Neuronal functions of clathrin-associated endocytic sorting adaptors – from molecules to disease
-
Natalia L. Kononenko
in vivo neuroanatomy to understand the role of membrane trafficking in the pathogenesis of neurodegeneration.and Volker Haucke
Abstract
Communication in the central nervous system is based on the transmission of electrical signals at specialized junctions between nerve cells termed synapses. During chemical neurotransmission, tiny membrane spheres called synaptic vesicles that are packed with neurotransmitters elicit a postsynaptic response by fusing with the presynaptic membrane and releasing their content into the synaptic cleft. Synaptic vesicle fusion is followed by the reuptake of the membrane by endocytosis and the local reformation of functional synaptic vesicles within the presynaptic compartment to sustain further rounds of neurotransmitter release. Here, we provide an overview of the clathrin-associated endocytic adaptor proteins that help to sort and recycle synaptic vesicles during presynaptic activity. These adaptors also serve additional functions in the turnover of defective or aged synaptic components and in the retrograde axonal transport of important signaling molecules by regulating the formation or transport of autophagosomes. Endocytic adaptors thus play multiple roles in the maintenance of synaptic function. Defects in their expression or function can lead to neurodegenerative and neurological diseases.
Zusammenfassung
Kommunikation im Zentralnervensystem basiert auf der Umwandlung elektrischer in chemische Signale an spezialisierten Kontaktstellen zwischen Nervenzellen, die Synapsen heißen. Während der chemischen Erregungsübertragung fusionieren winzige Membransphären, synaptische Vesikel genannt, welche mit Neurotransmitter Molekülen beladen sind, mit der präsynaptischen Membran, um so ihren Inhalt in den synaptischen Spalt freizusetzen und eine postsynaptische Antwort auszulösen. Der Fusion synaptischer Vesikel folgt die Wiederaufnahme der Membran und die lokale Rückbildung funktioneller synaptischer Vesikel im präsynaptischen Kompartiment, um weitere Runden der Neurotransmitterfreisetzung aufrecht zu erhalten. Hier geben wir einen Überblick über die mit Clathrin assoziierten endozytotischen Adaptoren, welche die Komponenten synaptischer Vesikel sortieren und recyceln, um so die korrekte Wiederherstellung funktioneller synaptischer Vesikel sicherzustellen. Diese Adaptoren üben ferner zusätzliche Funktionen im regulierten Umsatz defekter oder alter Komponenten der Synapse und im retrograden Transport wichtiger Signalmoleküle aus, indem sie die Bildung oder den Transport von Autophagosomen regulieren. Endozytotische Sortieradaptoren spielen demzufolge multiple Rollen bei der Aufrechterhaltung der synaptischen Funktion. Defekte in ihrer Expression oder Funktion könnten zu neurodegenerativen und neurologischen Krankheiten führen.
Introduction and objectives
The chemical way of neuronal communication involves the exchange of neurotransmitter messengers between neurons at specialized contact sites called synapses. Synapses are comprised of a presynaptic compartment that contains small synaptic vesicles (SVs), filled with neurotransmitter molecules such as glutamate or γ-aminobutyric acid (GABA), and an opposing postsynaptic compartment that harbors neurotransmitter receptors that control the activity of the postsynaptic cell. Neurotransmitter release is triggered by an action potential, which upon its arrival at the presynapse activates voltage-gated calcium channels enriched at a specialized presynaptic part called active zones (AZs). Resulting calcium influx elicits the rapid (in less than a millisecond) fusion of SVs docked at the AZ membrane to release their neurotransmitter content into the synaptic cleft (Sudhof, 2013). The fusion of a single SV thus is a correlate of a neurotransmitter quantum, proposed by Bernhard Katz in the early 1950s to be the physical unit of neurotransmission (Fatt and Katz, 1952). To prevent the expansion of the presynaptic plasma membrane and to locally replenish the pool of SVs, exocytosis is coupled to compensatory internalization of SV membranes and SV reformation by endocytosis (Kononenko and Haucke, 2015; Rizzoli, 2014; Saheki and De Camilli, 2012). Over the lifetime of a nerve cell, SVs undergo hundreds of cycles of exocytosis, endocytosis and SV reformation (Truckenbrodt et al., 2018). Naturally, this has to occur with a high fidelity as the failure to sort and retrieve SV components results in functional impairments of neurotransmission and causes malfunction of neuronal networks.
The objective of this review is to provide an up-to-date overview of proteins, which function as endocytic sorting adaptors at the synapse, and highlight their roles in the maintenance of synaptic function in health and disease.
Endocytosis in neurons
The ability to internalize pieces of the plasma membrane is not a unique property of neurons. In fact, neurons express many of the same proteins known to carry out different forms of endocytosis in non-neuronal cells. Those include clathrin-mediated endocytosis (CME) described in more detail below, macropinocytosis and other forms of clathrin-independent membrane internalization and phagocytosis that is mainly used by immune cells to engulf and digest large particles such as bacteria. Apart from cycles of exocytosis/endocytosis of SVs, neurons employ endocytosis to regulate their cell surface content, e.g. ion channels or nutrient transporters, and to regulate cell polarity and cell signaling processes. For example, developmental neurotrophin signaling is regulated by endocytic proteins such as endophilin (Barker et al., 2002; Burk et al., 2017), while the clathrin adaptors assembly protein (AP) complex-2 (AP-2) (Fiuza et al., 2017; Kastning et al., 2007; Kittler et al., 2005), Huntingtin-interacting protein 1 (HIP1) (Metzler et al., 2003, 2007) and endophilin (Zhang et al., 2017) have been implicated in determining the surface levels of postsynaptic ionotropic α‐amino‐3‐hydroxy‐5-methyl‐4‐isoxazolepropionic acid (AMPA)- or N-Methyl-D-aspartate (NMDA)-type glutamate and GABAA neurotransmitter receptors.
Work by us and others has suggested that neurons ensure the maintenance of functional SVs of the correct size and composition by initially performing clathrin-independent retrieval of SV membranes (Soykan et al., 2016, 2017; Watanabe et al., 2014), followed by sorting and recycling of SV components by CME (Heerssen et al., 2008; Kononenko et al., 2014). Clathrin-independent endocytosis of SV membranes requires linear F-actin filament polymerization by actin-nucleating formins as well as the activity of Bin/amphiphysin/Rvs (BAR) domain proteins such as endophilin, an upstream regulator of the lipid phosphatase synaptojanin, and the oligomeric GTPase dynamin (Ferguson et al., 2007; Soykan et al., 2017; Watanabe and Boucrot, 2017; Watanabe et al., 2018; Wu et al., 2016).
CME and endocytic protein sorting
Vesicle formation by CME allows selective internalization of plasma membrane proteins, followed by their sorting and incorporation into a newly generated vesicle, for example, SV in the case of neurons (Kononenko and Haucke, 2015; Rizzoli, 2014; Saheki and De Camilli, 2012) (Figure 1). It involves the formation of a characteristic lattice-like coat that is mainly comprised of the name-giving protein clathrin, a three-legged scaffold protein highly enriched in nerve terminals. In fact, clathrin was first purified from the brain by Barbara Pearse, more than 10 years after Keith Porter had described clathrin-coated vesicles and pits in electron micrographs from cells and tissues (Roth and Porter, 1964). Subsequent work over decades has unraveled the complex machinery underlying the formation of clathrin-coated vesicles during endocytosis in various cells and tissues.
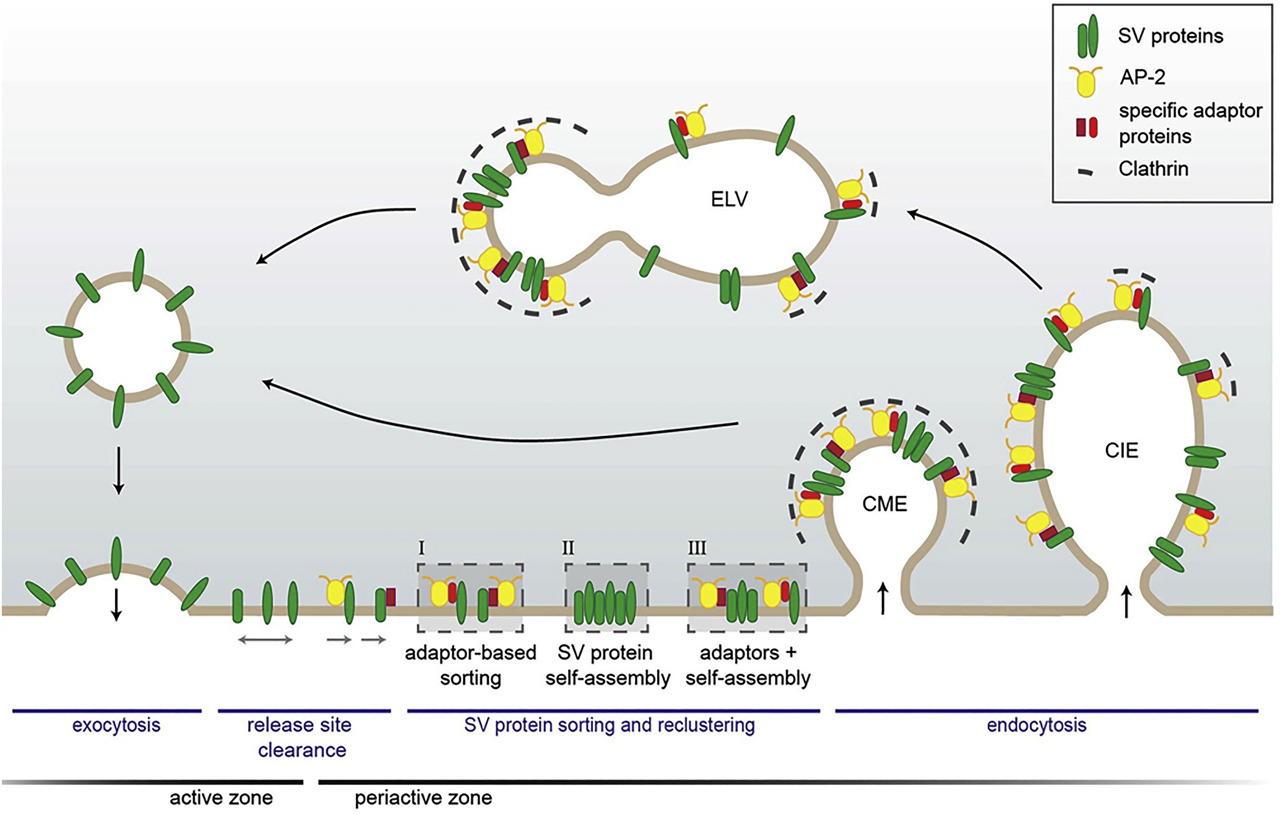
Model of SV protein sorting by endocytic adapters at the presynapse. After full collapse fusion, freely diffusing SV proteins are confined and recaptured by endocytic sorting adapters at the periactive zone. At the plasma membrane, SV proteins might either be clustered by AP-2 and additional cargo-specific adapter proteins (I); they might interact with each other and thereby self-assemble into clusters (II) or form mixed clusters of self-assembled SV proteins together with sorting adapters (III). These clusters can directly be endocytosed from the plasma membrane by CME to reform SVs. However, cargo-specific sorting proteins together with AP-2 and clathrin can also operate on endosome-like vacuoles (ELVs) after clathrin-independent endocytosis (CIE) to recycle SVs with correct protein composition. Reproduced from the study by Kaempf and Maritzen (2017). SV, synaptic vesicle; AP-2, assembly protein complex-2; CME, clathrin-mediated endocytosis.
Mechanistically, CME is initiated by the recruitment of early-acting endocytic proteins, termed adapters, such as Fer/Cip4 homology domain-only (FCHO) proteins, EPS15/ EPS15R, stonins and AP-2, a heterotetramer of two large (α, β2) and two small subunits (μ2, σ2), as well as curvature-inducing proteins (e.g. epsins and AP180 or clathrin assembly lymphoid myeloid [CALM] protein) to the plasma membrane. These adapters link clathrin to the underlying membrane via their association with charged plasma membrane lipids and couple the assembly of the clathrin coat with the selection of transmembrane cargo proteins, i.e. receptors and their ligands. The assembled endocytic clathrin coat progressively bends inward, into the direction of the cytoplasm, until eventually, the so-called endocytic pit is connected to the membrane only via a narrow stalk that is severed by mechanochemical forces executed by the protein dynamin, likely aided by other membrane-bending proteins such as endophilin, and the actin cytoskeleton (McMahon and Boucrot, 2011).
Endocytic adaptors are crucial in this process since they conduct the selection of membrane proteins destined for endocytosis. For example, specific adaptors enable liver cells to internalize the low-density lipoprotein receptors (i.e. these are then the “cargo” of the forming endocytic vesicle) to clear cholesterol from the circulation and loss of these adaptors causes hypercholesterolemia and atherosclerosis in humans (Mishra et al., 2002). A similar cargo-selective function is carried out by endocytic sorting adaptors in neurons, in particular during the exocytic/endocytic cycling of SVs that we will focus on now.
Neurons capitalize on endocytic sorting adaptors to reform functional SVs
The number of SVs available for fusion defines the efficacy of neurotransmitter release and fine-tunes neuronal function. A single SV is a complex organelle that contains several dozens of SV membrane proteins, many of which are present in just a few copies. These SV proteins are crucial for calcium-sensing, docking and fusion, endocytosis and other forms of membrane traffic at the presynapse (Takamori et al., 2006). Following calcium-triggered SV exocytosis, SV proteins are integrated into the presynaptic plasma membrane, from where they need to be removed by endocytosis. Although this compensatory endocytosis of collapsed SV membranes may not require clathrin coats per se, SV proteins must be sorted into the forming endocytic structure from which new SVs are eventually regenerated in a clathrin-mediated budding process that bears similarity to clathrin-coated vesicle formation in non-neuronal cells as first shown by Heuser and Reese (1973). A partially unsolved riddle is how synapses are capable of maintaining the composition of their SVs over multiple rapid rounds of exocytosis and endocytosis. Work in recent years has established a crucial role of endocytic adaptors in maintaining the protein composition of SVs by sorting their transmembrane proteins during the exo-endocytic cycle.
Multiple SV proteins have been shown to be recognized by specific dedicated endocytic sorting adaptors. The first specific adaptor for SV protein sorting was revealed by genetic screens in Drosophila (Grigliatti et al., 1973), where some mutant flies were reported to become paralyzed at elevated temperature as if they were “stoned”. The term “stoned” was used to describe the mutant locus, which subsequently was found to code, among others, for a protein called stoned B, a binding partner of SV calcium sensor protein synaptotagmin 1 (Maritzen et al., 2010; Phillips et al., 2010). Work by us and others identified stonin 2 as the mammalian paralog of Drosophila stoned B, which acts as a selective adapter for the retrieval of synaptotagmin 1 from the presynaptic plasma membrane (Diril et al., 2006; Jung et al., 2007; Kononenko et al., 2013). Interestingly, loss of stonin 2 does not affect the process of SV endocytosis per se but results in the selective accumulation of synaptotagmin 1 molecules at or near the presynaptic AZ. This function of stoned B/stonin 2 is conserved throughout evolution from worms and flies to humans (Jung et al., 2007; Maritzen et al., 2010; Mullen et al., 2012; Phillips et al., 2010). Recent work suggests that a related adaptor protein SGIP1 may serve a partially overlapping function in synaptotagmin 1 sorting at the synapse (Lee et al., 2019). In a similar way, it has been found that the SV soluble N–ethylmaleimide sensitive factor attachment protein receptor (SNARE) protein synaptobrevin/vesicle-associated membrane protein 2 (VAMP2) is recognized and sorted by a neuron-specific endocytic adaptor AP180 and its ubiquitous paralog CALM protein (Koo et al., 2015; Maritzen et al., 2012), while the vesicular glutamate transporter 1 required for the refilling of SVs with glutamate contains motifs recognized by endocytic proteins AP-2 and endophilin (Voglmaier et al., 2006).
How sorting of other SV proteins such as the vacuolar ATPase or SV2A/SV2B is accomplished remains unknown. For some SV proteins, piggy-back riding mechanisms involving the association with other SV proteins have been proposed. For example, sorting of synaptotagmin is chaperoned by its association with SV2A, which itself can bind to AP-2 (Kaempf et al., 2015), and synaptobrevin/VAMP2 is sorted in a complex with synaptophysin (Gordon and Cousin, 2016). An equally important but unresolved question is how the sorting by this diverse set of endocytic adaptors is coordinated to maintain the SV protein stoichiometry during multiple rounds of exo-endocytosis. Future studies will need to address this.
Noncanonical functions of endocytic adaptors in autophagy at the presynapse
Neurons like most other cells employ several strategies for removing damaged or misfolded proteins that include the ubiquitin-proteasome system, and the autophagy-lysosomal pathway. In autophagy, a double membrane organelle referred to as the autophagosome is formed from other membranes. The autophagosome delivers its engulfed cytoplasmic material to the lysosome for degradation (Ariosa and Klionsky, 2016). This function of autophagy is especially crucial in neurons, and defects of neuronal autophagy are associated with neurodegeneration and aging-associated memory decline (Gupta et al., 2013; Menzies et al., 2017). Recent work has suggested that synaptic autophagosome formation is crucially regulated by endocytic proteins (Murdoch et al., 2016, Soukup and Verstreken, 2017). For instance, endophilin, a possible sorting adaptor for the vesicular glutamate transporter 1, has been shown to be required for the stimulation-induced formation of autophagosomes. The switch of endophilin between a function in SV endocytosis and the formation of autophagosomes is regulated by its phosphorylation by the kinase LRRK2, a protein genetically linked to Parkinson’s disease (PD) (Arranz et al., 2015; Matta et al., 2012; Soukup and Verstreken, 2017). A similar switch between a role in SV endocytosis and autophagy has also been postulated for the endocytic adaptor AP-2, which has been shown to promote the retrograde axonal transport of autophagosomes that carry neurotrophin signals to the neuronal soma (Kononenko et al., 2017) (Figure 2). In summary, the study of SV endocytosis and the role of endocytic adaptors has revealed exciting and unexpected connections of endocytic adaptors for SV recycling to the autophagy.
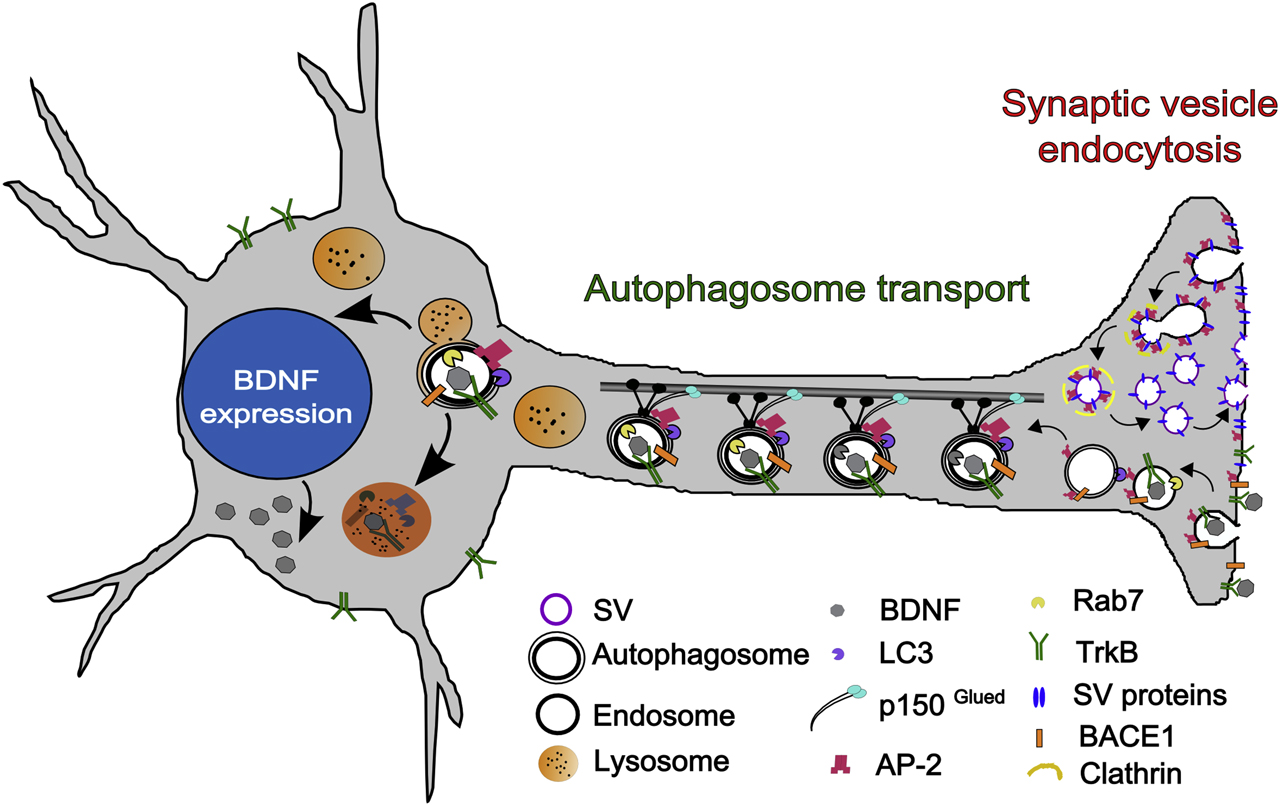
Hypothetical model explaining the dual role of AP-2 in SV recycling and autophagosome transport. At the presynapse, AP-2 is required to regenerate SVs from plasma membrane-derived endosomal vacuoles formed by clathrin-independent endocytosis of SV membranes postfusion. AP-2 may also aid sorting of select SV proteins at the plasma membrane. In addition, AP-2 serves a nonendocytic function in axonal trafficking of TrkB-containing signaling autophagosomes and in turnover of BACE1 via autophagy. SV, synaptic vesicle; AP-2, assembly protein complex-2.
Endocytic sorting adaptors in neurological disorders – where we go from here
Alterations in the function of endocytic sorting adaptors have been implicated in various forms of neurological disorders. How these relate to their functions in SV endocytosis or autophagy in most cases is unclear. For example, several endocytic adaptors, such as stonin 2, AP180 and CALM, has been linked to autism spectrum disorders (ASDs) and schizophrenia in humans (Ben-David and Shifman, 2012; Breedveld et al., 2010; Luan et al., 2011), although no mechanistic studies have been conducted to support this. SGIP1, a stonin 2-related adaptor for synaptotagmin 1 has been implicated in alcohol use disorder and as a factor affecting the human electroencephalogram, suggesting a role in the regulation of brain activity (Hodgkinson et al., 2010). Conversely, α-synuclein, a protein genetically linked to PD in humans has been found to play a role in SV recycling and the regulation of SV pool sizes (Vargas et al., 2014, 2017). Endocytic adaptors may also function at the presynapse to prevent the epilepsy. For example, loss of the endocytic proteins amphiphysin (Di Paolo et al., 2002), synaptojanin (Hardies et al., 2016), syndapin 1 (Koch et al., 2011) or the synaptobrevin/VAMP2 adaptor AP180 (Koo et al., 2015) in mice results in excitatory/inhibitory imbalance and seizures. Mutations in synaptojanin 1 have also been associated with early-onset Parkinsonism and generalized seizures in humans (Krebs et al., 2013). Moreover, recent work from us has uncovered that a missense mutation in one of the subunits of the AP-2 causes developmental and epileptic encephalopathy in children (Helbig et al., 2019). How exactly epilepsy arises in this case is unknown but may conceivably involve the missorting of SV proteins such as the vesicular GABA transporter, a known AP-2 cargo at inhibitory synapses.
The VAMP/synaptobrevin adaptors AP180 and CALM (PICALM) have also been genetically associated with Alzheimer’s disease (AD) (Gusareva et al., 2014). CALM expression is inversely correlated with levels of phospho-tau and the autophagosomal marker LC3 in the AD brain (Ando et al., 2016), where it may function to prevent the amyloid β generation by promoting the trafficking and autophagic degradation of crucial components of amyloidogenic pathway (Kanatsu et al., 2014; Tian et al., 2013; Zhao et al., 2015) and/or by regulating the sorting of endolysosomal VAMP/synaptobrevin required for functional autophagy (Moreau et al., 2014). Autophagosomes, in addition to their canonical role in degradation in all cells, may also promote survival by carrying neurotrophin signals to the neuronal soma (Deinhardt et al., 2006). We have shown that in neurons, the endocytic adaptor AP-2 serves an additional nonendocytic function in retrograde transport of neurotrophin-containing autophagosomes that depends on its ability to associate with autophagosome proteins and dynein motor proteins. Neuron-specific AP-2 knockout mice suffer from severe neurodegeneration and reduced neuronal complexity (Kononenko et al., 2017). AP-2 also may play a role in AD by promoting the degradation of BACE1, a protease known to function in amyloidogenic pathway to generate the toxic amyloid β isoforms (Bera et al., 2020) (see Figure 2). Finally, sorting nexins such as SNX27, an endosomal BAR domain protein associated with the retromer complex that acts downstream of endocytic clathrin adaptors, counteract neurodegeneration in PD by facilitating the recycling of receptors such as AMPA- and NMDA-type glutamate and serotonin receptors (Gallon et al., 2014; McMillan et al., 2016; Patel et al., 2018). Thus, endocytic sorting adaptors may counteract neurodegeneration by additionally promoting protein turnover via autophagy, while endosomal sorting adaptors such as SNX27 maintain synaptic function via recycling membrane cargo. We predict that future studies will uncover further novel associations between SV sorting adaptors and neurological disorders ranging from epilepsy and autism to neurodegeneration.
Glossary
- AP-2
-
Adaptor protein complex-2
- AZ
-
Presynaptic active zone
- Cargo
-
Proteins and lipids taken up from the plasma membrane and trafficked within the call
- CME
-
Clathrin-mediated endocytosis, a pathway canonically used by cells for uptake of nutrients
- GABA
-
γ-Aminobutyric acid
- LC3
-
Microtubule-associated protein 1 light chain 3
- Membrane retrieval
-
Endocytosis of plasma membrane
- SV
-
Synaptic vesicle
Funding source: Deutsche Forschungsgemeinschaft (DFG)
Award Identifier / Grant number: EXC 2030 - 390661388
Award Identifier / Grant number: KO5091/2-1
Funding source: Fritz Thyssen Foundation
Award Identifier / Grant number: Az. 10.18.1.036MN
Funding source: Reinhart-Koselleck-Program
Award Identifier / Grant number: HA2685/ 13-1
Funding source: Bundesministerium für Bildung und Forschung
Award Identifier / Grant number: Smartage 01GQ1420B
Award Identifier / Grant number: Smartage 01GQ1420C
About the authors

Natalia L. Kononenko received her Ph.D. in 2005 from the Koltzov Institute for Development Biology of Russian Academy of Sciences in Moscow, Russia, where she was originally trained in animal physiology. Following postdoctoral work in neuroanatomy at the Kavli Institute for Systems Neuroscience in Trondheim, Norway, she joined the group of Volker Haucke at the Freie Universität Berlin, where she used a combination of cell biology, imaging and genetic approaches to dissect the function of endocytic adaptors in the brain. Since 2016, she is a Research Group Leader in CECAD at the University of Cologne. Her lab integrates state-of-the-art genetic and cell biology approaches with live cell imaging, super-resolution microscopy and in vivo neuroanatomy to understand the role of membrane trafficking in the pathogenesis of neurodegeneration.

Volker Haucke received his Ph.D. summa cum laude in 1997 from the Biozentrum of the University of Basel, Switzerland, for his work on mitochondrial biogenesis in the group of Gottfried (Jeff) Schatz. Following postdoctoral work as a fellow of EMBO and the Human Frontier Science Program in the group of Pietro De Camilli at Yale University School of Medicine, he started his own laboratory at the University of Göttingen. He was appointed as a full professor of biochemistry at the Freie Universität Berlin in 2003. Since 2012, Volker Haucke is the director at the Leibniz Forschungsinstitut für Molekulare Pharmakologie (FMP) and professor of molecular pharmacology at the Freie Universität Berlin and a member of the NeuroCure Cluster of Excellence. The focus of research in his laboratory is the dissection of the molecular mechanisms of endocytosis and endolysosomal membrane dynamics and its role in the nervous system with a focus on neurotransmission.
Acknowledgements
We thank the Schram foundation for their generous support of our past activities and its service to the neuroscience community. The present work of N.L.K. is supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy—CECAD (EXC 2030–390661388), DFG (KO5091/2-1) and Fritz Thyssen Foundation (Az. 10.18.1.036MN).
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: V.H. acknowledges funding by Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy (EXC-2049–390688087), SFB 958 (projects A01 and A07) and the Reinhart-Koselleck-Program (HA2685/ 13-1 to V.H.), the Leibniz SAW program (SAW-2014-FMP-2 359, to V.H.) and the Bundesministerium für Bildung und Forschung to D.S. (Smartage 01GQ1420B) and V.H. (Smartage 01GQ1420C).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
Ando, K., Tomimura, K., Sazdovitch, V., Suain, V., Yilmaz, Z., Authelet, M., Ndjim, M., Vergara, C., Belkouch, M., Potier, M.C., et al. (2016). Level of PICALM, a key component of clathrin-mediated endocytosis, is correlated with levels of phosphotau and autophagy-related proteins and is associated with tau inclusions in AD, PSP and Pick disease. Neurobiol. Dis. 94, 32–43. https://doi.org/10.1016/j.nbd.2016.05.017.Search in Google Scholar PubMed
Ariosa, A.R. and Klionsky, D.J. (2016). Autophagy core machinery: overcoming spatial barriers in neurons. J. Mol. Med. 94, 1217–1227. https://doi.org/10.1007/s00109-016-1461-9.Search in Google Scholar PubMed PubMed Central
Arranz, A.M., Delbroek, L., Van Kolen, K., Guimaraes, M.R., Mandemakers, W., Daneels, G., Matta, S., Calafate, S., Shaban, H., Baatsen, P., et al. (2015). LRRK2 functions in synaptic vesicle endocytosis through a kinase-dependent mechanism. J. Cell Sci. 128, 541–552. https://doi.org/10.1242/jcs.158196.Search in Google Scholar PubMed
Barker, P.A., Hussain, N.K., and McPherson, P.S. (2002). Retrograde signaling by the neurotrophins follows a well-worn trk. Trends Neurosci. 25, 379–381. https://doi.org/10.1016/s0166-2236(02)02199-9.Search in Google Scholar PubMed
Ben-David, E. and Shifman, S. (2012). Networks of neuronal genes affected by common and rare variants in autism spectrum disorders. PLoS Genet. 8, e1002556. https://doi.org/10.1371/journal.pgen.1002556.Search in Google Scholar PubMed PubMed Central
Bera, S., Camblor-Perujo, S., Calleja Barca, E., Negrete-Hurtado, A., Racho, J., De Bruyckere, E., Wittich, C., Ellrich, N., Martins, S., Adjaye, J., et al. (2020). AP-2 reduces amyloidogenesis by promoting BACE1 trafficking and degradation in neurons. EMBO Rep. n/a, e47954. https://doi.org/10.15252/embr.20194795.Search in Google Scholar
Breedveld, G.J., Fabbrini, G., Oostra, B.A., Berardelli, A., and Bonifati, V. (2010). Tourette disorder spectrum maps to chromosome 14q31.1 in an Italian kindred. Neurogenetics 11, 417–423. https://doi.org/10.1007/s10048-010-0244-7.Search in Google Scholar PubMed PubMed Central
Burk, K., Murdoch, J.D., Freytag, S., Koenig, M., Bharat, V., Markworth, R., Burkhardt, S., Fischer, A., and Dean, C. (2017). EndophilinAs regulate endosomal sorting of BDNF-TrkB to mediate survival signaling in hippocampal neurons. Sci. Rep. 7, 2149. https://doi.org/10.1038/s41598-017-02202-4.Search in Google Scholar PubMed PubMed Central
Deinhardt, K., Salinas, S., Verastegui, C., Watson, R., Worth, D., Hanrahan, S., Bucci, C., and Schiavo, G. (2006). Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron 52, 293–305. https://doi.org/10.1016/j.neuron.2006.08.018.Search in Google Scholar PubMed
Di Paolo, G., Sankaranarayanan, S., Wenk, M.R., Daniell, L., Perucco, E., Caldarone, B.J., Flavell, R., Picciotto, M.R., Ryan, T.A., Cremona, O., et al. (2002). Decreased synaptic vesicle recycling efficiency and cognitive deficits in amphiphysin 1 knockout mice. Neuron 33, 789–804. https://doi.org/10.1016/s0896-6273(02)00601-3.Search in Google Scholar PubMed
Diril, M.K., Wienisch, M., Jung, N., Klingauf, J., and Haucke, V. (2006). Stonin 2 is an AP-2-dependent endocytic sorting adaptor for synaptotagmin internalization and recycling. Dev. Cell 10, 233–244. https://doi.org/10.1016/j.devcel.2005.12.011.Search in Google Scholar PubMed
Fatt, P. and Katz, B. (1952). Spontaneous subthreshold activity at motor nerve endings. J. Physiol. 117, 109–128. https://doi.org/10.1113/jphysiol.1952.sp004735.Search in Google Scholar
Ferguson, S.M., Brasnjo, G., Hayashi, M., Wolfel, M., Collesi, C., Giovedi, S., Raimondi, A., Gong, L.W., Ariel, P., Paradise, S., et al. (2007). A selective activity-dependent requirement for dynamin 1 in synaptic vesicle endocytosis. Science 316, 570–574. https://doi.org/10.1126/science.1140621.Search in Google Scholar PubMed
Fiuza, M., Rostosky, C.M., Parkinson, G.T., Bygrave, A.M., Halemani, N., Baptista, M., Milosevic, I., and Hanley, J.G. (2017). PICK1 regulates AMPA receptor endocytosis via direct interactions with AP2 alpha-appendage and dynamin. J. Cell Biol. 216, 3323–3338. https://doi.org/10.1083/jcb.201701034.Search in Google Scholar PubMed PubMed Central
Gallon, M., Clairfeuille, T., Steinberg, F., Mas, C., Ghai, R., Sessions, R.B., Teasdale, R.D., Collins, B.M., and Cullen, P.J. (2014). A unique PDZ domain and arrestin-like fold interaction reveals mechanistic details of endocytic recycling by SNX27-retromer. Proc. Natl. Acad. Sci. USA 111, E3604–E3613. https://doi.org/10.1073/pnas.1410552111.Search in Google Scholar PubMed PubMed Central
Gordon, S.L. and Cousin, M.A. (2016). The iTRAPs: Guardians of synaptic vesicle cargo retrieval during endocytosis. Front. Synaptic Neurosci. 8, https://doi.org/10.3389/fnsyn.2016.00001.Search in Google Scholar PubMed PubMed Central
Grigliatti, T.A., Hall, L., Rosenbluth, R., and Suzuki, D.T. (1973). Temperature-sensitive mutations in Drosophila melanogaster. Mol. Gen. Genet. MGG 120, 107–114. https://doi.org/10.1007/bf00267238.Search in Google Scholar PubMed
Gupta, V.K., Scheunemann, L., Eisenberg, T., Mertel, S., Bhukel, A., Koemans, T.S., Kramer, J.M., Liu, K.S., Schroeder, S., Stunnenberg, H.G., et al. (2013). Restoring polyamines protects from age-induced memory impairment in an autophagy-dependent manner. Nat. Neurosci. 16, 1453–1460. https://doi.org/10.1038/nn.3512.Search in Google Scholar PubMed
Gusareva, E.S., Carrasquillo, M.M., Bellenguez, C., Cuyvers, E., Colon, S., Graff-Radford, N.R., Petersen, R.C., Dickson, D.W., Mahachie John, J.M., Bessonov, K., et al. (2014). Genome-wide association interaction analysis for Alzheimer’s disease. Neurobiol. Aging 35, 2436–2443. https://doi.org/10.1016/j.neurobiolaging.2014.05.014.Search in Google Scholar PubMed PubMed Central
Hardies, K., Cai, Y., Jardel, C., Jansen, A.C., Cao, M., May, P., Djemie, T., Hachon Le Camus, C., Keymolen, K., Deconinck, T., et al. (2016). Loss of SYNJ1 dual phosphatase activity leads to early onset refractory seizures and progressive neurological decline. Brain 139, 2420–2430. https://doi.org/10.1093/brain/aww180.Search in Google Scholar PubMed PubMed Central
Heerssen, H., Fetter, R.D., and Davis, G.W. (2008). Clathrin dependence of synaptic-vesicle formation at the Drosophila neuromuscular junction. Curr. Biol. CB 18, 401–409. https://doi.org/10.1016/j.cub.2008.02.055.Search in Google Scholar PubMed PubMed Central
Helbig, I., Lopez-Hernandez, T., Shor, O., Galer, P., Ganesan, S., Pendziwiat, M., Rademacher, A., Ellis, C.A., Humpfer, N., Schwarz, N., et al. (2019). A recurrent missense variant in AP2M1 impairs clathrin-mediated endocytosis and causes developmental and epileptic encephalopathy. Am. J. Hum. Genet. 104, 1060–1072. https://doi.org/10.1016/j.ajhg.2019.04.001.Search in Google Scholar PubMed PubMed Central
Heuser, J.E. and Reese, T.S. (1973). Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J. Cell Biol. 57, 315–344. https://doi.org/10.1083/jcb.57.2.315.Search in Google Scholar PubMed PubMed Central
Hodgkinson, C.A., Enoch, M.-A., Srivastava, V., Cummins-Oman, J.S., Ferrier, C., Iarikova, P., Sankararaman, S., Yamini, G., Yuan, Q., Zhou, Z., et al. (2010). Genome-wide association identifies candidate genes that influence the human electroencephalogram. Proc. Natl. Acad. Sci. USA 107, 8695–8700. https://doi.org/10.1073/pnas.0908134107.Search in Google Scholar PubMed PubMed Central
Jung, N., Wienisch, M., Gu, M., Rand, J.B., Muller, S.L., Krause, G., Jorgensen, E.M., Klingauf, J., and Haucke, V. (2007). Molecular basis of synaptic vesicle cargo recognition by the endocytic sorting adaptor stonin 2. J. Cell Biol. 179, 1497–1510. https://doi.org/10.1083/jcb.200708107.Search in Google Scholar PubMed PubMed Central
Kaempf, N., Kochlamazashvili, G., Puchkov, D., Maritzen, T., Bajjalieh, S.M., Kononenko, N.L., and Haucke, V. (2015). Overlapping functions of stonin 2 and SV2 in sorting of the calcium sensor synaptotagmin 1 to synaptic vesicles. Proc. Natl. Acad. Sci. USA 112, 7297–7302. https://doi.org/10.1073/pnas.1501627112.Search in Google Scholar PubMed PubMed Central
Kaempf, N. and Maritzen, T. (2017). Safeguards of neurotransmission: endocytic adaptors as regulators of synaptic vesicle composition and function. Front. Cell. Neurosci. 11, 320. https://doi.org/10.3389/fncel.2017.00320.Search in Google Scholar PubMed PubMed Central
Kanatsu, K., Morohashi, Y., Suzuki, M., Kuroda, H., Watanabe, T., Tomita, T., and Iwatsubo, T. (2014). Decreased CALM expression reduces Abeta42 to total Abeta ratio through clathrin-mediated endocytosis of gamma-secretase. Nat. Commun. 5, https://doi.org/10.1038/ncomms4386.Search in Google Scholar PubMed
Kastning, K., Kukhtina, V., Kittler, J.T., Chen, G., Pechstein, A., Enders, S., Lee, S.H., Sheng, M., Yan, Z., and Haucke, V. (2007). Molecular determinants for the interaction between AMPA receptors and the clathrin adaptor complex AP-2. Proc. Natl. Acad. Sci. USA 104, 2991–2996. https://doi.org/10.1073/pnas.0611170104.Search in Google Scholar PubMed PubMed Central
Kittler, J.T., Chen, G., Honing, S., Bogdanov, Y., McAinsh, K., Arancibia-Carcamo, I.L., Jovanovic, J.N., Pangalos, M.N., Haucke, V., Yan, Z., et al. (2005). Phospho-dependent binding of the clathrin AP2 adaptor complex to GABAA receptors regulates the efficacy of inhibitory synaptic transmission. Proc. Natl. Acad. Sci. USA 102, 14871–14876. https://doi.org/10.1073/pnas.0506653102.Search in Google Scholar PubMed PubMed Central
Koch, D., Spiwoks-Becker, I., Sabanov, V., Sinning, A., Dugladze, T., Stellmacher, A., Ahuja, R., Grimm, J., Schuler, S., Muller, A., et al. (2011). Proper synaptic vesicle formation and neuronal network activity critically rely on syndapin I. EMBO J. 30, 4955–4969. https://doi.org/10.1038/emboj.2011.339.Search in Google Scholar PubMed PubMed Central
Kononenko, N.L., Classen, G.A., Kuijpers, M., Puchkov, D., Maritzen, T., Tempes, A., Malik, A.R., Skalecka, A., Bera, S., Jaworski, J., et al. (2017). Retrograde transport of TrkB-containing autophagosomes via the adaptor AP-2 mediates neuronal complexity and prevents neurodegeneration. Nat. Commun. 8, https://doi.org/10.1038/ncomms14819.Search in Google Scholar PubMed PubMed Central
Kononenko, N.L., Diril, M.K., Puchkov, D., Kintscher, M., Koo, S.J., Pfuhl, G., Winter, Y., Wienisch, M., Klingauf, J., Breustedt, J., et al. (2013). Compromised fidelity of endocytic synaptic vesicle protein sorting in the absence of stonin 2. Proc. Natl. Acad. Sci. USA 110, 23. https://doi.org/10.1073/pnas.1218432110.Search in Google Scholar PubMed PubMed Central
Kononenko, N.L. and Haucke, V. (2015). Molecular mechanisms of presynaptic membrane retrieval and synaptic vesicle reformation. Neuron 85, 484–496. https://doi.org/10.1016/j.neuron.2014.12.016.Search in Google Scholar PubMed
Kononenko, N.L., Puchkov, D., Classen, G.A., Walter, A.M., Pechstein, A., Sawade, L., Kaempf, N., Trimbuch, T., Lorenz, D., Rosenmund, C., et al. (2014). Clathrin/AP-2 mediate synaptic vesicle reformation from endosome-like vacuoles but are not essential for membrane retrieval at central synapses. Neuron 82, 981–988. https://doi.org/10.1016/j.neuron.2014.05.007.Search in Google Scholar PubMed
Koo, S.J., Kochlamazashvili, G., Rost, B., Puchkov, D., Gimber, N., Lehmann, M., Tadeus, G., Schmoranzer, J., Rosenmund, C., Haucke, V., et al. (2015). Vesicular synaptobrevin/VAMP2 levels Guarded by AP180 control efficient neurotransmission. Neuron 88, 330–344. https://doi.org/10.1016/j.neuron.2015.08.034.Search in Google Scholar PubMed
Krebs, C.E., Karkheiran, S., Powell, J.C., Cao, M., Makarov, V., Darvish, H., Di Paolo, G., Walker, R.H., Shahidi, G.A., Buxbaum, J.D., et al. (2013). The Sac1 domain of SYNJ1 identified mutated in a family with early-onset progressive Parkinsonism with generalized seizures. Hum. Mutat. 34, 1200–1207. https://doi.org/10.1002/humu.22372.Search in Google Scholar PubMed PubMed Central
Lee, S.E., Jeong, S., Lee, U., and Chang, S. (2019). SGIP1alpha functions as a selective endocytic adaptor for the internalization of synaptotagmin 1 at synapses. Mol. Brain 12, 41. https://doi.org/10.1186/s13041-019-0464-1.Search in Google Scholar PubMed PubMed Central
Luan, Z., Zhang, Y., Lu, T., Ruan, Y., Zhang, H., Yan, J., Li, L., Sun, W., Wang, L., Yue, W., et al. (2011). Positive association of the human STON2 gene with schizophrenia. Neuroreport 22, 288–293. https://doi.org/10.1097/wnr.0b013e328345ac22.Search in Google Scholar
Maritzen, T., Koo, S.J., and Haucke, V. (2012). Turning CALM into excitement: AP180 and CALM in endocytosis and disease. Biol Cell 104, 588–602. https://doi.org/10.1111/boc.201200008.Search in Google Scholar PubMed
Maritzen, T., Podufall, J., and Haucke, V. (2010). Stonins—specialized adaptors for synaptic vesicle recycling and beyond?. Traffic 11, 8–15. https://doi.org/10.1111/j.1600-0854.2009.00971.x.Search in Google Scholar PubMed
Matta, S., Van Kolen, K., da Cunha, R., van den Bogaart, G., Mandemakers, W., Miskiewicz, K., De Bock, P.J., Morais, V.A., Vilain, S., Haddad, D., et al. (2012). LRRK2 controls an EndoA phosphorylation cycle in synaptic endocytosis. Neuron 75, 1008–1021. https://doi.org/10.1016/j.neuron.2012.08.022.Search in Google Scholar PubMed
McMahon, H.T. and Boucrot, E. (2011). Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 12, 517–533. https://doi.org/10.1038/nrm3151.Search in Google Scholar PubMed
McMillan, K.J., Gallon, M., Jellett, A.P., Clairfeuille, T., Tilley, F.C., McGough, I., Danson, C.M., Heesom, K.J., Wilkinson, K.A., Collins, B.M., et al. (2016). Atypical parkinsonism-associated retromer mutant alters endosomal sorting of specific cargo proteins. J. Cell Biol. 214, 389–399. https://doi.org/10.1083/jcb.201604057.Search in Google Scholar PubMed PubMed Central
Menzies, F.M., Fleming, A., Caricasole, A., Bento, C.F., Andrews, S.P., Ashkenazi, A., Fullgrabe, J., Jackson, A., Jimenez Sanchez, M., Karabiyik, C., et al. (2017). Autophagy and neurodegeneration: pathogenic mechanisms and therapeutic opportunities. Neuron 93, 1015–1034. https://doi.org/10.1016/j.neuron.2017.01.022.Search in Google Scholar PubMed
Metzler, M., Gan, L., Wong, T.P., Liu, L., Helm, J., Liu, L., Georgiou, J., Wang, Y., Bissada, N., Cheng, K., et al. (2007). NMDA receptor function and NMDA receptor-dependent phosphorylation of huntingtin is altered by the endocytic protein HIP1. J. Neurosci. Off. J. Soc. Neurosci. 27, 2298–2308. https://doi.org/10.1523/jneurosci.5175-06.2007.Search in Google Scholar
Metzler, M., Li, B., Gan, L., Georgiou, J., Gutekunst, C.A., Wang, Y., Torre, E., Devon, R.S., Oh, R., Legendre-Guillemin, V., et al. (2003). Disruption of the endocytic protein HIP1 results in neurological deficits and decreased AMPA receptor trafficking. EMBO J. 22, 3254–3266. https://doi.org/10.1093/emboj/cdg334.Search in Google Scholar PubMed PubMed Central
Mishra, S.K., Watkins, S.C., and Traub, L.M. (2002). The autosomal recessive hypercholesterolemia (ARH) protein interfaces directly with the clathrin-coat machinery. Proc. Natl. Acad. Sci. USA 99, 16099–16104. https://doi.org/10.1073/pnas.252630799.Search in Google Scholar PubMed PubMed Central
Moreau, K., Fleming, A., Imarisio, S., Lopez Ramirez, A., Mercer, J.L., Jimenez-Sanchez, M., Bento, C.F., Puri, C., Zavodszky, E., Siddiqi, F., et al. (2014). PICALM modulates autophagy activity and tau accumulation. Nat. Commun. 5, https://doi.org/10.1038/ncomms5998.Search in Google Scholar PubMed PubMed Central
Mullen, G.P., Grundahl, K.M., Gu, M., Watanabe, S., Hobson, R.J., Crowell, J.A., McManus, J.R., Mathews, E.A., Jorgensen, E.M., and Rand, J.B. (2012). UNC-41/stonin functions with AP2 to recycle synaptic vesicles in Caenorhabditis elegans. PloS One 7, e40095. https://doi.org/10.1371/journal.pone.0040095.Search in Google Scholar PubMed PubMed Central
Murdoch, J.D., Rostosky, C.M., Gowrisankaran, S., Arora, A.S., Soukup, S.F., Vidal, R., Capece, V., Freytag, S., Fischer, A., Verstreken, P., et al. (2016). Endophilin-A deficiency induces the foxo3a-fbxo32 network in the brain and causes dysregulation of autophagy and the ubiquitin-proteasome system. Cell Rep. 17, 1071–1086. https://doi.org/10.1016/j.celrep.2016.09.058.Search in Google Scholar PubMed PubMed Central
Patel, D., Xu, C., Nagarajan, S., Liu, Z., Hemphill, W.O., Shi, R., Uversky, V.N., Caldwell, G.A., Caldwell, K.A., and Witt, S.N. (2018). Alpha-synuclein inhibits Snx3-retromer-mediated retrograde recycling of iron transporters in S. cerevisiae and C. elegans models of Parkinson’s disease. Hum. Mol. Genet. 27, 1514–1532. https://doi.org/10.1093/hmg/ddy059.Search in Google Scholar PubMed
Phillips, A.M., Ramaswami, M., and Kelly, L.E. (2010). Stoned. Traffic 11, 16–24. https://doi.org/10.1111/j.1600-0854.2009.00999.x.Search in Google Scholar PubMed
Rizzoli, S.O. (2014). Synaptic vesicle recycling: Steps and principles. EMBO J. 33, 788–822. https://doi.org/10.1002/embj.201386357.Search in Google Scholar PubMed PubMed Central
Roth, T.F. and Porter, K.R. (1964). Yolk protein uptake in the oocyte of the mosquito Aedes aegypti L. J Cell Biol 20, 313–332. https://doi.org/10.1083/jcb.20.2.313.Search in Google Scholar PubMed PubMed Central
Saheki, Y. and De Camilli, P. (2012). Synaptic vesicle endocytosis. Cold Spring Harb. Perspect. Biol. 4, https://doi.org/10.1101/cshperspect.a005645.Search in Google Scholar PubMed PubMed Central
Soukup, S.F. and Verstreken, P. (2017). EndoA/Endophilin-A creates docking stations for autophagic proteins at synapses. Autophagy 13, 971–972. https://doi.org/10.1080/15548627.2017.1286440.Search in Google Scholar PubMed PubMed Central
Soykan, T., Kaempf, N., Sakaba, T., Vollweiter, D., Goerdeler, F., Puchkov, D., Kononenko, N.L., and Haucke, V. (2017). Synaptic vesicle endocytosis occurs on multiple timescales and is mediated by formin-dependent actin assembly. Neuron 93, 854–866. https://doi.org/10.1016/j.neuron.2017.02.011.Search in Google Scholar PubMed
Soykan, T., Maritzen, T., and Haucke, V. (2016). Modes and mechanisms of synaptic vesicle recycling. Curr. Opin. Neurobiol. 39, 17–23. https://doi.org/10.1016/j.conb.2016.03.005.Search in Google Scholar PubMed
Sudhof, T.C. (2013). Neurotransmitter release: the last millisecond in the life of a synaptic vesicle. Neuron 80, 675–690. https://doi.org/10.1016/j.neuron.2013.10.022.Search in Google Scholar PubMed PubMed Central
Takamori, S., Holt, M., Stenius, K., Lemke, E.A., Gronborg, M., Riedel, D., Urlaub, H., Schenck, S., Brugger, B., Ringler, P., et al. (2006). Molecular anatomy of a trafficking organelle. Cell 127, 831–846. https://doi.org/10.1016/j.cell.2006.10.030.Search in Google Scholar PubMed
Tian, Y., Chang, J.C., Fan, E.Y., Flajolet, M., and Greengard, P. (2013). Adaptor complex AP2/PICALM, through interaction with LC3, targets Alzheimer’s APP-CTF for terminal degradation via autophagy. Proc. Natl. Acad. Sci. USA 110, 17071–17076. https://doi.org/10.1073/pnas.1315110110.Search in Google Scholar PubMed PubMed Central
Truckenbrodt, S., Viplav, A., Jahne, S., Vogts, A., Denker, A., Wildhagen, H., Fornasiero, E.F., and Rizzoli, S.O. (2018). Newly produced synaptic vesicle proteins are preferentially used in synaptic transmission. EMBO J. 37, https://doi.org/10.15252/embj.201798044.Search in Google Scholar PubMed PubMed Central
Vargas, K.J., Makani, S., Davis, T., Westphal, C.H., Castillo, P.E., and Chandra, S.S. (2014). Synucleins regulate the kinetics of synaptic vesicle endocytosis. J. Neurosci. Off. J. Soc. Neurosci. 34, 9364–9376. https://doi.org/10.1523/jneurosci.4787-13.2014.Search in Google Scholar
Vargas, K.J., Schrod, N., Davis, T., Fernandez-Busnadiego, R., Taguchi, Y.V., Laugks, U., Lucic, V., and Chandra, S.S. (2017). Synucleins have multiple effects on presynaptic Architecture. Cell Rep. 18, 161–173. https://doi.org/10.1016/j.celrep.2016.12.023.Search in Google Scholar PubMed PubMed Central
Voglmaier, S.M., Kam, K., Yang, H., Fortin, D.L., Hua, Z., Nicoll, R.A., and Edwards, R.H. (2006). Distinct endocytic pathways control the rate and extent of synaptic vesicle protein recycling. Neuron 51, 71–84. https://doi.org/10.1016/j.neuron.2006.05.027.Search in Google Scholar PubMed
Watanabe, S. and Boucrot, E. (2017). Fast and ultrafast endocytosis. Curr. Opin. Cell Biol. 47, 64–71. https://doi.org/10.1016/j.ceb.2017.02.013.Search in Google Scholar PubMed
Watanabe, S., Mamer, L.E., Raychaudhuri, S., Luvsanjav, D., Eisen, J., Trimbuch, T., Sohl-Kielczynski, B., Fenske, P., Milosevic, I., Rosenmund, C., et al. (2018). Synaptojanin and endophilin mediate neck formation during ultrafast endocytosis. Neuron 98, 1184–1197 e1186. https://doi.org/10.1016/j.neuron.2018.06.005.Search in Google Scholar PubMed PubMed Central
Watanabe, S., Trimbuch, T., Camacho-Perez, M., Rost, B.R., Brokowski, B., Sohl-Kielczynski, B., Felies, A., Davis, M.W., Rosenmund, C., and Jorgensen, E.M. (2014). Clathrin regenerates synaptic vesicles from endosomes. Nature 515, 228–233. https://doi.org/10.1038/nature13846.Search in Google Scholar PubMed PubMed Central
Wu, X.S., Lee, S.H., Sheng, J., Zhang, Z., Zhao, W.D., Wang, D., Jin, Y., Charnay, P., Ervasti, J.M., and Wu, L.G. (2016). Actin is crucial for all kinetically distinguishable forms of endocytosis at synapses. Neuron 92, 1020–1035. https://doi.org/10.1016/j.neuron.2016.10.014.Search in Google Scholar PubMed PubMed Central
Zhang, J., Yin, Y., Ji, Z., Cai, Z., Zhao, B., Li, J., Tan, M., and Guo, G. (2017). Endophilin2 interacts with GluA1 to mediate AMPA receptor endocytosis induced by oligomeric amyloid-beta. Neural Plast. 2017, 8197085. https://doi.org/10.1155/2017/8197085.Search in Google Scholar PubMed PubMed Central
Zhao, Z., Sagare, A.P., Ma, Q., Halliday, M.R., Kong, P., Kisler, K., Winkler, E.A., Ramanathan, A., Kanekiyo, T., Bu, G., et al. (2015). Central role for PICALM in amyloid-beta blood-brain barrier transcytosis and clearance. Nat. Neurosci. 18, 978–987. https://doi.org/10.1038/nn.4025.Search in Google Scholar PubMed PubMed Central
© 2020 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Editorial
- Editorial
- Review articles
- Synapses, networks, brain development – funding basic neuroscience research in Germany by the Schram Foundation
- Neuronal functions of clathrin-associated endocytic sorting adaptors – from molecules to disease
- Mechanisms of synaptic vesicle recycling provide a platform to explore mechanisms of neurodegeneration
- Optogenetic analyses of neuronal networks that generate behavior in Caenorhabditis elegans
- Direct contribution of angiogenic factors to neurodevelopment: a focus on angiopoietins
- The role of the dentate gyrus in mnemonic functions
- Presentation of scientific institutions
- Armin Schram: a sponsor of curiosity-driven brain research
- Nachrichten aus der Gesellschaft
- Nachrichten aus der Gesellschaft
Articles in the same Issue
- Frontmatter
- Editorial
- Editorial
- Review articles
- Synapses, networks, brain development – funding basic neuroscience research in Germany by the Schram Foundation
- Neuronal functions of clathrin-associated endocytic sorting adaptors – from molecules to disease
- Mechanisms of synaptic vesicle recycling provide a platform to explore mechanisms of neurodegeneration
- Optogenetic analyses of neuronal networks that generate behavior in Caenorhabditis elegans
- Direct contribution of angiogenic factors to neurodevelopment: a focus on angiopoietins
- The role of the dentate gyrus in mnemonic functions
- Presentation of scientific institutions
- Armin Schram: a sponsor of curiosity-driven brain research
- Nachrichten aus der Gesellschaft
- Nachrichten aus der Gesellschaft

