Synapses, networks, brain development – funding basic neuroscience research in Germany by the Schram Foundation
-
Dorothea Schulte
, Christian Rosenmund
and Eckart D. Gundelfinger
Abstract
Research driven solely by curiosity and the desire to understand fundamental principles of brain function. The freedom to address important questions with bold, sometimes risky experiments. A platform for open scientific exchange and discussions at highest academic level to provide new impulses to the field. And a growing number of scientists who share the passion for neuroscience and who join forces to tackle some of the big mysteries that surround the brain. These visions together with the deep conviction that basic research is the fundament needed for any progress in applied science motivated Dr. Armin Schram to create the foundation that carries his name. They are also the ideals that the foundation still pursues, and to date, 26 research proposals designed by individual researchers or small teams have been, or are, supported in this spirit. Here, we introduce the reader to the individual scientists who were awarded grants by the Schram Foundation over the years, highlight some of the many discoveries made in the course of their studies and list some of the key publications that arose from this work.
Zusammenfassung
Forschungsförderung, die sich der neurobiologischen Grundgenforschung auf höchstem wissenschaftlichem Niveau verpflichtet sieht, sowie ein Forum, das offene wissenschaftliche Diskussionen fördert, Impulse setzt und die Forschungslandschaft in Deutschland nachhaltig stärkt – das waren die Visionen, die Dr. Armin Schram zur Gründung der nach ihm benannten Stiftung bewegten. In diesem Geiste wurden seither 26 Projekte gefördert, die sich aus den unterschiedlichsten Blickwinkeln der Erforschung von Entwicklung, Funktion, Homöostase und Altern des Gehirns widmen. Im Folgenden umreißen wir einige der wichtigsten Entdeckungen, die dank Förderung durch die Schram-Stiftung möglich wurden, und stellen die vielfältigen Förderaktivitäten der Stiftung kurz vor.
Introduction
Working with animal models as diverse as mice, rats, chick, Mongolian gerbils, the fruit fly D. melanogaster or the nematode C. elegans, and drawing on a broad spectrum of techniques, projects supported by the Schram Foundation have tackled some of the central questions in molecular neuroscience: How is neuronal activity modulated at the level of individual synapses? How do neuronal networks form, become stabilized or adapt to ever-changing environmental conditions? How do genetic and epigenetic mechanisms influence nervous system development, homeostasis and aging? How do these building blocks cooperate to create what we call behavior? And finally, which techniques and methods are needed to accelerate neuroscientific research and how can they be developed?
Below, we have selected some of the most prominent discoveries, which were made with support of the Schram Foundation. This collection gives a good impression of the many activities of the foundation, yet it is far from complete. For a more comprehensive overview of the scientific output of research projects that had received support from the Schram Foundation, the reader is invited to visit the foundation’s homepage at https://www.schram-stiftung.de/ (see Table 1).
Research projects funded by the Schram-Foundation.
| 2004 |
| Sox9 vermittelte Genexpressionsänderungen als Ursache der Differenzierung neuraler Stammzellen zu zentralnervösen Gliazellen |
| Prof. Dr. Michael Wegner, Friedrich-Alexander-Universität Erlangen-Nürnberg, Emil-Fischer-Zentrum/Institut für Biochemie. |
| Caldendrin und Jacob – Eine Protein-Interaktion zur Kopplung synaptischer Ca 2+ - Signale an die dendritische Morphogenese? |
| Prof. Dr. Michael R. Kreutz und Dr. Christina Spilker, Leibniz-Institut für Neurobiologie, Magdeburg, Projektgruppe Neuroplastizität; aktuell: Leibniz-Institut für Neurobiologie Magdeburg und Zentrum für Molekulare Neurobiologie Hamburg (ZMNH). |
| RNA-Transport in Dendriten |
| Prof. Dr. Michael Kiebler, Medizinische Universität Wien, Abteilung für neuronale Zellbiologie; aktuell Biomedizinisches Zentrum München, Ludwig-Maximilians-Universität München. |
| 2006 |
| Die Rolle von Genexpressionsprogrammen beim Aufbau neuronaler Verschaltungen |
| Prof. Dr. Bernd Knöll, Eberhard-Karls-Universität Tübingen, Interfakultäres Institut für Zellbiologie, Abteilung Molekularbiologie; aktuell: Universität Ulm, Institut für Physiologische Chemie. |
| Regulation der molekularen, strukturellen und physiologischen Differenzierung durch physiologische elektrische Aktivitätsmuster im neonatalen Säugercortex |
| Prof. Dr. Heiko J. Luhmann, Johannes-Gutenberg-Universität Mainz, Institut für Physiologie und Pathophysiologie. |
| Prof. Dr. Volkmar Leßmann, Otto-von- Guericke-Universität Magdeburg, Institut für Physiologie. |
| Prof. Dr. Petra Wahle und Dr. Silke Patz, Ruhr-Universität Bochum, Allgemeine Zoologie und Neurobiologie. |
| Untersuchungen zur strukturellen Plastizität von Nervenzellverbindungen als Basis für Lern- und Gedächtnisprozesse |
| Prof. Dr. Britta Qualmann; Friedrich-Schiller-Universität Jena, Institut für Biochemie I. |
| Transkriptionelle Kontrolle der Entwicklung sympatischer und parasympatische Nervenzellen |
| Prof. Dr. Hermann Rohrer, Max-Planck-Institut für Hirnforschung, Frankfurt am Main. |
| 2009 |
| Molecular mechanisms underlying region-specific microcircuit formation in the brain |
| Prof. Dr. Thomas Hummel, Westfälische Wilhelms-Universität Münster, Institute of Neuro- and Behavior Biology und Universität Wien, Abteilung für Neurowissenschaften und Entwicklungsbiologie. |
| Rolle endozytischer Adaptor- und akzessorischer Proteine bei der Sortierung und Rezyklierung synaptischer Vesikelproteine |
| Prof. Dr. Volker Haucke, Freie Universität Berlin, Institut für Chemie und Biochemie; aktuell: Leibniz Forschungsinstitut für molekulare Pharmakologie, Berlin. |
| Optogenetics-assisted analysis of small neuronal networks and identification of novel proteins affecting recycling of synaptic vesicles in Caenorhabditis elegans |
| Prof. Dr. Alexander Gottschalk, Goethe-Universität Frankfurt am Main, Institut für Biochemie, Molekulare Membranbiologie und Neurobiologie. |
| The cellular mechanisms by which chromatin plasticity affects neuronal gene-expression in the ageing brain |
| Prof. Dr. André Fischer, European Neuroscience Institute (ENI) Göttingen; aktuell: Universitätsmedizin Göttingen und Deutsches Zentrum für Neurodegenerative Erkrankungen (DZNE) Göttingen. |
| 2011 |
| Kerntranslokation als Mechanismus der neuronalen Differenzierung |
| Prof. Dr. Jens C. Schwamborn, Universitätsklinikum Münster, Institut für Zellbiologie (ZMBE); aktuell: Université du Luxembourg, LCSB, Department of Developmental and Cellular Biology, Luxemburg. |
| Poly ADP Ribosylierung as novel control mechanism in adult and embryonic neurogenesis |
| Prof. Dr. Dorothea Schulte, Klinikum der Goethe-Universität Frankfurt am Main, Neurologisches Institut (Edinger Institut). |
| Role of the Perisynaptic Extracellular Matrix in Synaptic Plasticity and Network Activity |
| Dr. Renato Frischknecht, Leibniz-Institut für Neurobiologie (IfN) Magdeburg; aktuell: Friedrich-Alexander-Universität Erlangen-Nürnberg, Abteilung für Tierphysiologie. |
| Dissecting the dentate gyrus circuitry: Influence of dendritic versus perisomatic inhibition on network oscillations |
| Prof. Dr. Marlene Bartos, Albert-Ludwigs-Universität Freiburg i. B., Physiologisches Institut, Lichtenberg-Professur. |
| 2014 |
| Angiopoietine und ihre Tie-Rezeptoren in der Entwicklung neuronaler Netzwerke im Hippocampus |
| Prof. Dr. Carmen Ruiz de Almodovar, Ruprecht-Karls-Universität Heidelberg, Biochemiezentrum; aktuell: Medizinische Fakultät Mannheim der Universität Heidelberg. |
| Dynamische Membranen der Synapse: die Rolle subkompartimentaler Endosome in gesunden und kranken Nervenzellen |
| Dr. Ira Milosevic, European Neuroscience Institute (ENI) Göttingen. |
| Mechanismen von dendritischer Kv1.1-Inaktivierung, um “spike-timing”-abhängige synaptische Potenzierung zu bahnen |
| Prof. Dr. Oliver Marcus Schlüter, European Neuroscience Institute (ENI) Göttingen; aktuell: Universitätsmedizin Göttingen, Abteilung für Psychiatrie und Psychotherapie und Department of Neuroscience, University of Pittsburgh, Pittsburgh, PA, USA. |
| Neuronale Schaltkreise für Erleichterungslernen bei Drosophila |
| Dr. Ayse Yarali, Leibniz-Institut für Neurobiologie (IfN) Magdeburg. |
| 2017 |
| Regulierung der Genexpression in humanen induzierten Neuronen durch Faktoren der Musterbildung |
| Prof. Dr. Marisa Karow, Ludwig-Maximilians-Universität München, Biomedizinisches Centrum (BMC), Physiologische Genomik; aktuell: Friedrich-Alexander-Universität Erlangen-Nürnberg, Institut für Biochemie. |
| Determining the function of local inhibitory circuits in the synaptic dynamics of hippocampal pyramidal neurons during learning and memory |
| Dr. Alessio Attardo, Max-Planck-Institut für Psychiatrie, Dept. Stress Neurobiologie und Neurogenetik, München. |
| Chromatin und epigenetische Regulation während der neuronalen Migration |
| Dr. Tran Tuoc, Universitätsmedizin Göttingen, Institut für Neuroanatomie, Göttingen; aktuell: Ruhr-Universität Bochum, Medizinische Fakultät, Abteilung für Humangenetik. |
| α2δ-Untereinheiten spannungsgesteuerter Kalziumkanäle bestimmen die erregende und hemmende Konnektivität in neuronalen Netzwerken |
| Prof. Dr. Martin Heine, Leibniz-Institut für Neurobiologie (IfN), Magdeburg; aktuell: Johannes-Gutenberg-Universität Mainz, Institut für Entwicklungsbiologie und Neurobiologie. |
| 2020 |
| Untersuchung der Genregulation durch Polycomb-Proteine in neuralen Vorläuferzellen während der Entwicklung des humanen Neocortex |
| Dr. Mareike Albert, CRTD / DFG – Forschungszentrum, für Regenerative Therapien Dresden. |
| Regulation of synaptic vesicle biogenesis and degradation in neuronal transport: novel tools for studying the vesicle life cycle |
| Dr. Eugenio F. Fornasiero, Universitätsmedizin Göttingen, Institut für Neuro- und Sinnesphysiologie. |
| Structural, Molecular, and Functional determinants of enteroendocrine cell mediated gut-to-brain signaling |
| Dr. Cordelia Imig und Dr. Benjamin H. Cooper, Max-Planck-Institut für Experimentelle Medizin / Molecular Neurobiology, Göttingen, und University of Copenhagen, Department of Neuroscience, Kopenhagen, Dänemark. |
The basic interface of neuronal communication: the synapse
The central units for information transmission and processing in the brain are the chemical synapses, the contacts between neurons, which allow regulated neurotransmitter release from the presynapse and detection at the postsynaptic site. Several of the projects that were supported by grants from the Schram Foundation addressed the question of how synapses operate and how their activity changes to allow for plasticity and ultimately learning and memory. Many excitatory transmitter release sites utilizing glutamate as neurotransmitter contact spines, small protrusions from neuronal dendrites. Focusing on the BAR (Bin/Amphiphysin/Rvs)-domain protein syndapin I, the project led by Britta Qualmann (Figure 1) took a cell biological approach and examined how membrane shaping at these spines can be mediated by cytoskeletal forces and membrane-associated proteins. Syndapins partially insert into one leaflet of the cell membrane and can remodel membranes by scaffolding. They thus combine cytoskeletal and membrane shaping mechanisms. Britta Qualmann and her coworkers identified syndapin I as a crucial postsynaptic coordinator in the formation of excitatory synapses. Syndapin I–enriched membrane nanodomains thereby serve as important organizing platforms, which shape dendritic membrane areas into synaptic subcompartments (Schwintzer et al., 2011; Schneider et al., 2014).

Prof. Britta Qualmann discussing her results with Dr. Armin Schram during his visit at University Hospital Jena in 2011. Picture courtesy of Britta Qualmann (Foto: Riese/UKJ).
The project led by Volker Hauke dealt with the long-standing question of how synapses are kept up to speed (see also this issue). He and his coworkers focused on two complementary questions: First, how are key presynaptic components such as synaptic vesicles and active zone proteins formed, transported and assembled into nascent synapses? Second, how are synaptic vesicles regenerated after fusion? Among others, their work established that synaptic vesicles locally reform by adapter proteins that recognize specific components of the vesicle and sort them in a coordinated manner. Synapses thereby capitalize on clathrin-independent endocytosis and clathrin/AP-2–dependent reformation of synaptic vesicles from endosome-like vacuoles to maintain excitability (Kononenko et al., 2014).
The regulation of synaptic vesicle biogenesis and degradation is also addressed by the newly awarded grant to Eugenio Fornasiero. This project will develop new tools, based on protein stability measurements, imaging technologies and computational modeling, to decipher the precise molecular composition of synaptic vesicles and apply this knowledge to questions related to neuronal aging.
Membrane recycling mechanisms at the synapse were also at the center of the project headed by Ira Milosevic (see also this issue). Focusing on the key endocytic protein endophilin-A, she and her team described that, in addition to its essential role in endocytosis, endophilin-A has a role in the priming and fusion of secretory vesicles (Gowrisankaran et al., 2020). Endophilin-A deficiency causes dysregulation of autophagy and the ubiquitin-proteasome system (Murdoch et al., 2016). Synapses without endophilin-A accumulate clathrin-coated vesicles, an observation that led to the discovery that clathrin can control vesicle acidification by sterically blocking vacuolar ATPase activity (Farsi et al. 2018).
Besides membrane dynamics, the composition of the local extracellular matrix (ECM) at the synapse also profoundly influences synaptic function. Renato Frischknecht investigated the contribution of the perisynaptic ECM to network activity and memory formation. He and his colleagues observed that the perisynaptic ECM is modified during homeostatic plasticity and discovered activity-dependent mechanisms of ECM turnover. By training Mongolian gerbils in an auditory cortex–dependent discrimination and reversal learning task, they found that ECM removal promoted performance during reversal learning (Happel et al., 2014; Valenzuela et al., 2014). The local ECM at synapses thus contributes to neuronal network performance and memory consolidation.
Our ability to learn and memorize depends on internal brain states, such as attention and arousal, which are mediated by the action of neuromodulators. One such neuromodulator, noradrenaline, has long been known to facilitate NMDA (N-Methyl-D-Aspartat) receptor–dependent long-term synaptic potentiation (LTP), yet the precise mechanisms behind this effect have remained elusive. Supported by the Schram Foundation, Oliver Schlüter unraveled the identity of the potassium channel in the dendrite on which noradrenaline acts. Specifically, he discovered that the signaling scaffold protein SAP97 links the noradrenaline receptor beta2-adrenergic receptor to the inactivation of voltage-gated Kv1.1 potassium channels in the dendrite of hippocampal CA1 pyramidal neurons. This study provides a nice demonstration of how local changes in dendritic excitability can support the impact of NMDA-receptor activation during LTP (Liu et al., 2017).
Synapses do not work as isolated entities but must engage in continuous communication with the cell body and cell nucleus. Two of the first projects funded by the Schram Foundation addressed the mechanisms involved. Michael Kiebler discovered homologs of the invertebrate RNA-binding protein Staufen in mammals and made significant contributions to understanding their function at synapses. Supported by the Schram Foundation, he and his coworkers found that in rodent hippocampal neurons, Staufen 2 is critically involved in dendritic spine morphogenesis and contributes to memory formation and plasticity. Mechanistically, Staufen controls the transport and activity-dependent translation of mRNAs in distinct regions of the cell. Staufen proteins thereby facilitate locally restricted protein synthesis and consequently allow for spatially controlled adaptations within the cell (Fritzsche et al., 2013; Goetze et al., 2006; Heraud-Farlow et al., 2013).
Cellular events that lead to long-lasting memories require processes that occur in seconds but also on very long-time scales. That gene expression changes are involved has long been postulated. The project led by Michael Kreutz and Christina Spilker asked how synaptic events couple to transcriptional responses in the cell nucleus. They identified the neuronal Ca2+ sensor caldendrin, a postsynaptic density component, and Jacob, a caldendrin-binding partner, as key players in the communication from the dendrite to cell nucleus (Figure 2). Upon activation of NMDA-type glutamate receptors, Jacob is recruited to neuronal cell nuclei where it induces rapid transcriptional changes, which ultimately result in synaptic scaling and a drastically altered morphology of the dendritic tree. Caldendrin binds to Jacob’s nuclear localization signal in a Ca2+-dependent manner, thereby controlling Jacob’s ability to enter the cell nucleus. In addition, Michael Kreutz and his colleagues established that Jacob is phosphorylated by synaptic, but not extrasynaptic, NMDA-receptor activation and that Jacob’s differential phosphorylation determines whether NMDA-receptor activation promotes cell survival and enhances synaptic plasticity or induces cell death (Dieterich et al., 2008; Karpova et al., 2013).
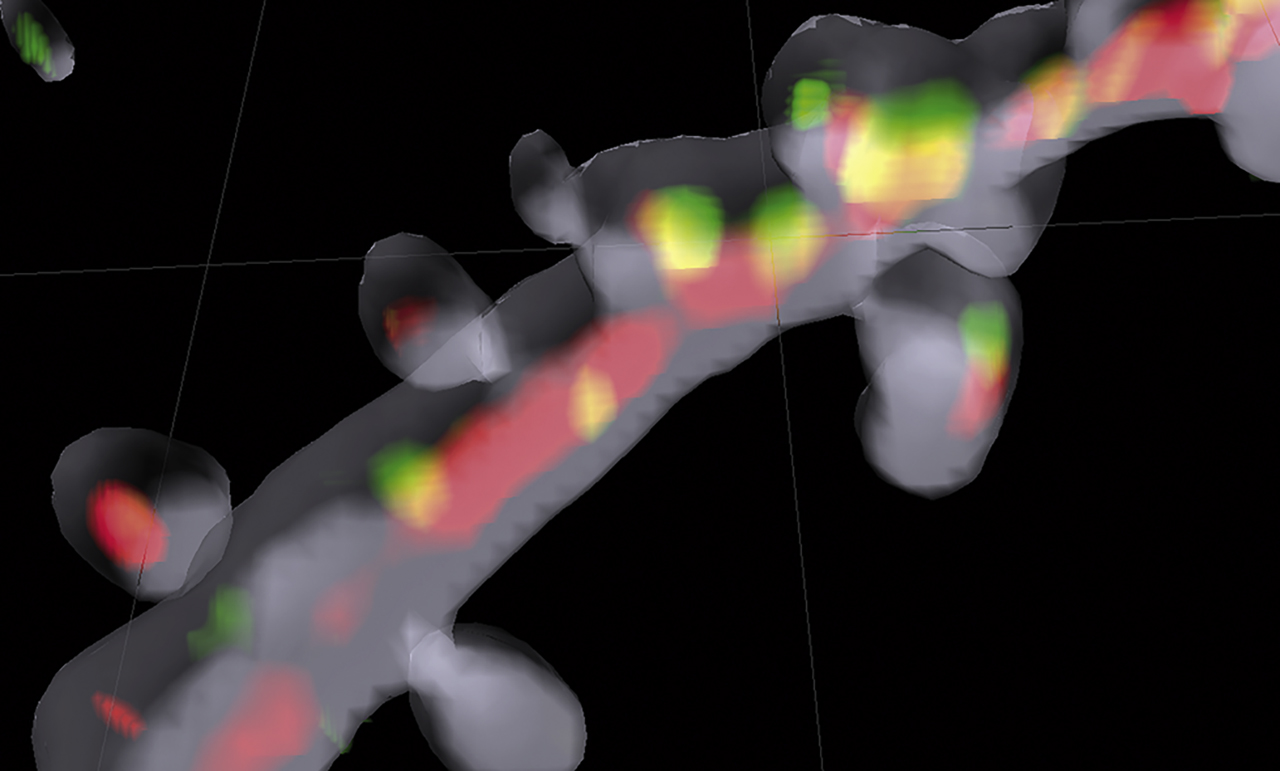
Communication between postsynapse and nucleus illuminated. 3D Imaris reconstruction of a dendritic segment filled with a volume marker (shown in gray) of a hippocampal pyramidal neuron. In red transport packages for importin-mediated long-distance transport are shown, and in green the synaptonuclear protein messenger Jacob can be seen on the way to the nucleus. Picture courtesy of Anna Karpova and Michael Kreutz, LIN, Magdeburg.
While the work highlighted above deals with the events taking place at synapses in the central nervous system, the grant recently awarded to the research team of Cordelia Imig and Benjamin H. Cooper enters truly new territories by dissecting fundamental synaptic signaling mechanisms at the synapse formed between enteroendocrine cells and sensory neurons. Enteroendocrine cells sense nutrients and metabolites in the gut and produce a range of gut hormones. Information exchange along the gut–brain axis is receiving increasing attention recently as it is crucial not only for feeding-related physiological responses, like appetite and satiety, but has also been linked to more complex traits such as anxiety-like behaviors.
You never walk alone: neuronal networks
Although the events taking place at individual synapses are the basis of learning and memory, it is the orchestrated activity of many neurons and the computational capacity of the resulting neuronal network that drives information processing and higher cognitive functions. Formation and stabilization of neuronal networks in rodents was investigated by several projects and from very different angles.
The strength of a given synapse in its neuronal network is primarily shaped by two parameters: the release probability of individual synaptic vesicles and the number of release sites that exist within each active zone. In his currently ongoing project, Martin Heine investigates how the composition and biochemical properties of voltage-gated calcium channels affect these processes. Focusing on CaV2.1, one of the major voltage-gated calcium channels responsible for fast synaptic transmission in the mammalian nervous system, he reported on the physiological consequences of alternative splicing of CaV2.1 transcripts, leading to channel isoforms with different intracellular domains. Depending on the nature of their intracellular domain, these alternative CaV2.1 isoforms exhibit diverse mobilities and dynamic organization within the presynaptic membrane, which alters the release probability of synaptic vesicles from these sites. This in turn profoundly affects the strength of synaptic transmission and consequently short-term plasticity and network properties (Heck et al., 2019). This study demonstrated not only that calcium channels at the presynapse are mobile and undergo permanent movements within nanodomains of the presynaptic membrane but also that alternative splicing of a single exon can have far-reaching consequences for the performance of the neuronal network as a whole (Heck et al., 2019; Figure 3). Calcium channels are crucial for neurotransmission, but are they also utilized to tune how excitation and inhibition in networks interact? Martin Heine and his team found that in the developing network the balance of excitation and inhibition is indeed regulated through varying the specific content of voltage-gated calcium channels (Bikbaev et al., 2020).
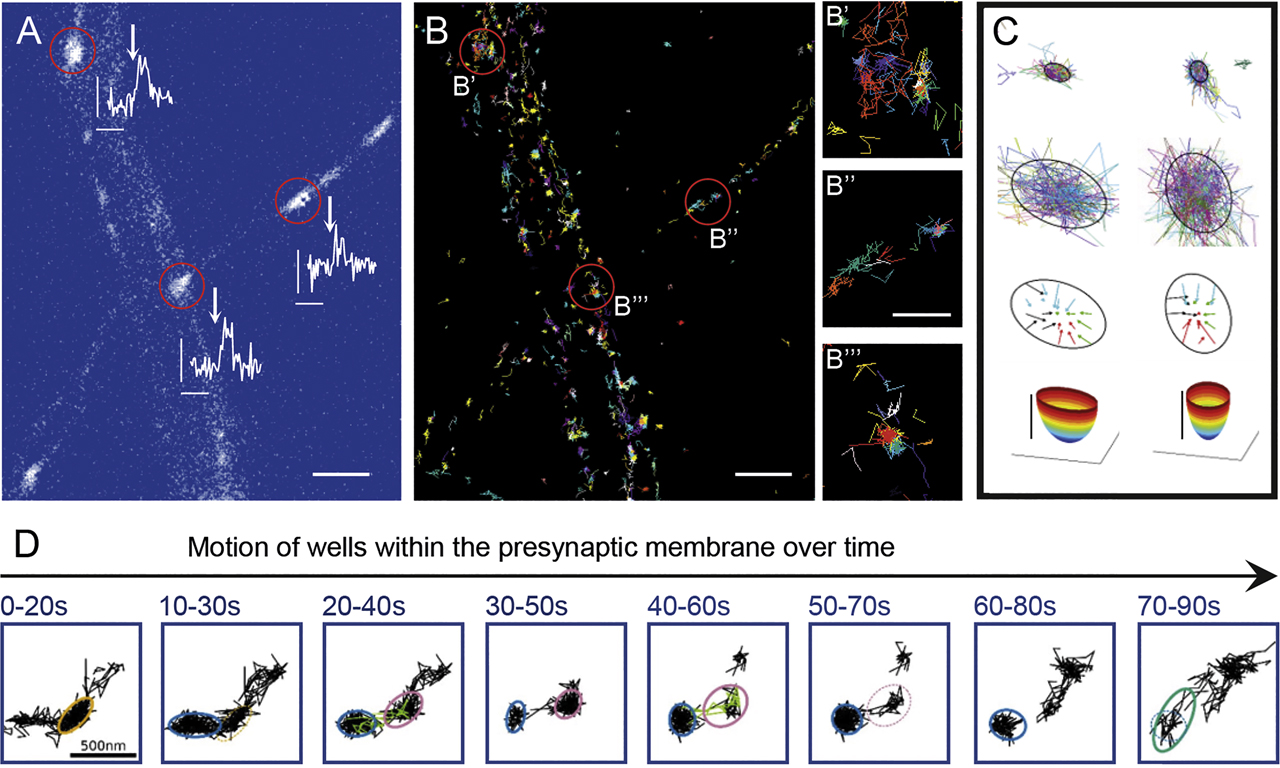
Local organization of voltage-gated calcium channels (CaV2.1) within the axonal membrane. (A) Axonal segments of rat hippocampal neurons expressing GCamp5::synaptophysin, the stimulation with four extracellular Action potential-like stimulations (scale bars 0.1 F/ΔF, 400 ms). (B) Trajectories of CaV2.1 channels; indicated are the synaptic locations (scale bar 2 µm). (B′–B′′′) higher magnifications of the synaptic regions marked in (B), demonstrating a mixed population of highly confined and mobile channels (scale bar 0.5 µm). (C) Examples of local confined CaV2.1 channels within energy wells keeping channels for a few 100 ms within the well. (D) In addition to motion inside the well, CaV2.1 channel wells move within the presynaptic membrane, disappearing and reappearing again. Picture courtesy of Martin Heine, Gutenberg University, Mainz.
GABAergic inhibitory interneurons play a key role in sculpting the representation of afferent information in principal cells. They are highly diverse and include the diversification in perisoma-inhibiting GABAergic interneurons, which control the timing and frequency of action potential generation in their target cells, and dendrite-targeting GABAergic cells whose functional characterization is lacking behind. Supported by the Schram Foundation, Marlene Bartos examined how dendrite-targeting GABAergic interneurons shape synaptic output properties in the dentate gyrus of mice (see also this issue). She and her coworkers discovered that one subtype of dendrite-targeting GABAergic interneurons, somatostatin-expressing cells, fall into different classes with distinct functional and dynamic synaptic output properties, which relate to the nature of their target cells. They undergo synaptic plasticity at their glutamatergic inputs, and the long-lasting potentiation of their inputs plays a key role in cognitive functions, like the recognition of replaced objects in the environment (Booker et al., 2020; Elgueta and Bartos, 2019).
In keeping with the saying ‘seeing is believing,’ Alessio Attardo has developed deep-brain 2-photon microscopy as a tool to visualize the dynamics of neuronal connections in living mice over weeks to months (Ulivi et al., 2019). Supported by the Schram Foundation, he currently applies this technique to the CA1 region of the hippocampal formation and tracks how the connectivity of excitatory and inhibitory neurons changes when animals undergo hippocampus-dependent learning tasks. Including optogenetics and chemogenetics, he also probes the effect of activation or inactivation of different classes of genetically defined local inhibitory neurons on synaptic dynamics, learning and memory.
Network formation viewed from a very different perspective was the topic of the project headed by Carmen Ruiz de Almodovar (see also this issue). As has become increasingly clear during the past decades, classical molecules that regulate neurodevelopment also play an important role in regulating the development of the vascular system. Adopting a converse approach, Carmen Ruiz de Almodovar asked whether angiogenic factors may also impinge on the nervous system. Although vascular endothelial growth factor (VEGF) and its receptor VEGFR2 were originally identified as angiogenesis-regulating receptor–ligand pair, VEGFR2 exhibits surprisingly restricted and dynamic expression on neurons of the CA3 region of the developing mouse hippocampus. Stimulation of VEGFR2-expressing hippocampal neurons with VEGF or targeted deletion of VEGFR2 in developing neurons both altered axonal branching and synapse formation. This finding established the prototypical angiogenic receptor VEGF as an important regulator of neuronal network formation (Luck et al., 2019).
A grant given to Petra Wahle, Silke Patz, Heiko Luhmann and Volkmar Leßmann dealt with the molecular, structural and physiological differentiation of the neonatal mammalian cortex. The Wahle and Patz groups identified which subunits of the AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid)-type glutamate receptors promote dendritic growth of cortical pyramidal cells and interneurons (Hamad et al., 2011) and showed that elevated levels of the proinflammatory cytokine Leukemia inhibitory factor (LIF) during brain development cause malfunctions of GABAergic neurons (Engelhardt et al., 2018). The Lessmann and Luhmann laboratories focused on the interplay of programmed cell death and developmental survival of neurons. Work on organotypic cultures showed that the activation of ionotropic glutamate receptors, GABA-A receptors, voltage-controlled calcium channels and electrical synapses (gap junctions) promotes the survival of neonatal cortical neurons (Golbs et al., 2011). This survival is mainly mediated by the brain-derived neurotrophic factor (BDNF) (Kuczewski et al., 2008).
Owing to the relative simplicity and accessibility of their nervous systems, invertebrates are excellent models to study neuronal network formation. The fruit fly D. melanogaster and the nematode C. elegans have so far taken the center stage in research projects funded by the Schram Foundation. In sharp contrast to the complex nervous systems of vertebrates, the nervous system of the adult C. elegans hermaphrodite has been fully mapped and the complete wiring diagram is known. Supported by funds from the Schram Foundation, Alexander Gottschalk developed a multispectral optical illumination system that allows precise spatiotemporal control over the activation of optogenetic tools in freely behaving animals (Stirman et al., 2012) (see also this issue). The term optogenetics refers to the targeted expression of genetically encoded light-sensitive ion channels or proton pumps, with the aim to functionally characterize single neurons or neuronal networks. Applying these tools to a nociceptive treatment regime, Alexander Gottschalk and his team dissected a neural circuit surrounding the neuron termed PVD and identified the channels, which by acting on PVD regulate behavioral outputs (Husson et al., 2012).
Painful events not only are answered by avoidance reactions but also establish memories. A neutral stimulus given close to a noxious experience can be remembered in opposite ways: Cues that precede pain or overlap with it are remembered as predictors of punishment and are later avoided. Cues that follow pain are perceived as relief and are therefore recalled positively. The project headed by Ayse Yarali examined the minimal circuit that supports the formation, storage and retrieval of these opposite memories in the mushroom bodies of D. melanogaster. These paired structures integrate multimodal inputs and fulfill important functions in learning and memory. Applying an optogenetic approach in fruit flies, Ayse Yarali’s team identified two types of dopaminergic neurons, each comprising one paired cell per hemisphere, which upon photostimulation evoke a reaction resembling the punishment-versus-relief memories that are reinforced by real noxious events (König et al., 2018).
While the two projects above dealt with the plasticity of already established neuronal networks, the project led by Thomas Hummel investigated the first steps of neuronal circuit formation during development. Among the great wonders of embryogenesis are the apparently self-organizing processes through which structure, order and complexity emerge. Thomas Hummel and coworkers discovered a simple but ingenious principle that drives this process. Opting for the D. melanogaster visual system as model, they found that the afferents of photoreceptor cells sequentially segregate into distinct layers of their target region depending on the relative time when the cells had undergone their final division. They identified a transcription factor, Sequoia, whose absolute protein load in individual photoreceptor cells reflects their relative birth order and which organizes growth cones in a dosage-sensitive manner. Small differences in the amount of Sequoia protein between individual photoreceptors organize their growth cones within the same layer, whereas large differences segregate growth cones between layers. The birth order of photoreceptor neurons thus establishes a prepattern, which dictates the assembly of synaptic connections during visual map formation (Kulkarni et al., 2016).
Finding one’s identity: cell fate specification
The performance of a neuronal network not only depends on the size, strength and kind of its synapses or the number and nature of its connections. Critically important for every network are the types of neurons it consists of and the glia that associate with them. Neuronal and glial cell types are highly diverse, differing in their size, morphology, and physiological and molecular properties. Understanding how individual cell types are produced at the right time and place and in the right relative proportions is therefore a key question in developmental neurobiology. Having been awarded one of the first Schram grants, Michael Wegner set out to decipher the transcription factors that control the generation of oligodendrocytes, the myelin-forming macroglia that facilitate the fast, saltatory nerve conduction characteristic of the vertebrate central nervous system. He uncovered a network of Sox-type transcription factors, centered around the Sox-family member SOX9, that allows for the timely progression of oligodendrocyte development in the spinal cord. He established that SOX9 is essential for gliogenesis and that it is required, jointly with SOX10, for survival and migration of oligodendroglial precursor cells (Finzsch et al., 2008). SOX9 and SOX10 regulate expression of the distantly related Sox5 and Sox6 genes, which in turn modulate the activity of Sox9 and Sox10 in a negative feedback loop and thereby determine the timing of oligodendroglial differentiation (Stolt et al., 2006). These studies shed light on the interdependent levels of transcriptional regulation that are needed to advance the production of a single cell type, myelinating oligodendrocytes.
A rather unexpected mechanism by which the development of myelinating oligodendrocytes is regulated in the corpus callosum of juvenile mice was revealed by the work of Bernd Knöll. He and his coworkers observed that targeted deletion of the transcription factor SRF in neurons interfered with oligodendrocyte development in a non–cell autonomous manner. Consistently, neuronal deletion of SRF resulted in myelination defects and axon degeneration, whereas forced activation of SRF in neurons affected the maturation of neighboring oligodendrocytes. Paracrine regulation of oligodendrogliogenesis by neuronal SRF involves two secreted molecules, connective tissue growth factor (CTGF), which is repressed by SRF, and insulin like growth factor 1 (IGF-1), which stimulates oligodendrocyte maturation but is antagonized by CTGF. This double-negative regulation places oligodendrocyte maturation under the control of nearby neurons (Stritt et al., 2009).
The network of transcription factors controlling autonomous nervous system development was investigated in the project led by Hermann Rohrer. The autonomous nervous system is derived from a transient cell population called neural crest. It regulates involuntary physiologic processes and contains three anatomical distinctions, the sympathetic, parasympathetic and enteric nervous system. Hermann Rohrer and his team demonstrated that the transcription factors AP-2α/AP-2β exert an early prespecifying function for sympathetic progenitor cells and a later survival function for sympathetic neurons (Schmidt et al., 2011). Likewise, transcription factors of the HoxB cluster exert an early influence on the prespecification of the sympathetic versus sensory neuron lineages of the neural crest and support and maintain the expression of sympathetic neuron genes (Huber et al., 2012).
Transcription factors bind enzymes, which chemically alter DNA or proteins, and recruit these enzymes to specific sites in the genome. This process, known as epigenetic modification, introduces heritable but reversible changes in DNA or histones, the building blocks of nucleosomes. For transcription to occur, nucleosomes must be destabilized on DNA by the activity of nucleosome-remodeling ATPases. Nucleosome remodeling and histone modifying activities jointly reorganize the chromatin structure in a way that either facilitates or inhibits gene expression. The Schram Foundation supported several projects that examined the effect of these activities on development and aging of the nervous system. Studying the role of the BAF (BRG1- or BRM-associated factor) nucleosome-remodeling complex in the developing mouse neocortex, Tran Tuoc discovered that nucleosome remodeling is closely integrated with the activity of histone demethylases during corticogenesis. He found that BAF complexes can simultaneously silence the expression of genes required for the proliferation of cortical progenitor cells and stimulate the expression of genes associated with the differentiation and migration of young neurons. Mechanistically, this involved recruitment of histone-demethylating enzymes with opposing functions, KDM6A/B and KDM1A, respectively. By acting both as activators and repressors of gene expression, BAF complexes thus ensure the generation of the appropriate numbers of neurons as well as their proper migration during cortical histogenesis (Narayanan et al., 2018; Nguyen et al., 2018) (Figure 4).
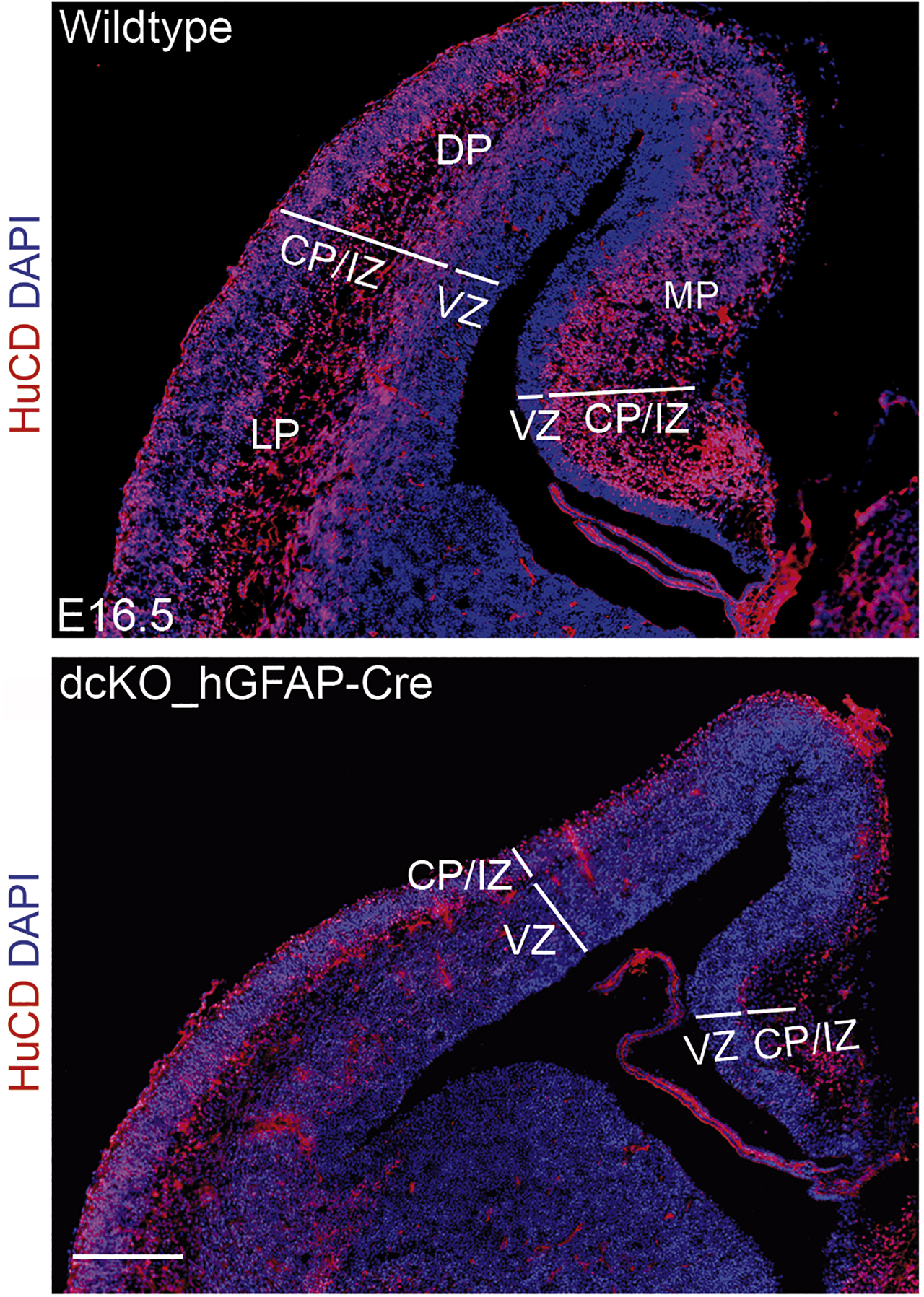
Loss of BAF155 and BAF170 in the early cortical anlage leads to a diminished thickness of the cortical plate at later embryonic stages. Immunofluorescence staining for the neuron-specific RNA-binding protein HuC/D (ELAV; red) in the cortices of E 16.5 wild-type mice and littermates double mutant for BAF155 and BAF170 under control of the human GFAP promoter. CP, cortical plate; DP, dorsal pallium; IZ, intermediate zone; LP, lateral pallium; MP, medial pallium; VZ, ventricular zone; Scale bar represents 100 mm. Picture courtesy of Tran Tuoc, Göttingen.
With the help of her recently awarded Schram grant, Mareike Albert will study the function(s) that Polycomb group (PcG) proteins, a family of histone-methylating enzymes and potent epigenetic repressors, have in the developing human cortex. In mice, PcG proteins contribute to all phases of cortical development. Yet, given the striking differences between the rodent and primate neocortex, lessons learned in murine models cannot be simply applied to humans. Mareike Albert will apply CRISPR/Cas9-based genome-editing tools to human brain organoids to functionally interrogate the role of histone methylation during human brain development.
The importance of epigenetic regulation for brain development cannot be discussed without acknowledging its role in aging. André Fischer established that age-associated memory impairment is tightly linked with altered epigenetic plasticity. In a study supported by the Schram Foundation, he discovered that aged mice display a specific deregulation of the epigenetic mark histone H4 lysine 12 (H4K12) acetylation and that this deregulation correlated with hippocampus-specific changes in gene expression programs associated with memory consolidation. Restoration of physiological H4K12 acetylation reinstated the expression of learning-induced genes and led to the recovery of cognitive abilities (Peleg et al., 2010). In another study, he and his team established that lysine acetyltransferase 2a (KAT2A), an enzyme that catalyzes the attachment of acetyl groups on histone and nonhistone proteins, regulates a highly interconnected gene expression network in the hippocampus and thereby impacts synaptic plasticity and long-term memory consolidation (Stilling et al., 2014).
Although most neurons in the central nervous system are generated during embryogenesis and in early postnatal life, a small but physiologically important number of neurons is continuously added during adulthood in a process known as adult neurogenesis. Production of neurons in the adult brain occurs in response to environmental stimuli and, hence, reflects the physiological state of the individuum. The molecular players that drive adult neurogenesis must therefore quickly and efficiently react to changing extrinsic cues. Dorothea Schulte examined how transcriptionally silent genes become activated when adult neural stem cells exit dormancy and begin to differentiate toward neurons. She and her team discovered a molecular cascade, involving the transcription factors MEIS2 and PBX1 and the nuclear enzyme PARP1, which induces the decompaction of transcriptionally silent chromatin at the regulatory regions of neuron-specific genes, thereby facilitating the rapid execution of neuronal gene expression programs (Hau et al., 2017). These chromatin dynamics are set into motion by the translocation of MEIS2 into the cell nucleus, which is controlled by MEIS2′ posttranslational modification downstream of signals from the stem cell niche (Kolb et al., 2018).
Temporal control over stem cell activation by nuclear translocation of a neurogenic cell fate determinant was also investigated in the project headed by Jens Christian Schwamborn. He demonstrated that the multifunctional protein TRIM32 undergoes differentiation-associated translocation into the nucleus when neural progenitors mature to olfactory bulb interneurons. TRIM32 participates in cytoplasmic and nuclear functions that are necessary for neuronal differentiation, consistent with the notion that its gradual nuclear accumulation reflects a gradual maturation of adult born neuroblasts (Hillje et al., 2013).
A fundamental question in cell biology is whether the acquisition of a given cell fate during embryonic development is fixed or reversible. Mounting evidence over the last years has shown that the forced expression of lineage-specific transcription factors in various differentiated cell types can promote the reversal of cellular fates, a process recognized as cellular reprogramming. In her ongoing project, Marisa Karow converted human pericytes, mural cells that wrap around blood vessels in the brain, into neurons by the overexpression of two neurogenic transcription factors, Ascl1 and Sox2. Using single-cell RNA sequencing to dissect transcriptome changes and reconstruct lineage reprogramming trajectories, Marisa Karow and colleagues discovered that successful reprogramming involves the recapitulation of developmental programs via stem cell–like intermediates (Karow et al., 2018).
Closing remarks
Owing to space limitations, this collection of results and concepts is inevitably incomplete. Nevertheless, it gives a brief but comprehensive overview over the many fundamental discoveries that research grants awarded by the Schram Foundation have made possible over the years. Also worth of note is that grants are predominantly given to young researchers, many of them at the transition from postdoctoral fellow to independent group leader or on the brink of taking their first academic position. In fact, in several cases the Schram Foundation gave the very first research funds to these projects and thereby contributed in an essential way to the start of new, long-term areas of research. As one Schram fellow put it, “There are many challenges associated with starting an independent group and developing the own research profile. With the Schram Stiftung backing my work, some challenges simply turned into opportunities.” Considering that most previous Schram fellows have taken permanent academic positions at domestic universities and institutions, the foundation’s impact on neuroscience research in Germany goes well beyond the immediate duration of the funded projects. It is thus fair to say that the Schram Foundation, during the relatively short time of its existence, has made remarkable contributions to the neuroscience research landscape in Germany. Undoubtedly it will continue to do so in the future.
About the authors

Dorothea Schulte is a professor of Experimental Neuropathology at the Edinger Institute of Goethe University Hospital in Frankfurt. She studied Biology at the University of Konstanz, where she also obtained her PhD in Molecular Genetics. After postdoctoral training at the Department of Genetics at Harvard Medical School in Boston, she returned to Germany to lead a research group in the Department of Neuroanatomy of the Max Planck Institute for Brain Research in Frankfurt. In 2011, she was awarded a research grant by the Schram Foundation and joined the Board of the foundation in 2016. Her research focusses on genetic and epigenetic regulation of neurogenesis.

Christian Rosenmund is a professor (W3) for Cellular Neurobiology at the Institute of Neurophysiology at the Charité - Center for Basic Sciences in Berlin. He initially studied Pharmacy in Frankfurt and performed his doctoral research at the Vollum Institute in Portland, USA, under supervision of Gary Westbrook from 1989 to 1993. He went to Charles Stevens at the Salk Institute, La Jolla, USA, for a postdoc and returned to Germany as a Helmholtz and Heisenberg fellow to the Department of Membrane Biophysics at Max Planck Institute for Biophysical Chemistry, Göttingen. In 2003, he went to the Departments of Neurosciences and Genetics at Baylor College of Medicine, Houston (USA) as an associate and full professor, before moving to Berlin in 2010. His research is focused on the cellular and molecular physiology and pathophysiology of central mammalian synapses. He joined the Board of the Schram Foundation in 2015.

Eckart D. Gundelfinger heads the Neurochemistry Department at the Leibniz Institute for Neurobiology (LIN) and is a professor at the Otto von Guericke University, Magdeburg. He studied Biology in Stuttgart, performed doctoral research at the MPI for Biology in Tübingen and was a postdoc at the EMBL in Heidelberg. In 1984, he joined the Betz laboratory at the Center for Molecular Biology Heidelberg (ZMBH), and from 1988 to 1993, he headed a BMFT/BMBF-funded group at the Center for Molecular Neurobiology, Hamburg (ZMNH). In 1992, he accepted his current position, and from 2010 to 2019, he was the managing scientific director of the LIN. His research is centered around the molecular structure and plasticity of brain synapses. He joined the Board of the Schram Foundation in 2009.
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: None declared.
-
Conflict of interest statement: The authors declare no conflicts of interest.
References
Bikbaev, A., Ciuraszkiewicz-Wojciech, A., Heck, J., Klatt, O., Freund, R., Mitlöhner, J., Enrile Lacalle, S., Sun, M., Repetto, D., Frischknecht, R., et al. (2020). Auxiliary α2δ1 and α2δ3 subunits of calcium channels drive excitatory and inhibitory neuronal network development. J. Neurosci. : Off. J. Soc. Neurosci. 40, 4824–4841, https://doi.org/10.1523/jneurosci.1707-19.2020.Search in Google Scholar PubMed PubMed Central
Booker, S.A., Harada, H., Elgueta, C., Bank, J., Bartos, M., Kulik, A., and Vida, I. (2020). Presynaptic GABAB receptors functionally uncouple somatostatin interneurons from the active hippocampal network. eLife 9, e51156, https://doi.org/10.7554/elife.51156.Search in Google Scholar
Dieterich, D.C., Karpova, A., Mikhaylova, M., Zdobnova, I., König, I., Landwehr, M., Kreutz, M., Smalla, K.-H., Richter, K., Landgraf, P., et al. (2008). Caldendrin-Jacob: A protein liaison that couples NMDA receptor signalling to the nucleus. PLoS Biol. 6, e34, https://doi.org/10.1371/journal.pbio.0060034.Search in Google Scholar PubMed PubMed Central
Elgueta, C. and Bartos, M. (2019). Dendritic inhibition differentially regulates excitability of dentate gyrus parvalbumin-expressing interneurons and granule cells. Nat. Commun. 10, 5561, https://doi.org/10.1038/s41467-019-13533-3.Search in Google Scholar PubMed PubMed Central
Engelhardt, M., Hamad, M.I.K., Jack, A., Ahmed, K., König, J., Rennau, L.M., Jamann, N., Räk, A., Schönfelder, S., Riedel, C., et al. (2018). Interneuron synaptopathy in developing rat cortex induced by the pro-inflammatory cytokine LIF. Exp. Neurol. 302, 169–180, https://doi.org/10.1016/j.expneurol.2017.12.011.Search in Google Scholar PubMed
Farsi, Z., Gowrisankaran, S., Krunic, M., Rammner, B., Woehler, A., Lafer, E.M., Mim, C., Jahn, R., and Milosevic, I. (2018). Clathrin coat controls synaptic vesicle acidification by blocking vacuolar ATPase activity. eLife 7, e32569, https://doi.org/10.7554/elife.32569.Search in Google Scholar
Finzsch, M., Stolt, C.C., Lommes, P., and Wegner, M. (2008). Sox9 and Sox10 influence survival and migration of oligodendrocyte precursors in the spinal cord by regulating PDGF receptor alpha expression. Development (Cambridge, England) 135, 637–646, https://doi.org/10.1242/dev.010454.Search in Google Scholar PubMed
Fritzsche, R., Karra, D., Bennett, K.L., Ang, F.Y., Heraud-Farlow, J.E., Tolino, M., Doyle, M., Bauer, K.E., Thomas, S., Planyavsky, M., et al. (2013). Interactome of two diverse RNA granules links mRNA localization to translational repression in neurons. Cell Rep. 5, 1749–1762, https://doi.org/10.1016/j.celrep.2013.11.023.Search in Google Scholar PubMed
Goetze, B., Tuebing, F., Xie, Y., Dorostkar, M.M., Thomas, S., Pehl, U., Boehm, S., Macchi, P., and Kiebler, M.A. (2006). The brain-specific double-stranded RNA-binding protein Staufen2 is required for dendritic spine morphogenesis. J. Cell Biol. 172, 221–231, https://doi.org/10.1083/jcb.200509035.Search in Google Scholar PubMed PubMed Central
Golbs, A., Nimmervoll, B., Sun, J.-J., Sava, I.E., and Luhmann, H.J. (2011). Control of programmed cell death by distinct electrical activity patterns. Cerebral Cortex (New York, N.Y.) 21, 1192–1202, https://doi.org/10.1093/cercor/bhq200.Search in Google Scholar PubMed
Gowrisankaran, S., Houy, S., Del Castillo, J.G.P., Steubler, V., Gelker, M., Kroll, J., Pinheiro, P.S., Schwitters, D., Halbsgut, N., Pechstein, A., et al. (2020). Endophilin-A coordinates priming and fusion of neurosecretory vesicles via intersectin. Nat. Commun. 11, 1266, https://doi.org/10.1038/s41467-020-14993-8.Search in Google Scholar PubMed PubMed Central
Hamad, M.I.K., Ma-Högemeier, Z.-L., Riedel, C., Conrads, C., Veitinger, T., Habijan, T., Schulz, J.-N., Krause, M., Wirth, M.J., Hollmann, M., et al. (2011). Cell class-specific regulation of neocortical dendrite and spine growth by AMPA receptor splice and editing variants. Development (Cambridge, England) 138, 4301–4313, https://doi.org/10.1242/dev.071076.Search in Google Scholar PubMed
Happel, M.F.K., Niekisch, H., Castiblanco Rivera, L.L., Ohl, F.W., Deliano, M., and Frischknecht, R. (2014). Enhanced cognitive flexibility in reversal learning induced by removal of the extracellular matrix in auditory cortex. Proc. Natl. Acad. Sci. U. S. A. 111, 2800–2805, https://doi.org/10.1073/pnas.1310272111.Search in Google Scholar PubMed PubMed Central
Hau, A.-C., Grebbin, B.M., Agoston, Z., Anders-Maurer, M., Müller, T., Groß, A., Kolb, J., Langer, J.D., Döring, C., and Schulte, D. (2017). MEIS homeodomain proteins facilitate PARP1/ARTD1-mediated eviction of histone H1. J. Cell Biol. 216, 2715–2729, https://doi.org/10.1083/jcb.201701154.Search in Google Scholar PubMed PubMed Central
Heck, J., Parutto, P., Ciuraszkiewicz, A., Bikbaev, A., Freund, R., Mitlöhner, J., Andres-Alonso, M., Fejtova, A., Holcman, D., and Heine, M. (2019). Transient confinement of CaV2.1 Ca2+-channel splice variants shapes synaptic short-term plasticity. Neuron 103, 66–79, e12, https://doi.org/10.1016/j.neuron.2019.04.030.Search in Google Scholar PubMed
Heraud-Farlow, J.E., Sharangdhar, T., Li, X., Pfeifer, P., Tauber, S., Orozco, D., Hörmann, A., Thomas, S., Bakosova, A., Farlow, A.R., et al. (2013). Staufen2 regulates neuronal target RNAs. Cell Rep. 5, 1511–1518, https://doi.org/10.1016/j.celrep.2013.11.039.Search in Google Scholar PubMed
Hillje, A.-L., Pavlou, M.A.S., Beckmann, E., Worlitzer, M.M.A., Bahnassawy, L., Lewejohann, L., Palm, T., and Schwamborn, J.C. (2013). TRIM32-dependent transcription in adult neural progenitor cells regulates neuronal differentiation. Cell Death Dis. 4, e976, https://doi.org/10.1038/cddis.2013.487.Search in Google Scholar PubMed PubMed Central
Huber, L., Ferdin, M., Holzmann, J., Stubbusch, J., and Rohrer, H. (2012). HoxB8 in noradrenergic specification and differentiation of the autonomic nervous system. Dev. Biol. 363, 219–233, https://doi.org/10.1016/j.ydbio.2011.12.026.Search in Google Scholar PubMed
Husson, S.J., Costa, W.S., Wabnig, S., Stirman, J.N., Watson, J.D., Spencer, W.C., Akerboom, J., Looger, L.L., Treinin, M., Miller, D.M., et al. (2012). Optogenetic analysis of a nociceptor neuron and network reveals ion channels acting downstream of primary sensors. Curr. Biol.: CB 22, 743–752, https://doi.org/10.1016/j.cub.2012.02.066.Search in Google Scholar PubMed PubMed Central
Karow, M., Camp, J.G., Falk, S., Gerber, T., Pataskar, A., Gac-Santel, M., Kageyama, J., Brazovskaja, A., Garding, A., Fan, W., et al. (2018). Direct pericyte-to-neuron reprogramming via unfolding of a neural stem cell-like program. Nat. Neurosci. 21, 932–940, https://doi.org/10.1038/s41593-018-0168-3.Search in Google Scholar PubMed PubMed Central
Karpova, A., Mikhaylova, M., Bera, S., Bär, J., Reddy, P.P., Behnisch, T., Rankovic, V., Spilker, C., Bethge, P., Sahin, J., et al. (2013). Encoding and transducing the synaptic or extrasynaptic origin of NMDA receptor signals to the nucleus. Cell 152, 1119–1133, https://doi.org/10.1016/j.cell.2013.02.002.Search in Google Scholar PubMed
Kolb, J., Anders-Maurer, M., Müller, T., Hau, A.-C., Grebbin, B.M., Kallenborn-Gerhardt, W., Behrends, C., and Schulte, D. (2018). Arginine methylation regulates MEIS2 nuclear localization to promote neuronal differentiation of adult SVZ progenitors. Stem Cell Rep. 10, 1184–1192, https://doi.org/10.1016/j.stemcr.2018.03.010.Search in Google Scholar PubMed PubMed Central
König, C., Khalili, A., Ganesan, M., Nishu, A. P., Garza, A.P., Niewalda, T., Gerber, B., Aso, Y., and Yarali, A. (2018). Reinforcement signaling of punishment versus relief in fruit flies. Learn. Mem. 25, 247–257, https://doi.org/10.1101/lm.047308.118.Search in Google Scholar PubMed PubMed Central
Kononenko, N.L., Puchkov, D., Classen, G.A., Walter, A.M., Pechstein, A., Sawade, L., Kaempf, N., Trimbuch, T., Lorenz, D., Rosenmund, C., et al. (2014). Clathrin/AP-2 mediate synaptic vesicle reformation from endosome-like vacuoles but are not essential for membrane retrieval at central synapses. Neuron 82, 981–988, https://doi.org/10.1016/j.neuron.2014.05.007.Search in Google Scholar PubMed
Kuczewski, N., Porcher, C., Ferrand, N., Fiorentino, H., Pellegrino, C., Kolarow, R., Lessmann, V., Medina, I., and Gaiarsa, J.-L. (2008). Backpropagating action potentials trigger dendritic release of BDNF during spontaneous network activity. J. Neurosci. : Off. J. Soc. Neurosci. 28, 7013–7023, https://doi.org/10.1523/jneurosci.1673-08.2008.Search in Google Scholar PubMed PubMed Central
Kulkarni, A., Ertekin, D., Lee, C.-H., and Hummel, T. (2016). Birth order dependent growth cone segregation determines synaptic layer identity in the Drosophila visual system. eLife 5, e13715.https://doi.org/10.7554/elife.13715.024.Search in Google Scholar
Liu, Y., Cui, L., Schwarz, M.K., Dong, Y., and Schlüter, O.M. (2017). Adrenergic gate release for spike timing-dependent synaptic potentiation. Neuron 93, 394–408, https://doi.org/10.1016/j.neuron.2016.12.039.Search in Google Scholar PubMed PubMed Central
Luck, R., Urban, S., Karakatsani, A., Harde, E., Sambandan, S., Nicholson, L., Haverkamp, S., Mann, R., Martin-Villalba, A., Schuman, E.M., et al. (2019). VEGF/VEGFR2 signaling regulates hippocampal axon branching during development. eLife 8, e49818. https://doi.org/10.7554/elife.49818.Search in Google Scholar PubMed PubMed Central
Murdoch, J.D., Rostosky, C.M., Gowrisankaran, S., Arora, A.S., Soukup, S.-F., Vidal, R., Capece, V., Freytag, S., Fischer, A., Verstreken, P., et al. (2016). Endophilin-A deficiency induces the Foxo3a-Fbxo32 network in the brain and causes dysregulation of autophagy and the ubiquitin-proteasome system. Cell Rep. 17, 1071–1086, https://doi.org/10.1016/j.celrep.2016.09.058.Search in Google Scholar PubMed PubMed Central
Narayanan, R., Pham, L., Kerimoglu, C., Watanabe, T., Castro Hernandez, R., Sokpor, G., Ulmke, P.A., Kiszka, K.A., Tonchev, A.B., Rosenbusch, J., et al. (2018). Chromatin remodeling BAF155 subunit regulates the genesis of basal progenitors in developing cortex. iScience 4, 109–126, https://doi.org/10.1016/j.isci.2018.05.014.Search in Google Scholar PubMed PubMed Central
Nguyen, H., Kerimoglu, C., Pirouz, M., Pham, L., Kiszka, K.A., Sokpor, G., Sakib, M.S., Rosenbusch, J., Teichmann, U., Seong, R.H., et al. (2018). Epigenetic regulation by BAF complexes limits neural stem cell proliferation by suppressing Wnt signaling in late embryonic development. Stem cell Rep. 10, 1734–1750, https://doi.org/10.1016/j.stemcr.2018.04.014.Search in Google Scholar PubMed PubMed Central
Peleg, S., Sananbenesi, F., Zovoilis, A., Burkhardt, S., Bahari-Javan, S., Agis-Balboa, R.C., Cota, P., Wittnam, J.L., Gogol-Doering, A., Opitz, L., et al. (2010). Altered histone acetylation is associated with age-dependent memory impairment in mice. Science (New York, N.Y.) 328, 753–756, https://doi.org/10.1126/science.1186088.Search in Google Scholar PubMed
Schmidt, M., Huber, L., Majdazari, A., Schütz, G., Williams, T., and Rohrer, H. (2011). The transcription factors AP-2β and AP-2α are required for survival of sympathetic progenitors and differentiated sympathetic neurons. Dev. Biol. 355, 89–100, https://doi.org/10.1016/j.ydbio.2011.04.011.Search in Google Scholar PubMed
Schneider, K., Seemann, E., Liebmann, L., Ahuja, R., Koch, D., Westermann, M., Hübner, C.A., Kessels, M.M., and Qualmann, B. (2014). ProSAP1 and membrane nanodomain-associated syndapin I promote postsynapse formation and function. J. Cell Biol. 205, 197–215, https://doi.org/10.1083/jcb.201307088.Search in Google Scholar PubMed PubMed Central
Schwintzer, L., Koch, N., Ahuja, R., Grimm, J., Kessels, M.M., and Qualmann, B. (2011). The functions of the actin nucleator Cobl in cellular morphogenesis critically depend on syndapin I. EMBO J. 30, 3147–3159, https://doi.org/10.1038/emboj.2011.207.Search in Google Scholar PubMed PubMed Central
Stilling, R.M., Rönicke, R., Benito, E., Urbanke, H., Capece, V., Burkhardt, S., Bahari-Javan, S., Barth, J., Sananbenesi, F., Schütz, A.L., et al. (2014). K-Lysine acetyltransferase 2a regulates a hippocampal gene expression network linked to memory formation. EMBO J. 33, 1912–1927, https://doi.org/10.15252/embj.201487870.Search in Google Scholar PubMed PubMed Central
Stirman, J.N., Crane, M.M., Husson, S.J., Gottschalk, A., and Lu, H. (2012). A multispectral optical illumination system with precise spatiotemporal control for the manipulation of optogenetic reagents. Nat. Protoc. 7, 207–220, https://doi.org/10.1038/nprot.2011.433.Search in Google Scholar PubMed PubMed Central
Stolt, C.C., Schlierf, A., Lommes, P., Hillgärtner, S., Werner, T., Kosian, T., Sock, E., Kessaris, N., Richardson, W.D., Lefebvre, V., et al. (2006). SoxD proteins influence multiple stages of oligodendrocyte development and modulate SoxE protein function. Dev. Cell 11, 697–709, https://doi.org/10.1016/j.devcel.2006.08.011.Search in Google Scholar PubMed
Stritt, C., Stern, S., Harting, K., Manke, T., Sinske, D., Schwarz, H., Vingron, M., Nordheim, A., and Knöll, B. (2009). Paracrine control of oligodendrocyte differentiation by SRF-directed neuronal gene expression. Nat. Neurosci. 12, 418–427, https://doi.org/10.1038/nn.2280.Search in Google Scholar PubMed
Ulivi, A.F., Castello-Waldow, T.P., Weston, G., Yan, L., Yasuda, R., Chen, A., and Attardo, A. (2019). Longitudinal two-photon imaging of dorsal hippocampal CA1 in live mice. JoVE, https://doi.org/10.3791/59598.Search in Google Scholar PubMed
Valenzuela, J.C., Heise, C., Franken, G., Singh, J., Schweitzer, B., Seidenbecher, C.I., and Frischknecht, R. (2014). Hyaluronan-based extracellular matrix under conditions of homeostatic plasticity. Phil. Trans. Roy. Soc. Lond. B Biol. Sci. 369, 20130606, https://doi.org/10.1098/rstb.2013.0606.Search in Google Scholar PubMed PubMed Central
© 2020 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Editorial
- Editorial
- Review articles
- Synapses, networks, brain development – funding basic neuroscience research in Germany by the Schram Foundation
- Neuronal functions of clathrin-associated endocytic sorting adaptors – from molecules to disease
- Mechanisms of synaptic vesicle recycling provide a platform to explore mechanisms of neurodegeneration
- Optogenetic analyses of neuronal networks that generate behavior in Caenorhabditis elegans
- Direct contribution of angiogenic factors to neurodevelopment: a focus on angiopoietins
- The role of the dentate gyrus in mnemonic functions
- Presentation of scientific institutions
- Armin Schram: a sponsor of curiosity-driven brain research
- Nachrichten aus der Gesellschaft
- Nachrichten aus der Gesellschaft
Articles in the same Issue
- Frontmatter
- Editorial
- Editorial
- Review articles
- Synapses, networks, brain development – funding basic neuroscience research in Germany by the Schram Foundation
- Neuronal functions of clathrin-associated endocytic sorting adaptors – from molecules to disease
- Mechanisms of synaptic vesicle recycling provide a platform to explore mechanisms of neurodegeneration
- Optogenetic analyses of neuronal networks that generate behavior in Caenorhabditis elegans
- Direct contribution of angiogenic factors to neurodevelopment: a focus on angiopoietins
- The role of the dentate gyrus in mnemonic functions
- Presentation of scientific institutions
- Armin Schram: a sponsor of curiosity-driven brain research
- Nachrichten aus der Gesellschaft
- Nachrichten aus der Gesellschaft

