, A., Luck, R., Marti, H.H., Ermakova, O., Rempel, E., Stoeckli, E.T., and Ruiz de Almodovar, C. (2017). Motor neurons control blood vessel patterning in the developing spinal cord. Nat. Commun. 8, 14583. https://doi.org/10.1038/ncomms14583.Suche in Google Scholar PubMed PubMed Central
Katoh, O., Tauchi, H., Kawaishi, K., Kimura, A., and Satow, Y. (1995). Expression of the vascular endothelial growth factor (VEGF) receptor gene, KDR, in hematopoietic cells and inhibitory effect of VEGF on apoptotic cell death caused by ionizing radiation. Cancer Res. 55, 5687–5692.Suche in Google Scholar
Kim, I., Kim, H.G., So, J.N., Kim, J.H., Kwak, H.J., and Koh, G.Y. (2000). Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3′-Kinase/Akt signal transduction pathway. Circ. Res. 86, 24–29. https://doi.org/10.1161/01.res.86.1.24.Suche in Google Scholar PubMed
Kim, K.T., Choi, H.H., Steinmetz, M.O., Maco, B., Kammerer, R.A., Ahn, S.Y., Kim, H.Z., Lee, G.M., and Koh, G.Y. (2005). Oligomerization and multimerization are critical for angiopoietin-1 to bind and phosphorylate Tie2. J. Biol. Chem. 280, 20126–20131. https://doi.org/10.1074/jbc.m500292200.Suche in Google Scholar
Kim, M., Allen, B., Korhonen, E.A., Nitschke, M., Yang, H.W., Baluk, P., Saharinen, P., Alitalo, K., Daly, C., Thurston, G., et al. (2016). Opposing actions of angiopoietin-2 on Tie2 signaling and FOXO1 activation. J. Clin. Invest. 126, 3511–3525. https://doi.org/10.1172/jci84871.Suche in Google Scholar
Koh, G.Y. (2013). Orchestral actions of angiopoietin-1 in vascular regeneration. Trends Mol. Med. 19, 31–39. https://doi.org/10.1016/j.molmed.2012.10.010.Suche in Google Scholar PubMed
Korhonen, E.A., Lampinen, A., Giri, H., Anisimov, A., Kim, M., Allen, B., Fang, S., D’Amico, G., Sipila, T.J., Lohela, M., et al. (2016). Tie1 controls angiopoietin function in vascular remodeling and inflammation. J. Clin. Invest. 126, 3495–3510. https://doi.org/10.1172/jci84923.Suche in Google Scholar
Kosacka, J., Figiel, M., Engele, J., Hilbig, H., Majewski, M., and Spanel-Borowski, K. (2005). Angiopoietin-1 promotes neurite outgrowth from dorsal root ganglion cells positive for Tie-2 receptor. Cell Tissue Res. 320, 11–19. https://doi.org/10.1007/s00441-004-1068-2.Suche in Google Scholar PubMed
Lim, J.S., Koh, G.Y., and Koh, J.Y. (2015). Angiopoietin-1 blocks neurotoxic zinc entry into cortical cells via PIP2 hydrolysis-mediated ion channel inhibition. Neurobiol. Dis. 81, 203–213. https://doi.org/10.1016/j.nbd.2014.11.001.Suche in Google Scholar PubMed
Liu, X.S., Chopp, M., Zhang, R.L., Hozeska-Solgot, A., Gregg, S.C., Buller, B., Lu, M., and Zhang, Z.G. (2009). Angiopoietin 2 mediates the differentiation and migration of neural progenitor cells in the subventricular zone after stroke. J. Biol. Chem. 284, 22680–22689. https://doi.org/10.1074/jbc.m109.006551.Suche in Google Scholar
Luck, R., Karakatsani, A., Shah, B., Schermann, G., Adler, H., Kupke, J., Tisch, N., Jeong, H., Müller, M.K., de Palma, M., et al. (unpublished). The angiopoietin-Tie2 pathway regulates Purkinje cell dendritogenesis in a cell-autonomous manner.Suche in Google Scholar
Luck, R., Urban, S., Karakatsani, A., Harde, E., Sambandan, S., Nicholson, L., Haverkamp, S., Mann, R., Martin-Villalba, A., Schuman, E.M., et al. (2019). VEGF/VEGFR2 signaling regulates hippocampal axon branching during development. Elife 8. https://doi.org/10.7554/elife.49818.Suche in Google Scholar PubMed PubMed Central
Macdonald, P.R., Progias, P., Ciani, B., Patel, S., Mayer, U., Steinmetz, M.O., and Kammerer, R.A. (2006). Structure of the extracellular domain of Tie receptor tyrosine kinases and localization of the angiopoietin-binding epitope. J. Biol. Chem. 281, 28408–28414. https://doi.org/10.1074/jbc.m605219200.Suche in Google Scholar PubMed
Mackenzie, F. and Ruhrberg, C. (2012). Diverse roles for VEGF-A in the nervous system. Development 139, 1371–1380. https://doi.org/10.1242/dev.072348.Suche in Google Scholar PubMed
Maisonpierre, P.C., Suri, C., Jones, P.F., Bartunkova, S., Wiegand, S.J., Radziejewski, C., Compton, D., McClain, J., Aldrich, T.H., Papadopoulos, N., et al. (1997). Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277, 55–60. https://doi.org/10.1126/science.277.5322.55.Suche in Google Scholar PubMed
Marteau, L., Pacary, E., Valable, S., Bernaudin, M., Guillemot, F., and Petit, E. (2011). Angiopoietin-2 regulates cortical neurogenesis in the developing telencephalon. Cereb Cortex 21, 1695–1702. https://doi.org/10.1093/cercor/bhq243.Suche in Google Scholar PubMed
Oh, N., Kim, K., Kim, S.J., Park, I., Lee, J.E., Seo, Y.S., An, H.J., Kim, H.M., and Koh, G.Y. (2015). A designed angiopoietin-1 variant, dimeric CMP-ang1 activates Tie2 and stimulates angiogenesis and vascular stabilization in N-glycan dependent manner. Sci. Rep. 5, 15291. https://doi.org/10.1038/srep15291.Suche in Google Scholar PubMed PubMed Central
Paredes, I., Himmels, P., and Ruiz de Almodovar, C. (2018). Neurovascular communication during CNS development. Dev. Cell 45, 10–32. https://doi.org/10.1016/j.devcel.2018.01.023.Suche in Google Scholar PubMed
Partanen, J., Armstrong, E., Makela, T.P., Korhonen, J., Sandberg, M., Renkonen, R., Knuutila, S., Huebner, K., and Alitalo, K. (1992). A novel endothelial cell surface receptor tyrosine kinase with extracellular epidermal growth factor homology domains. Mol. Cell Biol. 12, 1698–1707. https://doi.org/10.1128/mcb.12.4.1698.Suche in Google Scholar
Puri, M.C., Rossant, J., Alitalo, K., Bernstein, A., and Partanen, J. (1995). The receptor tyrosine kinase TIE is required for integrity and survival of vascular endothelial cells. EMBO J. 14, 5884–5891. https://doi.org/10.1002/j.1460-2075.1995.tb00276.x.Suche in Google Scholar PubMed PubMed Central
Reiss, Y., Droste, J., Heil, M., Tribulova, S., Schmidt, M.H.H., Schaper, W., Dumont, D.J., and Plate, K.H. (2007). Angiopoietin-2 impairs revascularization after limb ischemia. Circ. Res. 101, 88–96. https://doi.org/10.1161/circresaha.106.143594.Suche in Google Scholar PubMed
Rosa, A.I., Goncalves, J., Cortes, L., Bernardino, L., Malva, J.O., and Agasse, F. (2010). The angiogenic factor angiopoietin-1 is a proneurogenic peptide on subventricular zone stem/progenitor cells. J. Neurosci. 30, 4573–4584. https://doi.org/10.1523/jneurosci.5597-09.2010.Suche in Google Scholar
Ruiz de Almodovar, C., Coulon, C., Salin, P.A., Knevels, E., Chounlamountri, N., Poesen, K., Hermans, K., Lambrechts, D., Van Geyte, K., Dhondt, J., et al. (2010). Matrix-binding vascular endothelial growth factor (VEGF) isoforms guide granule cell migration in the cerebellum via VEGF receptor Flk1. J. Neurosci. 30, 15052–15066. https://doi.org/10.1523/jneurosci.0477-10.2010.Suche in Google Scholar
Ruiz de Almodovar, C., Fabre, P.J., Knevels, E., Coulon, C., Segura, I., Haddick, P.C., Aerts, L., Delattin, N., Strasser, G., Oh, W.J., et al. (2011). VEGF mediates commissural axon chemoattraction through its receptor Flk1. Neuron 70, 966–978. https://doi.org/10.1016/j.neuron.2011.04.014.Suche in Google Scholar
Saharinen, P., Kerkela, K., Ekman, N., Marron, M., Brindle, N., Lee, G.M., Augustin, H., Koh, G.Y., and Alitalo, K. (2005). Multiple angiopoietin recombinant proteins activate the Tie1 receptor tyrosine kinase and promote its interaction with Tie2. J. Cell Biol. 169, 239–243. https://doi.org/10.1083/jcb.200411105.Suche in Google Scholar
Saharinen, P., Leppanen, V.M., and Alitalo, K. (2017). SnapShot: angiopoietins and their functions. Cell 171, 724–724 e721. https://doi.org/10.1016/j.cell.2017.10.009.Suche in Google Scholar
Savant, S., La Porta, S., Budnik, A., Busch, K., Hu, J., Tisch, N., Korn, C., Valls, A.F., Benest, A.V., Terhardt, D., et al. (2015). The orphan receptor Tie1 controls angiogenesis and vascular remodeling by differentially regulating Tie2 in tip and stalk cells. Cell Rep. 12, 1761–1773. https://doi.org/10.1016/j.celrep.2015.08.024.Suche in Google Scholar
Schwarz, Q., Gu, C., Fujisawa, H., Sabelko, K., Gertsenstein, M., Nagy, A., Taniguchi, M., Kolodkin, A.L., Ginty, D.D., Shima, D.T., et al. (2004). Vascular endothelial growth factor controls neuronal migration and cooperates with Sema3A to pattern distinct compartments of the facial nerve. Genes Dev. 18, 2822–2834. https://doi.org/10.1101/gad.322904.Suche in Google Scholar
Seegar, T.C., Eller, B., Tzvetkova-Robev, D., Kolev, M.V., Henderson, S.C., Nikolov, D.B., and Barton, W.A. (2010). Tie1–Tie2 interactions mediate functional differences between angiopoietin ligands. Mol Cell 37, 643–655. https://doi.org/10.1016/j.molcel.2010.02.007.Suche in Google Scholar
Suri, C., Jones, P.F., Patan, S., Bartunkova, S., Maisonpierre, P.C., Davis, S., Sato, T.N., and Yancopoulos, G.D. (1996). Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87, 1171–1180. https://doi.org/10.1016/s0092-8674(00)81813-9.Suche in Google Scholar
Tanaka, M., Sato, A., Makino, M., and Tabira, T. (1993). Binding of an SJL T cell clone specific for myelin basic protein to SJL brain microvessel endothelial cells is inhibited by anti-VLA-4 or its ligand, anti-vascular cell adhesion molecule 1 antibody. J. Neuroimmunol. 46, 253–257. https://doi.org/10.1016/0165-5728(93)90256-x.Suche in Google Scholar
Valable, S., Bellail, A., Lesne, S., Liot, G., Mackenzie, E.T., Vivien, D., Bernaudin, M., and Petit, E. (2003). Angiopoietin-1-induced PI3-kinase activation prevents neuronal apoptosis. FASEB J. 17, 443–445. https://doi.org/10.1096/fj.02-0372fje.Suche in Google Scholar PubMed
Walchli, T., Wacker, A., Frei, K., Regli, L., Schwab, M.E., Hoerstrup, S.P., Gerhardt, H., and Engelhardt, B. (2015). Wiring the vascular network with neural cues: a CNS perspective. Neuron 87, 271–296. https://doi.org/10.1016/j.neuron.2015.06.038.Suche in Google Scholar PubMed
Ward, N.L., Putoczki, T., Mearow, K., Ivanco, T.L., and Dumont, D.J. (2005). Vascular-specific growth factor angiopoietin 1 is involved in the organization of neuronal processes. J. Comp. Neurol. 482, 244–256. https://doi.org/10.1002/cne.20422.Suche in Google Scholar PubMed
Witzenbichler, B., Maisonpierre, P.C., Jones, P., Yancopoulos, G.D., and Isner, J.M. (1998). Chemotactic properties of angiopoietin-1 and -2, ligands for the endothelial-specific receptor tyrosine kinase Tie2. J. Biol. Chem. 273, 18514–18521. https://doi.org/10.1074/jbc.273.29.18514.Suche in Google Scholar PubMed
Xu, M., Xu, H.H., Lin, Y., Sun, X., Wang, L.J., Fang, Z.P., Su, X.H., Liang, X.J., Hu, Y., Liu, Z.M., et al. (2019). LECT2, a ligand for Tie1, plays a crucial role in liver fibrogenesis. Cell 178, 1478–1492 e1420. https://doi.org/10.1016/j.cell.2019.07.021.Suche in Google Scholar PubMed
Yuan, H.T., Venkatesha, S., Chan, B., Deutsch, U., Mammoto, T., Sukhatme, V.P., Woolf, A.S., and Karumanchi, S.A. (2007). Activation of the orphan endothelial receptor Tie1 modifies Tie2-mediated intracellular signaling and cell survival. FASEB J. 21, 3171–3183. https://doi.org/10.1096/fj.07-8487com.Suche in Google Scholar PubMed
© 2020 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Editorial
- Editorial
- Review articles
- Synapses, networks, brain development – funding basic neuroscience research in Germany by the Schram Foundation
- Neuronal functions of clathrin-associated endocytic sorting adaptors – from molecules to disease
- Mechanisms of synaptic vesicle recycling provide a platform to explore mechanisms of neurodegeneration
- Optogenetic analyses of neuronal networks that generate behavior in Caenorhabditis elegans
- Direct contribution of angiogenic factors to neurodevelopment: a focus on angiopoietins
- The role of the dentate gyrus in mnemonic functions
- Presentation of scientific institutions
- Armin Schram: a sponsor of curiosity-driven brain research
- Nachrichten aus der Gesellschaft
- Nachrichten aus der Gesellschaft
Artikel in diesem Heft
- Frontmatter
- Editorial
- Editorial
- Review articles
- Synapses, networks, brain development – funding basic neuroscience research in Germany by the Schram Foundation
- Neuronal functions of clathrin-associated endocytic sorting adaptors – from molecules to disease
- Mechanisms of synaptic vesicle recycling provide a platform to explore mechanisms of neurodegeneration
- Optogenetic analyses of neuronal networks that generate behavior in Caenorhabditis elegans
- Direct contribution of angiogenic factors to neurodevelopment: a focus on angiopoietins
- The role of the dentate gyrus in mnemonic functions
- Presentation of scientific institutions
- Armin Schram: a sponsor of curiosity-driven brain research
- Nachrichten aus der Gesellschaft
- Nachrichten aus der Gesellschaft

