Abstract
A new 2D dibutyltin coordination polymer with 3,5-dinitrosalicylate and 4,4’-bipyridine ligands, [{Bu2Sn(3,5–(NO2)2–2–OC6H2COO)}2(4,4’-bpy)]n (1), has been synthesized and characterized both spectroscopically (IR, 1H, 13C, and 119Sn NMR) and a single-crystal X-ray diffraction analysis. The coordination geometry of tin atom in 1 is a distorted octahedron. 3,5-Dinitrosalicylate as doubly charged anion ligand adopts chelating-bridging mode to coordinate to tin atoms, and 4,4’-bipyridine further bridges the tin atoms to form a 2D herringbone-like network structure containing the 34-membered hexa-nuclear macrocycles.
Organotin carboxylates (such as R’COOSnR3 and (R’COO)2SnR2) are a kind of important organotin compounds, and their structures and biological activities have been receiving considerable attention (Banti et al., 2019; Chandrasekhar et al., 2013; Chen et al., 2020; Khan et al., 2020; Tian et al., 2019). Salicylic acid (H2L) has antiseptic and antifungal properties and is widely used in organic synthesis. Structurally, salicylic acid or substituted salicylic acid has two functional groups (COOH and OH), and can act as a monoanionic (HL–) or dianionic (L2–) ligand to coordinate to tin atom (Figure 1). The syntheses, structures, and biological activities of some organotin salicylates or substituted salicylates have been reported by several research groups (Basu Baul et al., 2006, 2018, 2019; Kundu et al., 2014; Liu et al., 2019; Zhang et al., 2016). However, only a few organotin salicylates with six-membered chelate ring formed by the oxygen atoms of both phenolate and carboxylate coordination to tin have been reported (Figure 1b) (Basu Baul et al., 2018, 2019; Kundu et al., 2014; Prabusankar and Murugavel, 2004; Tian et al., 2011). In organotin chemistry, the combination of a carboxylate ligand and N-donor ligand, such as 4,4’-bipyridine, is widely used for constructing the organotin coordination polymers. Basu Baul’s group used heteroditopic pyridylsalicylate ligands, such as 5-[(E)-2-(3 or 4-pyridyl)-1-diazenyl] salicylic acid, to construct the 1D and 2D organotin coordination polymers (Basu Baul et al., 2018, 2019). Chandrasekhar and Thirumoorthi synthesized the 1D triphenyltin coordination polymer by the reaction of 1,1’-ferrocenedicarboxylic acid with bis(triphenyltin) oxide in the presence of 4,4’-bipyridine (Chandrasekhar and Thirumoorthi, 2010). Kundu’s group prepared a 1D dibutyltin coordination polymer by the combination of 3,5-dinitrosalicylic acid and 6,6-bis(4-pyridinyloxy) cyclophosphazene ligands (Kundu et al., 2014). In this short communication, we report a new 2D dibutyltin coordination polymer (1) with 3,5-dinitrosalicylate and 4,4’-bipyridine ligands (see Scheme 1).
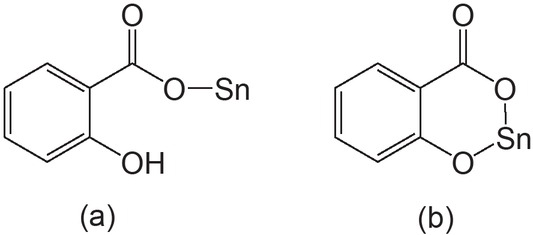
Coordination modes of salicylate ligand with tin atom in (a) monoanionic and (b) dianionic forms.
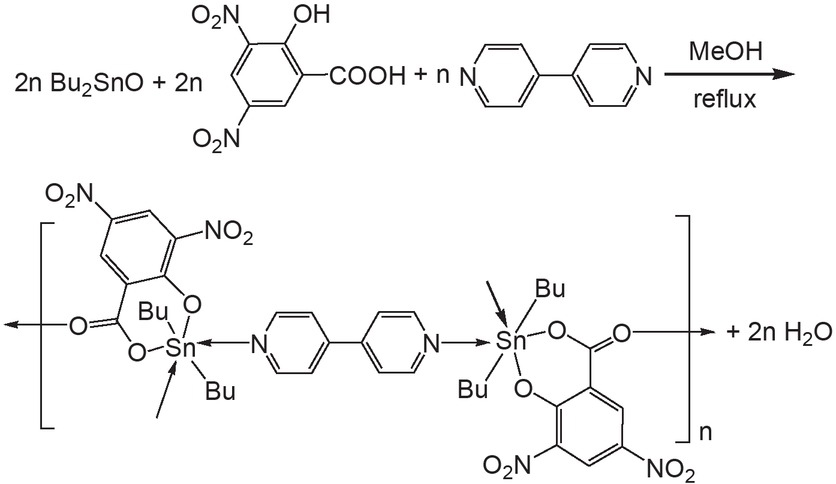
Synthesis of compound 1.
Coordination polymer 1 is obtained from the reaction of dibutyltin oxide, 3,5-dinitrosalicylic acid and 4,4’-bipyridine in the molar ratio 1:1:0.5 with a yield of 67% (Scheme 1). At room temperature, 1 is insoluble in chloroform and acetone, but soluble in DMSO. The NMR spectra in DMSO-d6 show the expected resonance absorption of 1H and 13C nuclei (see ESI). The position of the proton signals of 4,4’-bipyridine in 1 is identical to that of the free 4,4’-bipyridine ligand (see Figures S2 and S3 in Supplementary Material), indicating that 4,4’-bipyridine in 1 is released in DMSO-d6 solution. The 119Sn chemical shifts primarily depend on the coordination number and the type of the donor atoms bonded to tin atom (Holecek et al., 1983, 1986). Holecek and coworkers established for butyltin compounds that four-coordinate compounds have δ(119Sn) values in solution ranging from +200 to –60 ppm, five-coordinate compounds from –90 to –190 ppm and six-coordinate compounds from –210 to –400 ppm (Holecek et al., 1986). Compound 1 displays a single 119Sn resonance at –262 ppm, suggesting that the tin atom in 1 is six-coordinated in the coordination solvent DMSO-d6 and the six coordinated atoms come from two carbons of organic groups, a phenolic O and a carboxylic O of salicylate ligand, and two O atoms of two DMSO-d6.
The ν(C=N) band in 1 appears at 1606 cm–1, which is blue-shifted compared with the free 4,4′-bipyridine ligand (1589 cm–1), confirming the coordination of N atom to tin atom (Kondo et al., 1997; Ma et al., 2004; Shi et al., 2010). The difference between νas(COO) (1634 cm–1) and νs(COO) (1447 cm–1) (Δν = 187 cm–1) indicates the bidentate bridging coordination of the carboxylate group in the ligand (Deacon and Phillips, 1980; Tian et al., 2020).
In the crystalline state, as shown in Figures 2 and 3, compound 1 has a 2D herringbone-like network structure (Zang et al., 2006). The coordination geometry of tin atom is a distorted octahedron, and the six coordinated atoms come from two carbons [C(1) and C(5)] of butyl groups, one nitrogen N(1) of 4,4’-bipyridine and three oxygen atoms of two 3,5-dinitrosalicylate ligands, a phenolic O(1), and a carboxylic O(2) and a carbonyl O(3A) (symmetry code A: –x+1/2, y–1/2, –z+3/2) (Figure 2). The three bond angles around tin atom in trans-position, C(1)–Sn(1)–C(5), O(1)–Sn(1)–O(3A), and O(2)–Sn(1)–N(1), are 160.92(15), 159.57(8) and 164.60(8)°, respectively (Table 1).
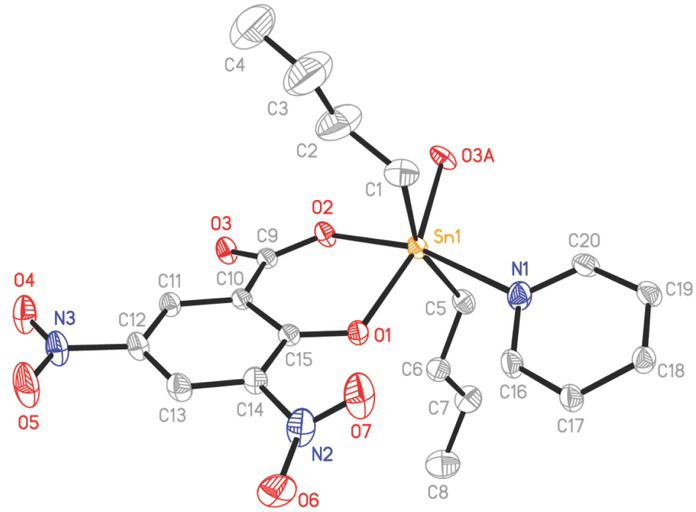
Perspective view of the asymmetric unit of compound 1. Ellipsoids are drawn at the 30% probability level. Hydrogen atoms are omitted for clarity. Symmetry code A: – x + 1/2, y – 1/2, – z + 3/2.
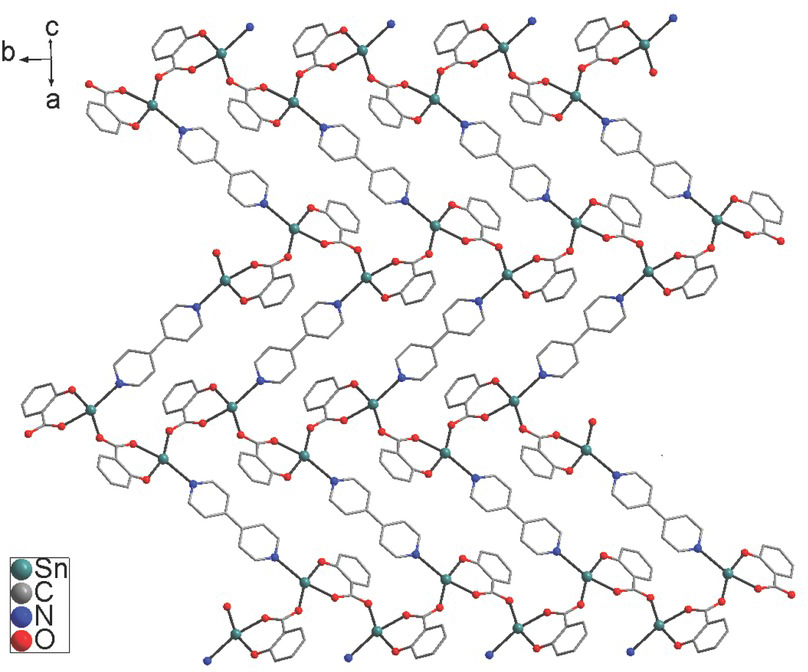
2D herringbone structure of 1 containing 34-membered hexa-nuclear macrocyclic rings. The nitro and butyl groups are omitted for clearity.
Selected bond lengths (Å) and angles (°) for 1.
| Sn(1)–C(1) | 2.101(3) |
| Sn(1)–C(5) | 2.118(3) |
| Sn(1)–N(1) | 2.548(3) |
| Sn(1)–O(1) | 2.1376(19) |
| Sn(1)–O(2) | 2.1428(19) |
| Sn(1)–O(3A) | 2.353(2) |
| C(1)–Sn(1)–C(5) | 160.92(15) |
| C(1)–Sn(1)–O(1) | 95.87(13) |
| C(5)–Sn(1)–O(1) | 98.38(11) |
| C(1)–Sn(1)–O(2) | 100.43(13) |
| C(5)–Sn(1)–O(2) | 94.13(12) |
| O(1)–Sn(1)–O(2) | 81.79(7) |
| C(1)–Sn(1)–O(3A) | 89.36(13) |
| C(5)–Sn(1)–O(3A) | 81.67(12) |
| O(1)–Sn(1)–O(3A) | 159.57(8) |
| O(2)–Sn(1)–O(3A) | 77.84(8) |
| C(1)–Sn(1)–N(1) | 83.52(13) |
| C(5)–Sn(1)–N(1) | 85.69(12) |
| O(1)–Sn(1)–N(1) | 83.00(8) |
| O(2)–Sn(1)–N(1) | 164.60(8) |
| O(3A)–Sn(1)–N(1) | 117.27(9) |
Symmetry code A: –x + 1/2, y– 1/2, –z + 3/2
Every dianionic 3,5-dinitrosalicylate ligand is bound to the tin atom in a chelating-bridging mode. The phenolate O(1) and carboxylate O(2) atoms coordinate to tin atom to produce a six-membered chelate ring with a O(1)–Sn(1)–O(2) angle of 81.79(7)°. The carbonyl O(3) atom bridges the other tin atom to form a 1D infinite chain with a Sn(1)–O(3A) bond of 2.353(2) Å, which is similar to that found in Bu2Sn(3,5–(NO2)2–2–OC6H2COO) (CH3OH) (Tian et al., 2011). In the chain, the neighboring Sn⋅⋅⋅Sn distance is 5.682(4) Å, and the Sn⋅⋅⋅Sn⋅⋅⋅Sn angle is 104.91(3)°. The C–O distances (1.245(3) and 1.259(3) Å) in carboxylate moiety do not differ significantly, which is the characteristic of the bridging coordination of carboxylic group (Chandrasekhar et al., 2002; Ma et al., 2006). The 1D chains are connected by the coordination of N(1) atom in 4,4’-bipyridine to tin atom to give rise to a 2D herringbone structural motif with a Sn(1)–N(1) bond of 2.548(3) Å. The 2D structure contains large 34-membered six-nuclear macrocycles with the maximum Sn⋅⋅⋅Sn distance of 19.715(4) Å. In the literatures (CSD version 5.41, Aug 2020), there is only one example of 2D organotin salicylate complexes, [Bu2Sn(5-(4-PyN=N)–2– OC6H3COO)]n (Basu Baul et al., 2018). In the case, the tin atoms are bridged by a carboxylate O atom (i.e. Sn-O→Sn) to form a 4-membered Sn2O2 ring. Thus, complex 1 is the first example of 2D organotin salicylate complex containing the carboxylate bridging coordination (i.e. Sn–O–C=O→Sn).
Experimental
All of the chemicals were obtained from Sinopharm Chemical Reagent Co., Ltd and Shanghai Darui Fine-chemical Co. Ltd (China). In the experiment, the measuring instruments used are as follows: Perkin Elmer 2400 Series II elemental analyzer (C, H, and N) (Perkin Elmer, Waltham, USA), Nicolet Nexus 470 FT-IR spectrophotometer (KBr pellets) (Thermo Nicolet Corporation, Madison, USA), Bruker Avance III HD 500 MHz NMR spectrometer (1H, 13C, and 119Sn) (Bruker Corporation, Switzerland).
Synthesis of 1
3,5-Dinitrosalicylic acid (0.228 g, 1.0 mmol), Bu2SnO (0.249 g, 1.0 mmol), and 50 mL anhydrous methanol were added to a 100 mL flask. Under continuous stirring, the reaction mixture was refluxed for 3 h, and turned into a yellow clear solution. A methanol solution (15 mL) of 4,4’-bipyridine (0.078 g, 0.5 mmol) was slowly added. The solution was refluxed for another hour and filtered when hot. The filtrate was evaporated slowly to obtain yellow block crystals. The yield of 1 was 0.362 g (67%), and m.p. >200°C. Anal. Found: C, 44.62; H, 4.36; N, 7.78. Calcd for C40H48N6O14Sn2: C, 44.72; H, 4.50; N, 7.82%. IR (KBr pellets, cm–1): 3091 (w), 2957 (w), 2929 (w), 2859 (w), 1634 [s, νas(COO)], 1606 [s, ν(C=N)], 1568 (m), 1532 (s), 1489 (m), 1447 [s, νs(COO)], 1412 (m), 1350 (s), 1325 (s), 1289 (s), 1169 (m), 1081 (m), 809 (s), 706 (w), 627 (w). 1H NMR (DMSO-d6, δ ): 0.78 (t, J = 7.2 Hz, 12H, CH3), 1.23 (sex, J = 7.2 Hz, 8H, CH2), 1.35–1.38 (m, 8H, CH2), 1.43-1.48 (m, 8H, CH2), 7.83 (d, J = 6.0 Hz, 4H, Py-H-3), 8.61 (d, J = 0.4 Hz, 2H, Sal-H-6), 8.72 (d, J = 6.0 Hz, 4H, Py-H-2), 8.82 (d, J = 0.4 Hz, 2H, Sal-H-4). 13C NMR (DMSO-d6, δ ): 165.36 (COO), 163.81 (Sal-C-2), 151.00 (Py-C-2), 144.82 (Py-C-4), 143.35 (Sal-C-5), 134.00 (Sal-C-3), 131.47 (Sal-C-6), 124.14 (Sal-C-4), 123.09 (Py-C-3), 121.72 (Sal-C-1), 30.60 (CH2), 27.19 (CH2), 25.97 (CH2), 13.93 (CH3). 119Sn NMR (DMSO-d6, δ ): –261.6.
X-ray crystallography
A yellow crystal suitable for X-ray diffraction was obtained from the methanol solution. The intensity data were collected at 295(2) K on a Bruker Smart Apex Diffractometer with graphite monochromated Mo-Kα radiation (0.71073 Å). The structure was solved by direct methods using SHELXS-97 (Sheldrick, 2008) and refined by full-matrix least squares on F2 using the SHELXL2014 program (Sheldrick, 2015). All non-hydrogen atoms were refined anisotropically, and hydrogen atoms were placed in calculated positions using the riding model. The disordered atoms (nitro group and pyridine ring) were split over two sites with a total occupancy of 1. In the refinement, the PART, DFIX, SIMU, and DELU instructions were used. Crystallographic data, refinement parameters, and the CCDC number are summarized in Table 2.
Crystallographic and refinement data for 1.
| Empirical formula | C20H24N3O7Sn |
| Formula weight | 537.11 |
| Crystal system | monoclinic |
| Space group | P21/n |
| a /Å | 13.2883(7) |
| b /Å | 9.0104(5) |
| c /Å | 18.6482(11) |
| β /(°) | 94.4050(10) |
| Volume /Å3 | 2226.2(2) |
| Z | 4 |
| Dc / (g⋅cm–3) | 1.603 |
| μ / mm–1 | 1.193 |
| F(000) | 1084 |
| θ range /(°) | 1.8–26.0 |
| Crystal size / mm | 0.50 × 0.42 × 0.40 |
| Tot. reflections | 13832 |
| Uniq. reflections, Rint | 4372, 0.022 |
| GOF on F2 | 1.023 |
| R1 indices [I>2σ(I)] | 0.030 |
| wR2 indices (all data) | 0.086 |
| Δρmin, Δρmax/(e·Å–3) | –0.456, 0.527 |
| CCDC No. | 2022568 |
References
Banti C.N., Hadjikakou S.K., Sismanoglu T., Hadjiliadis N., Anti-proliferative and antitumor activity of organotin(IV) compounds. An overview of the last decade and future perspectives. J. Inorg. Biochem., 2019, 194, 114-152, DOI: 10.1016/j.jinorgbio.2019.02.003.10.1016/j.jinorgbio.2019.02.003Search in Google Scholar
Basu Baul T.S., Rynjah W., Rivarola E., Lycka A., Holcapek M., Jirasko R., et al., Synthesis and characterization of bis[dicarboxylatotetr aorganodistannoxane] units involving 5-[(E)-2-(aryl)-1-diazenyl]-2-hydroxybenzoic acids: An investigation of structures by X-ray diffraction, NMR, electrospray ionization MS and assessment of in vitro cytotoxicity. J. Organomet. Chem., 2006, 691, 4850-4862, DOI: 10.1016/j.jorganchem.2006.08.005.10.1016/j.jorganchem.2006.08.005Search in Google Scholar
Basu Baul T.S., Chaurasiya A., Duthie A., Vasquez-Rios M.G., Höpfl H., Solvent and intermolecular nitrogen coordination dictated formation of self-assembled organostannane-macrocycles based on monomers and coordination polymers with unsymmetrical 5-[(E)-2-(4-pyridyl)-1-diazenyl]-2-hydroxybenzoate ligand: Structural topologies and dimensionality. J. Organomet. Chem., 2018, 872, 87-101, DOI: 10.1016/j. jorganchem.2018.07.028.10.1016/j.jorganchem.2018.07.028Search in Google Scholar
Basu Baul T.S., Chaurasiya A., Duthie A., Montes-Tolentino P., Höpfl H., Coordination-driven self-assembly of macrocycles and 1D or 2D coordination polymers using heteroditopic pyridyl-carboxylate ligands: the case study of 5-[(E)-2-(3-pyridyl)-1-diazenyl]-2-hydroxybenzoate in combination with {RnSn} (n = 2 and 3). Cryst. Growth Des., 2019, 19, 6656-6671, DOI: 10.1021/acs. cgd.9b01045.10.1021/acs.cgd.9b01045Search in Google Scholar
Chandrasekhar V., Thirumoorthi R., Coordination polymers containing ferrocene backbone. Synthesis, structure and electrochemistry. Dalton Trans., 2010, 39, 2684-2691, DOI: 10.1039/b922044e.10.1039/b922044eSearch in Google Scholar
Chandrasekhar V., Mohapatra C., Metre R.K., Reactions of 5-(pyridin-4-yl-methyleneamino)isophthalic acid with triorganotin oxides and chloride. Formation of one-dimensional- and two-dimensional-coordination polymers. Cryst. Growth Des., 2013, 13, 4607-4614, DOI: 10.1021/cg401201w.10.1021/cg401201wSearch in Google Scholar
Chandrasekhar V., Nagendran S., Baskar V., Organotin assemblies containing Sn-O bonds. Coord. Chem. Rev., 2002, 235, 1-52. DOI: 10.1016/S0010-8545(02)00178-9.10.1016/S0010-8545(02)00178-9Search in Google Scholar
Chen L., Wang Z., Qiu T., Sun R., Zhao Z., Tian L., et al., Synthesis, structural characterization and properties of triorganotin complexes of Schiff base derived from 3-aminobenzoic acid and salicylaldehyde or 2,4-pentanedione. Appl. Organomet. Chem., 2020, 34, e5790, DOI: 10.1002/aoc.5790.10.1002/aoc.5790Search in Google Scholar
Deacon G.B., Phillips R.J., Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev., 1980, 33, 227-250. DOI: 10.1016/S0010-8545(00)80455-5.10.1016/S0010-8545(00)80455-5Search in Google Scholar
Holecek J., Nadvornik M., Handlir K., 13C and 119Sn NMR spectra of dibutyltin(IV) compounds. J. Organomet. Chem., 1986, 315, 299-308. DOI: 10.1016/0022-328X(86)80450-8.10.1016/0022-328X(86)80450-8Search in Google Scholar
Holecek J., Nadvorník M., Handlir K., Lycka A., 13C and 119Sn NMR Study of some four- and five-coordinate triphenyltin compounds. J. Organomet. Chem., 1983, 241, 177-184, DOI: 10.1016/S0022-328X(00)98505-X.10.1016/S0022-328X(00)98505-XSearch in Google Scholar
Khan A., Parveen S., Khalid A., Shafi S., Recent advancements in the anticancer potentials of phenylorganotin(IV) complexes. Inorg. Chim. Acta, 2020, 505, 119464, DOI: 10.1016/j.ica.2020.119464Search in Google Scholar
Kondo M., Yoshitomi T., Seki K., Matsuzaka H., Kitagawa S., Three-dimensional framework with channeling cavities for small molecules: {[M2(4,4’-bpy)3(NO34⋅xH2O}n (M = Co, Ni, Zn). Angew. Chem. Int. Ed., 1997, 36, 1725-1727, DOI: 10.1002/anie.199717251.10.1002/anie.199717251Search in Google Scholar
Kundu S., Mohapatra C., Chandrasekhar V., Cyclophosphazene– organostannoxane hybrid motifs in polymeric and molecular systems. RSC Adv., 2014, 4, 53662-53664, DOI: 10.1039/c4ra09371b.10.1039/c4ra09371bSearch in Google Scholar
Liu J., Lin Y., Liu M., Wang S., Li Y., Liu X., et al., Synthesis, structural characterization and cytotoxic activity of triorganotin 5-(salicylideneamino)salicylates. Appl. Organomet. Chem., 2019, 33, e4715, DOI: 10.1002/aoc.4715.10.1002/aoc.4715Search in Google Scholar
Ma C., Zhang J., Zhang R., Syntheses, characterizations, and crystal structures of organotin(IV) chloride complexes with 4,4’-bipyridine. Heteroatom Chem., 2004, 15, 338-346, DOI: 10.1002/hc.20016.10.1002/hc.20016Search in Google Scholar
Ma C., Zhang Q., Zhang R., Wang D., Self-assembly of dialkyltin moieties and mercaptobenzoic acid into macrocyclic complexes with hydrophobic “pseudo-cage” or doubl-cavity structures. Chem. Eur. J., 2006, 12, 420-428, DOI: 10.1002/chem.200500590.10.1002/chem.200500590Search in Google Scholar PubMed
Prabusankar G., Murugavel R., Hexameric organotincarboxylates with cyclic and drum structures. Organometallics, 2004, 23, 5644-5647, DOI: 10.1021/om049584u.10.1021/om049584uSearch in Google Scholar
Sheldrick G.M., A short history of SHELX. Acta Crystallogr., 2008, A64, 112-122, DOI: 10.1107/S0108767307043930.10.1107/S0108767307043930Search in Google Scholar PubMed
Sheldrick G.M., SHELXL-Integrated space-group and crystal-structure determination. Acta Crystallogr., 2015, A71, 3-8, DOI: 10.1107/S2053273314026370.10.1107/S2053273314026370Search in Google Scholar PubMed PubMed Central
Shi X., Wang H., Li Y., Yang J., Chen L., Hui G., et al., Spectroscopic study of Co(II), Ni(II), Zn(II) complexes with 4,4’-bipyridine. Chem. Res. Chin. Univ., 2010, 26, 1011-1015.Search in Google Scholar
Tian L.-J., Chen L.-X., An W.-G., Liu X.-C., Diorganotin complexes of N-[4-(diethylamino)salicylidene]-(L)-tryptophane: syntheses, structures and properties. Chin. J. Struct. Chem., 2019, 38, 1977-1985, DOI: 10.14102/j.cnki.0254-5861.2011-2378.10.14102/j.cnki.0254-5861.2011-2378Search in Google Scholar
Tian L., Li F., Zheng X., Sun Y., Yan D., Tu L., Synthesis, characterization and in vitro cytotoxicity of diorganotin complexes of 3,5-dinitrosalicylic acid. Synth. React. Inorg. M., 2011, 41, 454-458, DOI: 10.1080/15533174.2011.568426.10.1080/15533174.2011.568426Search in Google Scholar
Tian L., Wang R., Zhang J., Zhong F., Qiu Y., Synthesis and structural characterization of dialkyltin complexes of N-salicylidene-L-valine. Main Group Met. Chem., 2020, 43, 138-146, DOI: 10.1515/mgmc-2020-0017.10.1515/mgmc-2020-0017Search in Google Scholar
Zhang S., Wang W., Liu Q., Zheng X., Tian L., Synthesis, crystal structure and antibacterial activity of tricyclohexyltin salicylates. Main Group Met. Chem., 2016, 39, 87-92, DOI: 10.1515/mgmc-2016-0014.10.1515/mgmc-2016-0014Search in Google Scholar
Zang S.Q., Su Y., Li Y.Z., Ni Z.P., Meng Q.J., Assemblies of a new flexible multicarboxylate ligand and d10 metal centers toward the construction of homochiral helical coordinationpolymers: structures, luminescence, and NLO-active properties. Inorg. Chem., 2006, 45, 174-180, DOI: 10.1021/ic051502m.10.1021/ic051502mSearch in Google Scholar PubMed
© 2020 Tian et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- An accelerated and effective synthesis of zinc borate from zinc sulfate using sonochemistry
- A new approach on lithium-induced neurotoxicity using rat neuronal cortical culture: Involvement of oxidative stress and lysosomal/mitochondrial toxic Cross-Talk
- Green synthesis and characterization of hexaferrite strontium-perovskite strontium photocatalyst nanocomposites
- Assessment of content and chemical forms of arsenic, copper, lead, and chromium in sewage sludge compost as affected by various bulking agents
- Preparation of skeletally diverse quinazoline-2,4(1H,3H)-diones using Na2SiO3/SnFe2O4 catalytic system through a four-component reaction
- Efficient photocatalytic degradation of organic dye from aqueous solutions over zinc oxide incorporated nanocellulose under visible light irradiation
- Synthesis of pyrimidines by Fe3O4@SiO2-L-proline nanoparticles
- Abnormally aggregation-induced emissions observed from hydrogen- and silyl-substituted siloles
- Organodiphosphines in PtP2X2 (X = As, Ge or Te) derivatives – Structural aspects
- Synthesis and structural characterization of dialkyltin complexes of N-salicylidene-L-valine
- Ultrasound-promoted solvent-free synthesis of some new α-aminophosphonates as potential antioxidants
- Occupational exposure in lead and zinc mines induces oxidative stress in miners lymphocytes: Role of mitochondrial/lysosomal damage
- Eccentric topological properties of a graph associated to a finite dimensional vector space
- Magnetically recoverable nanostructured Pd complex of dendrimeric type ligand on the MCM-41: Preparation, characterization and catalytic activity in the Heck reaction
- Short Communications
- The crystal structure of the first ether solvate of hexaphenyldistannane [(Ph3Sn)2 • 2 THF]
- New crystal structures of alkali metal tetrakis(pentafluorophenyl)borates
- s-Block metal scorpionates – A new sodium hydrido-tris(3,5-dimethyl-1-pyrazolyl)borate salt showing an unusual core stabilized by bridging and terminal O-bonded DMSO ligands
- Reduction of a 1,4-diazabutadiene and 2,2’-bipyridine using magnesium(I) compounds
- fac-Bis(phenoxatellurine) tricarbonyl manganese(I) bromide
- A new 2D dibutyltin coordination polymer with 3,5-dinitrosalicylate and 4,4’-bipyridine ligands
- Review
- Structures of Pt(0)P3, Pt(0)P4 and Pt(II)P4 – Distortion isomers
- Special Issue: Topological descriptors of chemical networks: Theoretical studies (Guest Editors: Muhammad Imran and Muhammad Javaid)
- Modified Zagreb connection indices of the T-sum graphs
- Topological properties of metal-organic frameworks
- Eccentricity based topological indices of siloxane and POPAM dendrimers
- On topological aspects of degree based entropy for two carbon nanosheets
- On multiplicative degree based topological indices for planar octahedron networks
- Computing entire Zagreb indices of some dendrimer structures
Articles in the same Issue
- Research Articles
- An accelerated and effective synthesis of zinc borate from zinc sulfate using sonochemistry
- A new approach on lithium-induced neurotoxicity using rat neuronal cortical culture: Involvement of oxidative stress and lysosomal/mitochondrial toxic Cross-Talk
- Green synthesis and characterization of hexaferrite strontium-perovskite strontium photocatalyst nanocomposites
- Assessment of content and chemical forms of arsenic, copper, lead, and chromium in sewage sludge compost as affected by various bulking agents
- Preparation of skeletally diverse quinazoline-2,4(1H,3H)-diones using Na2SiO3/SnFe2O4 catalytic system through a four-component reaction
- Efficient photocatalytic degradation of organic dye from aqueous solutions over zinc oxide incorporated nanocellulose under visible light irradiation
- Synthesis of pyrimidines by Fe3O4@SiO2-L-proline nanoparticles
- Abnormally aggregation-induced emissions observed from hydrogen- and silyl-substituted siloles
- Organodiphosphines in PtP2X2 (X = As, Ge or Te) derivatives – Structural aspects
- Synthesis and structural characterization of dialkyltin complexes of N-salicylidene-L-valine
- Ultrasound-promoted solvent-free synthesis of some new α-aminophosphonates as potential antioxidants
- Occupational exposure in lead and zinc mines induces oxidative stress in miners lymphocytes: Role of mitochondrial/lysosomal damage
- Eccentric topological properties of a graph associated to a finite dimensional vector space
- Magnetically recoverable nanostructured Pd complex of dendrimeric type ligand on the MCM-41: Preparation, characterization and catalytic activity in the Heck reaction
- Short Communications
- The crystal structure of the first ether solvate of hexaphenyldistannane [(Ph3Sn)2 • 2 THF]
- New crystal structures of alkali metal tetrakis(pentafluorophenyl)borates
- s-Block metal scorpionates – A new sodium hydrido-tris(3,5-dimethyl-1-pyrazolyl)borate salt showing an unusual core stabilized by bridging and terminal O-bonded DMSO ligands
- Reduction of a 1,4-diazabutadiene and 2,2’-bipyridine using magnesium(I) compounds
- fac-Bis(phenoxatellurine) tricarbonyl manganese(I) bromide
- A new 2D dibutyltin coordination polymer with 3,5-dinitrosalicylate and 4,4’-bipyridine ligands
- Review
- Structures of Pt(0)P3, Pt(0)P4 and Pt(II)P4 – Distortion isomers
- Special Issue: Topological descriptors of chemical networks: Theoretical studies (Guest Editors: Muhammad Imran and Muhammad Javaid)
- Modified Zagreb connection indices of the T-sum graphs
- Topological properties of metal-organic frameworks
- Eccentricity based topological indices of siloxane and POPAM dendrimers
- On topological aspects of degree based entropy for two carbon nanosheets
- On multiplicative degree based topological indices for planar octahedron networks
- Computing entire Zagreb indices of some dendrimer structures

