The role of the dentate gyrus in mnemonic functions
-
Jonas-Frederic Sauer
Venia Legendi in Physiology. In 2007, she was appointed as a full professor at the University of Aberdeen (UK) and received a Lichtenberg Professorship (W3) of the VW Foundation at Freiburg University in 2010. She is since 2018 the Director of the Institute for Physiology I at Freiburg University. Since 2014, she is a speaker of the Research Unit FOR2143 ‘Interneuron Plasticity,’ and she received the ERC Advanced Grant in 2018. Dr. Bartos laboratory examines the synaptic, cellular and network mechanisms, which underlie the formation of memory traces in the hippocampus. Her laboratory made seminal discoveries on the emergence, stability and reliability of memory during learning in the hippocampus and the mechanisms, which may underlie cognitive disorders in genetic models of schizophrenia and depression. Currently, she examines the role of inhibitory cells and interneuron plasticity in the establishment of memory engrams.
Abstract
The hippocampus is decisive for the storage of conscious memories. Current theories suggest that experience-dependent modifications in excitation–inhibition balance enable a select group of neurons to form a new cell association during learning which represents the new memory trace. It was further proposed that particularly GABAergic-inhibitory interneurons have a large impact on population activity in neuronal networks by means of their inhibitory output synapses. They synchronize active principal cells at high frequencies, thereby supporting their binding to cell assemblies to jointly encode information. However, how cell associations emerge in space and time and how interneurons may contribute to this process is still largely unknown. We started to address this fundamental question in the dentate gyrus (DG) as the input gate of the hippocampus, which has an indispensable role in conscious memory formation. We used a combination of in vivo chronic two-photon imaging of population activity in the DG and the hippocampal areas CA1–3 of mice exposed to a virtual reality, in which they perform a goal-oriented spatial memory tasks, with high-density in vivo recordings and multiple whole-cell recordings in acute slice preparations, to determine how memory engrams emerge during learning. We further examine how GABAergic interneurons may contribute to this process. We believe that these lines of research will add to a better understanding on the mechanisms of memory formation in cortical networks.
Zusammenfassung
Der Hippocampus ist für das Abspeichern bewusster Gedächtnisspuren entscheidend. Aktuelle Theorien besagen, dass erfahrungsabhängige Änderungen in dem Verhältnis von Exzitation zu Hemmung es auserwählten Neuronen ermöglichen neue Zellassoziationen während des Lernvorgans zu bilden, die die neue Gedächtnisspur repräsentieren. Es wird weiterhin angenommen, dass GABAerge hemmende Interneurone mit Hilfe ihrer inhibitorischen Ausgangsynapsen erheblichen Einfluss auf die Populationsaktivität neuronaler Netzwerke nehmen. Sie synchronisieren aktive Prinzipalzellen mit hohen Frequenzen und ermöglichen damit die funktionelle Kopplung aktiver Neurone zu Zellassoziationen, die gemeinsam Information kodieren. Wie sich diese Zellassoziationen zeitlich und räumlich ausbilden und welchen Beitrag hemmende Interneurone in diesem Prozess einnehmen ist allerdings weitgehend unklar. Wir begannen diese zentrale Frage im Gyrus Dentatus (DG) als der Eingangsregion des Hippocampus, der eine unersetzliche Rolle in der bewussten Gedächtnisbildung einnimmt, zu untersuchen. Wir setzen die zwei-Photonen angeregte bildliche Darstellung von Kalziumsignalen ein, um die Populationsaktivität von Prinzipalzellen im Hippocampus kopffixierter Mäuse, die zielorientierte räumliche Gedächtnisaufgaben in einer virtuellen Umgebung durchführen, zu messen und kombinieren die erfassten Daten mit elektrophysiologischen in vivo Ableitungen und simultanen Mehrfachableitungen von Neuronen in akuten Schnittpräparaten, um zu bestimmen, wie Gedächtnisspuren sich während des Lernprozesses ausbilden und welche Rolle Interneurone in diesem Prozess einnehmen. Wir sind davon überzeugt, dass diese multidisziplinäre Forschungsrichtung zu einem verbesserten Verständnis der Gedächtnisbildung in kortikalen Netzwerken beitragen wird.
Introduction and objectives
Our daily life depends on the processing and storing of a continuous stream of information, which enables us to rapidly adapt our behaviour to changes in our environment. Current theories of memory formation suggest that experience-dependent modifications in the balance between excitation and inhibition enable a selected group of neurons to form a new cell association during the learning process, which represents the newly formed memory trace, the engram (Buzsáki & Draguhn, 2004; Eichenbaum, 1993; Leutgeb et al., 2005; Neunuebel & Knierim, 2014). The functional changes at the level of synapses, single cells and cell populations that are associated with the learning process are, however, largely unknown. This is a crucial issue because impaired memory is a global problem implicated in various diseases including post-traumatic stress disorder, anxiety and depression and can have various causes such as stress, sleep deficit or medication (Kheirbek et al., 2012). In order to be able to effectively treat memory disorders, we first need to understand how new memories are formed in the central nervous system. We aim to address this question in the rodent dentate gyrus (DG) as the main input region of the hippocampus, functionally vital for the acquisition of new memories. Functional and lesion studies in animals proposed several memory functions for the DG, including spatial pattern separation (Gilbert et al., 2001; Neunuebel & Knierim, 2014; Treves & Rolls, 1994), pattern completion (Nakashiba et al., 2012), novelty detection (Hunsaker et al., 2008) and binding of sensory information or objects to a spatial context (Lee and Jung, 2017). However, only recent technical advances in neuroscience, particularly in two-photon calcium imaging of population activity with single-cell resolution, allowed us to study the activity of identified DG neuron types in behaving animals during learning on subsequent days (Danielson et al., 2016; Diamantaki et al., 2016; Hainmueller & Bartos, 2018, 2020). These data together with state-of-the-art in vitro recordings of interconnected cells (Bartos et al., 2002, 2007; Elgueta & Bartos, 2019; Savanthrapadian et al., 2014; Strüber et al., 2015, 2017), and in vivo high-density single-unit and local field potential recordings provided information on the synaptic, cellular and network mechanisms, which may underlie the emergence of DG-dependent memory traces. Thereby, this work massively propelled our understanding on the mnemonic functions of the DG. Here, we will focus on our recent published investigations on two main objectives:
Identification of the temporal and spatial emergence of learning-related cell associations representing new memories in the DG, and
Examination on the potential contribution of GABAergic inhibitory cells in the formation of memory engrams by synchronizing cell assemblies at high gamma (30–150 Hz) frequencies.
We will show that chronic two-photon calcium imaging in head-fixed mice enables us to perform a multiple-day spatial memory task in a virtual environment and to record neuronal activity from the same set of neurons in all major hippocampal subfields. We provide evidence that pyramidal cells in the hippocampal areas CA1–3 show precise and highly environment-specific but continuously changing representations of the learned spatial sceneries. In contrast, granule cells (GCs), the glutamatergic principal cells of the DG, have a spatial code that is stable over many days with low place or context specificity. Moreover, we show that fast-spiking parvalbumin-expressing interneurons (PVIs) in the DG contribute to the synchronization of cell assemblies and thereby may add to the encoding of contextual information.
Structure and function of the DG
Memories about our interactions with the environment are fundamental for our daily behavior. Conscious or ‘declarative’ memories can be divided into semantic memories including factual knowledge (e.g., Berlin is the capital of Germany), whereas episodic memories represent unique experiences (e.g., my first train ride to Berlin). Episodic memories associate individual events with the spatial and temporal context in which they were experienced. Memories must be first encoded as a permanent ‘engram,’ maintained and ‘consolidated’ over time (Tonegawa et al., 2018) for its subsequent recall and their usage in daily cognitive processes. The hippocampus, located in the temporal lobes of the brain (Figure 1), is crucial for declarative memories. Hippocampal principal cells encode with their activity spatially defined places, distinct elements of the environment or the association of elements with a context (e.g., blackboard in a lecture hall; O’Keefe & Dostrovsky, 1971). The hippocampus is divided into three areas, the CA1, the CA3 and the DG, which are interconnected by excitatory synapses, thereby forming the canonical trisynaptic path (Figure 1). The DG is situated between the entorhinal cortex and the CA3 area, forming the first stage of the trisynaptic circuit. Current theories propose that the DG receives a rich multimodal input from the entorhinal cortex which carries information on various modalities of the environment and its objects and translates the rich input stream into sparse and segregated (‘orthogonalized’) representations, a process called pattern separation (Leutgeb et al., 2007; Marr, 1971; Santoro, 2013; Treves & Rolls, 1994). By decorrelating the rich input stream into nonoverlapping sparse memories, the DG is proposed to allow a high resolution of information (Marr, 1971). Consistent with the sparse coding theory, GCs in the DG, discharge at low mean frequency (∼0.5 Hz; Pernía-Andrade & Jonas, 2014) and labeling studies of immediate early genes indicate that small differences in spatial environments are represented by nonoverlapping GC ensembles (Ramirez et al., 2013).
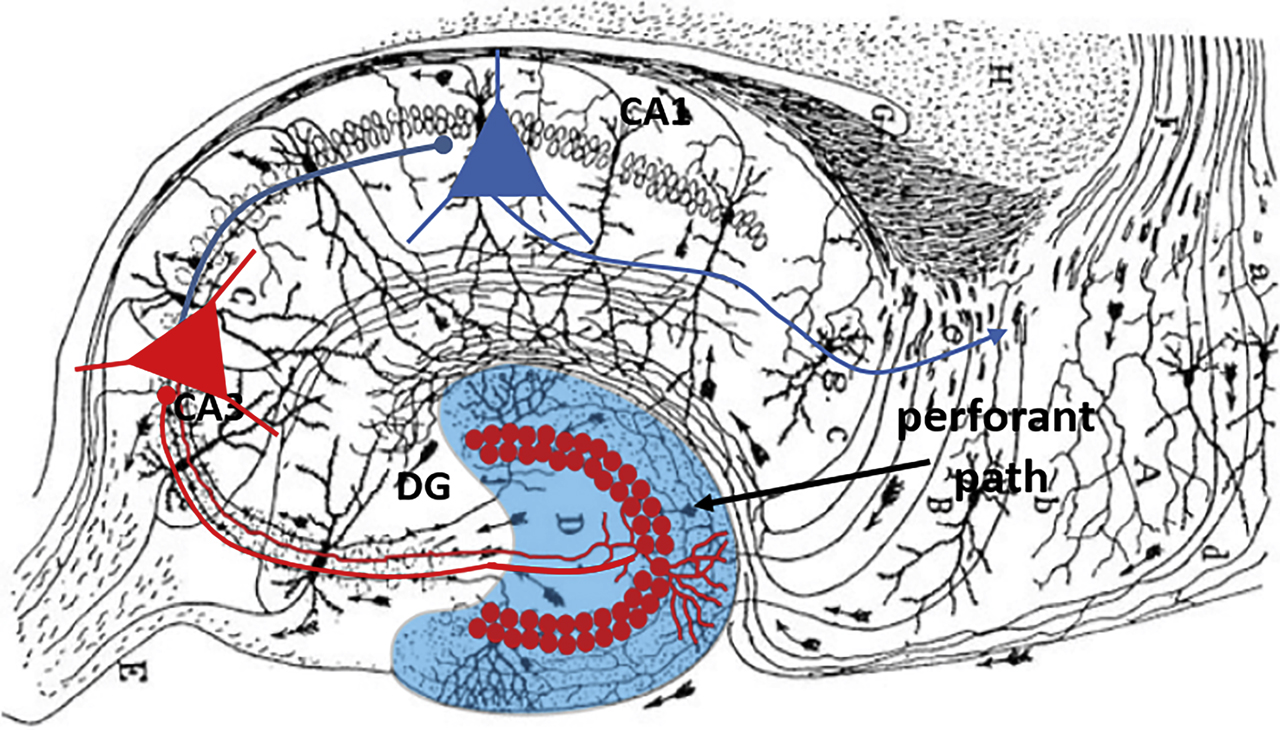
Hippocampal trisynaptic pathway.
Blue, dentate gyrus (DG); red circles, granule cell (GC) somata forming mossy fiber axons projecting to CA3. Pyramidal cells in CA3 (red triangles) project to CA1 (blue triangles) with axons forming the Schaffer collateral pathway.
The activity of GCs stays under tight inhibitory control of GABAergic interneurons. Among the various types of GABAergic cells in the DG (Hosp et al., 2014), particularly PVIs attracted highest attention owing to the strong perisomatic inhibition they provide onto GCs (Bartos et al., 2002, 2007; Vida et al., 2006). Enhanced activity of the entorhinal cortex onto GCs can induce long-lasting potentiation leading to input strengthening (Schmidt-Hieber et al., 2004) and enhanced activation of a selected group of GCs. They contact PVIs, which in turn provide feedback inhibition to the DG circuitry. Thus, once a selected group of GCs is recruited, this group will provide inhibition to the DG network and silence less-excited GCs. This mechanism may increase the signal-to-noise ratio in the network, ensure sparse coding and enhance the storage capacity of the DG. Thus, changes in excitation and inhibition on the level of single cells during learning may define who is the member of the cell assembly and who falls out.
Emergence of memory engrams in the DG
To study the emergence and dynamics of hippocampal memory engrams during the course of long-term learning, we established a virtual environment-based, goal-oriented learning task for head-fixed mice (Figure 2). By running on a spherical treadmill, mice move along a 4-m-long virtual linear track with four soymilk reward locations, displayed on monitors covering the mouse’s visual field. After >10 days of familiarization to this track (‘familiar’ context), imaging sessions started in which mice run alternatingly in this familiar context and additionally on a ‘novel’ linear track with only two reward sites and different visual cues. Thus, mice have to discriminate between the two novel and familiar contexts and to identify the new rewarded sites on the novel track. We showed that mice recognize the reward site by consistently licking more often inside versus outside the reward zone. Initially, the reward-related licking is lower in the novel than in the familiar context. These differences vanish with learning over the next subsequent ∼3 days, indicating that mice remember the new rewarded locations; they do learn (Hainmueller & Bartos, 2018). To measure the activity of hippocampal neurons, we inject unilaterally the recombinant adeno-associated virus (rAAV) encoding the fluorescent calcium indicator GCaMP6f in the hippocampus and panneuronally labelled cells of the DG, CA1 and CA3 of transgenic mice expressing the red fluorophore tdTomato (tdT) in PVIs. This allowed us to visually differentiate principal cells from PVIs. We chronically implanted an imaging window (Figure 2A and C) allowing us to image the same set of cells on subsequent days during learning (Dombeck et al., 2010).

2P-imaging of DG population activity in a virtual reality.
A–C Experimental design. Mice are head-fixed and run on a Styrofoam ball in a virtual reality presented with four monitors in front of the animal. The movement of the ball is detected and transferred in a movement of the virtual reality in real time. B Cranial window for DG imaging. C Linear track in the virtual reality. The ground and walls of the linear track are decorated with symbols, which visually allow the mouse to identify the virtual reality. The mouse is trained to obtain a reward at visually identifiable locations on the linear track. Lick frequency, running speed and pupil size are detected throughout the behavior. D rAAV-GCAMP6f expression in DG/CA1 of PV-tdT mice. White, GCAMP6f-expressing cells; red, PVIs. For DG and CA1 imaging, a cranial window was implanted above the hippocampus. For CA3 imaging, a cranial window was implanted above CA3 and the objective was tilted by 20°. E Ca2+ signals of a representative GC and a PVI; blue line, linear track. Red, Ca2+ transient in the GC. Note Ca2+ transients in PVIs have a slower time course than in GCs. F Activity in warm colors of a GC place cell recorded on 20 subsequent runs on the linear track.
Our data showed that consistent with previous findings, activity in the DG was sparse, markedly lower than the one in CA1 and CA3. Moreover, approximately 35% of these active neurons had place cell characteristics either in the familiar, novel or in both contexts (Hainmueller & Bartos, 2018). Place cells are active in a certain field of the world (O’Keefe & Burgess, 1996) and thereby encode with their activity a certain area of the linear track. Several of these place cells jointly formed a map of the virtual world. Pyramidal neurons in CA1–CA3 show precise and highly environment-specific but continuously changing representations of the learned spatial sceneries, whereas GCs of the DG had a spatial code that is stable over several days, with low place or environment specificity (Haimueller & Bartos, 2020). Finally, activity of DG GCs markedly declined on the first day of novelty exposure and then increased on the subsequent days, indicating that the memory engram emerged during the learning process. This decline in activity was not caused by PVIs (Hainmueller & Bartos, 2018) but more likely by reduced activity of the entorhinal cortex (Qin et al., 2018). Thus, our data showed that in contrast to previous views, spatial context representation is stable over time in the DG and the ability to discriminate between different contexts is low. In contrast, representation of spatial information is dynamic over time in the hippocampal CA1–CA3 and the ability to discriminate between spatial contexts is high. We therefore hypothesize that the DG provides a stable reference map of the global environment (e.g., lecture hall) to the hippocampus, in which it is combined with the temporally varying detailed contents (e.g., different students) constituting an experience.
GABAergic inhibition contributes to synchronization of cell assemblies
Elucidating the mechanisms underlying the formation of cell assemblies is crucial to understand the function of memory processes. In a behavioral task, in which rats have to repeatedly learn new spatial reward locations, local CA1 interneurons become associated with the emerging cell assembly, presumably by synaptic plasticity mechanisms that selectively strengthen excitatory connections among principal cells that encode the path to the reward locations (Dupret et al., 2013). In contrast, synaptic inputs onto interneurons from assemblies representing old target locations are weakened (Dupret et al., 2013). Thus, local GABAergic interneurons are considered to play an important role in the emergence of memory engrams during learning.
How do interneurons contribute to the organization of cell assembly firing? Oscillations of the local field potential are thought to provide temporal reference signals for assembly activity (Buzsáki & Draguhn, 2004). Gamma frequency oscillations (30–150 Hz) are a particularly interesting candidate in this regard. First, during exploratory behavior, gamma oscillations appear in the hippocampal formation typically nested in slower theta (6–12 Hz) oscillations. Second, gamma oscillations are prominently visible during cognitive efforts like the execution of spatial working memory (Yamamoto et al., 2014). Finally, the emergence of gamma activity depends on synaptic inhibition from and among GABAergic cells, particularly of PVIs (Sohal et al., 2009). Gamma activities can be further divided in two distinct functional components: low-gamma oscillations (30–75 Hz), which are thought to be generated in principle cell–interneurons synaptic loops, and high-gamma oscillations (75–150 Hz), which originate mostly from synaptic connections among interneurons (Bartos et al., 2002, 2007; Bieri et al., 2014; Colgin et al., 2009; Lasztóczi et al., 2016). Using current source density analysis applied to freely moving mice, we demonstrated that high-gamma oscillations are focal network phenomena, essentially occurring independently at several places within the DG, while low-gamma activities appear to be more distributed global network events (Figure 3; Strüber et al., 2017). These data suggest that local high-gamma activities might contribute to the segregation of active cell assemblies. Interestingly, using a combined in vitro whole-cell patch-clamp and computational approach, we could further show that the necessary prerequisites for the emergence of focal high-gamma activities might be embedded in the very fabric of the DG circuitry itself: GABAergic connections between PVIs show distinct distance-dependent changes in their basic properties, such as connection strength and decay kinetics, which support the occurrence of localized high-gamma activities in neuronal network models (Figure 3; Strüber et al., 2017). This functional configuration of the network might thus permit the parallel processing of spatially confined inputs by distinct gamma-modulated cell assemblies.

Measuring local gamma oscillations in the DG.
A Multisite local field potential (LFP) recording in the DG in vivo. Two LFP recording locations (A and B) are shown. Right, local high-gamma oscillations. Red colors show locally emerging oscillations as quantified by a focality index, which expresses how much the observed oscillation is restricted to a single electrode of the recording array. CSD: current source density. B In vitro paired whole-cell recordings between synaptically connected parvalbumin-positive interneurons (PIIs) reveal distance-dependent properties of amplitude and kinetics of inhibitory postsynaptic currents (IPSCs). The graph shows average IPSCs elicited by presynaptic action potentials (red) in a synaptically connected PII2 at a short intersomatic distance (<100 µm; black trace). After the recording was obtained, the recording pipette was removed and a second postsynaptic PII1 was recorded at a larger intersomatic distance (>100 µm; gray trace). Note: With increasing distance between presynaptic and postsynaptic partners, the amplitude and time course of IPSCs declines. C Structure of a network model composed by glutamatergic principal neurons (PNs, gray circles) and GABAergic inhibitory neurons (INs, black circles) with intersomatic distance of 50 µm between neighboring cells connected by synapses (lines with dots). D Network simulations with distance-dependent inhibition (bottom, red) create focal gamma oscillations upon excitatory stimulation with higher synchrony than in networks without distance-dependent inhibition (top, black). Individual dots represent individual action potentials generated by PNs (gray) or INs (black, red). Bottom trace, LFP trace. Note, network with distance-dependent inhibition shows highly synchronous LFP trace (bottom, red). Figure adapted from Strüber et al. (2017).
Conclusion and outlook
Our data propose that the DG provides a stable reference map of the global environment to the hippocampus, in which it can be flexibly associated with the temporally varying detailed contents such as objects or events that constitute a given experience in an environment (e.g., graduation ceremony on the Schlossberg in Freiburg). Activation of the map (e.g., by visiting the Schlossberg) may allow the reinstatement of that memory. DGs’ sparse activity supports the representation of a multitude of reference maps and associated experiences. We propose that among several mechanisms, distance-dependent inhibition in the DG supports the formation of multiple noninterfering reference maps.
Although in vivo Ca2+ imaging and electrophysiology propelled our understanding on how hippocampal circuits contribute to memory processes, it will be important to determine the dynamics of memory-bearing engrams over time. Particularly, what synaptic, cellular and network processes underlie the formation of memory engrams and their recall? Which roles do specialized classes of neurons such as the various GABAergic inhibitory neuron types play in shaping, maintaining and dissolving engrams? The increasing availability of advanced molecular, imaging and electrophysiological methods will put the field in the position to improve our understanding of encoding and recall of memories and thereby may open new avenues of study and eventually therapies for hippocampal dysfunction.
About the authors

Dr. Jonas-Frederic Sauer studied Molecular Medicine at the University of Freiburg, conducted his Ph.D. research at the University of Aberdeen (UK) and University of Freiburg (Ph.D. 2012) and investigated cellular and network defects in psychiatric disorders as a postdoctoral fellow in the laboratory of Prof. Bartos. With funding from the DFG (2018), EUCOR-the European Campus (2018) and the Else Kröner-Fresenius Stiftung (2020), he examines how neuronal oscillations organize network activity in the healthy and diseased brain.

Prof. Marlene Bartos studied Biology at the Technical University of Braunschweig and received her Ph.D. at the Technical University Munich 1994. With a DFG fellowship, she moved as postdoctoral fellow to the University of Pennsylvania, Philadelphia (USA). She was an assistant professor (C1) at the Albert-Ludwigs University in Freiburg where she received the Habilitation and Venia Legendi in Physiology. In 2007, she was appointed as a full professor at the University of Aberdeen (UK) and received a Lichtenberg Professorship (W3) of the VW Foundation at Freiburg University in 2010. She is since 2018 the Director of the Institute for Physiology I at Freiburg University. Since 2014, she is a speaker of the Research Unit FOR2143 ‘Interneuron Plasticity,’ and she received the ERC Advanced Grant in 2018. Dr. Bartos laboratory examines the synaptic, cellular and network mechanisms, which underlie the formation of memory traces in the hippocampus. Her laboratory made seminal discoveries on the emergence, stability and reliability of memory during learning in the hippocampus and the mechanisms, which may underlie cognitive disorders in genetic models of schizophrenia and depression. Currently, she examines the role of inhibitory cells and interneuron plasticity in the establishment of memory engrams.
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: None declared.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
Bartos, M., Vida, I., Frotscher, M., Meyer, A., Monyer, H., Geiger, J.R., and Jonas, P. (2002). Fast synaptic inhibition promotes synchronized gamma oscillations in hippocampal interneuron networks. Proc. Natl. Acad. Sci. U.S.A. 99, 13222–13227. https://doi.org/10.1073/pnas.192233099.Search in Google Scholar PubMed PubMed Central
Bartos, M., Vida, I., and Jonas, P. (2007). Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat. Rev. Neurosci. 8, 45–56. https://doi.org/10.1038/nrn2044.Search in Google Scholar PubMed
Bieri, K.W., Bobbitt, K.N., and Colgin, L.L. (2014). Slow and fast γ rhythms coordinate different spatial coding modes in hippocampal place cells. Neuron 82, 670–681. https://doi.org/10.1016/j.neuron.2014.03.013.Search in Google Scholar PubMed PubMed Central
Buzsáki, G. and Draguhn, A. (2004). Neuronal oscillations in cortical networks. Science 304, 1926–1929. https://doi.org/10.1126/science.1099745.Search in Google Scholar PubMed
Colgin, L.L., Denninger, T., Fyhn, M., Hafting, T., Bonnevie, T., Jensen, O., Moser, M.B., and Moser, E.I. (2009). Frequency of gamma oscillations routes flow of information in the hippocampus. Nature 462, 353–357. https://doi.org/10.1038/nature08573.Search in Google Scholar PubMed
Danielson, N.B., Kaifosh, P., Zaremba, J.D., Lovett-Barron, M., Tsai, J., Denny, C.A., Balough, E.M., Goldberg, A.R., Drew, L.J., Hen, R., et al. (2016). Distinct contribution of adult-born hippocampal granule cells to context encoding. Neuron 90, 101–112. https://doi.org/10.1016/j.neuron.2016.02.019.Search in Google Scholar PubMed PubMed Central
Diamantaki, M., Frey, M., Berens, P., Preston-Ferrer, P., and Burgalossi, A. (2016). Sparse activity of identified dentate granule cells during spatial exploration. eLife 5, e20252. https://doi.org/10.7554/eLife.20252.Search in Google Scholar PubMed PubMed Central
Dombeck, D.A., Harvey, C.D., Tian, L., Looger, L.L., and Tank, D.W. (2010). Functional imaging of hippocampal place cells at cellular resolution during virtual navigation. Nat. Neurosci. 13, 1433–1440. https://doi.org/10.1038/nn.2648.Search in Google Scholar PubMed PubMed Central
Dupret, D., O’Neill, J., and Csicsvari, J. (2013). Dynamic reconfiguration of hippocampal interneuron circuits during spatial learning. Neuron 78, 166–180. https://doi.org/10.1016/j.neuron.2013.01.033.Search in Google Scholar PubMed PubMed Central
Eichenbaum, H. (1993). Thinking about brain cell assemblies. Science 261, 993–994. https://doi.org/10.1126/science.8351525.Search in Google Scholar PubMed
Elgueta, C. and Bartos, M. (2019). Dendritic inhibition differentially regulates excitability of dentate gyrus parvalbumin-expressing interneurons and granule cells. Nat. Commun. 10, 5561. https://doi.org/10.1038/s41467-019-13533-3.Search in Google Scholar PubMed PubMed Central
Gilbert, P.E., Kesner, R.P., and Lee, I. (2001). Dissociating hippocampal subregions: Double dissociation between dentate gyrus and CA1. Hippocampus 11, 626–636. https://doi.org/10.1002/hipo.1077.Search in Google Scholar PubMed
Hainmueller, T. and Bartos, M. (2018). Parallel emergence of stable and dynamic memory engrams in the hippocampus. Nature 558, 292–296. https://doi.org/10.1038/s41586-018-0191-2.Search in Google Scholar PubMed PubMed Central
Hainmueller, T. and Bartos, M. (2020). Dentate gyrus circuits for encoding, retrieval and discrimination of episodic memories. Nat. Rev. Neurosci. 21, 153–168. https://doi.org/10.1038/s41583-019-0260-z.Search in Google Scholar PubMed PubMed Central
Hosp, J.A., Strüber, M., Vida, I., Jonas, P., and Bartos, M. (2014). Morpho-physiological criteria divide dentate gyrus interneurons into classes. Hippocampus 24, 189–203. https://doi.org/10.1002/hipo.22214.Search in Google Scholar PubMed PubMed Central
Hunsaker, M.R., Rosenberg, J.S., and Kesner, R.P. (2008). The role of the dentate gyrus, CA3a,b, and CA3c for detecting spatial and environmental novelty. Hippocampus 18, 1064–1073. https://doi.org/10.1002/hipo.20464.Search in Google Scholar PubMed
Kheirbek, M.A., Klemenhagen, K.C., Sahay, A., and Hen, R. (2012). Neurogenesis and generalization: A new approach to stratify and treat anxiety disorders. Nat. Neurosci. 15, 1613–1620. https://doi.org/10.1038/nn.3262.Search in Google Scholar PubMed PubMed Central
Lasztóczi, B. and Klausberger, T. (2016). Hippocampal place cells couple to three different gamma oscillations during place field traversal. Neuron 91, 34–40. https://doi.org/10.1016/j.neuron.2016.05.036.Search in Google Scholar PubMed
Lee, J.W. and Jung, M.W. (2017). Separation or binding? Role of the dentate gyrus in hippocampal mnemonic processing. Neurosci. Biobehav. Rev. 75, 183–194. https://doi.org/10.1016/j.neubiorev.2017.01.049.Search in Google Scholar PubMed
Leutgeb, S., Leutgeb, J.K., Barnes, C.A., Moser, E.I., McNaughton, B.L., and Moser, M.B. (2005). Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science 22, 619–623. https://doi.org/10.1126/science.1114037.Search in Google Scholar PubMed
Leutgeb, J.K., Leutgeb, S., Moser, M.-B., and Moser, E.I. (2007). Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science 315, 961–966. https://doi.org/10.1126/science.1135801.Search in Google Scholar PubMed
Marr, D. (1971). Simple memory: A theory for archicortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 262, 23–81. https://doi.org/10.1098/rstb.1971.0078.Search in Google Scholar PubMed
Neunuebel, J.P. and Knierim, J.J. (2014). CA3 retrieves coherent representations from degraded input: Direct evidence for CA3 pattern completion and dentate gyrus pattern separation. Neuron 81, 416–427. https://doi.org/10.1016/j.neuron.2013.11.017.Search in Google Scholar PubMed PubMed Central
Nakashiba, T., Cushman, J.D., Pelkey, K.A., Renaudineau, S., Buhl, D.L., McHugh, T.J., Rodriguez Barrera, V., Chittajallu, R., Iwamoto, K.S., McBain, C.J., et al. (2012). Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell 149, 188–201. https://doi.org/10.1016/j.cell.2012.01.046.Search in Google Scholar PubMed PubMed Central
O’Keefe, J. and Dostrovsky, J. (1971). The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 34, 171–175. https://doi.org/10.1016/0006-8993(71)90358-1.Search in Google Scholar PubMed
Pernía-Andrade, A.J. and Jonas, P. (2014). Theta-gamma-modulated synaptic currents in hippocampal granule cells in vivo define a mechanism for network oscillations. Neuron 81, 140–152. https://doi.org/10.1016/j.neuron.2013.09.046.Search in Google Scholar PubMed PubMed Central
Ramirez, S., Liu, X., Lin, P.A., Suh, J., Pignatelli, M., Redondo, R.L., Ryan, T.J., and Tonegawa, S. (2013). Creating a false memory in the hippocampus. Science 341, 387–391. https://doi.org/10.1126/science.1239073.Search in Google Scholar PubMed
Santoro, A. (2013). Reassessing pattern separation in the dentate gyrus. Front. Behav. Neurosci. 7, 1–4. https://doi.org/10.3389/fnbeh.2013.00096.Search in Google Scholar PubMed PubMed Central
Savanthrapadian, S., Meyer, T., Elgueta, C., Booker, S.A., Vida, I., and Bartos, M. (2014). Synaptic properties of SOM- and CCK-expressing cells in dentate gyrus interneuron networks. J. Neurosci. 34, 8197–8209. https://doi.org/10.1523/jneurosci.5433-13.2014.Search in Google Scholar
Schmidt-Hieber, C., Jonas, P., and Bischofberger, J. (2004). Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature 429, 184–187. https://doi.org/10.1038/nature02553.Search in Google Scholar PubMed
Sohal, V.S., Zhang, F., Yizhar, O., and Deisseroth, K. (2009). Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459, 698–702. https://doi.org/10.1038/nature07991.Search in Google Scholar PubMed PubMed Central
Strüber, M., Jonas, P., and Bartos, M. (2015). Strength and duration of perisomatic GABAergic inhibition depend on distance between synaptically connected cells. Proc. Natl. Acad. Sci. U.S.A. 112, 1220–1225. https://doi.org/10.1073/pnas.1412996112.Search in Google Scholar PubMed PubMed Central
Strüber, M., Sauer, J.-F., Jonas, P., and Bartos, M. (2017). Distance-dependent inhibition facilitates focality of gamma oscillations in the dentate gyrus. Nat. Commun. 8, 785. https://doi.org/10.1038/s41467-017-00936-3.Search in Google Scholar PubMed PubMed Central
Tonegawa, S., Morrissey, M.D., and Kitamura, T. (2018). The role of engram cells in the systems consolidation of memory. Nat. Rev. Neurosci. 19, 485–498. https://doi.org/10.1038/s41583-018-0031-2.Search in Google Scholar PubMed
Treves, A. and Rolls, E.T. (1994). Computational analysis of the role of the hippocampus in memory. Hippocampus 4, 374–391. https://doi.org/10.1002/hipo.450040319.Search in Google Scholar PubMed
O’Keefe, J. and Burgess, N. (1996). Geometric determinants of the place fields of hippocampal neurons. Nature 381, 425–428. https://doi.org/10.1038/381425a0.Search in Google Scholar PubMed
Qin, H., Fu, L., Hu, B., Liao, X., Lu, J., He, W., Liang, S., Zhang, K., Li, R., Yao, J., et al. (2018). A visual-cue-dependent memory circuit for place navigation. Neuron 99, 47–55. https://doi.org/10.1016/j.neuron.2018.05.021.Search in Google Scholar PubMed PubMed Central
Vida, I., Bartos, M., and Jonas, P. (2006). Shunting inhibition improves robustness of gamma oscillations in hippocampal interneuron networks by homogenizing firing rates. Neuron 49, 107–117. https://doi.org/10.1016/j.neuron.2005.11.036.Search in Google Scholar PubMed
Yamamoto, J., Suh, J., Takeuchi, D., and Tonegawa, S. (2014). Successful execution of working memory linked to synchronized high-frequency gamma oscillations. Cell 157, 845–857. https://doi.org/10.1016/j.cell.2014.04.009.Search in Google Scholar PubMed
© 2020 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Editorial
- Editorial
- Review articles
- Synapses, networks, brain development – funding basic neuroscience research in Germany by the Schram Foundation
- Neuronal functions of clathrin-associated endocytic sorting adaptors – from molecules to disease
- Mechanisms of synaptic vesicle recycling provide a platform to explore mechanisms of neurodegeneration
- Optogenetic analyses of neuronal networks that generate behavior in Caenorhabditis elegans
- Direct contribution of angiogenic factors to neurodevelopment: a focus on angiopoietins
- The role of the dentate gyrus in mnemonic functions
- Presentation of scientific institutions
- Armin Schram: a sponsor of curiosity-driven brain research
- Nachrichten aus der Gesellschaft
- Nachrichten aus der Gesellschaft
Articles in the same Issue
- Frontmatter
- Editorial
- Editorial
- Review articles
- Synapses, networks, brain development – funding basic neuroscience research in Germany by the Schram Foundation
- Neuronal functions of clathrin-associated endocytic sorting adaptors – from molecules to disease
- Mechanisms of synaptic vesicle recycling provide a platform to explore mechanisms of neurodegeneration
- Optogenetic analyses of neuronal networks that generate behavior in Caenorhabditis elegans
- Direct contribution of angiogenic factors to neurodevelopment: a focus on angiopoietins
- The role of the dentate gyrus in mnemonic functions
- Presentation of scientific institutions
- Armin Schram: a sponsor of curiosity-driven brain research
- Nachrichten aus der Gesellschaft
- Nachrichten aus der Gesellschaft

